Abstract
Myocardial infarction (MI) affects more than 8 million people in the United States alone. Due to the insufficient regeneration capacity of the native myocardium, one widely studied approach is cardiac tissue engineering, in which cells are delivered with or without biomaterials and/or regulatory factors to fully regenerate the cardiac functions. Specifically, in vitro cardiac tissue engineering focuses on using biomaterials as a reservoir for cells to attach, as well as a carrier of various regulatory factors such as growth factors and peptides, providing high cell retention and a proper microenvironment for cells to migrate, grow and differentiate within the scaffolds before implantation. Many studies have shown that the full establishment of a functional cardiac tissue in vitro requires synergistic actions between the seeded cells, the tissue culture condition, and the biochemical and biophysical environment provided by the biomaterials-based scaffolds. Proper electrical stimulation and mechanical stretch during the in vitro culture can induce the ordered orientation and differentiation of the seeded cells. On the other hand, the various scaffolds biochemical and biophysical properties such as polymer composition, ligand concentration, biodegradability, scaffold topography and mechanical properties can also have a significant effect on the cellular processes.
Keywords: Myocardial infarction, Cardiac tissue engineering, Cardiac differentiation, Biomaterials, Stem cell fate
Graphical abstract
Highlights
-
•
Cell therapy is an attractive approach for cardiac regeneration after myocardial infarction.
-
•
Biomaterials are used as cell carriers.
-
•
This review highlights how biochemical and biophysical properties of biomaterials affect cell fates.
1. Introductions
Cardiac diseases have become the leading cause of death throughout the world. There are around 2.5 million people die annually from severe cardiovascular diseases such as myocardial infarction (MI) and congestive heart failure (CHF) in the United States alone [1]. MI, commonly known as heart attack, is usually caused by the blood supply interruption due to a collection of lipids and/or white blood cells on the walls of the arteries. After MI, CHF may happen following inflammation, cardiomyocyte apoptosis, formation of the fibrous scars, and the stress burden increase of the surrounding myocardium tissues [2].
Myocardium functions can't fully be restored after MI or other serious cardiac diseases due to the insufficient regeneration capacity of cardiomyocytes. In normal state, cardiomyocytes in mammals or human beings can rarely divide. Even after an injury, the remaining cardiomyocytes have limited capacity to initiate the DNA synthesis and to re-enter the cell division cycles [3]. Thus one of the most crucial issues to cure cardiac diseases is to deliver proper types of cells to the infarcted locations and/or to induce the transplanted cells to differentiate into fully functional cardiomyocytes.
In order to reduce post-MI mortality, various surgical interventions such as mechanical circulatory devices and drugs have been developed in recent decades [4]. None of these methods, however, can fully restore the patients' cardiac functions [4]. Stem-cell therapy and cardiac tissue engineering have been thus studied in the goal of better maintaining myocardium function. The stem-cell therapy directly delivers cells into the infarcted myocardium. Cells that have been already utilized include induced pluripotent stem cells (iPSCs) [5], embryonic stem cells (ESCs) [6], mesenchymal stem cells (MSCs) [7], cardiosphere-derived cells (CDCs) [8], skeletal myoblasts [9] and cardiac stem cells (CSCs) [10]. Studies found that after the injection of bone marrow-derived cells, cardiomyocytes were found in the infarcted area of the heart and the cardiac function was partially improved [6], indicating that these cells may be capable of migrating to the infarcted locations and differentiating into cardiomyocytes or inducing the survived cardiomyocytes from other positions to the heart failure area [11]. Nevertheless, this method is still limited by the insufficient cell retention, survival, engraftment and differentiation rates. The leaking of cells during the delivery and apoptosis of the injected cells in the harsh ischemic environments have been suggested as possible reasons [12]. To better address these problems, cardiac tissue engineering could be an alternative strategy.
In cardiac tissue engineering, biomaterials and/or regulatory factors are combined together with the stem cells or cardiomyocytes to closely mimic the natural myocardium to improve the cell proliferation, migration and differentiation. Natural biomaterials including collagen [13], [14], [15], fibrin [12], matrigel [16], self-assembling peptide [17], decellularized extracellular matrix [18], [19], and synthetic polymers such as poly(lactide-co-glycolide) (PLGA) [20], polycaprolactone (PCL) [21], [22], poly(glycerol-sebacate) (PGS) [23], [24] and polyurethane (PU) [25], [26], [27] are now widely used in cardiac tissue engineering. These biomaterials should be biocompatible and biodegradable. Ideally, they should possess naturally occurring cardiac-tissue-like nanofibrous structures and anisotropic mechanical properties, providing an instructive microenvironment for the cells to attach, grow, migrate and differentiate. Some of these biomaterials can also be used to deliver protein, gene or RNAs together with the cells. Studies have discovered that by controlling the properties of the biomaterials such as the matrix stiffness [28], [29], [30], morphology [31], [32] or chemical properties [33], cardiac differentiation of the delivered stem cells can be significantly improved.
In this critical review, cardiac tissue engineering – especially the cell encapsulated scaffold-based cardiac tissue engineering– will be introduced and discussed. Biochemical and biophysical properties of the biomaterials that have been applied to induce stem cell fates, especially cell cardiac differentiation, as well as their advantages and limitations will be presented and discussed here.
2. Cell encapsulated scaffold-based cardiac tissue engineering
Cell encapsulated scaffold-based cardiac tissue engineering focuses on seeding cells on pre-formed 3D scaffolds and controlling the cell survival, proliferation and differentiation processes by precise adjustment of the various scaffold properties as well as the in vitro culture conditions before implanting in vivo. Among the various cell types used for cardiac tissue engineering, ESCs can differentiate into any cell type present in the heart, thus having the potential to fully regenerate the myocardium. However, there're two obstacles when applying ESCs to cardiac tissue engineering – immunological rejection response and ethic controversies [6]. In order to solve the ethic problem, iPSCs are introduced based on the fact that they can be obtained by treating differentiated cells such as fibroblasts with stemness-related genes without sacrificing an embryo [34], [35]. These reprogrammed cells gained pluripotency and have been found to be able to differentiate into functional cardiomyocytes. MSCs are proper candidate for cardiac therapy based on the fact that they are locally immunosuppressive [36] and can secrete paracrine growth factors for better myocardium regeneration at the same time [37]. On the other hand, CSCs can be isolated from human myocardium and expanded in vitro, suggesting the possibility of using autologous CSCs to minimum the immune rejections. CDCs can also be used autogeneically. They are isolated from the explant of exocardium biopsies [8] and have a quite fast proliferation rate. Other advantages of using CDCs include their high differentiation capability into cardiomyocytes both in vitro and in vivo compared with MSCs [8], [38].
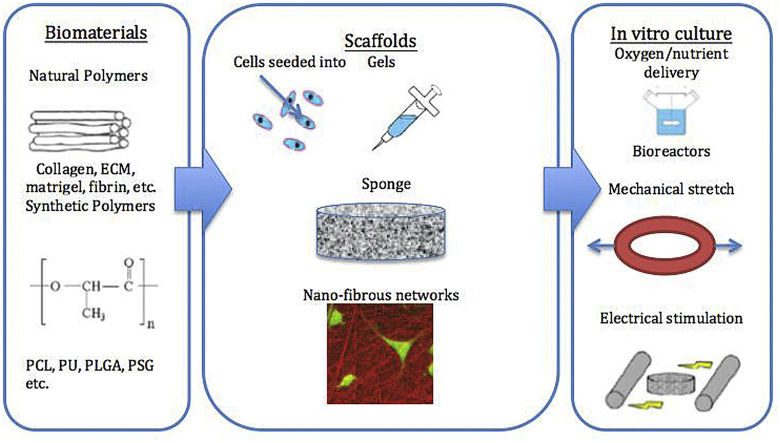
The basic requirement for the generated cardiac tissue constructs is to possess similar anisotropic mechanical properties – especially stiffness and flexibility – to natural myocardium, contain high densities of cells, and have enough thickness for clinical application (around 1.5 cm for human myocardium). Ideally, the implanted constructs should be able to integrate with the host tissues, deliver the electrical and mechanical signals within the matrix to the transplanted cells, pace synchronically with the surrounding tissues and promote angiogenesis for long-term improvement of the cardiac functions. The scaffold properties and the in vitro culture process both play important roles in the produced tissue constructs. Fig. 1 presents an overview of the cell encapsulated scaffold-based cardiac tissue engineering process.
Fig. 1.
An overview of cell encapsulated scaffold-based cardiac tissue engineering process: Cells are seeded into scaffolds made of natural-derived or synthetic polymers, and the tissue constructs are then cultured in vitro under specific conditions to develop into mature tissues before implantation in vivo.
2.1. Scaffold types
Scaffolds that have been applied in the field of cardiac tissue engineering can be divided into gel, foam and nanofibrous forms. The gel-form scaffolds can be easily bonded to the host tissues. Because of their injectability, they can be delivered by catheters to avoid large surgeries. The pre-formed foam and 3D nanofiber networks, on the other hand, can well mimic the structure of the natural myocardium extracellular matrix by precise control of the pore size, pore shape, fiber diameter and orientation.
Both natural-derived and synthetic gels can be used for cardiac regeneration. Collagen, for instance, as one of the main components in ECM of the myocardium as well as an essential structural support and mechanical property provider of the myocardium, has attracted significant attention as a matrix for cell delivery. While employing the collagen gels, cardiomyocytes or stem cells were seeded into the collagen solutions and tissue constructs were obtained by a simple gelation process [39], [40]. Studies showed that by mixing the collagen gel with rat neonatal cardiomyocytes, the contractility of the infarcted heart was gradually improved, and the maximum beating force was found 18 days after implantation [41]. The collagen gel was later further modified by matrigel, a basement membrane protein matrix, mixed with neonatal cardiomyocytes and cultured in vitro for 12 days before implanted into the infarcted hearts. Vascularization was observed within the tissue constructs 14 days after implantation. By four weeks, the heart wall thickness was dramatically increased and the myocardial dilation was significantly attenuated.
Foam is another widely applied scaffold form for cardiac tissue engineering. Large surface area and high porosity make them excellent structures for the cells to attach, migrate and communicate with each other. Furthermore, the interconnected pores also offer spaces for capillary growth. Foams can be prepared by biomaterial solution lyophilization [42], salt leaching [43] or microfabrication techniques to create complex geometries [44]. In order to induce the cell alignment, the foam scaffolds are usually designed with ordered channels or accordion-like honeycombs [44]. Interestingly, Engelmayr et al. found that the accordion-like scaffolds based on PGS possessed anisotropic mechanical properties closely matching the native heart tissues, with controllable elastic modulus by changing the polymer curing time. Moreover, better cell alignment and contractility inducible by electric field were also observed. One major limitation of the foam scaffolds is the difficulty to seed cells homogeneously within the 3-D scaffolds. Despite of the interconnected pores, cells usually have limited ability to migrate into the inner parts of the scaffolds due to the limited diffusion length of the nutrients and oxygen. In addition, a risk of residual chemicals may exist if harsh chemical conditions are required to produce the porous structures [45].
Nanofiber networks have also been utilized for cardiac tissue engineering because of their capacity of closely mimicking the nanoscale structure (collagen fibers within the myocardium are aligned nanofibers) and mechanical properties of natural myocardium. The highly ordered nanofibers can direct the cardiomyocytes alignment and contribute to anisotropic mechanical properties as desired. These scaffolds are usually fabricated by electrospinning, a drawing method through which micro or nanofibers are produced from polymer solutions with an electric field charging between an output needle and a collection area. By changing the polymer solution concentration and output speed, electric voltage or mandrel (collection part) velocity, one can control the many properties of the scaffolds including fiber diameter, density and alignment [46]. The nanofibrous networks have a large surface to volume ratio which can contribute to higher permeability, better cell migration and communication. However, it is always limited by the inhomogeneous distribution and limited culture depth of the seeded cells. Guan et al. carried out a method to produce 3D nanofibrous polyurethane scaffolds seeded with MSCs via electrospray/electrospinning approach [47]. Cells were successfully and homogeneously distributed within the full thickness of the scaffolds, and no significant difference in cell growth kinetics, cell morphology or MSC multipotency was found when comparing the electrosprayed MSCs with the non-electrosprayed ones. The fabricated scaffolds possessed a similar nanofibrous and anisotropic structure as well as stress-strain response to native porcine myocardium. Furthermore, 3-D alignment of the seeded cells within the scaffolds was obtained by stretching the constructs during the in vitro culture. Cardiac differentiation of MSCs was also upregulated, indicating the potential of aligned fibrous scaffolds for cardiac regeneration.
2.2. Biomaterial-properties guided cardiac differentiation of stem cells
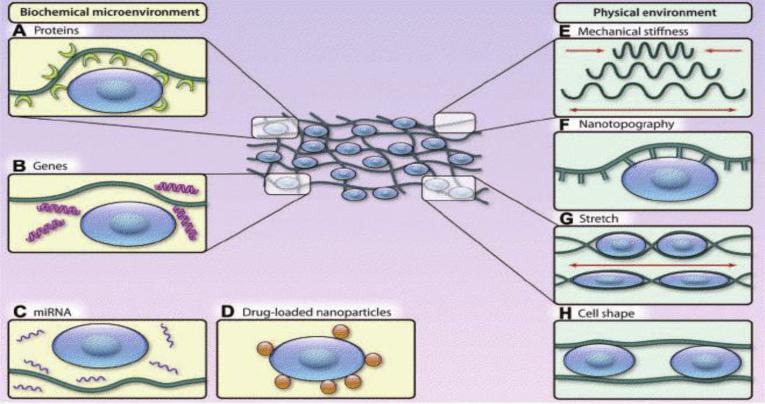
When using biomaterials to build up a native-like microenvironment for the seeded cells, both the chemical and physical properties of the biomaterial-based matrices/scaffolds are crucial for cell proliferation, differentiation and integration with the surviving myocardium tissues in damaged areas (Fig. 2). The physical features of scaffolds that could moderate stem cells fate include the various mechanical properties [28], [48] and scaffold morphology [49]. Additionally, the chemical properties of the applied biomaterials such as the ligand composition [50], special ligand concentration [51] and matrix biodegradability [52], [53] can also affect the cellular processes of the delivered stem cells.
Fig. 2.
Biochemical and biophysical microenvironment properties that affect the fate of transplanted stem cells [54].
2.2.1. Scaffold mechanical properties
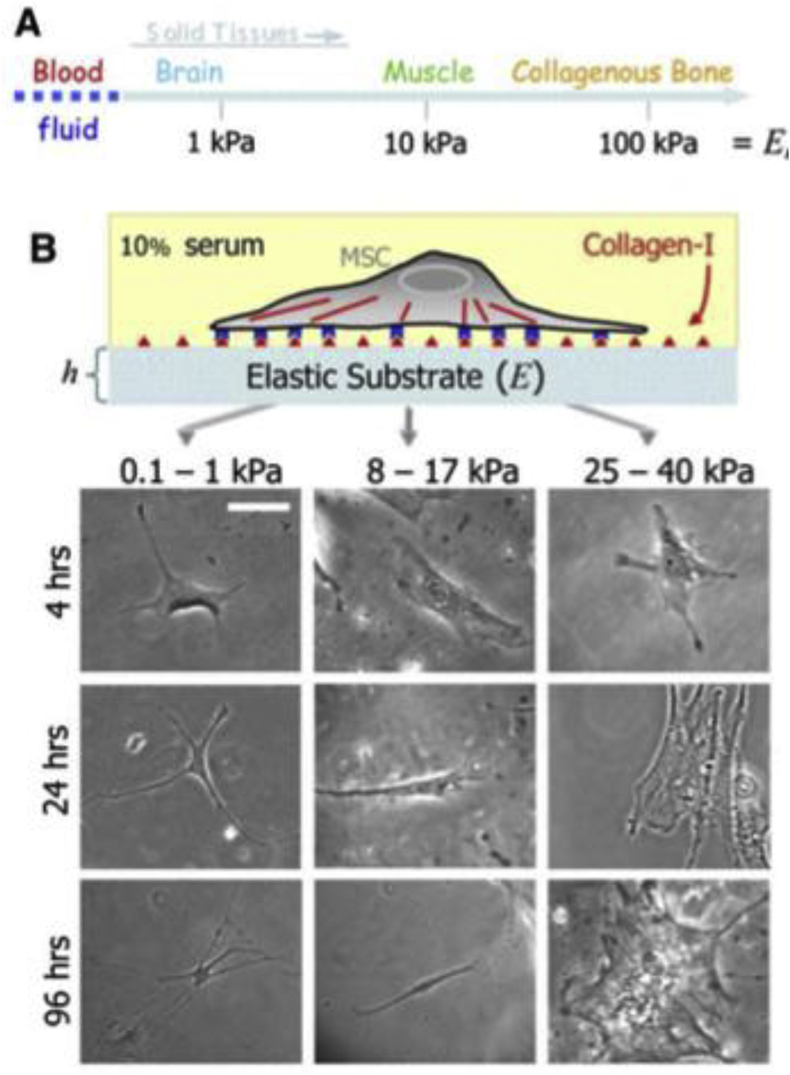
Decades ago, ECM was thought to be a passive component of the myocardium and only act as a support for the cells. However, studies have shown that the ECM patterns are changing in a specific manner during the embryonic development, and its component such as laminin, collagen or fibronectin are expressed differently in different stages [55]. Furthermore, the extracellular matrix mechanical properties were found to significantly affect the stem cell fates. Engler et al., for example, found that MSCs committed to the lineages specified by matrix elasticity instead of other factors after several weeks' culture in vitro. Soft matrices made of collagen-coated polyacrylamide that mimic brain's elasticity are neurogenic, stiffer gels with similar modulus as muscles are myogenic and relatively rigid matrices that mimic collagenous bones are osteogenic (Fig. 3) [28].
Fig. 3.
Matrix elasticity (A) and differentiation of seeded naive MSCs (B) [28].
Li et al. synthesized mechanically native-like thermosensitive hydrogels and successfully induced the cardiac differentiation of human mesenchymal stem cells (hMSCs) in vitro [56]. No significant difference in water content and oxygen permeability was found between these hydrogels with different moduli (16 kPa, 45 kPa, 65 kPa). All hMSCs survived during a 2-week in vitro culture period. Furthermore, 76% of hMSCs capsulated in hydrogel with higher modulus (65 kPa) expressed MYH6 and CTnI proteins, which are essential for cardiomyocytes to contract and relax, indicating the successful cardiac differentiation of MSCs into cardiomyocytes. This modulus-induced method possessed a much higher cardiac differentiation of hMSCs than traditional approaches such as co-culture hMSCs with cardiomyocytes [57] or using 5-azacytidine [58], demonstrating a feasibility of using matrix stiffness alone to induce stem cell cardiac differentiation. In Li's another study, the optimal hydrogel-collagen matrix modulus for CDCs cardiac differentiation was found to be 31 kPa [59]. As for ESCs, Kraehenbuehl et al. used PEG gels to induce their cardiac differentiation and found the optimal modulus was less than 1 kPa [60]. These distinguishing results may stem from the different natures of the cells and different chemical structures of the biomaterials.
On the other hand, Jacot et al. found that the elastic modulus of the Black Swiss mice epicardium underwent significant change from 12 ± 4 kPa as the embryonic value to 39 ± 7 kPa as the neonatal value, indicating that the modulus change might be a critical effect on the development of neonatal cardiomyocytes [61]. As a result, attempts have also been made to produce 3D matrix that could change its stiffness with time. For instance, Young et al. synthesized a hydrogel that stiffened from around 1 kPa–8 kPa over a period of 300 h by crosslinking thiolated-hyaluronic acid (HA) hydrogel with poly (ethylene glycol) diacrylate (PEGDA) [62]. This method was aimed to mimic the modulus change process of the native heart as the cells developed into mature adult cardiomyocytes. An increase of 3-fold in mature cardiac specific markers as well as around 60% more functional cardiac muscle fibers were observed for cells cultured within this dynamic gel compared with those in static polyacrylamide hydrogels, indicating the possibility of improving the cardiac regeneration by a precise control of the matrix stiffness over time.
Recently, except for the matrix stiffness effect, scientists found that other mechanical factors such as the material loss modulus [63], [64], relaxation rate [65] and the mechanical dosing [66] may also play an important role on stem cell fate control. Chaudhuri et al., for example, reported that faster MSCs spreading, proliferation and osteogenic differentiation were observed when they were cultured within 3D hydrogels with rapid relaxing rate (relaxing time of around 1 min), independently of the hydrogel's initial stiffness (17 kPa), degradation rate or available cell-adhesion ligands [65]. In another study, Yang et al. investigated the mechanical dosing effect on the stem cell fates using photo-tunable PEG hydrogels [66]. They found that an irreversible activation of YAP/TAZ and RUNX2 (pre-osteogenic transcription factor) happened when the cells were cultured on stiff hydrogel (E ∼10 kPa) long enough before transferred to soft hydrogel (E ∼2 kPa). These results highlighted not only the effect of scaffold mechanical properties on stem cell fate control, but also the profound influence from mechanical history/dynamics. Possible reasons of these effects include the local clustering of integrins, mediation of actomyosin contractility and nuclear localization of transcriptional factors. Researchers have found that certain signaling pathways such as Rho and Rac could be activated when the integrin clustering happened, which then contributed to the change of transcriptional factor activity and cell differentiation ultimately [65].
2.2.2. Scaffold morphology
Besides the influence of scaffold/matrix mechanical properties, morphology also plays a significant role in the myocardium regeneration process. In natural myocardium, cell may encounter various levels of topography from macro level such as the shape of blood vessels to micro level includes the projections and structure of other surrounding cells, and to nano level such as the collagen banding and protein conformation. All of these topographies have potential to influence cell behavior and functionalities. As mentioned above, pre-formed scaffolds such as porous foams or nanofibrous networks are widely used for cardiac tissue engineering. For porous foams, the interconnected pores provide cells with space to migrate and communicate as well as a supply of oxygen and nutrients, thus having a significant influence on the seeded cell distribution, density, migration and differentiation. For example, Wei et al. produced a porous genipin-based biological scaffold with a porosity of 91.2± 1.3% and a pore size of 130.5 ± 25.3 μm [67]. Shortly after inserting MSC multilayers into the scaffolds, the cells started to adhere to the scaffold to promote proliferation and migration. The tissue construct was then implanted in vivo using a rat myocardial infarction model after 7 days culture in vitro. The left ventricle cavity was beneficially reduced and the original pores were filled by cells with neo-connective tissue fibrils and neo-microvessles. Expressions of angiogenic cytokines (bFGF, vWF, and PDGF-B), cardiac protective factors (IGF-1 and HGF) and cardiac markers (Nkx2.5 and MEF2D) were also detected in transplanted MSCs.
As for the nanofibrous scaffolds, morphologies such as the fiber alignment and fiber diameter can also make a difference on stem cells fates. Ordered features such as parallel linear fibrous structures have been seen to be able to induce the cell actin cytoskeleton and the cell-matrix focal adhesion direction via the contact guidance phenomenon. Micro or nano fibers, on the other hand, are capable of regulating cell spreading, orientation, proliferation and differentiations, resulting from the fact that cells grown on them exhibit a higher cells aspect ratio and smaller projection than those grown on smooth substrates [49]. A good example is that Xu et al. successfully stimulated the cardiac differentiation of CDCs using an electrospun hydrogel/polyurethane fibrous scaffold as indicated in Fig. 4 [31]. The fabricated scaffolds reached fiber alignments above 45% and possessed anisotropic native-matched mechanical properties (macroscopic scaffold modulus between 50 kPa and 400 kPa). After 7 days' culture in vitro in spinner flasks, cells exhibited significant increase in cardiac differentiation at both gene and protein levels in constructs with relatively low scaffold modulus (50 kPa), low fiber alignment (45%) and high fiber volume fraction (85%). In another study, Sreerekha et al. developed a multiscale electrospun scaffold by electrospinning fibrin and poly(lactide-co-glycolide) (PLGA) simultaneously [68]. The fibrin nanofibers diameter ranged from 50 to 500 nm and the PLGA microfiber diameter was around 2–4 μm. Confocal images showed that this hierarchical structure allowed the cell migration into the interior parts of the scaffolds and promoted the uniform distribution of the seeded cells. Concurrently, cardiac specific proteins (α-sarcomeric actinin, troponin, tropomyosin, desmin, and ANP) were expressed in around 80% of the cells after 14 days' culture in vitro.
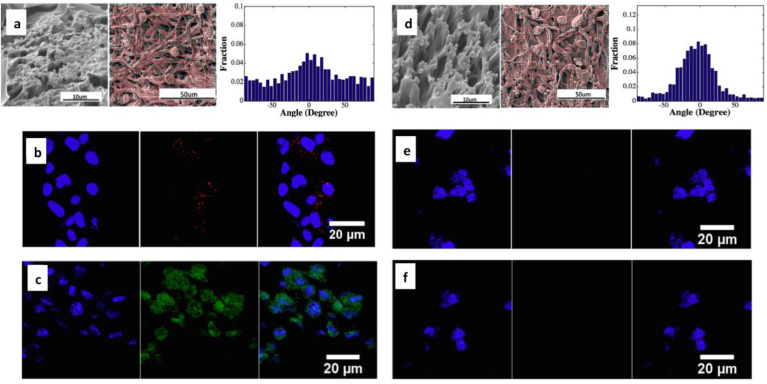
Fig. 4.
Different cell cardiac differentiation extents, as indicated by protein expressions of CX43 (b,e) and CTnI (c,f) were observed in CDCs seeded in scaffolds with different fiber densities, fiber orientations and mechanical properties (a, d) [31].
For other morphologies within the scaffolds, Tay et al. found that human MSCs could be controlled to differentiate into myocardial lineage by seeding them into fibronectin micropatterns on PLGA subtracts [69]. The micropatterns were made of 20 μm-wide stripes separated by 40 μm-wide grooves. Images showed that patterned cells exhibited elongated nuclei and cytoskeleton along the stripes, which suggested the possibility that cells may explore the topography cues and relay the information to nucleus to initiate a certain cellular process [70]. One explanation for the effect of topography on cells fate is that the various scaffold morphologies could influence the available surface area for protein adsorption, which restricts the ECM deposition and finally the number/shape of initial cell focal adhesion sites [70], and the change of cell focal adhesion sites could affect many cellular activities including cell attachment, migration, growth and differentiation via the mediation of signaling pathways.
2.2.3. Ligand composition and concentration
Cells receive much of the information in the form of biochemical cues from their local extracellular microenvironment. Protein signals, for instance, can bind to integrins, a transmembrane receptor that locates on the cell surface, and the associations between them can dictate the cell attachment, spread, proliferation and differentiate rates [71]. In cardiac tissue engineering, on top of the mechanical and structural support function, the chemical ligand of biomaterials make it possible for the scaffolds to serve as a delivery system of these biochemical regeneration cues to the infarcted myocardium. The strategies used to carry biomolecules include non-covalent immobilization (by electrostatic or hydrogen bonding), physical encapsulation and covalent bonding to the functional groups of the biomaterials. Growth factors such as hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF) [72], insulin-like growth factor 1 (IGF-1) [73], and transforming growth factor β (TGF-β) [55], [74] have already been found to have cardio-protective and cardiac differentiation effects, thus are capable of improving cardiac regeneration [75]. On the other hand, focal adhesions between cells and the extracellular matrix can be formed by bonding specific peptides such as the arginine-glycine-aspartic acid (RGD) [50], [76], [77] onto the biomaterials to promote cell-matrix interactions.
Padin-Iruegas et al. bonded biotinylated IGF-1, a cardiomyocyte division and differentiation factor, to the self-assembling peptide nanofibers and injected the mixture into infarcted rat myocardium together with cardiac progenitor cells (CPCs) [78]. In 24 h, CPCs with IGF-1 bonded nanofibers (NF-IGF-1) showed a higher cell proliferation rate and lower cell death than control groups (CPCs-only and NF-IGF-1 only). Additionally, CPCs in NF-IGF-1 group showed 32% and 230% higher differentiation into cardiomyocytes compared with CPCs-only and NF-IGF-1 only groups, respectively. A good example of applying the RGD-bonded biomaterial in cardiac tissue engineering was carried out by Tan's group [79]. RGD was coupled with self-assembling peptide RADA16 in order to enhance the marrow-derived cardiac stem cells (MCSCs) survival and differentiation. The scaffold underwent spontaneous assembling into well-ordered nanofibers with a diameter of around 10 nm. The results showed the RGD modified scaffold significantly decreased the MCSCs death in ischemic environment. 4 weeks after implantation, most of the cells in the RGD modified scaffolds expressed cardiac mature markers cTnI and CX-43, and integrated well with host tissue in aligned cell morphologies.
In addition to the ligand composition, the overall adhesion ligand concentration or bulk concentration is also of great importance. When seeding carcinoma stem cells on RGD-modified PEG-based hydrogel, for example, Kraehenbuehl et al. found that soft matrix with a modulus of 322 ± 64.2 Pa induced the initial cardiac muscle commitment [60]. However, further cardiac differentiation of the carcinoma stem cells was influenced by the linkage between integrin and RGD ligand. 6-fold increase of myosin heavy chain (MHC)-positive cells were obtained when the RGD was 100 mM as compared with cells in suspension. Other studies showed that the ligand bulk or spatial distribution is especially critical for protein delivery with chemotactic properties: a certain chemotactic gradient had to be developed to deliver these chemokines [80]. Fugetaxis, in which cells are repelled instead of attracted, may happen with a steep chemotactic gradient [81].
In addition to the ligand bulk concentration, the ligand line concentration, which can also be treated as local spacing, have been found to effect stem cell fate in other tissue engineering. Mooney et al., for examples, cultured MC3T3-E1 cells into RGD-modified alginate-based hydrogel with different RGD ligand spacing and successfully regulated osteoblast differentiation [51]. The proliferation rate of seeded cells was upregulated from 0.59 ± 0.08 to 0.73 ± 0.03 per day and the cell differentiation was dramatically promoted, as seen by a 4-fold increase of the secretion rate of the osteocalcin, a typical osteoblast differentiation marker in the late stage, with a ligand spacing from 78 nm to 36 nm. This study suggested the possibility of using proper ligand spacing based on certain biomaterials to induce cells cardiac differentiation for myocardium regeneration.
These experiments showed a great potential of the biomaterials, especially synthetic biomaterials used in cardiac tissue engineering, by taking use of their functional groups to bond biomolecules with specific properties to control over the cellular processes, especially the differentiation of transplanted cells within the scaffolds and finally develop a much more native-like and full functional tissue constructs for clinical use. One of the big challenges with this method lies in the fact that the biochemical cues in vivo are so complex that we are still quite far away from fully understanding it. Furthermore delivery of proteins to a receptor that is already activated by the endogenous proteins may be detrimental [82]. Finally, the factors should be targeted to specific cell types to avoid their possible toxic influence on other cells.
2.2.4. Material biodegradability
Optimally, the biomaterials used for cardiac tissue engineering should degrade in a controllable rate without causing toxicity. They should last long enough to guide the integration of transplanted cells with the host tissues, but not too long to inhibit the eventual cell–cell physiological coupling in the myocardium [83]. Traditional methods that have been applied to control the polymer degradation rate include varying cross-linking densities, molecular weight and combination of polymers with different degradation rates. While studying hMSCs in a covalently crosslinked hyaluronic acid (HA) hydrogel system that allows cell-mediated degradation, Khetan et al. found that cells exhibited high degree of spreading and exerted high tractions to the surrounding matrix, which contributed to a high cell differentiation [53]. Furthermore, after introducing non-degradable crosslinks into the matrix, a switch of hMSCs behavior and fate was observed through the block of cellular traction generation in a manner similar as direct pharamacological inhibition of myosin activities. These results indicated that stem cell fates within covalently crosslinked hydrogels may be dependent on the cell capability to generate tractions through the degradation of surrounding matrix to remodel focal adhesions and assemble cytoskeletal structures. As for the in vivo study, Xu et al. developed an biodegradable hydrogel based on thiolated collagen and multiple acrylate containing oligo (acryloyl carbonate)-b-poly(ethylene glycol)-b-oligo (acryloyl carbonate) copolymers and tested it in vivo using a rat infarction model [84]. Complete degradation of the hydrogel was discerned on day 3. In vivo results demonstrated that BMSC-encapsulated gels significantly reduced the infarct size, increased heart wall thickness and the ejection fraction 28 days after implantation. In another study, Fujimoto et al. developed a degradable hydrogel with elastic modulus of around 20 kPa and a degradation rate of 2 months in vivo for the treatment of MI [85]. The hydrogel was synthesized from N-isopropyl acrylamide (NIPAAm), acrylic acid (AAc) and hydroxyl ethyl methacrylate-poly(tremethylene carbonate) (HEMAPTMC). After 8 weeks, tissue ingrowth was observed in the injected hydrogel. In addition, left ventricular wall thickness and capillary density was dramatically increased in hydrogel group compared with the control group where PBS-only was injected. (see Fig. 5).
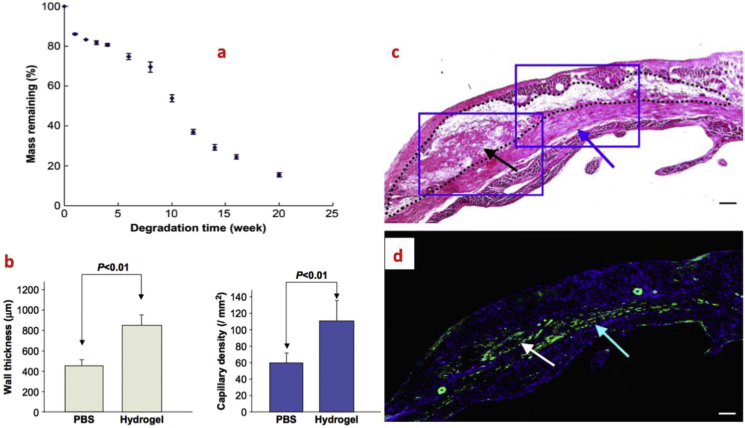
Fig. 5.
Use of biodegradable (a) hydrogels based on NIPAAm, AAc and HEMAPTMC for the treatment of MI. (b) Increased LV wall thickness and capillary density were observed 8 weeks after gel injection; (c) H&E staining; (d) Immunohistochemical staining (blue: nuclear, green: α-SMA). Both of the images indicated the infiltration of cells into the hydrogels (black dots are the injected hydrogel area) [85].
2.3. In vitro culture of scaffolds
As mentioned previously, the in vitro culture condition including the dynamic stretching, electrical stimulation and mass transfer is also an important aspect of tissue construct production. Bioreactors have been widely used to enhance the mass transfer between the tissue construct and its surrounding environment as well as to provide a controllable basic condition such as pH, temperature, oxygen and nutrient contents for the tissue constructs.
In static state, oxygen and nutrients can only diffuse to a thickness of around 100 μm within the constructs [43], which limits the size/thickness of non-vascularized tissue constructs. As a result, sophisticated bioreactors such as rotating, perfusion or spinner are applied to increase the mass transport. These flasks have been showed to enable sufficient oxygen and nutrient transports for cardiac tissues [86], [87]. However, the eddies generated by the turbulent flow in spinner flasks may be destructive for the seeded cells [88]. As for perfusion flasks, shear stresses are present during the perfusion process, which may lead to a final cell function difference [89]. Rotating flasks offer a dynamic environment with low shear stress and comparatively high mass transfer [90], but they cannot produce the necessary tensile stress that would occur in vivo [91]. Thus approaches such as placing oxygen carriers within the constructs [92], biomolecules modification [93] or pre-vascularization of the system [94] have been carried out to better supply the oxygen and nutrients for cells.
In native myocardium, the orderly pacing of electrical signals and macroscopic contraction are of great importance in the tissue organization and functionalization process. Radisic et al. found that by applying a 1 Hz, 5 V/cm, 2 ms monophasic square electrical wave, the cell alignment within the constructs was improved and the synchronic contraction was significantly increased by 7 times after 8 days' culture in vitro [95]. This study also suggested that the conductive and contractile properties of the tissue constructs might depend on the application time of the electrical initiation. If the electric stimulation is applied too early, the electric signals may inhibit cardiac protein accumulation and yield poor contractility. If applied too late, they may no longer have contribution to the functional assembly of the cells [96]. Studies have already found that the electric pulses can cause the hyperpolarization and depolarization of the cell ends, inducing cells to align according to the electrical field and to promote the formation of gap junction [97]. The increased gap junctions, at the same time, allow the cells to communicate with each other to transfer signals, which leads to a better synchronized contraction.
Considering the fact that the natural heart undergoes mechanical stretch while pumping blood, mechanical strains are also applied during the in vitro cultivation. Zimmerman et al. produced a neonatal rat cardiomyocyte-embedded tissue construct based on collagen/matrigel matrix and cultured the tissue constructs in vitro under a 110% uniaxial stretching at a frequency of 2 Hz [98]. Inter-connected and oriented cardiac muscle bundles were observed and the implanted tissue constructions showed contractile properties similar to natural myocardium. What's more, the engineered cardiac tissues showed non-delayed electrical coupling to the host tissues and also significantly prevented the chambers' further dilation, improving the cardiac functions after MI.
3. Conclusions and perspective
Cardiac tissue engineering has made huge strides in the past few decades to improve the potential methods available for patients with severe cardiac diseases compared with traditional strategies. Table 1 lists some of the experimental results in cardiac tissue engineering during the past few years. Despite of the fact that cell encapsulated scaffold-based cardiac tissue engineering has been successfully demonstrated to be able to improve the myocardium regeneration, and the various biophysical and biochemical cues mentioned above have been intensively studied these years to obtain a better control over the many cellular processes including the cell attachment, migration, division and differentiation, there are still some significant issues associated with this strategy. First, the tissue constructs cannot provide a native-like enough microenvironment for the development of a full functional cardiac tissue. Accordingly, biomaterials with matched mechanical properties (cardiac muscles are highly flexible and soft with elastic modulus around 1–140 kPa and fracture strain around 100% [71]) and similar fibrous structure with proper spatial and local micro and nano-topography distribution are in need.
Table 1.
Experimental results of some cardiac tissue engineering studies.
| Material type | Scaffold type | Biomolecules used | Cell type | Culture condition | Animal model | Improvement in cardiac function | Reference |
|---|---|---|---|---|---|---|---|
| Collagen | Gel | – | – | In vivo | Fischer rats | S.V. ↑, W. S. ↑, E.F. ↑ | [13] |
| Matrigel | Gel | – | Mouse ESCs | In vivo | Lewis rats | W.S. ↑, F.S. ↑, no LV dilation | [16] |
| RAD16-II peptide | Self-assembling nanofibers | IGF-1 | Mouse cardiomyocytes | In vivo | Sprague–Dawley rats | Activate Akt, C. D. ↑, increase myocyte cross-sectional area, decrease caspase-3 cleavage | [17] |
| Fibrin | Gel | – | Mouse myoblasts | In vivo | Sprague–Dawley rats | Angiogenesis ↑, decrease infarct scar size | [12] |
| PGS | Accordion-like honeycomb foam | – | Neonatal rat heart cells | Static in vitro culture with electric pulse | – | Native-like stiffness, electric field induced cell contractility, C.A. ↑ | [44] |
| PU | Nanofibers | – | Mouse MSCs | In vitro culture with constant stretch | – | Native-like structure and stress-strain response, C.A. ↑, C.D. ↑ | [100] |
| Collagen | Gel | – | Neonatal rats heart cells | In vitro culture with dynamic stretch before implantation | Wistar rats | Undelayed electrical coupling, prevent LV further dilution, W.S. ↑, F.S. ↑ | [98] |
| Collagen | Gel | RGD | Mouse cardiomyocytes | Static in vitro culture | – | High coupling yields, C.V. ↑, C.D. ↑, high cell contractility | [50] |
S.V. = stroke volume; W.S. = infarct wall thickness; E.F. = left ventricle ejection fraction; F.S. = fractional shortening; LV = left ventricle; C.D. = cardiac differentiation of transplanted cells; C.A. = cell alignment; C.V. = cell viability.
In addition, the transplanted cells within the scaffolds usually have limited engraft, growth and differentiation rates in the long term due to the ischemic environment in the infarcted myocardium, asking for the adequate angiogenesis within the scaffolds in vitro and/or vivo. In order to stimulate angiogenesis, endothelial cells, which are capable of forming blood vessels to provide oxygen and nutrients as well as exerting tropic and inotropic effects on cardiomyocytes [72], and various biomolecules such as stromal cell derived factor 1(SDF-1) [99] and vascular endothelial growth factor (VEGF) [6] were delivered by biomaterials. For instance, Segers et al. locally delivered protease-resistant SDF-1 by tethering it to self-assembling peptides and injected it into infarcted rat myocardium [73]. Capillary density was increased from 169 ± 42 to 283 ± 27 per mm2, and cardiac function, especially the ejection fraction, was increased significantly compared with control group in which native SDF-1 alone was delivered.
Finally, considering the large number of biomolecules that participate in the cardiac regeneration process, it is a big challenge to deliver all the signals at once. As a result, delivering key proteins at the right time and place is of great importance. Recently, various drug or proteins delivery or release systems including the pH-responsive release and oxidative stress-sensitive release were carried out based on the bioactivity and biodegradability of the applied biomaterials. A crucial aspect for the success of these systems will be the temporal orchestration as well as the direct correlations between the disease stages, release kinetics and therapeutic effects. With this in mind, a deep exploration of the stimuli change at different stages and precise design of biomaterials capable of detecting these changes with the corresponding sensing groups are essential.
The successful reestablishment of a favorable extracellular microenvironment for cardiac tissue regeneration asks for the synergistic actions between seeded cells, biomaterials-based scaffolds and multiple biomolecules such as growth factor and RGD peptide [75]. Despite of the problems listed above, significant progress on cell-seeded biomolecule-modified 3D scaffold application in cardiac tissue engineering has been witnessed during the past few decades. Additionally, researchers are gaining a better and deeper understanding of the effects of various physical and chemical cues such as scaffold matrix mechanical properties, micro or nano topography, biomaterial composition, ligand concentration and biodegradability on cell fates. Such an increasing understanding of the cells' response to their microenvironment provides an improved and encouraging insight for future studies into a more rational and proper design of the scaffolds to better regenerate the infarcted cardiac tissues.
Acknowledgments
This work was supported by National Science Foundation (1006734 and 1160122), National Institutes for Health (R01HL124122), American Heart Association (15GRNT25830058 and 13GRNT17150041), National Science Foundation of China (81471788), and Institute for Materials Research seed grant at The Ohio State University.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Rosamond W., Flegal K., Friday G., Furie K., Go A., Greenlund K. Heart disease and stroke statistics–2007 update: a report from the American heart association statistics committee and stroke statistics subcommittee. Circulation. 2007;115:e69–e171. doi: 10.1161/CIRCULATIONAHA.106.179918. [DOI] [PubMed] [Google Scholar]
- 2.Taylor D.A. Cell-based myocardial repair: how should we proceed? International J. Cardiol. 2004;95(Suppl. 1):S8–S12. doi: 10.1016/s0167-5273(04)90003-4. [DOI] [PubMed] [Google Scholar]
- 3.Segers V.F., Lee R.T. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 4.Li S.C., Wang L., Jiang H., Acevedo J., Chang A.C., Loudon W.G. Stem cell engineering for treatment of heart diseases: potentials and challenges. Cell Biol. Int. 2009;33:255–267. doi: 10.1016/j.cellbi.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 5.Nelson T.J., Martinez-Fernandez A., Yamada S., Perez-Terzic C., Ikeda Y., Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kraehenbuehl T.P., Ferreira L.S., Hayward A.M., Nahrendorf M., van der Vlies A.J., Vasile E. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials. 2011;32:1102–1109. doi: 10.1016/j.biomaterials.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amado L.C., Saliaris A.P., Schuleri K.H., St John M., Xie J.S., Cattaneo S. Cardiac repair with intramyocardial injection of allogeneic mesenchymal stem cells after myocardial infarction. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11474–11479. doi: 10.1073/pnas.0504388102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith R.R., Barile L., Cho H.C., Leppo M.K., Hare J.M., Messina E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 9.Murry C.E., Wiseman R.W., Schwartz S.M., Hauschka S.D. Skeletal myoblast transplantation for repair of myocardial necrosis. J. Clin. Invest. 1996;98:2512–2523. doi: 10.1172/JCI119070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey B., Izarra A., Alvarez R., Fischer K.M., Cottage C.T., Quijada P. Cardiac stem cell genetic engineering using the alphaMHC promoter. Regen. Med. 2009;4:823–833. doi: 10.2217/rme.09.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laflamme M.A., Murry C.E. Regenerating the heart. Nat. Biotechnol. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- 12.Christman K.L., Fok H.H., Sievers R.E., Fang Q., Lee R.J. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 13.Dai W., Wold L.E., Dow J.S., Kloner R.A. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J. Am. Coll. Cardiol. 2005;46:714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 14.Ahmadi A., Vulesevic B., Ruel M., Suuronen E.J. A collagen-chitosan injectable hydrogel improves vascularization and cardiac remodeling in a mouse model of chronic myocardial infarction. Can. J. Cardiol. 2013;29:S203–S204. [Google Scholar]
- 15.Elamparithi A., Punnoose A.M., Kuruvilla S. Electrospun type 1 collagen matrices preserving native ultrastructure using benign binary solvent for cardiac tissue engineering. Artif. Cells Nanomed. Biotechnol. 2015:1–8. doi: 10.3109/21691401.2015.1029629. [DOI] [PubMed] [Google Scholar]
- 16.Kofidis T., de Bruin J.L., Hoyt G., Lebl D.R., Tanaka M., Yamane T. Injectable bioartificial myocardial tissue for large-scale intramural cell transfer and functional recovery of injured heart muscle. J. Thorac. Cardiovasc. Surg. 2004;128:571–578. doi: 10.1016/j.jtcvs.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 17.Davis M.E., Hsieh P.C., Takahashi T., Song Q., Zhang S., Kamm R.D. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc. Natl. Acad. Sci. U. S. A. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Segers V.F., Lee R.T. Biomaterials to enhance stem cell function in the heart. Circ. Res. 2011;109:910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 19.Parker K.K., Ingber D.E. Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering. Philos. T R. Soc. B. 2007;362:1267–1279. doi: 10.1098/rstb.2007.2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu J.M., Wang X., Marin-Muller C., Wang H., Lin P.H., Yao Q. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009;9:325–341. doi: 10.1586/erm.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eschenhagen T., Didie M., Munzel F., Schubert P., Schneiderbanger K., Zimmermann W.H. 3D engineered heart tissue for replacement therapy. Basic research in cardiology. 2002;97(Suppl. 1):I146–I152. doi: 10.1007/s003950200043. [DOI] [PubMed] [Google Scholar]
- 22.Yeong W.Y., Sudarmadji N., Yu H.Y., Chua C.K., Leong K.F., Venkatraman S.S. Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 2010;6:2028–2034. doi: 10.1016/j.actbio.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 23.Radisic M., Park H., Martens T.P., Salazar-Lazaro J.E., Geng W.L., Wang Y.D. Pre-treatment of synthetic elastomeric scaffolds by cardiac fibroblasts improves engineered heart tissue. J. Biomed. Mater Res. A. 2008;86A:713–724. doi: 10.1002/jbm.a.31578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tallawi M., Zebrowski D.C., Rai R., Roether J.A., Schubert D.W., El Fray M. Poly(glycerol sebacate)/poly(butylene succinate-butylene dilinoleate) fibrous scaffolds for cardiac tissue engineering. Tissue Eng. Part C. Methods. 2015;21:585–596. doi: 10.1089/ten.tec.2014.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fujimoto K.L., Tobita K., Merryman W.D., Guan J., Momoi N., Stolz D.B. An elastic, biodegradable cardiac patch induces contractile smooth muscle and improves cardiac remodeling and function in subacute myocardial infarction. J. Am. Coll. Cardiol. 2007;49:2292–2300. doi: 10.1016/j.jacc.2007.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen P.H., Liao H.C., Hsu S.H., Chen R.S., Wu M.C., Yang Y.F. A novel polyurethane/cellulose fibrous scaffold for cardiac tissue engineering. Rsc Adv. 2015;5:6932–6939. [Google Scholar]
- 27.Baheiraei N., Yeganeh H., Ai J., Gharibi R., Ebrahimi-Barough S., Azami M. Preparation of a porous conductive scaffold from aniline pentamer-modified polyurethane/PCL blend for cardiac tissue engineering. J. Biomed. Mater. Res. A. 2015;103:3179–3187. doi: 10.1002/jbm.a.35447. [DOI] [PubMed] [Google Scholar]
- 28.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 29.Discher D.E., Sweeney L., Sen S., Engler A. Matrix elasticity directs stem cell lineage specification. Biophys. J. 2007:32a-a. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 30.Xu Y., Li Z., Li X., Fan Z., Liu Z., Xie X. Regulating myogenic differentiation of mesenchymal stem cells using thermosensitive hydrogels. Acta Biomater. 2015;26:23–33. doi: 10.1016/j.actbio.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Xu Y., Patnaik S., Guo X., Li Z., Lo W., Butler R. Cardiac differentiation of cardiosphere-derived cells in scaffolds mimicking morphology of the cardiac extracellular matrix. Acta Biomater. 2014;10:3449–3462. doi: 10.1016/j.actbio.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bashur C.A., Shaffer R.D., Dahlgren L.A., Guelcher S.A., Goldstein A.S. Effect of fiber diameter and alignment of electrospun polyurethane meshes on mesenchymal progenitor cells. Tissue Eng. Part A. 2009;15:2435–2445. doi: 10.1089/ten.tea.2008.0295. [DOI] [PubMed] [Google Scholar]
- 33.Lutolf M.P., Lauer-Fields J.L., Schmoekel H.G., Metters A.T., Weber F.E., Fields G.B. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: engineering cell-invasion characteristics. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5413–5418. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu J., Vodyanik M.A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J.L., Tian S. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Mangi A.A., Noiseux N., Kong D., He H., Rezvani M., Ingwall J.S. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat. Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 37.Gnecchi M., He H., Noiseux N., Liang O.D., Zhang L., Morello F. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20:661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 38.Johnston P.V., Sasano T., Mills K., Evers R., Lee S.T., Smith R.R. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation. 2009;120:1075–1083. doi: 10.1161/CIRCULATIONAHA.108.816058. 7 pp. following 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glowacki J., Mizuno S. Collagen scaffolds for tissue engineering. Biopolymers. 2008;89:338–344. doi: 10.1002/bip.20871. [DOI] [PubMed] [Google Scholar]
- 40.Eschenhagen T., Fink C., Remmers U., Scholz H., Wattchow J., Weil J. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. Faseb J. 1997;11:683–694. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 41.Zimmermann W.H., Schneiderbanger K., Schubert P., Didie M., Munzel F., Heubach J.F. Tissue engineering of a differentiated cardiac muscle construct. Circ. Res. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- 42.Shapiro L., Cohen S. Novel alginate sponges for cell culture and transplantation. Biomaterials. 1997;18:583–590. doi: 10.1016/s0142-9612(96)00181-0. [DOI] [PubMed] [Google Scholar]
- 43.Caspi O., Lesman A., Basevitch Y., Gepstein A., Arbel G., Habib I.H. Tissue engineering of vascularized cardiac muscle from human embryonic stem cells. Circ. Res. 2007;100:263–272. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 44.Engelmayr G.C., Jr., Cheng M., Bettinger C.J., Borenstein J.T., Langer R., Freed L.E. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat. Mater. 2008;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janik H., Marzec M. A review: fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C, Mater. Biol. Appl. 2015;48:586–591. doi: 10.1016/j.msec.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 46.Sill T.J., von Recum H.A. Electrospinning: applications in drug delivery and tissue engineering. Biomaterials. 2008;29:1989–2006. doi: 10.1016/j.biomaterials.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Guan J., Wang F., Li Z., Chen J., Guo X., Liao J. The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials. 2011;32:5568–5580. doi: 10.1016/j.biomaterials.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engler A.J., Griffin M.A., Sen S., Bonnemann C.G., Sweeney H.L., Discher D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166:877–887. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badami A.S., Kreke M.R., Thompson M.S., Riffle J.S., Goldstein A.S. Effect of fiber diameter on spreading, proliferation, and differentiation of osteoblastic cells on electrospun poly(lactic acid) substrates. Biomaterials. 2006;27:596–606. doi: 10.1016/j.biomaterials.2005.05.084. [DOI] [PubMed] [Google Scholar]
- 50.Schussler O., Coirault C., Louis-Tisserand M., Al-Chare W., Oliviero P., Menard C. Use of arginine-glycine-aspartic acid adhesion peptides coupled with a new collagen scaffold to engineer a myocardium-like tissue graft. Nat. Clin. Pract. Card. 2009;6:240–249. doi: 10.1038/ncpcardio1451. [DOI] [PubMed] [Google Scholar]
- 51.Lee K.Y., Alsberg E., Hsiong S., Comisar W., Linderman J., Ziff R. Nanoscale adhesion ligand organization regulates osteoblast proliferation and differentiation. Nano Lett. 2004;4:1501–1506. doi: 10.1021/nl0493592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qiu Y., Park K. Environment-sensitive hydrogels for drug delivery. Adv. Drug Deliv. Rev. 2001;53:321–339. doi: 10.1016/s0169-409x(01)00203-4. [DOI] [PubMed] [Google Scholar]
- 53.Khetan S., Guvendiren M., Legant W.R., Cohen D.M., Chen C.S., Burdick J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Segers V.F.M., Lee R.T. Biomaterials to enhance stem cell function in the heart. Circ. Res. 2011;109:910–922. doi: 10.1161/CIRCRESAHA.111.249052. [DOI] [PubMed] [Google Scholar]
- 55.Corda S., Samuel J.L., Rappaport L. Extracellular matrix and growth factors during heart growth. Heart Fail. Rev. 2000;5:119–130. doi: 10.1023/A:1009806403194. [DOI] [PubMed] [Google Scholar]
- 56.Li Z.Q., Guo X.L., Palmer A.F., Das H., Guan J.J. High-efficiency matrix modulus-induced cardiac differentiation of human mesenchymal stem cells inside a thermosensitive hydrogel. Acta Biomater. 2012;8:3586–3595. doi: 10.1016/j.actbio.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 57.Pijnappels D.A., Schalij M.J., Ramkisoensing A.A., van Tuyn J., de Vries A.A., van der Laarse A. Forced alignment of mesenchymal stem cells undergoing cardiomyogenic differentiation affects functional integration with cardiomyocyte cultures. Circ. Res. 2008;103:167–176. doi: 10.1161/CIRCRESAHA.108.176131. [DOI] [PubMed] [Google Scholar]
- 58.Balana B., Nicoletti C., Zahanich I., Graf E.M., Christ T., Boxberger S. 5-Azacytidine induces changes in electrophysiological properties of human mesenchymal stem cells. Cell Res. 2006;16:949–960. doi: 10.1038/sj.cr.7310116. [DOI] [PubMed] [Google Scholar]
- 59.Li Z., Guo X., Matsushita S., Guan J. Differentiation of cardiosphere-derived cells into a mature cardiac lineage using biodegradable poly(N-isopropylacrylamide) hydrogels. Biomaterials. 2011;32:3220–3232. doi: 10.1016/j.biomaterials.2011.01.050. [DOI] [PubMed] [Google Scholar]
- 60.Kraehenbuehl T.P., Zammaretti P., Van der Vlies A.J., Schoenmakers R.G., Lutolf M.P., Jaconi M.E. Three-dimensional extracellular matrix-directed cardioprogenitor differentiation: systematic modulation of a synthetic cell-responsive PEG-hydrogel. Biomaterials. 2008;29:2757–2766. doi: 10.1016/j.biomaterials.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 61.Jacot J.G., Martin J.C., Hunt D.L. Mechanobiology of cardiomyocyte development. J. Biomech. 2010;43:93–98. doi: 10.1016/j.jbiomech.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Young J.L., Engler A.J. Hydrogels with time-dependent material properties enhance cardiomyocyte differentiation in vitro. Biomaterials. 2011;32:1002–1009. doi: 10.1016/j.biomaterials.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cameron A.R., Frith J.E., Cooper-White J.J. The influence of substrate creep on mesenchymal stem cell behaviour and phenotype. Biomaterials. 2011;32:5979–5993. doi: 10.1016/j.biomaterials.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 64.Cameron A.R., Frith J.E., Gomez G.A., Yap A.S., Cooper-White J.J. The effect of time-dependent deformation of viscoelastic hydrogels on myogenic induction and Rac1 activity in mesenchymal stem cells. Biomaterials. 2014;35:1857–1868. doi: 10.1016/j.biomaterials.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 65.Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S.A., Weaver J.C. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat Mater. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang C., Tibbitt M.W., Basta L., Anseth K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wei H.J., Chen C.H., Lee W.Y., Chiu I., Hwang S.M., Lin W.W. Bioengineered cardiac patch constructed from multilayered mesenchymal stem cells for myocardial repair. Biomaterials. 2008;29:3547–3556. doi: 10.1016/j.biomaterials.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 68.Sreerekha P.R., Menon D., Nair S.V., Chennazhi K.P. Fabrication of electrospun poly (lactide-co-glycolide)-fibrin multiscale scaffold for myocardial regeneration in vitro. Tissue Eng. Part A. 2013;19:849–859. doi: 10.1089/ten.TEA.2012.0374. [DOI] [PubMed] [Google Scholar]
- 69.Tay C.Y., Yu H., Pal M., Leong W.S., Tan N.S., Ng K.W. Micropatterned matrix directs differentiation of human mesenchymal stem cells towards myocardial lineage. Exp. Cell Res. 2010;316:1159–1168. doi: 10.1016/j.yexcr.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 70.McNamara L.E., McMurray R.J., Biggs M.J., Kantawong F., Oreffo R.O., Dalby M.J. Nanotopographical control of stem cell differentiation. J. Tissue Eng. 2010;2010:120623. doi: 10.4061/2010/120623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thery M. Micropatterning as a tool to decipher cell morphogenesis and functions. J. Cell Sci. 2010;123:4201–4213. doi: 10.1242/jcs.075150. [DOI] [PubMed] [Google Scholar]
- 72.Wang Y., Haider H.K., Ahmad N., Xu M., Ge R., Ashraf M. Combining pharmacological mobilization with intramyocardial delivery of bone marrow cells over-expressing VEGF is more effective for cardiac repair. J. Mol. Cell. Cardiol. 2006;40:736–745. doi: 10.1016/j.yjmcc.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 73.Laflamme M.A., Chen K.Y., Naumova A.V., Muskheli V., Fugate J.A., Dupras S.K. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 74.Weist M.R., Wellington M.S., Bermudez J.E., Kostrominova T.Y., Mendias C.L., Arruda E.M. TGF-beta1 enhances contractility in engineered skeletal muscle. J. Tissue Eng. Regen. Med. 2013;7:562–571. doi: 10.1002/term.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hausenloy D.J., Yellon D.M. Cardioprotective growth factors. Cardiovasc Res. 2009;83:179–194. doi: 10.1093/cvr/cvp062. [DOI] [PubMed] [Google Scholar]
- 76.Choi W.S., Bae J.W., Lim H.R., Joung Y.K., Park J.C., Kwon I.K. RGD peptide-immobilized electrospun matrix of polyurethane for enhanced endothelial cell affinity. Biomed. Mater. 2008;3 doi: 10.1088/1748-6041/3/4/044104. 044104. [DOI] [PubMed] [Google Scholar]
- 77.Kidane A.G., Punshon G., Salacinski H.J., Ramesh B., Dooley A., Olbrich M. Incorporation of a lauric acid-conjugated GRGDS peptide directly into the matrix of a poly(carbonate-urea)urethane polymer for use in cardiovascular bypass graft applications. J. Biomed. Mater. Res. A. 2006;79:606–617. doi: 10.1002/jbm.a.30817. [DOI] [PubMed] [Google Scholar]
- 78.Padin-Iruegas M.E., Misao Y., Davis M.E., Segers V.F., Esposito G., Tokunou T. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation. 2009;120:876–887. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guo H.D., Cui G.H., Wang H.J., Tan Y.Z. Transplantation of marrow-derived cardiac stem cells carried in designer self-assembling peptide nanofibers improves cardiac function after myocardial infarction. Biochem. Bioph. Res. Co. 2010;399:42–48. doi: 10.1016/j.bbrc.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 80.Segers V.F., Lee R.T. Local delivery of proteins and the use of self-assembling peptides. Drug Discov. Today. 2007;12:561–568. doi: 10.1016/j.drudis.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Vianello F., Olszak I.T., Poznansky M.C. Fugetaxis: active movement of leukocytes away from a chemokinetic agent. J. Mol. Med. 2005;83:752–763. doi: 10.1007/s00109-005-0675-z. [DOI] [PubMed] [Google Scholar]
- 82.Banfi A., von Degenfeld G., Blau H.M. Critical role of microenvironmental factors in angiogenesis. Curr. Atheroscler. Rep. 2005;7:227–234. doi: 10.1007/s11883-005-0011-7. [DOI] [PubMed] [Google Scholar]
- 83.Davis M.E., Hsieh P.C., Grodzinsky A.J., Lee R.T. Custom design of the cardiac microenvironment with biomaterials. Circ. Res. 2005;97:8–15. doi: 10.1161/01.RES.0000173376.39447.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xu G., Wang X., Deng C., Teng X., Suuronen E.J., Shen Z. Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)-poly(ethylene glycol)-oligo(acryloyl carbonate) copolymer for functional cardiac regeneration. Acta Biomater. 2015;15:55–64. doi: 10.1016/j.actbio.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 85.Fujimoto K.L., Ma Z., Nelson D.M., Hashizume R., Guan J., Tobita K. Synthesis, characterization and therapeutic efficacy of a biodegradable, thermoresponsive hydrogel designed for application in chronic infarcted myocardium. Biomaterials. 2009;30:4357–4368. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carrier R.L., Rupnick M., Langer R., Schoen F.J., Freed L.E., Vunjak-Novakovic G. Perfusion improves tissue architecture of engineered cardiac muscle. Tissue Eng. 2002;8:175–188. doi: 10.1089/107632702753724950. [DOI] [PubMed] [Google Scholar]
- 87.Gerecht-Nir S., Radisic M., Park H., Cannizzaro C., Boublik J., Langer R. Biophysical regulation during cardiac development and application to tissue engineering. Int. J. Dev. Biol. 2006;50:233–243. doi: 10.1387/ijdb.052041sg. [DOI] [PubMed] [Google Scholar]
- 88.Papadaki M., Bursac N., Langer R., Merok J., Vunjak-Novakovic G., Freed L.E. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H168–H178. doi: 10.1152/ajpheart.2001.280.1.H168. [DOI] [PubMed] [Google Scholar]
- 89.Radisic M., Yang L., Boublik J., Cohen R.J., Langer R., Freed L.E. Medium perfusion enables engineering of compact and contractile cardiac tissue. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H507–H516. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- 90.Martin I., Wendt D., Heberer M. The role of bioreactors in tissue engineering. Trends Biotechnol. 2004;22:80–86. doi: 10.1016/j.tibtech.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 91.Bilodeau K., Mantovani D. Bioreactors for tissue engineering: focus on mechanical constraints. A comparative review. Tissue Eng. 2006;12:2367–2383. doi: 10.1089/ten.2006.12.2367. [DOI] [PubMed] [Google Scholar]
- 92.Radisic M., Park H., Chen F., Salazar-Lazzaro J.E., Wang Y., Dennis R. Biomimetic approach to cardiac tissue engineering: oxygen carriers and channeled scaffolds. Tissue Eng. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- 93.Li Z., Guo X., Guan J. A thermosensitive hydrogel capable of releasing bFGF for enhanced differentiation of mesenchymal stem cell into cardiomyocyte-like cells under ischemic conditions. Biomacromolecules. 2012;13:1956–1964. doi: 10.1021/bm300574j. [DOI] [PubMed] [Google Scholar]
- 94.Birla R.K., Borschel G.H., Dennis R.G., Brown D.L. Myocardial engineering in vivo: formation and characterization of contractile, vascularized three-dimensional cardiac tissue. Tissue Eng. 2005;11:803–813. doi: 10.1089/ten.2005.11.803. [DOI] [PubMed] [Google Scholar]
- 95.Radisic M., Park H., Shing H., Consi T., Schoen F.J., Langer R. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proc. Natl. Acad. Sci. U. S. A. 2004;101:18129–18134. doi: 10.1073/pnas.0407817101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tandon N., Cannizzaro C., Chao P.H., Maidhof R., Marsano A., Au H.T. Electrical stimulation systems for cardiac tissue engineering. Nat. Protoc. 2009;4:155–173. doi: 10.1038/nprot.2008.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wikswo J.P., Jr., Lin S.F., Abbas R.A. Virtual electrodes in cardiac tissue: a common mechanism for anodal and cathodal stimulation. Biophys. J. 1995;69:2195–2210. doi: 10.1016/S0006-3495(95)80115-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zimmermann W.H., Melnychenko I., Wasmeier G., Didie M., Naito H., Nixdorff U. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 99.Kondo K., Shintani S., Shibata R., Murakami H., Murakami R., Imaizumi M. Implantation of adipose-derived regenerative cells enhances ischemia-induced angiogenesis. Arter. Throm. Vas. 2009;29:61–U167. doi: 10.1161/ATVBAHA.108.166496. [DOI] [PubMed] [Google Scholar]
- 100.Guan J.J., Wang F., Li Z.Q., Chen J., Guo X.L., Liao J. The stimulation of the cardiac differentiation of mesenchymal stem cells in tissue constructs that mimic myocardium structure and biomechanics. Biomaterials. 2011;32:5568–5580. doi: 10.1016/j.biomaterials.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]