Abstract
The encapsulation of stem cells in a hydrogel substrate provides a promising future in biomedical applications. However, communications between hydrogels and stem cells is complicated; various factors such as porosity, different polymer types, stiffness, compatibility and degradation will lead to stem cell survival or death. Hydrogels mimic the three-dimensional extracellular matrix to provide a friendly environment for stem cells. On the other hand, stem cells can sense the surroundings to make the next progression, stretching out, proliferating or just to remain. As such, understanding the correlation between stem cells and hydrogels is crucial. In this Review, we first discuss the varying types of the hydrogels and stem cells, which are most commonly used in the biomedical fields and further investigate how hydrogels interact with stem cells from the perspective of their biomedical application, while providing insights into the design and development of hydrogels for drug delivery, tissue engineering and regenerative medicine purpose. In addition, we compare the results such as stiffness, degradation time and pore size as well as peptide types of hydrogels from respected journals. We also discussed most recently magnificent materials and their effects to regulate stem cell fate.
Keywords: Hydrogel, Stem cell, Biomaterial
Graphical abstract
Highlights
-
•
Hydrogels as Extracellular Matrix (ECM) mimics stem cells proliferation and differentiation.
-
•
Discuss how hydrogels interact with stem cells from the perspective of their biomedical applications.
-
•
Recent magnificent materials and their effects to regulate stem cells fate.
1. Introduction
In the past our understanding of biomaterials was quite a different view from the current understanding. Our views of biomaterials where dominated by the idea of an inert, inactive and non-viable substance for the use on living organisms. We now hold a greater prospective on the technical aspects and characterization of biomaterials and the need for them to interface with native tissue [1]. Hydrogels are three-dimensional systems with hydrophilic polymer chains [2] that link and have high water content [3], [4]. Because of hydrogels special traits, such as modifiable chemical properties, biocompatibility, elasticity, the capability to act as a growth medium and the ability to mimic the extracellular matrix (ECM), they have broad uses in biomedical research [5] that spans from drug delivery [6], [7] to regenerative medicine [3] to tissue engineering [8] and are gaining attention due to their ability to encapsulate cells. They are the subjects of numerous academic and industry projects/research [9], [10], [11], they have useful characteristics and their substrates allow for the influence of numerous variables [12], [13]. Hydrogels are often thought of in two categories, natural and synthetic. Natural hydrogels or naturally derived hydrogels consist of collagen, alginate, hyaluronic acid and chitosan to name a few [14]. These are increasing used in research as they exhibit desirable properties such as, biodegradability and therapeutic cell interactions [6]. On the other side of the spectrum, synthetic hydrogels may offer mechanical advantages such as strength and better elastic properties. Some examples of synthetic hydrogels are poly (ethylene glycol) commonly referred to as PEG, poly vinyl alcohol (PVA) and polyacrylamide (PAM). Each type of hydrogel, synthetic and natural, contain desirable traits and arrangements, that make them an encapsulating biomaterial [15] and are highly suitable, as such these combined traits are expressed in the form of hybrid hydrogels [16]. One such example is an alginate hydrogel, which can achieve high stiffness, one factor in the regulation of stem cell fate [17]. These hydrogels are commonly used in tissue regeneration and are often implemented in the form of injectable hydrogels [18]. The uses of these biomaterials are in an attempt to mimic native tissue [19], hence the term biomimetic hydrogel and often follow tissue characteristics, such as elasticity [10]. A spark in uses of hydrogels is in modifiable/tunable hydrogels [20] and this is where new kinds of hydrogels comes in, one of whom is elastomeric hydrogels that allow favorable stress related properties [21]. Another notable type of hydrogels is the environmental responsive hydrogel, which change to gel from external cues. One subset of this category is thermoresponsive hydrogels, which uses temperature as an activation of its abilities [22]. An important and main application in hydrogels as a bioactive material is the uses and effects of hydrogels in stem cell therapy [23]. In the field, this is often referred to as regulation of stem cell fate [17]. These hydrogels act as media to allow better viability of the stem cell and help in the proliferation [19] and retention [24] of the cells. In the span of decades of research and achievements, the scientific community has developed numerous advanced biomaterial systems composing of different properties and uses in clinical applications [25] for a wide range of medical complications all throughout the health related fields. Accomplishments can be attributed to a wide range of inter-disciplinary work, which have set the foundation for therapeutic strategies. The scope of this review covers the uses of hydrogels for the regulations and use of stem cell therapies in regenerative medicine, tissue engineering and other therapeutic applications.
2. Hydrogels as a bioactive material
2.1. Natural
Many polymers used for hydrogel fabrication comes from nature, including alginate, collagen, fibrin, chitosan, gelatin, hyaluronic acid among many others. These polymers have advantages of inherent biocompatibility, environmental sensitivity and are abundant in source [36]. Some of the natural polymer based constructs contains degradation moieties such as hydrolysable ester groups and enzyme-mediated hydrolytic amide groups. In addition, gels made from natural polymers present natural binding sites, which provide the interaction between cells and hydrogels. However, low stability, poor mechanical properties and rapid degradation rate are major disadvantages of the natural-based hydrogels [44]. In this section, we discussed some of the most commonly used natural polymers.
2.1.1. Alginate
Alginate is a polysaccharide extracted from brown algae, which is a natural anionic polymer electrolyte. It is a strictly linear copolymer that composed of two monosaccharides of α-L-guluronic acid (G) and β-D-mannuronic acid (M) respectively [26]. Because of its abundance, inexpensiveness and biocompatibility respectively, it is featured in a number of published articles, and its uses have been increasing in recent years [27], [28], [29]. Alginate has a characteristic that when it interacts with divalent ions, such as Ca2+, Sr2+ and Ba2+ it will become a dense three-dimensional structure called an “egg box” to improve the mechanical properties. However, it may be hard to control the degradation rate, as it is a considerable challenge for cationic polymers. The major disadvantages of natural materials are poor mechanical properties as well as cell adhesion [27]. One group developed a hybrid hydrogel comprised with alginate and poly ethylene glycol diacrylate (PEGDA) to achieve toughness greater than natural cartilage due to a PEG network with a high elasticity and reversible crosslinking qualities under deformation of alginate [30]. On the other hand, because of the poor adhesion property of alginate-based hydrogel, RGD sequences peptides are used to improve cell adhesion. Mooney group successfully developed a model where GRGDY-modified alginate hydrogel showed the cellular interaction between the hydrogel and mouse skeletal myoblasts [26]. In addition, other variables such as stiffness and porosity shows an effect in the cell’s fate, his group studies stress relaxation of the alginate-based hydrogels to regulate stem cell fate and activity. Interesting results present, that the hydrogel with faster relaxation property accommodates better cell spreading, differentiation and proliferation than slower relaxation. As such, the stress relaxation may be the key parameter to regulate stem cell fate for cell culture [31]. Surprisingly, the geometry of hydrogel is another significant factor effecting cells viability. One study founds out when using sphere alginate based hydrogel (Diameter = 1.5 mm) Fig. 1 can not only contain great biocompatibility but also restore blood-glucose control for above 180 days [32].
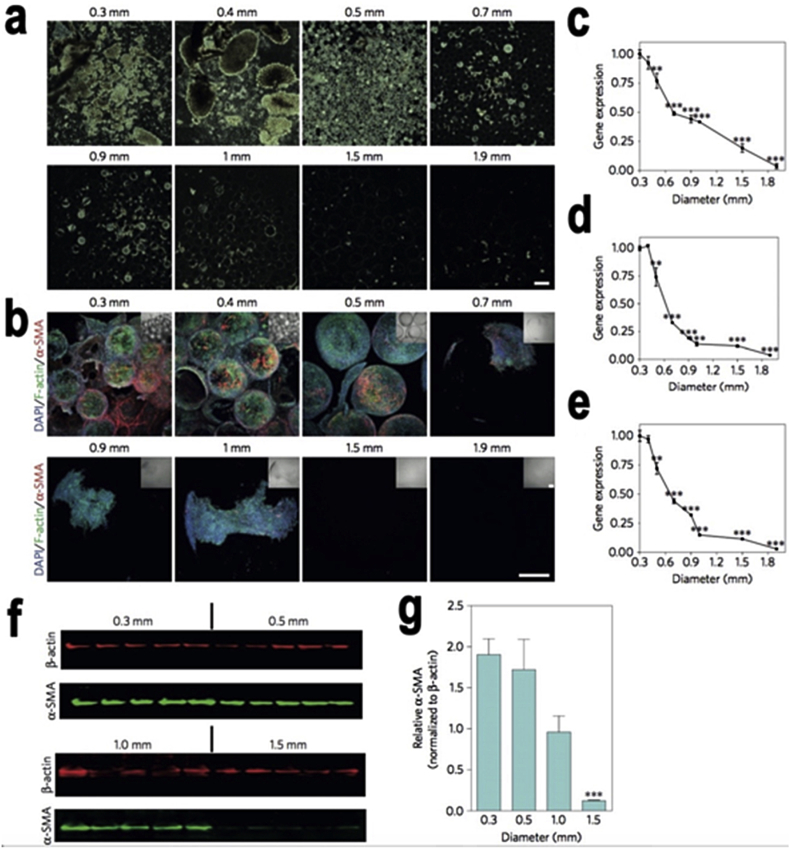
Fig. 1.
The geometry effects in cellular deposition and fibrosis formation. a) Dark-field phase contrast images: Increased sphere size inhibits cellular overgrowth. Scale bar, 2-mm. b) Confocal images of retrieved spheres: DAPI (highlighting cellular nuclei), phalloidin (highlighting F-actin) and α-SMA (highlighting myofibroblast cells) stained with immunofluorescence. Scale bar, 300 μm. (c–e), Gene expression analysis of fibrotic markers α-SMA, c) Collagen 1a1, d) Col1a1 and Collagen 1a2, e) Col1a2. f) Semiquantitative western blot analysis. g) Plot of analyzed band intensities. Copyright, Ref. [32], 2015, Nature Publishing Group.
2.1.2. Hyaluronic acid (HA)
Hyaluronic acid (HA), or hyaluronan, is a non-immune polysaccharide composing of repeating disaccharide units of α-1,4-D-glucuronic acid (GlcUA) and β-1,3-acetyl-D-glucosamine (GlcNAc) [33]. HA is a biocompatible, non-immunogenic non-sulfated anionic glycosaminoglycan that is distributed throughout the body like in connective tissues, synovial fluid, organs and the extracellular matrix of cartilage [34], [35]. More importantly, HA is a crucial component of the ECM and plays an essential role in wound repairing, cell signaling, angiogenesis, matrix organizations and morphogenesis [36], [37]. However, one of the primary challenges of HA is hyaluronidase, which causes a high degradation rate in vivo. One approach to overcome this obstacle is conjugating synthetic materials to improve toughness, durability and strength of HA, this has been published in recent years [38], [39]. Although the studies of 2D cell interactions with HA hydrogel has been wildly investigated, there were limited publications of encapsulation of cells within HA hydrogel [40] due to HA based hydrogels lack of cell adhesion. One research group found that differentiation of hMSCs within HA based hydrogel only dependents on degradation-mediated cellular traction. The results where in contradiction with 2D substrates [41]. On the other hand, HA-catechol functionalized hydrogel via oxidative crosslinking was invented, which had superior tissue adhesion, cell viability and reduce apoptosis [42] Fig. 2. HA is also known as a notable material for 3D printing due to its biocompatibility. Recently, one study developed a special HA based hydrogel ink technique called “Ghost writing”, which is a 3D printing procedure that can direct the writing of guest-host hydrogels, this is due to its reversible and non-covalent bonds. Moreover, these HA based hydrogels consist of a self-healing property that can be reformed after printing as well as printed with a complex structure at high resolution [43].
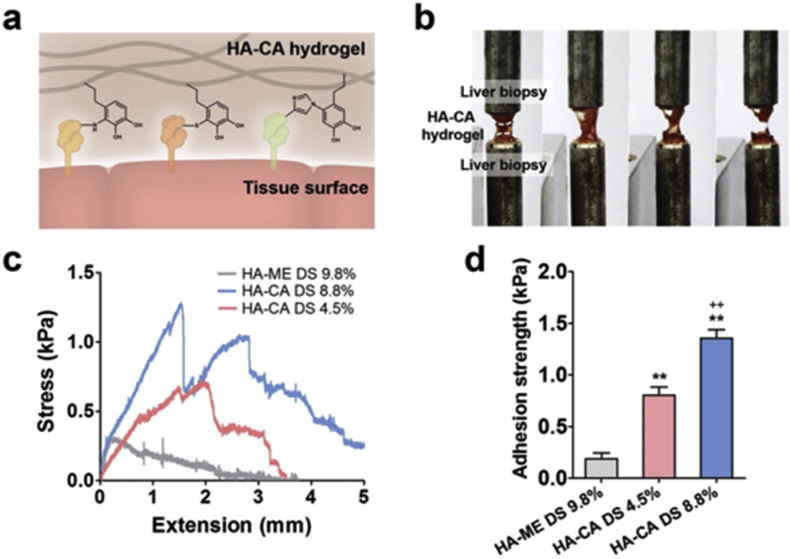
Fig. 2.
Analysis tissue adhesion of functionalized HA-CA hydrogel. a) Mechanism of the tissue adhesion of HA-CA hydrogel. b) Image of adhesion strength measurement of HA-CA hydrogel to liver tissue. c) Adhesion strength of HA-CA hydrogel to rat liver tissue by using UTM. d) Average adhesion strength of HA-CA and HA-ME hydrogel to rat liver tissue. Copyright, Ref. [42], 2015, Wiley.
2.1.3. Chitosan
Chitosan is a natural linear composition of β-(1,4)-linked D-glucosamine and N-acetyl-D-glucosamine units, which is derived from shrimps, crabs and insects. Different from other naturally occurring polysaccharides, chitosan has highly basic polysaccharides, which contains polyoxysalt formation; chelate metal ions as well as the ability to form film properties. In addition, chitosan has been wildly used in biomedical applications such as drug delivery [44], tissue engineering [45] and cancer diagnosis because of its biocompatibility, low toxicity and mechanical properties that can be tuned to match native ECM [46]. The value of pH plays an important role in chitosan; for instance, chitosan can only be dissolved in water when the pH is less than 6.2. Furthermore, gelation can spontaneously occur by altering the pH due to the balance among hydrogen bonding, the hydrophobic interactions and the interchain electrostatic interactions [47], [48]. A number of chitosan-based hydrogel studies have been published in the last decade [23], [49], especially for the injectable character [50]. However, a major obstacle of injectable hydrogels is the gelation time, if the gelation occurs too fast, blockage of the needle will occur during the injection process, if the gelation is too slow, cells, growth factors or drugs will be lost. In spite of this, the combination of the injectable and self-healing properties of a chitosan-based hydrogel was developed. The hydrogel contains intrinsic healing by means of dynamic covalent chemistry and appearing ∼81% recovery healing effect on neural development [51] Fig. 3.
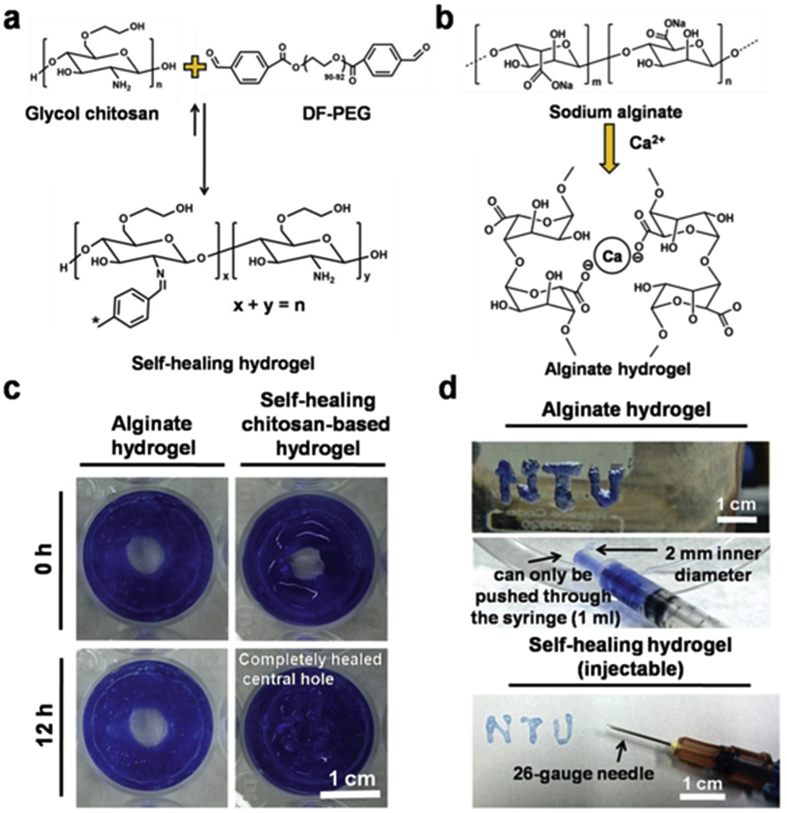
Fig. 3.
Self-healing Chitosan-based hydrogel. a) Mechanisum of Self-healing Chitosan-based hydrogel. b) Mechanisum of Sodium alginate based hydrogel (without self-healing property). c) Image of alginate and self-healing chitosan hydrogels. d) Injectable properties compared between self-healing chitosan based (can pass through a 26-gauge needle without clogging) and alginate based hydrogel (clogging within the needle). Copyright, Ref. [51], 2015, Wiley.
2.1.4. Collagen
Collagen is one of the major components of ECM protein existing in mammalian tissue. Collagen contains unique helix structure where three left handed polypeptide helices intertwine together into a right-handed triple helix, and at the ends of each helix peptide bonds are crosslink adjacent helices [52]. As such, collagen can be cross-linked by means of both physical and chemical methods. Reversible hydrogels can be formed via physical cross-linking but with poor mechanical properties. On the other hand, the chemically formed hydrogel contains better physical properties. Furthermore, because of its biocompatibility, biodegradable, and highly versatile natural collagen has been wildly used as a biomaterial in tissue engineering [53], [54], [55]. A study shows the metalloproteinase-7 functionalized collagen based hydrogel successfully improved the hMSCs viability and enhanced chondrogenic differentiation [54]. Collagen can be classified into type I, II III–XI, and more details about the collagen types can be found in other publications [56], [57]. In brief, type I collagen is derived from skin and tissues of vertebrate species and have been used in biomedical applications due to its support with the growth and differentiation of neurons in vitro [58], [59]. An investigation took place and showed that type I collagen based hydrogels can be covalently cross-linked to protein patterns to stimulate interactions between the gel surface and the cells [60]. Moreover, collagen I cooperates with polyacrylamide to create minimal matrix models of scars Fig. 4. Interestingly, this soft heterogeneous matrix exhibits less noise cell to cell than homogeneously stiff matrix [61].
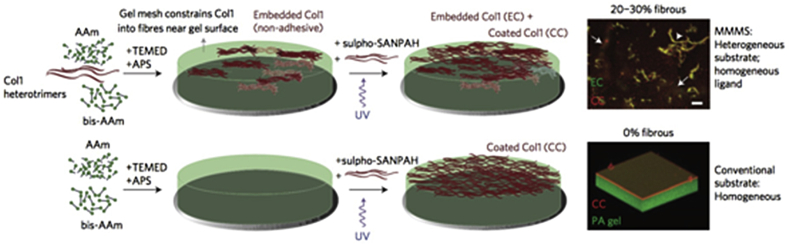
Fig. 4.
Collagen based minimal matrix model of scars (MMMS). Top left: MMMS matrix created by collagen I via free-radical polymerization of a polyacrylamide (PA). Top right: Fiber bundles of embedded collagen (EC, green) in coated-collagen (CC, red) 0.3 kPa PA hydrogel. Scale bar, 100 μm. Bottom left: Conventional collagen-I matrix attachment on PA hydrogel. Bottom right: PA hydrogel (green) with CC (red) reconstructed from confocal image stacks. Copyright Ref. [61], 2015, Nature Publishing Group.
2.1.5. Gelatin
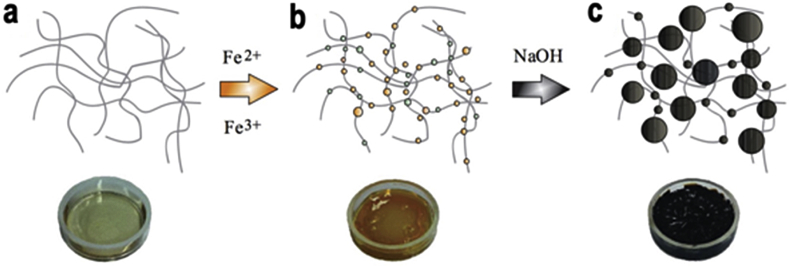
Gelatin is the one of most essential biopolymers, which is a soluble protein obtained by the irreversible process of partial hydrolysis of collagen [62], it can be derived from fish, insects and the skin of land animals. Gelatin is a polydisperse peptide with high molecular weight and because of its gelling and thickening characteristics is a popular material for food and biomedical use [63]. Gelatin hydrogels can be formed via chemically [64] and physically crosslinking methods [65]. Short degradation rates, poor mechanical properties and lack of thermal stability are major disadvantages of gelatin gels [66], [67]. As such, one research team developed a gelatin-based hydrogel, which combined magnetite nanoparticles within the gelatin hydrogels that have biocompatible and thermoreversible properties [65] Fig. 5. Moreover, there are two study groups [68], [69] were using photo-crosslinked methods, after the cooperation with methacrylate (GelMA) or free thiol moieties, not only to overcome defects but also appear to be great cell adhesion phenomenon. In addition, one group developed an interesting platform where GelMA hydrogels can be formed at room temperature with wide pH range and can contain simultaneously, drug-laden micro-droplets and encapsulation of stem cells under physiological conditions [70].
Fig. 5.
Gelatin corporate with magnetic nanoparticles. a) Gelatin hydrogel b) Hydrogel loaded with ferrous and ferric ions. c) Magnetic nanoparticles within the gelatin hydrogel via in situ co-precipitation. Copyright, Ref. [65], 2014, Wiley.
2.2. Synthetic
Different from natural polymers, the chemistry of synthetic polymers is tunable, accordingly the physicochemical and mechanical properties of synthetic polymer based hydrogels can be optimized. Additionally, diverse molecular weights, block structures and degradable linkages can lead to tunable properties contingent on the demands of mechanical properties and degradation rate [71]. Nevertheless, lack of adhesion sites, biocompatibility, and degradation products are some disadvantages of synthetic based hydrogels [72]. A few typically used synthetic based hydrogels were discussed in this review.
2.2.1. Poly acrylamide (PAM)
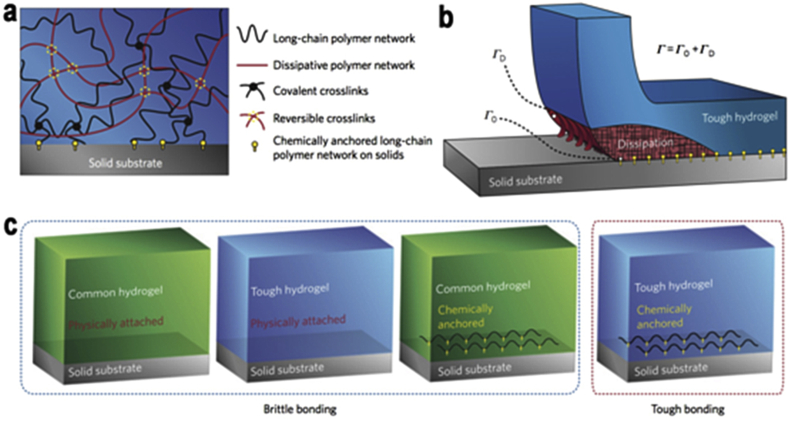
Poly acrylamide (PAM) based hydrogel have been wildly used in the biomedical fields, drug delivery and biosensor fluids in recent years. Herein, the most commonly used PAM based material is contact lenses due to its bioinert and hydrophilic properties [73], [74]. The unique tunable mechanical character is the one of the crucial factor that makes PAM stands out from other materials, the hydrogel strength can be tuned from less than 1000 pa to several Mpa [75]. Nevertheless, PAM contains minimum toxicity and poor cell adhesion, which are concerns in biomedical use. The incorporation with natural materials (e.g. alginate, collagen) and conjugation with peptides (e.g. RGD, FHRRIKA, IKVAV) becomes a necessary element of PAM based hydrogels [76]. For instance, Sun et al. developed a highly stretchable and tough hydrogel by integrating alginate and PAM to make a hybrid hydrogel by means of ionically and covalently crosslinked networks. The hybrid hydrogel can stretch 20 times than initial length as well as having a fracture toughness of nearly 9000 J/m2 [77]. In addition, poly (N-isopropyacrylamide) PNIPAM allows cell attachment, growth and spread in certain conditions because of its unique temperature response quality ∼ 37 °C [78], [79], [80]. On a similar note, they combined solid materials (e.g. metals, glass and silicon) and hydrogels to create non-porous surface materials. Classified as a high water content hydrogel, there may arise some attention because the devices will not be rigid but stretchable and flexible. However, the biggest hurdle is how to obtain adhesion between hydrogels and solid materials. Hyunwoo et al. found out there is an over 1000 J/m2 bonding between solid materials and PAM based hydrogels by using chemical anchorage results in enormous values of intrinsic work of adhesion as well as significant energy dissipation of the bulk hydrogels [16] Fig. 6. Rapid degradation property is a major issue of injectable PAM based hydrogel, to address that, in situ, a two step crosslinking mechanism is proposed, where first, encapsulated cells within soft gel are protected from the force of injection followed by in situ crosslinking to form a harden network in order to extend hydrogel degradation as well as protracts cell retention time as needed [24]. Recently, one study presents a functionalized NIPAAM based hydrogel with thermal-responsive tunable degradation and injectable properties. The NIPAAM hydrogel with a monomer methacrylic acid could easily adjust the degradation rate of NIPAAM based hydrogel [81].
Fig. 6.
Tough bonding of hydrogels to varied solids. a) Mechanisms of the hydrogel bonding to solid substrate. b) Schematic representation of chemical anchoring effects between solid substrate and tough hydrogel. (Γ0: Intrinsic work of adhesion & ΓD: The dissipation contributes to the total interfacial toughness. c) Schematics of diverse types of hydrogel-solid interfaces. Copyright, Ref. [16], 2016, Nature Publishing Group.
2.2.2. Poly ethylene glycol (PEG)
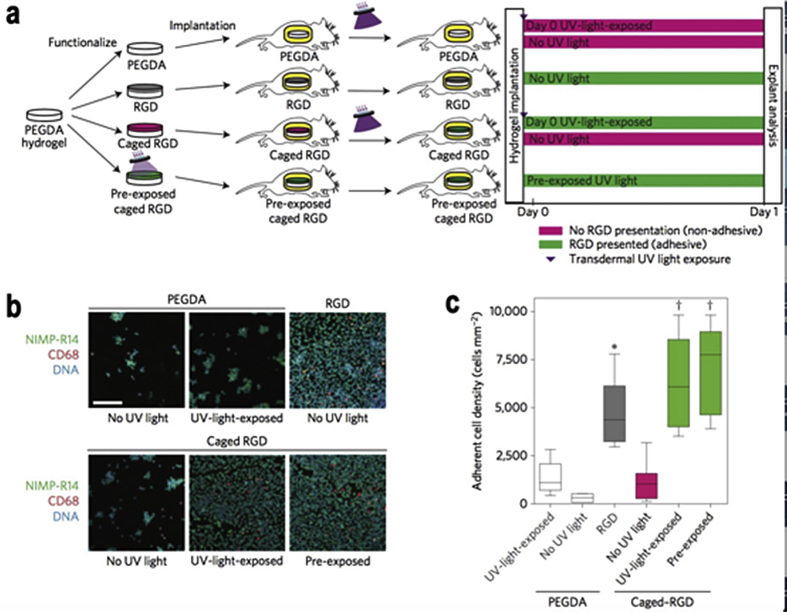
Polyethylene glycol (PEG) also known as polyoxyethylene (POE) or polyethylene oxide (PEO) is dependent on the synthesis conditions and molecular weight. PEG is a synthetic material with tremendous biocompatibility, low cost, water solubility and has been approved by the food and drug administration (FDA) [82]. As such, PEG plays a major role in a number of biomedical applications, from wound healing to drug delivery [83], [84], [85], [86], [87]. However, despite its biocompatibility, pure PEG based hydrogels or scaffolds do not support cell adhesion and proliferation. By incorporating RGD peptides or its derivatives into the PEG hydrogels backbone, it can promote cell adhesion which becomes a fundamental factor of PEG based hydrogel [88], [89] Fig. 7. Under physiological conditions, PEG based hydrogels simultaneously form by means of conjugation of functionalized multi arm PEG (e.g. Acrylate & Maleimide) with cysteine containing peptide sequences [90]. The mimicry of vascularization and organs is the goal of biomaterials, 3D printing can create an on demand structure as shown in a number of research papers that have been published in recent years [91], [92]. Herein, Alexandra group created a single “bioink method”, which is able to produce excitable, gel phase bioinks of natural/synthetic materials (especially PEG based hydrogels) and with cell-compatible quality [93]. On the other hand, to avoid surgery damage of implanted materials, noninvasive methods are needed; therefore, injectable hydrogels is the leading method in the biomedical field. However, a procedure for injection into the heart needs to be more delicate processes where the hydrogel should remain in an aqueous state at body temperature as well as quickly once in solution enter the tissue. The PEG-oxime system was developed, which is capable of forming hydrogels quickly upon injection into tissues via oxime crosslinking [39].
Fig. 7.
In vivo studies of cell adhesion. a) Time line for in vivo activation of cell adhesion. b) Hydrogels stained for adherent inflammatory cells. c) The Adherent cell density of various hydrogels formulation. Copyright, Ref. [88], 2015, Nature Publishing Group.
2.2.3. Poly vinyl alcohol (PVA)
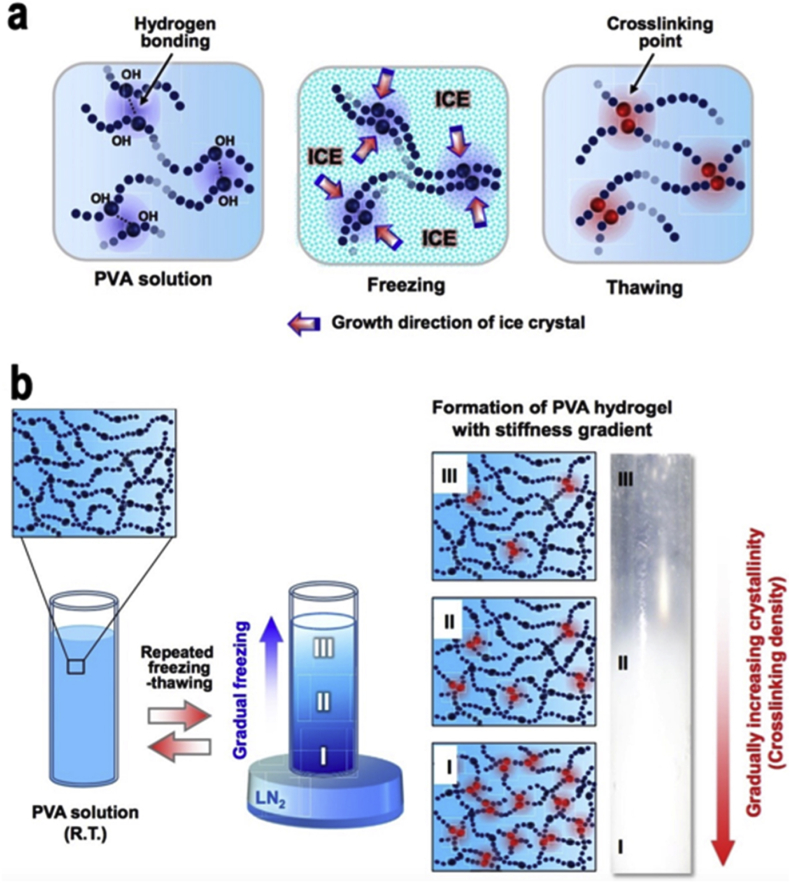
Poly (vinyl alcohol) (PVA) is a hydrolysis, alcoholysis or aminolysis product of poly (vinyl acetate) that contains a great biocompatibility non-toxic flexible design to hydrogel precursors [94], [95]. The water solubility of PVA depends on the degree of polymerization and the degree of hydrolysation. To dissolve fully hydrolyzed PVA (∼98%), the water needs to be heated about 90–100 °C and stirred for 2–4 h. On the other hand, the partial hydrolyzed PVA (∼87%) are easily soluble in water [96]. PVA is not degradable in most physiological conditions; however, the incorporation with hydrolytically labile ester can trigger the degradation of polymer networks at neutral pH [97]. Different than PEG; PVA with normally little cell adhesion properties could easily be modified with RGD in the backbone due to multiple hydroxyl groups [98], [99]. Herein, by means of site-specific conjugation through PVA terminal groups with RGD sequences, a peptide hydrogel was achieved, which contains microstructure and surface-adhered properties [98]. In addition, because of the nontoxicity that PVA hydrogel have, it can be formed via crystallite formation from freezing-thawing cycles without adding any crosslinker [100]. As such, the use of PVA based hydrogels is a promising biomaterial in the biomedical field. One interesting paper used PVA based hydrogel to investigate the substrate stiffness (1–24 kPa) for the purpose of regulating the differentiation of cells Fig. 8. The results demonstrated that stiffness at about 1 and 24 kPa PVA based hydrogels promote effective neurogenesis and osteogenesis of the cells respectively [101].
Fig. 8.
Mechanisms diagrams of PVA hydrogel. a) Freezing thawing method of PVA hydrogel formation. b) Formation of PVA based hydrogel with stiffness gradient. Copyright, Ref. [101], 2015, Elsevier.
3. Stem cells in tissue engineering/regeneration
Stem cells of different origin have been studied for numerous therapeutic applications, but a major problem has been the little cell retention rates in vivo, one notion to improve cell viability is using biomaterials, which can be used to encapsulate cells before transplantation [23]. When conducting research with biomaterials like hydrogels, it is imperative to think about mechanical and material properties of stem cells, a specific criterion one has to take into account that is of upmost importance that bulk matrix stiffness is an eminent key mechanical guide in stem cell differentiation [11]. Tissue engineering is mentioned frequently in these studies, when conducting research in this field, it is imperative to keep in mind; tissue engineering integrates cells as well as bioactive components in a distinct microenvironment [102], [103]. It still remains difficult to identify the supportive influence of ECM factors on cell behavior [104]. Hydrogels show some promising work and many studies and progress is shown in engineering hydrogels to mimic the ECM.
3.1. Neural stem cells (NSC)
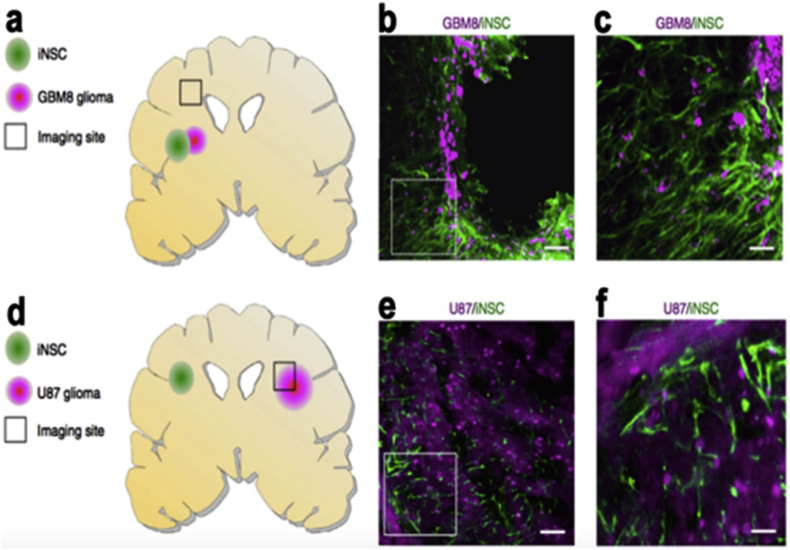
Neural stem/progenitor cells denoted as NSPCs, have great uses in biomedical applications and therapies, one example is in regenerative medicine [9], [12]. Neural stem cells (NSCs) are a kind of progenitor cell located in the nervous system with the property of generating into both neurons and glia, they are situated in the two adult neurogenic regions, the hippocampus and the subventricular zone [105]. Their capabilities can potentially alleviate tissue loss after brain injuries such as stroke or trauma [106]. As a study states, directing their differentiation to the needed phenotype within a defined matrix is difficult and with this notion in mind [9], they created an agarose modified hydrogel and in this matrix the results showed the ability of NSPCs to differentiate into the three primary cell types of the central nervous system (CNS), oligodendrocytes, astrocytes and neurons. These results established, that it is possible to direct the differentiation of NSPCs, with the help of growth factors. The applications of NSCs has numerous approaches and is modified and managed differently as shown by numerous studies, as such, one research team demonstrated that, transdifferentiation (denoted as TD) derived induced neural stem cells (iNSCs) are a useful therapeutic approach for brain cancer [107]. These iNSCs can be engineered to express a tumour necrosis factor generating ligand denoted as TRAIL and the stem cells as iNSC-sTR Fig. 9. And the same study concludes with in vivo testing, that iNSC-sTR treatment showed a decline in tumour growth and a shrinkage in GBM volumes 123-fold. Another approach for regenerative medicine with NSC is in neural tissue applications because of the poor regenerative capability of neural tissue. In the research, a peptide (IKVAV) modified hydrogel with encapsulated NSC, showed the development in regeneration of brain tissue. The studied concluded that the encapsulated NSCs could survive, proliferate and differentiate into neuronal or glial cells in a modified Peptide hydrogel [15].
Fig. 9.
The Migration of iNSCs to GBM in vivo. a–c) Assessing of iNSCs invading GBM, Fluorescent Imaging of the cryosections b–c) Images showing the colocalization of iNSCs (green) with invading GBMs (magenta) d–f) Assessing the iNSCs to far and set GBM. Copyright, Ref. [107], 2016, Nature Publishing Group.
3.2. Human mesenchymal stem cells (hMSC)
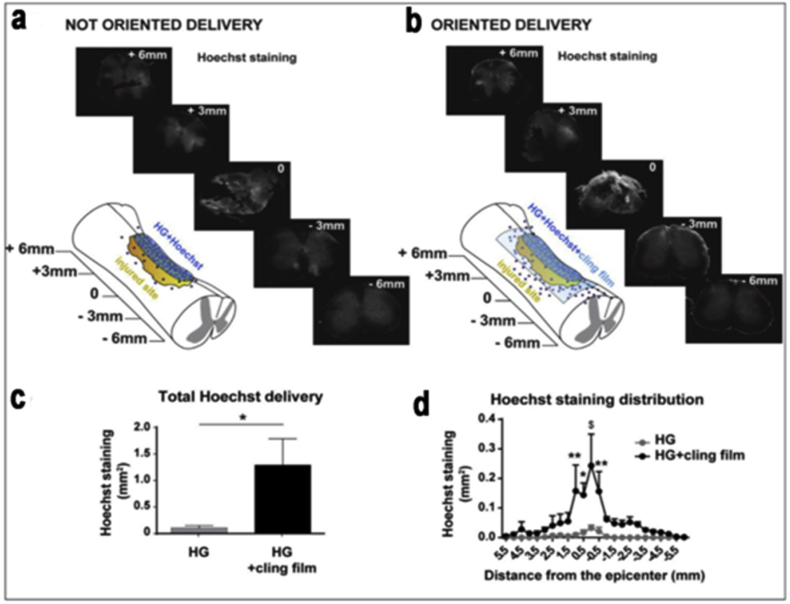
Mesenchymal stem cells (MSCs) have been extensively applied in the field of tissue regeneration, and MSCs derived from varying tissues show different properties [108]. Some examples of the research work in MSCs span great potential for the neural applications like stem cell therapy using human mesenchymal stem cells (hMSCs), which is an encouraging strategy in spinal cord injury applications Fig. 10. Noting though as with cell therapies there still lay limitations, but biomaterials that have the ability to encapsulate and sustain hMSCs show a reasonable approach to overcome these limitations, possibly improving the therapy. One study showed an agarose-carbomer based hydrogel, which aims to enhance hMSCs (by tackling various parameters like viability and density) by combining RGD tripeptide in order to enhance the ability of attaching and preserving hMSCs within the hydrogel [10]. And in the same study the biomimetic hydrogel was able to considerably immunomodulate the pro-inflammatory environment as well as increasing M2 macrophagic population. When conducting work using MSCs, it is essential to also analyze the biological interaction they have with the body outside of an engineering approach and more towards a mechanism biological understanding. One such study showed that MSCs and macrophages are central components of the stem cell niche and work in cooperation to control blood cell forming stem cell self-renewal. They also directly concluded that MSCs discharge micro RNA-containing exosomes that hinder macrophage activation [109]. This study is particularly significant because of the link they established with MSC survival and macrophage function, giving way to a context for the native immunomodulatory activity of MSCs. A research study showing these principals investigated the immune status of MSC and concluded, the immunomodulatory properties of mesenchymal stem/stromal cells could be used and implemented for the treatment of a wide range of inflammatory conditions [110]. ECM factors on hydrogels and its use in concert with MSCs is an important topic when discussing tissue engineering, a study used gradient-patterned methacrylate hydrogels, after several days exposure, differentiation was analyzed, and when density of fibronectin as well substrate stiffness were high, Osteogenic differentiation was detected [104]. The same study posted an Adipose-derived stem cell differentiation modeling, this model of stem cell differentiation as a function of stiffness was based on a previously described model.
Fig. 10.
In vivo delivery from HG RGD + ECM Profile release results in injured spinal cord. a) Oriented delivery. b) Post oriented delivery showing higher staining of solution delivered by HG RGD + ECM. c) Epicenter distribution (around). d) Comparing to the unsealed hydrogel. Copyright, Ref. [10], 2016, Elsevier.
3.3. Embryonic stem cell (ESC)/pluripotent stem cell (PSC)
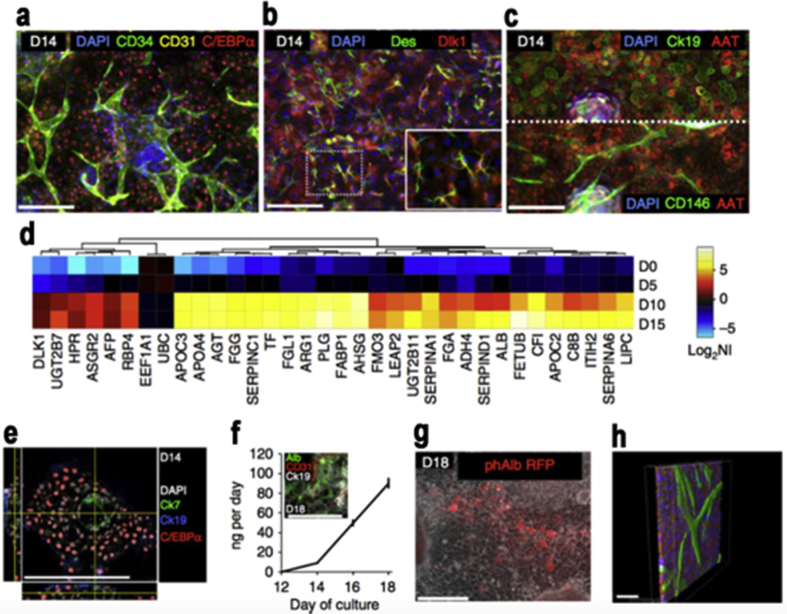
Pluripotent stem cell PSCs are derived from cells of the pre-implantation human embryo, hence embryonic stem cells or from somatic cells [111]. Reprogramming of somatic cells to a pluripotent state has greatly impacted our research of PSCs, the resulting cells are known as induced pluripotent stem cells or iPSCs [112]. Engineered multicellular systems based on human embryonic stem cells and human induced pluripotent stem cells are a widely studied research topic and have been shown in research for the formation vascular networks [113] Fig. 11. Human PSC is a superior choice in therapeutic research mainly because they are normal primary cell lines, they have an ability for unlimited self-renewal, and they have the potential to adopt any cellular fate [112]. One common application of PSCs is cardiac engineering [114], which involves vasculogenesis and angiogenesis. One study showed a chitosan (CS) based hydrogel that encapsulated ESC-ECs which are embryonic stem cell-derived endothelial cells (ESC-ECs), and then laden with VEGF-releasing microtubes, and when examined showed high cell survival and minimal cytotoxicity in vitro. When implanted into a mouse model, it induced robust cell retention, neovascularization via vasculogenesis and angiogenesis, strong cell retention as well as inducing recovery of blood flow in ischemic hindlimbs [23]. Another study used Peptide-modified (RGD) biomaterials which in this case influenced cell adhesion, survival and differentiation in vitro. The study also used Hyaluronan and methylcellulose hydrogel with a platelet-derived growth factor denoted as PDGF-A, which allowed early survival of grafted cells [115]. While many studies emphasis is on creating structures from a single germ layer, when conducting research one must keep in perspective organs in vivo encompass cells from multiple germ layers [113]. The use of embryonic/pluripotent stem cells is promising in this context because according to one study, Human induced pluripotent stem cells (hiPSCs) are able to differentiate in vitro to produce derivatives of the three primary germ layers [116]. To emphasize the importance of mechanical and materials testing while conducting research we show that in one study the researchers used HA-Tyr hydrogels and by fluctuating the concentrations they arranged hydrogels with different mechanical strength while studying the self-renewal properties of hESCs. The conclusions they observed was that both the mechanical strength and chemical composition were substantial metrics impacting cell proliferation and pluripotency. Their experimental data suggested that HA-Tyr hydrogel with a compressive modulus of 350 Pa aided the proliferation of hESCs at the pluripotent state in various conditioned medium [117].
Fig. 11.
Development of Fetal Liver functional tissue and non-parenchymal cells. a) Hepatocyte-like cells and vascular networks on the 14th day. b) Stellate-like cells. c) Hepatocyte-like cells. d) Up regulation of hepatic genes between 5th and 10th day. e) Bile duct-like channels and fetal hepatocyte-like cells developed. f) Greater and further maturation of liver-like phenotype. g) Albumin + cells. h) Confocal images of 3D reconstruction of AAT + hepatocyte-like cells (red) and endothelial-like cells (green). Copyright Ref. [113], 2016, Nature Publishing Group.
4. Hydrogel regulate stem cell fate
The relationship between hydrogels and stem cells is complex, and when conducting work with stem cells and hydrogels, it is important to understand the effects hydrogels have on stem cells and how certain factors can impact the hydrogels ability to regulate the stem cells fate. The connection of physical properties, as well as chemical properties of the materials, are respected as factors effecting stem cell fate [1]. Therefore, some aspects of hydrogels like biodegradability, stiffness, morphology and by-products play key roles in fate regulation and can influence stem cell differentiation and proliferation. These are topics of importance when working with hydrogels and stem cells. This section examines the relationships between the properties of the hydrogels and stem cell fate.
4.1. Biodegradability
One of the most essential factors to evaluate the merit of materials is biodegradability and it’s been well studied in past few years Table 1. For instance, when an implanted/injected hydrogel or scaffold is entered into the human body, the materials will remain inside permanently and without favorable degradation properties, it may exhibit long-term physiological and psychological damage. Moreover, degradation provides the space for cell stretching and proliferation, especially for synthetic based hydrogels due to pore size being much smaller than mammalian cells [118]. One study used the encapsulation of neural progenitor cells (NPCs) in alginate-based hydrogel to observe the proliferation property. They demonstrated the modified alginate hydrogel, which contains degradable (enzymatic) property that can significantly stimulate NPCs proliferation compared to alginate standard [119]. A similar study indicates that mouse mesenchymal stem cells (mMSC) can only spread in RGD modified HA based hydrogel with degradable character rather than in a non-degradable matrix [120]. Different from natural based hydrogels, synthetic hydrogels possess controllable degradation, which may vary based on the applications. In one study, PEG based photodegradable hydrogel was synthesized and showed great spreading of hMSCs [121]. PEG based hydrogels that degrade in response to hMSCs-secreted matrix metalloproteinase was also been studied [122]. Furthermore, the hybrid photodegradable hydrogel composed of gelatin and PEG not only appeared biocompatible and showed cell spreading but also better physical properties [123]. Biodegradability is a key for injectable hydrogel due to cells or drug release from the matrix [37]. Recently, Mooney group invented a void-forming alginate based hydrogel, in which mMSC are initially encapsulated in a nanoporous matrix and form pores in situ after injection. This unique technique possesses tremendous differentiation as well as locally control mMSC mediated tissue repair in situ [124].
Table 1.
Stem cell studies in hydrogel subtracts.
| Cell type | Material | Study | Degradation | Strength (kPa) | Ref |
|---|---|---|---|---|---|
| hMSCs | Alginate | Adjustable growth factor | 10–150 | [130] | |
| Alginate + PEG | 3D printing | 1500 j/m2 | [30] | ||
| Alginate | Enhance stem cell viability in implantation | 22 | [27] | ||
| Alginate | Cell reaction to stress relaxation | ∼7 days | 9–17 | [31] | |
| Gelatin | Bone regeneration | 2–6 weeks | 56–1250 | [147] | |
| Collagen | Scar modeling | ∼7 days | 0.3–40 | [61] | |
| HA | 3D printing & self-healing | ≥7 days | [43] | ||
| HA | Cell differentiation | ≥14 days | 4–95 | [41] | |
| PEG | 3D printing | 2–14 days | 1.12–589 pa | [93] | |
| PEG | Cell differentiation | 2–10 | [127] | ||
| PIC | Cell differentiation of gel stiffening | 0.2–0.4 | [11] | ||
| PEG | Control of protein presentation | 1–10 | [148] | ||
| PEG | Tissue regeneration | ∼4 h (50%) | 0.35–0.6 | [149] | |
| DexMA | Cell spreading to stiffness | 0.3–19 | [150] | ||
| Chitin | Tissue engineering | 41–138 Gpa | [151] | ||
| PEG | Cellular microenvironments | 13.6 | [121] | ||
| Collagen | Articular cartilage regeneration | ≥7 days | 2.5 | [54] | |
| Alginate | Bone regeneration | 5–110 | [124] | ||
| mMSCs | Gelatin | Delivery of stem cell | ≥15 days | 0.1–2.5 | [64] |
| Alginate + PAAM | Biocompatibility | Minimal | 19–30 | [73] | |
| PEG | New material of PEG dendrimer | 30–45 days | 0.2–6 | [76] | |
| PEG | Injectable scaffold | 0.030–1 | [152] | ||
| HA | Spreading, migration and proliferation of cell | 0.177–0.192 | [120] | ||
| hADSCs | HA | Tissue adhesive | ∼24 h | 0.18–1.35 | [42] |
| PAAM | Protect cell through injection | ≥21 days | 0.013–0.1 | [24] | |
| iPSCs | HA | Cell differentiation & viability after injection | 0.300–1 | [40] | |
| PEG | Cell differentiation | 0.3–1.2 | [89] | ||
| PAM | Stem cell self-renewal | ≥60 days | 0.7–10 | [128] | |
| NPCs | Alginate | Adjustable degradation rate | ≥7 days | [119] | |
| ASCs | PA&PDMS | Cell differentiation | 4–30 | [153] |
4.2. Stiffness
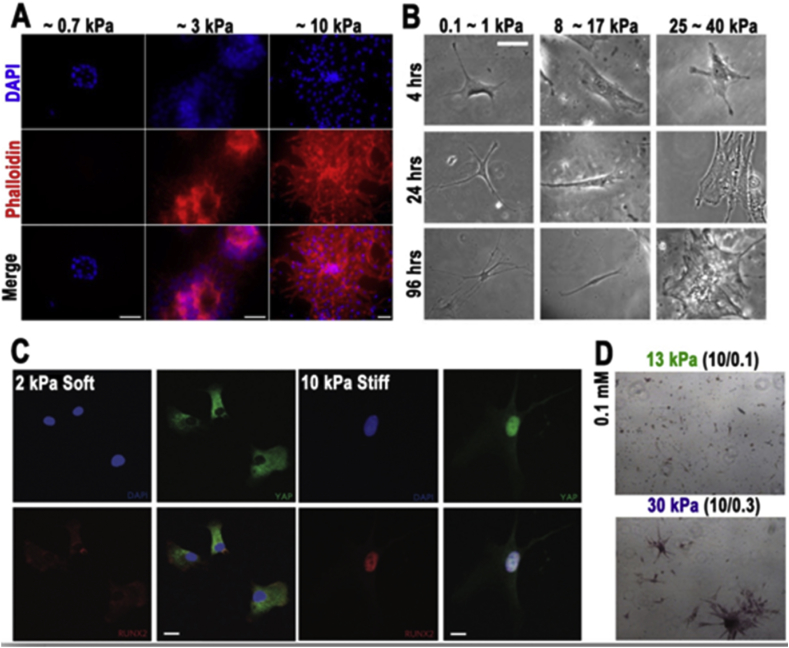
Materials stiffness plays a significant role in the regulation of stem cell fate such as adhesion, migration and differentiation Fig. 12. Polymer concentration and crosslinking density of the hydrogel are essential to effect hydrogel stiffness. The range of hydrogel stiffness can be very soft (<0.1 kPa), similar with viscous fluid or hard enough to compare with silicone (∼500 kPa). The differentiation of hMSCs seeded on the PAAM hydrogels surface was studied and the distinguishing results appeared in matrix stiffness from 1 to 100 kPa [125]. However, the cell adhesion of PAM hydrogels are not directly related to its stiffness but the ECM proteins [17], [126]. On a similar study of hydrogel stiffness and dosing, the influence of hMSCs fate while using PEG based hydrogel had the stiffness ranging from 2 to 10 kPa, this was comparable with tissue culture polystyrene (∼3 Gpa) [127]. In addition, one study developed a PAM based hydrogel conjugate with glycosaminoglycans (GAG)-binding peptide to study the correlation between substrate and human embryonic stem cells (hES). The Results show only stiffness (∼10 kPa) can maintain hES cells proliferation and pluripotency, which imply that hES cells can communicate with the substrate through GAG engagement [128]. A three-dimensional study of stem cell fate in hydrogel has also been conducted [126], [129], one remarkable study shows stress-stiffening-mediated control of hMSCs differentiation in polyisocyanopeptide-based hydrogel [11]. The conjugation of peptides with polymers can lean on both stiffness and adhesion while regulating stem cell fate at the same time. For instance, HA based hydrogel modified with RGD peptide has stiffness of (4–100 kPa) [41], alginate based hydrogels conjugated with RGD sequences peptide has the stiffness of (1–160 kPa) [129] and (10–150 kPa) [130]. Hydrogel stiffness has a strong affinity with the cell’s migration; one study demonstrated that physical properties of the cellar environment can directly effect epithelial growth as well as guild cell migration [131]. The similar result shows the stiffness is the majority factor of causing NSPCs migration in chitosan-based hydrogel [132]. (More comparison can find in Table 1).
Fig. 12.
Phenomenon of stem cell in various hydrogel stiffness. A) hMSCs seeding in Phototunable hydrogel on YAP and RUNX2 activation on soft and stiff hydrogels (2 and 10 kPa). Scale bar, 50 μm [128]. B) Adhesion of MSCs in various substrate stiffness. Scale bar, 20 mm [125]. C) Adhesion and spreading of hES cell of divers elasticity and stained for actin filaments. Scale bars, 20 μm [127]. D) Differentiation of ASCs in different hydrogel stiffness. Scale bar, 500 μm [153]. Copyright, Ref. [128], 2012, ACS. Copyright, Ref. [125], 2006 Cell Press. Copyright, Ref. [127], 2016, Nature Publishing Group. Adapted, with permission, from Ref. [153], 2016, Nature Publishing Group.
4.3. Morphology
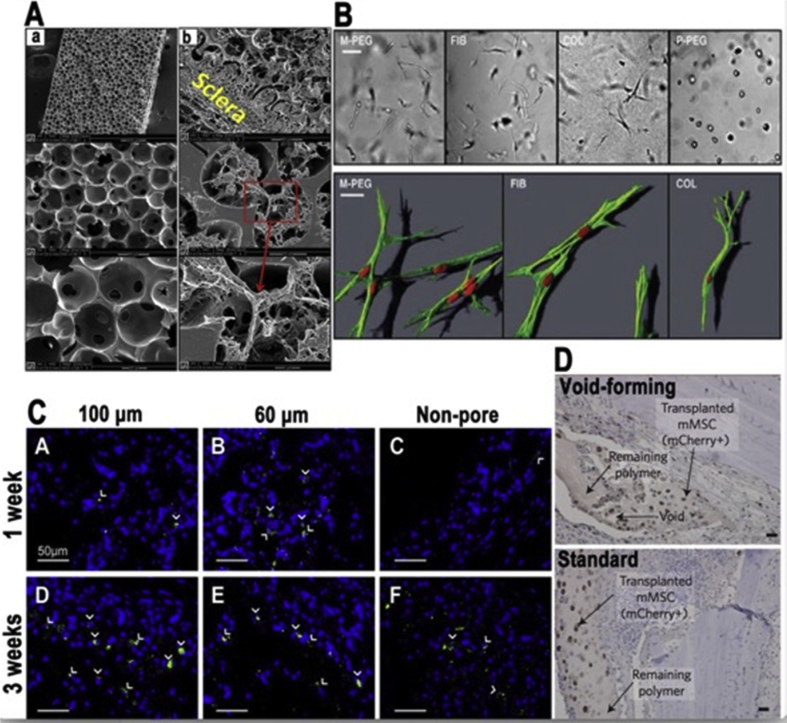
The ability to use stem cell therapies is limited by control over fate; this can be relatively evaded by the implementation of biomaterials with macroporous properties [124] Table 2. In the majority of scaffold materials, the porosity of the actual scaffold is significant in the guiding of tissue formation/function [2]. In the field of tissue engineering porous biomaterials are widely used and accepted as an important factor, and the porosity of materials is an important property in tissue engineering [133], [134]. When discussing the regulation of stem cell fate, physical properties of the materials used are of the upmost importance [17], [135]. One study showed that by using special techniques like sphere-templating fabrication, they were able to make controllable tough porous hydrogel scaffold, which supported proliferation and adhesion of the cell. This study compared hydrogels with their porous one and found that For many synthetic hydrogels the toughness ranges are about 104–105 J/m3 and the modified porous hydrogel scaffolds are about 1.4*107 J/m3 and 1.5*106 J/m3 respectively [134]. Another study presented that porous hydrogels showed transgene expression of mMSCs (mouse mesenchymal stem cell) [136]. The porous properties of biomaterials are crucial in revascularization and porous constructs have allowed vessel infiltration without the need for highly intensive matrix degradation [133]. One study compared degradable PEG hydrogels and their ECM imitating ability and determined that with the hydrogel, it allowed spindle-shaped cell morphologies comparable to natural fibrin, these studies point to a similar notion that porosity and more generally morphology can effect stem cell migration and ultimately stem cell fate Fig. 13. In another study, this team used porosity in their research in the form of a porous hydrogel for gene therapy. The study consisted of a nanoparticle customized porous PEG hydrogel to enhance lentivirus delivery for the gene therapy, and they conclude that the modified porous hydrogel was a good biomaterial, and had promise for many medical applications and therapies [137]. A study furthered showed the relationship between the size of pores and vascularization in PEG hydrogels and concluded that gels with a pore size of 25–50 μm showed cell invasion was limited to the external surface and gels with a greater pore size (100–150 μm) presented vascularized tissue formation [138]. As implied previously, the pore size and distribution are imperative aspects of a hydrogel matrix [139]. Because of the importance of pore size in tissue engineering, an investigation points out the optimized pore sizes in regeneration and it correlates as such, (5 μm) for neovascularization, (20–125 μm) for regeneration of adult mammalian skin, (100–350 μm) for regeneration of bone and (20 μm) for the ingrowth of hepatocytes [2]. Furthermore, in working with CNS tissue engineering the use of a macroporous scaffold with pores greater than 40 μm is needed [140]. Another study presented how printing extrinsic factors onto hydrogels helped in the regulation of stem cells fate and showed yet again the critical importance of pore size in these hydrogels [141]. Now we take a look from the perspective of stem cells and how stem cells react towards these factors. One study examines how hMSCs cooperate with hydrogels with different surface wrinkles like lamellar and showed that the stem cells adhere to wrinkles by changing to the pattern shape and differentiated, their conclusion clearly showed the influence of morphology on differentiation [142].
Table 2.
Applications of stem cells in concert with hydrogels.
| Cell type | Material | Study | Peptide | Pores (μm) | Ref |
|---|---|---|---|---|---|
| MSCs | Silk | Tissue engineering with silk hydrogels | RGD | ∼200 | [102] |
| PCL | A variation of pore size | RGD | 100–450 | [154] | |
| PEG | Injectable Biodegradable hydrogel deposits | ∼500 | [103] | ||
| HA | Functionalized hydrogel with mimetic peptide | N-Cadherin & RGD | [33] | ||
| NapFF-NO | Nitric oxide releasing hydrogels | FFGGG | [4] | ||
| CS | Affinity peptide bone matrix, with CS hydrogel | E7 | 30–80 | [135] | |
| Agarose | Biomimetic hydrogel delivers factors | RGD | [10] | ||
| PEG and CS | Controlling porosity of hydrogels | 180–240 | [2] | ||
| PEG | Evaluation of macroporous hydrogel | RGD | 100–600 | [7] | |
| NSCs | Agarose | The Effect of growth factor on NSCs | GRGD | [9] | |
| SAP | Encapsulated NSCs in SAP hydrogel | IKVAV | [15] | ||
| Gel-HPA | Effect of injectable hydrogel on proliferation | RGD | ∼3 | [106] | |
| Alginate | Promotion of oriented axonal regrowth | ∼27 | [12] | ||
| ESCs | PHEMA | Polymers for ES cell use | 100–500 | [8] | |
| PHEMA | Templates for cardiac tissue engineering | 20–80 | [114] | ||
| iPSCs | HA | Hydrogel promotes early survival of iPSC | RGD | [115] | |
| PEG | Reprogramming mouse fibroblasts | RGD | [13] | ||
| ASCs | HA | Thermosensitive injectable HA hydrogel | 3–20 | [5] |
Fig. 13.
Adhesion and migration of cells in diverse subtract. A) (a) Images of morphologies of the hydrogel scaffold prior to inserting. (b) Showing cell migration from tissues into the hydrogel scaffold, filling up the porous substantially [134]. B) M-PEG hydrogel (matrix metalloproteinases sensitive sequence) permits cell morphology which is comparable to natural fibrin/collagen gel. The P-PEG hydrogel (plasmin-sensitive sequence) stops the dispersal of cells [118]. C) Fluorescent staining of GFP of various hydrogels 100 μm (A, D), 60 μm (B, E) and Non-pore for (C, F). A–C for 1 weeks and D–F for 3weeks, this shows the transfected cells in the hydrogels [136]. D) Micrographs of tissues with void forming hydrogel (top), and standard hydrogel (bottom) [124]. Adapted, with permission, from Ref. [134], 2014 Elsevier. Copyright, Ref. [118], 2005 Biophysical Society. Copyright, Ref. [136], 2014, Elsevier. Copyright, Ref. [124], 2015, Nature Publishing Group.
4.4. By product of stem cell degradation
There is an emerging idea for the regulation of stem cells, it is degradation by-products, meaning that actual material can give sign messages to stem cells in the form of by-products. This is significant because cells develop a rich amount of information from their environment, like the materials that surround them [143]. These properties of hydrogels being responsive to the environment are sometimes referred to as smart biomaterials, which for example activate changes in molecular communications such as swelling and collapsing transitions [1]. On a further note, a multitude of smart materials have incorporated responsiveness to biological targets [144]. One study concluded the differentiation of (hMSCs) is guided by degradation-mediated cellular-traction, and traction, being contingent on stem cells degrading the surroundings while both creating focal adhesions as well as cytoskeletal constructions [41]. An indication that degradation can influence the differentiation of stem cells is the notion that various ions that are discharged affect phenotype; also the identification of degradable hydrogels also helps prove this concept, another idea is the ECM’s by-product can affect stem cells as pointed out that by-products of its degradation can be shown to control cell behavior by adjusting growth factors [17], [145]. And the degradation of ECM molecules, like hyaluronic acid and collagen has a record of influencing cell behavior.
5. Conclusion and prospective
Hydrogels are an attractive approach to therapeutic applications, when conducting research with hydrogels there are various factors, parameters and metrics one must take to achieve the desired results. When using hydrogels, the choice of the base should be made, whether or not it is natural or synthetic and the similarities and differences should be assessed, also the use of hybrid hydrogels and the characteristics associated must also be studied. The modifications of the material properties of hydrogels are crucial in biomedical applications and the properties have to be considered especially when seeding with stem cells. The stiffness, biodegradability and biocompatibility of the hydrogel should be evaluated, and the hydrogel has an interaction with the environment it is placed in, while regulating the fate of stem cells. The porosity of hydrogels should also be examined and the relationships between morphology and adhesion should also be formulated. One should take into perspective the possibility of poor mechanical factors and constancy of certain networks, and while limited, some corrections of these problems may include the uses of peptides [146]. Conducting research with hydrogels whether the scope is in tissue engineering or drug delivery requires interdisciplinary knowledge, stretching from material science to biology and chemistry. As in-depth looks of hydrogels would also encompass a study on the host’s response to the therapy, especially when using hydrogels seeded with stem cells. Going back to hydrogel choice, a good measure of determining which hydrogel to use is the cellular response; an ideal hydrogel is one that will completely degrade while simultaneously allowing cells to proliferate with complete compatibility to native tissue, and in this lies the challenge. As such, future work in the biomaterials field will consist of a more in depth study of the material properties of the hydrogels in use to create a “seamless” hydrogel in which it has tunable stiffness and strength, while maintaining mimics with tissue characteristics like having viscoelastic properties, as well as proper morphological structure. In addition, to incorporate a deeper biological classification, like the effects the seeded hydrogels have on the immune system in the host, possible cytotoxicity and other adverse effects. More importantly, a hydrogel with injectable and imaging properties that is crucial traits in the biomedical field due to noninvasive treatments and real-time tracking. Overall, to combine advantages of natural and synthetic polymers with tunable chemistry and structure, incorporate ECM elements, designable mechanical properties and adjustable degradation rate can lead to the “seamless” hydrogel.
References
- 1.Ulijn R.V., Bibi N., Jayawarna V., Thornton P.D., Todd S.J., Mart R.J., Smith A.M., Gough J.E. Bioresponsive hydrogels. Mater. Today. 2007;10:40–48. [Google Scholar]
- 2.Annabi N., Nichol J.W., Zhong X., Ji C., Koshy S., Khademhosseini A., Dehghani F. Controlling the porosity and microarchitecture of hydrogels for tissue engineering. Tissue Eng. Part B Rev. 2010;16:371–383. doi: 10.1089/ten.teb.2009.0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Annabi N., Tamayol A., Uquillas J.A., Akbari M., Bertassoni L.E., Cha C., Camci-Unal G., Dokmeci M.R., Peppas N.A., Khademhosseini A. 25th anniversary article: rational design and applications of hydrogels in regenerative medicine. Adv. Mater. 2014;26:85–124. doi: 10.1002/adma.201303233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao X., Liu Y., Gao J., Yang L., Mao D., Stefanitsch C., Li Y., Zhang J., Ou L., Kong D., Zhao Q., Li Z. Nitric oxide releasing hydrogel enhances the therapeutic efficacy of mesenchymal stem cells for myocardial infarction. Biomaterials. 2015;60:130–140. doi: 10.1016/j.biomaterials.2015.04.046. [DOI] [PubMed] [Google Scholar]
- 5.Tan H., Ramirez C.M., Miljkovic N., Li H., Rubin J.P., Marra K.G. Thermosensitive injectable hyaluronic acid hydrogel for adipose tissue engineering. Biomaterials. 2009;30:6844–6853. doi: 10.1016/j.biomaterials.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bae K.H., Lee F., Xu K., Keng C.T., Tan S.Y., Tan Y.J., Chen Q., Kurisawa M. Microstructured dextran hydrogels for burst-free sustained release of PEGylated protein drugs. Biomaterials. 2015;63:146–157. doi: 10.1016/j.biomaterials.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Keskar V., Marion N.W., Mao J.J., Gemeinhart R.A. In vitro evaluation of macroporous hydrogels to facilitate stem cell infiltration, growth, and mineralization. Tissue Eng. Part A. 2009;15:1695–1707. doi: 10.1089/ten.tea.2008.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kroupova J., Horak D., Pachernik J., Dvorak P., Slouf M. Functional polymer hydrogels for embryonic stem cell support. J. Biomed. Mater. Res. B Appl. Biomater. 2006;76:315–325. doi: 10.1002/jbm.b.30366. [DOI] [PubMed] [Google Scholar]
- 9.Aizawa Y., Leipzig N., Zahir T., Shoichet M. The effect of immobilized platelet derived growth factor AA on neural stem/progenitor cell differentiation on cell-adhesive hydrogels. Biomaterials. 2008;29:4676–4683. doi: 10.1016/j.biomaterials.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 10.Caron I., Rossi F., Papa S., Aloe R., Sculco M., Mauri E., Sacchetti A., Erba E., Panini N., Parazzi V., Barilani M., Forloni G., Perale G., Lazzari L., Veglianese P. A new three dimensional biomimetic hydrogel to deliver factors secreted by human mesenchymal stem cells in spinal cord injury. Biomaterials. 2016;75:135–147. doi: 10.1016/j.biomaterials.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 11.Das R.K., Gocheva V., Hammink R., Zouani O.F., Rowan A.E. Stress-stiffening-mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 2016;15:318–325. doi: 10.1038/nmat4483. [DOI] [PubMed] [Google Scholar]
- 12.Prang P., Muller R., Eljaouhari A., Heckmann K., Kunz W., Weber T., Faber C., Vroemen M., Bogdahn U., Weidner N. The promotion of oriented axonal regrowth in the injured spinal cord by alginate-based anisotropic capillary hydrogels. Biomaterials. 2006;27:3560–3569. doi: 10.1016/j.biomaterials.2006.01.053. [DOI] [PubMed] [Google Scholar]
- 13.Smith A.W., Hoyne J.D., Nguyen P.K., McCreedy D.A., Aly H., Efimov I.R., Rentschler S., Elbert D.L. Direct reprogramming of mouse fibroblasts to cardiomyocyte-like cells using Yamanaka factors on engineered poly(ethylene glycol) (PEG) hydrogels. Biomaterials. 2013;34:6559–6571. doi: 10.1016/j.biomaterials.2013.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S., Nakamoto T., Kawazoe N., Chen G. Engineering multi-layered skeletal muscle tissue by using 3D microgrooved collagen scaffolds. Biomaterials. 2015;73:23–31. doi: 10.1016/j.biomaterials.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 15.Cheng T.Y., Chen M.H., Chang W.H., Huang M.Y., Wang T.W. Neural stem cells encapsulated in a functionalized self-assembling peptide hydrogel for brain tissue engineering. Biomaterials. 2013;34:2005–2016. doi: 10.1016/j.biomaterials.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 16.Yuk H., Zhang T., Lin S., Parada G.A., Zhao X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2016;15:190–196. doi: 10.1038/nmat4463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy W.L., McDevitt T.C., Engler A.J. Materials as stem cell regulators. Nat. Mater. 2014;13:547–557. doi: 10.1038/nmat3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Purcell B.P., Lobb D., Charati M.B., Dorsey S.M., Wade R.J., Zellars K.N., Doviak H., Pettaway S., Logdon C.B., Shuman J.A., Freels P.D., Gorman J.H., 3rd, Gorman R.C., Spinale F.G., Burdick J.A. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat. Mater. 2014;13:653–661. doi: 10.1038/nmat3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva R., Fabry B., Boccaccini A.R. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials. 2014;35:6727–6738. doi: 10.1016/j.biomaterials.2014.04.078. [DOI] [PubMed] [Google Scholar]
- 20.Tasoglu S., Yu C.H., Gungordu H.I., Guven S., Vural T., Demirci U. Guided and magnetic self-assembly of tunable magnetoceptive gels. Nat. Commun. 2014;5:4702. doi: 10.1038/ncomms5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y., He W., Cao T., Guo H., Zhang Y., Li Q., Shao Z., Cui Y., Zhang X. Elastic, conductive, polymeric hydrogels and sponges. Sci. Rep. 2014;4:5792. doi: 10.1038/srep05792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsieh F.Y., Lin H.H., Hsu S.H. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials. 2015;71:48–57. doi: 10.1016/j.biomaterials.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 23.Lee S., Valmikinathan C.M., Byun J., Kim S., Lee G., Mokarram N., Pai S.B., Um E., Bellamkonda R.V., Yoon Y.S. Enhanced therapeutic neovascularization by CD31-expressing cells and embryonic stem cell-derived endothelial cells engineered with chitosan hydrogel containing VEGF-releasing microtubes. Biomaterials. 2015;63:158–167. doi: 10.1016/j.biomaterials.2015.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cai L., Dewi R.E., Heilshorn S.C. Injectable hydrogels with in situ double network formation enhance retention of transplanted stem cells. Adv. Funct. Mater. 2015;25:1344–1351. doi: 10.1002/adfm.201403631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornwell K.G., Landsman A., James K.S. Extracellular matrix biomaterials for soft tissue repair. Clin. Podiatr. Med. Surg. 2009;26:507–523. doi: 10.1016/j.cpm.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Rowley J.A., Madlambayan G., Mooney D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 27.Moshaverinia A., Chen C., Xu X., Ansari S., Zadeh H.H., Schricker S.R., Paine M.L., Moradian-Oldak J., Khademhosseini A., Snead M.L., Shi S. Regulation of the stem cell-host immune system interplay using hydrogel coencapsulation system with an anti-inflammatory drug. Adv. Funct. Mater. 2015;25:2296–2307. doi: 10.1002/adfm.201500055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayashi K., Sakamoto W., Yogo T. Smart ferrofluid with quick gel transformation in tumors for MRI-guided local magnetic thermochemotherapy. Adv. Funct. Mater. 2016;26:1708–1718. [Google Scholar]
- 29.Huang H., Choi J.K., Rao W., Zhao S., Agarwal P., Zhao G., He X. Alginate hydrogel microencapsulation inhibits devitrification and enables large-volume low-CPA cell vitrification. Adv. Funct. Mater. 2015;25:6839–6850. doi: 10.1002/adfm.201503047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong S., Sycks D., Chan H.F., Lin S., Lopez G.P., Guilak F., Leong K.W., Zhao X. 3D printing of highly stretchable and tough hydrogels into complex, cellularized structures. Adv. Mater. 2015;27:4035–4040. doi: 10.1002/adma.201501099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S.A., Weaver J.C., Huebsch N., Lee H.P., Lippens E., Duda G.N., Mooney D.J. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15:326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Veiseh O., Doloff J.C., Ma M., Vegas A.J., Tam H.H., Bader A.R., Li J., Langan E., Wyckoff J., Loo W.S., Jhunjhunwala S., Chiu A., Siebert S., Tang K., Hollister-Lock J., Aresta-Dasilva S., Bochenek M., Mendoza-Elias J., Wang Y., Qi M., Lavin D.M., Chen M., Dholakia N., Thakrar R., Lacik I., Weir G.C., Oberholzer J., Greiner D.L., Langer R., Anderson D.G. Size- and shape-dependent foreign body immune response to materials implanted in rodents and non-human primates. Nat. Mater. 2015;14:643–651. doi: 10.1038/nmat4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu M., Lin S., Sun Y., Feng Q., Li G., Bian L. Hydrogels functionalized with N-cadherin mimetic peptide enhance osteogenesis of hMSCs by emulating the osteogenic niche. Biomaterials. 2016;77:44–52. doi: 10.1016/j.biomaterials.2015.10.072. [DOI] [PubMed] [Google Scholar]
- 34.Burdick J.A., Prestwich G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011;23:H41–H56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deepa S.S., Carulli D., Galtrey C., Rhodes K., Fukuda J., Mikami T., Sugahara K., Fawcett J.W. Composition of perineuronal net extracellular matrix in rat brain: a different disaccharide composition for the net-associated proteoglycans. J. Biol. Chem. 2006;281:17789–17800. doi: 10.1074/jbc.M600544200. [DOI] [PubMed] [Google Scholar]
- 36.Allison D.D., Grande-Allen K.J. Review. Hyaluronan: a powerful tissue engineering tool. Tissue Eng. 2006;12:2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 37.Li Y., Rodrigues J., Tomas H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem. Soc. Rev. 2012;41:2193–2221. doi: 10.1039/c1cs15203c. [DOI] [PubMed] [Google Scholar]
- 38.Fisher S.A., Anandakumaran P.N., Owen S.C., Shoichet M.S. Tuning the microenvironment: click-crosslinked hyaluronic acid-based hydrogels provide a platform for studying breast cancer cell invasion. Adv. Funct. Mater. 2015;25:7163–7172. [Google Scholar]
- 39.Grover G.N., Braden R.L., Christman K.L. Oxime cross-linked injectable hydrogels for catheter delivery. Adv. Mater. 2013;25:2937–2942. doi: 10.1002/adma.201205234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lam J., Lowry W.E., Carmichael S.T., Segura T. Delivery of iPS-NPCs to the stroke cavity within a hyaluronic acid matrix promotes the differentiation of transplanted cells. Adv. Funct. Mater. 2014;24:7053–7062. doi: 10.1002/adfm.201401483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khetan S., Guvendiren M., Legant W.R., Cohen D.M., Chen C.S., Burdick J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shin J., Lee J.S., Lee C., Park H.-J., Yang K., Jin Y., Ryu J.H., Hong K.S., Moon S.-H., Chung H.-M., Yang H.S., Um S.H., Oh J.-W., Kim D.-I., Lee H., Cho S.-W. Tissue adhesive catechol-modified hyaluronic acid hydrogel for effective, minimally invasive cell therapy. Adv. Funct. Mater. 2015;25:3814–3824. [Google Scholar]
- 43.Highley C.B., Rodell C.B., Burdick J.A. Direct 3D printing of shear-thinning hydrogels into self-healing hydrogels. Adv. Mater. 2015;27:5075–5079. doi: 10.1002/adma.201501234. [DOI] [PubMed] [Google Scholar]
- 44.Suh J.K., Matthew H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: a review. Biomaterials. 2000;21:2589–2598. doi: 10.1016/s0142-9612(00)00126-5. [DOI] [PubMed] [Google Scholar]
- 45.Croisier F., Jérôme C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013;49:780–792. [Google Scholar]
- 46.Jayakumar R., Menon D., Manzoor K., Nair S.V., Tamura H. Biomedical applications of chitin and chitosan based nanomaterials—a short review. Carbohydr. Polym. 2010;82:227–232. [Google Scholar]
- 47.Chiu Y.L., Chen S.C., Su C.J., Hsiao C.W., Chen Y.M., Chen H.L., Sung H.W. pH-triggered injectable hydrogels prepared from aqueous N-palmitoyl chitosan: in vitro characteristics and in vivo biocompatibility. Biomaterials. 2009;30:4877–4888. doi: 10.1016/j.biomaterials.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 48.Cho J., Heuzey M.C., Begin A., Carreau P.J. Physical gelation of chitosan in the presence of beta-glycerophosphate: the effect of temperature. Biomacromolecules. 2005;6:3267–3275. doi: 10.1021/bm050313s. [DOI] [PubMed] [Google Scholar]
- 49.Wang H., Shi J., Wang Y., Yin Y., Wang L., Liu J., Liu Z., Duan C., Zhu P., Wang C. Promotion of cardiac differentiation of brown adipose derived stem cells by chitosan hydrogel for repair after myocardial infarction. Biomaterials. 2014;35:3986–3998. doi: 10.1016/j.biomaterials.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 50.Li L., Wang N., Jin X., Deng R., Nie S., Sun L., Wu Q., Wei Y., Gong C. Biodegradable and injectable in situ cross-linking chitosan-hyaluronic acid based hydrogels for postoperative adhesion prevention. Biomaterials. 2014;35:3903–3917. doi: 10.1016/j.biomaterials.2014.01.050. [DOI] [PubMed] [Google Scholar]
- 51.Tseng T.C., Tao L., Hsieh F.Y., Wei Y., Chiu I.M., Hsu S.H. An injectable, self-healing hydrogel to repair the central nervous system. Adv. Mater. 2015;27:3518–3524. doi: 10.1002/adma.201500762. [DOI] [PubMed] [Google Scholar]
- 52.Chevallay B., Herbage D. Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. Med. Biol. Eng. Comput. 2000;38:211–218. doi: 10.1007/BF02344779. [DOI] [PubMed] [Google Scholar]
- 53.Szot C.S., Buchanan C.F., Freeman J.W., Rylander M.N. 3D in vitro bioengineered tumors based on collagen I hydrogels. Biomaterials. 2011;32:7905–7912. doi: 10.1016/j.biomaterials.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parmar P.A., Chow L.W., St-Pierre J.P., Horejs C.M., Peng Y.Y., Werkmeister J.A., Ramshaw J.A., Stevens M.M. Collagen-mimetic peptide-modifiable hydrogels for articular cartilage regeneration. Biomaterials. 2015;54:213–225. doi: 10.1016/j.biomaterials.2015.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rafat M., Xeroudaki M., Koulikovska M., Sherrell P., Groth F., Fagerholm P., Lagali N. Composite core-and-skirt collagen hydrogels with differential degradation for corneal therapeutic applications. Biomaterials. 2016;83:142–155. doi: 10.1016/j.biomaterials.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 56.Ramachandran G. Springer Science & Business Media; 2013. Biochemistry of Collagen. [Google Scholar]
- 57.Maynes R. Elsevier; 2012. Structure and Function of Collagen Types. [Google Scholar]
- 58.O’Connor S.M., Stenger D.A., Shaffer K.M., Ma W. Survival and neurite outgrowth of rat cortical neurons in three-dimensional agarose and collagen gel matrices. Neurosci. Lett. 2001;304:189–193. doi: 10.1016/s0304-3940(01)01769-4. [DOI] [PubMed] [Google Scholar]
- 59.Joosten E.A., Veldhuis W.B., Hamers F.P. Collagen containing neonatal astrocytes stimulates regrowth of injured fibers and promotes modest locomotor recovery after spinal cord injury. J. Neurosci. Res. 2004;77:127–142. doi: 10.1002/jnr.20088. [DOI] [PubMed] [Google Scholar]
- 60.Hsiao T.W., Tresco P.A., Hlady V. Astrocytes alignment and reactivity on collagen hydrogels patterned with ECM proteins. Biomaterials. 2015;39:124–130. doi: 10.1016/j.biomaterials.2014.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dingal P.C., Bradshaw A.M., Cho S., Raab M., Buxboim A., Swift J., Discher D.E. Fractal heterogeneity in minimal matrix models of scars modulates stiff-niche stem-cell responses via nuclear exit of a mechanorepressor. Nat. Mater. 2015;14:951–960. doi: 10.1038/nmat4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pignatello R. InTech; 2011. Biomaterials Applications for Nanomedicine. [Google Scholar]
- 63.Kim Y.H., Furuya H., Tabata Y. Enhancement of bone regeneration by dual release of a macrophage recruitment agent and platelet-rich plasma from gelatin hydrogels. Biomaterials. 2014;35:214–224. doi: 10.1016/j.biomaterials.2013.09.103. [DOI] [PubMed] [Google Scholar]
- 64.Lee S.H., Lee Y., Chun Y.W., Crowder S.W., Young P.P., Park K.D., Sung H.J. Crosslinkable gelatin hydrogels for vasculogenic induction and delivery of mesenchymal stem cells. Adv. Funct. Mater. 2014;24:6771–6781. doi: 10.1002/adfm.201401110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Helminger M., Wu B., Kollmann T., Benke D., Schwahn D., Pipich V., Faivre D., Zahn D., Colfen H. Synthesis and characterization of gelatin-based magnetic hydrogels. Adv. Funct. Mater. 2014;24:3187–3196. doi: 10.1002/adfm.201303547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen P., Xia C., Mei S., Wang J., Shan Z., Lin X., Fan S. Intra-articular delivery of sinomenium encapsulated by chitosan microspheres and photo-crosslinked GelMA hydrogel ameliorates osteoarthritis by effectively regulating autophagy. Biomaterials. 2016;81:1–13. doi: 10.1016/j.biomaterials.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 67.Tsang K.M., Annabi N., Ercole F., Zhou K., Karst D., Li F., Haynes J.M., Evans R.A., Thissen H., Khademhosseini A., Forsythe J.S. Facile one-step micropatterning using photodegradable methacrylated gelatin hydrogels for improved cardiomyocyte organization and alignment. Adv. Funct. Mater. 2015;25:977–986. doi: 10.1002/adfm.201403124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fu Y., Xu K., Zheng X., Giacomin A.J., Mix A.W., Kao W.J. 3D cell entrapment in crosslinked thiolated gelatin-poly(ethylene glycol) diacrylate hydrogels. Biomaterials. 2012;33:48–58. doi: 10.1016/j.biomaterials.2011.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nichol J.W., Koshy S.T., Bae H., Hwang C.M., Yamanlar S., Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31:5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jeon O., Wolfson D.W., Alsberg E. In-situ formation of growth-factor-loaded coacervate microparticle-embedded hydrogels for directing encapsulated stem cell fate. Adv. Mater. 2015;27:2216–2223. doi: 10.1002/adma.201405337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Drury J.L., Mooney D.J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 72.Macaya D., Spector M. Injectable hydrogel materials for spinal cord regeneration: a review. Biomed. Mater. 2012;7:012001. doi: 10.1088/1748-6041/7/1/012001. [DOI] [PubMed] [Google Scholar]
- 73.Darnell M.C., Sun J.Y., Mehta M., Johnson C., Arany P.R., Suo Z., Mooney D.J. Performance and biocompatibility of extremely tough alginate/polyacrylamide hydrogels. Biomaterials. 2013;34:8042–8048. doi: 10.1016/j.biomaterials.2013.06.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernández E., López D., López-Cabarcos E., Mijangos C. Viscoelastic and swelling properties of glucose oxidase loaded polyacrylamide hydrogels and the evaluation of their properties as glucose sensors. Polymer. 2005;46:2211–2217. [Google Scholar]
- 75.Kandow C.E., Georges P.C., Janmey P.A., Beningo K.A. Polyacrylamide hydrogels for cell mechanics: steps toward optimization and alternative uses. Methods Cell Biol. 2007;83:29–46. doi: 10.1016/S0091-679X(07)83002-0. [DOI] [PubMed] [Google Scholar]
- 76.Wang Y., Zhao Q., Zhang H., Yang S., Jia X. A novel poly(amido amine)-dendrimer-based hydrogel as a mimic for the extracellular matrix. Adv. Mater. 2014;26:4163–4167. doi: 10.1002/adma.201400323. [DOI] [PubMed] [Google Scholar]
- 77.Sun J.Y., Zhao X., Illeperuma W.R., Chaudhuri O., Oh K.H., Mooney D.J., Vlassak J.J., Suo Z. Highly stretchable and tough hydrogels. Nature. 2012;489:133–136. doi: 10.1038/nature11409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ebara M., Yamato M., Hirose M., Aoyagi T., Kikuchi A., Sakai K., Okano T. Copolymerization of 2-carboxyisopropylacrylamide with N-isopropylacrylamide accelerates cell detachment from grafted surfaces by reducing temperature. Biomacromolecules. 2003;4:344–349. doi: 10.1021/bm025692t. [DOI] [PubMed] [Google Scholar]
- 79.Shi Y., Ma C., Peng L., Yu G. Conductive “smart” hybrid hydrogels with PNIPAM and nanostructured conductive polymers. Adv. Funct. Mater. 2015;25:1219–1225. [Google Scholar]
- 80.Li X., Serpe M.J. Understanding and controlling the self-folding behavior of poly (N-isopropylacrylamide) microgel-based devices. Adv. Funct. Mater. 2014;24:4119–4126. [Google Scholar]
- 81.Zhu Y., Jiang H., Ye S.H., Yoshizumi T., Wagner W.R. Tailoring the degradation rates of thermally responsive hydrogels designed for soft tissue injection by varying the autocatalytic potential. Biomaterials. 2015;53:484–493. doi: 10.1016/j.biomaterials.2015.02.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lutz J.F., Hoth A. Preparation of ideal PEG analogues with a tunable thermosensitivity by controlled radical copolymerization of 2-(2-methoxyethoxy)ethyl methacrylate and oligo(ethylene glycol) methacrylate. Macromolecules. 2006;39:893–896. [Google Scholar]
- 83.Zander Z.K., Hua G., Wiener C.G., Vogt B.D., Becker M.L. Control of mesh size and modulus by kinetically dependent cross-linking in hydrogels. Adv. Mater. 2015;27:6283–6288. doi: 10.1002/adma.201501822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lei Y., Segura T. DNA delivery from matrix metalloproteinase degradable poly(ethylene glycol) hydrogels to mouse cloned mesenchymal stem cells. Biomaterials. 2009;30:254–265. doi: 10.1016/j.biomaterials.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ni P., Ding Q., Fan M., Liao J., Qian Z., Luo J., Li X., Luo F., Yang Z., Wei Y. Injectable thermosensitive PEG-PCL-PEG hydrogel/acellular bone matrix composite for bone regeneration in cranial defects. Biomaterials. 2014;35:236–248. doi: 10.1016/j.biomaterials.2013.10.016. [DOI] [PubMed] [Google Scholar]
- 86.Berdichevski A., Shachaf Y., Wechsler R., Seliktar D. Protein composition alters in vivo resorption of PEG-based hydrogels as monitored by contrast-enhanced MRI. Biomaterials. 2015;42:1–10. doi: 10.1016/j.biomaterials.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 87.Del Bufalo F., Manzo T., Hoyos V., Yagyu S., Caruana I., Jacot J., Benavides O., Rosen D., Brenner M.K. 3D modeling of human cancer: a PEG-fibrin hydrogel system to study the role of tumor microenvironment and recapitulate the in vivo effect of oncolytic adenovirus. Biomaterials. 2016;84:76–85. doi: 10.1016/j.biomaterials.2016.01.030. [DOI] [PubMed] [Google Scholar]
- 88.Lee T.T., Garcia J.R., Paez J.I., Singh A., Phelps E.A., Weis S., Shafiq Z., Shekaran A., Del Campo A., Garcia A.J. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater. 2015;14:352–360. doi: 10.1038/nmat4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Caiazzo M., Okawa Y., Ranga A., Piersigilli A., Tabata Y., Lutolf M.P. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater. 2016;15:344–352. doi: 10.1038/nmat4536. [DOI] [PubMed] [Google Scholar]
- 90.Phelps E.A., Enemchukwu N.O., Fiore V.F., Sy J.C., Murthy N., Sulchek T.A., Barker T.H., Garcia A.J. Maleimide cross-linked bioactive PEG hydrogel exhibits improved reaction kinetics and cross-linking for cell encapsulation and in situ delivery. Adv. Mater. 2012;24:64–70. doi: 10.1002/adma.201103574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soman P., Kelber J.A., Lee J.W., Wright T.N., Vecchio K.S., Klemke R.L., Chen S. Cancer cell migration within 3D layer-by-layer microfabricated photocrosslinked PEG scaffolds with tunable stiffness. Biomaterials. 2012;33:7064–7070. doi: 10.1016/j.biomaterials.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hahn M.S., Miller J.S., West J.L. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv. Mater. 2006;18:2679–2684. [Google Scholar]
- 93.Rutz A.L., Hyland K.E., Jakus A.E., Burghardt W.R., Shah R.N. A multimaterial bioink method for 3D printing tunable, cell-compatible hydrogels. Adv. Mater. 2015;27:1607–1614. doi: 10.1002/adma.201405076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Peppas N.A., Hilt J.Z., Khademhosseini A., Langer R. Hydrogels in biology and medicine: from molecular principles to bionanotechnology. Adv. Mater. 2006;18:1345–1360. [Google Scholar]
- 95.Slaughter B.V., Khurshid S.S., Fisher O.Z., Khademhosseini A., Peppas N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009;21:3307–3329. doi: 10.1002/adma.200802106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chan L.W., Hao J.S., Heng P.W.S. Evaluation of permeability and mechanical properties of composite polyvinyl alcohol films. Chem. Pharm. Bull. 1999;10:1412–1416. [Google Scholar]
- 97.Thiele J., Ma Y., Bruekers S.M., Ma S., Huck W.T. 25th anniversary article: designer hydrogels for cell cultures: a materials selection guide. Adv. Mater. 2014;26:125–147. doi: 10.1002/adma.201302958. [DOI] [PubMed] [Google Scholar]
- 98.Chong S.F., Smith A.A., Zelikin A.N. Microstructured, functional PVA hydrogels through bioconjugation with oligopeptides under physiological conditions. Small. 2013;9:942–950. doi: 10.1002/smll.201201774. [DOI] [PubMed] [Google Scholar]
- 99.Millon L.E., Padavan D.T., Hamilton A.M., Boughner D.R., Wan W. Exploring cell compatibility of a fibronectin-functionalized physically crosslinked poly(vinyl alcohol) hydrogel. J. Biomed. Mater. Res. B Appl. Biomater. 2012;100:1–10. doi: 10.1002/jbm.b.31860. [DOI] [PubMed] [Google Scholar]
- 100.Stauffer S.R., Peppast N.A. Poly (vinyl alcohol) hydrogels prepared by freezing-thawing cyclic processing. Polymer. 1992;33:3932–3936. [Google Scholar]
- 101.Kim T.H., An D.B., Oh S.H., Kang M.K., Song H.H., Lee J.H. Creating stiffness gradient polyvinyl alcohol hydrogel using a simple gradual freezing-thawing method to investigate stem cell differentiation behaviors. Biomaterials. 2015;40:51–60. doi: 10.1016/j.biomaterials.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 102.Wang Y., Kim H.J., Vunjak-Novakovic G., Kaplan D.L. Stem cell-based tissue engineering with silk biomaterials. Biomaterials. 2006;27:6064–6082. doi: 10.1016/j.biomaterials.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 103.Park H., Temenoff J.S., Tabata Y., Caplan A.I., Mikos A.G. Injectable biodegradable hydrogel composites for rabbit marrow mesenchymal stem cell and growth factor delivery for cartilage tissue engineering. Biomaterials. 2007;28:3217–3227. doi: 10.1016/j.biomaterials.2007.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rape A.D., Zibinsky M., Murthy N., Kumar S. A synthetic hydrogel for the high-throughput study of cell-ECM interactions. Nat. Commun. 2015;6:8129. doi: 10.1038/ncomms9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Temple S. The development of neural stem cells. Nature. 2001;414:112–117. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 106.Lim T.C., Toh W.S., Wang L.S., Kurisawa M., Spector M. The effect of injectable gelatin-hydroxyphenylpropionic acid hydrogel matrices on the proliferation, migration, differentiation and oxidative stress resistance of adult neural stem cells. Biomaterials. 2012;33:3446–3455. doi: 10.1016/j.biomaterials.2012.01.037. [DOI] [PubMed] [Google Scholar]
- 107.Bago J.R., Alfonso-Pecchio A., Okolie O., Dumitru R., Rinkenbaugh A., Baldwin A.S., Miller C.R., Magness S.T., Hingtgen S.D. Therapeutically engineered induced neural stem cells are tumour-homing and inhibit progression of glioblastoma. Nat. Commun. 2016;7:10593. doi: 10.1038/ncomms10593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie Q., Wang Z., Huang Y., Bi X., Zhou H., Lin M., Yu Z., Wang Y., Ni N., Sun J., Wu S., You Z., Guo C., Sun H., Wang Y., Gu P., Fan X. Characterization of human ethmoid sinus mucosa derived mesenchymal stem cells (hESMSCs) and the application of hESMSCs cell sheets in bone regeneration. Biomaterials. 2015;66:67–82. doi: 10.1016/j.biomaterials.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 109.Phinney D.G., Di Giuseppe M., Njah J., Sala E., Shiva S., St Croix C.M., Stolz D.B., Watkins S.C., Di Y.P., Leikauf G.D., Kolls J., Riches D.W., Deiuliis G., Kaminski N., Boregowda S.V., McKenna D.H., Ortiz L.A. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015;6:8472. doi: 10.1038/ncomms9472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ankrum J.A., Ong J.F., Karp J.M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 2014;32:252–260. doi: 10.1038/nbt.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Trounson A., DeWitt N.D. Pluripotent stem cells progressing to the clinic. Nat. Rev. Mol. Cell Biol. 2016;17:194–200. doi: 10.1038/nrm.2016.10. [DOI] [PubMed] [Google Scholar]
- 112.Avior Y., Sagi I., Benvenisty N. Pluripotent stem cells in disease modelling and drug discovery. Nat. Rev. Mol. Cell Biol. 2016;17:170–182. doi: 10.1038/nrm.2015.27. [DOI] [PubMed] [Google Scholar]
- 113.Guye P., Ebrahimkhani M.R., Kipniss N., Velazquez J.J., Schoenfeld E., Kiani S., Griffith L.G., Weiss R. Genetically engineering self-organization of human pluripotent stem cells into a liver bud-like tissue using Gata6. Nat. Commun. 2016;7:10243. doi: 10.1038/ncomms10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Madden L.R., Mortisen D.J., Sussman E.M., Dupras S.K., Fugate J.A., Cuy J.L., Hauch K.D., Laflamme M.A., Murry C.E., Ratner B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. U. S. A. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fuhrmann T., Tam R.Y., Ballarin B., Coles B., Elliott Donaghue I., van der Kooy D., Nagy A., Tator C.H., Morshead C.M., Shoichet M.S. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials. 2016;83:23–36. doi: 10.1016/j.biomaterials.2015.12.032. [DOI] [PubMed] [Google Scholar]
- 116.Klawitter S., Fuchs N.V., Upton K.R., Munoz-Lopez M., Shukla R., Wang J., Garcia-Canadas M., Lopez-Ruiz C., Gerhardt D.J., Sebe A., Grabundzija I., Merkert S., Gerdes P., Pulgarin J.A., Bock A., Held U., Witthuhn A., Haase A., Sarkadi B., Lower J., Wolvetang E.J., Martin U., Ivics Z., Izsvak Z., Garcia-Perez J.L., Faulkner G.J., Schumann G.G. Reprogramming triggers endogenous L1 and Alu retrotransposition in human induced pluripotent stem cells. Nat. Commun. 2016;7:10286. doi: 10.1038/ncomms10286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xu K., Narayanan K., Lee F., Bae K.H., Gao S., Kurisawa M. Enzyme-mediated hyaluronic acid-tyramine hydrogels for the propagation of human embryonic stem cells in 3D. Acta Biomater. 2015;24:159–171. doi: 10.1016/j.actbio.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 118.Raeber G.P., Lutolf M.P., Hubbell J.A. Molecularly engineered PEG hydrogels: a novel model system for proteolytically mediated cell migration. Biophys. J. 2005;89:1374–1388. doi: 10.1529/biophysj.104.050682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ashton R.S., Banerjee A., Punyani S., Schaffer D.V., Kane R.S. Scaffolds based on degradable alginate hydrogels and poly(lactide-co-glycolide) microspheres for stem cell culture. Biomaterials. 2007;28:5518–5525. doi: 10.1016/j.biomaterials.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 120.Lei Y., Gojgini S., Lam J., Segura T. The spreading, migration and proliferation of mouse mesenchymal stem cells cultured inside hyaluronic acid hydrogels. Biomaterials. 2011;32:39–47. doi: 10.1016/j.biomaterials.2010.08.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kloxin A.M., Tibbitt M.W., Kasko A.M., Fairbairn J.A., Anseth K.S. Tunable hydrogels for external manipulation of cellular microenvironments through controlled photodegradation. Adv. Mater. 2010;22:61–66. doi: 10.1002/adma.200900917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schultz K.M., Kyburz K.A., Anseth K.S. Measuring dynamic cell-material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl. Acad. Sci. U. S. A. 2015;112:E3757–E3764. doi: 10.1073/pnas.1511304112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tamura M., Yanagawa F., Sugiura S., Takagi T., Sumaru K., Matsui H., Kanamori T. Optical cell separation from three-dimensional environment in photodegradable hydrogels for pure culture techniques. Sci. Rep. 2014;4:4793. doi: 10.1038/srep04793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huebsch N., Lippens E., Lee K., Mehta M., Koshy S.T., Darnell M.C., Desai R.M., Madl C.M., Xu M., Zhao X., Chaudhuri O., Verbeke C., Kim W.S., Alim K., Mammoto A., Ingber D.E., Duda G.N., Mooney D.J. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 2015;14:1269–1277. doi: 10.1038/nmat4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Engler A.J., Sen S., Sweeney H.L., Discher D.E. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 126.Trappmann B., Gautrot J.E., Connelly J.T., Strange D.G., Li Y., Oyen M.L., Cohen Stuart M.A., Boehm H., Li B., Vogel V., Spatz J.P., Watt F.M., Huck W.T. Extracellular-matrix tethering regulates stem-cell fate. Nat. Mater. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 127.Yang C., Tibbitt M.W., Basta L., Anseth K.S. Mechanical memory and dosing influence stem cell fate. Nat. Mater. 2014;13:645–652. doi: 10.1038/nmat3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Musah S., Morin S.A., Wrighton P.J., Zwick D.B., Jin S., Kiessling L.L. Glycosaminoglycan-binding hydrogels enable mechanical control of human pluripotent stem cell self-renewal. ACS Nano. 2012;6:10168–10177. doi: 10.1021/nn3039148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huebsch N., Arany P.R., Mao A.S., Shvartsman D., Ali O.A., Bencherif S.A., Rivera-Feliciano J., Mooney D.J. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat. Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jeon O., Alt D.S., Linderman S.W., Alsberg E. Biochemical and physical signal gradients in hydrogels to control stem cell behavior. Adv. Mater. 2013;25:6366–6372. doi: 10.1002/adma.201302364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Saez A., Ghibaudo M., Buguin A., Silberzan P., Ladoux B. Rigidity-driven growth and migration of epithelial cells on microstructured anisotropic substrates. Proc. Natl. Acad. Sci. U. S. A. 2007;104:8281–8286. doi: 10.1073/pnas.0702259104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leipzig N.D., Shoichet M.S. The effect of substrate stiffness on adult neural stem cell behavior. Biomaterials. 2009;30:6867–6878. doi: 10.1016/j.biomaterials.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 133.Xiao X., Wang W., Liu D., Zhang H., Gao P., Geng L., Yuan Y., Lu J., Wang Z. The promotion of angiogenesis induced by three-dimensional porous beta-tricalcium phosphate scaffold with different interconnection sizes via activation of PI3K/Akt pathways. Sci. Rep. 2015;5:9409. doi: 10.1038/srep09409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Teng W., Long T.J., Zhang Q., Yao K., Shen T.T., Ratner B.D. A tough, precision-porous hydrogel scaffold: ophthalmologic applications. Biomaterials. 2014;35:8916–8926. doi: 10.1016/j.biomaterials.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 135.Meng Q., Man Z., Dai L., Huang H., Zhang X., Hu X., Shao Z., Zhu J., Zhang J., Fu X., Duan X., Ao Y. A composite scaffold of MSC affinity peptide-modified demineralized bone matrix particles and chitosan hydrogel for cartilage regeneration. Sci. Rep. 2015;5:17802. doi: 10.1038/srep17802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tokatlian T., Cam C., Segura T. Non-viral DNA delivery from porous hyaluronic acid hydrogels in mice. Biomaterials. 2014;35:825–835. doi: 10.1016/j.biomaterials.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Thomas A.M., Gomez A.J., Palma J.L., Yap W.T., Shea L.D. Heparin-chitosan nanoparticle functionalization of porous poly(ethylene glycol) hydrogels for localized lentivirus delivery of angiogenic factors. Biomaterials. 2014;35:8687–8693. doi: 10.1016/j.biomaterials.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chiu Y.C., Cheng M.H., Engel H., Kao S.W., Larson J.C., Gupta S., Brey E.M. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials. 2011;32:6045–6051. doi: 10.1016/j.biomaterials.2011.04.066. [DOI] [PubMed] [Google Scholar]
- 139.Hoffman A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012;64:18–23. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 140.Li H., Wijekoon A., Leipzig N.D. 3D differentiation of neural stem cells in macroporous photopolymerizable hydrogel scaffolds. Plos One. 2012;7:e48824. doi: 10.1371/journal.pone.0048824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ilkhanizadeh S., Teixeira A.I., Hermanson O. Inkjet printing of macromolecules on hydrogels to steer neural stem cell differentiation. Biomaterials. 2007;28:3936–3943. doi: 10.1016/j.biomaterials.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 142.Guvendiren M., Burdick J.A. The control of stem cell morphology and differentiation by hydrogel surface wrinkles. Biomaterials. 2010;31:6511–6518. doi: 10.1016/j.biomaterials.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 143.Place E.S., Evans N.D., Stevens M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 144.Rosales A.M., Anseth K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016;1:15012. doi: 10.1038/natrevmats.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.O’Reilly M.S., Boehm T., Shing Y., Fukai N., Vasios G., Lane W.S., Flynn E., Birkhead J.R., Olsen B.R., Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 146.Balakrishnan B., Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011;111:4453–4474. doi: 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- 147.Neffe A.T., Pierce B.F., Tronci G., Ma N., Pittermann E., Gebauer T., Frank O., Schossig M., Xu X., Willie B.M., Forner M., Ellinghaus A., Lienau J., Duda G.N., Lendlein A. One step creation of multifunctional 3D architectured hydrogels inducing bone regeneration. Adv. Mater. 2015;27:1738–1744. doi: 10.1002/adma.201404787. [DOI] [PubMed] [Google Scholar]
- 148.DeForest C.A., Tirrell D.A. A photoreversible protein-patterning approach for guiding stem cell fate in three-dimensional gels. Nat. Mater. 2015;14:523–531. doi: 10.1038/nmat4219. [DOI] [PubMed] [Google Scholar]
- 149.Griffin D.R., Weaver W.M., Scumpia P.O., Di Carlo D., Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015;14:737–744. doi: 10.1038/nmat4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Baker B.M., Trappmann B., Wang W.Y., Sakar M.S., Kim I.L., Shenoy V.B., Burdick J.A., Chen C.S. Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments. Nat. Mater. 2015;14:1262–1268. doi: 10.1038/nmat4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Torres-Rendon J.G., Femmer T., De Laporte L., Tigges T., Rahimi K., Gremse F., Zafarnia S., Lederle W., Ifuku S., Wessling M., Hardy J.G., Walther A. Bioactive gyroid scaffolds formed by sacrificial templating of nanocellulose and nanochitin hydrogels as instructive platforms for biomimetic tissue engineering. Adv. Mater. 2015;27:2989–2995. doi: 10.1002/adma.201405873. [DOI] [PubMed] [Google Scholar]
- 152.Zhang J., Tokatlian T., Zhong J., Ng Q.K., Patterson M., Lowry W.E., Carmichael S.T., Segura T. Physically associated synthetic hydrogels with long-term covalent stabilization for cell culture and stem cell transplantation. Adv. Mater. 2011;23:5098–5103. doi: 10.1002/adma.201103349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wen J.H., Vincent L.G., Fuhrmann A., Choi Y.S., Hribar K.C., Taylor-Weiner H., Chen S., Engler A.J. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nat. Mater. 2014;13:979–987. doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhao Y., Tan K., Zhou Y., Ye Z., Tan W.S. A combinatorial variation in surface chemistry and pore size of three-dimensional porous poly(epsilon-caprolactone) scaffolds modulates the behaviors of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;59:193–202. doi: 10.1016/j.msec.2015.10.017. [DOI] [PubMed] [Google Scholar]