Improving the treatment of patients with advanced metastatic cancer is a daily challenge in clinical practice. The “renaissance” of immuno-oncology is mainly due to the success of the immune check-points inhibitors in prolonging the survival of metastatic cancer patients in different tumor types [1]. Very interestingly, some long lasting responses or even remissions have been reported [2].
Consequently, cancer immunotherapy became in the last decade, one of the major breakthrough in cancer treatment. Although active in monotherapy, anti-CTLA4 and anti-PD-1 monoclonal antibodies are not able to overcome every aspect of tumor immunosuppressive mechanisms. Several ongoing clinical trials attest that their combination may improve their clinical efficacy. Nevertheless, their associated autoimmune toxicities constitute a main reason of treatment discontinuation or abandon (NCT02477826, NCT03001882, NCT02982954 and NCT02231749) [3].
Thus, one of the main current challenge is how to improve cancer immunotherapy efficiency, without increasing autoimmune toxicities. An appropriate strategy may reside in a combinatorial approach able to overcome the numerous immunosuppressive networks of the tumor microenvironment (TME) [4] resulting in an “abscopal effect”, a rare phenomenon first described in patients treated with radiotherapy, characterized by regression of tumor outside the radiation field [5].
The reproduction of such systemic reaction requires at least two major steps, as described in our published model [6]. First, inducing apoptosis of tumor cells and second, reprogramming the TME into a TH1 polarized response through an IL-12-based immunotherapy. One adequate translational approach would be to replace radiotherapy by proapoptotic peptides (PAPs) that also offer the possibility of targeting specifically the tumor through a systemic administration [7]. PAPs are sequences of amino acids that have the ability to induce apoptosis after binding cell-surface receptors. They are composed of two functional domains: a targeting domain designed to recognize tumor cell surface and a pro-apoptotic domain designed to be non-toxic outside of cells but toxic when internalized.
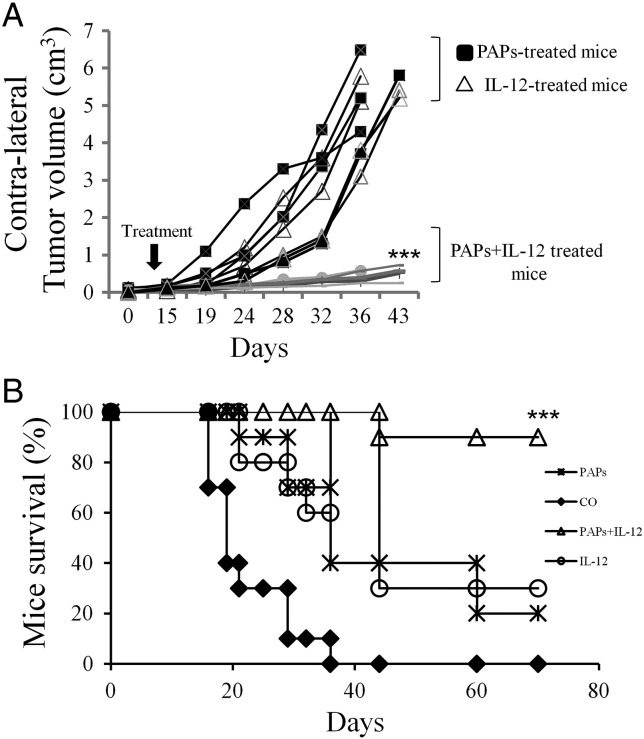
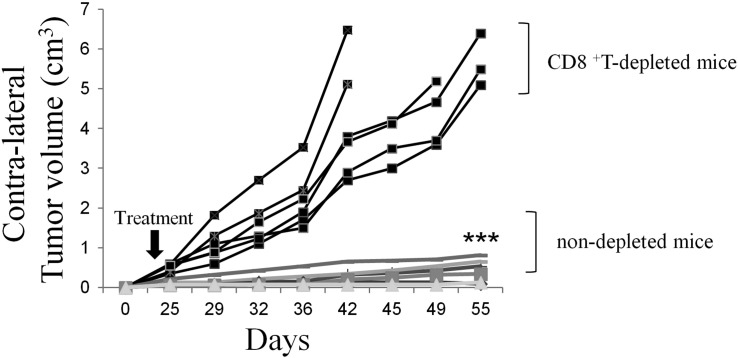
Here, we describe the combination of PAPs, composed of a protein transduction domain (PTD) peptide coupled to a killer peptide (KLAK) which disrupts eukaryotic mitochondrial membranes, along with an intratumor IL-12 based immunotherapy. This combination induces, in our murine model, an abscopal effect able to reduce massively the contralateral non-treated tumors (Figure 1A) and consequently to prolong dramatically the mice-bearing tumor survival (Figure 1B). In this report, we show that depletion of CD8+ T cells suppresses completely the abscopal effect on distant contralateral tumors, which in turn experience a dramatic progression (Figure 2A).
Figure 1.
The intratumor co-administration of IL-12 and proapoptotic peptides (PAPs) generates abscopal effect that reduces massively the contralateral tumor volume (A) and extends the lifespan (B) of BALB/c mice bearing CT26 colon cancer cells. Untreated CT26 tumors (seeded with 3 × 105 cells) were established in 6-wk.-old female BALB/c mice, as described previously [6], [9]. The different treatments were initiated when tumor reach a volume~ 0.3cm3. Only tumor on one side was co-injected with mouse recombinant IL-12 (R&D systems) (delivered at 10 μg/day for a period of 7 days) in the presence or absence of 300 μg (30 μl final) of PAPs (PTD/KLAK) [6] for a period of 7 days only. After that, the contralateral tumor (non-treated side) volume were evaluated twice weekly using a caliper. (A), contralateral tumors (non-treated side) in mice treated concomitantly with IL-12 and PAPs were significantly smaller (Student's t-test; P < .001) than untreated tumors (treated with PBS, control) or those treated with either IL-12 or PAPs alone. (B), mice treated concomitantly with IL-12 and PAPs survived longer than the untreated mice (control) or those treated with IL-12 or PAPs alone, as shown by a Kaplan- Meier survival plot (n = 14 animals/group; P < .05, log-rank test). All animals were bred and maintained according the guidelines of both the Federation of European Laboratory Animal Science Associations (Tamworth, U.K.) and the Animal Experimental Ethics Committee (Paris, France). Animals were used at between 6 and 20 wk. of age. Animals bearing tumors in excess of 20–25% of the body mass were killed.
Figure 2.
The in vivo depletion of CD8+ T cells abolishes the abscopal effect of PAPs and IL-12 in BALB/c mice bearing CT26 colon cancer cells. As described in Figure 1. Mice were depleted or not with H35.17.2 antibody (250 mg; obtained from American Type Culture Collection) respectively, 4 days before co-treatment with IL-12 and PAPs and repeated one time by week during a period of 30 days. After that, tumor volume were evaluated twice weekly using a caliper. The contralateral tumors in the depleted mice were significantly bigger compared to non-depleted mice (Student's t-test; P < .001).
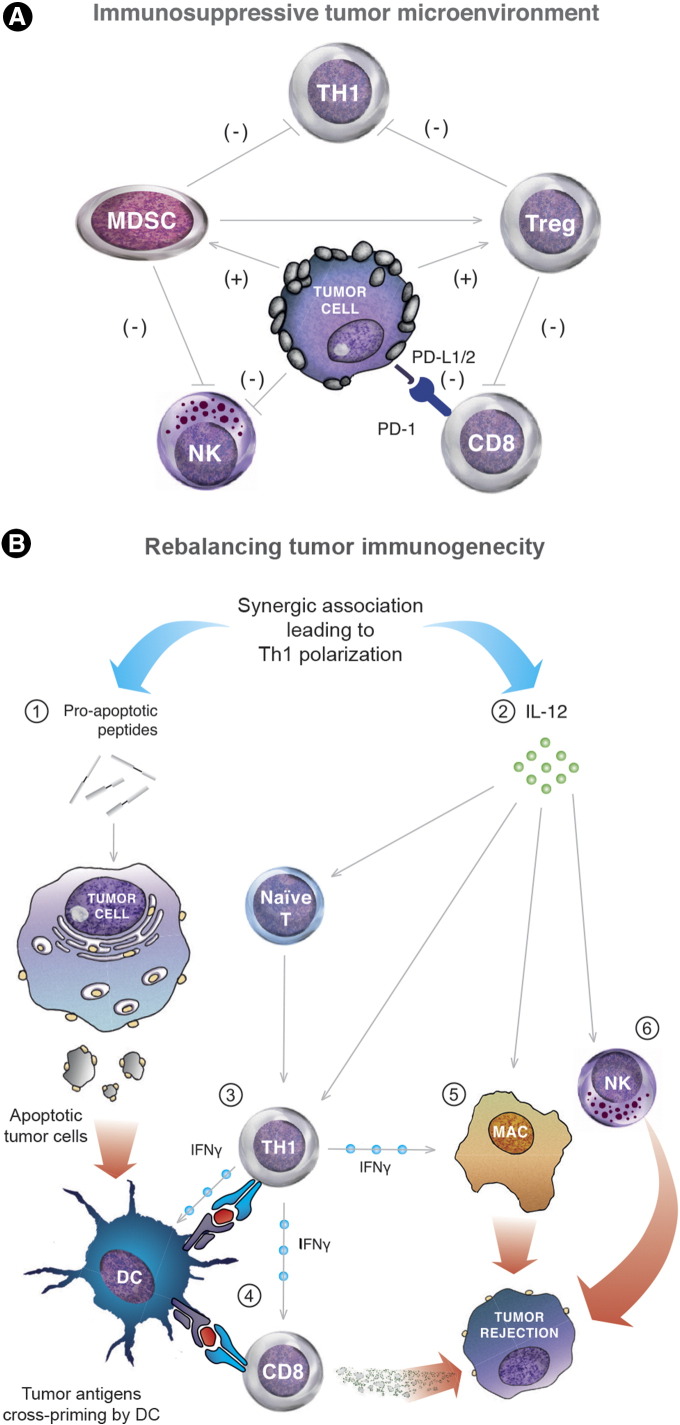
Our report shows for the first time the feasibility of inducing an abscopal effect through a synergistic combinatorial approach including locally administered PAPs and IL-12 based immunotherapy. This combination generates a potent antitumor immunity that acts as “in situ tumor vaccination” able to antagonize efficiently at multiple levels the tumor immunosuppressive environment (Figure 3A). The mechanisms implicate a rebalance of cytokine profile leading to a TH1-dominant immune response, through an IL-12 induced differentiation of naïve T cells [8], [9]. Consequently, immunosuppressive cytokines produced by TH2 pre-existing cells are reduced and cross presentation of dendritic cells (DCs) is enhanced by the up-regulation of interferon gamma (IFNγ) secretion from TH1 cells. This process also decreases the prevalence of immunosuppressive actors like myeloid-derived suppressor cells (MDSCs) and T lymphocytes regulators (Tregs). IL-12 also promotes the proliferation and the cytolytic activity of NK cells and effector CD8+ T-cells. However, without the microenvironment reprogramming conferred by IL-12 co-administration, the apoptotic cell death is not by itself sufficient to allow an effective tumor rejection. Together, all these synergistic modifications on tumor microenvironment elicit a potent antitumor immunity leading to the rejection of distant metastatic tumors (Figure 3B).
Figure 3.
(A) Immunosuppressive mechanisms of tumor microenvironment (TME). Malignant cells display multiple strategies and connection to escape immune system. The tumor stroma can recruit portent immunosuppressive cells, such as myeloid-derived suppressor cells (MDSC) and T regulator lymphocytes (Treg), which prevents, mainly through the secretion of immunosuppressive cytokines (as IL-10 and TGF-β)), the cytotoxic CD8+ T cells and NK cells from infiltrating efficiently the tumors. Additionally, tumor deployed another main immunosuppressive mechanism through the PD-L1/PD-1 interaction to induce tumor infiltrating lymphocytes (TILs) anergy and exhaustion. This local TME reprogramming is dramatically amplified by a systemic polarization of the T cell response towards a TH2 phenotype, limiting cytotoxic T-cells proliferation and activity as well as tumor recognition and elimination by NK cells. (B) Mechanisms underlying the “abscopal effect” elicited by an “in situ-tumor vaccination” approach combining pro-apoptotic peptides (PAPs) and IL-12-based immunotherapy. (1) Pro-apoptotic peptides (PAPs) induce tumor cell apoptosis, thus exposing surface proteins acting as potent “eat-me signal” for dendritic cells (DC). After phagocytosis, tumor-derived antigens are processed by DC and cross-presented to naïf T and B cells leading to the mounting of a “weak” antitumor response insufficient to elicit a full tumor rejection. (2) IL-12, a multitask cytokine, which is able to “dampening” the immunosuppressive TME network at multiple levels and especially by “suffocating” the immune suppressor cells of the tumor stroma, including Tregs, immature DCs and MDSCs. (3) This cytokine induces a TH1 cell differentiation of CD4+ T lymphocytes after antigen recognition and activates CD8+ effectors T-cells. (4) It stimulates their proliferation and increases the levels of interferon gamma (IFNγ) produced by TH1 cells, leading to an enhanced cytotoxic activity of CD8+ T cells and cross presentation capacity of DCs. (5) + (6) IL-12 directly activates macrophages and NK-cells thus promoting tumor rejection. In our murine model, this approach induced a local and distant tumor regression (i.e. abscopal effect). Reversing the immunosuppressive function is a required step to stimulate tumor-specific T cells. Specifically, the immunosuppressive TME will be converted from a TH2-dominated environment to a more TH1-like response. Thus, this in situ tumor vaccination protocol, which combines apoptotic peptides with IL- 12-based TH1 immunotherapy, allows for a strong synergetic anti-tumor immunity.
As a translational extension from this observation, we postulate that the replacement of locally administered PAPs by an intravenous infusion (as described in [7]) may result in the same abscopal effect. Such scenario will undeniably open the door to the “democratization” of the abscopal effect in the clinical practice, which may bring new hope for cancer patients refractory to immune check-point blockade therapies.
Author Contributions
MO conceived the study, performed the experiments and prepare the manuscript; FM prepared Figure 3; all authors wrote, commented and corrected the manuscript.
Acknowledgements
This work was supported by a grant from INSERM (Institut National de la santé et de la recherche médicale) and faculté de médecine Paris VI. The authors would like to thank Mr. Roger Guindon Montoya for its work on the Figure 3.
Ethics statement. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the European Union “European directive 86/609/EEC” and the French National Institutes of Health. The protocol was approved by the Committee on the Ethics of Animal Experiments of the University of Pierre et Marie Curie, Paris, France (Permit Number: A751301).
Footnotes
Conflict of interest. The authors declare no conflict of interest.
References
- 1.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsoone-Ulrich A, Jamme P, Alkeraye S, Dzwiniel V, Faure E, Templier C, Mortier L. Ipilimumab in anti-PD1 refractory metastatic melanoma: a report of eight cases. Melanoma Res. 2016;26:153–156. doi: 10.1097/CMR.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 3.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia A, Kumar Y. Cellular and molecular mechanisms in cancer immune escape: a comprehensive review. Expert Rev Clin Immunol. 2014;10:41–62. doi: 10.1586/1744666X.2014.865519. [DOI] [PubMed] [Google Scholar]
- 5.Mole RH. Whole body irradiation: radiobiology or medicine? Br J Radiol. 1953;26:234–241. doi: 10.1259/0007-1285-26-305-234. [DOI] [PubMed] [Google Scholar]
- 6.Deplanque G, Shabafrouz K, Obeid M. Can local radiotherapy and IL-12 synergise to overcome the immunosuppressive tumor microenvironment and allow "in situ tumor vaccination"? Cancer Immunol Immunother. 2017;66:833–840. doi: 10.1007/s00262-017-2000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obeid M. Anticancer activity of targeted proapoptotic peptides and chemotherapy is highly improved by targeted cell surface calreticulin-inducer peptides. Mol Cancer Ther. 2009;8:2693–2707. doi: 10.1158/1535-7163.MCT-09-0228. [DOI] [PubMed] [Google Scholar]
- 8.Smyth MJ, Taniguchi M, Street SE. The anti-tumor activity of IL-12: mechanisms of innate immunity that are model and dose dependent. J Immunol. 2000;165:2665–2670. doi: 10.4049/jimmunol.165.5.2665. [DOI] [PubMed] [Google Scholar]
- 9.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13:155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]