Abstract
Biodegradable orthopedic materials (BOMs) are used in rehabilitation and reconstruction of fractured tissues. The response of BOMs to the combined action of physiological stress and corrosion is an important issue in vivo since stress-assisted degradation and cracking are common. Although the degradation behavior and kinetics of BOMs have been investigated under static conditions, stress effects can be very serious and even fatal in the dynamic physiological environment. Since stress is unavoidable in biomedical applications of BOMs, recent work has focused on the evaluation and prediction of the properties of BOMs under stress in corrosive media. This article reviews recent progress in this important area focusing on biodegradable metals, polymers, and ceramics.
Keywords: Biodegradable orthopedic materials, External stress, Stress mode, Degradation rate, Mechanochemistry
Graphical abstract
Highlights
-
•
The response of biodegradable orthopedic materials to the combined action of physiological stress and corrosion was reviewed.
-
•
Physiological function stress to bone formation was reported.
-
•
Factors influencing the effects of stress and corrosion on biodegradable metals were discussed.
-
•
The response of biodegradable polymers to different stress mode was reported.
-
•
Degradation prediction of biodegradable biopolymers under stress was mentioned.
1. Introduction
Orthopedic biomaterials are commonly used in rehabilitation and reconstructing the mobility of millions of patients [1], [2] and recently, biodegradable materials have attracted much interest in orthopedics due to their degradability [3], [4], [5]. Biodegradable orthopedic materials (BOMs) include metals such as magnesium (Mg) alloys [6], polymers [7], ceramics [8], and composites. The mechanical properties of bone fixation implants must be adequate and match those of bone or tissues, otherwise early implant failure, secondary fracture, and other deleterious effects such as inflammation may occur.
Although the properties of BOMs is generally related to the microstructure and alloying elements [6], [9], [10], [11], [12], [13], [14], [15], the external physiological environment, especially stress and corrosive media, affects the behaviors as well. In vitro and clinical investigations have revealed the combined effects of stress and corrosion in early implant failure [16], [17], [18], [19]. For example, nearly 90% of the surface fracture on Ti-6Al-4V cementless hip prosthesis is caused by the combined effects of dynamic cyclic stress and corrosive media [20]. Tissue healing is sensitive to the implant properties which can be altered by the external environment and so it is important to study and understand the performance of BOMs under stress and in a corrosive medium. In this paper, recent progress in this area is reviewed in order to provide insights into the role of external stress in the degradation of BOMs and design of new orthopedic biomaterials.
2. Physiological stress
In order to understand the influence of external stress on BOMs, the physiological load modes are first described. Physiological stress in vivo varies with the activities, bone dimensions, and locations [21], [22], [23], [24] and multiple types of load may affect the activity [25], [26], [27], [28]. Table 1 shows the physiological load modes and magnitude for different activities. During normal walking, the peak axial compression force at the femur is about 1.12 body weight (BW) and the maximum bending moment is about 5 BW·cm [25]. Different walking speeds lead to different peak values. For example, the peak axial force when jogging is about 1.29 times that of normal walking [26]. The strain of bones under different activities is about 400 × 10−6 ∼ 2000 × 10−6 [22], suggesting the stress would be 0.8 Mpa–40 MPa for bones (considering the elastic modulus of the bone is 20 GPa). Furthermore, the load modes are different for different activities and bone types [27], [28]. Loadings are dynamic and the frequencies are different. For example, the frequency is 1–3 Hz during normal walking and goes up to 9–20 Hz during running [29].
Table 1.
Physiological load mode and magnitude of bones for different activities.
| Authors | Bones | Activities | Peak values of loads | |
|---|---|---|---|---|
| Duda et al. [25] | Femur | Walking | Axial force: ∼1.12 BW Bending moment: ∼5 BW·cm |
|
| Taylor et al. [26] | Femur | Jogging | Axial force: ∼3.6 BW | Bending moment: 8.5–9.8 BW·cm (antero-posterior axis) 4.7–7.6 BW·cm (medio-lateral axis) Axial torque: 0.2–1.3 BW·cm |
| Stair descending | Axial force: ∼3.1 BW | |||
| Walking | Axial force: ∼2.8 BW | |||
| Treadmill walking | Axial force: ∼2.75 BW | |||
| Stair ascending | Axial force:∼2.8 BW | |||
| Taylor et al. [27] | Femur | Walking (0.99–1.51 m/s) | Axial force:∼2.5 BW Shear force: 0.4–0.54 BW Axial torque:7 N m |
|
| Ascending stairs | Axial Force: ∼2.5 BW Axial torque:6.2 N m |
|||
| Descending stairs | Axial Force: ∼2.81 BW Axial torque:7.3 N m |
|||
| Rising from the chair | Axial Force: 2.09 BW Axial torque:7.9 N m |
|||
| Wehner et al. [28] | Tibia | Gait | Axial force: ∼4.7 BW Bending moment in the sagittal plane: ∼7.16 BW·cm |
|
| Gruber et al. [29] | Tibia | Rearfoot running | Impact shocking frequency: 9–20 Hz | |
| Forefoot running | Impact shocking frequency: 3–8 Hz | |||
The effects of physiological stress on bone formations have been studied [30], [31], [32], [33]. Generally, it is believed that dynamic stress can promote the formation and growth of bones, whereas static stress does not impose such effects. In fact, the study by Robling et al. [34] suggests that static loads suppress normal bone growth and the effects are different from those arising from dynamic stress. In another study [35], loads are applied on a porous coated implant based on the turkey ulna model and the effects of different dynamic loads on the bone ingrowth are studied. The results reveal that principal tensile or compressive strain is more important to bone adaptation, whereas shear strain has little effects. In this respect, dynamic compression, tension, and bending benefit bone healing. Thus, the behaviors of the implant materials under the stress condition, especially the dynamic stress condition, are significant for the implants.
3. Response of biodegradable metals to external stress
Biodegradable metals especially Mg alloys are considered next-generation metallic biomaterials [36]. As orthopedic biomaterials, Mg alloys have the following advantages [4], [37], [38]:
-
(i)
Mg alloys have a similar density and elastic modulus as human bones compared to conventional biometals such as stainless steel and Ti alloys.
-
(ii)
Mg is essential to human metabolism being a cofactor for many enzymes.
-
(iii)
The standard electrode potential of Mg at 25 °C is −2.37 Vnhe and Mg and Mg alloys can naturally degrade in vivo.
However, Mg alloys tend to degrade too rapidly in the physiological environment, especially under physiological stress [39], [40] and hence, it is critical to understand the degradation behavior of Mg alloys under various types of mechanical stress as well as in different corrosive media.
3.1. Stress-assisted degradation (SAD)
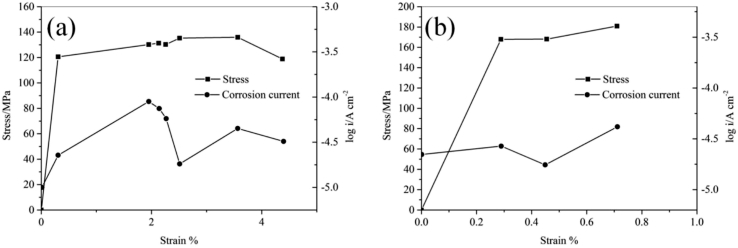
Many studies suggest stress would influence the degradation rate of magnesium alloys [41], [42], and this effect is denoted as the stress-assisted degradation (SAD) in this paper. It is reported that under cyclic loading, the corrosion rate of the die-cast AZ91D alloy in simulated body fluid (SBF) would be 7–8 times that in static SBF, while the corrosion rate of the extruded WE43 alloy is 4–12 times that in static SBF [43]. Studies based on electrochemical results [44], [45] suggest SAD is related to the magnitude of the applied stress. When the stress is below the yield strength of the Mg alloys, the degradation rate goes up with increasing applied stress. When the stress increases to above the yield strength, the anodic current density of the Mg alloys varies at dynamic recovery [44] as a function of the level of the plastic deformation [45] as shown in Fig. 1.
Fig. 1.
Anodic currents in the active state and stress as a function of strain [45]: (a) AM50 and (b) AZ91D.
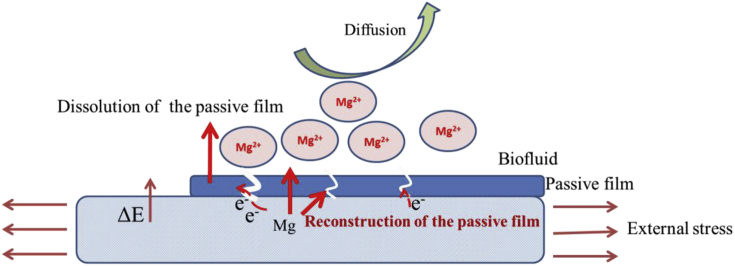
Generally, the increased degradation rates due to SAD can be explained depicted as follows and illustrated in Fig. 2:
-
(i)
Mechanochemistry - The stress applied to Mg alloys increases the internal surface energy and area and decreases the coherence energy of the solid [46]. Consequently, the activation energy of degradation increases thereby leading to accelerated degradation.
-
(ii)
Film cracking - During degradation, a hydroxide layer forms on the surface. However, it is unstable and external stress promotes initiation and propagation of microcracks in the layer [47] to exposing the Mg alloy substrate locally. The potential difference between the cathodic film and exposed Mg alloy substrate forms an electrochemical cell resulting in rapid degradation [48].
Fig. 2.
Schematic illustrating the mechanism of the stress-assisted degradation of Mg alloys [46], [47], [48].
3.2. Stress corrosion cracks (SCC)
Stress corrosion cracks (SCC) are particularly dangerous and complicated for Mg alloys. In particular, the magnesium alloy implant becomes embrittlement when subjected to external stress in the physiological environment. This sudden failure poses serious consequences ranging from tissue inflammation to removal of the failed implant [49]. In vitro results suggest that Mg alloys are more susceptible to SCC in the simulated physiological environment than air [50], [51], [52]. For example, aluminum-free magnesium alloys show obvious reduction in the ultimate tensile strength and elongation to failure in SBF compared to experiments conducted in air [52]. The SCC is considered to be related with the corrosion pitting. During immersion, the pitting depth increases under tensile stress and when a pit reaches a critical size, SCC is initiated due to the localized stress concentration SCC [49], [51]. Under a constant load, the stress increases with degradation and crack propagation, whereas in the constant displacement tests, the stress decreases due to the creep. In this respect, Mg alloys may be more susceptible to SCC under a constant load than constant displacement [53].
Moreover, the films formed during the degradation would influence the SCC. The study [54] about the SCC susceptibility of sand-cast AZ91 magnesium alloy in m-SBF shows SCC susceptibility is not substantial during the immersion. It is believed that this would be attributed to the hydroxyapatite film which shows faster regrowth than cracks propagate.
Hydrogen release and accumulation during Mg degradation may be detrimental to biomedical applications and in addition to the local basicity, the hydrogen bubbles may be trapped between the implant and tissues [3]. In fact, it has been demonstrated that hydrogen deteriorates the performance of Mg alloys by enhancing SCC [55], [56]. During degradation, hydrogen generated at the tip of a propagating crack reduces the cohesive strength of the magnesium alloy causing hydrogen embrittlement [55], [56].
3.3. Corrosion fatigue (CF)
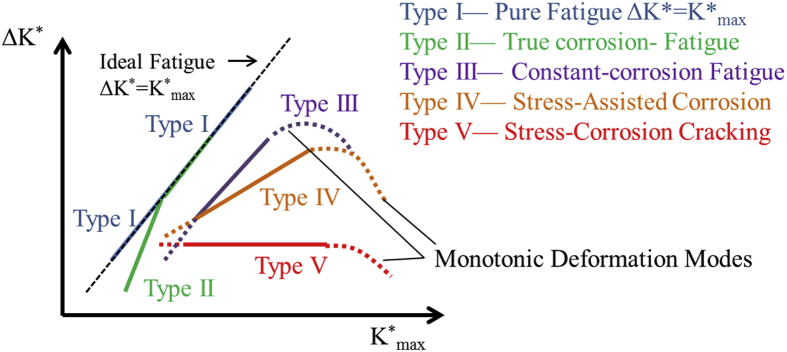
Fatigue fracture is common in engineering metals subjected to cyclic loading and in a corrosive environment, the fatigue strength of the metal is reduced. There are five fatigue crack growth modes as shown in Fig. 3 [57]. It could be seen that under the combined effects of stress and corrosive media, fatigue cracks propagate faster. Corrosion fatigue (CF) is of primary concern for metallic implants which commonly bear cyclic dynamic loads in vivo. The initial fatigue crack frequently occurs at the stress concentration area and manufacturing defects in the metallic devices such as holes, pits, and notches [39], [49]. Inclusions and corrosion pits may also be the crack initiation sites [43], [58], [59]. Gu et al. [43] have performed corrosion fatigue tests on AZ91D and WE43 in air and simulated body fluid (SBF). The fatigue strength shows significant reduction in SBF and fatigue cracks are initiated from the micropores in air and from corrosion pits in SBF, respectively. The corrosion pit propagation rate is influenced by the amount of stress, frequency, and cycle number [58], [59], [60], [61]. Based on the assumption that fatigue cracks are initiated at the corrosion pit, the pit growth rate can be determined by the following formula [62]:
| (1) |
where D is the pit depth, A is a constant calculated from the experiment, σ is the stress magnitude, N is the cycle number, and f is the frequency. A larger stress, smaller frequency, and larger cycle number increase the pit depth and therefore, it is important to conduct corrosion fatigue tests of biodegradable metals under actual dynamic cyclic loading conditions.
Fig. 3.
Five types of fatigue crack growth behavior [57].
3.4. Factors influencing the effects of stress and corrosion
There are many factors influencing the response of Mg implants to physiological stress, e.g. shape and geometry, alloying elements, and so on [12], [63]. Corrosion pitting is commonly initiated from sharp contours [49], [64] and alloying elements induce galvanic corrosion. It is well known that the sharp contours or the galvanic corrosion mainly causes the local stress concentration and their effects on the degradation could be attributed to SAD, SCC and CF. Thus, the following factors mainly focus on the stress factors (involving loading frequency and mode) and dissolved oxygen gas (one commonly neglected factor).
3.4.1. Loading frequency
The frequencies of loading which vary with the activities in vivo affect the time-dependent degradation of Mg alloys [60], [65]. Most of the investigations on SCC or CF have been carried out at high frequencies of 5–20 Hz [43], [58], [66] exceeding those in normal situations of 1–3 Hz in order to reduce the experimental time. It has been reported that frequency would influence the degradation behaviors. The study [65] about the fatigue crack growth of extruded AZ61 magnesium alloy at different loading frequency under the condition of 50 °C and 80% relative humidity (RH) is performed. The result shows that the sample with the loading frequency of 1 Hz has higher fatigue crack growth resistance than that at 10 Hz. Similar results have been reported from AZ61 in a NaCl solution [60]. In the low ΔK (stress intensity factor range) regime, the fatigue crack growth rate decreases with decreasing of frequency between 15 and 0.5 Hz and the frequency change does not affect the high ΔK regime. However, if the frequency is less than 0.05 Hz, the fatigue crack growth rates are higher than those at >0.5 Hz. Therefore, it is important to perform cyclic dynamic experiments on Mg alloys at a low frequency that can better simulate the actual condition in vivo. The suitable bending fatigue test on Mg alloys for temporary implants is suggested to be at 1 Hz [67], the normal walking frequency of adults, for 1 million cycles to represent the average human activity in one year.
3.4.2. Loading mode
Implants are subjected to complex and multi-axial loading in vivo. However, most dynamic cyclic loading tests have been conducted using constant loading in the single-tension or tension-compression mode, but implants experience step-wise dynamic loads during healing [68], [69], [70]. Moreover, the loads are seldom applied along the central long axial to provide the absolute axial force and bending moment in vivo. The stress distribution in the implants during bending is thus different from those in conventional tension or tension-compression tests. Implants suffer from critical fracture or degradation at the middle surface where there is the largest strain [71]. However, this phenomenon may not be observed from tension or tension-compression tests in which the stress is relatively uniform distributed. Therefore, it is important to conduct tests under realistic loading.
3.4.3. Dissolved oxygen gas
Most investigations on the influence of in vivo environment have been conducted using simulated solutions with adjusted ion concentrations and organic components in accordance with blood plasma [72], [73]. It has been demonstrated the ion concentration has significant influence on the degradation of Mg alloys by changing the composition and dissolution of the formed film [74], [75]. Another factor, dissolved oxygen (partial pressure pO2 in the body fluid is 28–78 mmHg [76]), has not been studied in-depth. During degradation, O2 takes part in the following cathode reaction:
| (2) |
The cathode reaction alters the degradation rate of Mg alloys and O2 in the solution undermines the fatigue properties of non-degradable metals [76], [77]. Morita et al. [76] have investigated the influence of a small amount of O2 in the body fluid on the corrosion fatigue behavior of 316 L stainless steel and noticed that remarkable deterioration in the fatigue durability. The results are consistent with those obtained from animals. Additionally, O2 is an inhibitor of hydrogen gas-accelerated fatigue crack growth [77] and it is important to fathom the effects of O2 in the solution on the degradation behavior of Mg alloys.
4. Response of biodegradable biopolymers to external stress
Degradable polymers are useful in temporary applications. However, it is imperative that the polymers are biocompatible and the degradation products are not toxic. Consequently, the choice of available materials is limited. Some common synthetic biodegradable polymers and applications are listed in Table 2 [78]. Among these materials, PLA, PGA, and their copolymers are most common.
Table 2.
Common synthetic biodegradable polymers and representative applications [78].
| Degradable polymers | Current major bioapplications |
|---|---|
| Synthetic degradable polyesters | |
| Polylactic acid (PLA), poly glycolic (PGA) and copolymers | Barrier membrances, drug delivery, guided tissue regeneration (in dental applicaitons), orthopedic applications, stents, staples, sutures, tissue engineering |
| Polyhydroxbutyrate (PHB), polygydroxyvalerate (PHV), and copolymers | Long-term drug delivery, orthopedic applications, stapes stents |
| Polycaprolactone | Long-term drug delivery, orthopedic applications, staples, stents |
| Polydioxanone | Fracture fixation in non-load-bearing bones, sutures, wound clip |
| Other synthetic degradable polymers | |
| Polyanhydrides | Drug delivery |
| Polycyanoacrylates | Adhesives, drug delivery |
| Poly(amino acids) and “pseudo”-poly(amino acids) | Drug delivery, tissue engineering, orthopedic applicaitons |
| Poly(ortho ester) | Drug delivery,stents |
| Polyphosphazenes | Blood contacting devices, drug delivery, skeletal reconstruction |
| Poly(propylene fumarate) | Orthopedic applications |
4.1. Influence of static stress
Although the influence of mechanical stress on the chemical reaction of polymers, namely mechanochemistry, has been widely investigated, there have been few reports about the degradation of biopolymers in vitro or in vivo under external stress. The influence of static tensile loading on polymers is commonly evaluated by the modified Arrhenius equation as follows [79], [80]:
| (3) |
where K0 is the Arrhenius frequency factor, K is the rate of bond-rupture events, EA is the activation energy, σ is the tensile stress, and α is the coefficient. Static tensile stress increases the activation energy leading to the accelerated degradation of the biopolymers.
Tensile loading is applied on an electrospun poly(l-lactide-co-glycolide) (PLGA) scaffold and the degradation behaviors are studied [81]. It shows that the molecular weight, thermal properties, and lactic acid release represent more extensive degradation compared to the absence of loading. Moreover, it seems the degradation of PLGA depends on the loading magnitude [82]. In the tensile stress range between 0.1 MPa and 0.5 MPa, a higher tensile stress results in faster degradation and a combined load further enhances degradation [83]. However, another study [84]shows the tensile loads have no effect on the degradation of PGLA (90/10 poly(glycolide-co-L-lactide)) but a higher temperature accelerates degradation. The difference may be attributed to the smaller applied load than those adopted in other studies, suggesting that there may be a critical load below which degradation is less severe.
The applied stress applied in the above works is commonly tensile loading but single static compression loading is not performed. According to Equation (3), the stress works on the system and the influence of the applied stress mode does not affect degradation. In particular, the function of compression loading should be similar to that of tensile loading. However, a study on photodegradation of polymers shows that compressive stress retards chain scission whereas tensile stress accelerates photodegradation [85]. The results suggest that stress affects not only the activation energy of degradation, but also diffusion of reagents [85] and structure and physical parameters of the polymers [86].
4.2. Influence of dynamic stress
The physiological stress is dynamic in vivo. The influence of dynamic stress on the degradation of biopolymers has been investigated and Table 3 summarizes the main results.
Table 3.
Influence of dynamic stress on the degradation behavior of biodegradable polymers.
| Polymers | Dynamic stress mode | Frequency | Main degradation effects |
|---|---|---|---|
| PLLA [87] | Compression | 1 Hz | No significant influence on the degradation in the early period and promote degradation in the following stage. |
| 70:30 PLGA [88] | Compression | 1 Hz | A faster reduction in mass, dimensions of the PLGA scaffolds, while the relative molecular weight decreased slower in the first week and faster in the following stages. |
| 50:50 PLGA [89] | Compression | 0.5 Hz | Lower molecular weight loss of the loaded specimens compared to the nonloaded specimens in a week immersion. |
| PEG-PLA [90] | Compression | 0.3 Hz, 1 Hz, and 3 Hz | The frequency has no influence at the low cross-linked gels while a higher frequency suggested a faster degradation at the high cross-linked gels. |
| PLLA [91] | Tension | 1 Hz | A faster degradation under load condition. |
| 50:50 PLGA [92] | Bending | 0.4 Hz | No significant influence on mass loss and molecular weight. |
4.2.1. Cyclic compression
The influence of compression on the degradation of biopolymers is complex. A faster reduction in mass and dimensions of the PLGA scaffolds have been observed under cyclic compression during immersion for 12 weeks compared to that under the static condition. The relative molecular weight decreases slowly during the first two weeks, following by faster degradation compared to that under static conditions [88]. A slower decrease of the molecular chain size during initial immersion has also been observed in the investigation of degradation of 50:50 PLGA in vitro [89]. This rate reduction arises from collapse of the pores in the implant due to dynamic compression loading. Another study [87] shows that cyclic compression does not affect degradation of PLLA in the early period, but promoted degradation is observed afterwards, suggesting that the influence of dynamic compression stress is related to the geometry of the specimens and the influence is more apparent in the later immersion stage. It should be mentioned that dynamic compression leads to enhanced fluid flow in and out of the specimen [89] consequently enhancing diffusion of small molecules and causing mass losses.
4.2.2. Cyclic tension
As mentioned in section 4.1, tension increases the degradation rate of polymers. Under cyclic tension, the enhanced fluid flow would further accelerate the degradation. Hayman et al. [91] have compared the degradation properties of PLLA under static loading (0.5 N and 1 N) and dynamic tensile loading (0.125–0.25 N) at 1 Hz and observed that a larger static load increases the degradation of mechanical properties and dynamic loading further accelerates degradation which is more noticeable at a later time.
4.2.3. Cyclic bending
Cyclic bending is a kind of complex load mode. One study [92] reports that cyclic bending has no significant influence on the mass loss and molecular weight change of PLGA in the 2-week interval, but protein release is enhanced. The increased protein release is attributed to cracks and poles caused by cyclic bending. However, the local degradation difference is not mentioned and the stress mode and magnitude is inhomogeneously distributed under bending while the outer section of the sample is under higher strain.
4.2.4. Effects of frequencies
The effects of the dynamic loading frequencies on the degradation behavior depend on the structure of the polymer. The study by Nicodemus et al. on PEG-PLA hydrogels suggests that the frequency causes no statistical differences in the degradation rates or bulk erosion profiles for the low cross-linked gels, but a higher frequency increases degradation in the higher cross-linked gels [90].
4.3. Degradation prediction
It is critical to know and predict the change in the implant properties with time in vivo. As aforementioned, the polymer geometry and composition vary too much for a reasonable comparison and it is difficult to identify the quantitative relationship between the degradation properties and external stress. Nonetheless, one viable approach is by using the stress-modified Arrhenius equation (3) to incorporate the stress factors into the degradation kinetics [80], so that the properties of biopolymers can be calculated according to the relationship between the molecular weight and strength [84], [93]. Another approach is to establish a mathematic model on the basis of the experiment results. A polynomial formula with nine constants is proposed describe the relationship between mass losses under different loads with time [82] and the simulation and experimental results are consistent. Since this model is only for the specific biopolymer, it is necessary to establish a more general model to the properties of loaded biopolymers.
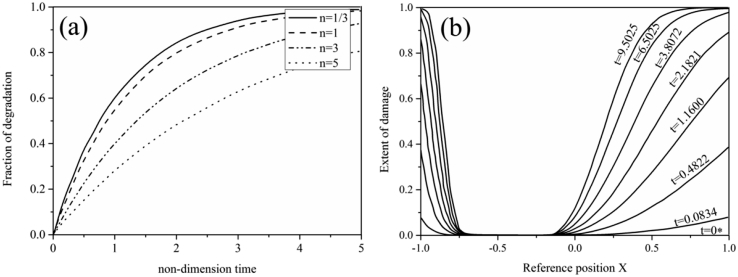
Another approach for the mode to predict degradation as a function of strain based on thermodynamics [71]. In this model, degradation is driven by strain alone while UV radiation, oxygen diffusion, and temperature are ignored. This model is used to predict the degradation of polymers under simple shearing and bending as shown in Fig. 4. This model describes the inhomogeneous degradation under pure bending quite well. The model is further developed for PLLA undergoing tensile deformation [94], [95]. A neo-Hookean material mode is adopted to describe the degradation by assuming the molecular weight reduction decreases the shear modulus of PLLA. However, there are still some deficiencies. For example, the self-catalytic effect which can affect the degradation rate is not considered.
Fig. 4.
Plots of the degradation fraction as a function of time [71] under (a) shear and (b) bending (b) loading with (a) indicating that degradation accelerates with decreasing the rate sensitivity index n and (b) showing that degradation is faster in the outer fibers since they are subjected to higher strain than those closer to the neutral axis.
5. Response of biodegradable ceramics to external stress
Although biodegradable ceramic materials such as calcium phosphate, glass ceramics, and hydroxyapatite are extensively used in coatings to promote bone growth and osseointegration [16], [96], [97], [98], they tend to have poor mechanical strength and low toughness [16], [96], [99]. In particular, their brittleness renders them more sensitive to the external stress which may spur crack initiation and propagation and increase the dissolution rate. A study [100] on the stress–corrosion crack growth (SCCG) of Si–Na–K–Mg–Ca–P–O bioactive glasses in a simulated human physiological environment shows the breakage of Si-O bonds is considered the dominant mechanism. Reis and Monteiro [101] have studied the structural changes in hydroxyapatite coatings plasma-sprayed on Ti-6Al-4V in aqueous media under cyclic bending. Dynamic bending increases dissolution of hydroxyapatite and immersion under static conditions for 2 years is equivalent to the cyclic test for 27.8 h in the same medium. In order to establish a quantitative model to evaluate the influence of stress, one common method [102] for bioglasses is the modified Arrhenius equation (3) and the enhanced degradation or breakage stems from the increased activation energy.
6. Conclusion
Biodegradable materials have attracted immense interest in orthopedics. During healing, physiological stress plays an important role in bone formation and there is evidence that physiological stress influences the degradation rate and service life of BOM. The lack of knowledge on the quantitative relationship between the degradation properties of the biodegradable materials and external stress undermines the design of optimal biomedical implants. The external stress mode, magnitude, and frequency affects the degradation behavior of BOMs. It is thus important to conduct tests in the actual simulated stress mode and establish a quantitative model to predict the performance of BOMs under physiological stress. In addition, the mechanical interactions between biodegradable implants and tissues during the healing process require more investigation.
Acknowledgments
This work was jointly supported by the National Natural Science Foundation of China (Grant No. 31570961), Scientific Research Foundation of Graduate School of Southeast University (YBJJ1525), and Hong Kong Research Grants Council (RGC) General Research Funds (GRF) No. 11301215.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Burger E.H., Klein-Nulend J., Veldhuijzen J.P. Mechanical stress and osteogenesis in vitro. J. Bone Miner. Res. 1992;7:S397–S401. doi: 10.1002/jbmr.5650071406. [DOI] [PubMed] [Google Scholar]
- 2.Sikavitsas V.I., Temenoff J.S., Mikos A.G. Biomaterials and bone mechanotransduction. Biomaterials. 2001;22:2581–2593. doi: 10.1016/s0142-9612(01)00002-3. [DOI] [PubMed] [Google Scholar]
- 3.Witte F. The history of biodegradable magnesium implants: a review. Acta Biomater. 2010;6:1680–1692. doi: 10.1016/j.actbio.2010.02.028. [DOI] [PubMed] [Google Scholar]
- 4.Staiger M.P., Pietak A.M., Huadmai J., Dias G. Magnesium and its alloys as orthopedic biomaterials: a review. Biomaterials. 2006;27:1728–1734. doi: 10.1016/j.biomaterials.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Tan L.L., Yu X.M., Wang P., Yang K. Biodegradable materials for bone repairs: a review. J. Mater. Sci. Technol. 2013;29:503–513. [Google Scholar]
- 6.Li N., Zheng Y. Novel magnesium alloys developed for biomedical application: a review. J. Mater. Sci. Technol. 2013;29:489–502. [Google Scholar]
- 7.Lasprilla A.J.R., Martinez G.A.R., Lunelli B.H., Jardini A.L., Filho R.M. Poly-lactic acid synthesis for application in biomedical devices — a review. Biotechnol. Adv. 2012;30:321–328. doi: 10.1016/j.biotechadv.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 8.Rezwan K., Chen Q.Z., Blaker J.J., Boccaccini A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials. 2006;27:3413–3431. doi: 10.1016/j.biomaterials.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 9.Song G.-L., Xu Z. Crystal orientation and electrochemical corrosion of polycrystalline Mg. Corros. Sci. 2012;63:100–112. [Google Scholar]
- 10.Liu Y., Liu D., You C., Chen M. Effects of grain size on the corrosion resistance of pure magnesium by cooling rate-controlled solidification. Front. Mater. Sci. 2015:1–7. [Google Scholar]
- 11.Zhou H., Lawrence J.G., Bhaduri S.B. Fabrication aspects of PLA-CaP/PLGA-CaP composites for orthopedic applications: a review. Acta Biomater. 2012;8:1999–2016. doi: 10.1016/j.actbio.2012.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Kirkland N.T., Birbilis N., Staiger M.P. Assessing the corrosion of biodegradable magnesium implants: a critical review of current methodologies and their limitations. Acta Biomater. 2012;8:925–936. doi: 10.1016/j.actbio.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Shi L., Huang Y., Yang L., Feyerabend F., Mendis C., Willumeit R. Mechanical properties and corrosion behavior of Mg–Gd–Ca–Zr alloys for medical applications. J. Mech. Behav. Biomed. 2015;47:38–48. doi: 10.1016/j.jmbbm.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S., Zhang X., Zhao C., Li J., Song Y., Xie C. Research on an Mg–Zn alloy as a degradable biomaterial. Acta Biomater. 2010;6:626–640. doi: 10.1016/j.actbio.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji H., Ikada Y. Properties and morphology of poly(l-lactide) 4. Effects of structural parameters on long-term hydrolysis of poly(l-lactide) in phosphate-buffered solution. Polym. Degrad. Stab. 2000;67:179–189. [Google Scholar]
- 16.Teoh S.H. Fatigue of biomaterials: a review. Int. J. Fatigue. 2000;22:825–837. [Google Scholar]
- 17.Bundy K., Marek M., Hochman R. In vivo and in vitro studies of the stress-corrosion cracking behavior of surgical implant alloys. J. Biomed. Mater. Res. 1983;17:467–487. doi: 10.1002/jbm.820170307. [DOI] [PubMed] [Google Scholar]
- 18.Chandra A., Ryu J.J., Karra P., Shrotriya P., Tvergaard V., Gaisser M. Life expectancy of modular Ti6Al4V hip implants: influence of stress and environment. J. Mech. Behav. Biomed. 2011;4:1990–2001. doi: 10.1016/j.jmbbm.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 19.Chandra A., Ryu J.J., Karra P., Shrotriya P., Weik T. Electrochemical dissolution of biomedical grade Ti6Al4V: influence of stress and environment. CIRP Ann. Manuf. Technol. 2009;58:499–502. [Google Scholar]
- 20.Chao J., López V. Failure analysis of a Ti6Al4V cementless HIP prosthesis. Eng. Fail. Anal. 2007;14:822–830. [Google Scholar]
- 21.Lanyon L.E. Using functional loading to influence bone mass and architecture: objectives, mechanisms, and relationship with estrogen of the mechanically adaptive process in bone. Bone. 1996;18:S37–S43. doi: 10.1016/8756-3282(95)00378-9. [DOI] [PubMed] [Google Scholar]
- 22.Lanyon L.E., Hampson W.G.J., Goodship A.E. Bone deformation recorded in vivo from strain gauges attached to human tibial shaft. Acta Orthop. Scand. 1975;46:256–268. doi: 10.3109/17453677508989216. [DOI] [PubMed] [Google Scholar]
- 23.Chao E.Y.S., Inoue N., Elias J.J., Aro H. Enhancement of fracture healing by mechanical and surgical intervention. Clin. Orthop. Relat. Res. 1998;355 doi: 10.1097/00003086-199810001-00018. [DOI] [PubMed] [Google Scholar]
- 24.Brånemark R., Öhrnell L.-O., Nilsson P., Thomsen P. Biomechanical characterization of osseointegration during healing: an experimental in vivo study in the rat. Biomaterials. 1997;18:969–978. doi: 10.1016/s0142-9612(97)00018-5. [DOI] [PubMed] [Google Scholar]
- 25.Duda G.N., Schneider E., Chao E.Y.S. Internal forces and moments in the femur during walking. J. Biomech. 1997;30:933–941. doi: 10.1016/s0021-9290(97)00057-2. [DOI] [PubMed] [Google Scholar]
- 26.Taylor S.J.G., Walker P.S. Forces and moments telemetered from two distal femoral replacements during various activities. J. Biomech. 2001;34:839–848. doi: 10.1016/s0021-9290(01)00042-2. [DOI] [PubMed] [Google Scholar]
- 27.Taylor S.J.G., Walker P.S., Perry J.S., Cannon S.R., Woledge R. The forces in the distal femur and the knee during walking and other activities measured by telemetry. J. Arthroplast. 1998;13:428–437. doi: 10.1016/s0883-5403(98)90009-2. [DOI] [PubMed] [Google Scholar]
- 28.Wehner T., Claes L., Simon U. Internal loads in the human tibia during gait. Clin. Biomech. 2009;24:299–302. doi: 10.1016/j.clinbiomech.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Gruber A.H., Boyer K.A., Derrick T.R., Hamill J. Impact shock frequency components and attenuation in rearfoot and forefoot running. J. Sport Health Sci. 2014;3:113–121. [Google Scholar]
- 30.Carter D.R., Van der Meulen M.C.H., Beaupré G.S. Mechanical factors in bone growth and development. Bone. 1996;18:S5–S10. doi: 10.1016/8756-3282(95)00373-8. [DOI] [PubMed] [Google Scholar]
- 31.Beaupied H., Lespessailles E., Benhamou C.-L. Evaluation of macrostructural bone biomechanics. Jt. Bone Spine. 2007;74:233–239. doi: 10.1016/j.jbspin.2007.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Skerry T.M. The response of bone to mechanical loading and disuse: fundamental principles and influences on osteoblast/osteocyte homeostasis. Arch. Biochem. Biophys. 2008;473:117–123. doi: 10.1016/j.abb.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 33.Burr D.B., Robling A.G., Turner C.H. Effects of biomechanical stress on bones in animals. Bone. 2002;30:781–786. doi: 10.1016/s8756-3282(02)00707-x. [DOI] [PubMed] [Google Scholar]
- 34.Robling A.G., Duijvelaar K.M., Geevers J.V., Ohashi N., Turner C.H. Modulation of appositional and longitudinal bone growth in the rat ulna by applied static and dynamic force. Bone. 2001;29:105–113. doi: 10.1016/s8756-3282(01)00488-4. [DOI] [PubMed] [Google Scholar]
- 35.Qin Y.-X., McLeod K.J., Guilak F., Rubin C.T., Chiang F.-P. Correlation of bony ingrowth to the distribution of stress and strain parameters surrounding a porous-coated implant. J. Orthop. Res. 1996;14:862–870. doi: 10.1002/jor.1100140604. [DOI] [PubMed] [Google Scholar]
- 36.Zheng Y.F., Gu X.N., Witte F. Biodegradable metals. Mat. Sci. Eng. R. 2014;77:1–34. [Google Scholar]
- 37.Xin Y., Hu T., Chu P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: a review. Acta Biomater. 2011;7:1452–1459. doi: 10.1016/j.actbio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Permana K.D., Shuib A., Ariwahjoedi B. A review of magnesium alloys for use in biodegradable cardiovascular stents. World Appl. Sci. J. 2014;30:375–381. [Google Scholar]
- 39.Jafari S., Harandi S., Singh Raman R.K. A review of stress-corrosion cracking and corrosion fatigue of magnesium alloys for biodegradable implant applications. JOM. 2015;67:1143–1153. [Google Scholar]
- 40.Unigovski Y., Eliezer A., Abramov E., Snir Y., Gutman E.M. Corrosion fatigue of extruded magnesium alloys. Mat. Sci. Eng. A. 2003;360:132–139. [Google Scholar]
- 41.Gutman E.M., Unigovski Y., Eliezer A., Abramov E. Mechanoelectrochemical behavior of pure magnesium and magnesium alloys stressed in aqueous solutions. J. Mater. Synth. Process. 2000;8:133–138. [Google Scholar]
- 42.Gutman E.M., Eliezer A., Unigovski Y., Abramov E. Mechanoelectrochemical behavior and creep corrosion of magnesium alloys. Mat. Sci. Eng. A. 2001;302:63–67. [Google Scholar]
- 43.Gu X.N., Zhou W.R., Zheng Y.F., Cheng Y., Wei S.C., Zhong S.P. Corrosion fatigue behaviors of two biomedical Mg alloys – AZ91D and WE43 – in simulated body fluid. Acta Biomater. 2010;6:4605–4613. doi: 10.1016/j.actbio.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 44.Gutman E.M. Metallurgiya; Moscow: 1974. Mechanochemistry of Metals and Corrosion Protection. 46. [Google Scholar]
- 45.Bonora P.L., Andrei M., Eliezer A., Gutman E.M. Corrosion behaviour of stressed magnesium alloys. Corros. Sci. 2002;44:729–749. [Google Scholar]
- 46.Baláž P. Mechanical activation in hydrometallurgy. Int. J. Min. Process. 2003;72:341–354. [Google Scholar]
- 47.Wang B., Gao J., Wang L., Zhu S., Guan S. Biocorrosion of coated Mg–Zn–Ca alloy under constant compressive stress close to that of human tibia. Mater. Lett. 2012;70:174–176. [Google Scholar]
- 48.Logan H.L. Film-rupture mechanism of stress corrosion. J. Res. Natl. Bur. Stand. 1952;48:99–105. [Google Scholar]
- 49.Singh Raman R.K., Jafari S., Harandi S.E. Corrosion fatigue fracture of magnesium alloys in bioimplant applications: a review. Eng. Fract. Mech. 2015;137:97–108. [Google Scholar]
- 50.Choudhary L., Singh Raman R.K. Mechanical integrity of magnesium alloys in a physiological environment: slow strain rate testing based study. Eng. Fract. Mech. 2013;103:94–102. [Google Scholar]
- 51.Choudhary L., Singh Raman R.K. Magnesium alloys as body implants: fracture mechanism under dynamic and static loadings in a physiological environment. Acta Biomater. 2012;8:916–923. doi: 10.1016/j.actbio.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 52.Choudhary L., Singh Raman R.K., Hofstetter J., Uggowitzer P.J. In-vitro characterization of stress corrosion cracking of aluminium-free magnesium alloys for temporary bio-implant applications. Mat. Sci. Eng. C. 2014;42:629–636. doi: 10.1016/j.msec.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 53.Rieck R., Atrens A., Smith I. The role of crack tip strain rate in the stress corrosion cracking of high strength steels in water. Metall. Trans. A. 1989;20:889–895. [Google Scholar]
- 54.Bobby Kannan M., Singh Raman R.K. Evaluating the stress corrosion cracking susceptibility of Mg–Al–Zn alloy in modified-simulated body fluid for orthopaedic implant application. Scr. Mater. 2008;59:175–178. [Google Scholar]
- 55.Song R.G., Blawert C., Dietzel W., Atrens A. A study on stress corrosion cracking and hydrogen embrittlement of AZ31 magnesium alloy. Mat. Sci. Eng. A. 2005;399:308–317. [Google Scholar]
- 56.Padekar B.S., Raja V.S., Raman R.K.S. Stress corrosion cracking of a wrought Mg–Mn alloy under plane strain and plane stress conditions. Eng. Fract. Mech. 2013;102:180–193. [Google Scholar]
- 57.Vasudevan A.K., Sadananda K. Classification of environmentally assisted fatigue crack growth behavior. Int. J. Fatigue. 2009;31:1696–1708. [Google Scholar]
- 58.Jafari S., Singh Raman R.K., Davies C.H.J. Corrosion fatigue of a magnesium alloy in modified simulated body fluid. Eng. Fract. Mech. 2015;137:2–11. [Google Scholar]
- 59.Zhao J., Gao L.L., Gao H., Yuan X., Chen X. Biodegradable behaviour and fatigue life of ZEK100 magnesium alloy in simulated physiological environment. Fatigue Fract. Eng. M. 2015;38:904–913. [Google Scholar]
- 60.Rozali S., Mutoh Y., Nagata K. Effect of frequency on fatigue crack growth behavior of magnesium alloy AZ61 under immersed 3.5 mass% NaCl environment. Mat. Sci. Eng. A. 2011;528:2509–2516. [Google Scholar]
- 61.He X., Wei Y., Hou L., Yan Z., Guo C. High-frequency corrosion fatigue behavior of AZ31 magnesium alloy in different environments. P I Mech. Eng. C J. Mech. 2014;228:1645–1657. [Google Scholar]
- 62.Bin Sajuri Z., Umehara T., Miyashita Y., Mutoh Y. Fatigue-life prediction of magnesium alloys for structural applications. Adv. Eng. Mater. 2003;5:910–916. [Google Scholar]
- 63.Chen Y., Xu Z., Smith C., Sankar J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014;10:4561–4573. doi: 10.1016/j.actbio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 64.Winzer N., Atrens A., Song G., Ghali E., Dietzel W., Kainer K.U. A critical review of the stress corrosion cracking (SCC) of magnesium alloys. Adv. Eng. Mater. 2005;7:659–693. [Google Scholar]
- 65.Sajuri Z., Miyashita Y., Mutoh Y. Effect of loading frequency on fatigue behavior of magnesium alloy in humid environment. J. Mek. 2006;22:115–131. [Google Scholar]
- 66.Bhuiyan M.S., Ostuka Y., Mutoh Y., Murai T., Iwakami S. Corrosion fatigue behavior of conversion coated AZ61 magnesium alloy. Mat. Sci. Eng. A. 2010;527:4978–4984. [Google Scholar]
- 67.Harandi S., Singh Raman R.K. Appropriate mechanochemical conditions for corrosion-fatigue testing of magnesium alloys for temporary bioimplant applications. JOM. 2015;67:1137–1142. [Google Scholar]
- 68.Perren S.M. Evolution of the internal fixation of long bone fractures. J. Bone Jt. Surg. Br. 2002;84:1093–1110. doi: 10.1302/0301-620x.84b8.13752. [DOI] [PubMed] [Google Scholar]
- 69.Mehboob H., Chang S.-H. Application of composites to orthopedic prostheses for effective bone healing: a review. Compos Struct. 2014;118:328–341. [Google Scholar]
- 70.Van Oosterwyck H., Duyck J., Vander Sloten J., Van der Perre G., De Coomans M., Lieven S. The influence of bone mechanical properties and implant fixation upon bone loading around oral implants. Clin. Oral Implants Res. 1998;9:407–418. doi: 10.1034/j.1600-0501.1996.090606.x. [DOI] [PubMed] [Google Scholar]
- 71.Rajagopal K.R., Srinivasa A.R., Wineman A.S. On the shear and bending of a degrading polymer beam. Int. J. Plast. 2007;23:1618–1636. [Google Scholar]
- 72.Xin Y., Hu T., Chu P.K. Influence of test solutions on in vitro studies of biomedical magnesium alloys. J. Electrochem. Soc. 2010;157:C238–C243. [Google Scholar]
- 73.Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27:2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 74.Xin Y., Hu T., Chu P.K. Degradation behaviour of pure magnesium in simulated body fluids with different concentrations of HCO3- Corros. Sci. 2011;53:1522–1528. [Google Scholar]
- 75.Zeng R.C., Hu Y., Guan S.K., Cui H.Z., Han E.-H. Corrosion of magnesium alloy AZ31: the influence of bicarbonate, sulphate, hydrogen phosphate and dihydrogen phosphate ions in saline solution. Corros. Sci. 2014;86:171–182. [Google Scholar]
- 76.Morita M., Sasada T., Nomura I., Wei Y., Tsukamoto Y. Influence of low dissolved oxygen concentration in body fluid on corrosion fatigue behaviors of implant metals. Ann. Biomed. Eng. 1992;20:505–516. doi: 10.1007/BF02368170. [DOI] [PubMed] [Google Scholar]
- 77.Somerday B.P., Sofronis P., Nibur K.A., San Marchi C., Kirchheim R. Elucidating the variables affecting accelerated fatigue crack growth of steels in hydrogen gas with low oxygen concentrations. Acta Mater. 2013;61:6153–6170. [Google Scholar]
- 78.Ratner B.D., Hoffman A.S., Schoen F., Lemons J.E. Elsevier; San Diego: 2004. Biomaterials Science: an Introduction to Materials in Medicine. [Google Scholar]
- 79.Davis D.A., Hamilton A., Yang J., Cremar L.D., Van Gough D., Potisek S.L. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature. 2009;459:68–72. doi: 10.1038/nature07970. [DOI] [PubMed] [Google Scholar]
- 80.Beyer M.K., Clausen-Schaumann H. Mechanochemistry: the mechanical activation of covalent bonds. Chem. Rev. 2005;105:2921–2948. doi: 10.1021/cr030697h. [DOI] [PubMed] [Google Scholar]
- 81.Li P., Feng X., Jia X., Fan Y. Influences of tensile load on in vitro degradation of an electrospun poly(l-lactide-co-glycolide) scaffold. Acta Biomater. 2010;6:2991–2996. doi: 10.1016/j.actbio.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 82.Guo M., Chu Z., Yao J., Feng W., Wang Y., Wang L. The effects of tensile stress on degradation of biodegradable PLGA membranes: a quantitative study. Polym. Degrad. Stab. 2016;124:95–100. [Google Scholar]
- 83.Fan Y.B., Li P., Zeng L., Huang X.J. Effects of mechanical load on the degradation of poly(d,l-lactic acid) foam. Polym. Degrad. Stab. 2008;93:677–683. [Google Scholar]
- 84.Deng M., Zhou J., Chen G., Burkley D., Xu Y., Jamiolkowski D. Effect of load and temperature on in vitro degradation of poly(glycolide-co-l-lactide) multifilament braids. Biomaterials. 2005;26:4327–4336. doi: 10.1016/j.biomaterials.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 85.Tong L., White J.R. Photo-oxidation of thermoplastics in bending and in uniaxial compression. Polym. Degrad. Stab. 1996;53:381–396. [Google Scholar]
- 86.Fan Y., Li P., Yuan X. Influence of mechanical loads on degradation of scaffolds. In: Lim C.T., Goh J.C.H., editors. 6th World Congress of Biomechanics (WCB 2010) August 1-6, 2010 Singapore. Springer; Berlin Heidelberg: 2010. pp. 549–552. [Google Scholar]
- 87.Zhao Y., Qiu D., Yang Y., Tang G., Fan Y., Yuan X. Degradation of electrospun poly(L-lactide) membranes under cyclic loading. J. Appl. Polym. Sci. 2012;124:E258–E266. [Google Scholar]
- 88.Yang Y., Tang G., Zhao Y., Yuan X., Fan Y. Effect of cyclic loading on in vitro degradation of poly(L-lactide-co-glycolide) scaffolds. J. Biomat. Sci. Polym. E. 2010;21:53–66. doi: 10.1163/156856209X410229. [DOI] [PubMed] [Google Scholar]
- 89.Thompson D.E., Agrawal C.M., Athanasiou K. The effects of dynamic compressive loading on biodegradable implants of 50-50% polylactic acid-polyglycolic acid. Tissue Eng. 1996;2:61–74. doi: 10.1089/ten.1996.2.61. [DOI] [PubMed] [Google Scholar]
- 90.Nicodemus G.D., Shiplet K.A., Kaltz S.R., Bryant S.J. Dynamic compressive loading influences degradation behavior of PEG-PLA hydrogels. Biotechnol. Bioeng. 2009;102:948–959. doi: 10.1002/bit.22105. [DOI] [PubMed] [Google Scholar]
- 91.Hayman D., Bergerson C., Miller S., Moreno M., Moore J.E. The effect of static and dynamic loading on degradation of PLLA stent fibers. J. Biomech. Eng. 2014;136:081006. doi: 10.1115/1.4027614. [DOI] [PubMed] [Google Scholar]
- 92.Arm D.M., Tencer A.F. Effects of cyclical mechanical stress on the controlled release of proteins from a biodegradable polymer implant. J. Biomed. Mater. Res. 1997;35:433–441. doi: 10.1002/(sici)1097-4636(19970615)35:4<433::aid-jbm3>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 93.Ward I.M., Sweeney J. John Wiley & Sons; New York: 2012. Mechanical Properties of Solid Polymers. [Google Scholar]
- 94.Soares J.S., Moore J.E., Jr., Rajagopal K.R. Constitutive framework for biodegradable polymers with applications to biodegradable stents. ASAIO J. 2008;54:295–301. doi: 10.1097/MAT.0b013e31816ba55a. [DOI] [PubMed] [Google Scholar]
- 95.Mollica F., Preziosi L., Rajagopal K.R. Birkhäuser Boston; Boston, MA: 2007. Modeling of Biological Materials. [Google Scholar]
- 96.Le Huec J.C., Schaeverbeke T., Clement D., Faber J., Le Rebeller A. Influence of porosity on the mechanical resistance of hydroxyapatite ceramics under compressive stress. Biomaterials. 1995;16:113–118. doi: 10.1016/0142-9612(95)98272-g. [DOI] [PubMed] [Google Scholar]
- 97.Burg K.J.L., Porter S., Kellam J.F. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21:2347–2359. doi: 10.1016/s0142-9612(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 98.Chen Q.Z., Thompson I.D., Boccaccini A.R. 45S5 Bioglass®-derived glass–ceramic scaffolds for bone tissue engineering. Biomaterials. 2006;27:2414–2425. doi: 10.1016/j.biomaterials.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 99.Nychka J.A., Mazur S.L.R., Kashyap S., Li D., Yang F. Dissolution of bioactive glasses: the effects of crystallinity coupled with stress. JOM. 2009;61:45–51. [Google Scholar]
- 100.Bloyer D.R., McNaney J.M., Cannon R.M., Saiz E., Tomsia A.P., Ritchie R.O. Stress–corrosion crack growth of Si–Na–K–Mg–Ca–P–O bioactive glasses in simulated human physiological environment. Biomaterials. 2007;28:4901–4911. doi: 10.1016/j.biomaterials.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Reis R.L., Monteiro F.J. Crystallinity and structural changes in HA plasma-sprayed coatings induced by cyclic loading in physiological media. J. Mater. Sci. Mater. Med. 1996;7:407–411. [Google Scholar]
- 102.Michalske T.A., Bunker B.C. A chemical kinetics model for glass fracture. J. Am. Ceram. Soc. 1993;76:2613–2618. [Google Scholar]