Abstract
Metal hypersensitivity (MHS) is a rare complication of total joint arthroplasty that has been linked to prosthetic device failure when other potential causes have been ruled out. The purpose of this review was to conduct an analysis of existing literature in order to get a better understanding of the pathophysiology, presentation, diagnosis, and management of MHS. It has been described as a type IV hypersensitivity reaction to the metals comprising prosthetic implants, often nickel and cobalt-chromium. Patients suffering from this condition have reported periprosthetic joint pain and swelling as well as cutaneous, eczematous dermatitis. There is no standard for diagnosis MHS, but tests such as patch testing and lymphocyte transformation testing have demonstrated utility, among others. Treatment options that have demonstrated success include administration of steroids and revision surgery, in which the existing metal implant is replaced with one of less allergenic materials. Moreover, the definitive resolution of symptoms has most commonly required revision surgery with the use of different implants. However, more studies are needed in order to understand the complexity of this subject.
Keywords: Total joint arthroplasty, Total knee arthroplasty, Total hip arthroplasty, Metal allergy, Metal hypersensitivity
1. Introduction
Despite the high success rate of total hip (THA) and total knee arthroplasty (TKA),1, 2 it is estimated that 10% to 20% of lower extremity total joint arthroplasties (TJA) annually are revision surgeries due to implant failure.3 The most common causes of failure include instability, infection, and stiffness for TKAs compared with dislocation and mechanical loosening for THAs.4, 5 However, in recent decades, attention has been drawn to metal hypersensitivity (MHS) as another possible cause of TJA failure.6
Metal hypersensitivity is a rare condition where the body develops an immunological reaction to the metallic portion of THA or TKA implants.6, 7 The frequency of cutaneous allergies to nickel, cobalt, and chromium in the general population, not related to arthroplasty, have been estimated to be 13%, 2%, and 1%, respectively, based on patch testing and blood analysis.8 Moreover, since these are the same metals that many THA and TKA components are made of, it is possible that patients who have these particular allergies may develop a reaction to these implants postoperatively. Patients who have MHS may present with periprosthetic joint pain and effusions, as well as a cutaneous, eczematous rash;9, 10, 11, 12 however, MHS is a diagnosis of exclusion, since the current methods of testing lack adequate sensitivity and validity.7
Although the condition is rare, the number of TJA patients that test positive for MHS has increased over the past two decades.1 The prevalence of cutaneous MHS in the general population is estimated to be 10% to 15%, while prevalence in patients with metallic implants may be as high as 25%.13, 14. Furthermore, the prevalence of cutaneous MHS in patients who had a malfunctioning prosthesis was estimated to be as high as 60%.13 However, the degree of association between MHS and TJA failure is currently unclear.15 Therefore, the purpose of this review was to evaluate the current literature for MHS related to TJA, specifically focusing on general allergic hypersensitivity reactions, in order to provide a better understanding of the pathology. We specifically reviewed: 1) basic science; 2) clinical syndromes; 3) diagnostic measures; and 4) management of TJA-related MHS.
1.1. Basic science
All metals experience some degree of corrosion when placed in contact with biological systems.13 Osteoclasts have been observed to proliferate and differentiate while adsorbed to prosthetic metallic surfaces, actively degrading the material and releasing ions into surrounding tissues and joint space.16 Metallic ions may then act as haptens, which interact with proteins to form antigenic complexes that stimulate the body’s inflammatory response.1
Metal hypersensitivity is defined as a type IV hypersensitivity reaction, which means that the body’s response is through a delayed cell-mediated response,1 where the antigenic complexes are first processed and presented to CD4+ T-lymphocytes by antigen presenting cells (APCs), which includes endothelial cells, macrophages, dendritic cells, or other immune cells found within synovial tissue.13, 14 The interaction between APCs and T-helper cells results in the subsequent activation of both CD4+ and CD8+ cells, as well as macrophages that release pro-inflammatory cytokines, including interleukin (IL)-1, IL-2, IL-6, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ,14, 17 which results in an adaptive immune response that may damage tissues and result in the symptoms associated with MHS.6 This mechanism of MHS differs from those seen with aseptic lymphocyte-dominated vasculitis-associated lesions and pseudotumors that occurs with adverse local tissue reactions that result from metal-on-metal THA implants.18, 19
Synovial fluid analysis of patients who have presented with MHS have been reported to have an increased concentrations of macrophages, polymorphonuclear leukocytes, and lymphocytes.20 Moreover, histologic analysis of the synovial membrane can demonstrate granulation tissue and fibrosis, along with numerous giant cells and calcification.20 Lymphocytic and plasma cell infiltrates in the surrounding synovial tissue have also been reported, and are indicative of a chronic inflammatory response that can be consistent with synovitis.7
1.2. Clinical syndromes
Metal hypersensitivity has been reported to be more common in women, and has been shown to occur between two months and two years postoperatively.6, 7 Patients typically present with periprosthetic synovitis and swelling, and less frequently with an eczematous dermatitis that may be local or generalized.7, 9, 10 The synovitis may present as pain that may be of burning quality, effusion, swelling, stiffness, and/or limited range of motion7, 9 and the dermatitis can be characterized as an erythematous, papular, pruritic, and scaly rash that may produce exudate.6, 11, 12
A study by Verma et al.12 reported on 15 TKA patients who developed cutaneous eczematic eruptions within 3 months of TKA. The rash was contained to the outer aspect of the knee in all cases, just lateral to the anterior midline incision.12 Similarly, Gao et al.21 reported on a case in which a TKA patient developed eczematous lesions on the skin surrounding the operative scar within 6 months after surgery. Given that these symptoms appeared postoperatively and all other potential causes had been ruled out, MHS was suspected.21 The lesions subsequently spread to the neck, buttocks, upper extremities, and ankles over the subsequent 3 months, and resulted in a chronic and recurrent dermatitis.21 Interestingly, there has been one reported case of a systemic response in which a TKA patient with suspected MHS developed a full-body dermatitis and alopecia.22
Although both TKA and THA patients may present with joint pain and swelling, the cutaneous reaction is not common among THA patients.23, 24 In a study that reported on 4 THA patients who had a suspected MHS, symptoms ranged from localized swelling to groin pain that worsened with walking.25 Additionally, osteolytic lesions in the proximity of the hip or knee implant may also be appreciated on radiographic images as a result of the inflammatory response and might result in aseptic loosening of the implant.6, 20, 24
1.3. Diagnosis
Metal hypersensitivity has been reported to be a diagnosis of exclusion,26 and should be considered when other causes of implant failure, including but not limited to infection and aseptic loosening, have been ruled out and inflammatory markers (CRP and ESR) and joint aspiration have demonstrated negative results.7, 27 Although no established standard for diagnosing MHS exists, skin patch testing, lymphocyte transformation testing, modified lymphocyte stimulation testing, and leukocyte migration inhibition testing have shown utility.1, 10, 13
Skin patch testing is performed in vivo by preparing aqueous solutions of various metals, incorporating each into petroleum jelly and each mixture is applied to patients’ skin via adhesive tape for up to 4 days.27 Then, the patches are removed and cutaneous reactions are graded on a severity scale based on the presence of erythema, edema, papules, or vesicles.28 In its ability to detect an allergy, patch testing has been shown to have a sensitivity and specificity of 77% and 71%, respectively.29 However, there are several drawbacks to skin patch testing, such as the immunologic response elicited in patch testing is mediated by intradermal Langerhans cells, whereas the MHS reaction in the joint space is mediated by lymphocytes and macrophages.27 Thus, it may not reliably predict the outcomes associated with TJA.1 For instance, there have been cases in which patch testing revealed that patients who had received conventional CoCr implants became sensitized to the component metals, and yet, did not display any symptoms of MHS.30 In addition, the results of skin patch testing are subjective, and therefore, the interpretation of the results can be difficult in terms of diagnosing MHS.20 Moreover, in vivo performance of this test may sensitize the patient to the tested metals.31 Despite these limitations, there is a consensus that preoperative screening should be performed in patients who have a history of metal allergy, such as through contact with jewelry or clothing accessories,1, 6 and if the patient tests positive for a metal that is present in the prosthetic component that is planned to be used, the use of a component made of hypoallergenic materials, such as titanium, zirconium or other ceramics, has been recommended.1, 32
Lymphocyte transformation testing (LTT) is an in vitro alternative to skin patch testing and is performed by adding the potential allergen to a sample of the patient’s blood and measuring the proliferation of lymphocytes in response.33 The principle advantage of LTT over patch testing is a higher sensitivity, which has been estimated to be between 55% and 95%.34, 35 Lymphocyte transformation testing also has no potential risk for sensitization as it is performed outside the body, and it produces a quantitative set of results, which offers more objectivity.6 Yet, there are several drawbacks to LTT such that it has been shown to have a limited specificity with no consensus as to the degree of specificity, despite the higher sensitivity relative to patch testing.31 In addition, LTT is also limited in the number of allergens that can be assessed at one time.33 Furthermore, while both skin patch testing and LTT are useful for evaluating patients for specific MHSs, they have not been shown to be reliable predictors of whether or not a patient will develop MHS following TJA.1 There is also no data on their reliability in predicting success of revision TJA using hypoallergenic materials in patients with symptomatic prosthetic joints who have tested positive for MHS using these tests.
Other in vitro tests that have been used to detect MHS include the modified lymphocyte stimulation test (mLST) and the leukocyte migration inhibition test.10, 13 The mLST is similar to LTT in that the proliferation of leukocytes is measured upon exposure to a potential antigen.10 In one study, patients who tested positive for chromium sensitivity by mLST were significantly (p < 0.05) more likely to develop eczema after TKA, which indicated that mLST may be useful in predicting clinical outcomes postoperatively.1. However, the number of studies that have evaluated its utility is limited. As for the leukocyte migration inhibition test, allergenicity is determined by measuring the rate at which leukocytes travel to a chemoattractant.13 In the presence of an allergen, this migration rate is decreased, and thus the leukocytes are said to be inhibited.13 However, this test has been found to have poor sensitivity, and is not recommended to be used alone when attempting to detect type IV hypersensitivity reactions.13
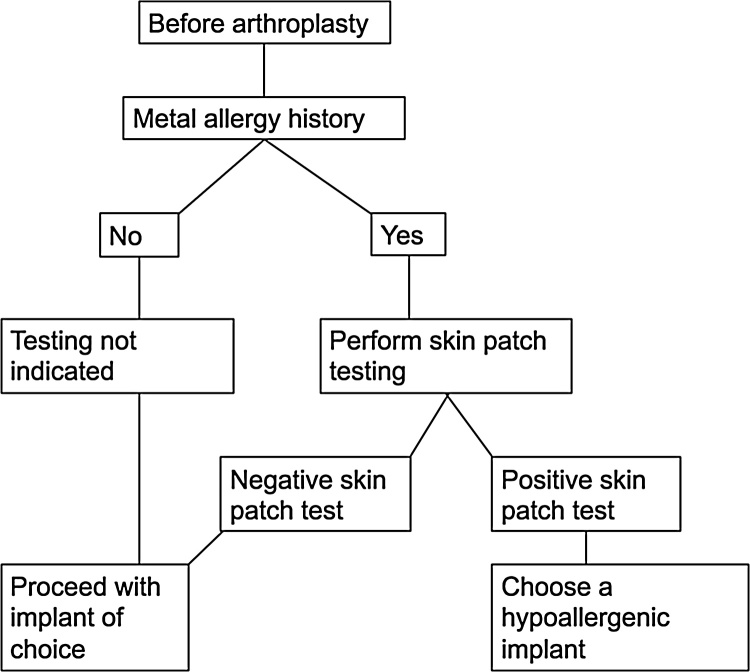
Among the variety of tests, skin patch testing has been considered to be an excellent initial test for evaluating potential allergens that cause contact dermatitis.33 Despite the limitations of these tests for accurately predicting MHS, it has been recommended that patients who have reported a history of metal allergy, such as through contact with jewelry or clothing accessories, undergo preoperative screening via skin patch testing.1, 33 If the patient tests positive for a metal that is present in the prosthetic component that is planned to be used, the use of a component made of hypoallergenic materials, such as titanium, zirconium, or other ceramics, has been recommended.1, 32 A diagnostic algorithm adapted from Mitchelson et al.6 is shown in Fig. 1.
Fig. 1.
Diagnostic algorithm for metal hypersensitivity (adapted from Mitchelson et al.6).
1.4. Management
Several studies have followed patients with preoperative confirmation of metal allergy following implantation of a metal-containing prostheses, reporting no patients with clinical signs of MHS36 and no correlation between MHS and prosthetic-related complications.37 A case in which staged bilateral THA for osteonecrosis of the femoral head was performed, using of zirconia-on-polyethylene bearing and metal-on-metal bearing, showed comparable osteolysis profiles.38 For cases of symptomatic MHS, short-term therapy has included topical steroids for cutaneous dermatitis and non-steroidal anti-inflammatory drugs (NSAIDs) or physical therapy for pain management in synovitis.39 In one study, administration of topical steroids cured the eczematous eruptions in 15 postoperative TKA patients within a period of 2 weeks without recurrence.12 However, definitive resolution of symptoms has most commonly required revision surgery where the implants are taken out and a more tolerable metal prosthesis is implanted.7
Oxidized zirconium (OxZr/Oxinium) implants have a ceramic outer coating, which minimizes the potential for corrosion and dissolution of metal ions into the surrounding tissues, and thereby, may limit the activation of an inflammatory response.14 A similar approach may also be taken with the use of conventional CoCr implants that are coated with a superficial layer of titanium nitride or zirconia nitride.32 Titanium coating could be utilized due to its reduced allergenicity when compared to other metals, yet its use has not been indicated for the majority of TJA patients due to higher associated costs.6
Case studies of patients who have MHS who underwent revision arthroplasty to prostheses comprised of OxZr, titanium or zirconium alloy, or CoCr coated with zirconium nitride or titanium nitride have shown resolution of symptoms within 2 months postoperatively.6, 9, 11, 32 In one case study, a TKA patient who had a chromium implant experienced complete resolution of chronic dermatitis within 2 months of revision surgery where a zirconium-niobium prosthesis was implanted, whereas corticosteroids had previously been ineffective.21 In a report by Thakur et al.9, 3 out of 5 patients experienced a complete reduction in pain after revision TKA from a CoCr implant to one comprised of OxZr and titanium, with the other 2 patients reported that they had only mild pain. Furthermore, all 5 patients experienced improved function in the affected knee, as measured by the Knee Society Score.9 Similar results have been reported among THA patients who underwent CoCr to ceramic revisions, with complete resolution of symptoms in all 4 patients.25
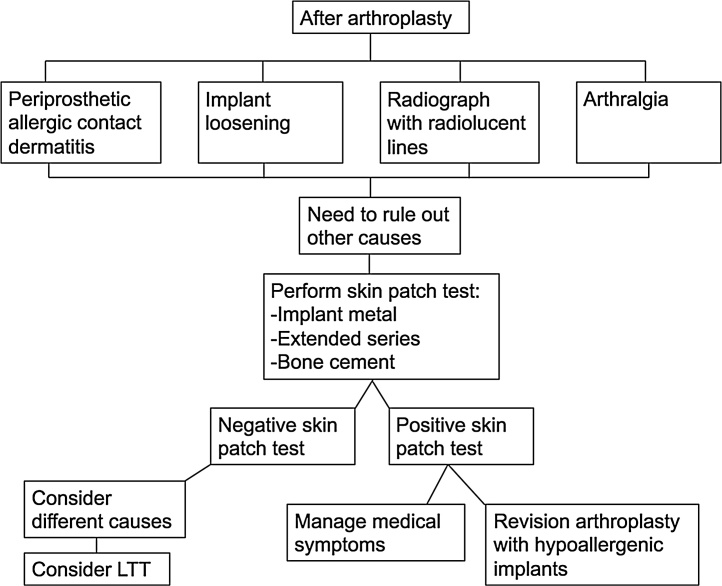
Additionally, if a patient who has MHS has previously had a metal-on-metal hip implant, revision arthroplasty to a metal-on-polyethylene prosthesis have been observed to release considerably fewer potential allergens.1 The availability of hypoallergenic TKA implants and their encouraging results have been reported by some studies.7, 32 Furthermore, Pinson et al.39 have speculated that desensitization may have a role in the future treatment of MHS, as some studies have demonstrated transient cessation of symptoms of sensitivity in patients who have taken daily doses of metals to which they were allergic. A treatment algorithm adapted from Mitchelson et al.6 is shown in Fig. 2.
Fig. 2.
Metal hypersensitivity treatment algorithm (adapted from Mitchelson et al.6).
2. Conclusion
Metal hypersensitivity is a rare condition that has been linked to TJA failure. It is a type IV hypersensitivity reaction that may present as a cutaneous eczematous rash or a painful synovitis in the affected joint. There is currently inconclusive evidence regarding the clinical utility of hypersensitivity testing in patients prior to undergoing TJA.14 Current evidence proposes recommendations against widespread screening for MHS prior to TJA, however, skin patch testing may be useful in patients who have reported a history of metal allergies.1 For patients who have MHS, it appears that definitive treatment can be achieved with revision arthroplasty to implants lacking the offending metals. However, the supporting data is scarce and largely anecdotal;7 therefore, further studies are needed in order to reach a definitive conclusion.
References
- 1.Granchi D., Cenni E., Giunti A., Baldini N. Metal hypersensitivity testing in patients undergoing joint replacement: a systematic review. Bone Joint J. 2012;94-B(8):1126–1134. doi: 10.1302/0301-620X.94B8.28135. [DOI] [PubMed] [Google Scholar]
- 2.Jones C.A., Beaupre L.A., Johnston D.W.C., Suarez-Almazor M.E. Total joint arthroplasties: current concepts of patient outcomes after surgery. Rheum Dis Clin North Am. 2007;33(1):71–86. doi: 10.1016/j.rdc.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Pacheco K.A. Allergy to surgical implants. J allergy Clin Immunol Pract. 2015;3(5):683–695. doi: 10.1016/j.jaip.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Le D.H., Goodman S.B., Maloney W.J., Huddleston J.I. Current modes of failure in TKA: infection, instability, and stiffness predominate. Clin Orthop Relat Res. 2014;472(7):2197–2200. doi: 10.1007/s11999-014-3540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozic K.J., Kamath A.F., Ong K. Comparative epidemiology of revision arthroplasty: failed THA poses greater clinical and economic burdens than failed TKA. Clin Orthop Relat Res. 2015;473(6):2131–2138. doi: 10.1007/s11999-014-4078-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchelson A.J., Wilson C.J., Mihalko W.M. Biomaterial hypersensitivity: is it real? Supportive evidence and approach considerations for metal allergic patients following total knee arthroplasty. Biomed Res Int. 2015;2015:137287. doi: 10.1155/2015/137287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lachiewicz P.F., Watters T.S., Jacobs J.J. Metal hypersensitivity and total knee arthroplasty. J Am Acad Orthop Surg. 2016;24(2):106–112. doi: 10.5435/JAAOS-D-14-00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schäfer T., Böhler E., Ruhdorfer S. Epidemiology of contact allergy in adults. Allergy. 2001;56(12):1192–1196. doi: 10.1034/j.1398-9995.2001.00086.x. [DOI] [PubMed] [Google Scholar]
- 9.Thakur R.R., Ast M.P., McGraw M., Bostrom M.P., Rodriguez J.A., Parks M.L. Severe persistent synovitis after cobalt-chromium total knee arthroplasty requiring revision. Orthopedics. 2013;36(4):e520–4. doi: 10.3928/01477447-20130327-34. [DOI] [PubMed] [Google Scholar]
- 10.Niki Y., Matsumoto H., Otani T. Screening for symptomatic metal sensitivity: a prospective study of 92 patients undergoing total knee arthroplasty. Biomaterials. 2005;26(9):1019–1026. doi: 10.1016/j.biomaterials.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 11.Thomsen M., Rozak M., Thomas P. Pain in a chromium-allergic patient with total knee arthroplasty: disappearance of symptoms after revision with a special surface- coated TKA — a case report. Acta Orthop. 2011;82(3):386–388. doi: 10.3109/17453674.2011.579521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Verma S.B., Mody B., Gawkrodger D.J. Dermatitis on the knee following knee replacement: a minority of cases show contact allergy to chromate, cobalt or nickel but a causal association is unproven. Contact Dermatitis. 2006;54(4):228–229. doi: 10.1111/j.0105-1873.2006.0775o.x. [DOI] [PubMed] [Google Scholar]
- 13.Hallab N., Merritt K., Jacobs J.J. Metal sensitivity in patients with orthopaedic implants. J Bone Joint Surg Am. 2001;83-A(3):428–436. doi: 10.2106/00004623-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 14.Kitagawa A., Chin T., Tsumura N., Iguchi T. Metal sensitivity in patients before and after total knee arthroplasty (TKA): comparison between ceramic surfaced oxidized zirconium and cobalt-chromium implants. Hypersensitivity. 2013;1(1) [Google Scholar]
- 15.Cousen P.J., Gawkrodger D.J. Metal allergy and second-generation metal-on-metal arthroplasties. Contact Dermatitis. 2012;66(2):55–62. doi: 10.1111/j.1600-0536.2011.01970.x. [DOI] [PubMed] [Google Scholar]
- 16.Cadosch D., Chan E., Gautschi O.P., Simmen H.P., Filgueira L. Bio-corrosion of stainless steel by osteoclasts-in vitro evidence. J Orthop Res. 2009;27(7):841–846. doi: 10.1002/jor.20831. [DOI] [PubMed] [Google Scholar]
- 17.Münch H.J., Jacobsen S.S., Olesen J.T. The association between metal allergy, total knee arthroplasty, and revision: study based on the Danish Knee Arthroplasty Register. Acta Orthop. 2015;86(3):378–383. doi: 10.3109/17453674.2014.999614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plummer D.R., Yi P.H., Jacobs J.J., Urban R.M., Moric M.M., Della Valle C.J. Aseptic lymphocytic-Dominated vasculitis-Associated lesions scores do not correlate with metal ion levels or unreadable synovial fluid white blood cell counts. J Arthroplasty. 2017;32(4):1340–1343. doi: 10.1016/j.arth.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Berend K.R., Morris M.J., Adams J.B., Lombardi A.V. Metal-on-metal hip arthroplasty: going, going, gone… − affirms. J Bone Joint Surg Br. 2012;94(11 (Suppl. A)):75–77. doi: 10.1302/0301-620X.94B11.30745. [DOI] [PubMed] [Google Scholar]
- 20.Gupta R., Phan D., Schwarzkopf R. Total knee arthroplasty failure induced by metal hypersensitivity. Am J Case Rep. 2015;16:542–547. doi: 10.12659/AJCR.893609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao X., He R.X., Yan S.G., Wu L.D. Dermatitis associated with chromium following total knee arthroplasty. J Arthroplasty. 2011;26(4):665. doi: 10.1016/j.arth.2010.06.002. [e13-665. e16] [DOI] [PubMed] [Google Scholar]
- 22.Post Z.D., Orozco F.R., Ong A.C. metal-sensitivity-after-tka-presenting-with-systemic-dermatitis-and-hair-loss. Orthopedics. 2013;36(4):e525–e528. doi: 10.3928/01477447-20130327-35. [DOI] [PubMed] [Google Scholar]
- 23.Zeng Y., Feng W. Metal allergy in patients with total hip replacement: a review. J Int Med Res. 2013;41(2):247–252. doi: 10.1177/0300060513476583. [DOI] [PubMed] [Google Scholar]
- 24.Willert H.G. Metal-on-Metal bearings and hypersensitivity in patients with artificial hip joints: a clinical and histomorphological study. J Bone Jt Surg. 2005;87(1):28–36. doi: 10.2106/JBJS.A.02039pp. [DOI] [PubMed] [Google Scholar]
- 25.Campbell P., Shimmin A., Walter L., Solomon M. Metal sensitivity as a cause of groin pain in metal-on-Metal hip resurfacing. J Arthroplasty. 2008;23(7):1080–1085. doi: 10.1016/j.arth.2007.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Thomas P., von der Helm C., Schopf C. Patients with intolerance reactions to total knee replacement: combined assessment of allergy diagnostics, periprosthetic histology, and peri-implant cytokine expression pattern. Biomed Res Int. 2015;2015:910156. doi: 10.1155/2015/910156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihalko WiM, Goodman S.B. AAOS Biomed Eng Biol Implant Committees; 2013. Metal Sensitivity Testing and Associated Total Joint Outcomes.https://www.aaos.org/cc_files/aaosorg/research/committee/biomed/bme_se_2013.pdf [Google Scholar]
- 28.Drake L.A., Dorner W., Goltz R.W. Guidelines of care for contact dermatitis. J Am Acad Dermatol. 1995;32(1):109–113. doi: 10.1016/0190-9622(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 29.Dickel H., Altmeyer P., Brasch J. New techniques for more sensitive patch testing? JDDG J der Dtsch Dermatologischen Gesellschaft. 2011;9(11):889–896. doi: 10.1111/j.1610-0387.2011.07671.x. [DOI] [PubMed] [Google Scholar]
- 30.Kręcisz B., Kieć-Świerczyńska M., Chomiczewska-Skóra D. Allergy to orthopedic metal implants — A prospective study. Int J Occup Med Environ Health. 2012;25(4):463–469. doi: 10.2478/S13382-012-0029-3. [DOI] [PubMed] [Google Scholar]
- 31.Hallab N.J., Jacobs J.J. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67:182–188. [PubMed] [Google Scholar]
- 32.Ajwani S.H., Charalambous C.P. Availability of total knee arthroplasty implants for metal hypersensitivity patients. Knee Surg Relat Res. 2016;28(4):312–318. doi: 10.5792/ksrr.16.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schalock P.C., Menné T., Johansen J.D. Hypersensitivity reactions to metallic implants − diagnostic algorithm and suggested patch test series for clinical use. Contact Dermatitis. 2012;66(1):4–19. doi: 10.1111/j.1600-0536.2011.01971.x. [DOI] [PubMed] [Google Scholar]
- 34.Cederbrant K., Hultman P., Marcusson J.A., Tibbling L. In vitro lymphocyte proliferation as compared to patch test using gold, palladium and nickel. Int Arch Allergy Immunol. 1997;112(3):212–217. doi: 10.1159/000237456. [DOI] [PubMed] [Google Scholar]
- 35.Hallab N.J., Caicedo M., Finnegan A., Jacobs J.J. Th1 type lymphocyte reactivity to metals in patients with total hip arthroplasty. J Orthop Surg Res. 2008;3(1):6. doi: 10.1186/1749-799X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carlsson A., Möller H. Implantation of orthopaedic devices in patients with metal allergy. Acta Derm Venereol. 1989;69(1):62–66. [PubMed] [Google Scholar]
- 37.Webley M., Kates A., Snaith M.L. Metal sensitivity in patients with a hinge arthroplasty of the knee. Ann Rheum Dis. 1978;37(4):373–375. doi: 10.1136/ard.37.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park Y.S., Moon Y.W., Lim S.J. Metal ion hypersensitivity in metal-on-Metal hip arthroplasty. In: Benazzo F., Falez F., Dietrich M., editors. Bioceramics and Alternative Bearings in Joint Arthroplasty. Steinkopff; Rome: 2006. pp. 57–63. [Google Scholar]
- 39.Pinson M.L., Coop C.A., Webb C.N. Metal hypersensitivity in total joint arthroplasty. Ann Allergy Asthma Immunol. 2014;113(2):131–136. doi: 10.1016/j.anai.2014.05.012. [DOI] [PubMed] [Google Scholar]