Abstract
Background
Nigella sativa (black cumin) and Cinnamomum cassia (Cinnamon) are an integral part of the Indian diet, and have also been sourced in the ayurveda, the traditional Indian system of medicine, for their medicinal properties. Both the herbs individually have been successfully evaluated for their preliminary antidiabetic potential.
Objective
Herein, we dived deeper into antidiabetic properties of these herbs, by investigating the combinatorial effect of both herbs, on parameters of diabetes and further, as an adjunct to metformin therapy, for assessing the pharmacodynamics of herb-drug interaction in diabetes mellitus. The objectives were to screen the combinatorial extract of Nigella sativa & Cinnamomum cassia’s (NSCCe) alone and in combination with metformin for its potential in mitigating symptoms of diabetes mellitus-alone, and as an adjunct therapy with metformin.
Materials and methods
Diabetes was induced in the animals by a single intraperitoneal injection of streptozotocin. Animals were divided into seven groups with 6 animals each: Vehicle control, Negative control, Positive control (Metformin 50 mg/kg), treatment groups 4 and 5 received NSCCe at the doses of 100 mg/kg and 200 mg/kg, respectively. Groups 6 and 7 received the same doses, in combination with Metformin (50 and 25 mg/kg). Following a 28-day dosing period, plasma glucose levels, lipid profile and renal function profile were evaluated. Histopathological examinations were performed to measure any morphological change in kidney, liver and pancreatic tissue.
Results
Combination of Nigella sativa & Cinnamomum cassia extracts significantly normalized plasma glucose levels, lipid profile and kidney function parameters, compared to the diabetic control group. Animals treated with the combinatorial extract and metformin showed more prominent effects on these parameters. Significant reversal in the pancreatic cell damage was observed on treatment with NSCCe.
Conclusion
This study generates evidence to support Nigella sativa & Cinnamomum cassia as an adjunctive in diabetes treatment protocols.
Keywords: Nigella sativa, Cinnamomum cassia, Combination therapy, Antidiabetic activity, Metformin, Streptozotocin, Type-I Diabetes
1. Introduction
Plant-based medicaments have served as the most important therapeutic agents to treat various diseases [1]. However, in recent time due to the increased incidence of the adverse drug reactions and economic burden of the modern system of medicine, there is a renewed and growing interest in the use of plant derived biologically active compounds as potent medicaments [1]. Diabetes mellitus is an endocrine disorder characterized by hyperglycaemia [2] and altered carbohydrate metabolism. In India, the number of people suffering from diabetes is expected to rise from 20 to 57 million in 2025 [2]. Type 1 diabetes mellitus (T1D) is one of the major factors contributing towards diabetes in youth, and children. As evident from the global epidemiological data, ≥ 85% of all diabetes cases in youth <20 years are attributed to T1D [3]. The incidence of T1D in adults is marginally lower than in children, however, about one fourth of persons diagnosed with T1D are adults [3]. Scrutinizing the recent Indian epidemiological data from the Registry of People with Diabetes [4] with Young Age at Onset (YDR), T1D seemed to be most prevalent cause of diabetes in youth, contributing over 60% of the cases. T1D is the result of autoimmune mediated destruction of β-cells which leads to dependence of the subject on exogenous insulin throughout his life. Current therapies utilized for T1D are very limited, and mostly centred to insulin administration. Insulin treatment is associated with a major drawback of glycaemic instability. Moreover, the repeated dose of parenteral insulin, and lack of efficient and feasible oral medications, make these therapies highly patient non-compliant. This is of prime concern in T1D, since considerable cases of such kind are reported in the pediatric population, and the therapy is life-long. Hence, search for alternative treatment areas, and research showing improvements in the disease parameters as compared to currently established treatments are always welcome. One such less explored but highly promising area is herbal medicine. The introduction of medicinal herbs that lower blood glucose level has brought a revolution in the field of medicine [5] and are considered as the next generation medicines for the management of diabetes [6]. WHO (2001) estimated that 80% of world population rely on medicinal plants for their primary health care needs [7]. The ethnobotanical information reveals that about 800 plants like Gymnema sylvestre, Brassica juncea, Hibiscus rosa sinesis, Lantana camara, Momordica charantia, Pterocarpus marsupium etc. possess anti-diabetic potential [8]. Nigella sativa, traditionally used as the component of food can reduce glucose absorption in intestine and hepatic gluconeogenesis [9]. Thymoquinone is an active component of Nigella sativa that is reported to reduce the blood glucose level as well as body weight [10]. Thus, the plant is a potent nutraceutical for the management of diabetes mellitus. On the other hand, methyl hydroxyl chalcone polymer (MHCP), a biologically active substance of Cinnamomum cassia was proposed to be effective insulin mimetic which activates the pathways leading to glucose utilisation in cells [11]. These herbs have been reported to delay carbohydrate digestion by competitively inhibiting α-glucosidase a membrane bound enzyme of the intestinal brush border [12]. Nigella sativa and C. cassia, both contain polyphenols which facilitates lipid and carbohydrate metabolism. It also controls hyperglycemia, dyslipidemia, insulin resistance, oxidative stress and stress sensitive signalling pathways and inflammatory pathways [13], [14]. Thus, the present study was undertaken to evaluate pharmacodynamic interaction of the combinatorial extract of Nigella sativa and C. cassia, (NSCCe) in T1D induced rats. The NSCCe was compared with metformin as standard, and was evaluated for α-glucosidase inhibitory activity, biochemical estimation such as glucose, lipids, creatinine, blood urea nitrogen, and histopathology.
2. Materials and methods
2.1. Chemicals
Streptozotocin (Batch number KL3456A) was purchased from SRL Laboratories, India. Metformin was received as gift sample from USV Ltd, Mumbai, India. Glucose estimation kit, triglyceride estimation kit, total cholesterol estimation kit, low density lipoprotein direct kit and high density lipoprotein direct kit were procured from Transasia Bio-medicals Ltd, Mumbai, India.
2.2. Plant material
The seeds of Nigella sativa & C. cassia were purchased from a local vendor in Vile Parle (East) Mumbai. The specimens of the plants were authenticated from Agharkar Research Institute, Pune & the voucher samples were kept for reference. The voucher no. is S-170 for Nigella sativa & S/B 114 for C. cassia.
2.3. Animals
Male Wistar rats weighing between 150 and 200 gm were procured from the animal house of SPP School of Pharmacy & Technology Management SVKM's (NMIMS). The animals were maintained in well ventilated room with 12 h light and 12 h dark cycle in polypropylene cages. Standard feed and tap water was provided ad libitum throughout the experimentation period. The study was designed according to the OECD guidelines, and the protocol of the research work approved by the CPCSEA and the Institutional Animal Ethics Committee (Protocol approval number: 1027/PO/a/07/CPCSEA).
2.4. Preparation of plant extracts
Nigella sativa (Family-Ranunculaceae) & C. cassia (Family Lauracea) were extracted by ethanol 95% v/v. The powdered seeds and bark of Nigella sativa and C. cassia, respectively were defatted by petroleum ether. Extraction was carried out in Soxhlet apparatus. The Soxhlet apparatus was heated at 65 °C for 6 h to prepare the extract of 100 gm of dried powdered Nigella sativa and C. cassia. The suspension was filtered and the residues were re-extracted. These different solvent extracts were pooled and concentrated in rotary flash evaporator at temperature 45 °C. Percentage yield of the extract was 4% for Nigella sativa and 5.2% for C. cassia.
2.4.1. Phytochemical screening of the extracts
Both extracts were screened for major phytochemical ingredients, namely tannins, flavonoids, phenolic compounds and saponins. using the methods of Khandelwal [15]. Analysis yielded the presence of phenolics, flavonoids and tannins.
2.4.2. Preparation of the combinatorial extract NSCCe
The extracts were then combined in 1:1 ratio by preparing a suspension in distilled water using 0.2% of sodium CMC as a suspending agent. The quantity was based on doses, either 200 mg/kg or 100 mg/kg [16], [17].
2.5. In vitro studies
2.5.1. α-glucosidase inhibitory activity
This test was undertaken to screen postprandial glucose excursion potential through α-glucosidase inhibitory activity of NS and CC extract per se and in combination (NSCCe). α-glucosidase inhibition plays an important role by delaying the carbohydrate metabolism in small intestine and hence decreasing the postprandial blood glucose and insulin levels.
The IC50 values for the extracts were calculated by extrapolation method [18].
2.6. Animal studies
2.6.1. Acute toxicity study [19].
Acute toxicity was performed using the ethanolic extract of Nigella sativa & C. cassia in female albino mice. From the three groups containing three female mice, the extract was administered orally at dose of 2000 mg/kg. Distilled water was administered to control group, orally by gavage. Animals were observed individually once during the first 30 min, periodically during the first 24 h, with special attention during the first 4 h, thereafter, for 14 days. If no mortality was recorded, it was planned to conduct a confirmatory test for another 14 days for validation of the results obtained in step 1. The dose was found to be safe, as confirmed with repetition of the test, and hence further animal studies were conducted.
2.6.2. Induction of T1D [20].
T1D was induced in overnight fasted male Wistar rats by single intraperitoneal injection of freshly prepared solution of streptozotocin (STZ) (45 mg/kg). STZ was dissolved in citrate buffer (pH = 4.5) and was administered to rats by intraperitoneal injection. Blood glucose was measured after 3 days of dosing. The animals with fasting blood glucose levels consistently for 4 consecutive readings above 300 mg/dl were considered diabetic and selected for further evaluation.
2.6.3. Experimental design
The animals were divided into seven groups consisting of 6 rats in each group respectively. The groups were as follows:
Group I: Vehicle Control.
Group II: STZ induced Diabetic rats (Negative control).
Group III: Metformin (50 mg/kg) treated STZ induced T1D rats.
Group IV: Low dose (100 mg/kg) NSCCe treated STZ induced T1D rats.
Group V: High dose (200 mg/kg) NSCCe treated STZ induced T1D rats.
Group VI: Metformin (50 mg/kg) + Low dose (100 mg/kg) NSCCe treated STZ induced T1D rats.
Group VII: Metformin (25 mg/kg) + High dose (200 mg/kg) NSCCe treated STZ induced T1D rats.
2.6.4. Biochemical estimations
Blood was withdrawn from the overnight fasted rats on 0, 14th, 28th day of the study. Plasma was separated and the plasma glucose level was estimated weekly while the lipid profile and kidney profile (Creatinine Level Estimation and Blood Urea Nitrogen Estimation) were evaluated on the 28th day, using commercially available kits by semi-automated bio analyser. The body weight and food consumption data were recorded weekly. At the end of the study, oral glucose tolerance test (OGTT) was conducted [20], [21].
2.6.5. Histopathology analysis
After 28 days the rats were sacrificed and liver, kidney and pancreas were preserved in 10% neutral buffered formalin for routine histopathological examination. The block of tissues were prepared by embedding in paraffin, sectioned and finally stained with haematoxylin and eosin (H, E) and were examined under light microscope. Evaluation of histopathology was performed by pathologist.
2.7. Statistical analysis
The differences among experimental and control groups were determined using the GraphPad INSTAT 3.0 software for windows. Comparisons among different groups were performed by analysis of variance (ANOVA). Statistically significant differences between control and experimental groups were assessed by Student's t-test and differences were considered significant when p < 0.05. All results are expressed as mean ± standard error of mean (SEM).
3. Results
3.1. In vitro studies (α-glucosidase inhibitory activity)
Thus, as evident from Fig. 1 α-glucosidase inhibitory activity of the extract and acarbose was found to be concentration dependent. The combinatorial extract NSCCe shows enhanced inhibiting effect than the individual extracts. Thus, the IC50 value for the combination was much lower than the individual extracts, indicating more potency of the combination (Table 1).
Fig. 1.
Comparative α-glucosidase inhibitory activity.
Table 1.
Comparative α-glucosidase inhibitory activity and IC50 values.
| Conc. | % Inhibition by Acarbose | % Inhibition by NS | % Inhibition by CC | % Inhibition by NS + CC |
|---|---|---|---|---|
| 50 | 13.64 | 2.5 | 4.2 | 9.9 |
| 100 | 21.5 | 5.1 | 10 | 17.9 |
| 200 | 34.6 | 22.0 | 24.6 | 38.5 |
| 400 | 44.5 | 38.0 | 50.0 | 67.8 |
| 800 | 56.6 | 62.0 | 79.5 | 88.4 |
| 1000 | 67.1 | 90.0 | 96.5 | 97.9 |
| IC50 = 560 ppm | IC50 = 610 ppm | IC50 = 360 ppm | IC50 = 260 ppm |
3.2. Acute toxicity study
Step 1: Acute toxicity studies revealed that the NSCCe was safe up to 2000 mg/kg of body weight and approximate LD50 was found to be more than 2000 mg/kg. Additionally, at the said dose no mortality, change in body weight, overt behaviour or clinical symptoms of toxicity were demonstrated (Table 2).
Table 2.
Acute toxicity study of NSCCe.
| Group | Dose | Sign of toxicity (ST/NB)a | Mortality (D/S)a |
|---|---|---|---|
| II NS | 2000 mg/kg | 0/3 | 0/3 |
| III CC | 2000 mg/kg | 0/3 | 0/3 |
| IV NSCCe | 2000 mg/kg | 0/3 | 0/3 |
ST: Sign of toxicity, NB: Normal Behaviour; D: No. of Deaths, S: Survive, NS: Nigella sativa, CC: C. cassia, NSCCe: Combinatorial extract.
Values are expressed as number of animals (n = 3).
Step 2: As no mortality was recorded, the acute toxicity study was repeated after a week, with the same doses (OECD 423 guidelines). (see Table 3).
Table 3.
Acute toxicity studies Step 2.
| Group | Dose (mg/kg) | Sign of toxicity (ST/NB) | Mortality (D/S) |
|---|---|---|---|
| I NS | 2000 | 0/3 | 0/3 |
| II CC | 2000 | 0/3 | 0/3 |
| III NSCCe | 2000 | 0/3 | 0/3 |
3.3. Biochemical estimation
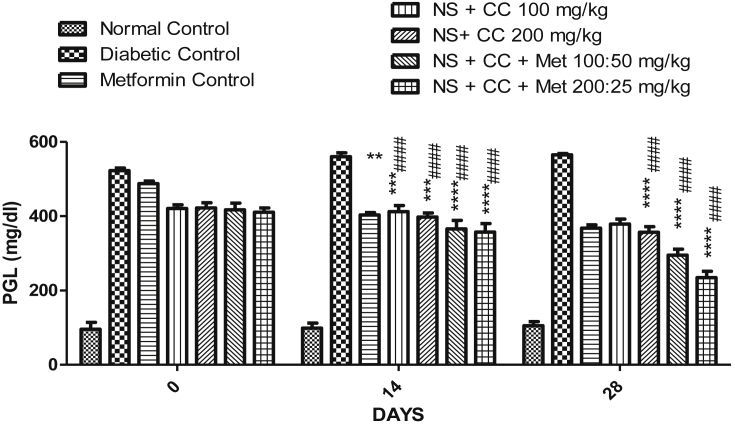
3.3.1. Plasma glucose level
The effect of NSCCe on plasma glucose level is shown in Fig. 2. Plasma glucose levels were determined on 0, 14 and 28 days of the study. The significant reduction in plasma glucose was observed in groups treated with NSCCe alone, in combination with metformin, when compared with the diabetic control. However, marked reduction in elevated glucose was observed in the groups treated with NSCCe in combination with metformin, with 29% and 42% reduction in the respective groups, at the end of the study. Additionally, on the 28th day, the percentage difference in the plasma glucose level was recorded to be more than 90% between the diabetes control group and NSCCe treated groups.
Fig. 2.
Effect of extract of Nigella sativa & C. cassia on plasma glucose level. **p < 0.05, ***p < 0.01, ***p < 0.001 were compared with the STZ (Diabetic) control group and #### p < 0.01 were compared with metformin group.
3.3.2. Lipid profile
Significant (p < 0.05) reduction in the LDL, total cholesterol and triglycerides was observed on the 28th day, in the NSCCe and metformin treated groups. The reduction in the LDL was most significant (p < 0.001) for the group treated with high dose extract and low dose metformin (Group VIII), as compared to the diabetic untreated rats. Although, lipid profiling is not of significance in this animal model, the results as assessed on the 28th day show marked difference in the treated and untreated groups (Fig. 3).
Fig. 3.
Comparison of lipid profile in various treatment groups on day 28. **p < 0.05, ***p < 0.01, ***p < 0.001 were compared with the STZ (Diabetic) control group and #### p < 0.01 were compared with metformin group.
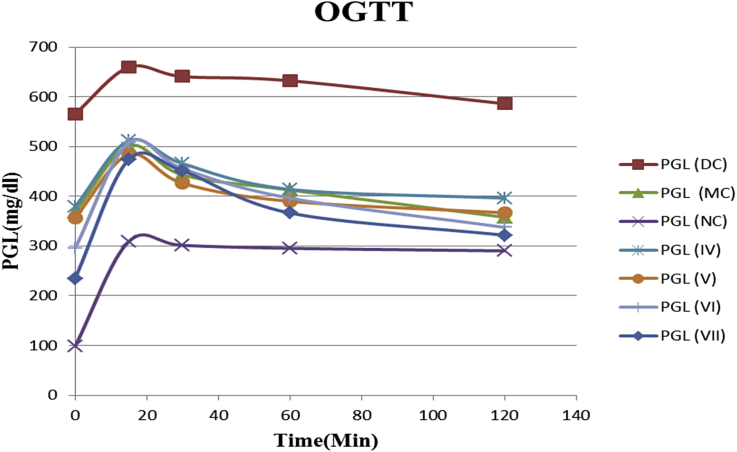
3.3.3. Oral glucose tolerance test
STZ administered groups demonstrated significant impairment in glucose tolerance to exogenously administered glucose (2 g/kg) as evident from elevated glycaemic level at 30, 60, 90 and 120 min post glucose challenge, compared with the vehicle treated control group (p < 0.05). However, treatment with NSCCe, alone and in combination with metformin significantly improved glucose tolerance (p < 0.001) to exogenously administered glucose (2 g/kg) after 60, 90 and 120 min interval on OGTT streptozotocin fed rats compared with the untreated control group. A noteworthy trend in glucose control was seen in the groups treated with the combination of high dose NSCCe with low dose metformin (Group VII) (Fig. 4).
Fig. 4.
Comparative results of OGTT. Terms and abbreviations: PGL: Plasma glucose level; NC: Normal control; DC: Diabetic control; MC: Metformin control; 1V-VIII: Individual group numbers.
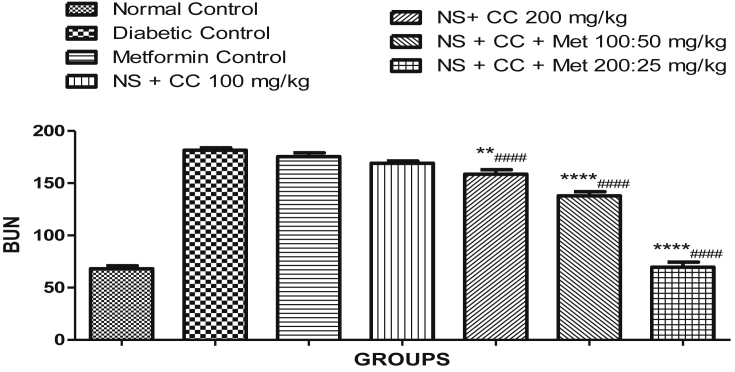
3.3.4. Kidney profile
The effect of NSCCe on kidney profile is shown in Fig. 5, Fig. 6. The kidney profile covered estimation of Creatinine Level (CL) and Blood Urea Nitrogen (BUN). As seen from the figure, both the parameters were on decreasing trends in the metformin and extract treated groups. As far as the creatinine level was concerned, the groups fed with the extracts alone as well as with combination of metformin showed significantly similar results as metformin, which showed significant improvement compared to the diabetic control groups.
Fig. 5.
Effect of extract of Nigella sativa & C. cassia on Kidney Profile (Creatinine). **p < 0.05, ***p < 0.01, ****p < 0.001 were compared with the STZ (diabetic) control group and #### p < 0.001 were compared with metformin group.
Fig. 6.
Effect of extract of Nigella sativa & C. cassia on Kidney Profile (BUN). **p < 0.05, ***p < 0.01, ****p < 0.001 were compared with the STZ (diabetic) control group and #### p < 0.001 were compared with metformin group.
As far as the BUN level was concerned, significant improvements were seen in the animal groups fed with the extracts either alone or in combination with metformin. The groups treated with the extracts showed significant reduction in BUN compared to metformin group as well as the diabetic control group.
3.3.5. Effect of NSCCE on body weight, feed and water intake
Sub acute treatment of NSCCe with or without metformin for 28 days lowered the food consumption and water intake significantly when compared with diabetic control (Fig. 8, Fig. 9). Treatment with NSCCe showed inhibition of reduction in body weight in streptozotocin induced diabetic animals. Diabetic untreated rats exhibited significant (p < 0.001) loss in body weight when compared with normal rats (Fig. 7). However, the decrease in body weight due to hyperglycemia was ameliorated by treatment with NSCCe along with metformin.
Fig. 8.
Daily feed intake as assessed on weekly basis.
Fig. 9.
Daily water intake as assessed on a weekly basis.
Fig. 7.
Comparative data on the weights of animals throughout the study.
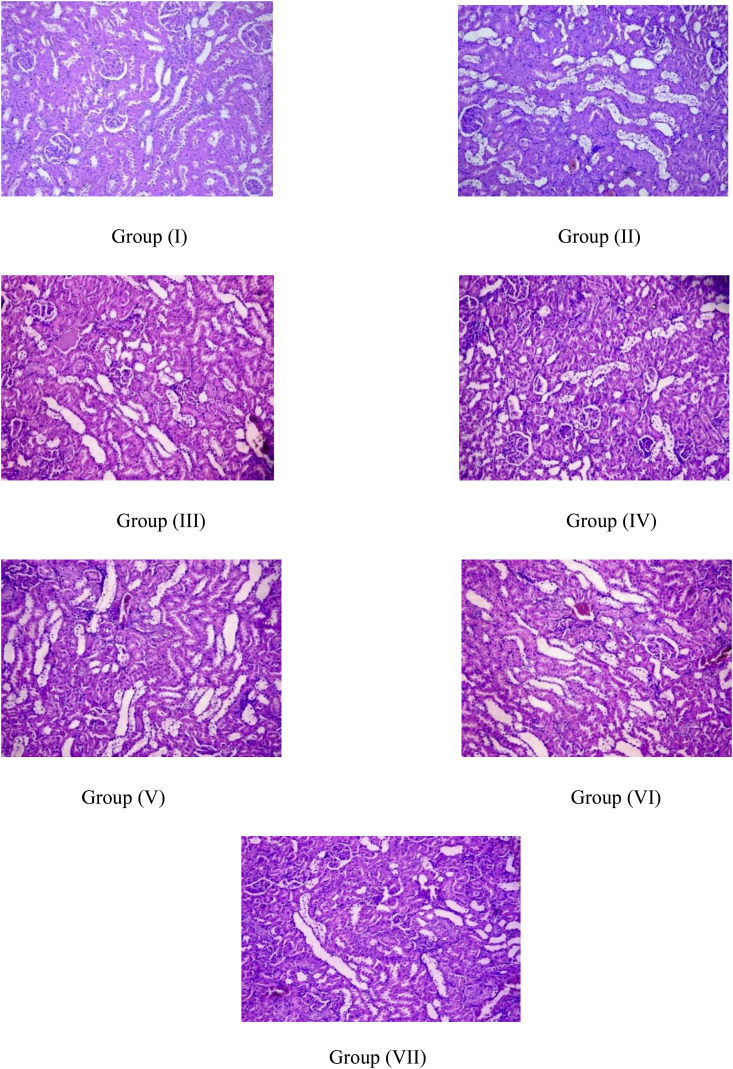
3.4. Histopathological studies
Histopathological studies [Fig. 10, Fig. 11, Fig. 12] showed the presence of degenerative changes and atrophy in diabetic rats as compared to the normal control. The comparative presence of beta cells of the islets of Langerhans and their size in pancreas was also found to be reduced in diabetic rats. Histopathological results exhibited significant improvement in pancreatic morphological changes treated with NSSCe per se and in combination with metformin. Additionally, the results with the extracts alone, and in combination with metformin showed better improvement in histopathology of pancreas as compared to the groups given metformin alone. Although, no remarkable difference in improvement was seen in the renal tissue as seen in those of the pancreas, a slight improvement in the vacuolar degeneration of tubular epithelium of the renal tissue was noted in the NSCCe treated groups.
Fig. 10.
Histopathological analysis of Pancreas.
Fig. 11.
Histopathological analysis of Kidney.
Fig. 12.
Histopathological analysis of Liver.
However, not much change was observed in the degenerated hepatocytes. Mild to moderate diffuse granular degeneration was noted in all the diabetic animals, irrespective of the treatment. Animals treated with the combination of NSCCe and metformin showed a comparatively lower degree of degeneration which, however, was not significantly distinct from other groups.
4. Discussion
T1D is an autoimmune disorder caused by pancreatic lymphocytic infiltration and beta cells destruction within the pancreatic islets of Langerhans by auto antibodies formed in the body. Due to destruction of beta cells in pancreas, its number and volume decreases which cause severe permanent insulin deficiency. The current treatment lines rely mostly on insulin therapy to ameliorate the disorder. However, metformin is gaining a role especially in controlling the hallmark symptoms such as hyperglycemia. Nigella sativa and C. cassia have reported glucose lowering activities in the previous literature [13], [14]. The evidence has shown different mechanisms of action of these extracts for glucose lowering and pancreas protective action individually. However, the studies emphasizing the combination of these extracts, their pharmacodynamic interaction with high and low dose metformin, and a permutation and combination of these factors on amelioration of various aspects of T1D have not been studied, which is the main objective and novelty of this manoeuvre.
This study aims to evaluate the therapeutic effect of NSCCe. We also aimed to investigate the effect of NSCCe in reducing the dose of metformin. Our study is based on the findings of previous studies that implicated the antidiabetic property of both Nigella sativa and C. cassia. However, reducing the doses and combining both of these extracts, along with metformin, and their pharmacological implications are not investigated previously.
To investigate the effects of combined administration of both the extracts over the extracts given individually, and to exploit the differing mechanism of actions of both the extracts, we initiated the study with a comparative α-glucosidase inhibitory activity. The results exhibited better IC50 values, and better inhibition of α-glucosidase by NSCCe, as compared to the respective individual extracts. Hence the combination NSCCe was considered and further studied in animals, to see whether the results produced in the former test are reproduced and translated into controlling disease parameters in diabetic animals. Hence, the half doses of each individual extract than those reported in the previous literature were considered, for investigating any possible synergism [11], [16]. The groups were given NSCCe in two doses, higher dose of 200 mg/kg and the same dose divided into half (100 mg/kg). T1D was induced using STZ which led to increased plasma glucose level, BUN and creatinine levels. Different treatment groups including metformin alone, two different doses (100 mg/kg and 200 mg/kg) of NSCCe per se and in combination with low dose and high dose of metformin (25 mg/kg and 50 mg/kg) were used. We observed significant reduction in plasma glucose level, in the groups treated with NSCCe alone and in combination with both low dose and high dose metformin. Thus, the highest degree of glucose lowering produced with low dose metformin and high dose NSCCe, suggests a synergistic effect between the two. Thus, implies reducing the daily dose of metformin, if combined with high dose NSCCe, owing to marked lowering of hyperglycemia, at par with high dose metformin given alone. Our results are in agreement with previous studies that showed antidiabetic efficacy of ethanolic extract of Nigella sativa [11], [22], [23]. However, more importantly is the effect produced by the combination, on regeneration of damaged organs such as pancreas, the effect that was more marked in the animals treated with NSCCe, even as compared to metformin alone. Post-induction of diabetes, there was a significant weight loss in all the groups compared to the normal control. Treatment with NSCCe with or without metformin reversed this state within 14 days of treatment, and preventing further weight loss as compared to the untreated diabetic group (p < 0.05). Untreated diabetic rats showed increased daily feed intake, as compared to the treated diabetic rats. Thus, the symptoms of diabetes viz. weight loss, and increased food and water intake, were alleviated on treatment with NSCCe. The normalization of elevated glucose levels, were also accompanied by improvement in the lipid parameters, especially LDL and total cholesterol. These findings were further corroborated when improved glycaemic control was observed in patients treated with NSCCe, when assessed by OGTT. Significant reduction in the LDL, triglycerides and cholesterol levels denote an ameliorating effect on symptoms of diabetes. This finding about lipid lowering would be further confirmed in specific models. However, primary evidence showing improvement in the lipid profile yield results in the favour of NSCCe. Thus, these findings support the diabetes alleviating potential of the combinatorial extract, alone and in combination with metformin, manifesting as improved glycaemic control, and normalizing cholesterol levels.
According to finding of Benhaddou-Andaloussi et al. [24], ethanolic extract of Nigella sativa has the property of regeneration and proliferation of pancreatic beta cells and can also enhance glucose uptake and induce secretion of insulin from beta cells in pancreas [20]. Studies also corroborate the hypoglycemic potential of C. cassia via different mechanisms including enhanced glucose uptake, delayed gastric emptying, insulin receptor activation [25], [26]. However, none of the studies mention the combined effect of these extracts and differing mechanisms of action, and whether these combined actions have any pharmacological implications, especially with synthetic drugs remains an area unsearched.
Diabetes is often associated with kidney disorder and renal failure [27], [28]. According to statistics approximately 20–30% of type-1 and type-2 diabetes patients suffer from diabetic nephropathy [25]. The levels of BUN and creatinine are elevated in case of impaired kidney function, diabetes, congestive cardiac failure and infection [28]. So we also evaluated BUN and creatinine level as they are considered as important markers of kidney disorder. Creatinine levels as well as BUN levels significantly improved in animals treated with NSCCe, either alone and in combination with metformin, most noteworthy was the observation that improvement in BUN levels were seen only in those groups subjected to treatment with NSCCe; the effect which was not marked even in the groups treated with metformin alone. This may be attributed to the antioxidant activity of the extracts. The polyphenols and flavonoids have been reported to be effective in reducing the oxidative stress and thus restoring kidney function [24], [25]. In this study, the preliminary phytochemical screening of Nigella sativa and C. cassia extract showed presence of flavonoids and phenolic compounds, to which this activity may be attributed [29].
As far as the histopathological studies were concerned, they showed degenerative changes and atrophy in the pancreatic, renal and the hepatic tissue of the rats treated with STZ. The comparative presence of islets of Langerhans and their size was also found to be reduced in diabetic rats. The treatment groups showed a significant improvement in the pancreatic tissue when compared to the diabetic animals. The results in groups given the extracts were better than the groups given metformin alone. Although, these results were more prominent with the pancreatic tissue, slight improvement in the vacuolar degeneration of tubular epithelium of the renal tissue was observed. However, no significant changes were noted in the degenerated hepatocytes. This may yield an important finding of cellular regeneration with the extracts, possibly correlating with an improvement in the respective organ function parameters, which are of a significant relevance in T1D.
Thus, the study showed signals for antidiabetic activity of the NSCCe, which may be used as an adjunct treatment with current antidiabetic drugs (in this case, metformin). Based on the preliminary findings, we can say that along with enhanced glucose and lipid control, the treatment with the extract reversed pancreatic cell damage. Thus, the combinatorial extract NSCCe demonstrated a protective effect on the islets plausibly by countering the immune function. However, further well planned studies in preclinical and clinical settings may be needed to reiterate these findings and, also to yield insights into the active components, mechanistic approach for antidiabetic activity, and pancreatic protection in T1D.
5. Conclusion
The present study demonstrated that the extract of Nigella sativa and C. cassia have potential to be utilized as an adjunct therapy with current antidiabetic agents due to better efficacy against hyperglycemia. The study also revealed a remarkable lipid lowering activity of low dose synthetic drug and herb combination than the individual extracts. Most importantly, the study demonstrated the capability of the combination to counter the immune function and render protection to islets, which is not exhibited by any other current therapies.
Sources of funding
This research was funded by the Department of Pharmacology, Shobhaben Pratapbhai Patel School of Pharmacy and Technology Management, SVKMs NMIMS, Mumbai-57.
Conflict of interest
None.
Acknowledgement
The authors would like to thank SPP School of Pharmacy and Technology Management, SVKM's NMIMS for funding and providing the facilities utilized in this project.
Footnotes
Peer review under responsibility of Transdisciplinary University, Bangalore.
References
- 1.Chandra A., Singh R.K., Tewari L. Antioxidative potential of herbal hypoglycemic agents in diabetes: an overview. SFRR Indian Bull. 2004;3:24–26. [Google Scholar]
- 2.Shajeela P.S., Kalpanadevi V., Mohan V.R. Potential antidiabetic, hypolipidaemic and antioxidant effects of Xanthosoma sagittifolium extract in alloxan induced diabetic rats. Int J Pharm Sci. 2013;5(1):27–31. [Google Scholar]
- 3.Consensus guidelines for the management of insulin-dependent (type 1) diabetes, European IDDM Policy Group 1993. Diabet Med. 1993;10(10):990–1005. [PubMed] [Google Scholar]
- 4.Praveen P.A., Madhu S.V., Mohan V., Das S., Kakati S., Shah N. Registry of youth Onset diabetes in India (YDR): rationale, recruitment, and current status. J Diabetes Sci Technol. 2016;10(5):1034–1041. doi: 10.1177/1932296816645121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marles R.J., Fransworth N.R. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2:137–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 6.Day C. Traditional plant treatments for diabetes mellitus: pharmaceutical foods. Br J Nutr. 1998;80:5–6. doi: 10.1017/s0007114598001718. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization . 2003. Traditional medicine. WHO fact sheet No. 134. Geneva: WHO revised. [Google Scholar]
- 8.Patel R., Ahirwar B., Ahirwar D. Current status of Indian medicinal plants with antidiabetic potential: a review. Asian Pac J Trop Biomed. 2011;1(2):S291–S298. [Google Scholar]
- 9.Afaf J.A., Muhamed T.O., Ariza A., Effat O. Immunomodulatory effect of Nigella sativa oil in the disease process of type 1 diabetic rats. Res J Pharm Biol Chem Sci. 2014;4(1):980–988. [Google Scholar]
- 10.Ilaiyaraja N., Khanum F. Nigella sativa L: a review of therapeutic applications. J Herb Med Toxicol. 2010;4(2):1–8. [Google Scholar]
- 11.Ranasinghe P., Perera S., Gunatilake M., Abeywardene E., Gunapala N., Premakumara S. Effects of Cinnamomum zeylanicum (Ceylon cinnamon) on blood glucose and lipids in a diabetic and healthy rat model. Pharmacogn Res. 2012;4(2):73–79. doi: 10.4103/0974-8490.94719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rchid H., Chevassus H., Nmila R., Guiral C., Petit P., Chokairi M. Nigella sativa seed extracts enhances glucose-induced insulin release from rat-isolated Langerhans islets. Fundam Clin Pharmacol. 2004;18(5):525–529. doi: 10.1111/j.1472-8206.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- 13.Kaatabi H., Bamosa A.O., Badar A., Al-Elq A., Hozaifa B.A., Lebda F. Nigella sativa improves glycemic control and ameliorates oxidative in patients with type 2 diabetes mellitus: placebo controlled participant blinded clinical trial. PLoS One. 2015;10:1–15. doi: 10.1371/journal.pone.0113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin B., Panickar K.S., Anderson R.A. Cinnamon: potential role in the prevention of insulin resistance, metabolic syndrome, and type 2 diabetes. J Diabetes Sci Technol. 2012;4(3):685–693. doi: 10.1177/193229681000400324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khandelwal K.R. 15th ed. Nirali Prakashan, Pune; Pune, India: 2006. Practical pharmacognosy; pp. 149–159. [Google Scholar]
- 16.Wahid Z., Sara Z., Bhatti H.A., Wajahat M., Khalid R., Abbas Ghulam. Cinnamomum cassia: an implication of serotonin reuptake inhibition in animal models of depression. Nat Prod Res. 2015;30(10):1212–1214. doi: 10.1080/14786419.2015.1047776. [DOI] [PubMed] [Google Scholar]
- 17.Abbasali A., Saeed N., Maryam M., Mohammad S., Seyed A.R., Seyed M.M. Nigella sativa seed decreases endothelial dysfunction in streptozotocin-induced diabetic rat aorta. Phytomed. 2016;6(1):67–76. [PMC free article] [PubMed] [Google Scholar]
- 18.Patel A., Kaur G., Meena C. α-glucosidase inhibitory activity of curcumin and its Comparison with combinatorial extract consisting of curcumin with piperine and quercetin. Pharmacol online. 2011;3:796–801. [Google Scholar]
- 19.OECD . OECD Guidelines for testing of chemicals. No. 423. Organization for economic cooperation and development; Paris, France: 2001. Acute oral toxicity test method. [Google Scholar]
- 20.Wu Jinzi, Yan Liang-Jun. Streptozotocin-induced type 1 diabetes in rodents as a model for studying mitochondrial mechanisms of diabetic β cell glucotoxicity. Diabetes Metab Syndr Obes. 2015;8:181–188. doi: 10.2147/DMSO.S82272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaur G., Meena C. Amelioration of obesity, glucose intolerance, and oxidative stress in high-fat diet and low-dose streptozotocin-induced diabetic rats by combination consisting of “curcumin with piperine and quercetin”. ISRN Pharmacol. 2012;2012:957283. doi: 10.5402/2012/957283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balbaa M., El-Zeftawy M., Ghareeb D., Taha N., Mandour A.W. Nigella sativa relieves the altered insulin receptor signaling in streptozotocin-induced diabetic rats fed with a high-fat diet. Oxidative Med Cell Longev. 2016;2016:2492107. doi: 10.1155/2016/2492107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanaa F.W., Seham A.H. Cytological and Histochemical Studies in Rat Liver and Pancreas during progression of streptozotocin induced diabetes and possible protection of certain natural antioxidants. J Nutr Food Sci. 2012;2(9):1–7. [Google Scholar]
- 24.Ali B., Martineau L.C., Danielle S., Vuong T., Leduc C., Erik J. Antidiabetic activity of Nigella sativa seed extract in cultured pancreatic β-cells, skeletal muscle cells, and adipocytes. Pharm Biol. 2008;46(1–2):96–104. [Google Scholar]
- 25.Tandon R., Gupta A., Ray A. Mechanism of action of Anti-diabetic property of Cinnamic acid, a principal active ingredient from the bark of Cinnamomum cassia. Int J Ther App. 2013;9:39–45. [Google Scholar]
- 26.Kirkham S., Akilen R., Sharma S., Tsiami A. The potential of cinnamon to reduce blood glucose levels in patients with type-2 diabetes and insulin resistance. Diabetes, Obes Metabolism. 2009;11:1100–1113. doi: 10.1111/j.1463-1326.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- 27.Stephanie E., Maalouf R., Jaffa A.A., Nassif J., Hamdy A., Rashid A. 20-HETE and EETs in diabetic nephropathy: a novel mechanistic pathway. PLoS One. 2013;8(8):1–10. doi: 10.1371/journal.pone.0070029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross J.L., de Azevedo M.J., Silveiro S.P., Canani L.H., Caramori M.L., Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28(1):164–176. doi: 10.2337/diacare.28.1.164. [DOI] [PubMed] [Google Scholar]
- 29.Nasri H., Rafieian-Kopaei M. Protective effects of herbal antioxidants on diabetic kidney disease. J Res Med Sci. 2014;19(1):82–83. [PMC free article] [PubMed] [Google Scholar]