Abstract
Background
Bone fragility and an increase in susceptibility to fracture osteoporosis is characterized by a reduction in bone mass and the micro-architectural deterioration of bone tissue. There is no previous study regarding the effect of Cinnamomum burmanini Blume on osteoporosis.
Objective
This study was aimed to investigate the effect of C. burmanini Blume on bone turnover marker, mineral elements, and mesostructure of ovariectomized rats.
Materials and methods
Thirty female Wistar rats were randomly divided into five groups, which included a control group (sham surgery), ovariectomy group (OVX), and ovariectomy groups in the presence of ethanolic extract of C. burmanini Blume (EECB) at doses of 12.5; 25; 50 mg/kg body weight (BW). Analysis of serum C-telopeptide collagen type I (CTX) and osteocalcin (OC) were done by enzyme-linked immunosorbent assay (ELISA). Tibia mineral elements and mesostructure were analyzed by X-ray Fluorescence and Scanning Electron Microscopy, respectively. In silico study was performed by modeling protein structure using SWISS-MODEL server and Ramachandran plot analysis.
Results
The increase in OC and CTX were significantly attenuated by treatments of EECB. Ovariectomy significantly decreased Cu/Zn ratio compared to sham-operated rats (p < 0.05). Mesostructure of ovariectomized rats was significantly different compared with the control group.
Conclusion
Cinnamon was able to normalize bone turnover markers, but, the mesostructure of hydroxyapatite crystal growth was achieved at the highest dose extract. In silico study showed that the active compound of EECB could not only support osteoclastogenesis process by decreasing the binding energy between RANKL and RANK, but also by inhibiting the interaction between OPG and RANK.
Keywords: Cinnamon, Bone turnover, Bone mesostructure, Osteoporosis
Highlights
-
•
Administration EECB significantly increased OC and CTX level compared to OVX group.
-
•
The ratio of Cu/Zn was lower significantly in OVX rats compared to sham-operated rats.
-
•
Hydroxyapatite crystal growth can reach at the highest dose of Cinnamon.
1. Introduction
Bone fragility and an increase in susceptibility to fracture osteoporosis are characterized by a reduction in bone mass and the micro-architectural deterioration of bone tissue [1]. The loss of ovarian function is the main factor for bone loss in aged females. The pathophysiology of this “ovary-related’ bone loss is not clear and cannot be simply explained by either increased bone resorption or decreased bone formation [2]. The model of bilaterally ovariectomized rats mimics the accelerated bone loss which manifests in postmenopausal women [3]. Intensified bone loss was manifested by a significant decrease in the mineral content/bone mass ratio [4]. Previous study shows that ovariectomized rats showed a significant gradual increase in serum calcium, phosphorus, zinc, and copper levels compared to the sham control [5], [6].
Estrogen replacement therapy (ERT) has been used as drug of choice for prevention of postmenopausal bone loss, but evidence indicates that long-term unopposed ERT may cause an increased risk of ovarian and endometrial cancer [7], [8]. Thus, the alternative therapeutic strategy with a proven efficacy and safety should be developed for the prevention and treatment of osteoporosis using medicinal plants with low side effects. Cinnamon is one of the well-known and oldest spices, which has been used for centuries in several cultures [9]. Although there are several origins of Cassia cinnamon, two kinds that are considered as the important are Chinese Cassia (Cinnamomum cassia Blume, syn. Cinnamomum aromaticum Nees) and Indonesian Cassia (Cinnamomum burmanini Blume). Chinese Cassia is cultivated in southern China, Burma, and Vietnam, whereas Indonesian cassia is mainly from Indonesia [10]. Cinnamon is the bark of Cinnamomum cassia and has been used as traditional folk herb to treat inflammation for thousands of years in Asia. It is also used in food industry as an antioxidant and spicy agent [11]. Previous research using cinnamon extract was shown its effect of increasing the estradiol level, LH secretion and in turn affecting the estrogen and progesterone synthesis [12]. Therefore, the aim of the current study was to clarify the effect of cinnamon on bone turnover marker, mineral elements, and mesostructure of ovariectomized rats in an osteoporosis model of rats.
2. Materials and methods
2.1. Preparation and extraction of cinnamon
Cinnamon was obtained from Materia Medica Garden, Batu, East Java, Indonesia. The plant was identified as Cinnamomum burmaninii by an officer from Materia Medica Garden, Batu, East Java, Indonesia. The preparation and extraction of cinnamon were performed according to the maceration extraction method [13]. One kilogram of dried cinnamon stem was extracted with distilled in 70% ethanol by a maceration method for five days, filtered and collected until there was colorless ethanol filtrate. The collected ethanol filtrate was evaporated using a rotatory evaporator to produce an ethanol extract of cinnamon. The ethanol extracts of cinnamon were stored at 4 °C.
2.2. High-performance liquid chromatography analysis
Methanol was filtered using membrane millipore with polytetrafluoroethylene (PTFE, Whatman® brand) and water was filtered using cellulose nitrate membrane. Placed in the HPLC eluent reservoir. HPLC tool analysis was balanced for appropriate conditions (optimum conditions): mobile phase of methanol: water = 8: 2, stationary phase was C – 18, flow rate was 0.75 ml/min. When HPLC tool achieved a straight baseline, the standard extracts were injected and measured. The standards, including eugenol 1.7 mg (Sigma Aldrich; Cas E51791), cardamom oil 1.4 mg (Sigma Aldrich, Cas W224111), coumarin 0.6 mg (Chengdu Biopurify Phytochemicals Ltd., Cas No: 91-64-5), trans-cinnamic acids 0.5 mg (Sigma Aldrich, Cas W228826) and cinnamon extract 4.1 mg of EECB were filtered using PTFE membrane and homogenized for 5 min. 20 ml of samples were injected into the HPLC column, and the chromatograms were detected using UV at a wavelength 254 nm. The experiment was performed triplicate.
2.3. Animal
Thirty, adult albino female Wistar rats, twelve months old, weighing 150–200 g were used in this study. The animals were acclimatized for 1 week to our laboratory conditions prior to experimental manipulation and were exposed to a 12-h light and 12-h dark cycle at room temperature of 24 °C. They had free access to standard laboratory chow and water ad libitum. The animals were randomly assigned into five groups: control group (SHAM) whose animal was sham ovariectomy, ovariectomy group (OVX) where animals received standard ovariectomy, and three ovariectomy groups which receiving the ethanolic extract of C. burmanini Blume (EECB) by gavage at dose 12.5 (OVX + EECB12.5); 25 (OVX + EECB25); and 50 (OVX + EECB50) mg/kg body weight (mg/kg BW). The EECB dose was determined based on previous research with some modification [14]. For the OVX rats, it was divided randomly after two weeks recovery from ovariectomy surgery. The animals received EECB by oral gavage for one month. The period of administration was conducted based on a previous experiment using cinnamon extract, in which one-month administration already showed the effect of its treatment [15], [16], [17], [18]. All animal procedures were approved by the ethical committee of the Medical Faculty, Lambung Mangkurat University prior the study.
2.4. Ovariectomy surgery procedure
Under ketamine (50 mg/kg) and xylazine (8 mg/kg) anesthesia, twenty four animals from the OVX groups underwent bilateral ovariectomy by ventral incisions while six were sham-operated (control) [19]. The treatment with extract was performed after two weeks recovery from ovariectomy surgery. Wound healing evaluation was conducted based on previous methods with some modification to decide the recovery time and to decide the treatment starting time [20]. The successful osteoporosis modeling animal was determined through identification for osteoblast gene expression using PCR and followed by histochemical staining (data not shown) based on previous research [21]. At the end of the experiment, animals in all groups were sacrificed. Serum and bone tissues were removed.
2.5. Tissue preparation
At the end of the treatment, rats in all groups were anesthetized; their blood was drawn by cardiac puncture. Both tibia and femur were collected, weighed, and later rinsed with physiological saline. All tibia samples were stored at glutaraldehyde until analyzed.
2.6. Analysis of bone turnover markers
The serum bone formation markers osteocalcin was measured using Rat Osteocalcin/Bone Gla Protein OT/BGP ELISA kits from NovaTeinBio, Inc (Cambridge, MA, USA). The serum bone resorption marker C-telopeptide of type I collagen (CTX) kit was purchased from NovaTeinBio, Inc (Cambridge, MA, USA) [22].
2.7. Analysis of bone mineral elements
Bone mineral element analysis was evaluated by X-Ray Fluorescence (XRF). For XRF analysis, the tibia bones were inserted into the bone tube, then put in the proper place in equipment. The processed bones were then analyzed at 10–20 kV accelerating voltages by an XRF (PANalytical MiniPAL 4). All procedures were done at Physic and Central Laboratory, Faculty of Mathematic and Natural Science, University of Malang [22].
2.8. Analysis of the bone cell number
Cellular parameters were obtained from decalcified sections of right femur bones. The bones were decalcified in the EDTA solution for five weeks and then dehydrated in graded concentrations of ethanol before being embedded in paraffin wax. The decalcified femur bones were sectioned at 5 microns thick using a microtome then stained with Hematoxylin and Eosin (H&E). These parameters were calculated under a light microscope (Olympus BX50, USA) interfaced with an image analyzer (Image Pro- Express, Media Cybernetics, USA) [23].
2.9. Analysis of bone mesostructure
Mesostructure analysis was evaluated by SEM. For SEM evaluation, femurs from all groups were cut vertically from the proximal metaphysis area. Then the femur bones were fixed with phosphate formalin buffer, dehydrated with graded concentration of ethanol and coated with gold and palladium. The processed bones were then analyzed at 20 kV accelerating voltages by SEM (FEI Inspect TM S50). All procedures were done at Physic and Central Laboratory, Faculty of Mathematics and Natural Science University of Malang [22].
2.10. In silico analysis
The compound structure of coumarin, eugenol, and trans-cinnamic acid (TCA) were obtained from a compound database of the National Center for Biotechnology Information (NCBI), United States National Library of Medicine (NLM), National Institutes of Health (NIH), and the protein sequences of RANK, RANKL, and OPG are NP_001258164.1; Q9ESE2.1; and O08727.1, respectively; retrieved from the sequence database of NCBI. Conversion *.sdf file of coumarin, eugenol, and TCA to become *.pdb file was done using Open Babel software [23], and modeling 3-D structure of RANK, RANKL, and OPG was predicted using the SWISS-MODEL web server [24], [25]. Protein structure was analyzed by Ramachandran plot using RAMPAGE web server (http://mordred.bioc.cam.ac.uk). Interaction analysis by docking method analyzed by using HEX 6.12 software [26], [27].
2.11. Ethics
This research has been approved by research ethics committee Faculty of Medicine University of Lambung Mangkurat, Banjarmasin, South Kalimantan, Indonesia.
2.12. Statistical analysis
The levels of bone turnover markers, the number of bone cells, and the level of tibia mineral elements are presented as mean ± standard deviation and differences between groups were analyzed using analysis of variance test using SPSS 16.0. p < 0.05 was considered statistically significant.
3. Results
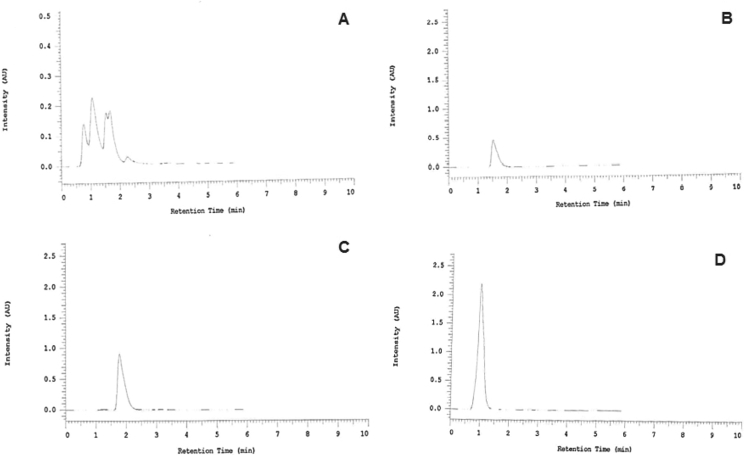
HPLC results (Fig. 1), based on absorption profile, retention time and spiking tests on the EECB (Table 1A), from the four standards showed that cardamom oil was not detected in the active compounds of EECB (figure not shown). However, trans-cinnamic acid, eugenol, and coumarin were detected among the active compounds in EECB (Fig. 1B–D). Based on the calculation, the highest concentration of active compounds in EECB was eugenol (7.3%), then followed by coumarin (3.2%) and trans-cinnamic acid (1.6%) respectively (Table 1B).
Fig. 1.
High-performance liquid chromatography (HPLC) profile of C. burmanini Blume and identification of active compounds using several kinds of standards. A) HPLC results from EECB showed several peaks; B) Identification of active compounds using coumarin acid as standard; C) Identification of active compounds using eugenol as standard; D) Identification of active compounds using trans-cinnamic acid as standard.
Table 1A.
Quantification of HPLC result from sample (EECB) and standard (coumarin, eugenol, and trans-cinnamic acid).
| Standard/Sample | RT | Peak | Sample (mg/5 ml) | Sample (μg/ml) |
|---|---|---|---|---|
| Eugenol | 1.78 | 6,997,404 | 1.7 | 340 |
| trans-Cinnamic | 0.98 | 14,783,984 | 0.5 | 100 |
| Coumarin | 1.53 | 3,156,667 | 0.6 | 120 |
| EECB | 1.07 | 1,931,865 | 4.1 | 820 |
| 1.67 | 1,232,999 | 4.1 | 820 | |
| 0.77 | 745,840 | 4.1 | 820 | |
| 1.54 | 689,570 | 4.1 | 820 |
Table 1B.
Concentration from three active compounds that identified in EECB.
| Standard | Content (μg/ml) | Content (%) |
|---|---|---|
| Eugenol (RT 1.78) | 59.91 | 7.3 |
| trans-Cinnamic (RT 0.98) | 13.07 | 1.6 |
| Coumarin (RT 1.53) | 26.21 | 3.2 |
Table 2 summarizes the bone turnover markers of the study. The levels of OC and CTX were significantly (p < 0.05) lower in OVX rats compared to the sham-operated group. The treatment with EECB at all doses significantly (p < 0.05) increased OC and CTX level compared to OVX group, to reach the similar level in the sham-surgery group.
Table 2.
Level of bone turn over markers in supplementation and control group (ng/ml).
| Sham | OVX | OVX + Cinnamon (mg/kg BW) |
|||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | |||
| CTX | 1.32 ± 0.23 | 1.03 ± 0.06a | 1.20 ± 0.03b | 1.22 ± 0.04b | 1.38 ± 0.10b |
| Osteocalcin | 2.14 ± 0.35 | 1.69 ± 0.14a | 2.32 ± 0.41b | 2.15 ± 0.21b | 2.73 ± 0.63b |
Values are presented as mean ± SD.
p < 0.05 in comparison with sham group.
p < 0.05 in comparison with ovariectomized group.
The number of osteoblast, osteoclast, and osteoblast/osteoclast ratio had no significant difference between groups, as shown in Table 3.
Table 3.
The number of bone cells in supplementation and control group (cells).
| Sham | OVX | OVX + Cinnamon (mg/kg BW) |
|||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | |||
| Osteoblast | 7.00 ± 7.00 | 40.00 ± 10.53 | 44.00 ± 11.53 | 48.33 ± 17.92 | 24.33 ± 4.04abcd |
| Osteoclast | 12.66 ± 7.23 | 34.66 ± 5.68 | 56.00 ± 18.73a | 40.00 ± 12.16 | 26.66 ± 9.86 |
| Osteocyte | 50.33 ± 13.86 | 78.00 ± 11.79 | 109.00 ± 39.39 | 75.66 ± 30.03 | 98.00 ± 23.89 |
| Ob/Oc ratio | 0.47 ± 0.24 | 1.14 ± 0.12 | 0.89 ± 0.54 | 1.32 ± 0.74 | 1.01 ± 0.43 |
Values are presented as mean ± SD.
p < 0.05 in comparison with sham group.
p < 0.05 in comparison with ovariectomy (OVX) group.
p < 0.05; in comparison with ovariectomy + 12.5 mg/kg BW of Cinnamon group.
p < 0.05; in comparison with ovariectomy + 25 mg/kg BW of Cinnamon group; Ob: osteoblast; Oc; osteoclast.
As shown in Table 4, the level of Ca, P, Fe, Cu, Zn, Ni, and Ca/P ratio did not reveal any significant difference in OVX groups than that in sham-operated group (p > 0.05). The Cu/Zn ratio was significantly lower in OVX rats compared to sham-operated rats (p < 0.05). There was no effect of extract treatment on the decrease the Cu/Zn ratio level.
Table 4.
Level of tibia mineral elements in supplementation and control group (%).
| Level (%) | Sham | OVX | OVX + Cinnamon (mg/kg BW) |
||
|---|---|---|---|---|---|
| 12.5 | 25 | 50 | |||
| Calcium | 87.33 ± 2.36 | 78.66 ± 14.70 | 84.51 ± 1.55 | 85.18 ± 0.88 | 85.88 ± 1.42 |
| Phosphorus | 6.21 ± 1.40 | 10.75 ± 5.25 | 11.12 ± 1.41 | 8.70 ± 3.59 | 9.60 ± 0.88 |
| Iron | 1.02 ± 0.06 | 0.73 ± 0.52 | 0.62 ± 0.10 | 0.88 ± 0.53 | 0.90 ± 0.20 |
| Copper | 0.33 ± 0.08 | 0.12 ± 0.07 | 0.09 ± 0.02 | 0.17 ± 0.13 | 0.23 ± 0.15 |
| Zinc | 0.78 ± 0.10 | 0.94 ± 0.39 | 0.67 ± 0.09 | 0.92 ± 0.23 | 0.70 ± 0.08 |
| Nickel | 0.21 ± 0.10 | 0.73 ± 0.83 | 0.10 ± 0.01 | 0.13 ± 0.07 | 0.30 ± 0.19 |
| Ca/P | 14.55 ± 3.35 | 12.03 ± 2.96 | 7.69 ± 1.12 | 11.51 ± 6.27 | 8.99 ± 0.79 |
| Cu/Zn | 0.43 ± 0.12 | 0.18 ± 0.01a | 0.13 ± 0.02a | 0.17 ± 0.08 | 0.32 ± 0.17 |
Values are presented as mean ± SD; Ca/P: calcium/phosphorus; Cu/Zn: copper/zinc; OVX: ovariectomy.
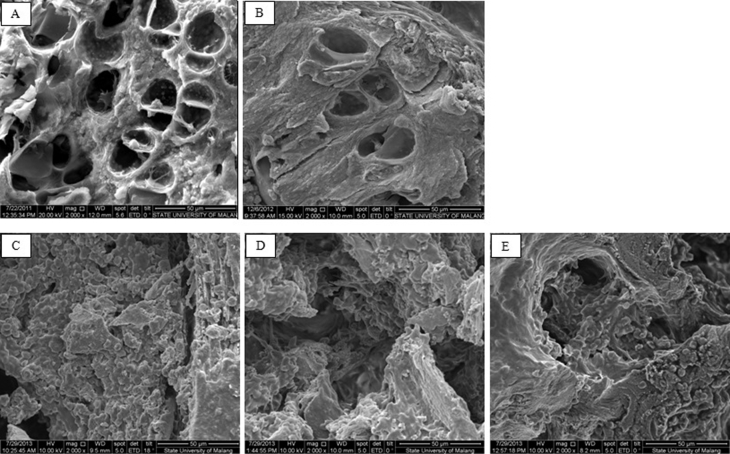
Mesostructure of sham-operated rats presented rod-like trabeculae with honeycomb appearance and minimal holes (A). Tibia mesostructure of ovariectomized rats was significantly different when compared with sham-operated rats. Trabecular breaking and stump structure contributed to a massive hole, and the loss of granule structure was observed in ovariectomized rats (B). Out of the 12.5, 50, and 100 mg/kg body weight doses of extract, only the highest dose induces the granule formation, as seen in Fig. 2.
Fig. 2.
Mesostructure of sham-operated rats (A) and ovariectomized rats (B). Mesostructure of sham-operated rats presented rod-like trabecules with honeycomb appearance and minimal holes (A). Mesostructure of tibia bone in ovariectomized was rats significantly different as compared to sham-operated rats. Trabecular breaking and stump structure, which contributed to massive hole, and the losing of granule structure was observed in ovariectomized rats (B). The trabecular surface of ovariectomized rats supplemented with EECB12.5 or EECB50 was not different compared with ovariectomized rats (C, D), and the granule structure was seen in the third dose group (E) (Scanning Electron Microscope, Magnification ×2000).
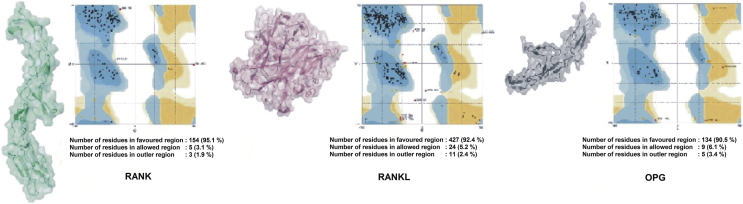
The modeling protein structure has an important role in studying the interaction between RANK, RANKL, and OPG. Protein 3-dimensional structure was generated using SWISS-MODEL server. From several predicted structures for RANK, RANKL, and OPG, the best model was selected after Ramachandran plot analysis. The best model was picked based on highest percentages of residues in most favored regions and lowest percentage scores in the outer region. The stereochemical quality of predicted protein structure was analyzed through residue-by-residue geometry and overall geometry of protein structures using the RAMPAGE server. Ramachandran plots were drawn for these protein structures. Analysis of Ramachandran plots showed the most favored regions which are indicated by dark blue patches, while bright blue areas show allowed regions (Fig. 3). It was observed that protein modeling of RANK, RANKL, and OPG give a good result that was shown by the high percentage of residues in the favored region. Protein 3-dimensional structure analysis is fundamental as the biological activity of a protein is accomplished by its 3-dimensional structure.
Fig. 3.
Ramachandran plot analysis and 3-D protein modeling of RANK, RANKL, and OPG.
Docking analysis by HEX software showed that binding energy (Table 5) for RANK and RANKL interaction was −742.59 kJ/mol. Coumarin, eugenol, and trans-cinnamic acid can reduce the energy required for interactive processes. In case of coumarin and eugenol, the lowest energy required for binding processes was obtained when coumarin and eugenol bind with RANKL before RANKL bind with its receptor, RANK. Docking analysis revealed that the lowest energy required for interaction between RANK and RANKL when coumarin and eugenol were present was −850.05 kJ/mol and 773.87 kJ/mol, respectively. In case of TCA, the lowest energy required for interaction between RANK and RANKL was obtained when RANK and RANKL bind with TCA before interaction (−841.61 kJ/mol).
Table 5.
Binding energy required for the interaction process.
| Coumarin | Eugenol | TCA | |
|---|---|---|---|
| RANKL | −750.88 kJ/mol | −768.91 kJ/mol | −834.33 kJ/mol |
| RANK–RANKL complex | −850.05 kJ/mol | −773.87 kJ/mol | −746.86 kJ/mol |
| OPG | −721.53 kJ/mol | −696.04 kJ/mol | −653.57 kJ/mol |
| RANKL–OPG complex | −649.09 kJ/mol | −687.59 kJ/mol | −642.78 kJ/mol |
Osteoprotegerin (OPG) has been known as an inhibitory molecule in the osteoclastogenesis process through competitive binding with RANK. Docking analysis revealed that the energy required for interaction between OPG and RANK in the normal condition without the active compound of EECB was −835.74 kJ/mol. Several active compounds of EECB including coumarin, eugenol, and TCA could increase the energy required for the binding process, which was −794.95 kJ/mol, −799.25 kJ/mol, and −784.92 kJ/mol, respectively. In all active compounds, the highest energy required for binding processes obtained when the active compound of EECB binds to RANK before OPG binds to it.
4. Discussion
In this study, we found several bioactive compounds in EECB, including non-phenolic (trans-cinnamic acid and coumarin), and phenolic (eugenol oil). Cinnamic acid has been identified as one of the bioactive compounds in Cinnamomum zeylanicum [28]. This study is consistent with previous study that chemical constituents of cinnamon extract are mostly cinnamyl alcohol, coumarin, cinnamic acid, cinnamaldehyde, anthocyanin, and essential oils together with constituents of sugar, protein, crude fats, pectin, and others [29]. An ultra-performance liquid chromatography coupled with a PDA detector and a mass spectrometry (UPLC-UV/MS) method has been developed to characterize coumarin, cinnamyl alcohol, cinnamaldehyde, cinnamic acid, eugenol and cinnamyl acetate [30].
It is well known that trabecular bone strength is determined not only by the quantity of composite material (mineral, protein and water) but also the quality materials (size, area, structural properties). Several properties, such as more abundant, thicker, well-connected, and plate-like trabecular, confer a stronger trabecular bone compartment [8], [31], [32], [33]. OVX in rats induces significant trabecular bone loss due to estrogen deficiency and subsequently increases bone turnover [34]. This includes increased rate of bone turnover with resorption exceeding formation and greater loss of cancellous than cortical bone [35]. Osteocalcin is a molecule exclusively produced by osteoblast as a marker for bone formation. Osteocalcin increases in osteoporosis of OVX rats, but another study found no significant difference of osteocalcin level in sham-operated compared with ovariectomized mice [36], [37], [38]. In this study, the levels of OC were significantly lower in ovariectomized rats than that in the sham-control group (p < 0.05), but the number of osteoblasts are not significantly different. This finding indicated that the activity of bone formation was reduced in ovariectomy although there was no change in the cell's quantity. Besides that, our result is similar with previous result by Zhang et al. [39], showing a significant decrease in OC level on OVX rats compared to control, but the measurement of OC on bone surface was higher than control. It is possible that the OC was not circulated yet. EECB significantly increases OC in ovariectomized rats to reach the similar level in the sham-operated group. This finding indicated that administration of EECB increases the activity of osteoblast. This study is consistent with previous studies reporting that C. cassia (C. cassia) stimulates bone formation by osteoblasts in vitro [18].
CTX is a reliable marker of the resorbing activity of osteoclast. Because resorption and therefore the total activity of osteoclast are increased after OVX, and owing to the fact that the absolute number of osteoclast is decreased, the remaining osteoclast must be substantially more active [40]. Previous studies found that higher plasma CTX concentrations in their OVX group than in sham operated animals, which indicated the increased bone turnover [41], [42]. In the present study, ovariectomized animals were found to have lower CTX level than sham controls (p < 0.05) in a number of osteoclasts and is not significantly different. EECB significantly increases CTX level at all doses compared with OVX rats (p < 0.05). In this study, low level of CTX in OVX groups may be correlated with a low level of osteocalcin. Osteocalcin act for recruitment and differentiation of circulating monocytes and osteoclast precursors, suggesting its role on osteoblast–osteoclast interaction and bone resorption. Besides, osteoclast was not sensitive to resorb bone areas which are deficient in osteocalcin [43], [44], [45]. In other sides, the knockout mice model for osteocalcin shows higher bone mineral density without any change in bone resorption and mineralization [46].
The study did not reveal any significant difference level of Ca, P, Fe, Cu, Zn, Ni, and Ca/P ratio in OVX groups than that in sham-operated group (p > 0.05). Only, the ratio of Cu/Zn was lower significantly in OVX rats compared to sham-operated rats (p < 0.05) and the administration of EECB50 could have normalized it but not have made a statistically significant difference. In Cu deficiency, the activity of lysyl oxidase in bony areas is greatly reduced, and it is presumed that this causes a reduction in collagen cross-linking that may influence the synthesis and stability of bone collagen and induce skeletal development disorders [47], [48]. Zn supports metabolism and growth of bones, increases bone density, inhibit bone loss, and is involved in bonding with organic structure [49], [50]. Reduction of Cu/Zn ratio in OVX rats indicated low collagen cross linking and EECB may have potential to repair it although insignificantly.
In silico analysis revealed that an active compound of EECB could support osteoclastogenesis process, not only by decreasing the binding energy between RANKL and RANK, but also by inhibiting the interaction between OPG and RANK. The lower binding energy required for the interaction could stabilize and facilitate the interaction process, and conversely, the higher binding energy required for interaction could decrease the stabilization of the interaction, thus leading to the inhibition of the interaction. This result is contradictory to previous studies which showed that C. zeylanicum inhibits RANKL-induced osteoclastogenesis [28].
Conclusion
We conclude that ovariectomy induces bone loss in the tibia. The administration of C. burmanini Blume at all doses are able to normalize serum bone turnover markers. Granule formation as a marker of hydroxyapatite crystal growth can reach at the highest dose of cinnamon. In silico study showed that an active compound of EECB could support osteoclastogenesis process, not only by decreasing the binding energy between RANKL and RANK, but also by inhibiting the interaction between OPG and RANK.
Sources of funding
None
Conflict of interest
None
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Zhao X., Wu Z., Zhang Y., Gao M., Yan Y., Cao P. Locally administrated perindopril improves healing in an ovariectomized rat tibial osteotomy model. Plos One. 2012;7(3):e33228. doi: 10.1371/journal.pone.0033228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang J., Lazarenko O.P., Blackburn M.L., Shankar K., Badger T.M. Feeding blueberry diets in early live prevent senescence of osteoblast and bone loss in ovariectomized adult female rats. Plos One. 2011;6(9):e24486. doi: 10.1371/journal.pone.0024486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Folwarczna J., Zych M., Trzeciak H.I. Effects of curcumin on the skeletal system in rats. Pharmacol Rep. 2010;62:900–909. doi: 10.1016/s1734-1140(10)70350-9. [DOI] [PubMed] [Google Scholar]
- 4.Pytlik M., Cegiela U., Folwrczna J., Janiec W., Pytlik W. Effects of retinol on development of osteopenic changes induced by bilateral ovariectomy in rats. Pol J Pharmacol. 2004;56:345–352. [PubMed] [Google Scholar]
- 5.Srikanta P., Nagarajappa S.H., Viswanatha G.L., Handral M., Subbanna R., Srinath R. Anti-osteoporotic activity of methanolic extract of an Indian herbal formula NR/CAL/06 in ovariectomized rats. J Chin Integr Med. 2011;(10):1125–1132. doi: 10.3736/jcim20111014. [DOI] [PubMed] [Google Scholar]
- 6.Liang H., Yu F., Tong Z., Huang Z. Effect of Cistanches Herba aqueous extract on bone loss in ovariectomized rat. Int J Mol Sci. 2011;12:5060–5069. doi: 10.3390/ijms12085060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palacios S. Advances in hormone replacement therapy: making the menopause manageable. BMC Women's Health. 2008;8:220. doi: 10.1186/1472-6874-8-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowring C.E., Francis R.M. National Osteoporosis Society's position statement on hormone replacement therapy in the prevention and treatment of osteoporosis. Menopause Int. 2011;17(2):63–65. doi: 10.1258/mi.2011.011012. [DOI] [PubMed] [Google Scholar]
- 9.Gruenwald J., Freder J., Armbruester N. Cinnamon and health. Crit Rev Food Sci Nut. 2010;50(9):822–834. doi: 10.1080/10408390902773052. [DOI] [PubMed] [Google Scholar]
- 10.Blahova J., Svobodova Z. Assessment of coumarin levels in ground cinnamon available in the Czech retail market. Sci World J. 2012;2012 doi: 10.1100/2012/263851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng X., Zhang Y., Gong Z., Huang C., Zang Y.Q. Improved insulin resistance and lipid metabolism by cinnamon extract through activation of peroxisome proliferator-activated receptors. PPAR Res. 2008;2008:9. doi: 10.1155/2008/581348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohammad P., Zienab B., Hossein K.J. The Effect of cinnamon extract on gonadotrop in changes (FSH& LH) in rats treated with gelophen. Biomed Pharmacol J. 2014;7(1):363–367. [Google Scholar]
- 13.Tiwari P., Kumar B., Kaur M., Kaur G., Kaur H. Phytochemical screening and extraction: a review. Int Pharm Sci. 2011;1(1):98–106. [Google Scholar]
- 14.Beejmohun V., Peytavy-Izard M., Mignon C., Muscente-Paque D., Deplanque X., Ripol C. Acute effect of Ceylon cinnamon extract on postprandial glycemia: alpha-amylase inhibition, starch tolerance test in rats, and randomized crossover clinical trial in healthy volunteers. BMC Complement Altern Med. 2014;14:351. doi: 10.1186/1472-6882-14-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dugoua J.J., Seely D., Perri D., Cooley K., Forelli T., Mills E. From type 2 diabetes to antioxidant activity: a systematic review of the safety and efficacy of common and cassia cinnamon bark. Can J Physiol Pharmacol. 2007;85:837–847. doi: 10.1139/Y07-080. [DOI] [PubMed] [Google Scholar]
- 16.Al-Jamal A., Rasheed I.N. Effects of cinnamon (Cassia zelynicum) on diabetic rats. Afr J Food Sci. 2010;4(9):615–617. [Google Scholar]
- 17.Eidi A., Mortazavi P., Bazargan M., Zaringhalam J. Hepatoprotective activity of cinnamon ethanolic extract against CCL4-induced liver injury in rats. EXCLI J. 2012;2012(11):495–507. [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K.H., Choi E.M. Stimulatory effects of extract prepared from the bark of Cinnamomum cassia blume on the function of osteoblastic MC3T3-E1 cells. Phytother Res. 2006;20:952. doi: 10.1002/ptr.1984. [DOI] [PubMed] [Google Scholar]
- 19.Yalin S., Sagir O., Comelekoglu U., Berkoz M., Eroglu P. Strontium ranelate treatment improves oxidative damage in osteoporotic rat model. Pharmacol Rep. 2011;63:396–402. doi: 10.1016/s1734-1140(12)70780-6. [DOI] [PubMed] [Google Scholar]
- 20.Parhizkar S., Ibrahim R., Latiff L.A. Incision choice in laparotomy: a comparison of two incision techniques in ovariectomy of rats. World Appl Sci J. 2008;4(4):537–540. [Google Scholar]
- 21.Sila-Asna M., Bunyaratvej A., Maeda S., Kitaguchi H., Bunyaratavej N. Osteoblast differentiation and bone formation gene expression in strontium-inducing bone marrow mesenchymal stem cell. Kobe J Med Sci. 2007;53(1):25–35. [PubMed] [Google Scholar]
- 22.Noor Z., Setiawan B. Subchronic inhaled particulate matter coal dust changes bone mesostructure, mineral element and turn over markers in rats. J Exp Integr Med. 2013;3(2):153–158. [Google Scholar]
- 23.O'Boyle N., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: an open chemical toolbox. J Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arnold K., Bordoli L., Kopp J., Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 25.Kiefer F., Arnold K., Kunzli M., Bordoli L., Schwede T. The SWISS-MODEL repository and associated resources. Nucleic Acids Res. 2009;37:387–392. doi: 10.1093/nar/gkn750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mustard D., Ritchie D.W. Docking essential dynamics eigenstructures. Prot Struct Funct Bioinform. 2005;60:269–274. doi: 10.1002/prot.20569. [DOI] [PubMed] [Google Scholar]
- 27.Macindoe G., Mavridis L., Venkatraman V., Devignes M.D., Ritchie D.W. HexServer: an FFT-based protein docking server powered by graphics processors. Nucleic Acids Res. 2010;38:445–449. doi: 10.1093/nar/gkq311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuji-Naito K. Aldehydic components of Cinnamon bark extract supresses RANKL-induced osteoclastogenesis through NFATc1 downregulation. Bioorg Med Chem. 2008;16(20):9176–9183. doi: 10.1016/j.bmc.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 29.Al-Dhubiab B.E. Pharmaceutical applications and phytochemical profile of Cinnamomum burmannii. Pharmacog Rev. 2012;6(12):125–131. doi: 10.4103/0973-7847.99946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y.H., Avula B., Zhao J., Smillie T.J., Nanayakkara N.P.D., Khan I.A. Characterization and Distribution of coumarin, cinnamaldehyde and related compounds in Cinnamomum spp. by UPLC-UV/MS combined with PCA. Planta Med. 2010;76 P31. [Google Scholar]
- 31.Mosekilde L., Ebbesen E.N., Tornvig L., Thomsen J.S. Trabecular bone structure and strength – remodelling and repair. J Musculoskelet Neuron Interact. 2000;1:25–30. [PubMed] [Google Scholar]
- 32.Burrows M., Liu D., Moore S., McKay H. Bone microstructure at the distal tibia provides a strength advantage to males in late puberty: a HR-pQCT study. J Bone Minl Res. 2010;25:1423–1432. doi: 10.1359/jbmr.091034. [DOI] [PubMed] [Google Scholar]
- 33.Ding M., Odgaard A., Danielsen C.C., Hvid I. Mutual associations among microstructural, physical and mechanical properties of human cancellous bone. J Bone Jt Surg Br. 2002;84:900–907. doi: 10.1302/0301-620x.84b6.11994. [DOI] [PubMed] [Google Scholar]
- 34.Cui Y., Bhandary B., Marahatta A., Lee G.H., Kim D.S., Chae S.W. Characterization of Salvia milthiorrhiza ethanol extracts as an anti-osteoporotic agent. BMC Complement Altern Med. 2011;11:120. doi: 10.1186/1472-6882-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Effendy N.M., Mohamed N., Muhammad N., Mohamad I.N., Shuid A.N. The effects of tualang honey on metabolism of postmenopaucal women. Evid Based Complement Altern Med. 2012:7. doi: 10.1155/2012/938574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lerner U.H. Bone remodeling in post-menopausal osteoporosis. J Dent Res. 2006;85(7):584–595. doi: 10.1177/154405910608500703. [DOI] [PubMed] [Google Scholar]
- 37.Hertrampf T., Schleipen B., Velders M., Laudenbach U., Fritzemeier K.H., Diel P. Estrogen receptor subtype-specific effects on markers of bone homeostasis. Mol Cell Endocrinol. 2008;291(1–2):104–108. doi: 10.1016/j.mce.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Banu J., Varela E., Bahadur A.N., Soomro R., Kazl N., Fernandes G. Inhibition of bone loss by Cissus quadrangularis in mice: a preliminary report. J Osteoporos. 2012:10. doi: 10.1155/2012/101206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Guan H., Li J., Fang Z., Chen W., Li F. Amlexanox suppresses osteoclastogenesis and prevent ovariectomy-induced bone loss. Sci Rep. 2015;5:13575. doi: 10.1038/srep13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Z., Dong J., Liu M., Li Y., Pan J., Liu H. Therapeutic effects of Cortex acanthopanacis aqueous extract on bone metabolism of ovariectimized rats. Evid Based Complement Altern Med. 2012:8. doi: 10.1155/2012/492627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rissanen J.P., Suominen M.I., Peng Z., Halleen J.M. Secreted tartrate-resistant acid phosphatase 5b is a marker of osteoclast number in human osteoclast cultures and the rat ovariectomy model. Calcif Tissue Int. 2008;82(2):108–115. doi: 10.1007/s00223-007-9091-4. [DOI] [PubMed] [Google Scholar]
- 42.Kim T.H., Jung J.W., Ha B.G., Hong J.M., Park E.K., Kim H.J. The effects of luteolin on osteoclast differentiation, function in vitro and ovariectomy-induced bone loss. J Nutr Biochem. 2011;22:8–15. doi: 10.1016/j.jnutbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 43.Mundy G.R., Poser J.W. Chemotactic activity of the γ-carboxyglutamic acid containing protein in bone. Calcif Tissue Int. 1983;35(2):164–168. doi: 10.1007/BF02405025. [DOI] [PubMed] [Google Scholar]
- 44.Villafán-Bernal J.R., Sánchez-Enríquez S., Muñoz-Valle J.F. Molecular modulation of osteocalcin and its relevance in diabetes. Int J Mol Med. 2011;28:283–293. doi: 10.3892/ijmm.2011.706. [DOI] [PubMed] [Google Scholar]
- 45.Yoon H.Y., Cho D.C., Yu S.H., Kim K.T., Jeon Y., Sung J.K. The change of bone metabolism in ovariectomized ratsl analyses of microCT scan and biochemical markers of bone turnover. J Korean Neurosurg Soc. 2012;51:323–327. doi: 10.3340/jkns.2012.51.6.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Glowacki J., Lian J.B. Impaired recruitment and differentiation of osteoclast progenitors by osteocalcin-deplete bone implants. Cell Differ. 1987;21(4):247–254. doi: 10.1016/0045-6039(87)90479-9. [DOI] [PubMed] [Google Scholar]
- 47.Siegel R.C., Page R.C., Martin G.R. The relative activity of connective tissue lysyl oxidase and plasma amine oxidase on collagen and elastin substrates. Biochim Biophys Acta. 1970;222:552–554. doi: 10.1016/0304-4165(70)90154-6. [DOI] [PubMed] [Google Scholar]
- 48.Lowe N.M., Lowe N.M., Fraser W.D., Jackson M.J. Is there a potential therapeutic value of copper and zinc for osteoporosis? Proc Nutr Soc. 2002;61:181–185. doi: 10.1079/PNS2002154. [DOI] [PubMed] [Google Scholar]
- 49.Holloway W.R., Collier F.M., Herbst R.E., Hodge J.M., Nicholson G.C. Osteoblast-mediated effects of zinc on isolated rat osteoclasts: inhibition of bone resorption and enhancement of osteoclast number. Bone. 1996;19:137–142. doi: 10.1016/8756-3282(96)00141-x. [DOI] [PubMed] [Google Scholar]
- 50.Aina V., Perardi A., Bergandi L., Malavasi G., Menabue L., Morterra C. Cytotoxicity of zinc-containing bioactive glasses in contact with human osteoblasts. Chem Biol Interact. 2007;167:207–218. doi: 10.1016/j.cbi.2007.03.002. [DOI] [PubMed] [Google Scholar]