Abstract
Through learning and practice, we can acquire numerous skills, ranging from the simple (whistling) to the complex (memorizing operettas in a foreign language). It has been proposed that complex learning requires a network of brain regions that interact with one another via white matter pathways. One candidate white matter pathway, the uncinate fasciculus (UF), has exhibited mixed results for this hypothesis: some studies have shown UF involvement across a range of memory tasks, while other studies report null results. Here, we tested the hypothesis that the UF supports associative memory processes and that this tract can be parcellated into subtracts that support specific types of memory. Healthy young adults performed behavioral tasks (two face-name learning tasks, one word pair memory task) and underwent a diffusion-weighted imaging scan. Our results revealed that variation in UF microstructure was significantly associated with individual differences in performance on both face-name tasks, as well as the word association memory task. A UF sub-tract, functionally defined by its connectivity between face-selective regions in the anterior temporal lobe and orbitofrontal cortex, selectively predicted face-name learning. In contrast, connectivity between the fusiform face patch and both anterior face patches had no predictive validity. These findings suggest that there is a robust and replicable relationship between the UF and associative learning and memory. Moreover, this large white matter pathway can be subdivided to reveal discrete functional profiles.
Keywords: uncinate fasciculus, white matter, diffusion imaging, faces, associative memory, orbitofrontal cortex, anterior temporal lobe
1. Introduction
Decades of research on declarative memory has helped identify brain regions involved in the formation and retrieval of memory traces. Regions known to be essential for episodic memory include the medial temporal lobes, mammillary bodies, and anterior nuclei of the thalamus, along with ventromedial portions of the frontal lobe. Regions known to be essential for semantic memory are more controversial, but include the anterior temporal lobes, the angular gyrus, as well as the inferior frontal gyri (Martin and Chao 2001; Rogers et al. 2006; Binder et al. 2009; Visser et al. 2010).
More recently, researchers have begun to investigate the essential role of structural connectivity – white matter fiber pathways – in this process. The information transmission properties of white matter pathways can be predicted by the function of the regions that they connect (Passingham et al. , 2002, Van Essen and Maunsell, 1983). For example, the fornix connects the hippocampus to the mammillary bodies and anterior thalamus, and it has a well established role in episodic memory (Gaffan 1992; Zahr et al. 2009; Metzler-Baddeley et al. 2011). The role of another limbic tract, the uncinate fasciculus (UF), is more controversial. Over 30 years ago, it was proposed that the UF plays an essential role in episodic memory: “The task of the uncinate fascicle will be to guide and channel this information flow to the prefrontal cortex and to transmit preprocessed information back to the temporal cortex for the final act of representation” (Markowitsch, 1982). This idea was based on the location of lesions causing retrograde amnesia in humans, as well as the location of this fiber pathway within the limbic system. The UF creates a direct structural connection between portions of the anterior and medial temporal lobes – the uncus, temporal pole, entorhinal cortex, perirhinal cortex, and amygdala – and inferior-lateral and polar aspects of the frontal lobes (e.g. orbitofrontal cortex (OFC) and BA 10; Catani, Dell’Acqua, & Thiebaut de Schotten, 2013; Von Der Heide, Skipper, Klobusicky, & Olson, 2013).
A small diffusion-weighted imaging (DWI) literature has linked microstructural variation of the UF to various types of episodic memory. For instance, performance on standardized neuropsychological memory tests, such as the California Verbal Learning Test (CVLT; Delis, Kramer, Kaplan, & Ober, 2000), the Weschler Memory Scale (WMS; Wechsler, 2009), and Doors and People (Baddeley, Emslie, & Nimmo-Smith, 1994) is significantly correlated with variation in UF microstructure (reviewed in Olson, Von Der Heide, Alm, & Vyas, 2015). However, other studies have failed to find an association between UF microstructure and episodic memory performance (e.g. Hirni, Kivisaari, Monsch, & Taylor, 2013; Mabbott et al., 2009; Metzler-Baddeley et al., 2011). Studies in which the UF is dissected (either in non-human primates or humans undergoing neurosurgery) have reported that some types of memory decline after UF destruction, while other mnemonic processes remain intact (reviewed in Von Der Heide, Skipper, Klobusicky, & Olson, 2013). Indeed, we previously reported a strong relationship between variation in UF microstructure and performance on two associative learning tasks, a face-name learning task and an object-location learning task. However, there was no significant relationship between the UF and performance on a face memory task called The Cambridge Face Memory Test (Alm et al. 2016).
The inconsistency in findings across the episodic memory and structural connectivity literature raises several questions. First, are the reported findings relating UF microstructure to memory performance robust and replicable? Some DWI studies are underpowered and others suffer from a range of methodological issues, such as collapsing across hemispheres, failing to control for important subject variables, such as participant sex, or using inappropriate statistical tests. Replications in this literature are rare.
Second, assuming the aforementioned results are replicable, does the UF support all types of learning and memory or only a subset of mnemonic processes? It is important to understand the specificity and generality of memory phenomena that can be linked to functionality of the UF. We previously identified two possible subsets. One possibility is that the UF’s role in long-term forms of memory is to adjudicate between competing memory representations at retrieval. Tasks with high retrieval competition tend to be difficult memory tasks, such as the recollection of proper names or the retrieval of stimuli in which features of the episode overlap with features of another encoded episode. Another possibility is that the UF’s role in memory is limited to associative or relational memory. This would be consistent with findings showing that a monkey’s ability to learn which of two visual objects is associated with a background scene is consistently disrupted after damage to the UF (Browning et al. 2005; Browning and Gaffan 2008).
Last, and most importantly, given that the UF is such a large white matter tract, is it possible that different streams or “sub-tracts” within the UF itself support distinct functions? As noted earlier, the function of white matter depends on the information processing capabilities of the gray matter “islands” that are bridged by the white matter tracts, and the anterior temporal lobes (ATLs) contain many distinct processing areas. For instance, both non-human primates and humans have face-selective cells in the ATLs (subsequently referred to as “face patches”), which are functionally interconnected with the extended face processing system. In humans, lesions to this region can affect the ability to recall proper names or retrieve biographical details. Moreover, the ATL face patch is relatively insensitive to perceptual manipulations of faces, but is highly sensitive to mnemonic manipulations of faces, such as familiarity, or the recollection of biographical details, suggesting that this brain region plays an important role in person memory (reviewed in Collins & Olson, 2014; Perrodin, Kayser, Abel, Logothetis, & Petkov, 2015). Even more anterior to the ATL face patch is the orbitofrontal cortex (OFC) face patch, which appears to play some role in the rewarding aspects of person representations (Troiani et al. 2016), although it is possible that the OFC face patch has other roles as well. We hypothesize that a UF sub-tract connecting the ATL and OFC face patches is preferentially associated with person memory.
The purpose of the present study was to test the three questions outlined above: (1) Do the findings linking the UF to associative learning and memory replicate? (2) Is the UF’s role in memory limited to associative learning and memory tasks? And most importantly, our third question (3) Do portions of the UF connecting the ATL face patch and OFC face patch play a particular role in person memory? We used Diffusion Weighted Imaging (DWI) and deterministic tractography to identify the UF and define white matter connectivity between the ATL and OFC face patches. To test Questions 1 and 3, in Experiment 1 participants learned face-name associations using a task that was superficially similar to the task we used in our prior study of the UF (Alm et al. 2016). Task differences included changes in the number of trials, trial timing, and the stimulus set. The faces were highly similar to one another and the names were common American names. Face-name associations were learned through trial and error. Experiment 2 was similar to Experiment 1, except a different set of faces and names were used. This task was designed to be easier than the task used in Experiment 1: the faces were more dissimilar to each other and the names were uncommon. The dependent measure of interest was overall learning accuracy for face-name pairs, and we examined how this performance relates to UF microstructure. To test Question 2, in Experiment 3, participants were required to remember a long list of word pairs. Later, they were tested on their ability to remember pairs of words or individual words from the studied list. The dependent measure of interest was memory for pairs vs. single words, and we examined how this relates to UF microstructure. Last, to test Question 3, we extracted a face-specific sub-tract of the UF and related this to face-name learning performance from Experiments 1 and 2. Connectivity between the Fusiform Face Area (FFA) and the OFC, and the FFA and ATL, was examined and for our purposes, served as control tracts.
2. Materials and Methods
2.1. Participants
A total of 30 healthy individuals (15 male, 15 female) between the ages of 18 and 29 (M = 21.30, SD = 2.55) participated in the present experiments. Six participants were excluded from analyses due to the presence of multivariate outliers (i.e. data exceeded the critical cut-off for Mahalanobis distance, χ2(4) = 9.49), leaving a final sample of 24 participants (12 male, 12 female, between the ages of 18 and 29; M = 21.58, SD = 2.70). Participants were undergraduate or graduate students at Temple University, right-handed, native English speakers, with normal to corrected-to-normal vision. Right-handedness was determined through self-report. All participants had no history of psychological or neurological disorders as ascertained by self-report and no MRI contraindications. Informed consent was obtained per the guidelines of the Institutional Review Board of Temple University, and participants received monetary compensation for participation in the experiments.
2.2. Study Protocol
Study participation occurred in two separate testing sessions, which occurred an average of one week apart. During the behavioral session, participants completed computerized tasks in the laboratory. Participants were tested individually in a well-lit room. Computerized tasks were programmed in E-Prime (Version 2.0 Professional) and presented on Dell computers. During a separate scanning session, diffusion-weighted MRI data, as well as high-resolution anatomical scans, were acquired at Temple University Hospital.
2.3. Behavioral Tasks
2.3.1. Experiment 1 and 2: Face-Name Associative Learning Tasks
Participants were instructed to learn face-name pairings to the best of their ability over the course of several learning blocks. On each trial, one face was presented at central fixation on a black background with two name options presented below the face. The task was to make a forced-choice decision about which name was correct within 4 s. Choices were made via key press. We considered each presentation of a learned face as a retrieval time point, since the correct associative information had to be retrieved to make a response.
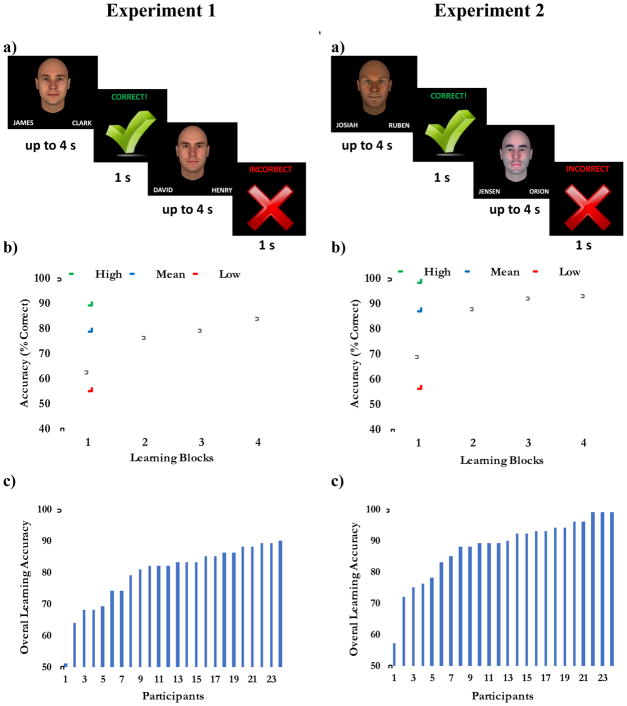
On the first presentation of each face-name pair, the correct name choice was not immediately apparent; therefore, participants were told to pay close attention to the feedback given after each trial to learn the correct associative pairings. Each face was repeated four times per block, thus allowing for the gradual learning of the correct pairs. Presentation order was randomized. The incorrect name choices, as well as the side of the screen on which the correct and incorrect name choices appeared were both randomized. Correct/incorrect feedback was given after each trial by presenting either a large green checkmark or a large red ‘X’ for 1 s. The face stimuli consisted of 36 unique male faces (18 unique males faces per experiment) from the Todorov randomly generated faces database (Oosterhof & Todorov, 2008; facegen.com). Faces were free from hair, eyeglasses, or other eye-catching features. Each face had a neutral expression. The name stimuli consisted of 72 names total obtained from the Social Security Administration “Top 1000 names for 2015” ranked on popularity (Social Security Administration, 2015). All names consisted of 5 or 6 letters. Each experiment’s stimuli consisted of 18 unique face-name pairs, learned over the course of 4 blocks, each block comprised of 72 trials. Each block was separated by a brief 30 s rest period. Each experiment consisted of 288 total trials and lasted approximately 20 minutes. Experiments 1 and 2 were identical in execution but differed in one crucial way: Experiment 1 had faces that were highly similar to each other paired with common American names (i.e. names that appeared in high ranks of the Social Security Administration Top 1000 list), while Experiment 2 used faces that were very dissimilar to one another and were paired with uncommon American names (i.e. names that appeared in the bottom ranks of the Top 1000 list). Thus, Experiment 2 was purposely designed to be easier and have lower retrieval demands. A schematic of the task design is depicted in Figure 1A.
Figure 1.
(a) Schematics of the associative learning tasks. In Experiment 1 (left), participants learned similar face-common name pairs, while in Experiment 2 (right), they learned dissimilar face-uncommon name pairs. (b) Behavioral performance plotted across learning blocks for each experiment. The blue line depicts mean accuracy (percent correct) for each learning block. Error bars represent standard error of the mean. The green and red lines depict learning curves for the participants with the highest and lowest performance, respectively. (c) Overall learning accuracy (percent correct on the last learning block) is plotted for each participant to display the individual variability among participants. The numbers on the x-axis are a rank for each participant.
2.3.2. Experiment 3: Associative and Single Word Memory Tasks
Participants were instructed to remember 56 word pairs (112 words total) (Kucera & Francis, 1967). On each trial, a word pair was presented on the screen for 4 s. Pairings were fixed, but the order of presentation and the side of the screen on which each word appeared was randomized. Following the studying of the word pairs, participants were asked to compete an associative and a single word memory task (task order counterbalanced). In the associative word memory task, participants were presented with 26 word pairs (52 words total) and were asked to make a choice of whether the pair was intact or rearranged. An intact pair consisted of two words that were presented together during the study phase, regardless of the side of the screen in which they were presented. By contrast, a rearranged pair consisted of two words both presented at some point during the study phase, but the words were never presented together as a pair. In the item memory tasks, 104 words were presented, one at a time, in the center of the screen. Fifty-two words were “old” words, while the other half were novel words not previously shown. Participants were asked to decide whether each word was new or old (e.g. previously studied). The study list was buffered by four trials (two at the beginning and two at the end) to control for primacy and recency effects.
2.4. Image Acquisition
MRI scanning was conducted at Temple University Hospital on a 3.0 T Siemens Verio scanner (Erlangen, Germany) using a Siemens twelve-channel phased-array head coil. DWI data were collected using a diffusion-weighted echo-planar imaging (EPI) sequence covering the whole brain. Salient imaging parameters were as follows: 55 axial slices, 2.5 mm slice thickness, approximately 2x2x2.5 in-plane spatial resolution, TR = 9,900 ms, TE = 95 ms, FOV = 240 mm2, matrix size = 122x122, b values of 0 and 1000 s/mm2 (one b0 image acquired), 64 non-collinear directions, single-shot acquisition. The DWI scan lasted approximately 11 minutes.
In addition to diffusion-weighted images, high-resolution anatomical images (T1-weighted 3D MPRAGE) were also collected for each participant with the following parameters: 160 axial slices, 1 mm slice thickness, TR = 1,900 ms, TE = 2.93 ms, inversion time = 900 ms, flip angle = 9o, FOV = 256 mm2. These anatomical images were co-registered to the diffusion images and used to draw regions of interest (ROIs).
2.5. DWI Preprocessing
The diffusion-weighted images were pre-processed using FSL (Smith et al. 2004) to correct for eddy currents and subject motion using an affine registration model. The b-vector matrix was adjusted based on rigid body registration, ensuring a valid computation of the tensor variables. Non-brain tissue was removed using FSL’s automated brain extraction tool (BET) (Jenkinson et al. 2005; Smith 2002), and a standard least squares diffusion tensor fitting model was then applied to the data. The diffusion tensor fitting provided estimates of fractional anisotropy (FA) and mean diffusivity (MD), as well as three eigenvectors and eigenvalues. FA values were calculated using the following equation: , where λ1, λ2, and λ3 represent the three eigenvalues respectively. MD was calculated by averaging the three eigenvalues. Finally, axial diffusivity (AD) was represented by the principal eigenvalue (λ1). These estimates were computed on individual voxels using a three-dimensional Gaussian distribution model that yielded a single mean ellipsoid for each voxel.
2.6. Tractography
Tractography was performed in native subject space using the Diffusion Toolkit and TrackVis software packages (Wang et al. 2007). The interpolated streamline algorithm (Conturo et al. 1999) was used to determine the branching and curving of the fiber tracts. Step length was fixed at 0.5 mm, and an angle threshold of 45 degrees was used to determine the termination point of the fiber tracts. This deterministic fiber tracking method is based on the determination that in one voxel, only one set of fibers can be present (Huang et al. 2004). Hence, it reconstructs fiber trajectories throughout the brain by tracking the direction of greatest diffusion in interpolated steps. The choice of the interpolated streamline algorithm was made based several studies comparing different deterministic and probabilistic tractography algorithms (Fillard et al. 2011; Feigl et al. 2014). A spline filter was used to smooth the tractography data.
A multiple ROI-based axonal tracking approach (Mori et al. 2002; Wakana et al. 2007; Thomas et al. 2011) was used to delineate the UF bilaterally. ROIs were drawn in subject native space using the high-resolution anatomical T1 images and the methods outlined by Thomas and colleagues (2011). For the UF, one ROI was drawn in the temporal lobe and included the portion of the temporal cortex that is anterior to the point at which the fornix descends to the mammillary bodies, while the second ROI was comprised of the portion of the frontal cortex located anterior to the rostrum of the callosum. A Boolean AND term was used to select only the fibers that passed through both of these seed regions of interest. To obtain connectivity between the ATL and OFC face patches, we created 10mm radius spherical ROIs in native space around the voxels with peak activation to faces, identified in prior studies by our laboratory (Von Der Heide et al. 2013b; Troiani et al. 2016). The reason for choosing ROIs of this size is empirical and lies in the individual differences of the location of brain activation to faces. The locus of brain activity when exposed to faces differed among participants for both the ATL and the OFC face patch. The maximum distance of the peak activations between participants in the x, y, and z planes was 20mm. Hence, we chose to use 10mm radius spherical ROIs to ensure that we capture the ATL and OFC face patches in all our participants. A Boolean AND term was used to select only the fibers that passed through both of the face patches. The resulting tract will subsequently be referred to as the “UF sub-tract”.
Mean FA, MD, AD and RD indices were subsequently extracted from the tracts of interest. To assess functional specificity in our tracts of interest, we measured white matter microstructure between a different set of face patches: FFA and the OFC, and between the FFA and ATL. The FFA mask was obtained from FSL’s Harvard-Oxford Cortical Structural Atlas (Frazier et al. 2005; Desikan et al. 2006; Makris et al. 2006; Goldstein et al. 2007). Due to individual differences of the FFA location, the mask we used was a combination of the Temporal Occipital Fusiform Cortex and the Occipital Fusiform Gyrus of FSL’s Harvard-Oxford Cortical Structural Atlas. We used FFA-ATL and FFA-OFC connectivity of the right hemisphere in our analysis because neural processing of faces has been found to be predominantly right lateralized (Rhodes 1985; Sergent et al. 1992; Kanwisher et al. 1997; Pyles et al. 2013). The resulting white matter tracts are presented in Figure 2. Additional information about streamline count, voxel count, volume, and length of all tracts can be found in the Supporting Information Appendix (Table S1).
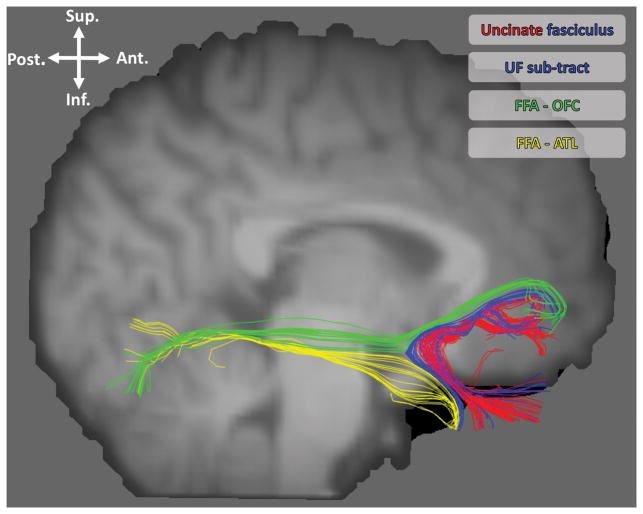
Figure 2.
Tractography delineating tracts discussed in this paper. Red and blue: the uncinate fasciculus; blue: the UF sub-tract linking the ATL face patch to the OFC face patch; green: the IFOF sub-tract connecting the FFA to the ofbitofrontal cortex; yellow: the ILF sub-tract connecting the fusiform face area (FFA) and the ATL.
2.7. Statistical Analyses
Statistical analyses were performed using SPSS (Version 23.0). Multiple linear regression analyses were used to examine the relationship between microstructure of the UF and performance on the three experimental tasks. Hemispheric differences were found in UF FA (t(23) = − 2 .23 , p = .03), as well as FA and AD in the UF sub-tract (t(23) = − 5.54, p < .001 and t(23) = − 5.70, p < .001, respectively). Additionally, hemispheric differences were found in FA and AD of the tracts connecting the FFA to the ATL face patch (t(23) = − 6.80, p < .001 and t(23) = − 6.46, p < .001), and trending hemispheric differences in MD and RD (t(23) = − 1.97, p = .061 and t(23) = − 6.46, p = .063). Thus, analyses were not collapsed across hemispheres.
In previous studies (Alm et al. 2015, 2016), we observed biological sex differences in the microstructural properties of the UF; therefore, we examined potential sex differences in this study as well. Indeed, several sex differences were present in the current sample. Specifically, compared to females, males exhibited increased values for AD in the left UF sub-tract (t(22) = 2.09, p = .048), and increased FA, MD, and AD in the right tracts connecting the FFA to the ATL face patch (t(22) = 2.52, p = .02, t(22) = 2.21, p = .04, t(22) = 3.09, p < .01). Because of these differences, sex was controlled for in the regressions.
We also examined potential age differences in white matter microstructure and cognitive scores. We found no significant effect of age (all p’s > .11), thus age was not controlled for in the regressions.
Separate regression models were constructed for each white matter index of interest. Predictors were entered simultaneously into the regression and each model consisted of three predictors: right and left white matter indices (FA, MD, AD, or RD) and sex.
In the first analysis using the whole UF, no correction for multiple comparisons was applied since we had a strong a priori prediction based on our prior findings showing that variation in UF microstructure predicted face-name learning in both the left and right hemispheres (Alm et al. 2016). In the second analysis using a UF sub-tract, we corrected the regression p-values for multiple comparisons using–Benjamini Hochberg false-discovery rate correction for multiple comparisons (Benjamini and Hochberg 1995).
Our study focuses on white matter microstructure and cognitive performance. To compliment our understanding of these results, we also examined white matter macrostructure - volume - to see whether we would observe the same results in a different white matter index. For the additional volume analysis see the Supporting Information Appendix (SI Methods, SI Results, SI Discussion, Tables S2 and S3).
3. Results
3.1. Behavioral Data
Behavioral data for Experiment 1, in which participants learned pairs of highly similar faces and common American names are presented in Figure 1. During the first learning block, performance was low (mean accuracy = 58.46%); however, by the last block, mean accuracy improved to 79.54%. A repeated measures ANOVA revealed a significant main effect of block, F(3,92) = 19.73, p < .001, and planned post-hoc t-tests revealed that accuracy significantly improved across learning blocks 1 and 2 (p < .001) and 3 and 4 (p = .002).
Similarly, in Experiment 2, in which participants learned pairs of highly dissimilar faces and uncommon American names, performance was low during the first learning block (mean accuracy = 63.29%), but by the last block, mean accuracy improved to 87.75%. A repeated measures ANOVA revealed a significant main effect of block, F(3,92) = 29.61, p < .001, and planned post-hoc t-test revealed that accuracy significantly improved across learning blocks 1 and 2 (p < .001), and 2 and 3 (p = .006).
To be consistent with prior studies (e.g. Alm et al., 2016), we used performance on Block 4 (B4 accuracy) as an overall measure of learning. To assess whether there was any relationship between performance on Experiment 1 and Experiment 2, we performed a Pearson correlation analysis on B4 learning accuracy between Experiments 1 and 2. We found that they were strongly correlated r(24) = .61, p = .002, indicating that participants who performed well in Experiment 1 tended to also perform well in Experiment 2.
In Experiment 3, (d’), a bias-free sensitivity index of signal-to-noise derived from the hit-rate and false-alarm rate, was computed as a measure of associative memory and item memory accuracy. To assess whether there was any relationship between performance on associative memory and item memory, we performed a Pearson correlation analysis on d’ for associative memory and item memory. We found that they were strongly correlated r(24) = .67, p < .001, indicating that participants who performed well in the associative memory task also performed well in the item memory task.
3.2. Do the findings linking the UF to associative learning and memory replicate?
For Experiments 1 and 2, regression models were constructed to predict overall learning (B4 accuracy). Predictors consisted of bilateral white matter indices (FA, MD, AD or RD) and sex, which was included as a control variable. Separate regressions were constructed for each white matter index. Results of the regression analyses are presented in Tables 1 and 2.
Table 1.
Summary of multiple linear regression models of the whole uncinate fasciculus predicting individual differences in overall learning (B4 accuracy). To the left is Experiment 1, testing similar face-common name associations; to the right is Experiment 2, testing dissimilar face-uncommon name associations.
| Experiment 1 (Similar faces – Common names) | Experiment 2 (Dissimilar faces – Uncommon names) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Predictor Variables | β | t-value | F | R2 | β | t-value | F | R2 |
| 3.60* | 0.35 | 4.92** | 0.43 | |||||
| Sex | 0.22 | 1.14 | 0.07 | 0.36 | ||||
| Right FA | 0.50* | 2.57* | 0.24 | 1.33 | ||||
| Left FA | 0.30 | 1.55 | 0.57** | 3.14** | ||||
| 1.58 | 0.19 | 5.41** | 0.45 | |||||
| Sex | 0.02 | 0.08 | −0.13 | −0.69 | ||||
| Right AD | −0.17 | −0.69 | −0.27 | −1.35 | ||||
| Left AD | 0.49* | 2.15* | 0.73** | 3.85** | ||||
| 2.22 | 0.25 | 1.51 | 0.18 | |||||
| Sex | −0.12 | −0.61 | −0.26 | −1.24 | ||||
| Right MD | −0.59* | −2.48* | −0.40 | −1.63 | ||||
| Left MD | 0.47 | 1.99 | 0.43 | 1.76 | ||||
| 1.52 | 0.19 | 0.78 | 0.11 | |||||
| Sex | 0.001 | 0.003 | −0.14 | −0.66 | ||||
| Right RD | −0.44 | −1.97 | −0.16 | −0.69 | ||||
| Left RD | 0.02 | 0.08 | −0.16 | −0.66 | ||||
p < .01,
p < .05.
B4: Block 4, FA: fractional anisotropy, AD: axial diffusivity, MD: mean diffusivity, RD: radial diffusivity, β: standardized regression coefficient.
Table 2.
Summary of multiple linear regression models of the whole uncinate fasciculus predicting individual differences in word associative and item memory (d’) on Experiment 3. To the left is the word associative memory; to the right is the word item memory.
| Word associative memory | Word item memory | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Predictor Variables | β | t-value | F | R2 | β | t-value | F | R2 |
| 2.14 | 0.24 | 3.05 | 0.31 | |||||
| Sex | 0.33 | 1.54 | 0.38 | 1.88 | ||||
| Right FA | 0.47* | 2.24* | 0.39 | 1.94 | ||||
| Left FA | 0.11 | 0.52 | 0.36 | 1.80 | ||||
| 1.84 | 0.22 | 2.24 | 0.25 | |||||
| Sex | 0.32 | 1.50 | 0.29 | 1.37 | ||||
| Right AD | 0.49 | 2.08 | 0.12 | 0.52 | ||||
| Left AD | −0.03 | −0.13 | 0.43 | 1.96 | ||||
| 0.18 | 0.03 | 0.53 | 0.07 | |||||
| Sex | 0.15 | 0.68 | 0.10 | 0.47 | ||||
| Right MD | 0.07 | 0.24 | −0.25 | −0.96 | ||||
| Left MD | −0.10 | −0.36 | 0.25 | 0.94 | ||||
| 0.46 | 0.06 | 0.78 | 0.11 | |||||
| Sex | 0.16 | 0.73 | 0.17 | 0.80 | ||||
| Right RD | −0.20 | −0.83 | −0.27 | −1.17 | ||||
| Left RD | −0.03 | −0.11 | −0.04 | −0.16 | ||||
p < .01,
p < .05.
FA: fractional anisotropy, AD: axial diffusivity, MD: mean diffusivity, RD: radial diffusivity, β: standardized regression coefficient.
The regression analyses revealed a significant relationship between microstructural properties of the UF and performance in Experiment 1, the difficult face-name learning task. In the left hemisphere, individual differences in AD (β = 0.49, t(20) = 2.15, p = 0.04) and MD (marginally) (β = 0.47, t(20) = 1.99, p = 0.06) were associated with overall learning, after controlling for sex. In the right hemisphere, individual differences in FA (β = 0.50, t(20) = 2.57, p = 0.02) and MD (β = −0.59 , t(20) = −2.48, p = 0.02) were significantly associated with overall learning, after controlling for sex. FA and AD indices exhibited a positive relationship with overall learning, such that higher microstructural values were associated with higher overall learning performance. The reverse relationship was demonstrated in right MD, such that lower MD values were associated with lower overall learning performance.
The analyses used in Experiment 1 were performed on the data from Experiment 2 in which easier face-name associations were learned. In the left hemisphere, individual differences in FA (β = 0.57, t(20) = 3.14, p = 0.005) and AD (β = 0.73, t(20) = 3.85, p = 0.001) were significantly associated with overall learning, after controlling for sex. No significant effects were observed in the right hemisphere. FA and AD indices exhibited the same positive relationship with overall learning as in Experiment 1.
3.3. Is the UF’s role in memory limited to associative learning and memory tasks?
To address this question, we used the behavioral data from the non-social verbal memory tasks. Regression analyses revealed a significant relationship between microstructural properties of the right UF and associative memory for words (FA: β = 0.47, t(20) = 2.24, p = 0.04; AD: β = 0.49, t(20) = 2.08, p = 0.051). No statistically significant effects were observed in the left hemisphere. In regards to memory for single words, item memory, there was a marginally significant effect in right FA (β = 0.39, t(20) = 1.94, p = 0.07) and left AD (β = 0.43, t(20) = 1.96, p = 0.06).
To compare the magnitudes of the associative and item memory model predictors, a z-test was performed using the respective regression coefficients for the significant findings (Paternoster et al. 1998). We found that the magnitude of the relationship between associative memory and FA of the right UF was not significantly greater than the relationship between item memory and FA of the right UF (z = 0.92, p = 0.36). We further compared the magnitudes of the similar and dissimilar face-name learning and item memory model predictors using the respective regression coefficients for the significant findings. We found that the magnitude of the relationship between similar faces-common names and AD of the left UF was significantly greater than the relationship between item memory and AD of the left UF (z = 1.99, p = 0.047). The same pattern was observed for dissimilar faces-uncommon names and item memory and the AD of the left UF (z = 3.65, p < 0.001) and for all other white matter indices (similar faces-common names – item memory: right UF FA (z = 2.39, p = 0.017), MD (z = −2.39, p = 0.017); dissimilar faces-uncommon names – item memory: left UF FA (z = 2.97, p < 0.01)).
Data from Experiments 1 and 2 revealed that the left and right UF play a key role in episodic memory, supporting learning and memory of face-name associations. These results are consistent with our prior findings on the UF and episodic memory (Alm et al. 2016). The results of Experiment 3, which used non-social verbal stimuli (word pairs), suggest that its role in episodic memory extends to non-social verbal stimuli. There was no difference in the magnitude of the relationship between associative word memory and FA of the right UF and the respective relationship for single word memory.
In the next set of analyses, we attempt to dissect the functionality of the UF. We tested the hypothesis that a portion of the UF, defined by its connectivity between face-specific cortex in the ATL and OFC, has some degree of category specificity for learning and memory tasks involving faces. We call this the “UF sub-tract.” To ascertain the specificity of any findings concerning this sub-tract, we examined the relationship between our behavioral indices and two other theoretically relevant sub-tracts connecting face-specific cortex in the fusiform gyrus (e.g. FFA) with the ATL and OFC, respectively. These sub-tracts would constitute portions of the inferior longitudinal fasciculus (ILF) and inferior frontal occipital fasciculus (IFOF).
3.4. Is there a UF Sub-tract Specialized for Face Memory?
The UF sub-tract was defined as the tracts connecting the ATL face patch to the OFC face patch in each hemisphere. The UF sub-tract was present in all participants. Compared to the entire UF, the UF sub-tract consisted of four to five times less streamlines, had half the voxel count, and was half the volume. Lengthwise, the two tracts were similar. Pairwise t-tests revealed a significant difference between the right UF and the whole UF sub-tract streamline count (t(23) = 6.79, p < 0.001), voxel count (t(23) = 7.05, p < 0.001), and volume (t(23) = 7.03, p < 0.001) while there was no significant difference in tract length (t(23) = −0.53, p = 0.60). Similarly, a significant difference was revealed between the left UF and the UF sub-tract streamline count (t(23) = 5.65, p < 0.001), voxel count (t(23) = 6.40, p < 0.001), and volume (t(23) = 6.40, p < 0.001) while there was no significant difference in tract length (t(23) = −0.78, p = 0.44).
The regression analyses revealed several significant relationships between microstructural properties of the UF sub-tract and overall performance in Experiments 1 and 2, after controlling for sex (see Tables 3 and 4 and Figure 3). Starting with Experiment 1, in the left hemisphere, higher FA and lower RD were associated with higher overall learning (FA: β = 0.64, t(20) = 2.79, p = 0.03; RD: β = −0.78, t(20) = −3.84, p = 0.02). Initially individual differences in left MD were also associated with overall learning; however, this effect did not survive correction for multiple comparisons (p = 0.03 uncorrected). No significant effects were found in the right hemisphere.
Table 3.
Summary of multiple linear regression models of the uncinate fasciculus sub-tract predicting individual differences in overall learning (B4 accuracy). To the left is Experiment 1, testing similar face-common name associations; to the right is Experiment 2, testing dissimilar face-uncommon name associations.
| Experiment 1 (Similar faces – Common names) | Experiment 2 (Dissimilar faces – Uncommon names) | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Predictor Variables | β | t-value | F | R2 | β | t-value | F | R2 |
| 5.12** | 0.43 | 7.71** | 0.54 | |||||
| Sex | −0.05 | −0.26 | −0.31 | −1.87 | ||||
| Right FA | 0.03 | 0.13 | −0.32 | −1.46 | ||||
| Left FA | 0.64* | 2.79* | 0.88** | 4.24** | ||||
| 0.80 | 0.11 | 0.68 | 0.09 | |||||
| Sex | 0.12 | 0.51 | −0.09 | −0.38 | ||||
| Right AD | −0.19 | −0.82 | 0.06 | 0.25 | ||||
| Left AD | 0.38 | 1.52 | 0.23 | 0.92 | ||||
| 2.49 | 0.27 | 3.33* | 0.33 | |||||
| Sex | −0.16 | −0.74 | −0.40 | −2.01 | ||||
| Right MD | −0.03 | −0.12 | 0.30 | 1.38 | ||||
| Left MD | −0.52 | −2.28 | −0.66** | −2.99** | ||||
| 4.32* | 0.39 | 5.44** | 0.45 | |||||
| Sex | −0.13 | −0.69 | −0.39 | −2.14 | ||||
| Right RD | 0.01 | 0.06 | 0.35 | 1.71 | ||||
| Left RD | −0.64* | −3.00* | −0.78** | −3.84** | ||||
p < .01,
p < .05 (after controlling for multiple comparisons).
B4: Block 4, FA: fractional anisotropy, AD: axial diffusivity, MD: mean diffusivity, RD: radial diffusivity, β: standardized regression coefficient.
Table 4.
Summary of multiple linear regression models of the uncinate fasciculus sub-tract predicting individual differences in word associative and item memory (d’) on Experiment 3. To the left is the word associative memory; to the right is the word item memory.
| Word associative memory | Word item memory | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Predictor Variables | β | t- value | F | R2 | β | t-value | F | R2 |
| 0.96 | 0.13 | 1.77 | 0.21 | |||||
| Sex | 0.21 | 0.93 | 0.14 | 0.65 | ||||
| Right FA | 0.27 | 0.90 | 0.05 | 0.18 | ||||
| Left FA | 0.09 | 0.33 | 0.40 | 1.48 | ||||
| 1.36 | 0.17 | 1.85 | 0.22 | |||||
| Sex | 0.30 | 1.32 | 0.34 | 1.54 | ||||
| Right AD | 0.08 | 0.38 | 0.03 | 0.14 | ||||
| Left AD | 0.38 | 1.56 | 0.47 | 2.00 | ||||
| 0.17 | 0.02 | 0.46 | 0.06 | |||||
| Sex | 0.13 | 0.54 | 0.07 | 0.31 | ||||
| Right MD | −0.03 | −0.10 | 0.06 | 0.23 | ||||
| Left MD | −0.05 | −0.20 | −0.24 | −0.92 | ||||
| 0.36 | 0.05 | 1.04 | 0.13 | |||||
| Sex | 0.14 | 0.58 | 0.07 | 0.33 | ||||
| Right RD | −0.07 | −0.26 | 0.06 | 0.23 | ||||
| Left RD | −0.13 | −0.50 | −0.37 | −1.46 | ||||
p < .01,
p < .05 (after controlling for multiple comparisons).
FA: fractional anisotropy, AD: axial diffusivity, MD: mean diffusivity, RD: radial diffusivity, β: standardized regression coefficient.
Figure 3.
(a) Tractography delineating the right uncinate fasciculus (UF; red and blue) and UF sub-tract (blue) in a sample participant. (b) Scatter plots of standardized residuals from the linear regression analyses illustrating the relationship between overall learning on the similar faces-common names task, dissimilar faces-uncommon names task, and associative memory word task (y-axis), and mean FA of the left UF sub-tract (x-axis).
The data from Experiment 2 were very consistent with the results from Experiment 1. Again, in the left hemisphere, higher FA (β = 0.88, t(20) = 4.24, p < 0.001), lower RD (β = −0.66, t(20) = −2.99, p = 0.003) and lower MD (β = −0.66, t(20) = −2.99, p = 0.02) were associated with higher overall learning of face-name associations. As in Experiment 1, no significant effects were found in the right hemisphere.
In order to know if this sub-tract has some degree of specificity for learning and memory tasks involving faces, we tested the relationship between memory for word pairs or single words from Experiment 3 and microstructural indices of the sub-tract. There were no statistically significant effects (see Table 4), suggesting some degree of specificity for learning and memory tasks involving faces.
Last, we asked whether there was tract specificity by examining the relationship between our behavioral indices and two other theoretically relevant sub-tracts, one connecting face-specific cortex in the fusiform gyrus (e.g. FFA) with the ATL and one connecting the FFA to the OFC. There were no statistically significant effects (See Supporting Information Appendix, Tables S4, S5).
4. General Discussion
Learning and memory constitute the foundation upon which complex cognition is built. Our capacity for rapid learning allows us to learn languages, mathematics, and a host of academic subjects. It also supports our social behavior, allowing us to remember the names, biographies, and personal anecdotes relevant to the many people we meet. In this study, we asked whether a specific white matter tract called the uncinate fasciculus (UF) is involved in our ability to learn associations. We previously observed a robust association between variation in UF microstructure and performance on a face-name learning task, as well as an object-location learning task (Alm et al. 2016). Thus, one goal of this study was to replicate and extend our prior finding. A second goal was to characterize the precise role of the UF in learning and memory; therefore, we tested the hypothesis that the UF’s involvement in learning and memory is limited to associative processing, such that memory for single items does not rely on this network. Third, and most importantly, we tested the hypothesis that a subregion of the UF, defined by its connections between cortical face patches, is specifically involved in forming and/or retrieving information about people.
Our first goal was met. We found that individual variability in UF microstructure was associated with performance on two distinct face-name learning tasks. This relationship was found in several white matter indices (FA, MD, AD, RD) and showed some bias towards the left hemisphere. This finding is consistent with the literature on the distinct roles the left and right anterior temporal lobes. The left anterior temporal lobe is associated with name retrieval while the right anterior temporal lobe is associated with retrieval of face and semantic information (for a review see Gainotti 2013). These findings replicate and extend those of our prior study using a different face-name learning task (Alm et al. 2016), and also a study by a different group who looked at face-place learning (Thomas et al. 2015). In the current study, 35% to 45% of the variance in face-name learning was accounted for by variation in UF white matter, which is similar to the effect sizes we reported previously of 29% to 43% (Alm et al., 2016). Taken together, these findings indicate that this effect is quite robust, surviving differences in stimuli, number of trials, subject demographics, and particulars of the DWI pipeline and analytic procedure.
Furthermore, we found a significant relationship between UF microstructure and memory for word pairs; the effect was only marginally significant for individual words. Prior studies have reported a relationship between the UF and performance on tasks that require the retrieval of single words or word pairs (reviewed in Olson, Von Der Heide, Alm, & Vyas, 2015). Our finding does not allow us to clearly address the issue of the UF and relational versus item memory. Additional power with an increased sample size would give a definitive answer to the question of the UF’s relationship with non-social verbal associations versus single items. At this point, we can say that the UF is involved in a range of associative memory tasks.
4.1. Is there a UF sub-tract with Person Memory Specificity?
Recently, researchers have been exploring the notion that white matter tracts connecting specialized gray matter regions support distinct cognitive processes. For instance, Gomez et al. (2015) used face- and place-specific functional ROIs to define seed regions and found distinct ventral stream tracts associated with face- and place-selective gray matter regions. There was a relationship between category-specific perceptual performance and the microstructural properties of these tracts local to the category-selective functional ROIs (within 10mm) but not for the entire white matter tracts. One possible interpretation is that different parts within a well-defined white matter tract support distinct functions.
Using a similar logic, in this study we began with the premise that large white matter tracts like the UF likely serve as the information conduit for a family of cognitive processes related to the functions of the neuron populations connected to one another by this tract. Here, we isolated a face-specific UF sub-tract, by examining specific connections from a face patch in the ATL to a face patch in the OFC.
Our results show that variation in the microstructure of this sub-tract was associated with performance on two face-name learning tasks. We examined the specificity of this finding in two ways. First, we tested the face specificity of this tract by assessing whether performance on a non-social memory task (Exp. 3, memory for word pairs or single words) correlated with microstructure of the sub-tract. There were no statistically significant effects. Second, we asked whether another tract, connecting the FFA and the ATL face patch, demonstrated a relationship with face-name task performance. This tract was chosen because of the obvious links to face processing. In addition, like the UF sub-tract, this tract enters the anterior temporal lobes (we used identical ATL seeds for both sub-tracts). Last, our group (Alm et al. 2016) and another group (Thomas et al., 2015) previously found that the ILF, which is presumably the large association fiber pathway to which the sub-tract belongs, had no relationship to face-name or face-scene learning rate. Our results are consistent with these findings, since we found no reliable relationship between FFA-ATL sub-tract microstructure and face-name associative learning. We also examined another control face tract, a structure connecting the FFA to the OFC face patch, which is presumably part of the IFOF. Again, no reliable relationships were observed between microstructure and face-name associative learning.
Other candidate regions to use in a “sub-tract” analysis include ventral v4 (color perception), the visual word form area, MT/v5 (motion perception), posterior STS (biological motion and body representations), regions in the inferior parietal lobe sensitive to numbers, the amygdala (emotion), regions in motor cortex that code for movements of particular body parts, and all primary sensory cortices. For instance, it’s possible that individuals with superior olfactory memory have altered white matter connectivity between the hippocampus and olfactory cortex in the uncus.
In the future, it will be important to see whether other types of social learning are related to UF sub-tracts. Some naturalistic examples include learning people’s biographies from a single telling, remembering people’s social status or personality traits, or even evoking implicit stereotypes (which are a type of semantic memory). Abnormalities in the UF have been reported in several psychiatric and neurological disorders characterized by impaired social cognition such as behavioral variant of frontotemporal dementia, anti-social personality disorder, and conduct disorder (reviewed in Von Der Heide, Skipper, Klobusicky, & Olson, 2013). Future research should examine socially-relevant learning and memory in these populations to see if abnormal UF microstructure relates to these behavioral indices.
4.2. Relationship to Prior Findings Literature
It has been proposed that the UF is part of the ventral language stream, playing an important, albeit non-essential, role in semantic memory retrieval (Duffau et al. 2009). However the relevant literature is inconsistent (reviewed by Von Der Heide et al., 2013). Some studies of individuals with semantic dementia or aphasia have implicated the UF in semantic retrieval (Harvey et al. 2013; Han et al. 2013), while other studies using electrical stimulation during neurosurgery suggest that the UF plays little to no role in semantic retrieval (Duffau et al. 2009) or only plays a role in retrieving proper names and unique entities (Mehta et al., 2016; Papagno et al., 2014). In a prior study, we found no relationship between microstructure of the UF and performance on a semantic retrieval task (Nugiel et al. 2016). Nevertheless, there is one semantic memory task that is consistently associated with the UF: proper name retrieval (Papagno et al. 2011; Nomura et al. 2013).
Most people find proper naming tasks very challenging. Indeed, a common memory complaints in older adults is difficulty recalling proper names (Leirer et al. 1990). This can be explained by the fact that face-name pairings are arbitrary; there is nothing in a person’s face or demeanor that provides a clue about what their name might be. Retrieving the correct proper name also evokes severe retrieval competition, given that many people may have the same first name (e.g. John Lennon, John Rockeller, John F. Kennedy, John the Baptiste, etc.). We previously suggested that the primary role of the UF in episodic memory is in adjudicating between competing representations at retrieval (Alm et al. 2016). We based this view on several key findings that emerged in our review of the literature (Olson et al. 2015). In non-human primates, performance in a task called “conditional rule learning” is impaired after UF transection (Bussey, Wise, & Murray, 2002; Gaffan, Gaffan, & Harrison, 1988; Parker & Gaffan, 1998). In this task, monkeys are shown many similar objects and their task is to correctly recall which one of the four choice locations was paired with a particular object. Locations are repeated each trial so there is a great deal of retrieval competition. In humans, tasks in which UF microstructural variation correlates with episodic memory performance tend to have high retrieval competition (e.g. verbal recall, proper name retrieval, associative learning and memory tasks). Performance on tasks with low competition, such as the Rey-Osterrieth, tend not to correlate with UF microstructure. The present results are broadly consistent with the retrieval competition idea, however further research will need to test this idea more specifically.
4.3. Limitations and Future Directions
Our study has two limitations. The first is methodological. The present study involves the use of tensor based models and deterministic tractography. These techniques cannot detect crossing fibers within a voxel. This is less of an issue when examining large, well-known fiber pathways such as the UF, which can be accurately reconstructed using the interpolated streamline algorithm as we have done in the present study. In addition, our image acquisition sequence only included a single nondiffusion-weighted image (b0 image). Future investigations should aim to collect multiple volumes with no diffusion that can be averaged together to increase the signal-to-noise ratio and correct potential EPI distortions.
The second has to do with our sample population. We tested a sample of neurologically normal undergraduates. Although our effect sizes were very robust, it would be wise to extend these findings to populations with memory complaints, such as older adults, individuals with traumatic brain injury, or individuals suffering from “chemobrain.” Studies with older populations have demonstrated that there are important age-related changes in the microstructure and the microstructure of the UF (Hasan et al. 2009, 2010). These populations would presumably show more variation in performance, and diffusion imaging could potentially provide a biomarker for impaired cognition. It would also be interesting to look at a population with social deficits, such as autism, to see whether there are deficits in face-name learning and other forms of social learning (such as imitation) that are attributable to alterations in the UF.
Future research should examine whether other UF sub-tracts, such as a tract beginning in perirhinal or secondary olfactory cortex (piriform), have distinct functional profiles. Given the logic that information transmission properties of white matter pathways is predicted by the function of the gray matter regions they connect (Passingham et al. , 2002, Van Essen and Maunsell, 1983), it is likely that the former sub-tract would support object associations while the later would support olfactory associations.
5. Conclusions
The results of the present investigation support the hypothesis that microstructural variation in the UF is associated with individual differences in associative memory performance. Significant relationships emerged across two different face-name learning tasks, as well as an associative word pair task. We also demonstrated that the UF can be further parcellated into a sub-tract that specifically supports associative memory for social stimuli. Therefore, the UF seems to play a critical role in supporting associative memory function, and the type of associative learning facilitated by the UF may depend on discrete sub-tracts within this long-range fiber pathway.
Supplementary Material
Acknowledgments
We would like to thank William Hampton and Linda Hoffman for assistance with participant testing. This work was supported by a National Institute of Health grant to I. Olson [RO1 MH091113]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. The authors declare no competing financial interests.
References
- Alm KH, Rolheiser T, Mohamed FB, Olson IR. Fronto-temporal white matter connectivity predicts reversal learning errors. Front Hum Neurosci. 2015;9:1–11. doi: 10.3389/fnhum.2015.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm KH, Rolheiser T, Olson IR. Inter-individual variation in fronto-temporal connectivity predicts the ability to learn different types of associations. Neuroimage. 2016;132:213–224. doi: 10.1016/j.neuroimage.2016.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995:289–300. [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning PGF, Easton A, Buckley MJ, Gaffan D. The role of prefrontal cortex in object-in-place learning in monkeys. Eur J Neurosci. 2005;22:3281–3291. doi: 10.1111/j.1460-9568.2005.04477.x. [DOI] [PubMed] [Google Scholar]
- Browning PGF, Gaffan D. Impairment in object-in-place scene learning after uncinate fascicle section in macaque monkeys. Behav Neurosci. 2008;122:477–482. doi: 10.1037/0735-7044.122.2.477. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Wise SP, Murray EA. Interaction of ventral and orbital prefrontal cortex with inferotemporal cortex in conditional visuomotor learning. Behav Neurosci. 2002;116:703–715. doi: 10.1037/0735-7044.116.4.703. [DOI] [PubMed] [Google Scholar]
- Catani M, Dell’Acqua F, Thiebaut de Schotten M. A revised limbic system model for memory, emotion and behaviour. Neurosci Biobehav Rev. 2013;37:1724–1737. doi: 10.1016/j.neubiorev.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M. A diffusion tensor imaging tractography atlas for virtual in vivo dissections. Cortex. 2008;44:1105–1132. doi: 10.1016/j.cortex.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Collins JA, Olson IR. Beyond the FFA: The role of the ventral anterior temporal lobes in face processing. Neuropsychologia. 2014;61:65–79. doi: 10.1016/j.neuropsychologia.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conturo TE, Lori NF, Cull TS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci. 1999;96:10422–10427. doi: 10.1073/PNAS.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Second Edition. 2000. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Duffau H, Gatignol P, Moritz-Gasser S, Mandonnet E. Is the left uncinate fasciculus essential for language? J Neurol. 2009;256:382–389. doi: 10.1007/s00415-009-0053-9. [DOI] [PubMed] [Google Scholar]
- Feigl GC, Hiergeist W, Fellner C, et al. Magnetic resonance imaging diffusion tensor tractography: evaluation of anatomic accuracy of different fiber tracking software packages. World Neurosurg. 2014;81:144–150. doi: 10.1016/j.wneu.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Fillard P, Descoteaux M, Goh A, et al. Quantitative evaluation of 10 tractography algorithms on a realistic diffusion MR phantom. Neuroimage. 2011;56:220–234. doi: 10.1016/j.neuroimage.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Frazier JA, Chiu S, Breeze JL, et al. Structural Brain Magnetic Resonance Imaging of Limbic and Thalamic Volumes in Pediatric Bipolar Disorder. Am J Psychiatry. 2005;162:1256–1265. doi: 10.1176/appi.ajp.162.7.1256. [DOI] [PubMed] [Google Scholar]
- Gaffan D. The role of the hippocampus-fornix-mammillary system in episodic memory. 2. Guilford Press; New York, NY, US: 1992. [Google Scholar]
- Gaffan EA, Gaffan D, Harrison S. Disconnection of the amygdala from visual association cortex impairs visual reward-association learning in monkeys. J Neurosci. 1988;8:3144–3150. doi: 10.1523/JNEUROSCI.08-09-03144.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G. Laterality effects in normal subjects’ recognition of familiar faces, voices and names. Perceptual and representational components. Neuropsychologia. 2013;51:1151–1160. doi: 10.1016/j.neuropsychologia.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Makris N, et al. Hypothalamic Abnormalities in Schizophrenia: Sex Effects and Genetic Vulnerability. Biol Psychiatry. 2007;61:935–945. doi: 10.1016/j.biopsych.2006.06.027. [DOI] [PubMed] [Google Scholar]
- Gomez J, Pestilli F, Witthoft N, et al. Functionally Defined White Matter Reveals Segregated Pathways in Human Ventral Temporal Cortex Associated with Category-Specific Processing. Neuron. 2015;85:216–227. doi: 10.1016/j.neuron.2014.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Ma Y, Gong G, et al. White matter structural connectivity underlying semantic processing: evidence from brain damaged patients. Brain. 2013;136:2952–2965. doi: 10.1093/brain/awt205. [DOI] [PubMed] [Google Scholar]
- Harvey DY, Wei T, Ellmore TM, et al. Neuropsychological evidence for the functional role of the uncinate fasciculus in semantic control. Neuropsychologia. 2013;51:789–801. doi: 10.1016/j.neuropsychologia.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Iftikhar A, Kamali A, et al. Development and aging of the healthy human brain uncinate fasciculus across the lifespan using diffusion tensor tractography. Brain Res. 2009;1276:67–76. doi: 10.1016/j.brainres.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan KM, Kamali A, Abid H, et al. Quantification of the spatiotemporal microstructural organization of the human brain association, projection and commissural pathways across the lifespan using diffusion tensor tractography. Brain Struct Funct. 2010;214:361–373. doi: 10.1007/s00429-009-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirni DI, Kivisaari SL, Monsch AU, Taylor KI. Distinct neuroanatomical bases of episodic and semantic memory performance in Alzheimer’s disease. Neuropsychologia. 2013;51:930–937. doi: 10.1016/j.neuropsychologia.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Huang H, Zhang J, van Zijl PCM, Mori S. Analysis of noise effects on DTI-based tractography using the brute-force and multi-ROI approach. Magn Reson Med. 2004;52:559–565. doi: 10.1002/mrm.20147. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Pechaud M, Smith S. BET2 -MR-Based Estimation of Brain, Skull and Scalp Surfaces. 2005. [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J Neurosci. 1997;17:4302–4311. doi: 10.1098/Rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leirer VO, Morrow DG, Sheikh JI, Pariante GM. Memory skills elders want to improve. Exp Aging Res. 1990;16:155–158. doi: 10.1080/07340669008251544. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Rovet J, Noseworthy MD, et al. The relations between white matter and declarative memory in older children and adolescents. Brain Res. 2009;1294:80–90. doi: 10.1016/j.brainres.2009.07.046. [DOI] [PubMed] [Google Scholar]
- Makris N, Goldstein JM, Kennedy D, et al. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr Res. 2006;83:155–171. doi: 10.1016/j.schres.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ. Thalamic mediodorsal nucleus and memory: A critical evaluation of studies in animals and man. Neurosci Biobehav Rev. 1982;6:351–380. doi: 10.1016/0149-7634(82)90046-X. [DOI] [PubMed] [Google Scholar]
- Martin A, Chao LL. Semantic memory and the brain: structure and processes. Curr Opin Neurobiol. 2001;11:194–201. doi: 10.1016/S0959-4388(00)00196-3. [DOI] [PubMed] [Google Scholar]
- Mehta S, Inoue K, Rudrauf D, et al. Segregation of anterior temporal regions critical for retrieving names of unique and non-unique entities reflects underlying long-range connectivity. Cortex. 2016;75:1–19. doi: 10.1016/j.cortex.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metzler-Baddeley C, Jones DK, Belaroussi B, et al. Frontotemporal connections in episodic memory and aging: A diffusion MRI tractography study. J Neurosci. 2011;31:13236–13245. doi: 10.1523/JNEUROSCI.2317-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S, Kaufmann WE, Davatzikos C, et al. Imaging cortical association tracts in the human brain using diffusion-tensor-based axonal tracking. Magn Reson Med. 2002;47:215–223. doi: 10.1002/mrm.10074. [DOI] [PubMed] [Google Scholar]
- Nomura K, Kazui H, Tokunaga H, et al. Possible roles of the dominant uncinate fasciculus in naming objects: a case report of intraoperative electrical stimulation on a patient with a brain tumour. Behav Neurol. 2013;27:229–234. doi: 10.3233/BEN-110249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugiel T, Alm KH, Olson IR. Individual differences in white matter microstructure predict semantic control. Cogn Affect Behav Neurosci. 2016;16:1003–1016. doi: 10.3758/s13415-016-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson IR, Von Der Heide RJ, Alm KH, Vyas G. Development of the uncinate fasciculus: Implications for theory and developmental disorders. Dev Cogn Neurosci. 2015;14:50–61. doi: 10.1016/j.dcn.2015.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oosterhof NN, Todorov A. The functional basis of face evaluation. Proc Natl Acad Sci. 2008;105:11087–11092. doi: 10.1073/pnas.0805664105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagno C, Casarotti A, Comi A, et al. Long-term proper name anomia after removal of the uncinate fasciculus. Brain Struct Funct. 2016;221:687–694. doi: 10.1007/s00429-014-0920-8. [DOI] [PubMed] [Google Scholar]
- Papagno C, Miracapillo C, Casarotti A, et al. What is the role of the uncinate fasciculus? Surgical removal and proper name retrieval. Brain. 2011;134:405–414. doi: 10.1093/brain/awq283. [DOI] [PubMed] [Google Scholar]
- Parker A, Gaffan D. Memory after frontal/temporal disconnection in monkeys: conditional and non-conditional tasks, unilateral and bilateral frontal lesions. Neuropsychologia. 1998;36:259–271. doi: 10.1016/S0028-3932(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Paternoster R, Brame R, Mazerolle P, Piquero A. Using the correct statistical test for the equality of regression coefficients. Criminology. 1998;36:859–866. doi: 10.1111/j.1745-9125.1998.tb01268.x. [DOI] [Google Scholar]
- Perrodin C, Kayser C, Abel TJ, et al. Who is That? Brain Networks and Mechanisms for Identifying Individuals. Trends Cogn Sci. 2015;19:783–796. doi: 10.1016/j.tics.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyles JA, Verstynen TD, Schneider W, Tarr MJ. Explicating the Face Perception Network with White Matter Connectivity. PLoS One. 2013;8:e61611. doi: 10.1371/journal.pone.0061611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G. Lateralized processes in face recognition. Br J Psychol. 1985;76:249–271. doi: 10.1111/j.2044-8295.1985.tb01949.x. [DOI] [PubMed] [Google Scholar]
- Rogers TT, Hocking J, Noppeney U, et al. Anterior temporal cortex and semantic memory: Reconciling findings from neuropsychology and functional imaging. Cogn Affect Behav Neurosci. 2006;6:201–213. doi: 10.3758/CABN.6.3.201. [DOI] [PubMed] [Google Scholar]
- Sergent J, Signoret J-L, Bruce V, Rolls ET. Functional and Anatomical Decomposition of Face Processing: Evidence from Prosopagnosia and PET Study of Normal Subjects. Philos Trans R Soc London B Biol Sci. 1992;335:55–62. doi: 10.1098/rstb.1992.0007. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Thomas C, Avram A, Pierpaoli C, Baker C. Diffusion MRI properties of the human uncinate fasciculus correlate with the ability to learn visual associations. Cortex. 2015;72:65–78. doi: 10.1016/j.cortex.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Thomas C, Humphreys K, Jung K-J, et al. The anatomy of the callosal and visual-association pathways in high-functioning autism: a DTI tractography study. Cortex. 2011;47:863–873. doi: 10.1016/j.cortex.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troiani V, Dougherty CC, Michael AM, Olson IR. Characterization of Face-Selective Patches in Orbitofrontal Cortex. Front Hum Neurosci. 2016;10:279. doi: 10.3389/fnhum.2016.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M, Embleton KV, Jefferies E, et al. The inferior, anterior temporal lobes and semantic memory clarified: Novel evidence from distortion-corrected fMRI. Neuropsychologia. 2010;48:1689–1696. doi: 10.1016/j.neuropsychologia.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Klobusicky E, Olson IR. Dissecting the uncinate fasciculus: disorders, controversies and a hypothesis. Brain. 2013a;136:1692–1707. doi: 10.1093/brain/awt094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Heide RJ, Skipper LM, Olson IR. Anterior temporal face patches: A meta-analysis and empirical study. Front Hum Neurosci. 2013b;7:1–18. doi: 10.3389/fnhum.2013.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36:630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Benner T, Sorensen AG, Wedeen VJ. Diffusion Toolkit : A Software Package for Diffusion Imaging Data Processing and Tractography. Proc 15th Sci Meet Int Soc Magn Reson Med; 2007. p. 3720. [Google Scholar]
- Wechsler D. Wechsler Memory Scale (WMS-IV) 2009. [Google Scholar]
- Zahr NM, Rohlfing T, Pfefferbaum A, Sullivan EV. Problem solving, working memory, and motor correlates of association and commissural fiber bundles in normal aging: A quantitative fiber tracking study. Neuroimage. 2009;44:1050–1062. doi: 10.1016/j.neuroimage.2008.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.