Graphical abstract
Cardioprotective activity of saponins via different molecular targets. 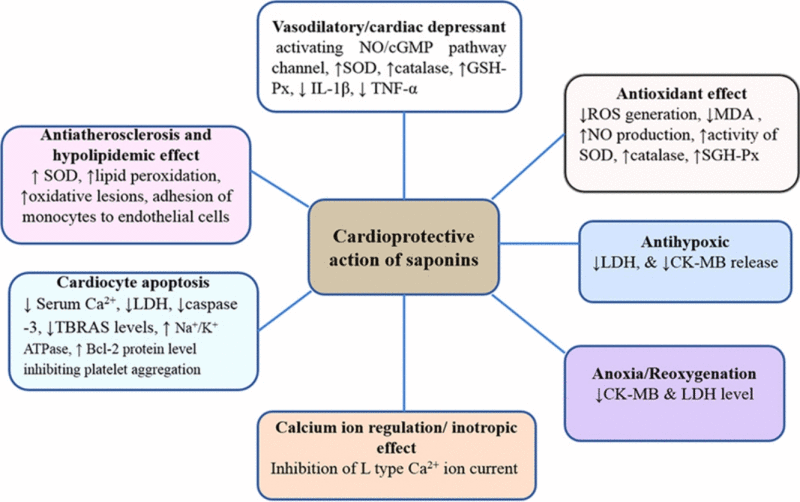
Keywords: Bioavailability, Cardioprotective activity, Saponins, Structure activity relationship
Abstract
Cardiovascular diseases are the leading cause of death, accounting about 31% deaths globally in 2012. The major risk factors causing cardiovascular diseases are coronary atherosclerosis, hyperlipidemia, myocardial infarction, and stroke. The dominating cause of cardiovascular diseases is accredited to our modern lifestyle and diet. Medicinal plants have been used for the prevention and treatment of cardiovascular diseases from centuries. The in built chirality and chemical space of natural products have been playing an important role in providing leads and templates for pharmacophore synthesis. This review highlights one of the important naturally occurring class saponins and their role in cardioprotection along with structural characteristics and pharmacological effects such as antioxidant, Ca2+ ion regulation, antiapoptotic, antiatherosclerosis, antihyperlipidemic, hypocholesterolemic, angiogenic, vasodilatory, and hypotensive. The characteristic cholesterol lowering, hemolytic, and anticoagulant properties of the saponins prompted us to select as one of the natural products class for cardioprotection. This review covers the most updated information on saponins related to their cardioprotective effects, mechanism of action, bioavailability, and structure activity relationship.
1. Introduction
Cardiovascular diseases (CVDs) are the leading cause of death worldwide and according to World Health Organization about 17.5 million people died from CVDs in the year 2012.1 Hyperlipidemia and hypercholesterolemia are the two major risks for CVDs along with atherosclerosis, coronary heart disease, coronary artery disease, coronary calcium, stroke, myocardial infarction, peripheral arterial disease, and arrhythmias.2, 3 Medicinal plants have been a rich source of lead molecules for the treatment of CVDs including atherosclerosis, angina pectoris, congestive heart failure, systolic hypertension, cerebral insufficiency, and arrhythmia.4, 5, 6 Reserpine drugs, the first effective treatment for hypertension and digitoxin for congestive heart failure were derived from the plants Rauwolfia serpentina (snakeroot) and Digitalis species, respectively.7 Natural products of different classes such as flavonoids/phenolics (resveratrol, genistein, catechin, apigenin, ellagic acid, and aspirin), organosulphur (sulforaphane), cardiac glycosides (oleandrin), terpenoids (arjunolic acid and gymnemic acid), steroids (diosgenin), omega-3 fatty acids, and pigments (lycopene and carotenoids) have been studied for their cardioprotective potential.4, 8, 9, 10, 11, 12
Although medicinal plants have been extensively studied for the treatment of CVDs from centuries, only a few natural products derived drugs are available so far. In a recent update on natural products as a source of new drugs stated that about 13 cardiotonic drugs has been approved for CVDs in duration of year 1981–2014 out of which 3 were semisynthetic modified natural product derivatives, 2 total synthetic drugs, 3 natural product mimics synthetic drugs and 5 total synthetic drugs having natural product derived pharmacophore.13 This study showed the gap of the research findings of plant based cardioprotective molecules as well as alluring our attention towards medicinal plants in search of new cardioprotective molecules.
In last two decades, saponins have been extensively researched and reviewed by different research groups for their isolation, structural elucidation, distribution, biosynthesis, classification, commercial and pharmacological importance in the form of pure compound as well as saponin enriched crude extract (total saponin).14, 15, 16, 17, 18 The present review summarizes pharmacological importance of plant-based saponins in cardiovascular disorders along with their mechanism of action and structure activity relationship (SAR) studies. Also, the physicochemical properties of saponins and cardiotonic drugs have been evaluated through in silico method to understand their bioavailability and pharmacokinetics.
2. Saponins and cardioprotection
Saponins are high molecular weight amphiphilic compounds having triterpenoid and/or steroid aglycon as lipophilic moiety and sugars (usually glucose, rhamnose, glucuronic acid, arabinose, and xylose) as hydrophilic moiety.16, 19 Saponins are distributed in plants, fungi, and marine organisms such as starfish and sea cucumbers.17, 20 The commercial importance of saponins came into existence in 1960 by the synthesis of sex hormone progesterone as a first oral contraceptive from diosgenin (derived from saponin named dioscin).21 Ginseng has been emerged as one of the most explored natural product for cardioprotection due to its active constituent saponins.22, 23 Several saponin-enriched medicinal plants such as Allium species, Terminalia arjuna, Clematis species, Glycyrrhiza glabra, Ilex cornuta, Crataegus oxyacantha, and Astragalus membranaceus have been well studied for their cardioprotective potential.24, 25, 26 Natural products e.g. digoxin, ouabain, digitoxin, acetyldigitoxin, rostafuroxin, deslanoside, atorvastatin (from fungal metabolite mevastatin), vorapaxar (himbacine analogue from Galbulimima baccata), cardiac glycoside, and pyridoxal-5-phosphate (a vitamin B6 derivative) have been found as lead candidates in cardioprotection.27 Phytosterols such as diosgenin and its derivatives are well renowned cardioprotective agents that lower serum cholesterol in the intestinal tract by inhibiting cholesterol absorption.2 Overall, the structural similarities with cardioactive phytosterols along with interesting pharmacological effects such as hemolytic or permeabilization of cell membrane, antilipemic, serum cholesterol lowering and anticoagulant prompted to explore the importance of saponins in cardioprotection.15, 16, 18, 28, 29
3. Cardioprotective activity of saponins
Different pharmacological effects including antioxidant, antihypoxic, anoxia/reoxygenation, Ca2+ ion regulation or calcium antagonist, cardiocyte apoptosis, vasodilatory effect, angiogenesis, inotropic and others have been compiled to explore the cardioprotective potential of saponins.
3.1. Antioxidant activity
The intracellular oxidative damage caused by the increased production of reactive oxygen species (ROS) is considered as a major cause of CVDs. The ROS toxicity at the time of reperfusion causes myocardial ischemia/reperfusion (I/R) injury by xanthine decomposition in mitochondria, increase in cellular accumulation of lipid peroxides, depletion of endogenous antioxidants and overloading of Ca2+ ions.30, 31 The cardioprotective role of steroidal saponins from Allium chinensis attenuates the increased malondialdehyde (MDA) formation and nitric oxide (NO) release compared to nimodipine, a clinically approved calcium channel blocker against oxidative injury.32 Saponins of Allium species, I. cornuta and Dioscorea or yam plants have been best studied against hydrogen peroxide (H2O2)-mediated oxidative injuries by generating highly reactive hydroxyl radicals.33, 34, 35 Saponins, for example, glycyrrhizic acid, asperosaponin VI, elatoside C, tribulosin, platycodin D, astragaloside IV, protodioscin, and trillin are known to increase the activity of several antioxidant enzymes like super oxide dismutase (SOD), catalase and glutathione peroxidase (GSH-Px), which work at cellular defense against ROS induced cardiac damage.24, 36, 37, 38, 39, 40, 41, 42
Clematichinenoside, a triterpenoid saponin from the roots of Clematis chinensis exhibited cardioprotective effects in ischemia/reperfusion injury via antioxidant effect by restoring the balance between inducible NO synthase and endothelial NO synthase.43, 44 The pre-administration of clematichinenoside (8 mg/kg, 16 mg/kg, 32 mg/kg) significantly reduces the infarct size to 32 ± 6%, 29 ± 7% and 26 ± 4% (p < 0.05, p < 0.05 and p < 0.01 vs. model group), respectively compared to standard tanshinone IIA (16 mg/kg, 24 ± 4%, p < 0.01). Clematichinenoside attenuates infarct size, decreases low-density lipoprotein (LDL), creatine kinase and MDA level, increases SOD activity as well as improves hemodynamic indexes.44 A triterpenoid saponin, sasanquasaponin from Chinese traditional herb Camellia oleifera Abel. induces cardioprotection against ischemia-reperfusion (I/R) injury possibly via activation of bradykinin–NO pathway followed by the suppression of ROS release.31 Another saponin from Chinese herb A. membranaceus named astragaloside IV (3-O-β-d-xylopyranosyl-6-O-β-d-glucopyranosyl-cycloastragenol) has been reported for anti-ischemic properties.45 Astragaloside IV induces the activity of SOD and NO along with increase in coronary flow by reducing the infarct size (in vivo).46
Steroidal saponin ophiopogonin D from the tubers of Ophiopogon japonicus have been used to treat inflammation and cardiovascular disorders also exhibited antioxidant effect against H2O2 injured human umbilical vein endothelial cells (HUVEC). Ophiopogonin D restores the cellular total antioxidative capacity, inhibits release of inflammatory cytokines and enzymatic activities (catalase, HO-1 and caspase) possibly via ERK pathway.47 Pretreatment of ophiopogonin D reduces the doxorubicin-induced excessive autophagy due to the reduction of ROS generation and thus protects the heart against doxorubicin-induced damage.48 These results suggest ophiopogonin D can be useful in the combination therapy with anticancer drugs causing cardiotoxicity.
Elatoside C from Aralia elata inhibited mitochondrial ROS overproduction and maintained [Ca2+]i homeostasis in cardiac I/R injury through the activation of reperfusion injury salvage kinase (Akt and ERK1/2) and survivor activating factor enhancement JAK2/STAT3 pathways.49 The improved myocardial performance of elatoside C suggests its application during cardiac surgery and ischemic heart diseases. The pretreatment of another saponin, acanthopanax senticoside B (200 μg/mL and 400 μg/mL) from Acanthopanax senticosus protects cardiomyocytes against H2O2 induced oxidative damage by increasing the activity of catalase, GSH-Px, SOD, levels of reduced glutathione and increase in cell viability.50 The saponins of I. cornuta exhibited protective effects against H2O2-mediated myocardial cell injury whereas did not show DPPH free radical scavenging activity (<30% at 200μM).26, 35 The antioxidant activity results suggested that the protective effects of saponins were not directly associated with their free radical scavenging effect and warrant to investigate further.
3.2. Antihypoxic activity and anoxia/reoxygenation (A/R)
The reduced oxygen supply mainly causes ischemic injuries. A steroidal saponin named fistulosaponin A from Welsh onion (Allium fistulosum) seeds exhibited cardioprotective effects against hypoxia-induced HUVEC injury with a cell viability of 59.5 ± 3.0%, 76.3 ± 3.3%, 80.1 ± 3.6%, 82.7 ± 4.1% and 86.3 ± 4.6% using Diao-xin-xue-kang (an extract of the roots of Dioscorea anthaica Prain et Burkill) as positive control with cell viability of 65.8 ± 3.3%, 69.6 ± 2.7%, 75.8 ± 1.8%, 79.9 ± 2.9% and 81.0 ± 3.4% at 0.5μM, 1.0μM, 5μM, 10μM and 50μM.51 In Tibet, Clematis tangutica is used for invigorating blood circulation, prevention, and treatment of cardiac diseases in clinical practices. Triterpenoid saponins of C. tangutica possess cardioprotective effects by decreasing the serum levels of lactate dehydrogenase (LDH) and creatine kinase-MB (CK-MB) against hypoxia-induced cell damage. As the serum levels of CK-MB and LDH are indicators of myocardial injury. C. tangutica saponins like lematangosides, cauloside D and asperosaponin VI at 0.05mM concentration showed anti-myocardial ischemic effects against LDH and CK-MB release with ED50 values ranging from 75.77μM to 127.22μM in A/R-induced cell damage.43 In another study, C. tangutica saponins exhibited anti-ischemic activities with ED50 values in the range of 75.77–127.22μM using diltiazem hydrochloride as positive control (ED50 = 21.04 ± 0.84μM, LDH and ED50 = 15.17 ± 0.35μM CK-MB) against LDH and CK-MB release.44
3.3. Ca2+ ion regulation/calcium antagonist activity
Calcium ion (Ca2+) is an important second messenger that regulates various cellular processes like NO-synthase activation on increasing the cytosolic Ca2+ levels in endothelial cells. Also, the cardiac I/R or hypoxia damage are associated with the Ca2+ overloading.52 Saponins from A. macrostemon B bulbs named macrostemonoside B, macrostemonoside M, macrostemonoside N and (25R)-26-O-β-d-glucopyranosyl-22-hydroxy-furost-3β,26β-diol-3-O-β-d-glucopyranosyl(1-2)-β-d-galactopyranoside (10 μm/L) increased the Ca2+ ion mobilization higher than potassium chloride and also increased the function of cardiac muscles in isolated cardiomyocytes of Guinea pig.53 Macrostemonoside E was found as a promising compound in the treatment of heart failure. Another furostanol saponin, methyl protodioscin from D. collettii var. hypoglauca upregulated the sodium and calcium pump and maintains the low calcium in cardiomyocytes.54 Also a saponin monomer named DT-13 [(25(R,S)-ruscogenin 1-O-[β-d-glucopyranosyl (1→2)][β-d-xylopyranosyl (1→3)]-β-d-fructopyranoside)] from dwarf lily turf tubers (O. japonicus) possess potent cardioprotective effects both in normal and hypoxia condition in adult rat ventricular myocytes.55, 56 DT-13 at 0.001 μm/L, 0.01 μm/L, 0.1 μm/L, 1.0 μm/L and 5.0 μm/L inhibited the current density of L-type calcium current (ICa,L) by 14.59 ± 3.18% (n = 5, p < 0.05), 27.01 ± 5.12% (n = 5, p < 0.05), 36.82 ± 6.97% (n = 5, p < 0.05), 62.76 ± 8.17% (n = 5, p < 0.001) and 77.13 ± 7.15%, respectively.55 The cardioprotective effect of DT-13 might be the direct inhibition of L type Ca2+ currents via voltage-gated calcium channels.
3.4. Cardiocyte apoptosis
Apoptosis is observed as one of the cause of heart failure in response to several pathological effects like ischemia, I/R, hypoxia, calcium excess, oxidative stress, gene induction and doxorubicin toxicity.57 Cardiotoxicity caused by anticancer drug, for example, doxorubicin is due to the oxidative damage of cellular components and formation of free radicals. Pretreatment of gymnemic acid phospholipid complex prevented the doxorubicin-induced cardiotoxicity in rats, improves the heart to body weight ratio, decreases serum Ca2+, LDH, myocardial caspase-3, thiobarbituric acid reactive substances (TBRAS) levels, as well as increases Na+/K+ ATPase and antioxidant enzyme levels. Further, the internucleosomal DNA laddering prevention suggests the anti-apoptotic effect of gymnemic acid phospholipid ester.58
Cardiocyte apoptosis is attributed to the functional deterioration of ischemia/hypoxia cardiomyopathies. A triterpenoid saponin, hecogenin-3-O-β-d-glucopyranosyl (1→4)-β-d-galactopyranoside from Tribulus terrestris attenuated apoptosis in NaCN digested rat ventricular cardiomyocytes.59 Hecogenin glycoside decreases the intracellular free Ca2+ ion concentration, increases Bcl-2 protein levels and activated translocation of protein kinase (PKCɛ)-mediated cellular signaling transduction pathways. Dioscin, a natural steroidal saponin of Dioscorea species has been traditionally used in China to prevent coronary diseases. Moreover, dioscin also prevented ischemia/perfusion induced injury in H9c2 cells via mitochondrial apoptotic pathway by reducing oxidative stress.60 Additionally the cardioprotective potential of dioscin was also supported by its antithrombotic effects by improving anticoagulation activity and inhibiting platelet aggregation.61
3.5. Anti-atherosclerosis and hypolipidemic activity
Atherosclerosis is a chronic inflammatory disorder usually initiated by the adhesion of monocytes to the vascular endothelium.62 Saponins like reinioside C, ginsenoside Rb1, Rg1, Re, and R1 are reported to inhibit monocyte-endothelial cell adhesion.63 Reinioside C from the roots of Chinese herb Polygala fallax shows promising hypolipidemic effect due to the protective effects on oxidative lesions induced by oxidized low-density lipoprotein (OxLDL), inhibiting cholesteryl ester accumulation in macrophages as well as decreasing [Ca2+]i and smooth muscle cells proliferation.64 Reinioside C inhibited OxLDL-induced [Ca2+]i elevation in smooth muscle cells by 35.1% compared to calcium antagonist verapamil (41.3% at 10−6 mol/L) and suggests that reinioside C can partially abolish the OxLDL action by inhibiting the calcium influx into macrophages. Further, reinioside C inhibited the adhesion of monocytes to endothelial cells via nicotinamide adenine dinucleotide phosphate (NADPH) oxidase/ROS/nuclear factor-kappa B (NF-κB) pathway.65
Natural products including dietary fibers, herbal formulations/extracts, plant sterols and yeast extracts have been used as anti-hyperlipidemic agent.42, 66 The steroidal constituents such as dioscin, diosgenin, protodioscin, and trillin from the rhizomes of Dioscorea nipponica showed anti-hyperlipidemic activity by improving the levels of lipid peroxidation and SOD activity mediated through anti-lipase mechanism.41, 42 The anti-hyperlipidemic potential of D. nipponica emerges it as a food supplement against several cardiovascular disorders in future.
3.6. Vasodilatory activity
Luna-Vazquez et al (2013) have reviewed 270 plant derived metabolites as vasodilator along with their possible mechanism of action.6 Authors further concluded that the activation of NO/cGMP pathway and/or blocking of voltage-dependent calcium channels is the major vasodilatory mechanism of action for different classes of compounds including saponins. Bacoside A3 and bacopaside II belong to class jujubogenin and pseudojujubogenin, respectively isolated from Ayurvedic medicinal plant Bacopa monnieri possess blood pressure reducing effect by releasing NO from endothelium and influences smooth muscle Ca2+ ion homeostasis.67
Ginsenosides Rb1 and Re from P. ginseng were reported to possess cardiac depressive response at ventricular myocyte level through NO-mediated vasodilation.22 The cardiac depressant activity of ginsenosides has clinical applications in the treatment of cardiovascular disorders like hypertension and heart failure.
3.7. Inotropic activity
Saponins are known to interact with cholesterol and generate sub-skinning condition that can modify the electrical and mechanical properties of cardiac muscles. Enomoto et al (1986) studied the positive inotropic effect of saponins of Panax species and concluded that modification of calcium channel might be involved in this effect.68 Saponins with positive inotropic effect were showed haemolysis in rabbit erythrocytes. A cardioactive steroidal saponin containing rare neogetogenin as aglycon named cistocardin from Ayurvedic medicinal plant Tribulus cistoides (thistle of the hot country) exhibited strong positive inotropic effect at 10−6M to 10−5M concentration in papillary muscles of Guinea pigs.69
3.8. Angiogenic activity
Angiogenesis is the formation of new vessels from existing one and has importance in the diseases with insufficient blood vessels formation such as peripheral and coronary ischemia and infarction, chronic wounds failure and ulcers. Panaxtriol (PTS), a designed extract of P. notoginseng enriched with three major bioactive saponins notoginsenosides R1 (11%), gensenoside Rg1 (50%) and gensenoside Re (6%) is reported to possess pro-angiogenic effect and thereby used to treat ischemic stroke in China.70, 71 A study in middle cerebral artery occlusion (MACO) rat model showed in vivo efficacy of PTS against cerebral ischemic injury by enhancing cerebral blood flow, angiogenesis, and modulating cytokines vascular endothelial growth factor and angiopoietin-1 expression.71 The protective effect of PTS is mediated through the activation of Shh signaling pathway mechanism. In addition, the ginsenosides (R1, Rg1 and Re) were individually evaluated in different models and emerges as promising pro-angiogenic agents.72, 73 The results suggest PTS and its active saponins can be used in therapeutic angiogenesis such as ischemic stroke.
3.9. Other pharmacological activities
The cardioprotective potential of saponins have been evaluated for several other pharmacological effects including fibrinolytic activity of endothelial cells, antiplatelet and/or antithrombotic, endothelial protectant, contractile effect, bradycardiac effect, cardiac depressant and cyclic AMP phosphodiesterase inhibitory activity. Saponin β-Ascin from Aesculus hippocastanum seeds showed improved endothelium dysfunction and contractile effect in oxidative stressed rat aortic rings that can be useful in the treatment of venous insufficiency and limiting side effects, respectively.74
Ginsenoside Ro, an oleane saponin of P. ginseng inhibits αIIb/β3-mediated fibrinogen binding mediated by cyclic adenosine monophosphate (cAMP)-dependent phosphorylation of vasodilator-stimulated phosphoprotein (Ser157) and might be responsible to prevent the platelet aggregation-mediated thrombotic diseases.75 Interestingly, the cardiac tissue regeneration effect has also been studied for few saponins e. g. ginsenoside Rg1 and Rb1, tanshinone IIA, astragaloside IV, salvianolic acid B, and cardiogenin (Niga-ichigoside F1).76 The cardiac regeneration activity of saponins can be used as a tool for the effective treatment of injured hearts and other body organs.77
4. Bioavailability of saponins
The high molecular weight and poor membrane permeability of saponins make them low bioavailable, and hence restricting saponins as a drug candidate. The poor bioavailability of saponins might be a reason for lesser studies on its pharmacological mechanism of action. During the last decade, total saponins and isolated pure saponins of the plants have been evaluated for their pharmacokinetic studies. Saponins like clematichinenoside AR, astragaloside IV, methylprotodioscin and ginsenosides have been studied for their pharmacokinetics and metabolites identification. The oral bioavailability of ginsenosides Ra3, Rb1 and Rd was found as 0.1–0.2%, whereas ginsenosides Re, Rg1, and notoginsenoside R1 of 0.2–0.6%.78
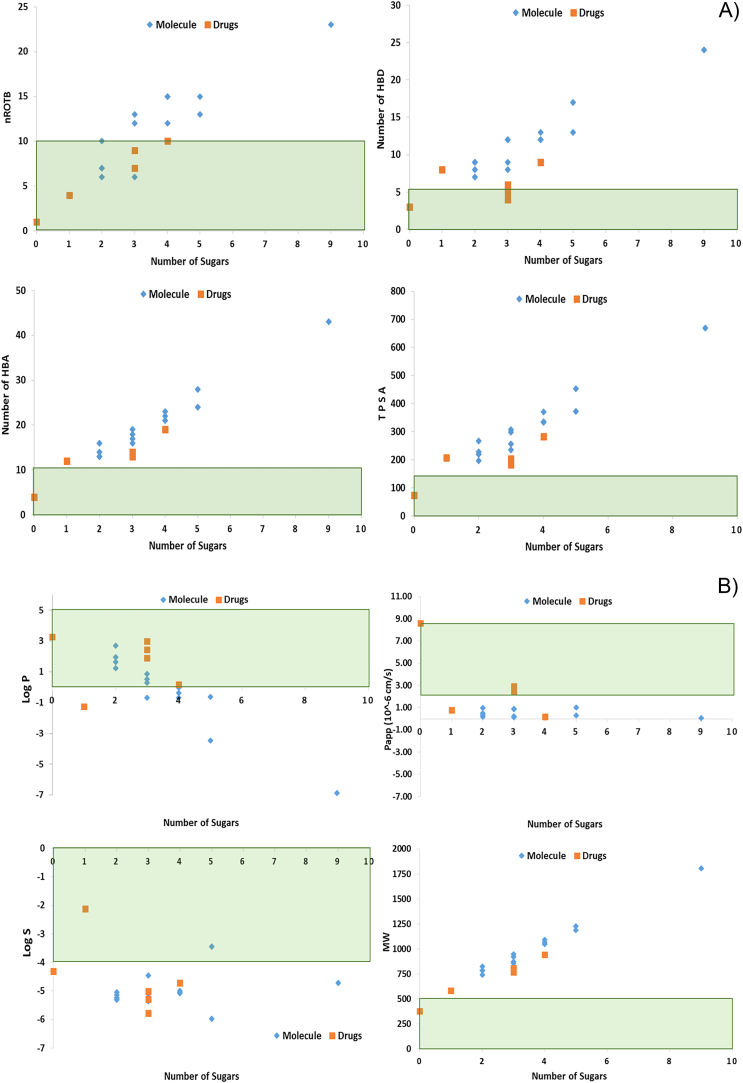
Because of the poor bioavailability and permeability of saponins, the calculated important physicochemical parameters of saponins and natural products derived cardiotonic drugs were compared to understand the intestinal absorption and bioavailability of saponins through in silico approach. The key physicochemical properties including predicted lipophilicity (log P), topological polar surface area (TPSA), hydrogen bond donor (HBD), hydrogen bond acceptor (HBA) and number of rotatable bonds (nRotB) responsible for bioavailability were calculated and given in Table 1 for 14 selected cardioprotective saponins and 6 Food and Drug Administration (FDA) approved natural product derived cardiotonic drugs.79 It is evident from in silico analysis that the deviation from key drug-like physicochemical properties is the primary reason for poor bioavailability of the saponins, such as high molecular weight (>500 Da); large number of rotatable bond, nRotB (>10) responsible for molecular flexibility; high HBA (>10); high HBD (>5); large TPSA (>140 Å2) and low lipophilicity (Log P) that associated with poor membrane permeability.
Table 1.
Physicochemical Properties of Cardioprotective Saponins and Cardiotonic Drugs
| Saponins | Sugar count | MW | nRotb | HBA | HBD | TPSA | Log P | Log S | Papp (10−6 cm/s) |
|---|---|---|---|---|---|---|---|---|---|
| Acanthopanax senticosides B | 5 | 1187 | 13 | 24 | 13 | 372 | −0.62 | −5.97 | 0.299 |
| Astragaloside IV | 2 | 785 | 7 | 14 | 9 | 228 | 1.23 | −5.04 | 0.501 |
| Clematichinenoside | 9 | 1808 | 23 | 43 | 24 | 669 | −6.88 | −4.72 | 0.072 |
| DT-13 | 3 | 871 | 7 | 17 | 9 | 256 | 0.31 | −5.08 | 0.845 |
| Elatoside C | 4 | 1089 | 12 | 23 | 13 | 371 | −0.36 | −5.04 | 0.170 |
| Ginsenoside Re | 3 | 947 | 12 | 18 | 12 | 298 | 0.54 | −5.11 | 0.215 |
| Ginsenoside Rg3 | 2 | 785 | 10 | 13 | 9 | 219 | 2.69 | −5.3 | 0.959 |
| Glycyrrhizic acid | 2 | 823 | 7 | 16 | 8 | 267 | 1.63 | −5.14 | 0.170 |
| Gypenoside XLIX | 4 | 1047 | 15 | 21 | 12 | 334 | −0.01 | −5.07 | 0.205 |
| Methyl protodioscin | 4 | 1063 | 15 | 22 | 12 | 335 | −0.68 | −4.99 | 0.138 |
| Ophiopogonin D | 3 | 855 | 6 | 16 | 8 | 236 | 0.87 | −5.36 | 0.881 |
| Platycodin D | 5 | 1225 | 15 | 28 | 17 | 453 | −3.45 | −3.44 | 0.998 |
| Timosaponin A-III | 2 | 741 | 6 | 13 | 7 | 197 | 1.96 | −5.24 | 0.359 |
| Timosaponin B II | 3 | 921 | 13 | 19 | 12 | 307 | −0.68 | −4.46 | 0.155 |
| Cardiotonic drugs | |||||||||
| Acetyldigitoxin | 3 | 807 | 9 | 14 | 4 | 189 | 2.98 | −5.77 | 2.931 |
| Deslanoside | 4 | 943 | 10 | 19 | 9 | 282 | 0.19 | −4.71 | 0.197 |
| Digitoxin | 3 | 765 | 7 | 13 | 5 | 183 | 2.46 | −5.29 | 2.729 |
| Digoxin | 3 | 781 | 7 | 14 | 6 | 203 | 1.89 | −5.01 | 2.404 |
| Ouabain | 1 | 585 | 4 | 12 | 8 | 207 | −1.24 | −2.13 | 0.771 |
| PST-2238 | 0 | 375 | 1 | 4 | 3 | 74 | 3.27 | −4.31 | 8.610 |
In silico assessment of aqueous solubility (log S) suggested that most of the saponins could be defined as moderately to good soluble (Fig. 1). Log S value of saponins ranges from −5.97 to −3.44, which were significantly greater than the predicted apparent membrane permeability (Papp Caco-2 cell). However, the solubility was shown to decrease for saponins with fewer sugar moieties attached in the similar aglycon skeleton and similar observations have been reported on ginsenosides.80 The LogS values of similar skeleton of timosaponin B II and timosaponin A-III is −4.46 and −5.24, respectively which corresponds to their number of sugar count 3 and 2. On the other hand, most of the saponins have poor membrane permeability (Papp Caco-2 cell), while cardiotonic drugs have membrane permeability ranging from 0.197 × 10−6 cm/s to 8.610 × 10−6 cm/s suggesting the good membrane permeability and hence better bioavailability of the cardiotonic drugs compared to saponins. As shown in Fig. 1, almost all saponins have unfavorable physicochemical traits and violating the drug likeness rules, which could be influenced by the increasing number of sugar moieties. Also, cardiotonic drugs are not following all thresholds of key physicochemical properties for drug likeness. While on comparison of saponins with cardiotonic drugs, >90% cardiotonic drugs follow the optimal property ranges for nRotB (responsible for molecular flexibility), HBD, and lipophilicity (Log P). Saponins have shown very high molecular weight ranging from 741 Da to 1808 Da, high HBD count from 7 to 24, and high TPSA from 197 Å2 to 669 Å2. Further, on reducing the number of sugar moieties in saponins could decrease the hydrogen-bonding capacity (HBA and HBD), molecular mass as well as TPSA but also reduces their solubility (Fig. 1).
Fig. 1.
Relationship between the sugar substitution in cardioprotective saponins, drugs and their physicochemical properties that responsible for bioavailability.
5. Structure activity relationships (SAR)
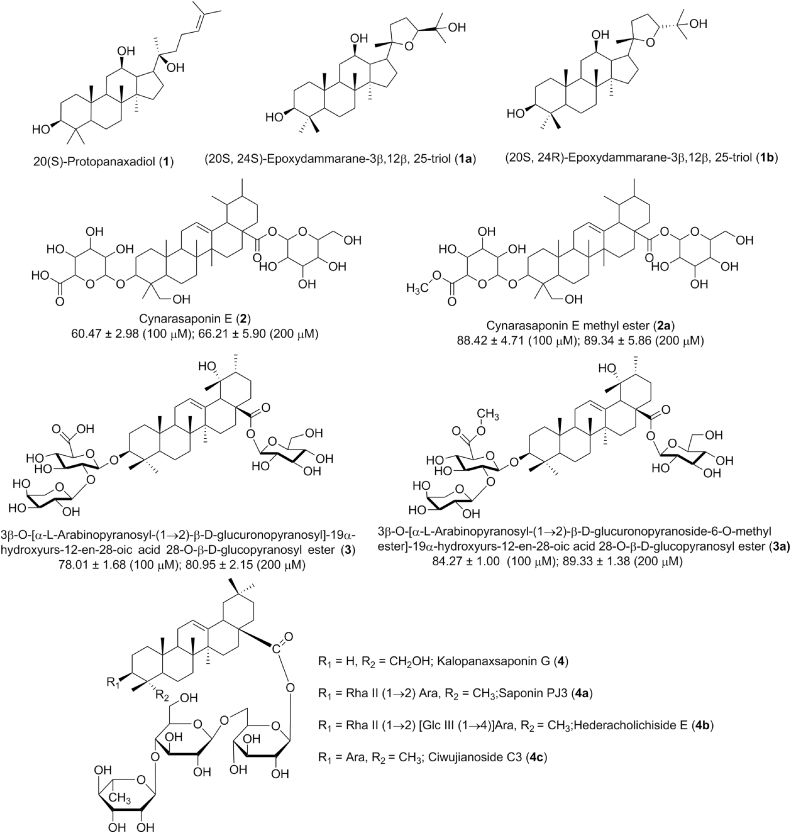
Saponins have been well studied for their cardioprotective potential along with their mechanism of action, while very few reports are available on discussing their SAR. Saponins are glycosides of triterpenoids or steroids and glycosidation makes saponins more complex and poor bioavailable. In few available reports, aglycon was found as better cardioprotective agents than their respective glycoside.43, 32 The importance of esterification of sugar moiety in saponin has discussed here with given parent and their respective ester derivatives in Fig. 2.
Fig. 2.
Structures of saponins for structure activity relationship (SAR).
Wang et al (2010) have discussed the structure function relationship of 20(S)-protopanaxadiol along with its two synthetic epimeric analogues (20S,24S)-epoxydammarane-3β,12β,25-triol and (20S,24R)-epoxydammarane-3β,12β,25-triol, and found that the configuration at C-24 position of furan ring was responsible for the cardioprotective effect of protopanaxadiol saponins against isoproterenol induced myocardial injury as shown in Fig. 2. Further, the 20(S)-protopanaxadiol (C24-25 alkene) and its 24(R)-epoxy epimeric derivatives were found to be active against myocardial ischemic injury by enhancing the antioxidant activity of heart tissues, whereas 24S-epoxy derivative was inactive due to the configuration of C-24 of furan ring.81 These SAR suggest the importance of stereochemistry of the saponins in cardioprotection. Another two separate studies on the glucuronopyranosyl methyl ester derivatives of ursane triterpenoid saponins of I. cornuta were found to have better cardioprotective effects than their parent non-methylated saponins against H2O2 induced cardiomyocyte injury along with increased cell viability at 25μM.35, 26 These results suggest that the esterification on glucuronic acid moiety at C-3 position of ursane saponins improve the cardioprotective effect of methylated saponins, as shown in Fig. 2. Sugar linkage position and residue numbers also played cardioprotective role e.g., anti-atherosclerosis activity of different ginsenosides, as mentioned in Table 1, ginsenoside Re having 2 sugar units showing better physicochemical properties versus ginsenoside Rg3 with 3 sugar moieties.22, 80 Another study by Zhang and coworkers on six bisdesmosidic triterpenoid saponins was found that the presence of a free hydroxyl group at C-3 position in bisdesmosidic saponin (kalopanaxsaponin G) plays an important role in cardioprotection.43 Whereas other bisdesmosidic saponins with reduced-cardioprotective activity despite having similar aglycon and sugar chain at C-28 further suggest that the chemical class and sequence of oligosaccharide chain at C-3 position played an important role in cardioprotection.
Recently, Quynh Vo et al have evaluated the SAR on the hemolytic potential of triterpenoid saponins and sapogenins, and suggest that not only the complexity of sugar moieties but also the types and stereochemical configurations of functional groups at different positions as well as the skeleton types (oleanane, ursane, and dammarane) are important structural features affecting the hemolytic potential. They found that the oleanane-type sapogenins had stronger hemolytic effects than those of the ursane and dammarane types as well as the presence of polar regions on sapogenins significantly enhanced hemolysis.82 Overall, the available SAR studies on saponins concluded that functionalization at aglycon as well as in sugar linkage position, derivatization of sugar moiety, and sugar residue number played an important role in the cardioprotection. However, investigation of the biological activity of natural saponins along with their semi-synthetic analogues in different bioassay models is required in depth understanding of the importance of functionalities, stereochemistry, number of sugar moieties, and their linkage position of saponins in cardioprotection.
6. Conclusions and future prospect
Present review summarizes the importance of naturally occurring saponins, their role in cardioprotection, bioavailability, and SAR. The cardioprotective effects of plant based saponins can be a good source of nutraceutical in life style diseases. The saponin enriched diets such as alfalfa, onion, garlic, soybean, and ginseng have been found to inhibit the intestinal absorption of cholesterol with reduction of serum cholesterol. Saponins such as astragaloside IV, gymnemic acid, and ophiopogonin D are effective in protecting heart from cardiotoxicity induced by anticancer drugs such as doxorubicin. Glycyrrhizin and ginsenosides from edible herbs can be used as a health supplement in routine intake for cardiac protection. DT-13, dioscin, astragaloside IV, glycyrrhizin, ophiopogonin D and trillin possess strong cardioprotective activity and need to be explored further in clinical applications. The ginsenosides Rg1, Re, Rb, Rc, and Rg3 have vasorelaxant activity by inhibiting Ca2+ influx via receptor-operated Ca2+ channels in vascular smooth muscle cells, and ginsenoside Rg3 emerges as the most potent vasodilatory agent to open the Ca2+ activated K+ channel. The in silico analyses have shown that most of the saponins have poor membrane permeability and unfavorable physicochemical properties, whereas cardiotonic drugs have shown the optimal membrane permeability and hence better bioavailability of the cardiotonic drugs compared to saponins.
In past few decades, several natural products derived cardioprotective agents have been evaluated in clinical trials and unfortunately very few are successful so far. Therefore, there is a need to further exploration of natural products in search of new cardioprotective agents in view of life style changes and global emergence of chronic diseases like cancer and diabetes. Besides having good pharmaceutical and nutraceutical value in lowering of serum cholesterol, there is no FDA approved saponin based drug available in the market so far. Although total saponins (in crude form) have been well explored for cardiac protection, very few reports on cardioprotection of pure saponin warrant further study. The search for pharmacologically active saponins will be useful in the development of dietary supplements as well as potential therapeutic agents against hyperlipidemia and drug induced cardiovascular disorders. Moreover, the potent antioxidant and cholesterol lowering effects of saponins across the literatures, further attract its attention as a preventive medicine for cardiovascular disorders.
Conflict of interest
The authors have no conflict of interest to declare for this publication.
Acknowledgement
The authors are thankful to the Director, CIMAP-CSIR, Lucknow, India.
Contributor Information
Deepika Singh, Email: deepika.sh25@yahoo.com.
Prabir Kumar Chaudhuri, Email: pkchaudhuri_2000@rediffmail.com.
References
- 1.World Health Organization (WHO) 2016. Cardiovascular diseases (CVDs) [Google Scholar]
- 2.Genser B., Silbernagel G., De Backer G., Bruckert E., Carmena R., Chapman M.J. Plant sterols and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2012;33:444–451. doi: 10.1093/eurheartj/ehr441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao D. Challenges associated with elucidating the mechanisms of the hypocholesterolaemic activity of saponins. J Funct Foods. 2016;23:52–65. [Google Scholar]
- 4.Shukla S.K., Gupta S., Ojha S.K., Sharma S.B. Cardiovascular friendly natural products: a promising approach in the management of CVD. Nat Prod Res. 2010;9:873–898. doi: 10.1080/14786410903417378. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad V.U., Noorwala M., Mohammad F.V., Sener B., Gilani A.H., Aftab K. Symphytoxide A, a triterpenoid saponin from the roots of Symphytum officinale. Phytochemistry. 1993;32:1003–1006. doi: 10.1016/0031-9422(93)85244-l. [DOI] [PubMed] [Google Scholar]
- 6.Luna-Vazquez F.J., Ibarra-Alvarado C., Rojas-Molina A., Rojas-Molina I., Zavala-Sanchez M.A. Vasodilator compounds derived from plants and their mechanisms of action. Molecules. 2013;18:5814–5857. doi: 10.3390/molecules18055814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mashour N.H., Lin G.I., Frishman W.H. Herbal medicine for the treatment of cardiovascular disease: clinical considerations. Arch Intern Med. 1998;158:2225–2234. doi: 10.1001/archinte.158.20.2225. [DOI] [PubMed] [Google Scholar]
- 8.Sugihara Y., Nojima H., Matsuda H., Murakami T., Yoshikawa M., Kimura I. Antihyperglycemic effects of gymnemic acid IV, a compound derived from Gymnema sylvestre leaves in streptozotocin-diabetic mice. J Asian Nat Prod Res. 2000;2:321–327. doi: 10.1080/10286020008041372. [DOI] [PubMed] [Google Scholar]
- 9.Ojewole J.A. Cardiovascular effects of mollic acid glucoside, a 1α-hydroxycycloartenoid saponin extractive from Combretum molle R Br ex Don (Combretaceae) leaf. Cardiovasc J Afr. 2008;19:128–134. [PMC free article] [PubMed] [Google Scholar]
- 10.Manivannan J., Shanthakumar J., Silambarasan T., Balamurugan E., Raja B. Diosgenin, a steroidal saponin, prevents hypertension, cardiac remodeling and oxidative stress in adenine induced chronic renal failure rats. RSC Adv. 2015;5:19337–19344. [Google Scholar]
- 11.Testai L., Martelli A., Cristofaro M., Breschi M.C., Calderone V. Cardioprotective effects of different flavonoids against myocardial ischaemia/reperfusion injury in Langendorff-perfused rat hearts. J Pharm Pharmacol. 2013;65:750–756. doi: 10.1111/jphp.12032. [DOI] [PubMed] [Google Scholar]
- 12.Muller L., Caris-Vevrat C., Lowe G., Bohm V. Lycopene and its antioxidant role in the prevention of cardiovascular diseases—a critical review. Crit Rev Food Sci Nutr. 2016;56:1868–1879. doi: 10.1080/10408398.2013.801827. [DOI] [PubMed] [Google Scholar]
- 13.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981–2014. J Nat Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 14.Lacaille-Dubois M.A., Wagner H. A review of the biological and pharmacological activities of saponins. Phytomedicine. 1996;4:363–386. doi: 10.1016/S0944-7113(96)80081-X. [DOI] [PubMed] [Google Scholar]
- 15.Lacaille-Dubois M.A., Wagner H. Bioactive saponins from plants: an update. Stud Nat Prod Chem. 2000;21:633–687. [Google Scholar]
- 16.Sparge S.G., Light M.E., Staden J.V. Biological activities and distribution of plant saponins. J Ethnopharmacol. 2004;94:219–243. doi: 10.1016/j.jep.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Obsbourn A., Goss R.J., Field R.A. The saponins-polar isoprenoids with important and diverse biological activities. Nat Prod Rep. 2011;28:1261–1268. doi: 10.1039/c1np00015b. [DOI] [PubMed] [Google Scholar]
- 18.Cheok C.Y., Salman H.A.K., Sulaiman R. Extraction and quantification of saponins: a review. Food Res Int. 2014;59:16–40. [Google Scholar]
- 19.Vicken J.P., Heng L., de Groot A., Gruppen H. Saponins, classification and occurrence in the plant kingdom. Phytochemistry. 2007;68:275–297. doi: 10.1016/j.phytochem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Gafni J., Munsch J.A., Lam T.H., Catlin M.C., Costa L.G., Molinski T.F. Xestospongins: potent membrane permeable blockers of the inositol 1,4,5-triphosphate receptor. Neuron. 1997;19:723–733. doi: 10.1016/s0896-6273(00)80384-0. [DOI] [PubMed] [Google Scholar]
- 21.Balandrin M.F. vol. 404. Plenum Press; New York: 1996. Commercial utilization of plant-derived saponins: an overview of medicinal, pharmaceutical, and industrial applications; pp. 1–14. (Saponins used in traditional and modern medicine: advances in experimental medicine and biology). [DOI] [PubMed] [Google Scholar]
- 22.Scott G.I., Colligan P.B., Ren B.H., Ren J. Ginsenosides Rb1 and Re decrease cardiac contraction in adult rat ventricular myocytes: role of nitric oxide. Br J Pharmacol. 2001;134:1159–1165. doi: 10.1038/sj.bjp.0704377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi Y., Han B., Yu X., Qu S., Sui D. Ginsenoside Rb3 ameliorates myocardial ischemia reperfusion injury in rats. Pharm Biol. 2011;49:900–906. doi: 10.3109/13880209.2011.554845. [DOI] [PubMed] [Google Scholar]
- 24.Haleagrahara N., Vakkey J., Chakravarthi S. Cardioprotective effects of glycyrrhizic acid against isoproterenol-induced myocardial ischemia in rats. Int J Mol Sci. 2011;12:7100–7113. doi: 10.3390/ijms12107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang R., Fang W., Han D., Sha L., Wei J., Liu L. Clematichinenoside attenuates myocardial infarction in ischemia/reperfusion injury both in vivo and in vitro. Plant Med. 2013;79:1289–1297. doi: 10.1055/s-0033-1350671. [DOI] [PubMed] [Google Scholar]
- 26.Li S., Zhao J., Liu Y., Chen Z., Xu Q., Khan I.A. New triterpenoid saponins from Ilex cornuta and their protective effects against H2O2-induced myocardial cell injury. J Agric Food Chem. 2014;62:488–496. doi: 10.1021/jf4046667. [DOI] [PubMed] [Google Scholar]
- 27.Butler M.S. Natural products to drugs: natural product-derived compounds in clinical trial. Nat Prod Rep. 2008;25:475–516. doi: 10.1039/b514294f. [DOI] [PubMed] [Google Scholar]
- 28.Dini I., Tenore G.C., Dini A. Saponins in Ipomoea batatas tubers: isolation, characterization, qualification and antioxidant properties. Food Chem. 2009;113:411–419. [Google Scholar]
- 29.Basu N., Rastogi R.P. Triterpenoid saponins and sapogenins. Phytochemistry. 1967;6:1249–1270. [Google Scholar]
- 30.Misra M.K., Sarwat M., Bhakuni P., Tuteja R., Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–RA219. [PubMed] [Google Scholar]
- 31.Liao Z., Yin D., Wang W., Zeng G., Liu D., Chen H. Cardioprotective effect of sasanquasaponin preconditioning via bradykinin–NO pathway in isolated rat heart. Phytother Res. 2009;23:1146–1153. doi: 10.1002/ptr.2767. [DOI] [PubMed] [Google Scholar]
- 32.Ren G., Qiao H.X., Yang J., Zhou C.X. Protective effects of steroids from Allium chinensis against H2O2-induced oxidative stress in rat cardiac H9c2 cells. Phytother Res. 2010;24:404–409. doi: 10.1002/ptr.2964. [DOI] [PubMed] [Google Scholar]
- 33.Kuroda M., Mimaki K., Kameyama A., Sashida Y., Nikaido T. Steroidal saponins from Allium chinense and their inhibitory activities on cyclic AMP phosphodiesterase and Na+/K+ ATPase. Phytochemistry. 1995;40:1071–1076. doi: 10.1016/0031-9422(95)00423-5. [DOI] [PubMed] [Google Scholar]
- 34.Tang Y.N., He X.C., Ye M., Huang H., Chen H.L., Peng W.L. Cardioprotective effect of total saponins from three medicinal species of Dioscorea against isoprenaline-induced myocardial ischemia. J Ethnopharmacol. 2015;175:451–455. doi: 10.1016/j.jep.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 35.Wang W., Zhao J., Li S., Lu Y., Liu Y., Xu Q. Five new triterpenoidal saponins from the roots of Ilex cornuta and their protective effects against H2O2-induced cardiomyocytes injury. Fitoterapia. 2014;99:40–47. doi: 10.1016/j.fitote.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Li C., Liu Z., Tian J., Li G., Jiang W., Zhang G. Protective roles of asperosaponin VI, a triterpene saponin isolated from Dipsacus asper Wall on acute myocardial infarction in rats. Eur J Pharmacol. 2010;627:235–241. doi: 10.1016/j.ejphar.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Li C., Gao Y., Tian J., Xing Y., Zhu H., Shen J. attenuates cardiac dysfunction, myocardial fibrosis in a rat model of chronic myocardial infarction. Food Chem Toxicol. 2012;50:1432–1438. doi: 10.1016/j.fct.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 38.Wang M., Meng X.B., Yu Y.L., SunGB, Xu X.D., Zhang X.P. Elatoside C protects against hypoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes through the reduction of endoplasmic reticulum stress partially depending on STAT3 activation. Apoptosis. 2014;19:1727–1735. doi: 10.1007/s10495-014-1039-3. [DOI] [PubMed] [Google Scholar]
- 39.Zhang S., Li H., Yang S.J. Tribulosin protects rat hearts from ischemia/perfusion injury. Acta Pharmacol Sin. 2010;31:671–678. doi: 10.1038/aps.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu J., Yang G., Zhu W., Wen W., Zhang F., Yuan J. Anti-atherosclerotic activity of platycodin D derived from roots of Platycodon grandiflorum in human endothelial cells. Biol Pharm Bull. 2012;35:1216–1221. doi: 10.1248/bpb.b-y110129. [DOI] [PubMed] [Google Scholar]
- 41.Wang T., Choi R.C., Li J., Li J., Bi C.W., Zang L. Antihyperlipidemic effect of protodioscin, an active ingredient isolated from the rhizomes of Dioscorea nipponica. Planta Med. 2010;76:1642–1646. doi: 10.1055/s-0030-1249960. [DOI] [PubMed] [Google Scholar]
- 42.Wang T., Choi R.C., Li J., Bi C.W., Ran W., Chen X. Trillin, a steroidal saponin isolated from the rhizomes of Dioscorea nipponica, exerts protective effects against hyperlipidemia and oxidative stress. J Ethnopharmacol. 2012;139:214–220. doi: 10.1016/j.jep.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 43.Zhang W., Wang X., Tang H., Wang M., Ji L. Triterpenoid saponins from Clematis tangutica and their cardioprotective activities. Fitoterapia. 2013;84:326–331. doi: 10.1016/j.fitote.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 44.Zhang W., Yao M.N., Tang H.F., Tian X.R., Wang M.C., Ji L.J. Triterpenoid saponins with anti-myocardial ischemia activity from the whole plants of Clematis tangutica. Planta Med. 2013;79:673–679. doi: 10.1055/s-0032-1328541. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W., Zhang C., Liu R., Li H., Zhang J., Mao C. Quantitative determination of Astragaloside IV, a natural product with cardioprotective activity, in plasma, urine and other biological samples by HPLC coupled with tandem mass spectrometry. J Chromatogr B. 2005;822:170–177. doi: 10.1016/j.jchromb.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 46.Zhang W.D., Chen H., Zhang C., Liu H.L., Chen H.Z. Astragaloside IV from Astragalus membranaceus shows cardioprotection during myocardial ischemia in vivo and in vitro. Planta Med. 2006;72:4–8. doi: 10.1055/s-2005-873126. [DOI] [PubMed] [Google Scholar]
- 47.Qian J., Jiang F., Wang B., Yu Y., Zhang X., Yin Z. Ophiopogonin D prevents H2O2-induced injury in primary human umbilical vein endothelial cells. J Ethnopharmacol. 2010;128:438–445. doi: 10.1016/j.jep.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y.Y., Meng C., Zhang X.M., Yuan C.H., Wen M.D., Chen Z. Ophiopogonin D attenuates doxorubicin-induced autophagic cell death mitochondrial damage in vitro and in vivo. J Pharmacol Exp Ther. 2015;352:166–174. doi: 10.1124/jpet.114.219261. [DOI] [PubMed] [Google Scholar]
- 49.Wang M., Sun G.B., Zhang J.Y., Luo Y.L., Xu X.D., Meng X.B. Elatoside C protects heart from ischemia/reperfusion injury through the modulation of oxidative stress and intracellular Ca2+ homeostasis. Int J Cardiol. 2015;185:167–176. doi: 10.1016/j.ijcard.2015.03.140. [DOI] [PubMed] [Google Scholar]
- 50.Liang Q., Yu X., Qu S., Xu H., Sui D. Acanthopanax senticosides B ameliorates oxidamage induced by hydrogen peroxide in cultured neonatal rat cardiomyocytes. Eur J Pharmacol. 2010;627:209–215. doi: 10.1016/j.ejphar.2009.10.055. [DOI] [PubMed] [Google Scholar]
- 51.Lai W., Wu Z., Lin H., Li T., Sun L., Chai Y. Anti-ischemia steroidal saponins from the seeds of Allium fistulosum. J Nat Prod. 2010;73:1053–1057. doi: 10.1021/np900815p. [DOI] [PubMed] [Google Scholar]
- 52.Stamm C., del Nido P.J. Protein kinase C and myocardial calcium handing during ischemia and reperfusion: lesions learned using Rhod-2-spectrofluorometry. Thorac Cardiovasc Surg. 2004;52:127–134. doi: 10.1055/s-2004-817978. [DOI] [PubMed] [Google Scholar]
- 53.Chen H.F., Wang N.L., Sun H.L., Yang B.F., Yao X.S. Novel furostanol saponins from the bulbs of Allium macrostemon B. and their bioactivity on [Ca2+]i increase induced by KCl. J Asian Nat Prod Res. 2006;8:21–28. doi: 10.1080/10286020500172533. [DOI] [PubMed] [Google Scholar]
- 54.Ning Z., Li Y., Zhang R. Effects of methyl protodioscin on [Ca2+]i and ATPase activity in cardiomyocytes and analysis of mechanisms. Zhongguo Zhong Yao Za Zhi. 2010;35:80–83. [PubMed] [Google Scholar]
- 55.Tao J., Wang H., Zhou H., Li S. The saponin monomer of dwarf lilyturf tuber, DT-13 reduces L-type calcium currents during hypoxia in adult rat ventricular myocytes. Life Sciences. 2005;77:3021–3030. doi: 10.1016/j.lfs.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 56.Tao J., Wang H., Chen J., Xu H., Li S. Effects of saponin monomer 13 of dwarf lilyturf tuber L-type calcium currents in adult rat ventricular myocytes. Am J Chin Med. 2005;33:797–806. doi: 10.1142/S0192415X05003417. [DOI] [PubMed] [Google Scholar]
- 57.Sharove V.G., Sabbah H.N., Shimovama H., Goussey A.V., Lesch M., Goldstein S. Evidence of cardiocytes apoptosis in myocardium of dogs with chronic heart failure. Am J Pathol. 1996;148:141–149. [PMC free article] [PubMed] [Google Scholar]
- 58.Pathan R.A., Bhandari U., Javed S., Nag T.C. Anti-apoptotic potential of gymnemic acid phospholipid complex pretreatment in Wistar rats with experimental cardiomyopathy. Indian J Exp Biol. 2012;50:117–127. [PubMed] [Google Scholar]
- 59.Sun W., Li H., Yang S.J. A triterpene saponin from Tribulus terrestris attenuates apoptosis in cardiocyte via activating PKC signaling transduction pathway. J Asian Nat Prod Res. 2008;10:39–48. doi: 10.1080/10286020701275846. [DOI] [PubMed] [Google Scholar]
- 60.Qin J., Kang Y., Xu Z., Zang C., Fang B., Liu X. Dioscin prevents the mitochondrial apoptosis and attenuates oxidative stress in cardiac H9c2 cells. Drug Res (Stuttg) 2014;64:47–52. doi: 10.1055/s-0033-1349101. [DOI] [PubMed] [Google Scholar]
- 61.Li H., Huang W., Wen Y., Gong G., Zhao Q., Yu G. Anti-thrombotic activity and chemical characterization of steroidal saponins from Dioscorea zingiberensis C.H. Wright. Fitoterapia. 2010;81:1147–1156. doi: 10.1016/j.fitote.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 62.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 63.Chai H., Wang Q., Huang L., Xie T., Fu Y. Ginsenoside Rb1 inhibits tumor necrosis factor-alpha-induced vascular cell adhesion molecule-1 expression in human endothelial cells. Biol Pharm Bull. 2008;31:2050–2056. doi: 10.1248/bpb.31.2050. [DOI] [PubMed] [Google Scholar]
- 64.Li H., Wang Q.J., Zhu D.N., Yang Y. Reinioside C, triterpene saponin of Polygala aureocauda Dunn, exerts hypolipidemic effect on hyperlipidemic mice. Phytother Res. 2008;22:159–164. doi: 10.1002/ptr.2262. [DOI] [PubMed] [Google Scholar]
- 65.Bai Y.P., Hu C.P., Chen M.F., Xu K.P., Tan G.S., Shi R.-Z. Inhibitory effect of reinioside C on monocyte-endothelial cell adhesion induced by oxidized low-density lipoprotein via inhibiting NADPH oxidase/ROS/NF-B pathway. Naunyn-Schmied Arch Pharmacol. 2009;380:399–406. doi: 10.1007/s00210-009-0450-8. [DOI] [PubMed] [Google Scholar]
- 66.Yamada K., Tokunaga Y., Ikeda A., Ohkura K., Kaku-Ohkura S., Mamiya S. Effect of dietary fiber on the lipid metabolism and immune function of aged Sprague-Dawley rats. Biosci Biotechnol Biochem. 2003;67:429–433. doi: 10.1271/bbb.67.429. [DOI] [PubMed] [Google Scholar]
- 67.Kamkaew N., Scholfield C.N., Ingkaninan K., Maneesai P., Parkington H.C., Tare M. Bacopa monnieri and its constituents is hypotensive in anaesthetized rats and vasodilator in various artery types. J Ethanopharmacol. 2011;137:790–795. doi: 10.1016/j.jep.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 68.Enomoto Y., Ito K., Kawagoe Y., Morio Y., Yaasaki Y. Positive inotropic action of saponins on isolated atrial and papillary muscles from the guinea-pig. Br J Pharmac. 1986;88:259–267. doi: 10.1111/j.1476-5381.1986.tb09494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Achenbach H., Hubner H., Brandt W., Reiter M. Cardioprotective steroid saponins and other constituents from the aerial parts of Tribulus cistoides. Phytochemistry. 1994;35:1527–1543. doi: 10.1016/s0031-9422(00)86890-9. [DOI] [PubMed] [Google Scholar]
- 70.Yu L.C., Chen S.C., Chang W.C., Huang Y.C., Lin K.M., Lai P.H. Stability of angiogenic agents, gensenoside Rg1 and Re, isolated from Panax ginseng: in vitro and in vivo studies. Int J Pharm. 2007;328:168–176. doi: 10.1016/j.ijpharm.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 71.Hui Z., Sha D.J., Wang S.L., Li C.S., Qian J., Wang J.Q. Panaxtriol saponins promotes angiogenesis and enhances cerebral perfusion after ischemic stroke in rats. BMC Complement Altern Med. 2017;17:70. doi: 10.1186/s12906-017-1579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cheung L.W.T., Leung K.W., Wong C.K.C., Wong R.N.S., Wong A.S.T. Ginsenoside-Rg1 induces angiogenesis via non-genomic crosstalk of glucocorticoid receptor and fibroblast growth factor receptor-1. Cardiovasc Res. 2011;89:419–425. doi: 10.1093/cvr/cvq300. [DOI] [PubMed] [Google Scholar]
- 73.Bin-rui Y., Si-jia H., Ming-Yuen Lee S., Wei-hong C., Jian-bo W., Zhe-rui Z. Pro-angiogenic activity of Notoginsenoside R1 in human umbilical vein endothelial cells in vitro and in a chemical-induced blood vessel loss model of Zebrafish in vivo. Chin J Integr Med. 2016;22:420–429. doi: 10.1007/s11655-014-1954-8. [DOI] [PubMed] [Google Scholar]
- 74.Carrasco O.F., Vidrio H. Endothelium protectant and contractile effects of the antivaricose principle escin in rat aorta. Vasc Pharmacol. 2007;47:68–73. doi: 10.1016/j.vph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 75.Shin J.H., Kwon H.W., Cho H.-J., Rhee M.H., Park H.-J. Vasodilator-stimulated phosphoprotein-phosphorylation by ginsenoside Ro inhibits fibrinogen binding to IIb/b3 in thrombin-induced human platelets. J Ginseng Res. 2016;40:359–365. doi: 10.1016/j.jgr.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhou Z., Li D., Zhou H., Lin X., Li C., Tang M. Plants and their bioactive compounds with the potential to enhance mechanism of inherited cardiac regeneration. Planta Med. 2015;81:637–647. doi: 10.1055/s-0035-1545946. [DOI] [PubMed] [Google Scholar]
- 77.Liang H.C., Chen C.T., Chang Y., Huang Y.C., Chen S.C., Sung H.W. Loading of a novel angiogenic agent, ginsenoside Rg1 in an acellular biological tissue for tissue regeneration. Tissue Eng. 2005;5–6:835–846. doi: 10.1089/ten.2005.11.835. [DOI] [PubMed] [Google Scholar]
- 78.Yu K., Chen F., Li C. Absorption, disposition, and pharmacokinetics of saponins from Chinese medicinal herbs: what do we know and what do we need to know more? Curr Drug Metab. 2012;13:577–598. doi: 10.2174/1389200211209050577. [DOI] [PubMed] [Google Scholar]
- 79.Meanwell N.A. Improving drug candidates by design: a focus on physicochemical properties as a means of improving compound disposition and safety. Chem Res Toxicol. 2011;24:1420–1456. doi: 10.1021/tx200211v. [DOI] [PubMed] [Google Scholar]
- 80.Liu H., Yang J., Du F., Gao X., Ma X., Huang Y. Absorption and disposition of ginsenosides after oral administration of Panax notoginseng extract to rats. Drug Metab Dispos. 2009;37:2290–2298. doi: 10.1124/dmd.109.029819. [DOI] [PubMed] [Google Scholar]
- 81.Wang T., Meng Q., Zhang J., Bi Y., Jiang N. Study on the structure–function relationship of 20(S)-panaxadiol and its epimeric derivatives in myocardial injury induced by isoproterenol. Fitoterapia. 2010;81:783–787. doi: 10.1016/j.fitote.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 82.Quynh Vo N.N., Fukushima E.O., Muranaka T. Structure and hemolytic activity relationships of triterpenoid saponins and sapogenins. J Nat Med. 2017;71:50–58. doi: 10.1007/s11418-016-1026-9. [DOI] [PubMed] [Google Scholar]