Abstract
Treatment of HIV has long faced the challenge of high mutation rates leading to rapid development of resistance, with ongoing need to develop new methods to effectively fight the infection. Traditionally, early HIV medications were designed to inhibit RNA replication and protein production through small molecular drugs. Peptide based therapeutics are a versatile, promising field in HIV therapy, which continues to develop as we expand our understanding of key protein-protein interactions that occur in HIV replication and infection. This review begins with an introduction to HIV, followed by the biological basis of disease, current clinical management of the disease, therapeutics on the market, and finally potential avenues for improved drug development.
Keywords: HIV, HIV treatment, Drug development, Peptide therapeutic, Peptide inhibitor
Abbreviations: HIV, human immunodeficiency virus; AIDS, acquired immunodeficiency syndrome; CDC, Centers for Disease Control and Prevention; FY, fiscal year; R&D, research and development; FDA, US Food and Drug Administration; HCV, hepatitis C Virus; ART, antiretroviral therapy; HAART, highly active antiretroviral therapy; NRTI, Nucleoside/Nucleotide Reverse Transcriptase Inhibitors; NNRTI, Non-nucleoside reverse transcriptase inhibitors; INSTI, Integrase strand transfer inhibitors; RT, reverse transcriptase; LEDGF, lens epithelium-derived growth factor
Graphical abstract
This review discusses the biological basis of HIV, the current clinical management of the disease, the therapeutics developed to treat HIV, and the potential strategies for improving HIV drug development. It explores new methods for developing HIV therapeutics that aim to produce effective drugs in the treatment of HIV. Specifically, it looks at peptide inhibitors currently being researched that show promise for HIV treatment.
Highlights
-
•
HIV hard to control, manage, and infectious with billions spent on research for prevention and treatment.
-
•
This review summarizes the biological basis of HIV, current management, and improved drug development.
-
•
It explores peptide inhibitors as a promising new therapeutic for HIV treatment.
1. Introduction
The human immunodeficiency virus (HIV) is one of the hardest to control, medically manage, and lethal infectious diseases. HIV is a major public health problem globally and domestically. The World Health Organization estimates 37 million people living with HIV or acquired immunodeficiency syndrome (AIDS) worldwide in 2014 with Sub-Saharan Africa accounting for almost 70% of new global HIV infections [1]. About 44,000 people become infected with HIV each year in the United States [2], 350,000 in Asia, 25,000 in the Middle East & North Africa, 94,000 in Latin America, 88,000 in West and Central Europe and North America, 110,000 in Eastern Europe and Central Asia, and 12,000 in the Caribbean [3]. The CDC estimates about 1.2 million people in the United States were living with HIV at the end of 2012 [4]. Of those people, about 12.8% do not know they are infected [5]. The number of new infections continues to rise, particularly in women. HIV treatment is costly. Estimated cost per patient per year in 2010 was $13,251 [6] in the United States, impeding HIV treatment in low-resource settings [7]. Therefore, HIV is still one of the hottest topics in basic science, clinical, and public health research.
Given the magnitude of this pandemic, numerous global efforts have been gathered to fund research needed for HIV prevention and treatment. Over $15 billion has been devoted from 2000 to 2014 [8]. The cumulative HIV/AIDS treatment costs from 1996 to 2010 are estimated to be $242 billion. FY2016 US funding for domestic HIV research is $2.8 billion [9]. North America provides the vast majority of HIV prevention R&D investment (90.9%) [8].
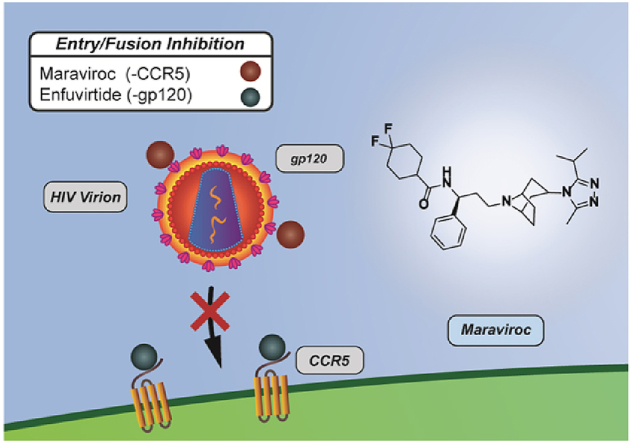
Peptide therapeutics are composed of short amino acid sequences that target protein-protein interactions, such as the critical interaction between the host cell receptors and HIV glycoproteins required for viral entry into host cells. Peptide based therapies offer several advantages over small molecule based therapies, including versatility, high potency, and lower side effects [10]. Peptide/Protein based therapeutics can bind a domain more specifically and effectively than small molecule drugs, as it has larger surface area and stronger interaction between the target domain and the drug [11]. It also has fewer medication interactions and toxicity, as it has more specific binding to target domains and is metabolized into nontoxic amino acids [12]. Peptide therapeutics are less likely to encounter drug resistance, as it requires much more drastic changes in the viral structure for the virus to develop resistance against a peptide. However, peptide based therapeutics face challenges, such as poor in vivo stability and difficulty of forming oral formulations [13]. Currently, two peptide based therapeutics are being used for HIV treatment: Enfuvirtide, a fusion inhibitor that binds and blocks conformational change in gp41 [14], and Maraviroc, an entry inhibitor which blocks binding of gp120 to CCR5 [15], with others under investigation for FDA approval.
Treatment of HIV has advanced significantly over the past 3 decades. This review will briefly discuss the etiology of HIV [16], medical management [17], and approved drugs commonly used [18]. An interesting avenue that has gained much traction for HIV treatment is the development of HIV virion inhibitor peptides. The subsequent focus of this review will be to discuss the fundamental facets of peptide based therapeutics, specifically those currently in research. Finally, we provide insight into how these and other novel therapeutics may change the way we treat HIV. Overall, to be an effective drug in the treatment of HIV the following are required: 1) Cost-effective synthesis, 2) high potency with minimal side effects, 3) easy administration to enhance patient compliance, and 4) educational outreach for disease management and prevention.
2. The biological basis of HIV
2.1. Basics of HIV
The origin of HIV has not been clearly understood [19], with wide speculation that includes its evolution from the simian immunodeficiency virus [20]. HIV is the virus that can lead to AIDS. HIV infects and destroys CD4 cells (T cells), and undermines the human immune system in AIDS. In general, HIV cannot be cured at present and therefore requires life-long treatment. When not treated, undermined immunity surrenders to co-infections, such as HCV (hepatitis C Virus) [21], tuberculosis [22], other sexually transmitted infections [23], cytomegalovirus [24], and papillomavirus [25]. It can also result in age associated morbidities, such as myocardial infection and cancer [26], and eventually leading to significant patient mortality.
HIV is highly heterogeneous, mutates quickly, and can be latent for over 10 years, increasing the difficulty in prevention and treatment [27]. Although uncommon, there has been at least one report of a long-term control for HIV reported using CCR5 Delta32/Delta32 stem cell transplantation that is resistant to HIV-1 [28], [29]. A number of research efforts are ongoing to find a cure for HIV [30], [31]. Efforts have focused on finding treatment and prevention methods through vaccine and other methods of prophylaxis, including anti-retroviral drugs like Tenofovir in topical applications such as vagina gel and ring [32], [33], [34], in oral pre-exposure prophylaxis [35], and in implants [36]. Oral pre-exposure prophylaxis has shown effective protection for men who have sex with men by reducing HIV incidence by 44% [37], but not for heterosexual women, potentially due to low pill adherence or low drug concentrations at the genital tract [38]. Preclinical studies have also shown significant reduction of HIV infection by topical pre-exposure prophylaxis [39].
2.2. Clinical treatment and procedures
Antiretroviral therapy (ART) is the treatment for HIV infection. Multidrug regimens are used to reduce the progression of disease to AIDS, occurrence of opportunistic infections, hospitalizations, and death. There are currently more than 25 antiretroviral medications available in 5 drug categories (discussed below). Although a small portion of these are recommended for initial therapy, continuous assessment of the patient for adverse effects and toxicities as well as adherence guides medication choice. Multiple comparative clinical trials have shown that combination therapy consisting of 2 nucleoside reverse transcriptase inhibitors and a third agent from another class is the most effective treatment. The other classes used in initial treatment are non-nucleoside reverse transcriptase inhibitors, protease inhibitors, and integrase strand transfer inhibitors [40].
2.3. Clinical presentation of HIV
HIV infection can present early as a mononucleosis-like illness; however, many affected individuals are asymptomatic. Estimates for those who are asymptomatic with HIV are between 10 and 60%, but it is hard to estimate because most diagnoses are made after a symptom has led to a work up. Those who have an acute infection, also known as acute retrovirus syndrome, develop symptoms two to four weeks after infection. However, incubation of up to 10 months has been reported [41]. Symptoms of acute HIV infection include fever, lymphadenopathy, sore throat, rash, myalgia, arthralgia, and headache. None of these symptoms are specific, but the presence of these features for an extended duration or with associated mucocutaneous ulcers is suggestive. Many patients experience nausea, diarrhea, anorexia, and weight loss. Patients can present with aseptic meningitis and rarely self-limited encephalopathy. The peripheral nervous system can also be affected. Opportunistic infections (OIs) usually occur in the later course of the infection and rarely occur during early infection with the transient lymphopenia [42]. Oral and esophageal candidiases are the most commonly occurring OIs. Other OIs in early HIV include cytomegalovirus (CMV) colitis, proctitis, hepatitis, pneumocystis jiroveci pneumonia, and cryptosporidiosis.
The chronic period of HIV infection is the time from acute infection to a CD4 count of <200, and it usually lasts 8–10 years. AIDS is diagnosed when CD4 reaches <200 cells/μL or with the presence of an AIDS defining illness, which includes OIs, recurrent infections, lymphoma of brain, and invasive cervical cancer [43]. Mucocutaneous candidiasis, oral hairy leukoplakia, seborrheic dermatitis, and herpetic infections occur with greater frequency when the CD4 cell count is < 200 cells/μL. Eosinophilic folliculitis, xerosis, prurigo nodularis, molluscum contagiosum, bacillary angiomatosis, exacerbation of psoriasis, and severe scabies are associated with AIDS. Anemia, leukopenia, lymphopenia, or thrombocytopenia is present in 40% of those with CD4 < 200 cells/μL. Polyclonal hyperglobulinemia is another hematologic aberration.
2.4. Severity of HIV and associated diseases
OIs usually occur at CD4 levels <200 cells/μL and less often at levels above that, with approximately 10% of patients developing an AIDS-defining diagnosis with a CD4 count ≥200 cells/μL [44]. Disseminated M. avium infection and CMV infections occur predominantly with a CD4 cell count <50 cells/μL. In the absence of antiretroviral therapy (ART), the median time to an AIDS-defining condition in someone with a CD4 cell count < 200 cells/μL is estimated at 12–18 months [45].
In the absence of ART, the average survival of patients with a CD4 < 50 cells/μL is 12–18 months. Most patients who die of AIDS-related complications have CD4 < 50 cells/μL [46]. Above CD4 of 50 cells/μL there is an average of one HIV-related death per 96.7 patient-years of observation as compared with one death per 2.5 patient-years of observation after the CD4 count had fallen below this level (P < 0.0001) [47].
2.5. Clinical management of HIV
ART is initiated in nearly all HIV infected patients and is usually started immediately after the initial assessment. Most patients benefit from the effects of ART regardless of CD4 count. However, there is a small subset of patients known as “HIV controllers” who maintain very low HIV RNA levels without ART therapy in which no clear benefit has been demonstrated. The urgency of initiating ART depends on CD4 count as well as the presence of opportunistic infections. Patients with CD4 counts in normal ranges, >500 cells/μL may elect to defer therapy, but they should be counseled as to the benefits of ART regardless of CD4 count. Starting ART at CD4 counts of <350 cells/μL and especially <200 cells/μL has significant reduction in mortality and morbidity. The higher the CD4 count at time of initiation the higher the long term CD4 count will be, which is associated with better long term outcomes [48].
2.6. Morbidity and mortality rates of patients
One study showed a 50% reduction in morbidity and mortality in those who initiated ART versus those who did not [40]. The overall outcome however depended on the level of immunodeficiency, as indicated by CD4 count at the time of treatment initiation. The hazard ratio for mortality for treated versus untreated are: 0.29 for CD4 < 100 cells/μL, 0.33 for 100–199 cells/μL, 0.38 for 200–349 cells/μL, 0.55 for 350–599 cells/μL, and 0.77 for >/ = 500 cells/μL. Another study showed that after 5 years of ART, the mortality of patients who initiated ART with low CD4 levels and begins to converge with those with intermediate to high CD4 levels. When opportunistic infections are present, early initiation of ART within 14 days leads to a 50% reduction in morbidity and mortality as compared to late initiation of ART after 14 days.
3. Therapeutic treatments of HIV
3.1. Approved therapeutics on market
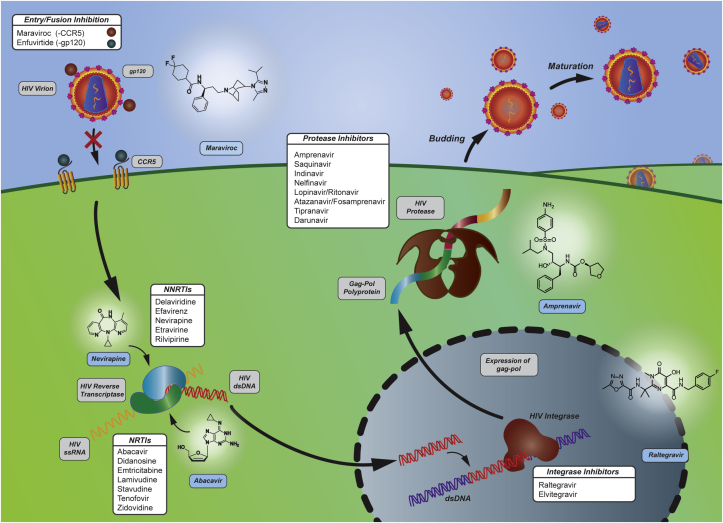
More than 25 antiretroviral drugs from 5 therapeutic classes are available for the management of HIV with over 30 FDA approved in single and multi-class combination agents [49], [50], [51]. Therapeutics currently on the market aim to suppress the virus replication below the level of detection (<50 RNA copies/ml) and to reconstitute immunity by increasing CD4+ T cells [52]. These therapeutics can be classified into 5 categories based on their mechanisms [53], [54]. 1) Entry inhibitors and 2) integrase inhibitors are two new categories of anti-retroviral therapeutics [14], [55], [56]. 3) Nucleoside/nucleotide reverse transcriptase inhibitors, 4) non-nucleoside reverse transcriptase inhibitors, and 5) protease inhibitors were the targets of first generation HIV therapeutics. These are summarized schematically in Fig. 1.
Fig. 1.
HIV proliferation targets and their anti-retroviral drugs. Targets for anti-retroviral drugs include entry inhibition, reverse transcription, genome integration, and protease inhibition. Contemporary HIV treatments consist of administering multiple drugs (cocktails) to inhibit multiple phases of the HIV life-cycle.
3.2. Entry/fusion inhibitor
The entry and fusion process of HIV into the host cell is a complicated process involving several protein-protein interactions that can be drug targets. Entry of HIV into host cells involves several viral and host proteins [57], which are reviewed in detail elsewhere [58]. Briefly, the first step of HIV entry is attachment – HIV surface protein gp120 or host proteins integrated in the HIV membrane typically bind to target cell membranes of CD4+ T cells [59]. The CD4 transmembrane glycoprotein domain on target T cells attaches to the CD4 binding site on gp120. This induces a conformational change in gp120, exposing its co-receptor binding sites [60]. The co-receptors include but are not limited to CCR5 and CXCR4 on target T cells, which bind to their respective binding sites on gp120 that are now exposed [61]. Fusion of the viral and host membranes begins as co-receptor binding induces the second conformational change in the HIV-1 envelope. As a result, gp41 is exposed and inserts into the host cell membrane [62]. As mechanisms of entry and fusion are being elucidated, inhibitors have been discovered that target different steps in the process.
Entry inhibitors prevent HIV from entering host cells. These differ from integrase inhibitors, which prevent viral DNA from integrating into host genome as detailed below [63]. A variety of compounds including small molecules and antibody-based inhibitors have been tested against gp120 and CD4 binding [64], [65], [66]. However, this approach has been shown to be ineffective with HIV isolates [67]. Development of entry inhibitors face this challenge due to the variability of the viral env gene that codes for gp120 and gp41 and the variability of co-receptors. With respect to blocking binding of co-receptors, an FDA approved entry inhibitor, Maraviroc (approved in 2007), targets CCR5. It is an inverse agonist of CCR5, which allosterically binds the CCR5 receptor and stabilizes the inactive conformation of CCR5 [68]. Maraviroc blocks binding of viral envelope protein gp120 to its CCR5 co-receptor in order to prevent further steps of membrane fusion necessary for viral entry [15]. Another prime example of an entry/fusion inhibitor is Enfuvirtide, a 36 amino acid peptide (approved in 2003) [69], [70], [71]. Enfuvirtide binds to a region of the HIV-1 envelope protein gp41 involved in the fusion of viral membrane and host cell membrane [14].
Although two drugs have been approved by the FDA in this category, they face challenges and still need to be improved upon. Maraviroc is only effective for HIV strains that utilize CCR5 for infection, but not CXCR4-tropic or CXC4/CCR5 bitropic (dualtropic) HIV strains [72], [73]. Inhibition of the CCR-5 co-receptor may interfere with the normal function of CCR5 as a chemokine receptor, which is required in inflammatory responses to infections [73], [74]. Enfuvirtide has a very limited window of action when gp41 is exposed after a conformational change in HIV envelope structure during the entry/fusion process. As a result, a high concentration of Enfuvirtide must be maintained in the patient [73]. To maintain this concentration, Enfuvirtide requires dosing at 90 mg subcutaneously twice daily, which is very difficult to achieve in resource-limited settings or with good patient compliance. Notwithstanding, skin sensitivity and side effects commonly associated with high dosages limit utility. Viral mutation leads to limitations of Maraviroc and Enfuvirtide due to the nature of their targets. Therefore, new compounds in development potentially target other proteins in the entry/fusion process and avoid limitations associated with CCR5 and gp41 [75], [76], [77], [78].
3.3. Nucleoside/nucleotide reverse transcriptase inhibitors (NRTIs) [79]
NRTIs were the first antiretroviral drugs that appeared on the market [80]. NRTIs are analogs of native nucleoside substrates that target reverse transcriptase. Reverse transcriptase (RT) is a viral enzyme that catalyzes the transcription of viral genomic RNA into double-stranded proviral DNA [81]. The heterodimer of RT is important for incorporation of the viral genome into the host's genome. RT has two catalytic activities: DNA polymerase activity, which synthesizes DNA from RNA, and DNA and ribonuclease H activity that cleaves the RNA strand in the DNA-RNA duplex formed after DNA polymerase activity [82]. Seven approved NRTIs are on the market: Abacavir, Didanosine, Emtricitabine, Lamivudine, Stavudine, Tenofovir, and Zidovudine [83]. After being phosphorylated by host cellular kinases, NRTIs compete with the natural substrate - endogenous deoxyguanosine triphosphate [84]. NRTIs add to and cap the growing viral DNA chain. NRTIs not only target reverse transcriptase in RNA-dependent DNA synthesis but also inhibit DNA-dependent DNA synthesis, preventing production of both (−) and (+) strands of viral DNA. Significant toxicity is a major challenge faced by NRTIs, which compromises treatment effectiveness [85]. For example, Stavudine was once part of first-line anti-viral regimens, but then its recommended dosage was reduced [86] until the use of Stavudine was limited due to its well-recognized toxicities [87]. Mitochondrial toxicity results from NRTI inhibition of mitochondrial DNA polymerase and manifests as myopathy, neuropathy, hepatic failure, and lactic acidosis [88], [89]. Current management of side effects is through symptomatic treatment and reducing dosage regimens [90]. Consequently, drugs in this pipeline specifically require mitochondrial toxicity testing in preclinical phases of development [91].
3.4. Non-nucleoside reverse transcriptase inhibitors (NNRTIs) [92]
NNRTIs are the most popular first-line HIV treatment regimens both in the United States and world-wide [93], [94]. Some FDA approved NNRTIs include Delavirdine, Efavirenz, Nevirapine, Etravirine, and Rilvipirine. NNRTIs also target reverse transcriptase but are allosteric inhibitors. Allosteric binding of NNRTIs to HIV-1 RT [95] induces conformational changes in substrate binding site of reverse transcriptase, reducing its activity [96]. Challenges to NNRTIs are a low genetic barrier to drug resistance and cross-resistance even from a single mutation [97]. Drug resistance is another major challenge faced by first-generation NRTIs, causing most virologic failures of first-line regimens. As a result, NNRTIs are not recommended for second-line regimens in order to avoid the risk of resistance [98], [99], [100]. Such problems have been partially improved upon by second-generation NRTIs whose conformational flexibly allows them to interact with new residues in the binding pocket or with the main chain residues, which are less likely to mutate by single side chain residues [101], [102]. Consequently, second-generation NNRTIs can act on first-generation NNRTI resistant HIV strains. In fact, the second-generation NNRTIs, Rilpivirine and Etravirine, are second-line drugs in resource-limited countries. They are indicated in patients for whom Efavirenz or Nevirapine fail due to their different resistance profiles (low cross-resistance) and suggested high genetic barrier brought on by their different structures [98], [103]. Increasing resistance to both NRTIs and NNRTIs that decrease drug-RT binding has been observed in low-income and middle-income countries. Therefore, new drugs are urgently needed for emerging drug resistant HIV strains [104]; drugs that do not come with major side effects including skin rash, liver toxicity, and gastrointestinal disturbance [105]. Development of new NNRTIs is currently in multiple stages, aiming to target resistant HIV strains and reduce side effects [98].
3.5. Protease inhibitors
Protease inhibitors are a central part of highly active antiretroviral therapy (HAART), accounting for 10 out of 26 FDA approved anti-HIV therapeutics. Protease inhibitors target the viral encoded aspartyl protease, which is essential for viral maturation and infectivity [106]. Proteases are important enzymes in many biological processes and catalyze the hydrolysis of peptide bonds with high selectivity through activated water molecules or activated thioesters. HIV Protease in particular is an aspartic protease that cleaves gag-pol (polypeptide precursors) and gag-polyprotein precursors into smaller proteins (e.g. reverse transcriptase, RNase H and integrase) that are modified and assembled into new viruses [106], [107]. Gag-pol and gag polyprotein precursors are translated from the viral pol and gag gene [108]. These genes are important for the formation of gag-pol and gag, which are important precursors for enzymes synthesis. FDA approved protease inhibitors are competitive inhibitors to viral proteases. Some FDA approved therapies include Saquinavir, Indinavir, Nelfinavir, Amprenavir, Lopinavir/Ritonavir, Atazanavir and Fosamprenavir, Tipranavir, and Darunavir. Saquinavir was the first FDA approved drug for the treatment of AIDS, and it mimics the tetrahedral intermediate of the proteolytic reaction catalyzed by viral protease [109]. Next generation protease inhibitors have been developed by modifying the structure of previous protease inhibitors. Most protease inhibitors share similar mechanisms of action by mimicking the substrate transition state in the proteolytic reaction [106]. They are competitive inhibitors and all but one (Nelfinavir) are peptidomimetics of the polyprotein cleavage site of the viral protease [110]. The disadvantage of protease inhibitors include the necessity for costly life-long treatment, off-target side effects, toxicity, and adherence [111]. First-generation protease inhibitors were described as highly peptidic and thus suffer from high instability and poor bioavailability [112]. Second-generation inhibitors were designed to address pharmacokinetic stability, side effects, and drug resistance of first-generation protease inhibitors [112]. Side effects to first-generation protease inhibitors include gastrointestinal distress, nausea, diarrhea, abdominal pain, and most commonly lipodystrophy caused by altering adipocyte metabolism [110], [113]. A significant disadvantage is the extensive drug-drug interactions of protease inhibitors with other drugs, making protease inhibitors undesirable for patients on other drug regimens [98].
3.6. Integrase inhibitors [105]
Integrase inhibitors target the integration process of HIV viral DNA into host DNA. This is a two-step process mediated by the retroviral enzyme integrase: 1) cut viral DNA and 2) join viral DNA into host DNA, termed DNA strand transfer [114], [115]. Integration is an essential step in establishing irreversible and chronic infection [116]. Integrase strand transfer inhibitors (INSTIs) are competitive inhibitors to integrase that bind to the host DNA binding site and inhibit DNA strand transfer activity [117], [118]. Current integrase inhibitors target the strand transfer step of the integration process by binding to the enzyme active site and disengaging it from the viral DNA [119]. The first-generation INSTIs, Raltegravir and Elvitegravir [120] were derived from modification of the monoketo acid motif of quinolone antibiotics with chelating ability [121], [122]. The development of the first INSTI Raltegravir started with the discovery of a β diketo acid moiety with selective inhibitory activity against DNA strand transfer reactions [123]. Raltegravir had the disadvantage of a low genetic barrier to mutation and frequent dosing (twice daily) [98]. Research efforts have been focused on optimizing interactions between INSTIs and integrase, and it has resulted in the development of small molecules similar to Raltegravir. For example, Elvitegravir only requires once per day dosing. However, as Raltegravir-resistant HIV mutations emerge, integrase inhibitors with mechanisms similar to first-generation INSTIs are likely to fall victim to the mutational competence of HIV [120]. The second-generation INSTI Dolutegravir was developed as drug resistant mutations emerged towards first-generation INSTIs [55]. Compared to the first-generation INSTIs, Dolutegravir improves binding with viral DNA through its more flexible linker region [119], and has shown fewer drug-drug interactions and side effects [124]. Most frequently reported adverse effects are headache (around 2%) and insomnia (around 3%) [124] (see Table 1, Table 2).
Table 1.
Antiretroviral medications.
| Medication | Target | Type | Usage | Toxicity |
|---|---|---|---|---|
| Enfuvirtide | gp41 | Fusion inhibitor | Binds & blocks conformational change in gp41 | Significant |
| Maraviroc | gp120 | Entry inhibitor | Blocks binding of gp120 to CCR5 | hepatotoxic |
| Abacavir | RT | NRTI | Inhibit DNA-dependent synthesis | + |
| Didanosine | RT | NRTI | Inhibit DNA-dependent synthesis | ++++ |
| Emtricitabine | RT | NRTI | inhibit DNA-dependent synthesis | + |
| Lamivudine | RT | NRTI | Inhibit DNA-dependent synthesis | + |
| Stavudine | RT | NRTI | Inhibit DNA-dependent synthesis | ++++ |
| Tenofovir | RT | NRTI | Inhibit DNA-dependent synthesis | + |
| Zidovudine | RT | NRTI | Inhibit DNA-dependent synthesis | ++ |
| Zalcitabine | RT | NRTI | inhibit DNA-dependent synthesis | ++++ |
| Raltegravir | Enzyme active site | Integrase inhibitor | Inhibit DNA strand transfer activity | Adverse effects |
| Elvitegravir | Enzyme active site | Integrase inhibitor | Inhibit DNA strand transfer activity | Adverse effects |
| Dolutegravir | Enzyme active site | Integrase inhibitor | Inhibit DNA strand transfer activity | Adverse effects |
| Efavirenz | RT | NRTI & NtRTI | Induce conformational change of RT | Toxic effects to CNS |
| Saquinavir | Aspartyl protease | Protease inhibitor | Viral protease inhibitor | GI toxicity |
| Ritonavir | Aspartyl protease | Protease inhibitor | Viral protease inhibitor | GI toxicity |
| Darunavir | Aspartyl protease | Protease inhibitor | Viral protease inhibitor | GI toxicity |
| Indinavir | Aspartyl protease | Protease inhibitor | Viral protease inhibitor | GI toxicity |
| Tipranavir | Aspartyl protease | Protease inhibitor | Viral protease inhibitor | GI toxicity |
| Fosamprenair | Aspartyl protease | Protease inhibitor | Viral protease inhibitor | GI toxicity |
| Nelfinavir | Aspartyl protease | Protease inhibitor | Viral protease inhibitor | GI toxicity |
| Atazanavir | Aspartyl protease | Protease inhibitor | Viral protease inhibitor | GI toxicity |
| Lopinavir | Aspartyl protease | Protease inhibitor | Viral protease inhibitor | GI toxicity |
| Amprenavir | Aspartyl protease | Protease inhibitor | Viral protease inhibitor | GI toxicity |
++++ Strongest association with mitochondrial toxicity, +weakest association with mitochondrial toxicity; Enfuvirtide has common adverse events which include pain, erythema, pruritus, and induration. Central nervous system (CNS) toxicity involves abnormal mood, delusions, and insomnia; Gastrointestinal toxicity (GI) includes abdominal pain, nausea, emesis, diarrhea; Adverse effects: nausea, dizziness, headache, insomnia, and fatigue [271], [272].
Table 2.
Peptide inhibitors.
| Peptide | Amino acid sequence | Type | Target | Reference |
|---|---|---|---|---|
| Anti-gp120 | DGGNSNNESEIFRPGGGDMRDN | Entry inhibitor | HIV-1/gp120 | [162] |
| Anti-CCR5 | YQVSSPIYDINYYTSEPCQKINVKQIAA | Entry inhibitor | Co-receptor CCR5 | [163] |
| PIE12-trimer | HPXXCDYPEWQWLCXXELGK | Entry inhibitor | HIV gp41 N-trimer pocket | [159] |
| GTKWLTEWIPLTAEAEC | RT inhibitor | HIV-1 RT | [179] | |
| G12 | GI-p-benzoylphenylalanine-FVSL | Protease inhibitor | ε-amino group of Lys 14 of HIV protease | [205] |
| E1P47 | WILEYLWKVPFDFWRGV | Entry inhibitor | HIV-1 Fusion Peptide | [168] |
| p7 | KETWETWWTE | RT inhibitor | Dimerization of RT | [182] |
| Apam (2)-Tyr-Glu-T (4)-OH | RT inhibitor | HIV-1 protease dimer interface | [180] | |
| Vpr 57-71 | VEAIIRILQQLLFIH | RT inhibitor | HIV-1 IN & RT | [183] |
| Vpr 61-75 | IRILQQLLFIHFRIG | RT inhibitor | HIV-1 IN & RT | [183] |
| NYAD-1 | HITFEDLLDYYGP-NH2 | Gag inhibitor | HIV-1 Gag polyprotein | [245] |
| Vif peptide | LITPKKIKPPLPSVT | Vif inhibitor | HIV-1 Vif | [234] |
| p27 | PQITLRKKRRQRRRPPQVSFNFCTLNF | Protease inhibitor | WT & PI resistant HIV-1 protease | [190], [207] |
| Tetrameric peptide 10 | ((ILPWKWPWWPWPP)2K)2K-NH2 | Integrase inhibitor | HIV-1 integrase | [270] |
| Tetrameric RIN-25 | ((ILPWKWPWWPWPP)2K)2K | Integrase inhibitor | HIV-1 integrase | [209] |
3.7. Disadvantages of current therapeutics [125]
Drug resistance is a major problem with current HIV therapeutics for patients failing therapy and therapy-naive patients due to the genetic diversity of HIV. 1 to 10 mutations may be generated every viral replication cycle [54]. Therefore the constant evolution of HIV demands constant evolution of HIV therapeutics. The prevalence of HIV-1 mutated strains that are resistant to one or more antiretroviral inhibitors or drug classes remains one of the leading causes of treatment failure among patients with HIV/AIDS [105], [126], [127]. NNRTIs are particularly susceptible to small mutations because single nucleotide changes can result in high-level resistance with only a slight loss of replicative fitness [54]. Resistance to therapeutics undermines the efficacy of HAART as multidrug resistant strains evolve. Currently available therapeutics used in HAART also need to be improved because of serious side effects. NRTI and NNRTIs have been reported to induce a variety of adverse effects: neuropathy, lactic acidosis, pruritus, fatigue, nausea, and myalgia [107], [125], [128]. Abacavir, for example, causes an immune-mediated hypersensitivity reaction in 5% of the patients [129]. Injection site reactions are the most common adverse events associated with Enfuvirtide [130], which requires twice daily subcutaneous administration due to the high concentration that needs to be maintained in the body (discussed above in 3.2.). Protease inhibitors have shown unbearable toxicity [106]: metabolic syndrome, dyslipidemia, insulin-resistance, lipodystrophy/lipoatrophy, and cardiovascular and cerebrovascular toxicity [131], [132], [133], [134]. Additionally, they have shown poor oral bioavailability and poor penetration across the blood brain barrier [52]. Lowering toxicity of new HIV drugs has been predicted to improve life expectancy and compliance for many patient groups [135]. Inability of drugs currently employed in HAART to reach latent viral reservoirs leads to the necessity for life-long, consistent treatment [136], and the rapid relapse after any non-compliance [137]. Current drugs have poor targeting ability and short residence time, contributing to the latent viral reservoirs [138]. Thus, prolonged and consistent treatment also contributes to the build-up of toxicity and emergence of resistant HIV strains. Nanotechnologies have been proposed to solve this problem [138]. Nanotechnologies have been employed to improve drug oral or i.v. formulation for better bioavailability [139], [140], [141], [142], [143], targeted release [144], [145], [146], or long-term action through sustained release [147]. Among the three nanotechnology applications in HIV treatment, peptides have been mostly studied for cell targeting [148], [149], [150] and cell penetration [114], [151] in delivery of small molecules and/or biologics for treatment and prevention of HIV. The synthetic and chemical approach to HIV treatment has left much to be desired in the ways a technology can overcome current drug resistances, reduce toxic effects, and improve ease of administration for wider acceptance, compliance, and application in global settings, especially in resource-limited areas [18], [152], [153]. Short peptides/proteins are a new field that may hold promise for the treatment of HIV, especially for inhibiting newly discovered protein-protein interaction targets due to its greater affinity than small molecules to these weak binding pockets.
4. Targets of peptide inhibitors [154], [155], [156]
4.1. Peptide inhibitors
Peptide inhibitors share similar targets to currently approved therapeutics and have also been applied to other targets. The targets that have been investigated include entry/fusion, reverse transcriptase, protease and integrase. HIV therapeutics are now generally discovered through 1) high-throughput compound screening with virus-specific assays, 2) optimization of lead compounds, and 3) rational drug design based on the structure of viral proteins [54]. Peptide inhibitors are usually designed based on 1) viral or host protein targets and protein-protein interactions and 2) screening of peptide libraries. Structures are then subject to virus specific assays. Peptide/protein inhibitors hold great promise to improving upon the drug-resistance of small molecular antiviral therapeutics because resistance to macromolecules like peptides and proteins requires evolutionary mutations or co-receptor changes that are a lot less likely than single nucleotide changes in NNRTI-resistant mutations [111], [125], [157], [158], [159]. Thanks to Qureshi et al., a database of HIV inhibiting peptides has been developed and is constantly updated [160]. Peptides in development, summarized in previous reviews and databases [156], [160], are summarized below according to their targets.
4.2. Entry and virus cell fusion [125]
Peptide inhibitors that target the entry process inhibit one or more key proteins including gp41, gp120, coreceptors (CCR5, CXCR4, APJ), and CD4 [77], [111], [125]. Currently as the biological understanding of the entry process increases, a number of design and modification schemes have been presented, and a large number of prospective fusion peptides have emerged [161]. The most promising peptides and proteins that inhibit the entry and fusion process function as antibodies that neutralize the virus or as direct inhibitors by binding viral or host proteins. Antibodies include anti-gp120 monoclonal antibodies and smaller derivatives [162], [163], anti-CD4 antibodies, and anti-CCR5 monoclonal antibodies. Direct inhibitors include soluble gp120 receptors as gp120 inhibitors, synthetic and natural gp41 inhibitors [159], [164], [165], [166], synthetic anti-CCR5 peptides [167], CXCR4 inhibitors, and multi-functional inhibitors (lectins and defensins) [125]. Another class of peptide inhibitors targeting the entry/fusion process mimics the E2 envelope glycoprotein and the NS5A phosphoprotein from GB virus C (GBV-C), which has been shown to inhibit HIV entry [168]. Molecules under investigation share similar limitations to the peptide fusion inhibitor Enfuvirtide - poor bioavailability [169], [170] and high cost of production [171], [172]. Some protease inhibitors show adverse side effects, such as an inflammatory response induced by anti-CCR5 peptides [125], [172]. High dosage is needed for current anti-gp120 antibodies [173]. Due to such limitations, microbicides were used as an alternative to oral administration or injection for several promising peptide entry inhibitors (T20 (Enfuvirtide) [174], T1249 [175], L′644 [176] and Sifuvirtide [177]).
4.3. Reverse transcriptase (RT)
Peptide mimics of reverse transcriptase subunits have been found to inhibit heterodimerization and conformational changes during the formation of reverse transcriptase [81], [178], [179], [180], [181]. Examples include residues 395–404 of RT [182] and Paw [179]. Peptide mimics of other RT binding viral proteins, such as a Vpr protein-derived peptide, also hold promise as peptide inhibitors [183]. Other peptide inhibitors for RT include peptides derived from ribonuclease [184], [185], polyarginine transporter molecules [186], N-methylated peptides that bind RNA [187], protein targeting RTC [188], and anti-fungal peptides [189]. Similarly to protease and integrase peptide inhibitors, RT peptide inhibitors also face the problem of low potency (high IC50 values) and thus have not advanced to in vivo evaluation or clinical studies. There are much fewer publications on RT peptide inhibitors than on entry peptide inhibitors, integrase peptide inhibitors, and protease peptide inhibitors, which is partially due to the success of small molecules in inhibiting RT.
4.4. Protease inhibitors
Peptide inhibitors have also been shown to inhibit protease dimerization. The dimerization interface of protease is created by the conserved active site triad, Asp-Thr-Gly, and four antiparallel beta sheets of the C-terminus and N-terminus of each monomer [190], [191], [192]. Short peptides were synthesized according to the N and C terminus of the protease, which prevents the protease monomer from associating with another monomer. As dimerization is necessary for proteolytic activity, prevention of dimerization effectively inhibits protease activity [178], [193]. To improve upon the weak inhibitory potency of C-terminus and N-terminus mimics [194], [195], [196], flexible linkers and rigid scaffolds have been used, as well as side chain tethering (intercalating “molecular tongs”) [113], [197], [198], [199], [200] and terminal modification with lipophilic groups and alkyl chains [201], [202], [203], [204]. Interfacial peptides can also serve as irreversible inhibitors by covalently associating with protease [205], [206]. Another type of peptide inhibitor that targets dimerization is the fusion of N-terminal HIV-1 protease peptide with cell permeable domain of the HIV-1 Tat protein, which is the transactivator of the virus [190], [207]. Peptide-based protease inhibitors require modulation to overcome the weak binding potency of inhibitors with the protein-protein interaction interface, which increases the complexity of drug design involving optimization of covalent modifications of peptide chains [198]. Prevalence of and similarities between families of proteases in the human body increases the standard for protease inhibitors in recognizing and inhibiting viral proteases and penetrating cell membrane [208]. Meanwhile, peptides show great promise as protease inhibitors because of their versatility in targeting parts of proteases and protease-binding proteins other than the catalytic domain and binding sites that are similar between human and viral proteases.
4.5. Integrase inhibitors
Protein-protein interactions involving integrase, including integrase dimerization and integrase-substrate and allosteric cofactor interactions have been investigated for peptide inhibitor designs [81], [105], [209]. The first group of peptides was derived from the dimer surface of integrase inhibitors with the intent to disrupt integrase inhibitors' dimerization or to inhibit their enzymatic activity [178], [209], [210], [211], [212], [213], [214], [215]. Lead peptides were then improved in design with D-amino acids to prevent degradation. Advancement in the understanding of protein-protein interactions involving integrase has enabled more targeted drug designs. The second group of peptides were derived from integrase inhibitor-binding proteins in order to inhibit integrase inhibitor interaction with host proteins (cofactors such as LEDGF/p75 [216], [217], [218], [219]) [220] or viral proteins [221], [222], [223], such as viral reverse transcriptase [224], Vpr protein [183], and Rev protein. Besides designing inhibitory peptides from integrase inhibitors and integrase inhibitor-binding proteins, inhibitory peptides have been found through screening of phage display libraries or with yeast two-hybrid systems. Antimicrobial peptides showing integrase inhibitory activities include Indolicidin, a host cell defense tridecapeptide [225]. Protein-protein interaction inhibitors, including dimerization inhibitors, have been shown to be relatively week inhibitors with IC50 in the range of 1–250 μM [209]. The advantage of macromolecules, such as peptides and proteins, over small molecule drugs is that they can potentially be optimized in designing shallow binding pockets, such as protein-protein interactions. Since current lead peptide inhibitors are only tested in vitro assays, potency needs to be optimized before in vivo assays and clinical studies. Peptide interface inhibitors of protease and integrase have been studied since the last century [193], [196] from biochemical standpoints but have yet to come close to testing in animals. As interface inhibitors need to bind the target protein tightly enough to outcompete its natural binding partner, it is challenging to design peptides that mimic the binding partner yet have significantly higher binding affinity to achieve clinically meaningful potency [226]. Alternatively, next generation designs of small molecule drugs have been proposed using peptides as starting points [209], [222], [223].
4.6. Others
Other HIV inhibitors include those designed based on other specific targets [227], [228], [229] and through screening of existing peptide libraries [230], [231], [232], [233]. Target-based design of HIV inhibitors have involved targeting: 1) interactions between viral Tat protein and host TAR protein [227], [228], 2) polyproline interfaces of viral infectivity factor for the multimerization of viron infectivity factor proteins [234], 3) the budding of HIV after replication in host cells [235], [236], 4) viral gene expression [237], 5) viron maturation (gag-derived peptides [229], inhibiting gag processing), 6) multi-stage infectivity (CD4 antigen-based anti-receptor peptides [238]), 7) unidentified GBV-C E2 protein [239], [240], 8) syncytium formation [241], [242], 9) viral Vpr and rev proteins [243], [244], 10) viral assembly [245], [246], [247], and 11) alpha-glucosidase [248]. Antimicrobial peptides have been derived from a variety of species, such as amphibians, or through antimicrobial databases screened for anti-HIV activity [230]. They have been shown to inhibit HIV infections of T cells and disrupt HIV envelope and viral core proteins [230], [231], [232], [233]. A prime example is caerin antimicrobial peptides [230]. Anti-HIV antimicrobial peptides have been developed in vitro thus far, and more research is needed to evaluate the suppressing or enhancing effects, as well as chemotactic effects of antimicrobial peptides on the native immune system [249]. Physiological toxicity is a major drawback antimicrobial peptides [250].
4.7. Promising strategies in development
As drug resistance continues to emerge, new drug classes (integrase and virus entry inhibitors) and new drugs in old classes (NRTI and NNRTIs) that are active against drug-resistant HIV strains are needed to combat drug-resistant HIV strains [251], [252]. Because of viral resistance to integrase strand transfer inhibitors targeting the catalytic site, new generations of HIV inhibitors not based on the catalytic triad, such as allosteric integrase inhibitors (ALLINIs), can target INSTI-resistant viruses [105].
Peptide inhibitor design starts from designing peptides according to certain regions of viral or host proteins, screening in peptide libraries for anti-HIV activities, and screening of the entire span of viral proteins and protein-protein interactions that can generate lead peptides needed to be refined [183]. For integrase inhibitors, it is easier to design peptide inhibitors than small molecule inhibitors for preventing protein-protein interactions because it is easier to use peptides to mimic existing binding sites [209]. Limitations faced by peptide inhibitors before clinical trials include bioavailability, instability, and high cost of production [111]. Peptides are polar compounds, and cell penetration is necessary for most targets except for entry/fusion inhibitors, which requires lipophilicity [11]. The inability to penetrate cell membranes has halted development of many peptide based therapeutics [245]. Cell penetration can be aided by nanotechnologies discussed earlier in the review, including conjugating peptides with a cell penetrating peptide derived from HIV Tat protein [253]. Peptide/protein drugs are very prone to degradation in vivo and therefore generally are susceptible to GI first pass metabolism [11]. This instability of peptide/protein drugs and poor oral bioavailability necessitates subcutaneous, intramuscular, or intravenous delivery. Using peptides as a starting point, small molecule drugs can be designed for systemic administration [254]. Alternatively, peptide/protein inhibitors can be used in topical applications, such as peptide/protein based microbicides [125]. Unlike other small molecules, peptide fusion inhibitors may be adsorbed slowly and could extend their half-life at mucosal sites [168]. The high cost of production due to costly expression systems in bacteria, yeast, or mammalian systems has been one of the major limitations for several promising peptide inhibitors [125].
5. Discussion
HIV therapeutics have developed significantly over the past few decades. Clinically, decreasing the spread of HIV begins with education. Rationale approaches given the socioeconomic and societal considerations of the patient population encourage the use of preventative approaches such as pre exposure prophylaxis (PrEP). Once infection is established, the current standard of care is combination of ART initiation with two nucleoside reverse transcriptase inhibitors and a third agent from a different drug category. This is followed by monitoring for any adverse events, toxicity, adherence issues, or drug resistance and making appropriate changes in medication.
The primary deficiencies of current HIV therapeutics in development are administration difficulties, adverse effects, and emerging resistance. Peptide and protein drugs are an exciting new field in medicinal chemistry, as evident by more than 1000 peptide-based drugs that have reached the market [255]. The development of peptide based inhibitors of HIV infection have the benefit of higher potency, higher selectivity, lower toxicity, fewer side effects than small molecules, predictable metabolism, shorter time to market, lower attrition rates, standard synthetic protocols, targeted designs to a broad range of targets, lower accumulation in tissues, higher chemical and biological diversity, and lower chances of developing drug resistance [11], [256].
An interesting approach to improve target cell interaction is to increase the epitope presentation of peptide based drugs [257]. In a recent study based on angiogenesis for the treatment of peripheral artery disease, a novel self-assembling peptide therapeutic, termed SLanc, has shown the potential for improved angiogenesis [258]. The potential mechanism of action is increased peptide epitope presentation that enhances angiogenic receptor activation and signaling [258]. Similarly, the potential for self-assembling peptides to present inhibitory signals at high epitope density on the surface of T cells or virons, may allow for smaller dosing regimens and higher efficacy [259]. One useful application for self-assembling peptides is in vaginal microbicide for prevention of HIV [260]. Using a self-assembling peptide hydrogel to present inhibitory signals reduces dosing and costs, which are significant challenges to be solved in HIV microbicide development [261]. Another strategy is conjugating self-assembling peptides with mimetic peptides derived from the viral envelope proteins or receptors that neutralize HIV particles [262], [263]. Self-assembling peptides conjugated with mimetic peptides in vivo can potentially bind and aggregate HIV particles. The third potential of conjugation of a self-assembling peptide with HIV peptide inhibitors involves using self-assembling peptide to improve bioavailability and stability, as shown in PEGylation of peptide inhibitors [49], [264]. Such conjugation may protect peptide inhibitors from rapid degradation and clearance from the circulation by self-assembly. Conjugation of small molecule and peptide drugs to polymers may allow slow and constant release of drugs and avoid spikes in the blood plasma [265]. Slow, steady release can be further tuned by the length and self-assembling properties of peptide scaffolds and results in decreased dosage and improved patient compliance [266], [267], [268], [269]. Overall, conjugation of self-assembling peptides presents an opportunity to significantly improve major limitations of peptide-based drugs, including unsatisfactory pharmacokinetic properties, rapid metabolism, poor solubility, and poor bioavailability.
6. Conclusion
HIV is a difficult disease to manage with high rates of morbidity and mortality. There are 37 million infected individuals worldwide and 12.8% of them do not know they are infected. Given the magnitude of this pandemic numerous research efforts looking for prevention and treatment of HIV are made with $15 billion being devoted from 2000 to 2004. We aimed to discuss HIV viron inhibitor peptides as a potential effective therapeutic. HIV requires life-long treatment. ART is the treatment for HIV: 2 nucleoside reverse transcriptase inhibitors and a third agent from another class is the most effective treatment. The other classes used in initial treatment are non-nucleoside reverse transcriptase inhibitors, protease inhibitors, and integrase strand transfer inhibitors. HIV can present as an early mononucleosis-like illness or can be asymptomatic early on. Chronic infection occurs from the time of acute infection to a CD4 count of <200 cells/μL. AIDS is defined as a CD4 count of less than 200 cells/μL or the presence of an AIDS defining illness.
ART is recommended for all patients immediately after initial assessment regardless of CD4 count. More than 25 antiretroviral from six therapeutic classes are available. A variety of compounds have been tested against gp120 and CD4 binding. Development of these compounds faces challenges due to the variety in the env gene that codes for gp120 and gp41 and the variety of co-receptors. With regards to co-receptors, CCR5 is blocked by Maraviroc, which blocks binding of gp120 to CCR5. Enfuvirtide binds gp41. These are the two FDA drugs approved as entry/fusion inhibitors and each have their own limitations. Novel agents are looking to target proteins other than CCR5 and gp41. NRTIs were the first antiretroviral drugs available. These agents are complicated by associated toxicity. NNRTIs are the most utilized first line treatment and are faced by the challenge of a low genetic barrier to drug resistance. Protease inhibitors are complicated by being costly and having side effects, as well as drug-drug interactions. Integrase inhibitors block the incorporation of HIV DNA to host DNA. Drug resistance is one of the major challenges to current therapeutics. There are also a variety of toxicities and side effects. They lack good targeting ability and have a short residence time. Short peptides used to inhibit newly discovered protein-protein interaction targets are an area of research that holds promise for novel therapeutics.
Peptide inhibitors share similar targets to currently approved therapeutics as well as other targets. They hold promise on improving drug resistance issues. Many entry and fusion inhibitor models exist for peptides. There are fewer publications on peptide reverse transcriptase inhibition. Peptide inhibitors for integrase inhibition have been investigated. For integrase inhibitors, peptide inhibitors are better than small molecules to target protein-protein interactions. They are limited by bioavailability, instability, and high cost of production.
Competing interests
No authors report competing interests with the current work.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.World Health Organization . 2015. HIV/AIDS Fact Sheet N°360. [Google Scholar]
- 2.Center for Disease Control and Prevention . 2016. Basic Statistics. [Google Scholar]
- 3.Avert . 2015. HIV and AIDS Regional Overview. [Google Scholar]
- 4.Center for Disease Control and Prevention . 2016. HIV Basics. [Google Scholar]
- 5.Center for Disease Control and Prevention . 2016. HIV Testing. [Google Scholar]
- 6.Gebo K.A., Fleishman J.A., Conviser R., Hellinger J., Hellinger F.J., Josephs J.S. Contemporary costs of HIV health care in the HAART era. AIDS (London, Engl.) 2010;24:2705–2715. doi: 10.1097/QAD.0b013e32833f3c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumarasamy N. The impact of antiretroviral therapy in resource-limited settings and current HIV therapeutics. Oral Dis. 2016;22:42–45. doi: 10.1111/odi.12458. [DOI] [PubMed] [Google Scholar]
- 8.AIDS Vaccine Advocacy Coalition . 2015. HIV Prevention Research & Development Investment in 2014. [Google Scholar]
- 9.The Henry J. 2015. Kaiser Family Foundation. U.S. Federal Funding for HIV/AIDS: the President's FY 2016 Budget Request. [Google Scholar]
- 10.Fosgerau K., Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov. today. 2015;20:122–128. doi: 10.1016/j.drudis.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Craik D.J., Fairlie D.P., Liras S., Price D. The future of peptide-based drugs. Chem. Biol. drug Des. 2013;81:136–147. doi: 10.1111/cbdd.12055. [DOI] [PubMed] [Google Scholar]
- 12.Olmez E.O., Akbulut B.S. 2012. Protein-peptide Interactions Revolutionize Drug Development: Chapter. [Google Scholar]
- 13.Choonara B.F., Choonara Y.E., Kumar P., Bijukumar D., du Toit L.C., Pillay V. A review of advanced oral drug delivery technologies facilitating the protection and absorption of protein and peptide molecules. Biotechnol. Adv. 2014;32:1269–1282. doi: 10.1016/j.biotechadv.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Matthews T., Salgo M., Greenberg M., Chung J., DeMasi R., Bolognesi D. Enfuvirtide: the first therapy to inhibit the entry of HIV-1 into host CD4 lymphocytes. Nat. Rev. Drug Discov. 2004;3:215–225. doi: 10.1038/nrd1331. [DOI] [PubMed] [Google Scholar]
- 15.Dorr P., Westby M., Dobbs S., Griffin P., Irvine B., Macartney M. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maartens G., Celum C., Lewin S.R. HIV infection: epidemiology, pathogenesis, treatment, and prevention. Lancet. 2014;384:258–271. doi: 10.1016/S0140-6736(14)60164-1. [DOI] [PubMed] [Google Scholar]
- 17.Siegfried N., Uthman O.A., Rutherford G.W. Optimal time for initiation of antiretroviral therapy in asymptomatic, HIV-infected, treatment-naive adults. Cochrane Libr. 2010;(3):1–29. doi: 10.1002/14651858.CD008272.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kumarasamy N., Krishnan S. Beyond first-line HIV treatment regimens: the current state of antiretroviral regimens, viral load monitoring, and resistance testing in resource-limited settings. Curr. Opin. HIV AIDS. 2013;8:586–590. doi: 10.1097/COH.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 19.Lu D., Wu H., Lu Y., Lu T. The origins of HIV. Adv. Pharmacoepidemiol Drug Saf. 2015;4:e136. [Google Scholar]
- 20.Burke D.S. Recombination in HIV: an important viral evolutionary strategy. Emerg. Infect. Dis. 1997;3:253. doi: 10.3201/eid0303.970301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Crowell T.A., Berry S.A., Fleishman J.A., LaRue R.W., Korthuis P.T., Nijhawan A.E. Impact of hepatitis coinfection on healthcare utilization among persons living with HIV. JAIDS J. Acquir. Immune Defic. Syndromes. 2015;68:425–431. doi: 10.1097/QAI.0000000000000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Naidoo K., Grobler A.C., Deghaye N., Reddy T., Gengiah S., Gray A. Cost-effectiveness of initiating antiretroviral therapy at different points in TB treatment in hiv-TB coinfected ambulatory patients in South Africa. JAIDS J. Acquir. Immune Defic. Syndromes. 2015;69:576–584. doi: 10.1097/QAI.0000000000000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poudel K.C., Poudel-Tandukar K., Palmer P.H., Mizoue T., Jimba M., Kobayashi J. Coinfection of sexually transmitted infections among hiv-positive individuals cross-sectional results of a community-based positive living with HIV (POLH) study in Nepal. J. Int. Assoc. Providers AIDS Care (JIAPAC) 2015 doi: 10.1177/2325957415614644. [DOI] [PubMed] [Google Scholar]
- 24.Freeman M.L., Mudd J.C., Shive C.L., Younes S.-A., Panigrahi S., Sieg S.F. CD8 T-Cell expansion and inflammation linked to CMV coinfection in ART-treated HIV infection. Clin. Infect. Dis. 2016;62:392–396. doi: 10.1093/cid/civ840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beachler D.C., Sugar E.A., Margolick J.B., Weber K.M., Strickler H.D., Wiley D.J. Risk factors for acquisition and clearance of oral human papillomavirus infection among HIV-infected and HIV-uninfected adults. Am. J. Epidemiol. 2015;181:40–53. doi: 10.1093/aje/kwu247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Althoff K.N., McGinnis K.A., Wyatt C.M., Freiberg M.S., Gilbert C., Oursler K.K. Comparison of risk and age at diagnosis of myocardial infarction, end-stage renal disease, and non-AIDS-defining cancer in HIV-infected versus uninfected adults. Clin. Infect. Dis. 2015;60:627–638. doi: 10.1093/cid/ciu869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levy J.A. Dispelling myths and focusing on notable concepts in HIV pathogenesis. Trends Mol. Med. 2015;21:341–353. doi: 10.1016/j.molmed.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Allers K., Hütter G., Hofmann J., Loddenkemper C., Rieger K., Thiel E. Evidence for the cure of HIV infection by CCR5Δ32/Δ32 stem cell transplantation. Blood. 2011;117:2791–2799. doi: 10.1182/blood-2010-09-309591. [DOI] [PubMed] [Google Scholar]
- 29.Hütter G., Nowak D., Mossner M., Ganepola S., Müßig A., Allers K. Long-term control of HIV by CCR5 delta32/delta32 stem-cell transplantation. N. Engl. J. Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 30.Allers K., Schneider T. CCR5Δ32 mutation and HIV infection: basis for curative HIV therapy. Curr. Opin. Virol. 2015;14:24–29. doi: 10.1016/j.coviro.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 31.Tebas P., Stein D., Tang W.W., Frank I., Wang S.Q., Lee G. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. N. Engl. J. Med. 2014;370:901–910. doi: 10.1056/NEJMoa1300662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Date A.A., Shibata A., Goede M., Sanford B., La Bruzzo K., Belshan M. Development and evaluation of a thermosensitive vaginal gel containing raltegravir+ efavirenz loaded nanoparticles for HIV prophylaxis. Antivir. Res. 2012;96:430–436. doi: 10.1016/j.antiviral.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johnson T.J., Clark M.R., Albright T.H., Nebeker J.S., Tuitupou A.L., Clark J.T. A 90-day tenofovir reservoir intravaginal ring for mucosal HIV prophylaxis. Antimicrob. Agents Chemother. 2012;56:6272–6283. doi: 10.1128/AAC.01431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moss J.A., Srinivasan P., Smith T.J., Butkyavichene I., Lopez G., Brooks A.A. Pharmacokinetics and preliminary safety study of pod-intravaginal rings delivering antiretroviral combinations for HIV prophylaxis in a macaque model. Antimicrob. Agents Chemother. 2014;58:5125–5135. doi: 10.1128/AAC.02871-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.García-Lerma J.G., Paxton L., Kilmarx P.H., Heneine W. Oral pre-exposure prophylaxis for HIV prevention. Trends Pharmacol. Sci. 2010;31:74–81. doi: 10.1016/j.tips.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 36.Gunawardana M., Remedios-Chan M., Miller C.S., Fanter R., Yang F., Marzinke M.A. Pharmacokinetics of long-acting tenofovir alafenamide (GS-7340) subdermal implant for HIV prophylaxis. Antimicrob. Agents Chemother. 2015;59:3913–3919. doi: 10.1128/AAC.00656-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant R.M., Lama J.R., Anderson P.L., McMahan V., Liu A.Y., Vargas L. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N. Engl. J. Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karim S.S.A., Kashuba A.D., Werner L., Karim Q.A. Drug concentrations after topical and oral antiretroviral pre-exposure prophylaxis: implications for HIV prevention in women. Lancet. 2011;378:279. doi: 10.1016/S0140-6736(11)60878-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh U.M., Dobard C., Sharma S., Cong M.E., Jia H., Martin A. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J. Virol. 2009;83:10358–10365. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gunthard H.F., Aberg J.A., Eron J.J., Hoy J.F., Telenti A., Benson C.A. Antiretroviral treatment of adult HIV infection: 2014 recommendations of the international antiviral Society-USA panel. JAMA: J. Am. Med. Assoc. 2014;312:410–425. doi: 10.1001/jama.2014.8722. [DOI] [PubMed] [Google Scholar]
- 41.Ridzon R., Gallagher K., Ciesielski C., Ginsberg M.B., Robertson B.J., Luo C.C. Simultaneous transmission of human immunodeficiency virus and hepatitis C virus from a needle-stick injury. N. Engl. J. Med. 1997;336:919–922. doi: 10.1056/NEJM199703273361304. [DOI] [PubMed] [Google Scholar]
- 42.Gupta K.K. Acute immunosuppression with HIV seroconversion. N. Engl. J. Med. 1993;328:288–289. doi: 10.1056/NEJM199301283280419. [DOI] [PubMed] [Google Scholar]
- 43.From the Centers for Disease Control and Prevention 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. JAMA: J. Am. Med. Assoc. 1993;269:729–730. [PubMed] [Google Scholar]
- 44.Taylor J.M., Sy J.P., Visscher B., Giorgi J.V. CD4+ T-cell number at the time of acquired immunodeficiency syndrome. Am. J. Epidemiol. 1995;141:645–651. doi: 10.1093/oxfordjournals.aje.a117480. [DOI] [PubMed] [Google Scholar]
- 45.From the Centers for Disease Control and Prevention Projections of the number of persons diagnosed with AIDS and the number of immunosuppressed HIV-infected persons–United States, 1992-1994. JAMA: J. Am. Med. Assoc. 1993;269:733. [PubMed] [Google Scholar]
- 46.Yarchoan R., Venzon D.J., Pluda J.M., Lietzau J., Wyvill K.M., Tsiatis A.A. CD4 count and the risk for death in patients infected with HIV receiving antiretroviral therapy. Ann. Intern Med. 1991;115:184–189. doi: 10.7326/0003-4819-115-3-184. [DOI] [PubMed] [Google Scholar]
- 47.Phillips A.N., Elford J., Sabin C., Bofill M., Janossy G., Lee C.A. Immunodeficiency and the risk of death in HIV infection. JAMA: J. Am. Med. Assoc. 1992;268:2662–2666. [PubMed] [Google Scholar]
- 48.Group I.S.S., Lundgren J.D., Babiker A.G., Gordin F., Emery S., Grund B. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N. Engl. J. Med. 2015;373:795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danial M., Klok H.A. Polymeric anti-hiv therapeutics. Macromol. Biosci. 2015;15:9–35. doi: 10.1002/mabi.201400298. [DOI] [PubMed] [Google Scholar]
- 50.Deeks S.G., Phillips A.N. Clinical review: HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. Bmj. 2009;338:288–292. doi: 10.1136/bmj.a3172. [DOI] [PubMed] [Google Scholar]
- 51.U.S. Food and Drug Administration Get Illness/Condition Information. 2015. [Google Scholar]
- 52.Esté J.A., Cihlar T. Current status and challenges of antiretroviral research and therapy. Antivir. Res. 2010;85:25–33. doi: 10.1016/j.antiviral.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 53.U.S. Department of Health and Human Services . 2015. Overview of HIV Treatments. [Google Scholar]
- 54.Arts E.J., Hazuda D.J. HIV-1 antiretroviral drug therapy. Cold Spring Harb. Perspect. Med. 2012;2:a007161. doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rhee S.-Y., Liu T.F., Kiuchi M., Zioni R., Gifford R.J., Holmes S.P. Natural variation of HIV-1 group M integrase: implications for a new class of antiretroviral inhibitors. Retrovirology. 2008;5:74. doi: 10.1186/1742-4690-5-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Clercq E. New developments in anti-HIV chemotherapy. Il Farm. 2001;56:3–12. doi: 10.1016/s0014-827x(01)01007-2. [DOI] [PubMed] [Google Scholar]
- 57.Meanwell N., Kadow J. Inhibitors of the entry of HIV into host cells. Curr. Opin. drug Discov. Dev. 2003;6:451–461. [PubMed] [Google Scholar]
- 58.Tilton J.C., Doms R.W. Entry inhibitors in the treatment of HIV-1 infection. Antivir. Res. 2010;85:91–100. doi: 10.1016/j.antiviral.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 59.Wilen C.B., Tilton J.C., Doms R.W. Springer; 2012. Molecular Mechanisms of HIV Entry. Viral Molecular Machines; pp. 223–242. [DOI] [PubMed] [Google Scholar]
- 60.Wilen C.B., Tilton J.C., Doms R.W. HIV: cell binding and entry. Cold Spring Harb. Perspect. Med. 2012;2:a006866. doi: 10.1101/cshperspect.a006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan Q., Zhu Y., Li J., Chen Z., Han G.W., Kufareva I. Structure of the CCR5 chemokine receptor–HIV entry inhibitor maraviroc complex. Science. 2013;341:1387–1390. doi: 10.1126/science.1241475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Promsri S., Ullmann G.M., Hannongbua S. Molecular dynamics simulation of HIV-1 fusion domain-membrane complexes: insight into the N-terminal gp41 fusion mechanism. Biophys. Chem. 2012;170:9–16. doi: 10.1016/j.bpc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Esté J.A., Telenti A. HIV entry inhibitors. Lancet. 2007;370:81–88. doi: 10.1016/S0140-6736(07)61052-6. [DOI] [PubMed] [Google Scholar]
- 64.Mann A., Friedrich N., Krarup A., Weber J., Stiegeler E., Dreier B. Conformation-dependent recognition of HIV gp120 by designed ankyrin repeat proteins provides access to novel HIV entry inhibitors. J. Virol. 2013;87:5868–5881. doi: 10.1128/JVI.00152-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sangphukieo A., Nawae W., Laomettachit T., Supasitthimethee U., Ruengjitchatchawalya M. Computational design of hypothetical new peptides based on a cyclotide scaffold as HIV gp120 inhibitor. PloS One. 2015;10:e0139562. doi: 10.1371/journal.pone.0139562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu L., Yu F., Cai L., Debnath A., Jiang S. Development of small-molecule HIV entry inhibitors specifically targeting gp120 or gp41. Curr. Top. Med. Chem. 2016;16(10):1074–1090. doi: 10.2174/1568026615666150901114527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daar E.S., Li X.L., Moudgil T., Ho D.D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc. Natl. Acad. Sci. U. S. A. 1990;87:6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Garcia-Perez J., Rueda P., Staropoli I., Kellenberger E., Alcami J., Arenzana-Seisdedos F. New insights into the mechanisms whereby low molecular weight CCR5 ligands inhibit HIV-1 infection. J. Biol. Chem. 2011;286:4978–4990. doi: 10.1074/jbc.M110.168955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cai L., Jiang S. Development of peptide and small-molecule HIV-1 fusion inhibitors that target gp41. ChemMedChem. 2010;5:1813–1824. doi: 10.1002/cmdc.201000289. [DOI] [PubMed] [Google Scholar]
- 70.Pan C., Liu S., Jiang S. HIV-1 gp41 fusion intermediate: a target for HIV therapeutics. J. Formos. Med. Assoc. 2010;109:94–105. doi: 10.1016/S0929-6646(10)60029-0. [DOI] [PubMed] [Google Scholar]
- 71.Eggink D., Berkhout B., Sanders W., Rigier Inhibition of HIV-1 by fusion inhibitors. Curr. Pharm. Des. 2010;16:3716–3728. doi: 10.2174/138161210794079218. [DOI] [PubMed] [Google Scholar]
- 72.Wagner T.A., Frenkel L.M. Potential limitation of CCR5 antagonists: drug resistance more often linked to CXCR4-utilizing than to CCRS-utilizing HIV-1. AIDS (London, Engl.) 2008;22:2393. doi: 10.1097/QAD.Ob013e328312c72c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bahrami S, Tolstrup M, Ryttergmrd MD, Pedersen FS, Ostergaard LJ. Bivalent molecules for hiv entry inhibition. Google Patent; 2010.
- 74.Ariel A., Fredman G., Sun Y.-P., Kantarci A., Van Dyke T.E., Luster A.D. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat. Immunol. 2006;7:1209–1216. doi: 10.1038/ni1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sakaida H., Hori T., Yonezawa A., Sato A., Isaka Y., Yoshie O. T-tropic human immunodeficiency virus type 1 (HIV-1)-derived V3 loop peptides directly bind to CXCR-4 and inhibit T-tropic HIV-1 infection. J. Virol. 1998;72:9763–9770. doi: 10.1128/jvi.72.12.9763-9770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maselko M., Ward C., Pastey M. A RhoA-derived peptide inhibits human immunodeficiency virus-1 entry in vitro. Curr. HIV Res. 2011;9:1–5. doi: 10.2174/157016211794582605. [DOI] [PubMed] [Google Scholar]
- 77.Zou M.X., Liu H.Y., Haraguchi Y., Soda Y., Tatemoto K., Hoshino H. Apelin peptides block the entry of human immunodeficiency virus (HIV) FEBS Lett. 2000;473:15–18. doi: 10.1016/s0014-5793(00)01487-3. [DOI] [PubMed] [Google Scholar]
- 78.VanCompernolle S.E., Taylor R.J., Oswald-Richter K., Jiang J., Youree B.E., Bowie J.H. Antimicrobial peptides from amphibian skin potently inhibit human immunodeficiency virus infection and transfer of virus from dendritic cells to T cells. J. Virol. 2005;79:11598–11606. doi: 10.1128/JVI.79.18.11598-11606.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cihlar T., Ray A.S. Nucleoside and nucleotide HIV reverse transcriptase inhibitors: 25 years after zidovudine. Antivir. Res. 2010;85:39–58. doi: 10.1016/j.antiviral.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 80.Lange J., Ananworanich J. The discovery and development of antiretroviral agents. Antivir. Ther. 2014;19:5–14. doi: 10.3851/IMP2896. [DOI] [PubMed] [Google Scholar]
- 81.d Soultrait V., Desjobert C., Tarrago-Litvak L. Peptides as new inhibitors of HIV-1 reverse transcriptase and integrase. Curr. Med. Chem. 2003;10:1765–1778. doi: 10.2174/0929867033457007. [DOI] [PubMed] [Google Scholar]
- 82.Engelman A., Mizuuchi K., Craigie R. HIV-1 DNA integration: mechanism of viral DNA cleavage and DNA strand transfer. Cell. 1991;67:1211–1221. doi: 10.1016/0092-8674(91)90297-c. [DOI] [PubMed] [Google Scholar]
- 83.U.S. Department of Veterans Affairs.
- 84.Yuen G.J., Weller S., Pakes G.E. A review of the pharmacokinetics of Abacavir. Clin. Pharmacokinet. 2012;47:351–371. doi: 10.2165/00003088-200847060-00001. [DOI] [PubMed] [Google Scholar]
- 85.Lewis W., Day B.J., Copeland W.C. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat. Rev. Drug Discov. 2003;2:812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- 86.World Health Organization . 2006. Antiretroviral therapy for hiv infection in adults and adolescents: Recommendations for a Public Health Approach. ed2006. [PubMed] [Google Scholar]
- 87.World Health Organization . 2010. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach. ed2010. [PubMed] [Google Scholar]
- 88.Claessens Y.-E., Chiche J.-D., Mira J.-P., Cariou A. Bench-to-bedside review: severe lactic acidosis in HIV patients treated with nucleoside analogue reverse transcriptase inhibitors. Crit. care. 2003;7:226. doi: 10.1186/cc2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Margolis A.M., Heverling H., Pham P.A., Stolbach A. A review of the toxicity of HIV medications. J. Med. Toxicol. 2014;10:26–39. doi: 10.1007/s13181-013-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee H., Hanes J., Johnson K.A. Toxicity of nucleoside analogues used to treat AIDS and the selectivity of the mitochondrial DNA polymerase. Biochemistry. 2003;42:14711–14719. doi: 10.1021/bi035596s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dykens J.A., Will Y. The significance of mitochondrial toxicity testing in drug development. Drug Discov. Today. 2007;12:777–785. doi: 10.1016/j.drudis.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 92.de Béthune M.-P. Non-nucleoside reverse transcriptase inhibitors (NNRTIs), their discovery, development, and use in the treatment of HIV-1 infection: a review of the last 20 years. Antivir. Res. 1989–2009;2010(85):75–90. doi: 10.1016/j.antiviral.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 93.McKinnell J.A., Willig J.H., Westfall A.O., Nevin C., Allison J.J., Raper J.L. Antiretroviral prescribing patterns in treatment-naive patients in the United States. AIDS Patient Care STDs. 2010;24:79–85. doi: 10.1089/apc.2009.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Horberg M.A., Klein D.B. An update on the use of Atripla in the treatment of HIV in the United States. HIV/AIDS Res. Palliat. Care. 2010;2:135–140. doi: 10.2147/hiv.s6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kohlstaedt L., Wang J., Friedman J., Rice P., Steitz T. Crystal structure at 3.5 A resolution of HIV-1 reverse transcriptase complexed with an inhibitor. Science. 1992;256:1783–1790. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 96.Peletskaya E.N., Kogon A.A., Tuske S., Arnold E., Hughes S.H. Nonnucleoside inhibitor binding affects the interactions of the fingers subdomain of human immunodeficiency virus type 1 reverse transcriptase with DNA. J. Virol. 2004;78:3387–3397. doi: 10.1128/JVI.78.7.3387-3397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Desai M., Iyer G., Dikshit R.K. Antiretroviral drugs: critical issues and recent advances. Indian J. Pharmacol. 2012;44:288–298. doi: 10.4103/0253-7613.96296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Usach I., Melis V., Peris J.-E. Non-nucleoside reverse transcriptase inhibitors: a review on pharmacokinetics, pharmacodynamics, safety and tolerability. J. Int. AIDS Soc. 2013:16. doi: 10.7448/IAS.16.1.18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hill A., McBride A., Sawyer A.W., Clumeck N., Gupta R.K. Resistance at virological failure using boosted protease inhibitors versus nonnucleoside reverse transcriptase inhibitors as first-line antiretroviral therapy—implications for sustained efficacy of ART in resource-limited settings. J. Infect. Dis. 2013;207:S78–S84. doi: 10.1093/infdis/jit112. [DOI] [PubMed] [Google Scholar]
- 100.Tang M.W., Shafer R.W. HIV-1 antiretroviral resistance. Drugs. 2012;72:e1–e25. doi: 10.2165/11633630-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sluis-Cremer N., Temiz N.A., Bahar I. Conformational changes in HIV-1 reverse transcriptase induced by nonnucleoside reverse transcriptase inhibitor binding. Curr. HIV Res. 2004;2:323. doi: 10.2174/1570162043351093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Campiani G., Ramunno A., Maga G., Nacci V., Fattorusso C., Catalanotti B. Non-nucleoside HIV-1 reverse transcriptase (RT) inhibitors: past, present, and future perspectives. Curr. Pharm. Des. 2002;8:615–657. doi: 10.2174/1381612024607207. [DOI] [PubMed] [Google Scholar]
- 103.Hamers R.L., Sigaloff K.C., Wensing A.M., Wallis C.L., Kityo C., Siwale M. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin. Infect. Dis. 2012 doi: 10.1093/cid/cis254. cis254. [DOI] [PubMed] [Google Scholar]
- 104.Bartlett J.A., Shao J.F. Successes, challenges, and limitations of current antiretroviral therapy in low-income and middle-income countries. Lancet Infect. Dis. 2009;9:637–649. doi: 10.1016/S1473-3099(09)70227-0. [DOI] [PubMed] [Google Scholar]
- 105.Wainberg M.A., Mesplède T., Quashie P.K. The development of novel HIV integrase inhibitors and the problem of drug resistance. Curr. Opin. Virol. 2012;2:656–662. doi: 10.1016/j.coviro.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 106.Lv Z., Chu Y., Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV/AIDS (Auckl. NZ) 2015;7:95. doi: 10.2147/HIV.S79956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pierson T.C., Doms R.W., Pöhlmann S. Prospects of HIV-1 entry inhibitors as novel therapeutics. Rev. Med. Virol. 2004;14:255–270. doi: 10.1002/rmv.435. [DOI] [PubMed] [Google Scholar]
- 108.Jacks T., Power M.D., Masiarz F.R., Luciw P.A., Barr P.J., Varmus H.E. Characterization of ribosomal frameshifting in HIV-1 gag-pol expression. Nature. 1988;331:280–283. doi: 10.1038/331280a0. [DOI] [PubMed] [Google Scholar]
- 109.Brik A., Wong C.-H. HIV-1 protease: mechanism and drug discovery. Org. Biomol. Chem. 2003;1:5–14. doi: 10.1039/b208248a. [DOI] [PubMed] [Google Scholar]
- 110.Pokorná J., Machala L., Řezáčová P., Konvalinka J. Current and novel inhibitors of HIV protease. Viruses. 2009;1:1209–1239. doi: 10.3390/v1031209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.von Recum H.A., Pokorski J.K. Peptide and protein-based inhibitors of HIV-1 co-receptors. Exp. Biol. Med. 2013;238:442–449. doi: 10.1177/1535370213480696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ghosh A.K., Anderson D.D., Mitsuya H. The FDA approved HIV-1 protease inhibitors for treatment of hiv/AIDS. Burger's Med. Chem. Drug Discov. 2010:1–74. [Google Scholar]
- 113.Ghosh A.K., Osswald H.L., Prato G. Recent progress in the development of HIV-1 protease inhibitors for the treatment of hiv/AIDS. J. Med. Chem. 2016;59(11):5172–5208. doi: 10.1021/acs.jmedchem.5b01697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou J., Neff C.P., Liu X., Zhang J., Li H., Smith D.D. Systemic administration of combinatorial dsiRNAs via nanoparticles efficiently suppresses HIV-1 infection in humanized mice. Mol. Ther. 2011;19:2228–2238. doi: 10.1038/mt.2011.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Temesgen Z., Siraj D.S. Raltegravir: first in class HIV integrase inhibitor. Ther. Clin. Risk Manag. 2008;4:493–500. doi: 10.2147/tcrm.s2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shimura K., Kodama E.N. Elvitegravir: a new HIV integrase inhibitor. Antivir. Chem. Chemother. 2009;20:79–85. doi: 10.3851/IMP1397. [DOI] [PubMed] [Google Scholar]
- 117.Espeseth A.S., Felock P., Wolfe A., Witmer M., Grobler J., Anthony N. HIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integrase. Proc. Natl. Acad. Sci. 2000;97:11244–11249. doi: 10.1073/pnas.200139397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Engelman A., Cherepanov P. The structural biology of HIV-1: mechanistic and therapeutic insights. Nat. Rev. Microbiol. 2012;10:279–290. doi: 10.1038/nrmicro2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hare S., Smith S.J., Métifiot M., Jaxa-Chamiec A., Pommier Y., Hughes S.H. Structural and functional analyses of the second-generation integrase strand transfer inhibitor dolutegravir (S/GSK1349572) Mol. Pharmacol. 2011;80:565–572. doi: 10.1124/mol.111.073189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Serrao E., Odde S., Ramkumar K., Neamati N. Raltegravir, elvitegravir, and metoogravir: the birth of. Retrovirology. 2009;6:25. doi: 10.1186/1742-4690-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elvitegravir Shinkai H. A new HIV-1 integrase inhibitor for antiretroviral therapy. Success. Drug Discov. 2015:113–128. [Google Scholar]
- 122.Dewdney T.G. 2013. Biochemical, Structural, and Drug Design Studies of Multi-drug Resistant Hiv-1 Therapeutic Targets. [Google Scholar]
- 123.Espeseth A.S., Felock P., Wolfe A., Witmer M., Grobler J., Anthony N. HIV-1 integrase inhibitors that compete with the target DNA substrate define a unique strand transfer conformation for integrase. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11244–11249. doi: 10.1073/pnas.200139397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Osterholzer D.A., Goldman M. Dolutegravir: a next-generation integrase inhibitor for treatment of HIV infection. Clin. Infect. Dis. 2014;59:265–271. doi: 10.1093/cid/ciu221. [DOI] [PubMed] [Google Scholar]
- 125.Fumakia M., Yang S., Gu J., Ho E.A. Protein/peptide-based entry/fusion inhibitors as anti-HIV therapies: challenges and future direction. Rev. Med. Virol. 2016;26:4–20. doi: 10.1002/rmv.1853. [DOI] [PubMed] [Google Scholar]
- 126.Wensing A.M., van Maarseveen N.M., Nijhuis M. Fifteen years of HIV Protease Inhibitors: raising the barrier to resistance. Antivir. Res. 2010;85:59–74. doi: 10.1016/j.antiviral.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 127.Brenner B.G., Wainberg M.A., Turner D. HIV-1 drug resistance: can we overcome? Expert Opin. Biol. Ther. 2002;2:751–761. doi: 10.1517/14712598.2.7.751. [DOI] [PubMed] [Google Scholar]
- 128.Fukunaga K., Tsutsumi H., Mihara H. Cell differentiation on disk-and string-shaped hydrogels fabricated from Ca2+-responsive self-assembling peptides. Biopolymers. 2015;106:476–483. doi: 10.1002/bip.22756. [DOI] [PubMed] [Google Scholar]
- 129.Martin M.A., Kroetz D.L. Abacavir pharmacogenetics–from initial reports to standard of care. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2013;33:765–775. doi: 10.1002/phar.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bean P. New drug targets for HIV. Clin. Infect. Dis. 2005;41:S96–S100. doi: 10.1086/429504. [DOI] [PubMed] [Google Scholar]
- 131.Bozzette S.A., Ake C.F., Tam H.K., Chang S.W., Louis T.A. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N. Engl. J. Med. 2003;348:702–710. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 132.Hruz P.W. HIV protease inhibitors and insulin resistance: lessons from in vitro, rodent and healthy human volunteer models. Curr. Opin. HIV AIDS. 2008;3:660. doi: 10.1097/COH.0b013e3283139134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kotler D.P. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. JAIDS J. Acquir. Immune Defic. Syndromes. 2008;49:S79–S85. doi: 10.1097/QAI.0b013e318186519c. [DOI] [PubMed] [Google Scholar]
- 134.Soontornniyomkij V., Umlauf A., Chung S.A., Cochran M.L., Soontornniyomkij B., Gouaux B. HIV protease inhibitor exposure predicts cerebral small vessel disease. AIDS (London, Engl. 2014;28:1297–1306. doi: 10.1097/QAD.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khademi A., Braithwaite R.S., Saure D., Schaefer A.J., Nucifora K., Roberts M.S. Should expectations about the rate of new antiretroviral drug development impact the timing of HIV treatment initiation and expectations about treatment benefits? PloS one. 2014;9:e98354. doi: 10.1371/journal.pone.0098354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alexaki A., Liu Y., Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr. HIV Res. 2008;6:388. doi: 10.2174/157016208785861195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Richman D.D., Margolis D.M., Delaney M., Greene W.C., Hazuda D., Pomerantz R.J. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- 138.Sharma P., Garg S. Pure drug and polymer based nanotechnologies for the improved solubility, stability, bioavailability and targeting of anti-HIV drugs. Adv. Drug Deliv. Rev. 2010;62:491–502. doi: 10.1016/j.addr.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 139.Müller R., Jacobs C., Kayser O. Nanosuspensions as particulate drug formulations in therapy: rationale for development and what we can expect for the future. Adv. drug Deliv. Rev. 2001;47:3–19. doi: 10.1016/s0169-409x(00)00118-6. [DOI] [PubMed] [Google Scholar]
- 140.Van Eerdenbrugh B., Froyen L., Martens J., Blaton N., Augustijns P., Brewster M. Characterization of physico-chemical properties and pharmaceutical performance of sucrose co-freeze–dried solid nanoparticulate powders of the anti-HIV agent loviride prepared by media milling. Int. J. Pharm. 2007;338:198–206. doi: 10.1016/j.ijpharm.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 141.Fakes M.G., Vakkalagadda B.J., Qian F., Desikan S., Gandhi R.B., Lai C. Enhancement of oral bioavailability of an HIV-attachment inhibitor by nanosizing and amorphous formulation approaches. Int. J. Pharm. 2009;370:167–174. doi: 10.1016/j.ijpharm.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 142.Dou H., Morehead J., Destache C.J., Kingsley J.D., Shlyakhtenko L., Zhou Y. Laboratory investigations for the morphologic, pharmacokinetic, and anti-retroviral properties of indinavir nanoparticles in human monocyte-derived macrophages. Virology. 2007;358:148–158. doi: 10.1016/j.virol.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 143.Shaik N., Pan G., Elmquist W.F. Interactions of pluronic block copolymers on P-gp efflux activity: experience with HIV-1 protease inhibitors. J. Pharm. Sci. 2008;97:5421–5433. doi: 10.1002/jps.21372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.LÖBENBERG R., KREUTER J. Macrophage targeting of azidothymidine: a promising strategy for AIDS therapy*. AIDS Res. Hum. Retroviruses. 1996;12:1709–1715. doi: 10.1089/aid.1996.12.1709. [DOI] [PubMed] [Google Scholar]
- 145.Shah L.K., Amiji M.M. Intracellular delivery of saquinavir in biodegradable polymeric nanoparticles for HIV/AIDS. Pharm. Res. 2006;23:2638–2645. doi: 10.1007/s11095-006-9101-7. [DOI] [PubMed] [Google Scholar]
- 146.Kuo Y.-C., Kuo C.-Y. Electromagnetic interference in the permeability of saquinavir across the blood–brain barrier using nanoparticulate carriers. Int. J. Pharm. 2008;351:271–281. doi: 10.1016/j.ijpharm.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 147.Baert L., van‘t Klooster G., Dries W., François M., Wouters A., Basstanie E. Development of a long-acting injectable formulation with nanoparticles of rilpivirine (TMC278) for HIV treatment. Eur. J. Pharm. Biopharm. 2009;72:502–508. doi: 10.1016/j.ejpb.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 148.Dutta T., Garg M., Jain N.K. Targeting of efavirenz loaded tuftsin conjugated poly (propyleneimine) dendrimers to HIV infected macrophages in vitro. Eur. J. Pharm. Sci. 2008;34:181–189. doi: 10.1016/j.ejps.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 149.Dutta T., Jain N.K. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim. Biophys. Acta (BBA)-General Subj. 2007;1770:681–686. doi: 10.1016/j.bbagen.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 150.Borgmann K., Rao K.S., Labhasetwar V., Ghorpade A. Efficacy of Tat-conjugated ritonavir-loaded nanoparticles in reducing HIV-1 replication in monocyte-derived macrophages and cytocompatibility with macrophages and human neurons. AIDS Res. Hum. Retroviruses. 2011;27:853–862. doi: 10.1089/aid.2010.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhou J., Shu Y., Guo P., Smith D.D., Rossi J.J. Dual functional RNA nanoparticles containing phi29 motor pRNA and anti-gp120 aptamer for cell-type specific delivery and HIV-1 inhibition. Methods. 2011;54:284–294. doi: 10.1016/j.ymeth.2010.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hosseinipour M.C., Gupta R.K., Van Zyl G., Eron J.J., Nachega J.B. Emergence of HIV drug resistance during first-and second-line antiretroviral therapy in resource-limited settings. J. Infect. Dis. 2013;207:S49–S56. doi: 10.1093/infdis/jit107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sigaloff K.C., Kayiwa J., Musiime V., Calis J.C., Kaudha E., Mukuye A. Short communication: high rates of thymidine analogue mutations and dual-class resistance among HIV-infected Ugandan children failing first-line antiretroviral therapy. AIDS Res. Hum. Retroviruses. 2013;29:925–930. doi: 10.1089/AID.2012.0218. [DOI] [PubMed] [Google Scholar]
- 154.Andreola M. Therapeutic potential of peptide motifs against HIV-1 reverse transcriptase and integrase. Curr. Pharm. Des. 2009;15:2508–2519. doi: 10.2174/138161209788682244. [DOI] [PubMed] [Google Scholar]
- 155.Du Q.-S., Xie N.-Z., Huang R.-B. Recent development of peptide drugs and advance on theory and methodology of peptide inhibitor design. Med. Chem. 2015;11:235–247. doi: 10.2174/1573406411666141229163355. [DOI] [PubMed] [Google Scholar]
- 156.Chandra K., Maes M., Friedler A. Interactions of HIV-1 proteins as targets for developing anti-HIV-1 peptides. Future Med. Chem. 2015;7:1055–1077. doi: 10.4155/fmc.15.46. [DOI] [PubMed] [Google Scholar]
- 157.Nedellec R., Coetzer M., Lederman M.M., Offord R.E., Hartley O., Mosier D.E. “Resistance” to PSC-RANTES revisited: two mutations in human immunodeficiency virus type 1 HIV-1SF162 or simian-human immunodeficiency virus SHIVSF162-p3 do not confer resistance. J. Virol. 2010;84:5842–5845. doi: 10.1128/JVI.01907-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nedellec R., Coetzer M., Lederman M.M., Offord R.E., Hartley O., Mosier D.E. Resistance to the CCR5 inhibitor 5P12-RANTES requires a difficult evolution from CCR5 to CXCR4 coreceptor use. PloS One. 2011;6:e22020. doi: 10.1371/journal.pone.0022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Welch B.D., Francis J.N., Redman J.S., Paul S., Weinstock M.T., Reeves J.D. Design of a potent D-peptide HIV-1 entry inhibitor with a strong barrier to resistance. J. Virol. 2010;84:11235–11244. doi: 10.1128/JVI.01339-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Qureshi A., Thakur N., Kumar M. HIPdb: a database of experimentally validated HIV inhibiting peptides. PloS one. 2013;8:e54908. doi: 10.1371/journal.pone.0054908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Jun Tan J., Ting Ma X., Liu C., Yi Zhang X., Xin Wang C. The current status and challenges in the development of fusion inhibitors as therapeutics for HIV-1 infection. Curr. Pharm. Des. 2013;19:1810–1817. doi: 10.2174/1381612811319100005. [DOI] [PubMed] [Google Scholar]
- 162.Wang L.-X., Mellon M., Bowder D., Quinn M., Shea D., Wood C. Escherichia coli surface display of single-chain antibody VRC01 against HIV-1 infection. Virology. 2015;475:179–186. doi: 10.1016/j.virol.2014.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang M.-Y., Borges A.R., Ptak R.G., Wang Y., Dimitrov A.S., Alam S.M. Taylor & Francis; 2010. Potent and Broad Neutralizing Activity of a Single Chain Antibody Fragment against Cell-free and Cell-associated HIV-1. MAbs; pp. 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bellows M.L., Taylor M.S., Cole P.A., Shen L., Siliciano R.F., Fung H.K. Discovery of entry inhibitors for HIV-1 via a new de novo protein design framework. Biophysical J. 2010;99:3445–3453. doi: 10.1016/j.bpj.2010.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Forssmann W.-G., Stoll M., Adermann K., Albrecht U., Tillmann H.-C., Barlos K. Short-term monotherapy in HIV-infected patients with a virus entry inhibitor against the gp41 fusion peptide. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3001697. 63re3-re3. [DOI] [PubMed] [Google Scholar]
- 166.Naito T., Izumi K., Kodama E., Sakagami Y., Kajiwara K., Nishikawa H. SC29EK, a peptide fusion inhibitor with enhanced alpha-helicity, inhibits replication of human immunodeficiency virus type 1 mutants resistant to enfuvirtide. Antimicrob. Agents Chemother. 2009;53:1013–1018. doi: 10.1128/AAC.01211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Liu Q., Cimbro R., Guzzo C., Zhang P., Miao H., Ryk D. P-D4 Tyrosine-sulfated peptides from the gp120 V2 domain block HIV-1 entry through CCR5 Mimicry. JAIDS J. Acquir. Immune Defic. Syndromes. 2016;71:93. [Google Scholar]
- 168.Gómara M., Sánchez-Merino V., Paús A., Merino-Mansilla A., Gatell J., Yuste E. Definition of an 18-mer synthetic peptide derived from the GB virus C E1 protein as a new HIV-1 entry inhibitor. Biochim. Biophys. Acta (BBA)-General Subj. 2016 Jun;1860(6):1139–1148. doi: 10.1016/j.bbagen.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 169.Lalezari J.P., Bellos N.C., Sathasivam K., Richmond G.J., Cohen C.J., Myers R.A. T-1249 retains potent antiretroviral activity in patients who had experienced virological failure while on an enfuvirtide-containing treatment regimen. J. Infect. Dis. 2005;191:1155–1163. doi: 10.1086/427993. [DOI] [PubMed] [Google Scholar]
- 170.Martin-Carbonero L. Discontinuation of the clinical development of fusion inhibitor T-1249. AIDS Rev. 2004;6:61. [PubMed] [Google Scholar]
- 171.Chen W., Dimitrov D.S. Monoclonal antibody-based candidate therapeutics against HIV type 1. AIDS Res. Hum. Retroviruses. 2012;28:425–434. doi: 10.1089/aid.2011.0226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Choi W.-T., Nedellec R., Coetzer M., Colin P., Lagane B., Offord R.E. CCR5 mutations distinguish N-terminal modifications of RANTES (CCL5) with agonist versus antagonist activity. J. Virol. 2012;86:10218–10220. doi: 10.1128/JVI.00353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Moldt B., Rakasz E.G., Schultz N., Chan-Hui P.-Y., Swiderek K., Weisgrau K.L. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc. Natl. Acad. Sci. 2012;109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Jenabian M.A., Saidi H., Charpentier C., Van Herrewege Y., Son J.C., Schols D. In vitro synergistic activity against CCR5-tropic HIV-1 with combinations of potential candidate microbicide molecules HHA, KRV2110 and enfuvirtide (T20) J. Antimicrob. Chemother. 2009;64:1192–1195. doi: 10.1093/jac/dkp380. [DOI] [PubMed] [Google Scholar]
- 175.Murphy D.J., Amssoms K., Pille G., Clarke A., O'Hara M., van Roey J. Sustained release of the candidate antiretroviral peptides T-1249 and JNJ54310516-AFP from a rod insert vaginal ring. Drug Deliv. Transl. Res. 2016:1–9. doi: 10.1007/s13346-015-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Harman S., Herrera C., Armanasco N., Nuttall J., Shattock R.J. Preclinical evaluation of the HIV-1 fusion inhibitor L'644 as a potential candidate microbicide. Antimicrob. Agents Chemother. 2012;56:2347–2356. doi: 10.1128/AAC.06108-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li L., Ben Y., Yuan S., Jiang S., Xu J., Zhang X. Efficacy, stability, and biosafety of sifuvirtide gel as a microbicide candidate against HIV-1. PloS One. 2012;7:e37381. doi: 10.1371/journal.pone.0037381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sluis-Cremer N., Tachedjian G. Modulation of the oligomeric structures of HIV-1 retroviral enzymes by synthetic peptides and small molecules. Eur. J. Biochem. 2002;269:5103–5111. doi: 10.1046/j.1432-1033.2002.03216.x. [DOI] [PubMed] [Google Scholar]
- 179.Agopian A., Gros E., Aldrian-Herrada G., Bosquet N., Clayette P., Divita G. A new generation of peptide-based inhibitors targeting HIV-1 reverse transcriptase conformational flexibility. J. Biol. Chem. 2009;284:254–264. doi: 10.1074/jbc.M802199200. [DOI] [PubMed] [Google Scholar]
- 180.Divita G., Restle T., Goody R.S., Chermann J.C., Baillon J.G. Inhibition of human immunodeficiency virus type 1 reverse transcriptase dimerization using synthetic peptides derived from the connection domain. J. Biol. Chem. 1994;269:13080–13083. [PubMed] [Google Scholar]
- 181.Divita G., Baillon J.G., Rittinger K., Chermann J.C., Goody R.S. Interface peptides as structure-based human immunodeficiency virus reverse transcriptase inhibitors. J. Biol. Chem. 1995;270:28642–28646. doi: 10.1074/jbc.270.48.28642. [DOI] [PubMed] [Google Scholar]
- 182.Morris M.C., Robert-Hebmann V., Chaloin L., Mery J., Heitz F., Devaux C. A new potent HIV-1 reverse transcriptase inhibitor a synthetic peptide derived from the interface SUBUNIT domains. J. Biol. Chem. 1999;274:24941–24946. doi: 10.1074/jbc.274.35.24941. [DOI] [PubMed] [Google Scholar]
- 183.Gleenberg I.O., Herschhorn A., Hizi A. Inhibition of the activities of reverse transcriptase and integrase of human immunodeficiency virus type-1 by peptides derived from the homologous viral protein R (Vpr) J. Mol. Biol. 2007;369:1230–1243. doi: 10.1016/j.jmb.2007.03.073. [DOI] [PubMed] [Google Scholar]
- 184.Yuan S., Yan J., Ye X., Wu Z., Ng T. Isolation of a ribonuclease with antiproliferative and HIV-1 reverse transcriptase inhibitory activities from Japanese large brown buckwheat seeds. Appl. Biochem. Biotechnol. 2014;175:2456–2467. doi: 10.1007/s12010-014-1438-5. [DOI] [PubMed] [Google Scholar]
- 185.Zhang R., Tian G., Zhao Y., Zhao L., Wang H., Gong Z. A novel ribonuclease with HIV-1 reverse transcriptase inhibitory activity purified from the fungus Ramaria formosa. J. Basic Microbiol. 2015;55:269–275. doi: 10.1002/jobm.201300876. [DOI] [PubMed] [Google Scholar]
- 186.Pemmaraju B.P., Malekar S., Agarwal H.K., Tiwari R.K., Oh D., Doncel G.F. Design, synthesis, antiviral activity, and pre-formulation development of poly-L-arginine-fatty acyl derivatives of nucleoside reverse transcriptase inhibitors. Nucl. Nucl. Nucleic Acids. 2015;34:1–15. doi: 10.1080/15257770.2014.945649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hilimire T.A., Bennett R.P., Stewart R.A., Garcia-Miranda P., Blume A., Becker J. N-methylation as a strategy for enhancing the affinity and selectivity of RNA-binding peptides: application to the HIV-1 frameshift-stimulating RNA. ACS Chem. Biol. 2015;11:88–94. doi: 10.1021/acschembio.5b00682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lin M.-H., Apolloni A., Cutillas V., Sivakumaran H., Martin S., Li D. A mutant tat protein inhibits HIV-1 reverse transcription by targeting the reverse transcription complex. J. Virol. 2015;89:4827–4836. doi: 10.1128/JVI.03440-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang H.X., Ng T.B. Ascalin, a new anti-fungal peptide with human immunodeficiency virus type 1 reverse transcriptase-inhibiting activity from shallot bulbs. Peptides. 2002;23:1025–1029. doi: 10.1016/s0196-9781(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 190.Davis D.A., Brown C.A., Singer K.E., Wang V., Kaufman J., Stahl S.J. Inhibition of HIV-1 replication by a peptide dimerization inhibitor of HIV-1 protease. Antivir. Res. 2006;72:89–99. doi: 10.1016/j.antiviral.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 191.Todd M.J., Semo N., Freire E. The structural stability of the HIV-1 protease. J. Mol. Biol. 1998;283:475–488. doi: 10.1006/jmbi.1998.2090. [DOI] [PubMed] [Google Scholar]
- 192.Sayer J.M., Aniana A., Louis J.M. Mechanism of dissociative inhibition of HIV protease and its autoprocessing from a precursor. J. Mol. Biol. 2012;422:230–244. doi: 10.1016/j.jmb.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zutshi R., Franciskovich J., Shultz M., Schweitzer B., Bishop P., Wilson M. Targeting the dimerization interface of HIV-1 protease: inhibition with cross-linked interfacial peptides. J. Am. Chem. Soc. 1997;119:4841–4845. [Google Scholar]
- 194.Zhang Z.-Y., Poorman R., Maggiora L., Heinrikson R., Kezdy F. Dissociative inhibition of dimeric enzymes. Kinetic characterization of the inhibition of HIV-1 protease by its COOH-terminal tetrapeptide. J. Biol. Chem. 1991;266:15591–15594. [PubMed] [Google Scholar]
- 195.Babé L.M., Rosé J., Craik C.S. Synthetic “interface” peptides alter dimeric assembly of the HIV 1 and 2 proteases. Protein Sci. 1992;1:1244–1253. doi: 10.1002/pro.5560011003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Schramm H.J., Boetzel J., Büttner J., Fritsche E., Göhring W., Jaeger E. The inhibition of human immunodeficiency virus proteases by ‘interface peptides’. Antivir. Res. 1996;30:155–170. doi: 10.1016/0166-3542(96)00940-0. [DOI] [PubMed] [Google Scholar]
- 197.Bowman M.J., Chmielewski J. Sidechain-linked inhibitors of HIV-1 protease dimerization. Bioorg. Med. Chem. 2009;17:967–976. doi: 10.1016/j.bmc.2008.02.060. [DOI] [PubMed] [Google Scholar]
- 198.Dufau L., Marques Ressurreição A.S., Fanelli R., Kihal N., Vidu A., Milcent T. Carbonylhydrazide-based molecular tongs inhibit wild-type and mutated HIV-1 protease dimerization. J. Med. Chem. 2012;55:6762–6775. doi: 10.1021/jm300181j. [DOI] [PubMed] [Google Scholar]
- 199.Vidu A., Dufau L., Bannwarth L., Soulier J.L., Sicsic S., Piarulli U. Toward the first nonpeptidic molecular tong inhibitor of wild-type and mutated HIV-1 protease dimerization. ChemMedChem. 2010;5:1899–1906. doi: 10.1002/cmdc.201000308. [DOI] [PubMed] [Google Scholar]
- 200.Bannwarth L., Kessler A., Pèthe S., Collinet B., Merabet N., Boggetto N. Molecular tongs containing amino acid mimetic fragments: new inhibitors of wild-type and mutated HIV-1 protease dimerization. J. Med. Chem. 2006;49:4657–4664. doi: 10.1021/jm060576k. [DOI] [PubMed] [Google Scholar]
- 201.Schramm H.J., Rosny E.D., Reboud-Ravaux M., Büttner J., Dick A., Schramm W. Lipopeptides as dimerization inhibitors of HIV-1 protease. Biol. Chem. 1999;380:593–596. doi: 10.1515/BC.1999.076. [DOI] [PubMed] [Google Scholar]
- 202.Dumond J., Boggetto N., Schramm H.J., Schramm W., Takahashi M., Reboud-Ravaux M. Thyroxine-derivatives of lipopeptides: bifunctional dimerization inhibitors of human immunodeficiency virus-1 protease. Biochem. Pharmacol. 2003;65:1097–1102. doi: 10.1016/s0006-2952(02)01622-2. [DOI] [PubMed] [Google Scholar]
- 203.Bannwarth L., Rose T., Dufau L., Vanderesse R., Dumond J., Jamart-Grégoire B. Dimer disruption and monomer sequestration by alkyl tripeptides are successful strategies for inhibiting wild-type and multidrug-resistant mutated HIV-1 proteases†. Biochemistry. 2008;48:379–387. doi: 10.1021/bi801422u. [DOI] [PubMed] [Google Scholar]
- 204.Lee S.G., Chmielewski J. Cross-linked peptoid-based dimerization inhibitors of HIV-1 protease. ChemBioChem. 2010;11:1513–1516. doi: 10.1002/cbic.201000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Young T.S., Young D.D., Ahmad I., Louis J.M., Benkovic S.J., Schultz P.G. Evolution of cyclic peptide protease inhibitors. Proc. Natl. Acad. Sci. 2011;108:11052–11056. doi: 10.1073/pnas.1108045108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zutshi R., Chmielewski J. Targeting the dimerization interface for irreversible inhibition of HIV-1 protease. Bioorg. Med. Chem. Lett. 2000;10:1901–1903. doi: 10.1016/s0960-894x(00)00369-3. [DOI] [PubMed] [Google Scholar]
- 207.Davis D.A., Tebbs I.R., Daniels S.I., Stahl S.J., Kaufman J.D., Wingfield P. Analysis and characterization of dimerization inhibition of a multi-drug-resistant human immunodeficiency virus type 1 protease using a novel size-exclusion chromatographic approach. Biochem. J. 2009;419:497–506. doi: 10.1042/BJ20082068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Farady C.J., Sun J., Darragh M.R., Miller S.M., Craik C.S. The mechanism of inhibition of antibody-based inhibitors of membrane-type serine protease 1 (MT-SP1) J. Mol. Biol. 2007;369:1041–1051. doi: 10.1016/j.jmb.2007.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Maes M., Loyter A., Friedler A. Peptides that inhibit HIV-1 integrase by blocking its protein–protein interactions. FEBS J. 2012;279:2795–2809. doi: 10.1111/j.1742-4658.2012.08680.x. [DOI] [PubMed] [Google Scholar]
- 210.Zhao L., O'Reilly M.K., Shultz M.D., Chmielewski J. Interfacial peptide inhibitors of HIV-1 integrase activity and dimerization. Bioorg. Med. Chem. Lett. 2003;13:1175–1177. doi: 10.1016/s0960-894x(03)00040-4. [DOI] [PubMed] [Google Scholar]
- 211.Sourgen F., Maroun R.G., Frere V., Bouziane M., Auclair C., Troalen F. A synthetic peptide from the human immunodeficiency virus type-1 integrase exhibits coiled-coil properties and interferes with the in vitro integration activity of the enzyme. Correlated biochemical and spectroscopic results. Eur. J. Biochem./FEBS. 1996;240:765–773. doi: 10.1111/j.1432-1033.1996.0765h.x. [DOI] [PubMed] [Google Scholar]
- 212.Rosenbluh J., Hayouka Z., Loya S., Levin A., Armon-Omer A., Britan E. Interaction between HIV-1 Rev and integrase proteins: a basis for the development of anti-HIV peptides. J. Biol. Chem. 2007;282:15743–15753. doi: 10.1074/jbc.M609864200. [DOI] [PubMed] [Google Scholar]
- 213.Maroun R.G., Krebs D., Roshani M., Porumb H., Auclair C., Troalen F. Conformational aspects of HIV-1 integrase inhibition by a peptide derived from the enzyme central domain and by antibodies raised against this peptide. Eur. J. Biochem./FEBS. 1999;260:145–155. doi: 10.1046/j.1432-1327.1999.00130.x. [DOI] [PubMed] [Google Scholar]
- 214.Maroun R.G., Gayet S., Benleulmi M.S., Porumb H., Zargarian L., Merad H. Peptide inhibitors of HIV-1 integrase dissociate the enzyme oligomers. Biochemistry. 2001;40:13840–13848. doi: 10.1021/bi011328n. [DOI] [PubMed] [Google Scholar]
- 215.Levin A, Hayouka Z, Friedler A, Loyter A. HIV-1 integrase derived peptides and compositions. US Patent 20,160,032,263; 2016.
- 216.Al-Mawsawi L.Q., Christ F., Dayam R., Debyser Z., Neamati N. Inhibitory profile of a LEDGF/p75 peptide against HIV-1 integrase: insight into integrase–DNA complex formation and catalysis. FEBS Lett. 2008;582:1425–1430. doi: 10.1016/j.febslet.2008.02.076. [DOI] [PubMed] [Google Scholar]
- 217.Christ F., Debyser Z. The LEDGF/p75 integrase interaction, a novel target for anti-HIV therapy. Virology. 2013;435:102–109. doi: 10.1016/j.virol.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 218.Tsiang M., Jones G.S., Hung M., Samuel D., Novikov N., Mukund S. Dithiothreitol causes HIV-1 integrase dimer dissociation while agents interacting with the integrase dimer interface promote dimer formation. Biochemistry. 2011;50:1567–1581. doi: 10.1021/bi101504w. [DOI] [PubMed] [Google Scholar]
- 219.Rhodes D.I., Peat T.S., Vandegraaff N., Jeevarajah D., Newman J., Martyn J. Crystal structures of novel allosteric peptide inhibitors of HIV integrase identify new interactions at the LEDGF binding site. Chembiochem. 2011;12:2311–2315. doi: 10.1002/cbic.201100350. [DOI] [PubMed] [Google Scholar]
- 220.Hayouka Z., Hurevich M., Levin A., Benyamini H., Iosub A., Maes M. Cyclic peptide inhibitors of HIV-1 integrase derived from the LEDGF/p75 protein. Bioorg. Med. Chem. 2010;18:8388–8395. doi: 10.1016/j.bmc.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 221.Zawahir Z., Neamati N. Inhibition of HIV-1 integrase activity by synthetic peptides derived from the HIV-1 HXB2 Pol region of the viral genome. Bioorg. Med. Chem. Lett. 2006;16:5199–5202. doi: 10.1016/j.bmcl.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 222.Suzuki S., Urano E., Hashimoto C., Tsutsumi H., Nakahara T., Tanaka T. Peptide HIV-1 integrase inhibitors from HIV-1 gene products. J. Med. Chem. 2010;53:5356–5360. doi: 10.1021/jm1003528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Suzuki S., Maddali K., Hashimoto C., Urano E., Ohashi N., Tanaka T. Peptidic HIV integrase inhibitors derived from HIV gene products: structure-activity relationship studies. Bioorg Med. Chem. 2010;18:6771–6775. doi: 10.1016/j.bmc.2010.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Oz Gleenberg I., Avidan O., Goldgur Y., Herschhorn A., Hizi A. Peptides derived from the reverse transcriptase of human immunodeficiency virus type 1 as novel inhibitors of the viral integrase. J. Biol. Chem. 2005;280:21987–21996. doi: 10.1074/jbc.M414679200. [DOI] [PubMed] [Google Scholar]
- 225.Marchand C., Krajewski K., Lee H.F., Antony S., Johnson A.A., Amin R. Covalent binding of the natural antimicrobial peptide indolicidin to DNA abasic sites. Nucleic acids Res. 2006;34:5157–5165. doi: 10.1093/nar/gkl667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Wells J.A., McClendon C.L. Reaching for high-hanging fruit in drug discovery at protein–protein interfaces. Nature. 2007;450:1001–1009. doi: 10.1038/nature06526. [DOI] [PubMed] [Google Scholar]
- 227.Lalonde M.S., Lobritz M.A., Ratcliff A., Chamanian M., Athanassiou Z., Tyagi M. Inhibition of both HIV-1 reverse transcription and gene expression by a cyclic peptide that binds the Tat-transactivating response element (TAR) RNA. PLoS Pathog. 2011;7:e1002038. doi: 10.1371/journal.ppat.1002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Davidson A., Leeper T.C., Athanassiou Z., Patora-Komisarska K., Karn J., Robinson J.A. Simultaneous recognition of HIV-1 TAR RNA bulge and loop sequences by cyclic peptide mimics of Tat protein. Proc. Natl. Acad. Sci. 2009;106:11931–11936. doi: 10.1073/pnas.0900629106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Niedrig M., Gelderblom H.R., Pauli G., Marz J., Bickhard H., Wolf H. Inhibition of infectious human immunodeficiency virus type 1 particle formation by Gag protein-derived peptides. J. General Virol. 1994;75(Pt 6):1469–1474. doi: 10.1099/0022-1317-75-6-1469. [DOI] [PubMed] [Google Scholar]
- 230.VanCompernolle S., Smith P.B., Bowie J.H., Tyler M.J., Unutmaz D., Rollins-Smith L.A. Inhibition of HIV infection by caerin 1 antimicrobial peptides. Peptides. 2015;71:296–303. doi: 10.1016/j.peptides.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 231.Caffrey M. HIV envelope: challenges and opportunities for development of entry inhibitors. Trends Microbiol. 2011;19:191–197. doi: 10.1016/j.tim.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wang G., Watson K.M., Peterkofsky A., Buckheit R.W., Jr. Identification of novel human immunodeficiency virus type 1-inhibitory peptides based on the antimicrobial peptide database. Antimicrob. Agents Chemother. 2010;54:1343–1346. doi: 10.1128/AAC.01448-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang G., Watson K.M., Buckheit R.W., Jr. Anti-human immunodeficiency virus type 1 activities of antimicrobial peptides derived from human and bovine cathelicidins. Antimicrob. Agents Chemother. 2008;52:3438–3440. doi: 10.1128/AAC.00452-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Yang B., Gao L., Li L., Lu Z., Fan X., Patel C.A. Potent suppression of viral infectivity by the peptides that inhibit multimerization of human immunodeficiency virus type 1 (HIV-1) Vif proteins. J. Biol. Chem. 2003;278:6596–6602. doi: 10.1074/jbc.M210164200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tavassoli A., Lu Q., Gam J., Pan H., Benkovic S.J., Cohen S.N. Inhibition of HIV budding by a genetically selected cyclic peptide targeting the Gag-TSG101 interaction. ACS Chem. Biol. 2008;3:757–764. doi: 10.1021/cb800193n. [DOI] [PubMed] [Google Scholar]
- 236.Waheed A.A., Freed E.O. Peptide inhibitors of HIV-1 egress. ACS Chem. Biol. 2008;3:745–747. doi: 10.1021/cb800296j. [DOI] [PubMed] [Google Scholar]
- 237.Wachinger M., Kleinschmidt A., Winder D., von Pechmann N., Ludvigsen A., Neumann M. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. general Virol. 1998;79(Pt 4):731–740. doi: 10.1099/0022-1317-79-4-731. [DOI] [PubMed] [Google Scholar]
- 238.Nara P.L., Hwang K.M., Rausch D.M., Lifson J.D., Eiden L.E. CD4 antigen-based antireceptor peptides inhibit infectivity of human immunodeficiency virus in vitro at multiple stages of the viral life cycle. Proc. Natl. Acad. Sci. U. S. A. 1989;86:7139–7143. doi: 10.1073/pnas.86.18.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Xiang J., McLinden J.H., Kaufman T.M., Mohr E.L., Bhattarai N., Chang Q. Characterization of a peptide domain within the GB virus C envelope glycoprotein (E2) that inhibits HIV replication() Virology. 2012;430:53–62. doi: 10.1016/j.virol.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Xiang J., McLinden J.H., Chang Q., Jordan E.L., Stapleton J.T. Characterization of a peptide domain within the GB virus C NS5A phosphoprotein that inhibits HIV replication. PLoS One. 2008;3:e2580. doi: 10.1371/journal.pone.0002580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Owens B.J., Anantharamaiah G.M., Kahlon J.B., Srinivas R.V., Compans R.W., Segrest J.P. Apolipoprotein A-I and its amphipathic helix peptide analogues inhibit human immunodeficiency virus-induced syncytium formation. J. Clin. Investig. 1990;86:1142–1150. doi: 10.1172/JCI114819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Nehete P.N., Arlinghaus R.B., Sastry K.J. Inhibition of human immunodeficiency virus type 1 infection and syncytium formation in human cells by V3 loop synthetic peptides from gp120. J. Virol. 1993;67:6841–6846. doi: 10.1128/jvi.67.11.6841-6846.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yao X.-J., Lemay J., Rougeau N., Clément M., Kurtz S., Belhumeur P. Genetic selection of peptide inhibitors of human immunodeficiency virus type 1 Vpr. J. Biol. Chem. 2002;277:48816–48826. doi: 10.1074/jbc.M207982200. [DOI] [PubMed] [Google Scholar]
- 244.Zhuang X., Stahl S.J., Watts N.R., DiMattia M.A., Steven A.C., Wingfield P.T. A cell-penetrating antibody fragment against HIV-1 rev has high antiviral activity characterization of the paratope. J. Biol. Chem. 2014;289:20222–20233. doi: 10.1074/jbc.M114.581090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang H., Zhao Q., Bhattacharya S., Waheed A.A., Tong X., Hong A. A cell-penetrating helical peptide as a potential HIV-1 inhibitor. J. Mol. Biol. 2008;378:565–580. doi: 10.1016/j.jmb.2008.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Sticht J., Humbert M., Findlow S., Bodem J., Müller B., Dietrich U. A peptide inhibitor of HIV-1 assembly in vitro. Nat. Struct. Mol. Biol. 2005;12:671–677. doi: 10.1038/nsmb964. [DOI] [PubMed] [Google Scholar]
- 247.A.K. Debnath, H. Zhang, Q. Zhao, Stabilized therapeutic small helical antiviral peptides, Google Patents, 2015, US Patent US8940864 B2, Priority date: Oct 5, 2006
- 248.Cabras T, Casoli C, Castagnola M, Inzitari R, Longhi R, Messana I, et al., Antiviral peptides. Google Patents, 2012, US Patent US 20140038884 A1, Priority date: Jan 28, 2011.
- 249.Conlon J.M., Mechkarska M., Lukic M.L., Flatt P.R. Potential therapeutic applications of multifunctional host-defense peptides from frog skin as anti-cancer, anti-viral, immunomodulatory, and anti-diabetic agents. Peptides. 2014;57:67–77. doi: 10.1016/j.peptides.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 250.Aoki W., Ueda M. Characterization of antimicrobial peptides toward the development of novel antibiotics. Pharmaceuticals. 2013;6:1055–1081. doi: 10.3390/ph6081055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Johnson V.A., Calvez V., Gunthard H., Paredes R., Pillay D., Shafer R.W. Update of the drug resistance mutations in HIV-1. Top. Antivir. Med. March 2013;2013(21):6–14. [PMC free article] [PubMed] [Google Scholar]
- 252.Pham Q.D., Wilson D.P., Law M.G., Kelleher A.D., Zhang L. Global burden of transmitted HIV drug resistance and HIV-exposure categories: a systematic review and meta-analysis. AIDS. 2014;28:2751–2762. doi: 10.1097/QAD.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 253.Torchilin V.P. Tat peptide-mediated intracellular delivery of pharmaceutical nanocarriers. Adv. Drug Deliv. Rev. 2008;60:548–558. doi: 10.1016/j.addr.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 254.Otvos L. Peptide-based drug design: here and now. Peptide-Based Drug Design. 2008:1–8. doi: 10.1007/978-1-59745-419-3_1. [DOI] [PubMed] [Google Scholar]
- 255.Vlieghe P., Lisowski V., Martinez J., Khrestchatisky M. Synthetic therapeutic peptides: science and market. Drug Discov. today. 2010;15:40–56. doi: 10.1016/j.drudis.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 256.Peters B.M., Shirtliff M.E., Jabra-Rizk M.A. Antimicrobial peptides: primeval molecules or future drugs? PLoS Pathog. 2010;6:e1001067. doi: 10.1371/journal.ppat.1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Tang A., Kopecek J. Presentation of epitopes on genetically engineered peptides and selection of lymphoma-targeting moieties based on epitope biorecognition. Biomacromolecules. 2002;3:421–431. doi: 10.1021/bm015606+. [DOI] [PubMed] [Google Scholar]
- 258.Kumar V.A., Taylor N.L., Shi S., Wang B.K., Jalan A.A., Kang M.K. Highly angiogenic peptide nanofibers. ACS Nano. 2015;9:860–868. doi: 10.1021/nn506544b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Liu W., Chen Y.H. High epitope density in a single protein molecule significantly enhances antigenicity as well as immunogenicity: a novel strategy for modern vaccine development and a preliminary investigation about B cell discrimination of monomeric proteins. Eur. J. Immunol. 2005;35:505–514. doi: 10.1002/eji.200425749. [DOI] [PubMed] [Google Scholar]
- 260.Easterhoff D., DiMaio John T.M., Doran Todd M., Dewhurst S., Nilsson Bradley L. Enhancement of HIV-1 infectivity by simple, self-assembling modular peptides. Biophysical J. 2011;100:1325–1334. doi: 10.1016/j.bpj.2011.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Ramessar K., Rademacher T., Sack M., Stadlmann J., Platis D., Stiegler G. Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. Proc. Natl. Acad. Sci. 2008;105:3727–3732. doi: 10.1073/pnas.0708841104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Chiang J.J., Gardner M.R., Quinlan B.D., Dorfman T., Choe H., Farzan M. Enhanced recognition and neutralization of HIV-1 by antibody-derived CCR5-mimetic peptide variants. J. Virol. 2012;86:12417–12421. doi: 10.1128/JVI.00967-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Chevigne A., Delhalle S., Counson M., Beaupain N., Rybicki A., Verschueren C. Isolation of an HIV-1 neutralizing peptide mimicking the CXCR4 and CCR5 surface from the heavy-chain complementary determining region 3 repertoire of a viremic controller. AIDS. 2016;30:377–382. doi: 10.1097/QAD.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 264.Danial M., van Dulmen T.H., Aleksandrowicz J., Potgens A.J., Klok H.A. Site-specific PEGylation of HR2 peptides: effects of PEG conjugation position and chain length on HIV-1 membrane fusion inhibition and proteolytic degradation. Bioconjugate Chem. 2012;23:1648–1660. doi: 10.1021/bc3002248. [DOI] [PubMed] [Google Scholar]
- 265.Pechar M., Pola R., Laga R., Braunova A., Filippov S.K., Bogomolova A. Coiled coil peptides and polymer-peptide conjugates: synthesis, self-assembly, characterization and potential in drug delivery systems. Biomacromolecules. 2014;15:2590–2599. doi: 10.1021/bm500436p. [DOI] [PubMed] [Google Scholar]
- 266.Webber M.J., Appel E.A., Meijer E.W., Langer R. Supramolecular biomaterials. Nat. Mater. 2015;15:13–26. doi: 10.1038/nmat4474. [DOI] [PubMed] [Google Scholar]
- 267.Webber M.J., Khan O.F., Sydlik S.A., Tang B.C., Langer R. A perspective on the clinical translation of scaffolds for tissue engineering. Ann. Biomed. Eng. 2015;43:641–656. doi: 10.1007/s10439-014-1104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Chertok B., Webber M.J., Succi M.D., Langer R. Drug delivery interfaces in the 21st century: from science fiction ideas to viable technologies. Mol. Pharm. 2013;10:3531–3543. doi: 10.1021/mp4003283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kumar V.A., Wang B.K., Kanahara S.M. Rational design of fiber forming supramolecular structures. Exp. Biol. Med. (Maywood) 2016 May;241(9):899–908. doi: 10.1177/1535370216640941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Krajewski K., Marchand C., Long Y.Q., Pommier Y., Roller P.P. Synthesis and HIV-1 integrase inhibitory activity of dimeric and tetrameric analogs of indolicidin. Bioorg Med. Chem. Lett. 2004;14:5595–5598. doi: 10.1016/j.bmcl.2004.08.061. [DOI] [PubMed] [Google Scholar]
- 271.Margolis A.M., Heverling H., Pham P.A., Stolbach A. A review of the toxicity of HIV medications. J. Med. Toxicol. 2014;10:26–39. doi: 10.1007/s13181-013-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Danial M., Klok H.A. Polymeric anti-HIV therapeutics. Macromol. Biosci. 2015;15:9–35. doi: 10.1002/mabi.201400298. [DOI] [PubMed] [Google Scholar]