Abstract
The strength of titanium scaffolds with the introduction of high porosity decreases dramatically and may become inadequate for load bearing in biomedical applications. To simultaneously meet the requirements of biocompatibility, low elastic modulus and appropriate strength for orthopedic implant materials, it is highly desirable to develop new biocompatible titanium based materials with enhanced strength. In this study, we developed a niobium pentoxide (Nb2O5) reinforced titanium composite via powder metallurgy for biomedical applications. The strength of the Nb2O5 reinforced titanium composites (Ti-Nb2O5) is significantly higher than that of pure titanium. Cell culture results revealed that the Ti-Nb2O5 composite exhibits excellent biocompatibility and cell adhesion. Human osteoblast-like cells grew and spread healthily on the surface of the Ti-Nb2O5 composite. Our study demonstrated that Nb2O5 reinforced titanium composite is a promising implant material by virtue of its high mechanical strength and excellent biocompatibility.
Keywords: Titanium-niobium pentoxide composite, Particulate-reinforcement, Orthopedic implant, Mechanical property, Biocompatibility
Graphical abstract

Highlights
-
•
Developed novel Ti-Nb2O5 composites by powder metallurgy for biomedical applications.
-
•
The Ti-Nb2O5 composite shows significantly higher strength than pure titanium.
-
•
The Ti-Nb2O5 composite exhibits excellent biocompatibility and cell adhesion.
-
•
The Ti-Nb2O5 composite is a promising implant material.
1. Introduction
Titanium (Ti) has shown the greatest potential to be the material basis of load-bearing implant applications due to its excellent mechanical strength, biocompatibility over alternative biomaterials, such as polymers and ceramics [1], [2]. It also possesses a relatively low elastic modulus and excellent corrosion resistance compared with other metal biomaterials, such as SUS316L stainless steel and Co–Cr–Mo alloys. However, pure titanium used today is in its solid forms and often much stiffer than human bone as the elastic modulus of titanium implants is much higher than that of natural bone [1], [2]. This mismatch of elastic modulus causes stress shielding, leading to implant loosening and eventual failure [1], [3].
The elastic modulus of titanium can be tailored through the introduction of a porous structure [4], [5]. A porous structure may also provide new bone tissue ingrowth ability and vascularization, which make porous titanium as a promising scaffold biomaterial for bone tissue engineering. However, the strength of porous titanium decreases dramatically with the introduction of porosity, and becomes lower than that of natural bone when with high porosity [3], [5]. To be a successful implant, it is necessary to simultaneously meet the requirements of low elastic modulus, appropriate strength and excellent biocompatibility for load-bearing implant materials.
Particulate-reinforced metal matrix composites (MMCs) are known to provide a higher strength and hardness, and better wear resistance, compared to unreinforced metals [6], [7], [8]. The appropriate selection of reinforcement and metal matrix systems are of critical importance for ensuring a property combination of biocompatibility, high strength and high wear resistance. Volume fraction, shape, size, and orientation of the reinforcement phase in metal matrix play an essential role in determining the properties of MMCs. A wide range of metal systems and reinforcements are available for MMCs, including, for example, Al, Mg, Ni, Ti, Cu, Fe, etc., and reinforcement systems in the form of whiskers, fibers, and particulates, for instance, boron, carbon fiber, carbon nanotubes, Kevlar, silicon carbide and various metal oxides. Particulate-reinforced metal matrix composites have attracted extensive attention as a result of their relatively low costs and characteristic isotropic properties.8 Silicon carbide, boron carbide, titanium carbide, titanium diboride, zirconium diboride, and aluminum oxide are commonly used to produce reinforced titanium composites [9]. However, for particulate-reinforced MMCs for biomaterials applications, they should have not only enhanced mechanical properties but also excellent biocompatibility. Silicon carbide, boron carbide, titanium diboride, zirconium diboride, and aluminum oxide may be of insufficient biocompatibility according to their Material Safety Data Sheets [10]. To ensure the particulate-reinforced MMCs with excellent biocompatibility, the selected particle reinforcements should be biocompatible [11], [12], [13].
Niobium pentoxide (Nb2O5) is considered biocompatible [10]. The titanium alloy coated with Nb2O5 by using a sol–gel technique can significantly improve its biocompatibility, bioactivity and corrosion resistance [14], [15]. It has been reported that particulate-reinforced titanium metal matrix composites using metal oxides as reinforcements, such as TiO2, SiO2, ZrO2 and SrO, show promising mechanical properties and excellent biocompatibility for orthopedic implant materials [16], [17], [18]. In this study, to simultaneously achieve high strength and excellent biocompatibility for load-bearing applications, we attempt reinforcing titanium metal matrix with Nb2O5 particulate to fabricate a Ti-Nb2O5 composite for biomedical applications. The mechanical properties and the microstructure of the particulate-reinforced Ti-Nb2O5 composite were evaluated using compression tests and scanning electron microscopy (SEM). The in vitro biocompatibility and cell adhesion behavior of the Ti-Nb2O5 composite were assessed by using human osteoblast-like SaOS2 cells.
2. Experimental methods
Processing methods for MMCs play a crucial role in the properties of the final titanium-metal oxide composites. Powder metallurgy has several advantages in the processing of metal matrix composites since it can synthesize a wider range of compositions with relatively low costs and characteristic isotropic properties [3], [5]. Typical powder metallurgy process to fabricate MMCs is schematically shown in Fig. 1.
Fig. 1.
Schematic illustration of powder metallurgy process in the fabrication of particulate reinforced metal matrix composites (MMCs).
In this study, titanium powder (purity of 99.8%, average particle size ∼ 45 μm, Atlantic Equipment Engineers, USA), and Nb2O5 particles (purity of 99.9%, average particle size < 45 μm, Sigma-Aldrich, Australia) were used as the starting materials. The morphologies of the starting materials are shown in Fig. 2. Powder mixtures for the niobium pentoxide reinforced titanium composites containing 2%, 3%, and 4% of Nb2O5 (weight percent, hereafter) were blended in a planetary ball mill for 2 h at a weight ratio (balls to powder) of 10:1 and a rotation speed of 100 rpm. The mixtures of titanium powder and Nb2O5 particles were consolidated using a cold presser at 300 MPa and then sintered at 1000 °C in a vacuum furnace at 10−6 torr to obtain the MMCs. Titanium composite disc samples with a diameter of 9 mm and thickness of 2 mm were cut from the sintered products for microstructure characterization, phase analysis, and in vitro biocompatibility assessments. Cylinder composite samples with a diameter of 3 mm and a height of 6 mm were used for evaluating the mechanical properties under compression. Compressive tests were performed at room temperature at an initial strain rate of 10−3 s−1 using an Instron universal tester equipped with a video extensometer (Instron 5567, Norwood, MA). Characterization of the microstructures of the Ti-Nb2O5 composites was performed by scanning electron microscopy (Quanta 200, USA).
Fig. 2.
Morphologies of starting materials: (a) Ti powder and (b) Nb2O5 powder.
Osteoblast-like cells (SaOS2, a human osteosarcoma cell line with osteoblastic properties, Barwon Biomedical Research, Geelong Hospital, Victoria, Australia) were used to assess the in vitro biocompatibility of the Ti-Nb2O5 composites based on a direct contact method [3], [11]. SaOS2 cells were cultured in a modified minimum essential medium (MMEM) at 37 °C in a humidified atmosphere of 5% CO2 in air. MMEM composed of minimum essential medium (MEM, Gibco, Invitrogen, Mulgrave, VIC, Australia), 10% fetal bovine serum (Bovogen Biologicals, Essendon, Victoria, Australia), 1% non-essential amino acid (Sigma-Aldrich, Castle Hill, NSW, Australia), 10,000 units/mL penicillin-10,000 μg/mL streptomycin (Gibco), and 0.4% amphostat B (In Vitro Technologies, Auckland, New Zealand). The culture medium was changed every 3 days. When cells become confluent, they were harvested by using 0.1% Trypsin-5 mM EDTA (Sigma-Aldrich, Australia) and collected for use.
For the in vitro assessment, all samples were sterilized in an autoclave. The samples were then placed in a 48-well cell culture plate with each well containing one sample covered with 200 μl MMEM. SaOS2 cells were seeded at a density of 5 × 103 cells per well. After incubating the cells in a humidified atmosphere with 5% CO2 in air at 37 °C for 7 d, MTS assay was performed to measure the cell number in the wells. The respective medium in each well was replaced by 150 μl phenol red free MEM. MTS/PMS solution of 50 μl was added in each well and the cell culture plate was incubated for 1 h at 37 °C in a humidified atmosphere of 5% CO2 in air. Then, 100 μl from each well was transferred to a 96-well plate for absorbance reading at 490 nm using a spectrophotometer (GENios Pro, Tecan, Mannedorf, Switzerland).
The cell morphology of the osteoblast-like cells (SaOS2) on the surfaces of the Ti-Nb2O5 composite samples was observed using a confocal microscope (Leica SP45, Germany) and scan electron microscopy (SEM). For confocal microscopy observation, the cell-seeded discs after cell culture were fixed in 2% paraformaldehyde and permeabilised with 0.2% (v/v) triton-X100 in phosphate-buffered saline (PBS) (Sigma-Aldrich, Australia) each for 10 min at room temperature. The discs were then incubated with 1% phalloidin and 4′-6-Diamidino-2-phenylindole (DAPI) overnight at 4 °C. Three washes by PBS were included in between each of the steps mentioned above. The stained samples were stored in PBS until required and confocal microscopy observations of these samples were conducted within a week of staining. For SEM observation, the cell-seeded discs after cell culture were fixed in 3.9% glutaraldehyde for 1 h at room temperature. Then the cells were dehydrated through sequential washings in 60, 70, 80, 90, 95, and 100% ethanol solutions for 10 min each. After that, the samples were chemically dried using hexamethyldisilazane (HMDS) and coated with gold for SEM observation.
All cell culture experiments were conducted in triplicates. Results were expressed as mean ± standard deviation (SD). One-way ANOVA (SPSS 14.0 for windows) was applied to determine the statistical significance of the differences observed between groups. p < 0.05 was considered as statistically significant. The optical density (OD) values for all samples were converted to the respective viable cell counts using a standard curve and a cell viability ratio (CVR) for every sample was calculated using the following equation:
| (1) |
3. Results and discussion
Fig. 3 shows the X-ray diffraction patterns of the Ti-Nb2O5 composites with different Nb2O5 contents. The Ti-Nb2O5 composites showed mainly the sharp peaks attributable to α-Ti. The peak intensities of the reinforcing phase Nb2O5 increased with increasing of Nb2O5 addition to the composites. It can been seen that there was no new phase formed after the vacuum sintering of the Ti-Nb2O5 powder mixtures.
Fig. 3.
XRD patterns of Ti-Nb2O5 composites with (a) 0, (b) 2%, (c) 3%, and (d) 4% of Nb2O5.
Fig. 4 shows the microstructure of Ti-Nb2O5 composites with different Nb2O5 additions and EDX analysis of Ti/4% Nb2O5 composite. It can be seen from Fig. 4(a) – (c) that the average grain size did not change significantly with the increasing of Nb2O5 addition from 2% to 4%. The porosity of the Ti-Nb2O5 composites was 4%, 3% and 2% for the composite with the Nb2O5 addition of 2%, 3% and 4%, respectively. The porosity difference for the composites with different percentage of particulate reinforcement can be considered negligible. Fig. 4(d) and (e) show the EDX spectra of the spots of the matrix and around a Nb2O5 particle which indicate the compositions. The boundary of the pore displays rich Nb contents and the grain matrix contains very low Nb content. It suggests that the Nb2O5 particles (Fig. 4 (a)–(c)) were almost removed during the grinding and polishing processes, leaving behind the pores.
Fig. 4.
Microstructure of Ti-Nb2O5 composite with: (a) 2%, (b) 3%, and (c) 4% of Nb2O5; (d) EDX spectrum indicating the composition of “y” as marked in the matrix in (c); (e) EDX spectrum indicating the composition of “z” as marked in the boundary of the pore in (c).
The mechanical properties of the Ti-Nb2O5 composites are shown in Fig. 5. It can be seen that the compressive yield strengths of the titanium matrix composites with 2%, 3% and 4% of Nb2O5 additions are 1245 ± 52, 1310 ± 54 and 1409 ± 58 MPa, respectively, which are higher than that of pure titanium fabricated by powder metallurgy (1046 ± 50 MPa) [16]. The compressive yield strength of the Ti-Nb2O5 composites increased with increasing Nb2O5 addition. However, the ultimate strains of the Ti-Nb2O5 composites were lower than that of pure titanium, which indicate that the addition of Nb2O5 particulates reduced the ductility of the Ti-Nb2O5 composites. Also, the ultimate strains of the Ti/Nb2O5 composites decreased with increasing Nb2O5 addition. The Ti-Nb2O5 composites with 2, 3, and 4% of Nb2O5 addition showed an ultimate strain of 22 ± 2, 15 ± 1.9 and 13 ± 1.5%, respectively.
Fig. 5.
Mechanical properties of the Ti-Nb2O5 composites.
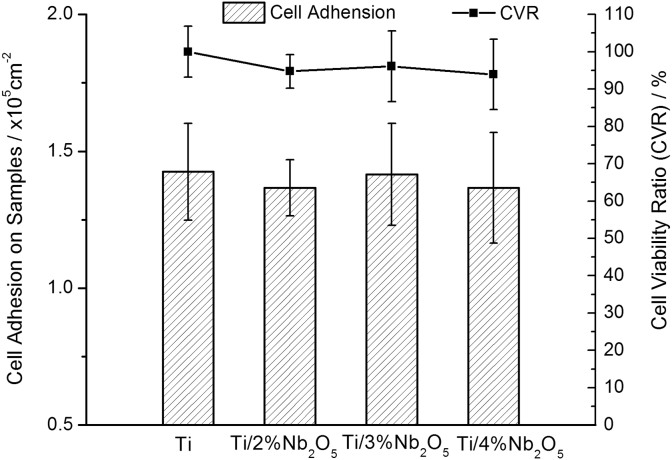
The biocompatibility of an implant material plays an important role in ensuring a successful implantation. To assess the biocompatibility and cell response of the Ti-Nb2O5 composites, in vitro assessments were carried out using human osteoblast-like SaOS2 cells. Fig. 6 shows the cell proliferation and variability of osteoblast-like SaOS2 cells on the composite disc samples after cell culture for 7 d. It can be seen that all the Ti-Nb2O5 composites exhibited a similar or comparable cell proliferation and variability compared to pure titanium, which indicated that the Ti-Nb2O5 composites in this study are nontoxic and of excellent biocompatibility.
Fig. 6.
Cell adhesion and variability of osteoblast-like SaOS2 cells on the Ti-Nb2O5 composites after cell culture for 7 d.
As Ti/4%Nb2O5 composite contained the highest content of Nb2O5, the investigation of cell adhesion morphology on its surface was carried out through the confocal microscopy and SEM. The confocal micrographs of SaOS2 cells on the surface of the Ti/4%Nb2O5 composite after cell culture for 1 and 7 d are shown in Fig. 7. The insert in Fig. 7(b) shows the SEM image of the cell morphologies after culturing for 7 d. It can be seen that the SaOS2 cells attached, spread, and grew healthily on the surfaces of the Ti/4%Nb2O5 composite. At the early stage of cell culture for 1 d (Fig. 7(a)), it can be seen that only limited numbers of SaOS2 cells attached to Ti/4%Nb2O5 composite surface; however, after culturing for 7 d, the cell numbers increased substantially. It is also noticeable that there were cells spreading with extracellular matrix on Ti/4%Nb2O5 composite surface (Fig. 7(b)), which indicated the Ti/4%Nb2O5 composite possesses excellent biocompatibility. The result of cell morphology and attachment observation is consistent with that of MTS assay.
Fig. 7.
Cell morphologies of osteoblast-like SaOS2 cells on the Ti/4%Nb2O5 composite after cell culture for: (a) 1 d and (b) 7 d.
4. Conclusions
This study investigated the microstructure, the mechanical properties and the biocompatibility of particulate-reinforced Ti-Nb2O5 composites (MMCs) fabricated via powder metallurgy. The strength of the MMCs is significantly higher than that of pure titanium. Cell culture assessment revealed that the MMCs exhibit excellent biocompatibility. Human osteoblast-like SaOS2 cells grow and spread healthily on the surfaces of the MMCs. This study illustrated the feasibility of using the particulate-reinforced Ti-Nb2O5 composites as orthopedic implant materials.
Acknowledgments
This research is financially supported by the National Health and Medical Research Council (NHMRC) through GNT1087290.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Long M., Rack H.J. Titanium alloys in total joint replacement—a materials science perspective. Biomaterials. 1998;19:1621–1639. doi: 10.1016/s0142-9612(97)00146-4. [DOI] [PubMed] [Google Scholar]
- 2.Niinomi M. Recent metallic materials for biomedical applications. Metall. Mater. Trans. A. 2002;33:477–486. [Google Scholar]
- 3.Li Y., Xiong J., Wong C.S., Hodgson P.D., Wen C. Ti6Ta4Sn alloy and subsequent scaffolding for bone tissue engineering. Tiss. Eng. A. 2009;15:3151–3159. doi: 10.1089/ten.TEA.2009.0150. [DOI] [PubMed] [Google Scholar]
- 4.Gibson L.G., Ashby M.F. Cambridge University Press; Cambridge, U.K: 1997. Cellular Solids: Structure and Properties. [Google Scholar]
- 5.Wen C., Yamada Y., Shimojima K., Chino Y., Hosokawa H., Mabuchi M. Novel titanium foam for bone tissue engineering. J. Mater. Res. 2002;17:2633–2639. [Google Scholar]
- 6.Krasnowski M., Dąbrowski J. Ti-Y2O3 composites with nanocrystalline and microcrystalline matrix. Metall. Mater. Trans. A. 2012;43:1376–1381. [Google Scholar]
- 7.Liu Y.B., Liu Y., Tang H.P., Wang B., Liu B. Reactive sintering mechanism of Ti + Mo2C and Ti + VC powder compacts. J. Mater. Sci. 2011;46:902–909. [Google Scholar]
- 8.Ranganath S. Review on particulate-reinforced titanium matrix composites. J. Mater. Sci. 1997;32(1):1–16. [Google Scholar]
- 9.Munir K.S., Kingshott P., Wen C. Carbon nanotube reinforced titanium metal matrix composites prepared by powder metallurgy—a review. Crit. Rev. Solid State Mater. Sci. 2014;40:38–55. [Google Scholar]
- 10.Yamamoto A., Honma R., Sumita M. Cytotoxicity evaluation of 43 metal salts using murine fibroblasts and osteoblastic cells. J. Biomed. Mater. Res. 1998;39:331–340. doi: 10.1002/(sici)1097-4636(199802)39:2<331::aid-jbm22>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 11.Li Y., Wong C., Xiong J., Hodgson P., Wen C. Cytotoxicity of titanium and titanium alloying elements. J. Dent. Res. 2010;89:493–497. doi: 10.1177/0022034510363675. [DOI] [PubMed] [Google Scholar]
- 12.Geurtsen W. Biocompatibility of dental casting alloys. Crit. Rev. Oral Biol. Med. 2002;13:71–84. doi: 10.1177/154411130201300108. [DOI] [PubMed] [Google Scholar]
- 13.Brune D. Metal release from dental biomaterials. Biomaterials. 1986;7:163–175. doi: 10.1016/0142-9612(86)90097-9. [DOI] [PubMed] [Google Scholar]
- 14.Velten D., Eisenbarth E., Schanne N., Breme J. Biocompatible Nb2O5 thin films prepared by means of the sol–gel process. J. Mater. Sci. Mater. Med. 2004;15:457–461. doi: 10.1023/b:jmsm.0000021120.86985.f7. [DOI] [PubMed] [Google Scholar]
- 15.Mazur M., Kalisz M., Wojcieszak D., Grobelny M., Mazur P., Kaczmarek D., Domaradzki J. Determination of structural, mechanical and corrosion properties of Nb2O5 and (NbyCu 1-y)Ox thin films deposited on Ti6Al4V alloy substrates for dental implant applications. Mater. Sci. Eng. C. 2015;47:211–221. doi: 10.1016/j.msec.2014.11.047. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y., Wong C., Wen C., Hodgson P., Li Y. Ti-SrO metal matrix composites for bone implant materials. J. Mater. Chem. B. 2014;2:5854–5861. doi: 10.1039/c4tb00372a. [DOI] [PubMed] [Google Scholar]
- 17.Li Y., Han C., Zhu X., Wen C., Hodgson P. Osteoblast cell response to nanoscale SiO2/ZrO2 particulate-reinforced titanium composites and scaffolds by powder metallurgy. J. Mater. Sci. 2012;47:4410–4414. [Google Scholar]
- 18.Han C., Li Y., Wu X., Ren S., San X., Zhu X. Ti/SiO2 composite fabricated by powder metallurgy for orthopedic implant. Mater. Des. 2013;49:76–80. [Google Scholar]