Abstract
Background
The medicinal uses of plants are in many cases based exclusively on traditional knowledge without enough scientific evidences. Different parts of Moringa oleifera were traditionally used for the treatment of wide variety of ailments including arthritis and joints pain. The present study had been designed to evaluate the anti-arthritic and anti-nociceptive activities of ethanol extract of Moringa leaves, this being the most abundant plant part suitable for commercial mass production of botanical medicinal products.
Methods
Complete Freund's adjuvant (CFA)-induced arthritis in rats was used as disease model. CFA-induced inflammatory paw edema, body weight, arthritic index, X-ray radiography, hematological parameters, and walk track and locomotion analysis were all evaluated for the assessment of disease progression. In addition to that, anti-nociceptive activity was examined at different dose levels in both normal and arthritic-induced rats using Eddy's hot plate and tail flick thermal analgesia.
Results
The analysis of various arthritic assessment parameters used in this study revealed that Moringa extract has a considerable effect in preventing development or ameliorate arthritis disease severity. Moreover, the ethanol extract of Moringa leaves revealed significant anti-nociceptive activity at in both normal and CFA-induced arthritis rats in a dose-dependent manner.
Conclusion
Ethanol extract of Moringa leaves appears to be a really promising as analgesic and arthritis medication, but a larger and more detailed preclinical and clinical studies especially in human is highly recommended.
Keywords: Anti-nociceptive, Complete Freund's adjuvant (CFA), Moringa and Rheumatoid arthritis (RA)
1. Introduction
Rheumatoid arthritis (RA) is a chronic systemic inflammatory disorder usually affecting the symmetrical bilateral joints.1 Uncontrolled RA characterized by progressive damages of synovial, cartilage, and bone is associated, probably, with extra-articular signs.2, 3 RA may possibly progress to severe disability with direct negative impacts on life style and increase in mortality rate.4, 5 The overall prevalence of clinically diagnosed RA was 0.5–2% of the population with higher prevalence in developed countries.6 All ages are susceptible to develop RA, but the incidence increased significantly in people aged over 40 years, especially women who are two to three times more susceptible to RA than men.7 The ultimate goal of RA treatment is to stop or at least minimize the joint damage, alleviate pain, and maintain normal joint functions.8 As RA is a chronic disease, it needs lifelong treatment. Medications such as disease modifying anti-rheumatoid drugs (DMARDs), non-steroidal anti-inflammatory drugs (NSAIDs), and corticosteroids help in reducing inflammation of the joint(s) and decreasing pain but, unfortunately, 30% of the patients failed to respond to the treatment9 in addition to the high cost and a lot of serious adverse effects.10 Therefore, more patients prefer using natural product remedies.11
Medicinal plants and herbs are a source of unlimited variety of phytochemicals that continuously proved to be effective in preventing, treating, or ameliorating different health conditions. Moringa oleifera Lam is one of the broadly used plants in traditional medicine. M. oleifera is the most cultivated species of a mono-generic family, the Moringaceae, native to the sub-Himalayan tracts of India, Pakistan, Bangladesh, Afghanistan, and Malaya.12 All parts of Moringa tree possess medicinal properties, but the leaves with its exceptional richness of biologically active phytoconstituents is the most useful part.13, 14 Among other therapeutic effects of Moringa, in vivo anti-RA activity has been positively highlighted in seed, flower, root, and stem bark.15, 16, 17 To the best to our knowledge, only one published study on anti-RA activity of Moringa leaves was published18 using methanol as extraction solvent with few approaches to verify the anti-arthritic activity, that is, no X-ray imaging, no walk track analysis and not involving anti-nociceptive activity.
The problem associated with the medical uses of natural products is the lack of scientific evidence supporting the claimed uses, which, in many cases, were based solely on a traditional knowledge. This fact meets a critical need for in vivo evaluation of the tradition claimed by using laboratory animal model. For RA, there are several induced and simultaneous animal models used for the evaluation of anti-arthritic efficacy of new natural and synthetic drugs. Complete Freund's adjuvant (CFA)-induced arthritis is a scientifically justified standard experimental procedure for the induction of chronic immune-pathological RA in laboratory animals with similar cellular immunity response and pathological mechanism as in human.19 In addition to that, this disease model has other advantages such as being very predictive in testing of the efficacy of novel compounds as anti-RA, superior disease manifestation, and disease progression than other arthritis-induced models of up to 100% incidence rate, low inter- and intra-variables among tested animals, which allows the use of fewer number of laboratory animals comparing to other arthritis induced models.20, 21
To evaluate anti-rheumatoid activity of ethanol extract of Moringa leaves, CFA-induced RA in Sprague-Dawley male rats was used as disease model. In addition to that, the anti-nociceptive activity was also evaluated in both normal and arthritic-induced rats.
2. Methods
2.1. Animals
Animal experimental protocol was approved by Animal Ethics Committee USM (AECUSM) of Univeristi Sains Malaysia, School of Pharmaceutical Sciences (USM/Animal Ethics Approval/2016/(103)(807)). Healthy Sprague-Dawley male rats, 8–10 weeks old and weighing 150–200 g, were used for the study. Animals were housed in polypropylene cages (3 animals per cage) maintained under standard condition (12 h light, 12 h dark cycle; 27 ± 3°C, 35–50% humidity), fed with standard pellet diet and free access to water (ad libitium), and allowed to acclimatize for seven days before starting the experiment.
2.2. Animals and experimental design
The animals were randomly assigned into five different groups of six animals per group (n = 6): group I (Normal control), no CFA injection; group II (CFA-control), no treatment-only vehicle; group III, given indomethacin 2.5 mg/kg/day as reference standard; group IV (E500), given 500 mg/kg/day of Moringa extract; and group V (E250), given 250 mg/kg/day of Moringa extract. The daily oral dose for all treatment groups (groups III–V) and CFA-control group was administered at 8–9 am and the measurements were conducted at 2 pm.
2.3. Induction of arthritis
Induction of experimental immunological arthritis was as described by Nair et al19 with minor modifications in group classification. Briefly, hind limbs of all groups except group I were shaved, sterilized by 70% (v/v) alcohol and then 0.1 mL of CFA containing 10 mg/mL of heat-killed M. tuberculosis was injected into subplantar of the left hind paw of each animal under mild anesthesia with diethyl ether. The time of adjuvant injection was referred as day 0. The daily oral doses of vehicle/Moringa extract/indomethacin were started on day 0 and continued to day 21 post injection.
2.4. Assessment of arthritis
2.4.1. Effect of Moringa extract on CFA-induced arthritic paw edema
The edema of both hind paws was observed on days 0, 3, 6, 9, 12, 15, 18, and 21 post CFA injection using digital micrometer gauge. The percentage increase in paw edema was calculated by the following formula:
where To is the mean of paw thickness at day 0 and Tt is the mean of paw thickness at a particular time. The results were statistically compared to the CFA-control group.
2.4.2. Effect of Moringa extract on body weight gain
Animal's body weight for all groups was monitored starting from day 0 and repeated on days 3, 6, 9, 12, 15, 18 and 21 post CFA injection. The percent weight change was calculated using the following formula:
where Wt is the weight of animal at time t and Wo is the weight of animal on day 0. The result was statistically compared to both normal control and CFA-control groups.
2.4.3. Arthritic index
Development and severity of induced arthritis were evaluated by a visual scoring system of the clinical signs and symptoms on scale of 0–4 per limb as described by Vijayalaxmi et al9 where 0: no change, 1: slight swelling and erythema of the limb, 2: mild swelling and erythema of the limb, 3: gross swelling and erythema of the limb, and 4: gross deformity and inability of the limb. A score of the both hind limb was counted and a score more than 1 exhibits the arthritis whereas a maximum score of the arthritis is 8. The measurement was started on day 3 post CFA injection and repeated on days 6, 9, 12, 15, 18, and 21 post injection.
2.4.4. Hematology profile
On day 21, post CFA injection blood was withdrawn through cardiac puncture from all groups under mild anesthesia with diethyl ether and kept in a suitable blood collection tubes (BD Vacutainer, Becton Dickinson, USA). The hematological parameters including hemoglobin content (HGB), total red blood cell count (RBC), % packed cell volume (% PCV), total white blood cells count (WBC) were evaluated immediately after blood sample collection using an automated hematology analyzer (Beckman Coulter Blood Analyzer, California, USA). Erythrocyte sedimentation rate (ESR) was determined by the Wintrobe method.
2.4.5. Walking track analysis
Walking track analysis was first described by de Medinaceli et al22 as an approach to combine gait analysis and the temporal and spatial relationship of one footprint to another during walking, that is, analysis of changes in locomotion in addition to analysis of functional behavior. The rat's footprints for all treatment groups were collected by immersion of the rat's hind paws in ink and allowing the animal to walk over paper sheet fixed on a confined walkway corridor, 8.7 cm wide by 43 cm long, with a dark shelter at the end. After two or three conditioning trials, the animals walked steadily to the dark shelter. The procedure was repeated on day 0 before CFA injection, and days 14 and 21 post injection. The obtained data from rat's footprint were used to calculate the functional index (FI) using the following equation:
where ETOF and NTOF: experimental (injected paw) and normal paw time to opposite foot, EPL and NPL: experimental and normal paw print length, ETS and NTS: experimental and normal total paw spreading, and EIT and NIT: experimental and normal intermediary toes spreading. Normal value was in range −11 to +11%, where 0 represents totally normal and 100 represents totally impaired.
2.4.6. X-ray radiographic assessment
On day 22 post CFA-injection, radiographs were taken with X-ray apparatus (PHILIPS Diagnose X-ray, Amsterdam, Netherlands). The focal film distance was 60 cm at 55 kVp and 3 mA. The severity of the joint deformation was blindly scored according to the extent of osteoporosis, joint spaces, soft tissue inflammation, subchondral erosion, and joint ankylosis on a scale of 0–4 as described by Vijayalaxmi et al.9 The highest possible score is 20 per paw. X-ray images were analyzed and separately scored by two certified radiologists who were blinded to the treatment groups.
2.5. Anti-nociceptive effect of ethanol extract of M. oleifera leaves
For normal rats, two thermal analgesia methods were used to evaluate the anti-nociceptive activity of ethanol extract of Moringa leaves, that is, Eddy's hot plate method and tail flick method, whereas for arthritic rats, only tail flick was used due to the unsuitability of hot plate method in CFA-induced arthritis model.23
2.5.1. Anti-nociceptive effect of ethanol extract of M. oleifera leaves on normal rats
In normal rats experiment, 36 Sprague-Dawley male rats were designated into six groups of six rats each (n = 6): group I, given 1 mL of 1% (w/v) CMC in water (vehicle); group II, given indomethacin 5 mg/kg body weight (standard drug); and groups III to VI, given ethanol extract of Moringa leaves at doses 125, 250, 500, and 750 mg/kg body weight.
2.5.1.1. Eddy's hot plate thermal analgesia method
First, the animal was placed on the hot plate and allowed to acclimatize for three to five minutes; then, start the instrument with a cut-off period of 18 seconds and 55 ± 0.1°C maximum heat to avoid harming the animals and observe the reaction time (in seconds) and the time taken by the animal to start paw licking or jumping, which is considered as baseline. The procedure was repeated 30, 60, 90, 120 and 180 minutes after oral administration of calculated dose of Moringa extract/indomethacin/vehicle. At each measuring time, the test was repeated three times for each animal with 5-minute intervals. The latency time was calculated using the following equation:
where Tt is the time taken by the rat to respond at particular measurement time and To is the time taken by the rat to respond at time 0.
2.5.1.2. Tail flick analgesiometer
The tail flick test using digital analgesiometer was performed as described by Rani and Gupta24 with minor modification in cutoff time. Rat was held gently with one hand and the tail tip end (1–3 cm from the most end of the tail) was placed on the source of radiant heat and the response (tail flicking) was elicited by applying the radiant heat to the surface of the tail. A cutoff response time was 20 seconds to avoid harming the animal or cause tissue injury. First, the animal was placed on the tail flick analgesiometer; start the instrument to observe the responding time (in seconds), which was considered as baseline. The procedure was repeated 30, 60, 90, 120, and 180 minutes after oral administration of calculated dose of Moringa extract/indomethacin/vehicle. At each measuring time, the test was repeated three times for each animal with 5-minute intervals. The latency time was calculated using the following equation:
where Tt is the time taken by the rat to move his tail at particular measurement time and To is the time taken by the rat to move his tail at time 0.
2.5.2. Anti-nociceptive effect of ethanol extract of M. oleifera leaves on CFA-induced rat
The same procedure and test parameters of tail flick analgesiometer used in normal rats experiment except the time of measurements were also used for the evaluation of analgesic effect of ethanol extract of Moringa leaves on arthritic rats at two dose levels of 250 and 500 mg/kg body weight. The measurement of latency time was started on day 0 before CFA injection (considered as baseline) and repeated on days 3, 6, 9, 12, 15, 18, and 21 post CFA injection after the specified daily oral dose of Moringa extract/indomethacin/vehicle.
2.6. Data analysis
All the data were expressed as mean ± SEM and statistically analyzed by IBM-SPSS version 20 software using one-way ANOVA followed by post hoc Dunnett-t test (2-sides) at different variance levels.
3. Results
3.1. Effect of Moringa extract on CFA-induced arthritic paw edema
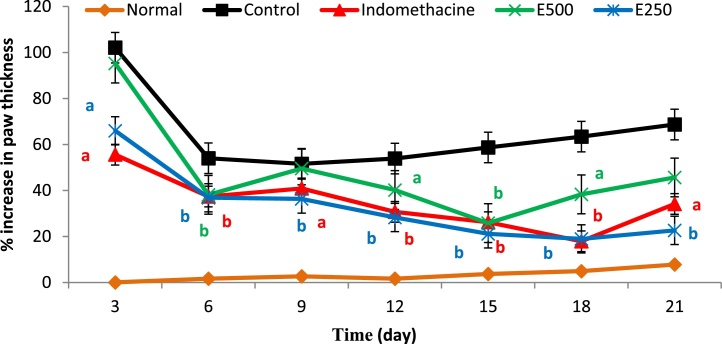
The activity of ethanol extract of Moringa leaves at doses 250 and 500 mg/kg body weight and indomethacin to inhibit inflammatory paw edema was examined against CFA-control group and found to be significant at p < 0.001 and p < 0.05 respectively (Fig. 1). Highest percent of inhibition was expressed by Moringa extract at a dose of 250 mg/kg, especially at chronic phase at 70.80% and even more active than indomethacin (70.48%).
Fig. 1.
Percent increase in paw edema in CFA-induced arthritis in rats of CFA-control (no treatment), indomethacin at dose 2.5 mg/kg/day, E500 and E250 groups that were given Moringa leaves extract at dose 500 and 250 mg/kg/day, respectively. a: significant at p < 0.05 and b: significant at p < 0.001.
3.2. Effect of Moringa extract on animal's body weight
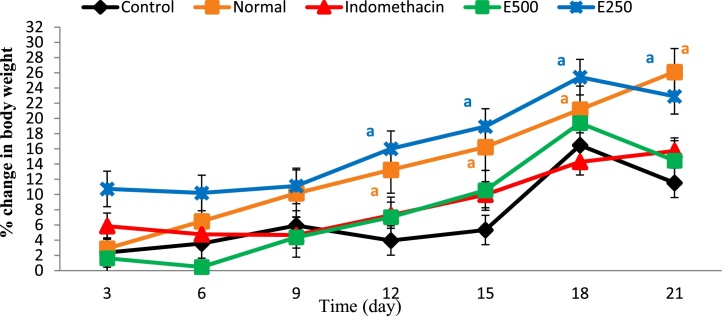
Treatment groups that were given crude extract at dose 250 mg/kg showed a significant increase in body weight at p < 0.05, whereas at dose of 500 mg/kg the increase in body weight was non-significant as compared to CFA-control group (Fig. 2). Indomethacin group also showed a non-significant weight gain in spite of being almost at constant rate increase. Normal group showed a steady increase in body weight. All treatment groups and CFA-control group showed a biphasic pattern, in that, a continuous increase in body weight started on day 6 until day 18 (established chronic phase) and then the weight started to decrease.
Fig. 2.
Percent increase in body weight of normal control, disease control, and treatment groups. E500: the animal group that was given Moringa leaf extract at dose 500 mg/kg and E250: the animal group that was given Moringa extract at dose 250 mg/kg. a: significant at p < 0.05.
3.3. Arthritic index
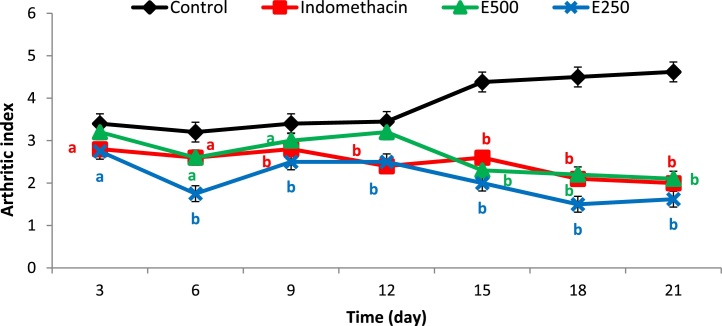
Recording of arthritic score for both hind paws was started on day 3 post CFA injection by measuring paw thickness, ankle and knee diameter, swelling and redness of paw's phalanges, and signs of inflammation on the eye, mouth, nose, ear, and tail. A significant decrease in arthritic index (p < 0.001) was recorded in indomethacin group and groups that were given Moringa extract at both doses compared to CFA-control group (Fig. 3). The animal group that was given Moringa extract at dose 250 mg/kg body weight showed better arthritic index, that is, less clinical signs of inflammation and arthritis than animal groups that were given either indomethacin or Moringa extract at dose 500 mg/kg body weight.
Fig. 3.
Arthritic index for disease control group, indomethacin group, and two animal groups (E500 and E250) that were given Moringa extract at doses of 500 and 250 mg/kg body weight. a: significant at p < 0.05 and b: significant at p < 0.01.
3.4. Hematology profile
Hematological parameters including HGB, RBC, PCV, WBC, and ESR were measured for all animal groups including normal control group (Table 1). All the evaluated hematological parameters of CFA-control group except WBC showed an out of normal range results. This abnormal results suggested the development of iron deficiency anemia, which is one of the clinical manifestations of RA. For HGB, RBC, and PCV, all treatment groups including the group that were given indomethacin were comparable to normal group, within normal range and significantly different (p < 0.05 and 0.001) from CFA-control group. The animal group that was given Moringa extract at dose 250 mg/kg showed the highest effects with a significant effect at p < 0.001 compared to CFA-control group. For WBC, only animal groups that were given either indomethacin or Moringa extract at dose 500 mg/kg showed a significant effect (p < 0.05) compared to CFA-control. For ESR test, all treatment groups showed a significant effect (p < 0.001 and 0.05) compared to CFA- control group.
Table 1.
Effects of Ethanol Extract of Moringa oleifera Leaves on Some Hematologic Parameters in CFA-induced RA Rats
| Hematology parameter (normal range) | Group |
||||
|---|---|---|---|---|---|
| CFA-control (mean ± SEM) | Normal (mean ± SEM) | Indomethacin (mean ± SEM) | E500 (mean ± SEM) | E250 (mean ± SEM) | |
| HGB (g/dL) (13.5–18.4)* | 12.25 ± 0.787 | 13.60 ± 0.147a | 13.30 ± 0.450a | 13.67 ± 0.606a | 13.87 ± 0.359b |
| PCV (%) (38.9–54.9)* | 35.75 ± 0.0.016 | 39.50 ± 0.008a | 39.50 ± 0.015a | 40.3 ± 0.028a | 41.0 ± 0.006b |
| RBC (1012/L) (7.8–10.2)* | 7.08 ± 0.385 | 7.86 ± 0.088b | 7.83 ± 0.366a | 7.53 ± 0.619 | 7.95 ± 0.232a |
| WBC (109/L) (5.9–19.0)* | 12.59 ± 0.538 | 9.70 ± 2.199a | 7.75 ± 1.222a | 7.60 ± 2.368a | 9.76 ± 0.741 |
| ESR (mm/h) (0.5–1.45)** | 2.04 ± 0.038 | 1.12 ± 0.131a | 0.45 ± 0.047b | 0.75 ± 0.129b | 0.84 ± 0.117b |
3.5. Walking track analysis using de Medinaceli method
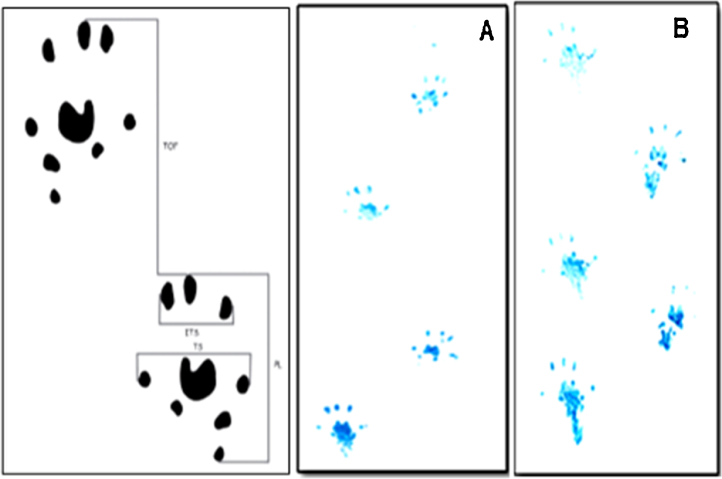
The calculated results of SFI (Fig. 4 and Table 2) clearly reveal normal values for all treatment groups, whereas in CFA-control group, the results showed an out of normal values after three weeks of arthritis.
Fig. 4.
de Medinaceli walk track analysis of CFA-control rat. (A) Footprints of rat on day 0 and (B) footprints of rat on day 21 post arthritis induction.
Table 2.
Effects of Ethanol Extract of Moringa oleifera Leaves on Functional Index (mean ± SEM) and Locomotion in CFA-induced RA in Rats
| Group | Day 0 | Day 14 | Day 21 |
|---|---|---|---|
| CFA-control | 1.67 ± 1.513 | −1.41 ± 1.987 | −18.22 ± 6.192 |
| Indomethacin | −4.11 ± 1.921 | −1.55 ± 6.121 | −5.88 ± 2.603 |
| E500 | 2.93 ± 3.895 | −0.23 ± 3.847 | −3.32 ± 4.967 |
| E250 | −1.80 ± 4.430 | −3.06 ± 4.265 | −1.38 ± 3.869 |
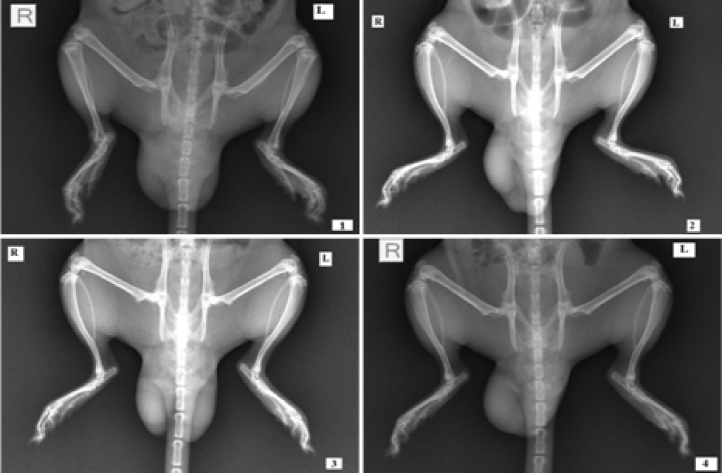
3.6. X-ray radiography assessment
In CFA-control group, a soft tissue swelling along with the reduction of the joint spaces was observed, which implies the subchondral erosion in arthritic condition (Fig. 5). The average radiographic score of CFA-control group was 9.66 ± 1.16. The standard drug indomethacin (2.5 mg/kg) treated group and Moringa extract treated groups at 500 and 250 mg/kg dose for 21 days duration have an average radiographic scores of 3.17 ± 1.08, 3.84 ± 1.23, and 3.34 ± 1.41, respectively. Statistical analysis of the obtained scores (mean ± SEM) revealed a significant effect (p < 0.001) of indomethacin and both doses of Moringa extract.
Fig. 5.
X-ray radiographs for hind paws of CFA-induced arthritis rats on day 22 post induction. 1: CFA-control; 2: animal given indomethacin; 3: animal given Moringa extract at dose 500 mg/kg, and 4: animal given Moringa extract at dose 250 mg/kg.
3.7. Analgesic effect of M. oleifera on normal rats
3.7.1. Eddy's hot plate
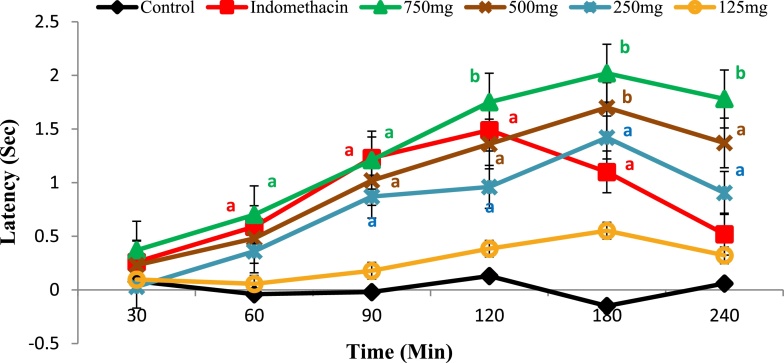
Ethanol extract of Moringa leaves showed a significant anti-nociceptive activity in a dose-dependent manner at dose 250, 500, and 750 mg/kg body weight compared to control group (p < 0.05 and 0.001), while at dose of 125 mg/kg body weight, the effect was found to be nonsignificant. The anti-nociceptive activity of Moringa extract was started at minute 90 after oral administration and reached its peak activity at minute 180 and then the effect started to decline. In indomethacin group, the activity started earlier at minute 60 and reached its peak at minute 120 and then the effect started to decline (Fig. 6).
Fig. 6.
Effect of ethanol extract of Moringa oleifera leaves at different dose levels on latency time of normal rats measured using Eddy's hot plate thermal analgesia method. A 750 mg, 500 mg, 250 mg and 125 mg are animal groups given Moringa extract at doses of 750, 500, 250 and 125 mg/kg body weight. a: significant at p < 0.05 and b: significant at p < 0.001.
3.7.2. Tail flick thermal analgesia
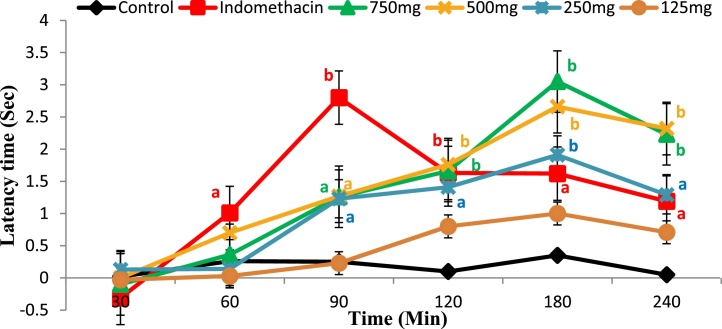
Ethanol extract of Moringa leaves at dose 250, 500 and 750 mg/kg body weight showed a significant anti-nociceptive activity (p < 0.05 and 0.001) in a dose-dependent manner compared to control group, whereas at dose of 125 mg/kg body weight, the effect was found to be nonsignificant. The analgesic activity of Moringa extract was started at minute 90 after oral administration and reached its peak activity at minute 180 and then started to decline, whereas in indomethacin group, the activity started earlier at minute 60 and reached its peak at minute 90 (Fig. 7).
Fig. 7.
Effect of ethanol extract of Moringa oleifera leaves at different dose levels on latency time of normal rats measured using tail flick thermal analgesia method. A 750 mg, 500 mg, 250 mg and 125 mg are animal groups given Moringa extract at doses of 750, 500, 250 and 125 mg/kg body weight. a: significant at p < 0.05 and b: significant at p < 0.001.
3.8. Anti-nociceptive effect of M. oleifera on CFA-induced arthritis
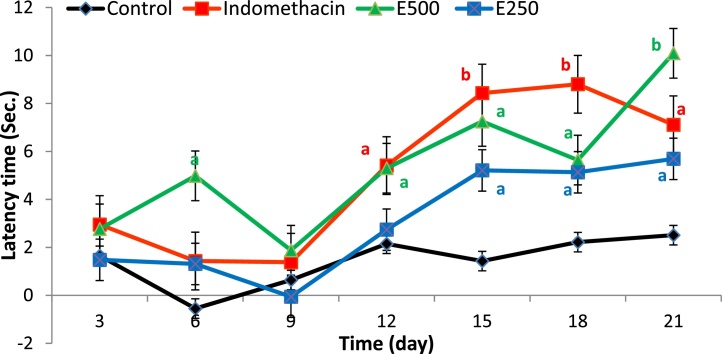
Two animal groups, that is, indomethacin and the group that received 500 mg/kg Moringa extract, showed a significant analgesic activity (p < 0.001 and 0.05, respectively) compared to CFA-control group (Fig. 8). For indomethacin group, an obvious anti-nociceptive activity was observed on day 12 post CFA-induction, that is, after the end of acute disease phase and reached its peak activity on day 18; thereafter, a slight decrease in anti-nociceptive activity was observed. On the other hand, the animal group that received Moringa extract at dose 500 mg/kg showed that an earlier significant anti-nociceptive activity than indomethacin and peak activity was on day 21, suggesting a good anti-inflammatory and anti-arthritic activity during acute phase and preventing development or, at least, mitigating severity of chronic RA.
Fig. 8.
Effect of ethanol extract of Moringa oleifera leaves at doses 500 and 250 mg/kg on latency time of CFA-induced arthritis rats measured using tail flick thermal analgesia method. A 500 mg and 250 mg are animal groups given Moringa extract at doses of 500 and 250 mg/kg body weight. a: significant at p < 0.05 and b: significant at p < 0.001.
4. Discussion
RA is a heterogeneous pathological condition associated with elevation of certain pro-inflammatory and inflammatory mediators including IL-1, IL-6, and TNF-a, which, if not suppressed, will increase infiltration of macrophages into the inflamed site with excessive production of auto-antibodies.27 Another important mechanism in the pathogenesis of RA is the increase in the production of free radicals by the activated macrophages and neutrophils.11 Both in vivo and in vitro studies of anti-inflammatory and anti-oxidant activity of Moringa leaves extract indicated a significant inhibition of nitric oxide (NO) production by macrophage cells, decrease in serum level of IL-1, IL-6, and TNF-a in addition to inhibition of COX2 pathway by inhibition of PGE2 production.28, 29, 30The results of the current study showed that Moringa extract has an inhibitory activity against inflammatory paw edema in both acute and chronic phases of CFA-induced arthritis. Interestingly, Moringa extract at low dose of 250 mg/kg illustrated stronger inhibitory activity against inflammatory paw edema than higher dose of 500 mg/kg. Other researchers like Sudha et al31 reported that low dose of methanol extract of Moringa leaves (250 mg/kg) was more effective than higher dose (750 mg/kg) as immune-modulatory effects in mice. Moreover, Leone and Alessandro32 reported that ethanol extract of Moringa leaves show an inhibitory activity against collagen-induced inflammatory paw edema in dose non-dependent pattern. One suggested explanation was the presence of phytates and other antinutrient phytoconstituents, which can hamper the absorption and consequently the activity of certain bioactive phytocompounds.12 Other explanation related the lower activity of higher dose to oversaturation of the dissolution medium in rat's stomach leading to precipitation of the bioactive phytoconstituent(s). The higher concentrations achieved in the in vivo dissolution media, beyond the equilibrium solubility, create driving forces for the nucleation and crystallization of the stable phases. Eventually, the stable phases will nucleate and crystallize. However, certain time is required before the depletion of concentrations in solution followed by re-dissolution of precipitated phytoconstituents.
The significant increase in body weight of the animal group that was given Moringa extract at dose 250 mg/kg reflects the safety, low toxicity, and less adverse effects compared to the animal group that was given indomethacin. The non-significant increase in body weight of the animal group that was given indomethacin may be due to ulcerative and gastric disturbance side effects of indomethacin. All groups other than normal group shows biphasic pattern, in that, a continuous increase in body weight started at day 6 until day 18 (established chronic phase) the weight decreased.
The animal groups that were given either indomethacin or Moringa extract showed less inflammatory and arthritis symptoms with statistically significant decrease in arthritic index. This result suggests that Moringa extract is effective in the prevention of RA development.
Iron deficiency anemia is one of the hematological symptoms of RA. In addition to its anti-inflammatory activity, Moringa leaves contain highest levels of essential amino acids, iron, and vitamins such as B complex32 which are essential for the treatment of anemia. The administration of ethanol extract of Moringa leaves to normal rats reported to significantly increase HGB level with no effect on WBC level.33 The level of WBC of all treatment groups and normal control group was much less than CFA-group. The level of WBC appears not to be affected by Moringa extract or the dose level. Other factors such as standard housing, age, sex and sex hormones level, and the level of hygiene maintained in the animal facilities all should be considered in the evaluation of erythrocyte and leukocyte profiles. In contrast, the study done by Martínez-González et al34 reported that the methanol extract of Moringa leaves at doses 400 and 800 mg/kg caused significant increase in the level of WBC counts and its differentials.
The results of walking track analysis using de Medinaceli's method point out the development of chronic RA in CFA-control group and the affect of chronic RA on locomotion and the change in gait behavior. In contrast, the animal groups that were given indomethacin or Moringa extract showed no considerable change in locomotion and gait behavior with FI within normal range. This result suggested that both indomethacin and Moringa extract successfully prevented the development of RA or at least ameliorate the severity of the disease.
Radiographic imaging in RA is a useful diagnostic tool, which is able to specify the severity of the disease. Soft tissue swelling and inflammation is the earlier sign, whereas prominent morphologic changes such as subchondral erosions and narrowing of joint spaces are late signs and can be observed only in the developed stages of the disease. Moringa extract at both doses had noticeably prevented joint destruction and soft tissue damages.
The ethanol extract of Moringa leaves revealed a noticeable anti-nociceptive activity in both normal and CFA-induced arthritis rats. In normal rats, Moringa extract illustrated a statistically significant anti-nociceptive effect in a dose-dependent manner in both the methods used. In arthritic rats, a progressive recuperation was observed in rats receiving Moringa extract that was more pronounced and significant at dose 500 mg/kg. This finding suggests that Moringa extract effectively decreases the inflammatory-associated pain, and not only central action but also peripheral inhibition of the prostaglandins-mediated potential of analgesic action of bradikinins were involved. Similar results were previously observed by Manaheji et al17 with a methanol extract of combined roots and leaves of Moringa in three arthritic models in rats and by Martínez-González et al34 with an ethanol extract of Moringa leaves in a collagen-induced RA.
In conclusion, this study provides the scientific evidence of the effectiveness of ethanol extract of Moringa leaves as analgesic, anti-inflammatory, and anti-arthritic medication supporting the common traditional beliefs and uses. For sure, a more preclinical and clinical study, especially in human subjects, is very essential and highly recommended. Moreover, the use of leaves offers a good option for a resource management that is more abundant and easy to get all the year around. Encouraging the use of leaves instead of seeds, flowers, or roots would maintain the remedial benefits involving pain and inflammation without affecting the proliferation of this source.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
The authors acknowledge Vet Dr Ng Yeong Sheng, Hope Veterinary Centre for his assistance in X-ray radiography. They would also like to thank Mr Rosli Hassan, Chief Lab. Assistant, Discipline of Pharmacology, School of Pharmaceutical Sciences, USM and Dr Saifullahi Abubakar for their cooperation and help in monitoring and in the evaluation of animals disease development.
References
- 1.Rajaram C., Reddy K.R., Chandra K.B. Evaluation of anti-arthritic activity of Caesalpinia pulcherrima in freund's complete adjuvant induced arthritic rat model. J Young Pharm. 2015;7:128–132. [Google Scholar]
- 2.Shi F., Zhou D., Ji Z., Xu Z., Yang H. Anti-arthritic activity of luteolin in Freund's complete adjuvant-induced arthritis in rats by suppressing P2X4 pathway. Chem Biol Interact. 2015;226:82–87. doi: 10.1016/j.cbi.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 3.Bhalekar M.R., Upadhaya P.G., Nalawade S.D., Madgulkar A.R., Kshirsagar S.J. Anti-rheumatic activity of chloroquine-SLN gel on wistar rats using complete freund's adjuvant (CFA) model. Indian J Rheumatol. 2015;10:58–64. [Google Scholar]
- 4.Gomes R.P., Bressan E., Silva T.M., Gevaerd S.M., Tonussi C.R., Domenech S.C. Standardization of an experimental model suitable for studies on the effect of exercise on arthritis. Einstein (São Paulo) 2013;11:76–82. doi: 10.1590/S1679-45082013000100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kapetanovic M., Lindqvist E., Geborek P., Saxne T., Eberhard K. Long-term mortality rate in rheumatoid arthritis patients with disease onset in the 1980s. Scand J Rheumatol. 2011;40:433–438. doi: 10.3109/03009742.2011.573503. [DOI] [PubMed] [Google Scholar]
- 6.Goulielmos G.N., Zervou M.I., Myrthianou E., Burska A., Niewold T.B., Ponchel F. Genetic data: the new challenge of personalized medicine, insights for rheumatoid arthritis patients. Gene. 2016;583:90–101. doi: 10.1016/j.gene.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Crowson C.S., Matteson E.L., Myasoedova E., Michet C.J., Ernste F.C., Warrington K.J. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:633–639. doi: 10.1002/art.30155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guruprasad B., Chaudhary P., Choedon T., Kumar V.L. Artesunate ameliorates functional limitations in Freund's complete adjuvant-induced monoarthritis in rat by maintaining oxidative homeostasis and inhibiting COX-2 expression. Inflammation. 2015;38:1028–1035. doi: 10.1007/s10753-014-0067-z. [DOI] [PubMed] [Google Scholar]
- 9.Vijayalaxmi A., Bakshi V., Begum N. Anti-arthritic and anti inflammatory activity of beta caryophyllene against Freund's complete adjuvant induced arthritis in wistar rats. Bone Rep Recomm. 2015;1:1–10. [Google Scholar]
- 10.Zampeli E., Vlachoyiannopoulos P.G., Tzioufas A.G. Treatment of rheumatoid arthritis: unraveling the conundrum. J Autoimmun. 2015;65:1–18. doi: 10.1016/j.jaut.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Gutiérrez-Rebolledo G.A., Galar-Martínez M., García-Rodríguez R.V., Chamorro-Cevallos G.A., Hernández-Reyes A.G., Martínez-Galero E. Antioxidant effect of Spirulina (Arthrospira) maxima on chronic inflammation induced by Freund's complete adjuvant in rats. J Med Food. 2015;18:865–871. doi: 10.1089/jmf.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gopalakrishnan L., Doriya K., Kumar D.S. Moringa oleifera: a review on nutritive importance and its medicinal application. Food Sci Hum Well. 2016;5:49–56. [Google Scholar]
- 13.Teixeira E.M.B., Carvalho M.R.B., Neves V.A., Silva M.A., Arantes-Pereira L. Chemical characteristics and fractionation of proteins from Moringa oleifera Lam. leaves. Food Chem. 2014;147:51–54. doi: 10.1016/j.foodchem.2013.09.135. [DOI] [PubMed] [Google Scholar]
- 14.Coppin J.P., Xu Y., Chen H., Pan M.H., Ho C.T., Juliani R. Determination of flavonoids by LC/MS and anti-inflammatory activity in Moringa oleifera. J Func Foods. 2013;5:1892–1899. [Google Scholar]
- 15.Mahajan S.G., Mali R.G., Mehta A.A. Protective effect of ethanolic extract of seeds of Moringa oleifera Lam. against inflammation associated with development of arthritis in rats. J Immunotoxicol. 2007;4:39–47. doi: 10.1080/15476910601115184. [DOI] [PubMed] [Google Scholar]
- 16.Mahajan S.G., Mehta A.A. Anti-arthritic activity of hydroalcoholic extract of flowers of Moringa oleifera Lam in wistar rats. J Herbs Spices Med Plants. 2009;15:149–163. [Google Scholar]
- 17.Manaheji H., Jafari S., Zaringhalam J., Rezazadeh S., Taghizadfarid R. Analgesic effects of methanolic extracts of the leaf or root of Moringa oleifera on complete Freund's adjuvant-induced arthritis in rats. Zhong Xi Yi Jie He Xue Bao. 2011;9:216–222. doi: 10.3736/jcim20110216. [DOI] [PubMed] [Google Scholar]
- 18.Kumar V., Verma A., Ahmed D., Sachan N.K., Anwar F., Mujeeb M. Fostered antiarthritic upshot of Moringa oleifera Lam stem bark extract in diversely induced arthritis in wistar rats with plausible mechanism. Int J Pharma Sci Res. 2013;4:3894–3901. [Google Scholar]
- 19.Nair V., Singh S., Gupta Y. Evaluation of disease modifying activity of Coriandrum sativum in experimental models. Indian J Med Res. 2012;135:240–248. [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkins P., Armstrong R., Boden T., Garside P., Knight K., Lilley E. Applying refinement to the use of mice and rats in rheumatoid arthritis research. Inflammopharmacol. 2015;23:131–150. doi: 10.1007/s10787-015-0241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuncel J., Haag S., Hoffmann M.H., Yau A.C., Hultqvist M., Olofsson P. Animal models of rheumatoid arthritis. (I) Pristane-induced arthritis in the rat. PLOS ONE. 2016;11:e0155936. doi: 10.1371/journal.pone.0155936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Medinaceli L., Wyatt R.J., Freed W.J. Peripheral nerve reconnection: mechanical, thermal, and ionic conditions that promote the return of function. Exp Neurol. 1983;81:469–487. doi: 10.1016/0014-4886(83)90276-5. [DOI] [PubMed] [Google Scholar]
- 23.Sandkühler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–758. doi: 10.1152/physrev.00025.2008. [DOI] [PubMed] [Google Scholar]
- 24.Rani S., Gupta M.C. Evaluation and comparison of antinociceptive activity of aspartame with sucrose. Pharmacol Rep. 2012;64:293–298. doi: 10.1016/s1734-1140(12)70767-3. [DOI] [PubMed] [Google Scholar]
- 25.Petterino C., Argentino-Storino A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp Toxicol Pathol. 2006;57:213–219. doi: 10.1016/j.etp.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Ihedioha J.I., Okafor C., Ihedioha T.E. The haematological profile of the Sprague-Dawley outbred albino rat in Nsukka, Nigeria. Anim Res Int. 2004;1:125–132. [Google Scholar]
- 27.Dinesh L., Karthik R., Gayathri N., Sivasudha T. Advancement in contemporary diagnostic and therapeutic approaches for rheumatoid arthritis. Biomed Pharmacother. 2016;79:52–61. doi: 10.1016/j.biopha.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Fard M.T., Arulselvan P., Karthivashan G., Adam S.K., Fakurazi S. Bioactive extract from Moringa oleifera inhibits the pro-inflammatory mediators in lipopolysaccharide stimulated macrophages. Pharmacogn Mag. 2015;11:S556. doi: 10.4103/0973-1296.172961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arulselvan P., Tan W.S., Gothai S., Muniandy K., Fakurazi S., Esa N.M. Anti-inflammatory potential of ethyl acetate fraction of Moringa oleifera in downregulating the NF-κB signaling pathway in lipopolysaccharide-stimulated macrophages. Molecules. 2016;21:1452. doi: 10.3390/molecules21111452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajanandh M., Satishkumar M., Elango K., Suresh B. Moringa oleifera Lam a herbal medicine for hyperlipidemia: a pre-clinical report. Asian Pac J Trop Dis. 2012;2:S790–S795. [Google Scholar]
- 31.Sudha P., Asdaq S.M.B., Dhamingi S.S., Chandrakala G.K. Immunomodulatory activity of methanolic leaf extract of Moringa oleifera in animals. Indian J Physiol Pharmacol. 2010;54:133–140. [PubMed] [Google Scholar]
- 32.Leone A., Spada A., Battezzati A., Schiraldi A., Aristil J. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Int J Mol Sci. 2015;16:12791–12835. doi: 10.3390/ijms160612791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ujah O., Ujah I., Johnson J., Oka V. Effect of ethanolic leaf extract of Moringa olifera leaf on haematological and biochemical parameters of wistar rats. J Nat Product Plant Resourc. 2013;3:10–14. [Google Scholar]
- 34.Martínez-González C.L., Martínez L., Martínez-Ortiz E.J., González-Trujano M.E., Déciga-Campos M., Ventura-Martínez R. Moringa oleifera, a species with potential analgesic and anti-inflammatory activities. Biomed Pharmacother. 2017;87:482–488. doi: 10.1016/j.biopha.2016.12.107. [DOI] [PubMed] [Google Scholar]