Abstract
Background
Low concentration of trace elements has been associated with poor prognosis and mortality in HIV infection.
Methods
A cross sectional study was conducted among 100 HIV-infected subjects (70 were on ART treatment, while 30 were ART naïve). Fifty (50) apparently healthy controls were enrolled. Concentration of serum levels of zinc and copper was done using atomic absorption spectrometric method, while complete blood count was determined using automated blood analyzer. CD4+ T-cell count was done using cyflow cytometer.
Aim and setting
The aim of this study was to investigate the level of some trace elements and some hematological parameters of HIV-seropositive subjects attending University of Calabar Teaching Hospital Clinic as well as prevalence of trace elements deficiency and anemic status and compare same with HIV-seronegative control.
Results
Mean serum zinc, CD4+ T-cell count, Hb, PCV, RBC, MXD, were significantly (p < 0.05) reduced in the HIV-infected subjects, while copper/zinc ratio, MCV, MCH and platelet count were significantly (p < 0.05) raised in the HIV-infected subjects. The serum Cu level was comparable (p > 0.05) with the control. ART treatment had no effect on all the parameters assessed except CD4+ T-cell count. Twenty five percent (25%), 3% and 56% of the HIV-infected subjects were zinc deficient, copper deficient and anemic, respectively. Gender was found as a predictor of zinc deficiency. Copper and zinc showed weak positive correlation with CD4+ T-cell count.
Conclusion
ART treatment did not complement zinc status in HIV infection while improving CD4+ T-cell count, hence the need to consider supplementation.
Keywords: Anemia, CD4 count, HIV, Serum zinc, Serum copper
1. Introduction
Trace elements are micronutrients that are essential for normal body metabolism.1 They are required in minute amount by living organisms. They are essential for the host defense against infection2 and act as activators in controlling biological functions.3 Changes in the levels of micronutrients and its effects have been described in inflammatory responses, cancer cases, as well as parasitic and viral infections.4, 5, 6 The human immune function has been reported to depend on nutritional status.7, 8 Serum trace elements such as copper and zinc have been reported to be useful in the diagnosis of viral hepatic disease.9
Zinc is an integral part of more than 200 enzymes (metallo-enzymes) and play a crucial role in nucleic acid metabolisms, cell replication, tissue repairs, and growth. On one hand, its deficiency leads to severe alteration of the thymic function and subsequent loss of T-cell-mediated responses and increased susceptibility to infectious diseases.10, 11 On the other hand, copper functions as a scavenger of free radicals in biological membranes and structures via its presence in cytosolic erythrocyte superoxide dismutases.12
HIV/AIDS, despite the campaigns, has remained a public health concern in Sub-Saharan Africa, where an estimated 25.8 million adults and children are infected.13 HIV patients have a large variety of physiological alterations at every level of the disease. These complications in synergy with related pathologies give rise to different nutritional problems.14
The present study sets off to investigate the usefulness of copper, zinc, copper–zinc relationship and some hematological parameters in managing HIV/AIDS patients.
2. Methods
2.1. Study design and subjects
In this cross-sectional study, a total of 150 subjects were enrolled. Seventy (70) of these were HIV-infected subjects on anti-retroviral therapy (ART), while 30 were HIV ART naïve subjects attending the HIV clinic, University of Calabar Teaching Hospital. Fifty (50) apparently healthy HIV seronegative individuals were recruited as controls. All subjects were residents in Calabar, Cross River State, Southern Nigeria. All subjects were between the age range of 18–65 years. Ethical approval was obtained from the University of Calabar Teaching Hospital Medical Ethics Committee. Informed consent was obtained from the subjects prior to the study.
2.2. Blood collection
Blood samples were collected from the patients after overnight fasting via the antecubital vein and separated into EDTA and plain containers for complete blood count/CD4+T cell count and micronutrient assay respectively. The blood samples in plain container were centrifuged at 3500 × g for 10 minutes to obtain serum. The complete blood count and CD4+ T-cell count were analyzed immediately while the sera for copper and zinc analysis were frozen at −20°C and transported under cold chain to international Institute of Tropical Agriculture, Ibadan, Oyo State, Nigeria for analysis.
2.3. Complete blood count and CD4+ T-cell assay
The complete blood count was performed using Sysmex KX-21N by Sysmex Corporation Kobe, Japan. The analysis was done following the manufacturers instruction. CD4+ T-cell count was analyzed using Partex cyflow cytometer by Partec Cyflow, Germany. After booting the machine, 20 mL of CD4+ T-cell count PEmAb was added to a Rohren tube followed by 20 μL of well-mixed EDTA blood sample. Both were mixed and incubated in the dark for 15 minutes at room temperature. This was followed by addition of 800 μL of the CD4+ T-cell count buffer. The mixtures were mixed and read on the cyflow by plugging the sample tube to the sample port of the cyflow.
2.4. Determination of trace elements
The trace elements were assayed using atomic absorption spectrophotometric method using atomic absorption spectrophotometer by Buck Scientific (Model 205), USA. One thousand microliter of the serum were pipetted into the test tubes followed by equal volume of 10% trichloroacetic acid (TCA). Paraffin was used to cover the top of the tubes which after mixing were left to stand for 10 minutes. The tubes were than centrifuged. The supernatants were then transferred to another tube using Pasteur pipette where they were then aspirated into the spectrophotometer. Wave length of 324.8 nm and 213.9 nm were used for copper and zinc estimations, respectively.15 All other settings were operated under conditions recommended by the manufacturers.
2.5. Statistical analysis
Data were analyzed using SPSS version 22 Package (SPCC Inc., Chicago, IL, USA). Categorical data were summarized into frequency and proportions while continuous data were expressed as means and standard deviations. One sample Kolmogorov–Smirnov test was used to assess the normality of the data. The complete blood count and trace element values were normally distributed in both the test and control subjects, hence parametric procedure. Comparison of the hematological parameters and serum trace elements between the test and control were performed using independent t-test while comparison among the various age ranges were performed using one-way ANOVA. Logistic regression was used to determine factors associated with copper and zinc deficiencies. p-values less than 0.05 (p < 0.05) were considered statistically significant.
3. Result
The mean age of the HIV-infected subjects was 35.97 ± 10.32 years, while that of the control was 29.84 ± 7.38 years. Thirty five percent (35%) of the HIV-infected subjects were males while the females constituted 65%. Of the 100 HIV-infected subjects analyzed for zinc, copper, and hematological parameters, 25(25%), 3(3%), and 56(56%) were zinc deficient, copper deficient and anemic, respectively. Only 9% of the controls were anemic (Table 1). The highest prevalence of zinc deficiency 17/35 (48.57%) was found among the males while the highest prevalence of copper deficiency 2/65 (3.08%) was found among the females (Table 5).
Table 1.
Demographic, Clinical and Trace Element Data of HIV-infected Subjects and Healthy Control
| Parameter | HIV subjects | Control |
|---|---|---|
| n = 100 | n = 50 | |
| Age | 35.97 ± 10.32a | 29.84 ± 7.38a |
| Gender | ||
| Male | 35 (35.00) | 27 (54.0) |
| Female | 65 (65.00) | 23 (46.0) |
| Zinc deficiencyb | 25 (25.00) | 0 (0.00) |
| Copper deficiencyc | 3 (3.00) | 0. (0.00) |
| Anemiad | 56 (56.00) | 9 (9.00) |
Mean ± S.D.
Cutoff according to National Health and Nutrition Examination Survey II.
Cutoff according to Schneider et al (2017).
Cutoff according to WHO Report (1989).38
Values in parenthesis () represents percentage (%).
Table 5.
Factors Associated With Zinc and Copper Deficiencies in HIV Subjects
| Parameters | N. deficient (%) | Odd ratio | 95% CI | p-value |
|---|---|---|---|---|
| Zinc | ||||
| Continuous variables | ||||
| Age | 0.888 | 0.764–1.032 | 0.121 | |
| Hb | 0.714 | 0.296–1.720 | 0.452 | |
| CD4 | 1.003 | 0.996–1.011 | 0.426 | |
| Categorical variables | ||||
| Male | 17 (48.57) | 0.191 | 0.72–0.503 | 0.001 |
| Female | 8 (12.31) | |||
| Anemic | 13 (23.21) | 1.175 | 0.474–2.911 | 0.728 |
| Nonanemic | 12 (27.27) | |||
| CD4 < 200cells/mL | 9 (28.13) | 0.786 | 0.303–2.039 | 0.621 |
| CD4 > 200 cells/mL | 16 (23.53) | |||
| Copper | ||||
| Continuous variables | ||||
| Age | 1.022 | 0.956–1.092 | 0.530 | |
| Hb | 0.885 | 0.586–1.337 | 0.563 | |
| CD4 | 1.001 | 0.998–1.004 | 0.692 | |
| Categorical variables | ||||
| Male | 1 (2.86) | 1.085 | 0.254–4.630 | 0.913 |
| Female | 2 (3.08) | |||
| Anemic | 3 (5.36) | 0.583 | 0.137–2.477 | 0.465 |
| Nonanemic | 0 (0.00) | |||
| CD4 < 200 cells/mL | 1 (3.13) | 0.935 | 0.218–4.005 | 0.928 |
| CD4 > 200 cells/mL | 2 (2.94) | |||
N = absolute number; Hb = hemoglobin; CD = cluster of differentiation.
Table 2 shows copper, zinc, CD4+ T-cell count and some hematological parameters of HIV-infected subjects and the seronegative control. The CD4+ T-cell count, hemoglobin (Hb), packed cell volume (PCV), Red blood cell count (RBC), mean cell hemoglobin (MCH), platelet count, mixed cells (MXD), zinc, Cu/Zn ratio differ significantly between HIV-infected subjects and seronegative control (p < 0.05). Mean serum zinc, CD4+ T-cell count, Hb, PCV, RBC, and MXD, were significantly (p < 0.05) reduced in the HIV-infected subjects, while copper/zinc ratio, MCV, MCH, and platelet count were significantly (p < 0.05) raised in the HIV-infected subjects. The serum Cu level was comparable (p > 0.05) with the control.
Table 2.
Zinc, Copper, CD4+ T-cells Count and Hematological Parameters of HIV-infected and Control Subjects.
| Parameters | Subjects |
p-value | |
|---|---|---|---|
| HIV Subjects | Control subjects | ||
| N = 100 | N = 50 | ||
| Mean ± S.D. | Mean ± S.D. | ||
| Hematology | |||
| CD4 (cells/mL) | 332.28 ± 228.87 | 784.04 ± 283.27 | <0.01 |
| Hb (g/dL) | 12.10 ± 1.95 | 13.75 ± 1.62 | <0.01 |
| PCV (%) | 38.05 ± 5.50 | 43.49 ± 4.78 | <0.01 |
| MCV (fL) | 96.41 ± 11.09 | 89.18 ± 6.57 | <0.01 |
| RBC (×1012/L) | 4.02 ± 0.64 | 4.91 ± 0.66 | <0.01 |
| MCH (pg) | 30.40 ± 4.76 | 28.23 ± 2.59 | <0.01 |
| MCHC (g/dL) | 31.29 ± 1.91 | 31.63 ± 1.19 | 0.19 |
| WBC (×109/L) | 4.81 ± 1.34 | 5.16 ± 1.16 | 0.10 |
| Platelet (×109/L) | 241.24 ± 83.24 | 212.72 ± 62.10 | 0.02 |
| NEUT (×109/L) | 2.22 ± 0.99 | 2.18 ± 0.87 | 0.81 |
| LYM (×109/L) | 2.06 ± 0.74 | 2.34 ± 0.60 | 0.14 |
| MXD (×109/L) | 0.52 ± 0.45 | 0.67 ± 0.31 | 0.02 |
| Trace elements | |||
| Zinc (μg/dL) | 73.94 ± 10.18 | 121.49 ± 9.86 | <0.01 |
| Copper (μg/dL) | 112.60 ± 9.47 | 106.88 ± 9.81 | 0.36 |
| Cu/Zn ratio | 1.89 ± 1.65 | 0.93 ± 1.11 | 0.03 |
N = absolute number; Hb = hemoglobin; PCV = packed cell volume; MCV = mean corpuscular volume; RBC = red blood cell; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; WBC = white blood cell; NEUT = neutrophil; LYM = lymphocyte; MXD = mixed cell; CD = cluster of differentiation.
Table 3 shows zinc, copper, and CD4+T-cell count and some hematological parameters of HIV-infected subjects on ART and HIV-infected ART naïve subjects. All parameters did not differ significantly (p > 0.05) excluding MCV and MCH (that where significantly higher in the ART group) and RBC and neutrophil count (that were significantly lower in the ART group).
Table 3.
Zinc, Copper, CD4+T-cells Count and Hematological Parameters of HIV-infected Patients on Anti-retroviral Therapy and Those not on Anti-retroviral Therapy
| Parameters | HIV subjects |
p-value | |
|---|---|---|---|
| HIV subjects on ART | HIV ART-naïve subjects | ||
| (N = 70) | (N = 30) | ||
| Mean ± SD | Mean ± SD | ||
| Hematology | |||
| CD4 (cells/mL) | 336.11 ± 215.97 | 323.33 ± 252.81 | 0.81 |
| Hb (g/dL) | 12.17 ± 1.93 | 11.93 ± 1.96 | 0.58 |
| PCV (%) | 38.54 ± 4.93 | 36.89 ± 6.40 | 0.22 |
| MCV (fL) | 98.65 ± 11.13 | 91.19 ± 8.81 | 0.00 |
| RBC (×1012/L) | 3.93 ± 0.58 | 4.23 ± 0.73 | 0.05 |
| MCH (pg) | 31.23 ± 4.92 | 28.47 ± 3.65 | <0.01 |
| MCHC (g/dl) | 31.44 ± 1.88 | 30.96 ± 1.92 | 0.26 |
| WBC (×109/L) | 4.76 ± 1.4 | 4.92 ± 1.16 | 0.56 |
| Platelet (×109/L) | 237.54 ± 79.34 | 249.87 ± 89.83 | 0.52 |
| NEUT(×109/L) | 2.09 ± 0.98 | 2.54 ± 0.93 | 0.04 |
| LYM (×109/L) | 2.13 ± 0.79 | 1.90 ± 0.57 | 0.11 |
| MXD (×109/L) | 0.51 ± 0.48 | 0.56 ± 0.36 | 0.57 |
| Trace elements | |||
| Zinc (μg/dL) | 74.76 ± 10.04 | 72.02 ± 10.25 | 0.23 |
| Copper (μg/dL) | 112.57 ± 9.10 | 112.62 ± 10.11 | 0.98 |
| Cu/Zn ratio | 1.87 ± 0.17 | 1.93 ± 1.6 | 0.208 |
N = absolute number; Hb = hemoglobin; PCV = packed cell volume; MCV = mean corpuscular volume; RBC = red blood cell; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; WBC = white blood cell; NEUT = neutrophil; LYM = lymphocyte; MXD = mixed cell; CD = cluster of differentiation.
Table 4 shows the zinc, copper, and CD4+ T-cell count of the HIV-seropositive subjects based on duration of treatment. The values did not differ significantly (p > 0.05) among the various durations of treatments.
Table 4.
Zinc, Copper, CD4+ T-cells and Some Hematological Parameters of HIV-infected Subjects Based on Duration of Treatment
| Parameters | Duration of treatment |
p-value | |||
|---|---|---|---|---|---|
| 0–2 years | 3–5 years | 6–8 years | 9–12 years | ||
| (N = 31) | (N = 24) | (N = 12) | (N = 3) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | ||
| Hematology | |||||
| CD4 (cells/mL) | 278.58 ± 171.25 | 355.04 ± 262.97 | 430.25 ± 200.38 | 402.67 ± 247.13 | 0.04 |
| Hb (g/dL) | 12.51 ± 1.82 | 11.95 ± 1.28 | 12.03 ± 1.28 | 10.94 ± 0.33 | 0.49 |
| PCV (%) | 39.50 ± 4.81 | 38.00 ± 5.83 | 38.03 ± 3.00 | 35.00 ± 0.94 | 0.38 |
| MCV (fL) | 100.26 ± 10.11 | 96.46 ± 12.53 | 100.33 ± 10.86 | 92.93 ± 5.57 | 0.47 |
| RBC (×1012/L) | 3.95 ± 0.60 | 3.98 ± 0.65 | 3.82 ± 0.40 | 3.77 ± 0.16 | 0.82 |
| MCH (pg) | 27.58 ± 4.67 | 30.43 ± 5.47 | 31.88 ± 4.74 | 29.13 ± 1.55 | 0.63 |
| MCHC (g/dL) | 31.48 ± 11.92 | 31.33 ± 11.35 | 31.58 ± 10.37 | 31.33 ± 10.17 | 0.98 |
| WBC (×109/L) | 4.70 ± 1.58 | 4.81 ± 1.42 | 4.61 ± 0.83 | 5.53 ± 1.61 | 0.78 |
| Platelet (×109/L) | 233.32 ± 75.19 | 246.76 ± 78.11 | 240.33 ± 74.95 | 196 ± 76.38 | 0.75 |
| NEUT (×109/L) | 2.06 ± 0.94 | 2.11 ± 1.16 | 1.93 ± 0.53 | 2.87 ± 201.78 | 0.53 |
| LYM (×109/L) | 2.11 ± 0.80 | 2.04 ± 0.75 | 2.40 ± 0.72 | 2.03 ± 0.90 | 0.63 |
| MXD (×109/L) | 0.35 ± 0.55 | 0.57 ± 0.43 | 0.3 ± 0.34 | 0.63 ± 0.24 | 0.41 |
| Trace elements | |||||
| Zinc (μg/dL) | 77.40 ± 10.60 | 72.56 ± 8.98 | 72.72 ± 9.83 | 73.10 ± 11.66 | 0.28 |
| Copper (μg/dL) | 115.28 ± 4.35 | 101.80 ± 9.00 | 99.70 ± 9.56 | 97.7 ± 9.56 | 0.17 |
| Zn/Cu ratio | 1.74 ± 0.17 | 1.70 ± 0.16 | 1.67 ± 0.18 | 1.69 ± 0.19 | 0.90 |
N = absolute number; Hb = hemoglobin; PCV = packed cell volume; MCV = mean corpuscular volume; RBC = red blood cell; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; WBC = white blood cell; NEUT = neutrophil; LYM = lymphocyte; MXD = mixed cell; CD = cluster of differentiation.
Table 5 shows factors associated with zinc and copper deficiencies when the variables were controlled by using logistic regression. Gender was the only variable shown to be statistically significant as explanatory to zinc deficiency, while none of the variables had an association with copper deficiency.
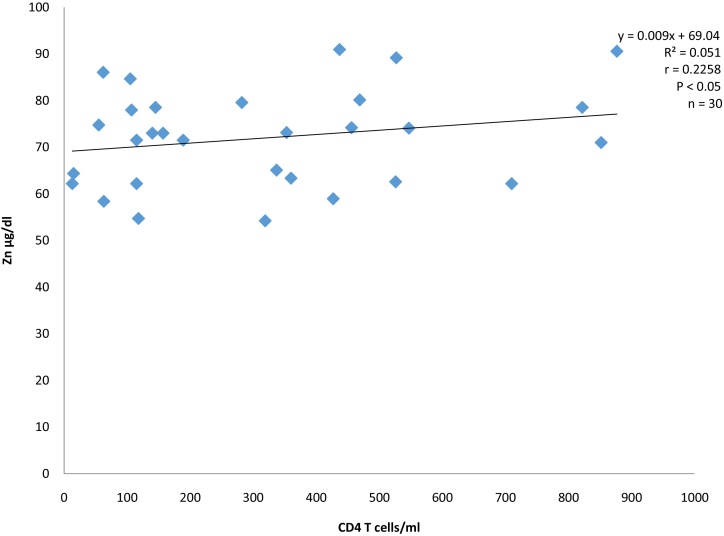
Serum zinc level showed a weak positive correlation (r = 0.226) with CD4+T-cell count (Fig. 1).
Fig. 1.
Correlation between Zinc and CD4 T cells of HIV infected anti-retroviral drugs naïve subjects.
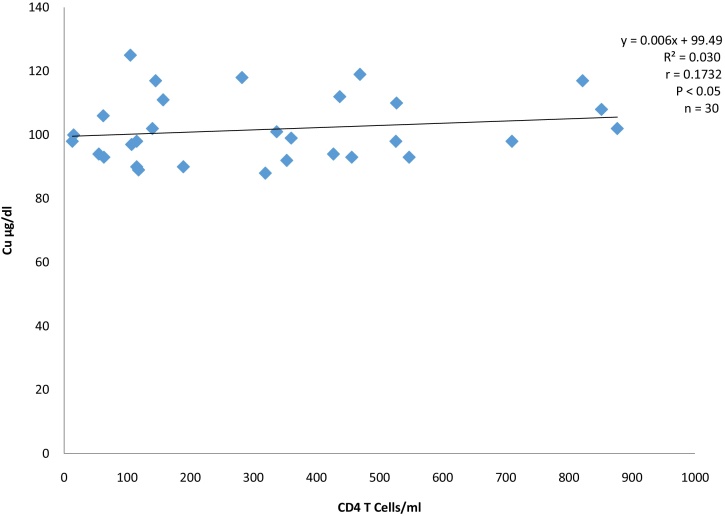
Serum copper level also showed weak positive correlation (r = 0.173) with CD4+T-cell count (Fig. 2).
Fig. 2.
Correlation between Copper and CD4 T cells of HIV infected anti-retroviral drugs naïve subjects.
4. Discussion
The present study demonstrated that HIV-infected subjects had significantly lower zinc concentration when compared with the control subjects. This observation is in agreement with previous reports.16, 17 This finding may be attributed to high demand in zinc because HIV nucleocapsid and integrase proteins that are essential for assembly of infectious virions contain zinc fingers that require zinc for normal structure and functioning.18, 19, 20 Zinc deficiency is associated with impaired immune function21, 22 and an increased susceptibility to infection. This assertion could be corroborated by the weak positive correlation of copper to CD4+T-cell count observed in this study. More so, zinc deficiency may result due to malnutrition. One of the factors responsible for malnutrition in an HIV-infected person is reduced appetite, which could be due to difficulty in ingesting food as a result of infections such as oral thrush or oesophagitis caused by Candida, a common opportunistic infection in HIV-infected people and fever, side effects of medicines, or depression. Poor absorption of nutrients may be due to accompanying diarrhea which may be because of bacterial infections such as Salmonella or Mycobacterium avium intercellular; viral such as CMV or parasitic infections such as Giardia, Cryptosporidium parvum, and Enterocytozoon bieneusi; due to nausea/vomiting as a side effect of medications used to treat HIV or opportunistic infections. About 30–50% of HIV patients in developed and nearly 90% in developing countries complain of diarrhea and malabsorption.23 However, this finding is at variance with report of Ndagije et al24 who reported no significant difference in the zinc status of HIV-infected children and non HIV-infected children. Although the exact reason for this disparity is not clear, but it may be related to the design of the study as both the HIV-infected and non HIV-infected children were malnourished without further stratification to determine the contributing role of disease progression and malnutrition to the zinc status. This study recorded zinc deficiency prevalence of 25% using cutoff value of <70 μg/dL.25 This finding is similar to previous report of 23% and 20% from studies in Germany26 and South Africa,27 respectively, using 12.5 μmol/L (81.63 μg/dL) and 10.7 μmol/L (69.87 μg/dL) cut offs, respectively. However, this finding is lower than 53% zinc deficiency among HIV subjects earlier reported in Addis Ababa, Ethiopia28 using 10.7 μmol/L cutoff. Regional disparity in zinc intake may account for the difference. The serum zinc level of the HIV-infected ART-naïve subjects was comparable to the HIV-infected subjects on ART. This finding is in consonance with previous report.26 The ART treatment and its duration seem to have no effect on zinc level. Furthermore, the association between gender and serum zinc level was statistically significant (p < 0.05). This shows gender to be predictor/risk factor of zinc deficiency.
Serum copper in this study did not differ between the HIV-infected subjects and the HIV seronegative subjects. This finding is in consonance with another study in Ethiopia12. However, this observation is in contrast with previous reports14, 29 that found significant higher serum copper in HIV-seropositive subjects. This disparity might be due to the fact that the seropositive subjects in the present study were not stratified into stages of the diseases progression, but rather, duration of treatment to ascertain if the majority of the subjects were in the acute or chronic phase of the disease. Copper has been established as an acute phase reactant and its level in serum have been shown to change significantly in a range of acute and chronic infective, inflammatory, and neoplastic processes owing to increased reproductive of ceruloplasmin.30, 31 Serum copper has been shown to return to normality after overcoming the initial acute phase of the associated disease.14 The present study observed a very low copper deficiency among the HIV seropositive subjects (3%) using <90 μg/dL32 cutoff. This finding is line with previous study.33 ART treatment did not significantly affect copper status of the HIV subjects. This finding is consistent with previous study.34 We did not find any association between serum copper and gender, age, anemic status, and CD4+ status as risk factors.
This study observed a significantly raised Cu/Zu ratio in HIV-infected subjects when compared with the control. This finding is in agreement with previous observation.33 This is as a result of the markedly decrease in serum zinc and corresponding increase in serum copper. The concentration of copper and zinc in the serum of healthy humans has been reported to be proportional to each other but vary during infection. The determination of the ratio has been considered helpful in diagnosing many diseases, observing their transformations and reflecting the nutritional status of zinc in human body better than its content in the serum.35, 36 We did not find any difference between the Cu/Zn ratio of the HIV-infected subjects on ART and HIV-infected ART-naïve subjects.
As expected, this study observed a significantly lower CD4+ T-cell count in the HIV-infected subjects when compared with the control. This finding is consistent with older reports.17, 37 This finding is reinforced by the progressive increase in CD4+ T-cell count as years of treatment increase. The hallmark of HIV infection and subsequently AIDS pathogenesis is a progressive depletion of CD4+ T-cell populations in close association with progressive impairment of cellular immunity and increasing susceptibility to opportunistic infections.38
Hematological indices provide physiological information on the blood picture and the reticuloendothelial system.39 This study observed significant reduction in the hemoglobin, packed cell volume, total red blood cell count and significant increase in the red cell indices (MCV and MCH) of the HIV-seropositive subjects. This observation is in agreement with report by another author.40 We observed anemia prevalence of 56% among the HIV-infected subjects using hemoglobin cutoff of 12 g/dL and 13 g/dL for females and males, respectively.41 This high prevalence of anemia observed in this study is similar to previous findings of 60.1%,42 67.4%,43 and 64.0%44 in other studies in Nigeria. In contrast, some authors have reported higher values of 80%, and 71% in Nigeria45 and Iran,46 respectively. More so, lower values of 39%, 19%, 31% and 12% have been reported for African American women, white women, African American men and white men, respectively in a study in United States.46 This disparity could be attributed to racial difference.46 Anemia occurs often in HIV seropositive humans, but its multifactorial origin complicates its differential diagnosis and adequate treatment, hence, the etiology often remains unclear.47 Among the several attempts to explain the mechanisms leading to anemia in HIV is postulation of direct infection of the erythroid progenitors. However, this explanation has remained a hypothesis without prove. More so, soluble factors such as HIV proteins and cytokines have been suggested to inhibit the growth of hematopoietic cells in the bone marrow of HIV-infected patients. Furthermore, opportunistic complications may contribute to anemia in large number of HIV-infected patients.48
The limitation of the study is that the HIV-infected subjects were not stratified based on phase of the disease, but on the duration of treatment. Hence care should be taken in extrapolating the findings.
In conclusion, this study demonstrated high level of zinc deficiency and anemia. More so, the zinc status of the HIV-infected subjects on ART treatment was comparable with HIV-infected subjects that are ART naïve. This showed that the ART treatment does not complement zinc status rather than the CD4+ T-cell count of the HIV-infected subjects. ART treatment centers on boosting the immune system as shown by increased CD4+ T-cell count, while the subjects may still suffer zinc deficiency amid improved CD4+ T-cell count. Hence, zinc supplementation should be considered while offering ART treatment as well as consideration of prevalent anemia to prevent zinc deficiency and anemia.
Author's contributions
A.E.A. conceived the study; A.E.A. and O.I.M. designed the study; O.H.U. and A.E.A. performed the statistical analysis; A.E.A., O.I.M., A.S.O., E.E., and F.U. performed the experiments; A.E.A., O.H.U., and E.E.R. prepared the manuscript; all authors read and approved the final manuscript.
Conflict of interest
The authors declares no conflict of interest.
References
- 1.Drain P.K., Kupka R., Mugusi F., Fawzi W.W. Micronutrients in HIV positive persons receiving active anti-retroviral therapy. Am J Clin Nutr. 2007;85:333–345. doi: 10.1093/ajcn/85.2.333. [DOI] [PubMed] [Google Scholar]
- 2.Soundravally R., Sherin J., Agieshkumar B.P., Daisy M.S., Cletus C., Narayanan P. Serum levels of copper and iron in Dengue fever. Rev Inst Med Trop Sao Paulo. 2015;57:315–320. doi: 10.1590/S0036-46652015000400007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaturvedi U.C., Shrivastava R., Upreti R.R. Viral infection and trace elements: a complex interaction. Curr Sci. 2004;87:1536–1554. [Google Scholar]
- 4.Fris H., Michaelsen K.F. Micronutrients and HIV infections: a review. Eur J Clin Nut. 1998;52:157–163. doi: 10.1038/sj.ejcn.1600546. [DOI] [PubMed] [Google Scholar]
- 5.Haraguchi Y., Sakurai H., Hussain S., Anner B.M., Hoshimo H. Inhibition of HIV-1 infection by zinc group metal compounds. Antiviral Res. 1999;43:123–133. doi: 10.1016/s0166-3542(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 6.McMillan D.C., Talwar D., Sattar N., Underwood M., O’Reilly D.S., McArdle C. The relationship between reduced vitamin antioxidant concentrations and the systemic inflammatory response in patients with common solid tumors. Clin Nutr. 2002;21:161–164. doi: 10.1054/clnu.2001.0527. [DOI] [PubMed] [Google Scholar]
- 7.Kupka R., Muguzi F., Abound S., Msamaga G.I., Finkelstein J.L., Spicgelman D. Randomized double blind, placebo controlled trial of selenium supplements among HIV-infected pregnant women in Tanzania: effects on maternal and child outcomes. Am J Clin Nutr. 2008;87:1802–1808. doi: 10.1093/ajcn/87.6.1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibeh I.N., Abuo A., Isitua C.C. Studies on trace elements metabolism in HIV/AIDS disease in Nigeria. Prime J Microbiol Res. 2012;2:86–92. [Google Scholar]
- 9.Linn C.C., Huang J.F., Tsai L.Y., Huang Y.L. Selenium, iron, copper and zinc levels and copper-to-zinc ratios in serum of patients at different stages of viral hepatic diseases. Biol Trace Elem Res. 2006;109:5–24. doi: 10.1385/BTER:109:1:015. [DOI] [PubMed] [Google Scholar]
- 10.WHO: World Health Organization . 1996. Trace elements in human nutrition and health. [Google Scholar]
- 11.Failla M.L. Trace elements and host defense: recent advances and continuing challenges. J Nutr. 2003;133:14435–14475. doi: 10.1093/jn/133.5.1443S. [DOI] [PubMed] [Google Scholar]
- 12.Amare B., Tafess K., Moges F., Moges B., Yabutani T., Ota F. Levels of serum zinc, copper and copper/zinc ratio in patients with diarrhea and HIV infection in Ethiopia. Vitam Trace Elem. 2011;1:101. [Google Scholar]
- 13.Joint United Nations Program on HIV/AIDS/World Health Organization (UWAIDS/WHO) 2005. AIDS epidemic update.
- 14.Moreno T., Artacho R., Navarro M., Perez A., Ruiz-Lopez M.D. Serum copper concentration in HIV-infected patients and relationship with other biochemical indices. Sci Total Environ. 1998;217:21–26. doi: 10.1016/s0048-9697(98)00158-2. [DOI] [PubMed] [Google Scholar]
- 15.Parker M.M., Humoller F.L., Mahler D.J. Determination of copper and zinc in biological fluids. Clin Chem. 1967;13:40–48. [PubMed] [Google Scholar]
- 16.Anyabolu H.C., Adejuyigbe E.A., Adeodu O.O. Serum micronutrient status of Haart-Naïve, HIV infected children. A case control study. AIDS Res Treat. 2014:351043. doi: 10.1155/2014/351043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nsonwu-Anyanwu A.C., Egbe E.R., Agu C.E., Ofors S.J., Usoro C.A., Essien I.A. Nutritional indices and cardiovascular risk factors in HIV infection in Southern Nigeria. J Immunol Microbiol. 2017;2:34–42. [Google Scholar]
- 18.Bobat R., Coovadia H., Stephen C., Naidoo K.L., Mckerrow N., Black R.E. Safety and efficacy of zinc supplementation for children with HIV-I infection in South Africa: a randomized double-blind placebo-controlled trial. Lancet. 2005;366:1862–1867. doi: 10.1016/S0140-6736(05)67756-2. [DOI] [PubMed] [Google Scholar]
- 19.Tranchov V., Decimo D., Pechoux C., Lener D., Rogemond V., Berthoux L. Role of the N-terminal zinc finger of HIV-1 nucleocapsid protein in virus structure and replication. J Virol. 1998;72:4442–4447. doi: 10.1128/jvi.72.5.4442-4447.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorelick R.J., Gagliardi T.D., Bosche W.J., Wiltrout T.A., Coren L.V., Chabot D.J. Strict conservation of the retroviral nucleocapsid protein zinc finger is strongly influenced by its role in viral infection processes: characterization of HIV-1 particles containing mutant nucleocapsid zinc-cordinating sequence. Virology. 1999;256:92–104. doi: 10.1006/viro.1999.9629. [DOI] [PubMed] [Google Scholar]
- 21.Shankar A.H., Prasad A.S. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–464S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 22.Walker F.C., Black R.E. Zinc and the risk of infectious disease. Am Rev Nutr. 2004;24:255–275. doi: 10.1146/annurev.nutr.23.011702.073054. [DOI] [PubMed] [Google Scholar]
- 23.Duggal S., Chugh T.D., Duggal A.K. HIV and malnutrition: effects on the immune system. Clin Dev Immunol. 2012 doi: 10.1155/2012/784740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ndagije F., Baribwira C., Coulter J.R. Micronutrient and T-cell subsets: a comparison between HIV-infected and uninfected, severely malnourished Rwandan children. Ann Trop Pediatr. 2007;27:269–275. doi: 10.1179/146532807X245652. [DOI] [PubMed] [Google Scholar]
- 25.Hotz C., Peerson J.M., Brown K.H. Suggested lower cutoffs of serum zinc concentration for assessing zinc status: reanalysis of the Second National Health and Nutrition Examination Survey data (1976–1980) Am J Clin Nutr. 2003;78:756–764. doi: 10.1093/ajcn/78.4.756. [DOI] [PubMed] [Google Scholar]
- 26.Wellinghausen N., Kern W.V., Jochle W., Kern P. Zinc serum level in human immunodeficiency virus-infected patients in relation to immunological status. Biol Elem Res. 2000;73:139–149. doi: 10.1385/BTER:73:2:139. [DOI] [PubMed] [Google Scholar]
- 27.Visser M.E., Maartens G., Kossew G., Hussey G.D. Plasma vitamin A and zinc levels in HIV-infected adults in Cape Town, South Africa. Br J Nutr. 2003;89:475–482. doi: 10.1079/BJN2002806. [DOI] [PubMed] [Google Scholar]
- 28.Fufa H., Umeta M., Taffese S., Mokhtar N., Aguenaou H. Nutritional and immunological status and their associations among HIV infected adults in Addis Ababa, Ethiopia. Food Nutr Bull. 2009;30:227–232. doi: 10.1177/156482650903000303. [DOI] [PubMed] [Google Scholar]
- 29.Graham N.H.M., Sorensen D., Odaka N. Relationship of serum copper and zinc levels to HIV-1 seropositivity and progression to AIDS. J Acquired Immune Defic Syndr. 1991;4:976–980. [PubMed] [Google Scholar]
- 30.Beisel W.R. Trace elements in infectious process. Med Clin North Am. 1976;60:831–849. doi: 10.1016/s0025-7125(16)31864-8. [DOI] [PubMed] [Google Scholar]
- 31.Powanda M.C. Changes in the body balances of nitrogen and other key nutrients: description and underlying mechanisms. Am J Clin Nutr. 1977;30:1254–1268. doi: 10.1093/ajcn/30.8.1254. [DOI] [PubMed] [Google Scholar]
- 32.Schneider J.M., Fuji M.L., Lamp C.L., Lonnerdal B.O. The prevalence of low serum zinc and copper levels and dietary Habits associated with serum zinc and copper in 12- to 36-months old children from low-income families at risk for iron deficiency. J Am Diet Assoc. 2007;107:1924–1929. doi: 10.1016/j.jada.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 33.Kassu A., Yabutani T., Mahmud Z.H., Muhamad A., Nguyen N., Huong B.T.M. Alterations in the serum levels of trace elements in tuberculosis and HIV infection. Eur J Clin Nutr. 2006;60:580–586. doi: 10.1038/sj.ejcn.1602352. [DOI] [PubMed] [Google Scholar]
- 34.Akinola F.F., Akinjinmi A.A., Oguntibeju O.O. Effects of combined antiretroviral therapy on selected trace elements and CD4+ T-cell count in HIV positive persons in African setting. J AIDS Clin Res. 2012;3:1–5. [Google Scholar]
- 35.Falchuk K.H. Disturbances in trace elements. In: Fauci A.C., Braunwald E., Isselbacher K.J., Wilson J.D., Martin J.B., Kasper D.L., Hauser S.L., Longo D.L., editors. Harrison's principle of Internal Medicine. McGraw-Hill; New York: 1998. pp. 489–492. [Google Scholar]
- 36.Hua-Dong L.U., Zhi-Qiang W., Yu-Rong P., Tian-Wang K. Comparison of Cu, Zn and Se content between healthy people and patients in high, middle and low incidence areas of gastric cancer of Fugian province. World J Gastroenterol. 1999;5:84–86. doi: 10.3748/wjg.v5.i1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ekwempu A.I., Ekwempu C.C., Ikeh E., Agaba E. Comparison of CD4+ cell count in pregnant HIV-seropositive and HIV-seronegative Nigerian women. Lab Med. 2012;43:168–171. [Google Scholar]
- 38.Okoye A.A., Picker L.J. CD4+ T-cell depletion in HIV infection: mechanisms of immunological failure. Immunol Rev. 2014;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okoroiwu H.U., Okafor I.M., Uko E.K., Atangwho I.J. Some hematological parameters of Wistar rats treated with Chromolaena odorata leave extracts. J Biol Res. 2017;90:51–55. [Google Scholar]
- 40.Ositadinma I.M., Odozi E.B., Meludu S.C., Okeke C.O. Effects of HIV infection on some haematological parameters and immunoglobulin levels in HIV patients in Benin City, Southern Nigeria. J HIV Retrovirus. 2016;2:2. [Google Scholar]
- 41.WHO: World Health Organization . World Health Organization; Geneva: 1968. Nutritional anemias. Report of WHO scientific group. Technical Report Series; No. 405. Available at http://whqlibdoc.who.int/trs/WHO_TRS_405.pdf. [Google Scholar]
- 42.Omoregie R., Omokaro E.U., Palmer O., Ogefere H.O., Egbeobauwaye A., Adegue J.E. Prevalence of anemia among HIV-infected patients in Benin city, Nigeria. Tanzan J Health Res. 2009;11:1–4. doi: 10.4314/thrb.v11i1.43242. [DOI] [PubMed] [Google Scholar]
- 43.Anyabolu E. Prevalence and associated factors of anemia in treatment-naïve HIV-positive subjects in Southeast Nigeria. Am J Med Sci Med. 2016;4:41–46. [Google Scholar]
- 44.Pennap G.R., Abubakar K. Prevalence of anemia among Human Immunodeficiency virus infected patients Accessing Healthcare in Federal Medical Centre Keffi, Nigeria. Int J Trop Dis Health. 2015;10:1–7. [Google Scholar]
- 45.Erhabor O., Ejele O.A., Nwauche C.A., Buseri F.I. Some hematological parameters in human immunodeficiency virus (HIV) infected Africans: the Nigerian perspective. Niger J Med. 2005;14:33–38. doi: 10.4314/njm.v14i1.37132. [DOI] [PubMed] [Google Scholar]
- 46.Meidani M., Razae F., Maracy M.R., Avijgan M., Tayeri K. Prevalence, severity and related factors of anemia in HIV/AIDS patients. J Res Med Sci. 2012;17:138–142. [PMC free article] [PubMed] [Google Scholar]
- 47.Creagh T., Mildvan D. The anemia prevalence study group, program and abstract of the 40th annual meeting of the Infectious Disease Society of America (Chicago) VAinfectious Disease Society of America; Alexandria: 2002. Greater prevalence of anemia in women and African Americans with HIV/AIDS in HAART era: a study of 10,000 patients (abstract 475) [cap 127] [Google Scholar]
- 48.Redgrave B.E., Stone S.E., French M.A., Krueger R., James I.R., Price P. The effect of combination antiretroviral drug therapy on CD5 B-cells, B-cell activation and hypergammaglobulinaemia in HIV-1 infected patients. HIV Med. 2005;6:307–312. doi: 10.1111/j.1468-1293.2005.00312.x. [DOI] [PubMed] [Google Scholar]