Abstract
Objective
To clarify the relationship between depression and heart rate variability (HRV) in a sample of twins. Reduced HRV, a measure of autonomic dysfunction, has been linked to depression but many studies have inadequately controlled for familial and environmental factors. Furthermore, little is known about whether depression and HRV share common genetic pathways.
Methods
We performed power spectral analysis on 24-hour ambulatory electrocardiograms in 288 middle-aged male twins. Log-normalized ultra low, very low, low, high frequency, and total power were calculated. A lifetime history of major depressive disorder (MDD) was determined, using the Structured Clinical Interview for Psychiatry Disorders, and current depressive symptoms were measured with the Beck Depression Inventory. Mixed-effect regression models were used to account for intrapair variability and estimate within-pair effects at the same time controlling for potential confounders.
Results
Both current depressive symptoms and a history of MDD were significantly associated with lower HRV. There was a graded effect, and power in each frequency band was 29% to 36% lower in the lowest band compared with the highest BDI category. All HRV measures except high frequency remained significantly associated with current depressive symptoms in multivariable analysis, but not with lifetime history of MDD. When analyses were stratified by zygosity, a significant within-pair association between BDI score and HRV was found in the dizygotic but not in the monozygotic twins, suggesting a genetic influence on the association.
Conclusions
A shared, genetically influenced biological pathway underlies the association between depression and lower HRV. These two phenotypes may be the expression of a generalized neurobiological perturbation.
Keywords: autonomic function, electrophysiology, genetic factors, psychosocial factors, risk factors
INTRODUCTION
Depression is associated with increased morbidity and mortality in individuals with (1) and without (2) cardiac disease but the mechanisms underlying these effects are unclear. One of the proposed explanations is dysregulation of the autonomic nervous system in depressed individuals, with increased sympathetic tone and/or decreased parasympathetic modulation (3).
Heart rate variability (HRV) analysis, a measure of beat-to-beat heart rate fluctuations over time, is a widely used method for the assessment of autonomic modulation of the heart. Reduced HRV may reflect either increased sympathetic or decreased parasympathetic tone (4,5) and is a consistent predictor of cardiac events and mortality in patients with acute myocardial infarction (6–8) and in unselected community populations (9–13). Patients with coronary artery disease (CAD) show an association between depression, or depressive symptoms, and reduced HRV (14–17), with few exceptions (18). However, among persons drawn from the community who are predominantly free of CAD, this relationship is less established (19–21). Even among depressed psychiatric patients compared with controls, the results are not consistent (22–25).
A limitation of many previous studies is that uncontrolled physiological and environmental factors may profoundly influence HRV. For example, high- and low-frequency power HRV may be affected by changes in respiration that accompany every day activities, such as talking or mental tasks (26), which, in turn, may be influenced by depression. Long-range HRV (ultra low-frequency power) is influenced by physical activity (27), another factor likely to be affected by depression. It is therefore important to standardize environmental situations when examining HRV data (28). Furthermore, previous research has not examined whether familial factors, such as family background and genetic factors, play a role in the association between depression and HRV. Because both depression (29) and HRV (30,31) are associated with social position, the latter may confound the association. Furthermore, because both depression (32) and HRV (33,34) are influenced by genes, it is possible that the association is genetically modulated. These two phenotypes could be, at least in part, under common genetic control, for example, expressions of a generalized neurobiological dysregulation.
In a sample of monozygotic (MZ) and dizygotic (DZ) male twins, we sought to clarify the relationship between depression and HRV. Twins provide unique opportunities to study associations at the same time controlling for shared environmental or genetic factors, because twin siblings share genes (50%, on average, if DZ, and all if MZ), maternal factors, and early familial environment. First, if the association between depression and HRV is due to environmental factors that are matched between twins, such as activity level during examination and family social class, then it should be found only between and not within, twin pairs. Second, if the association is due to genetic factors, it should not be found within MZ depression-discordant twin pairs, who are 100% matched on genetic background. However, if the association is due to nonfamilial/nongenetic factors, it should be found within both MZ and DZ depression-discordant twin pairs. We hypothesized that depressive symptoms would be associated with lower HRV but that the association is due, in part, to shared genes.
METHODS
Subjects
The Twins Heart Study (THS) is an investigation of psychological, behavioral, and biological risk factors for subclinical cardiovascular disease using twins (35–37). Twins were selected from the Vietnam Era Twin (VET) Registry (38), which includes 7369 middle-aged male-male twin pairs both of whom served in the United States military during the time of the Vietnam War. The characteristics of the VET Registry were previously reported (39).
THS included 360 twins from the VET Registry, of which 102 pairs were MZ and 78 pairs were DZ, all born between 1946 and 1956 (>90% of the twins in the VET registry fell into this range). The twins were free of a self-reported previous diagnosis of cardiovascular disease based on survey data collected in 1990 (40), including a previous diagnosis of heart attack/ myocardial infarction, coronary heart disease, angina, congestive heart failure or stroke, or previous coronary angioplasty or coronary bypass surgery. From this group, we randomly sampled two groups of twin pairs: one group included depression-discordant twins, where one member of the pair had a lifetime history of major depressive disorder (MDD) assessed with the Diagnostic Interview Schedule (41) and the other did not; the second group of twins included pairs where neither had a history of MDD. Our main goal was to conduct co-twin control studies in subjects discordant for MDD or depressive symptoms. As concordant twin pairs for depression do not provide information in the co-twin control design, they were not selected.
Once selected, twin pairs were examined together at the Emory University General Clinical Research Center (GCRC) between March 2002 and March 2006, where the twins had a comprehensive history and physical examination and were queried again about previous diagnoses of cardiovascular diseases that might have occurred from the time of the initial screen in 1990. Because cardiovascular disease could have developed after depression in this sample, we did not automatically exclude twins who reported cardiovascular disease after 1990, but we chose to perform sensitivity analyses with and without these twins included in the analysis. The GCRC admission lasted about 27 hours and the entire data collection for the study occurred during this time. The two twins maintained an identical schedule during their study participation in the GCRC. Activity was limited to leisurely ambulation within the Emory facilities, and all assessment, including ambulatory electrocardiographic (ECG) monitoring, began and ended at the same time. Zygosity information by means of DNA typing was available for all but 11 twin pairs. The zygosity of these remaining 11 pairs was based on questionnaires supplemented by blood group typing abstracted from military records (39), a method that in our sample had an accuracy of 94%. This protocol was approved by the Institutional Review Board at Emory University and informed consent was obtained from all subjects.
Assessment of Depression and Other Psychiatric Diagnoses
We administered the Beck Depression Inventory-II (BDI-II) (42), a standardized scale providing a continuous measure of depressive symptoms. This self-report instrument includes 21 symptom items and the twins rated the severity of each symptom from 0 to 3. The BDI is used extensively with community samples and has satisfactory test-retest reliability and internal consistency (43). There is no uniform recommendation on how to categorize the BDI-II score. Cut-points of 10, 14, 20, and 29 have been suggested for increasing severity of depressive symptoms (42,44). We also administered the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) (SCID) (45) to classify subjects based on a lifetime history of MDD. However, because 78% of the major depressive episodes were remote (>1 year before the assessment), our analysis focused on the current level of depressive symptoms measured with the BDI. The SCID also provided a diagnosis of other psychiatric disorders, including a lifetime history of posttraumatic stress disorder (PTSD), and a lifetime history of alcohol, drug abuse, or dependence. Current substance abuse and other psychiatric diagnoses were extremely infrequent.
Measurement of HRV
Twins wore an ambulatory ECG monitor (Holter, GE Marquette SEER digital system, Little Chalfont, UK) for 24 hours. Both twins in a pair were studied at the same time and their recording times, schedule, and activity level during the recording were matched. HRV data were analyzed following published methodology (6,8). Holter recordings were digitally sampled and analyzed, using a GE Medical/Marquette system. Each tape was manually processed and edited. A list of R-R intervals with annotations denoting normal beats, types of ectopics, and noise was saved and later transferred to a Sun workstation for further processing; the analysis used customized software written locally at Yale University and incorporating methods described in the literature (4). The file was first edited to remove ectopics and noise, and gaps were filled in by interpolation. The R-R interval time series was sampled, using a boxcar window (46), to give 1024 samples per 5 minutes (3.41333 Hz) to generate a uniformly spaced instantaneous heart rate time series. The heart rate spectrum was computed, using a fast Fourier transform (FFT) with a Parzen window. The FFT routines were optimized for sequences with lengths equal to multiples of small primes (6,46), giving flexibility in processing patient files of differing duration. The power spectrum was corrected for attenuation due to windowing and sampling (47). Because long-term autonomic function was the goal of this study, the FFT was performed on the 24-hour R-R interval file. The power spectrum was integrated over four discrete frequency bands (6): ultra low frequency (ULF) <0.0033 Hz; very low frequency (VLF) 0.0033 to <0.04 Hz; low frequency (LF) 0.04 to <0.15 Hz; and high frequency (HF) 0.15 to <0.40 Hz. Total power (TPow), incorporating the full spectrum <0.40 Hz, was also measured. The ratio of LF to HF, which may represent sympathovagal balance, was also calculated. Twins whose recordings showed >20% interpolation or <18 recorded hours were excluded from the analysis.
Other Measurements
A medical history and a physical examination were obtained from all twins from a research nurse. Weight and height were used to calculate body mass index. Abdominal and hip circumferences were also measured to derive the waist-hip ratio. Systolic and diastolic blood pressures were measured by mercury sphygmomanometer on the right arm with the twin in a sitting position after 10 minutes of rest; the average of two measurements 5 minutes apart was used in the analysis. Venous blood samples were drawn for the measurements of glucose and lipid profile after an overnight fast. Total triglycerides were determined by enzymatic methods (Beckman Coulter Diagnostics, Fullerton, California). Direct high-density lipoprotein and low-density lipoprotein (LDL) cholesterol were measured with homogeneous assays (Equal Diagnostics, Exton, Pennsylvania). Glucose was measured on the Beckman CX7 chemistry autoanalyzer. Physical activity was assessed with a modified version of the Baecke Questionnaire of Habitual Physical Activity used in the Atherosclerosis Risk in Communities Study (48), a 16-question instrument documenting level of physical activity at work, during sports and nonsports activities. The global physical activity score was used in the analysis. Cigarette smoking was classified into current versus never or past smoker. A history of coronary heart disease was defined as a previous diagnosis of myocardial infarction or angina pectoris, or previous coronary revascularization procedures. Diabetes mellitus was defined as having a fasting glucose level of >126 mg/dl or being treated with antidiabetic medications. Information on current use of other medications was also collected.
Statistical Analysis
In initial descriptive analyses, we compared means and percents of study factors between MZ and DZ twins. The p values were corrected for the correlation between co-twins using generalized estimating equations (GEE) for categorical variables and mixed-effects models for continuous variables.
To examine the association between depression and HRV, two sets of analyses were performed: a) between individuals, in which all twins were eligible for inclusion regardless of whether their brother was available for analysis; and b) within twin pairs. In the analysis between individual twins, we used mixed-effects regression models and accounted for the twin pairs using a random effect term for each pair. Because the BDI score was skewed and because we were interested in the dose-response pattern between severity of depressive symptoms and HRV, the BDI score was categorized using cut-points of increasing severity at 10, 14, and 20 (42,44). Category midpoints were used for analysis. Statistical analyses were performed on log-transformed data, as is customary with HRV data which are not normally distributed. To display the actual magnitude of the difference, percentages were based on nontransformed values by using the following formula: [1−(expβ)] × 100 (%). Analyses were repeated after adjusting for potential confounding factors.
To avoid model overfitting, for each HRV variable we constructed a “base” model that included behavioral and medical history factors that were either significantly related to depression, or were a priori considered clinically relevant risk factors. These included current cigarette smoking, waist-hip ratio, habitual physical activity, history of diabetes, and history of coronary heart disease. Next, we considered additional potential correlates of HRV, including marital status, LDL cholesterol, number of caffeinated beverages per day, heart rate over 24 hours, current use of antidepressants, current use of β blockers, history of alcohol abuse, history of drug abuse, and PTSD. However, none of these factors materially changed the study estimates when included in the models. Adjusting for age also did not affect the results; the sample had a narrow age range, and age was a redundant factor in within-pair analyses. Furthermore, adjusting for the sampling frame (MDD discordant versus non-MDD pairs) did not alter the results.
Next, we performed within-pair analyses, which examined differences in HRV between co-twins in each pair. The within-pair effects are inherently controlled for demographic, shared familial, and early environmental influences; in addition, daily activities and other environmental factors during the ambulatory ECG recording are controlled because co-twins were examined at the same time and under identical conditions. We fitted mixed-effects models adapted for twin research (49), which allow for partitioning within- and between-pair differences in the dependent variable (HRV) as a function of the independent variables. In these models, the within-pair parameter is the individual twin variation from the twin pair average. This coefficient is identical to the coefficient from a model that fits the absolute difference between the co-twins (49). Although these models allowed us to include all twins in the analysis to fully use the data, only discordant twin pairs contributed to the within-pair analyses. Twins were considered discordant if they fell into different BDI categories. Discordance was also analyzed as a dichotomous variable by classifying a twin as either having a higher or a lower BDI category than his brother, and also as an ordinal variable counting the number of BDI categories that the co-twins differed for.
The last set of analyses involved testing whether there was a genetic influence underlying the association between BDI score and HRV, by adding to the model a term for the interaction between zygosity and the within-pair depression effect. If a larger difference in HRV is found within BDI-discordant DZ pairs than within BDI-discordant MZ pairs (i.e., a significant interaction is present), this suggests that genetic factors play a role. This concept may not be obvious to those who are unfamiliar with twin studies and behavioral genetics. If any trait or association between traits is due to genes, then MZ twins cannot differ because they are genetically identical. This is similar to other confounders in epidemiological studies, which are eliminated when the groups are matched for the confounding variable. DZ pairs, however, only share on average 50% of their genetic material. Thus, if the association between BDI score and HRV is modulated by genes, it will be found within DZ discordant twin pairs but not within MZ discordant pairs. The interaction between zygosity and the within-pair effect for BDI tested whether there was a significant difference in HRV within DZ pairs but not within MZ pairs, thus providing a test for genetic confounding. To better describe differences by zygosity, we also fitted separate models for MZ and DZ twins. Similar analyses were conducted for lifetime history of MDD. To facilitate the interpretation of results, we derived heritability estimates for HRV variables using the intraclass correlation coefficient (ICC) in MZ and DZ twin pairs. The ICC was calculated as the variance due to the clustering variable divided by the sum of variance due to the clustering variable and the remaining variance using unconditional means models (50). Because of our sampling frame (MDD discordant and non-MDD pairs), we calculated heritability before and after excluding the MDD-discordant pairs. Because the results were overall similar, we report heritability estimates for the overall sample.
RESULTS
Of the 360 THS twins, 288 (80%), including 115 pairs and 58 singletons (resulting from exclusion of the co-twin) had usable ambulatory ECG data (≥ 18 hours of recording with at least 80% noninterpolated intervals) and were included in the analysis. Of these, 239 had a BDI score of < 10; 17 had a score between 10 and 13; 18 had a score between 14 and 19; and 14 had a score of ≥20. In addition, 61 twins (21%) had a lifetime history of MDD; however, only 7 twins met the criteria for current depressive episode according to the DSM-IV criteria. Furthermore, 62% experienced only a single lifetime major depressive episode, and 78% of the reported episodes were in the distant past (>1 year before examination). Except for a slightly older age and lower LDL level in the DZ twins, there were no significant differences in demographic characteristics and risk factors according to zygosity (Table 1).
TABLE 1.
Distribution of Demographic, Behavioral and Coronary Risk Factors in Monozygotic and Dizygotic Twins
| MZ n = 167 |
DZ n = 121 |
p* | |
|---|---|---|---|
| Age, years, mean ± SD | 54.0 ± 2.8 | 54.8 ± 2.8 | .04 |
| Greater than high school education, % | 31.7 | 21.5 | .09 |
| Married, % | 78.4 | 81.0 | .58 |
| Systolic blood pressure, mm Hg, mean ± SD | 129.5 ± 16.1 | 126.8 ± 15.2 | .24 |
| Diastolic blood pressure, mm Hg, mean ± SD | 81.1 ± 11.2 | 78.8 ± 9.3 | .14 |
| Heart rate (24-hour monitoring), beats/min, mean ± SD | 65.8 ± 8.9 | 67.3 ± 9.5 | .25 |
| LDL cholesterol, mg/dl, mean ± SD | 127.8 ± 34.5 | 115.5 ± 32.3 | .01 |
| HDL cholesterol, mg/dl, mean ± SD | 38.4 ± 9.6 | 38.4 ± 9.4 | .92 |
| Diabetes, % | 8.3 | 10.6 | .57 |
| Body mass index, mean ± SD | 29.0 ± 4.3 | 29.7 ± 5.0 | .25 |
| Waist-hip ratio, mean ± SD | 0.942 ± 0.063 | 0.947 ± 0.060 | .59 |
| Current smoker, % | 18.6 | 16.5 | .65 |
| Physical activity (Baecke) score, mean ± SD | 7.4 ± 1.8 | 7.2 ± 1.8 | .27 |
| Number of caffeinated beverages in typical day, mean ± SD | 4.7 ± 5.5 | 4.1 ± 3.5 | .33 |
| Number of alcoholic beverages in typical week, mean ± SD | 4.5 ± 8.1 | 4.7 ± 7.4 | .71 |
| Prior coronary heart disease, % | 6.6 | 10.7 | .26 |
| Lifetime history of PTSD, % | 6.0 | 5.0 | .76 |
| Lifetime history of alcohol abuse or dependence, % | 41.3 | 42.1 | .88 |
| Lifetime history of drug abuse or dependence, % | 13.8 | 9.9 | .37 |
| Taking antidepressant medications, % | 13.2 | 15.7 | .54 |
| Taking β blocker medications, % | 7.2 | 6.6 | .86 |
MZ = monozygotic; DZ = dizygotic; SD = standard deviation; LDL = low-density lipoprotein; HDL = high-density lipoprotein; PTSD = posttraumatic stress disorder.
p values are obtained from mixed models for continuous variables or generalized estimating equations (GEE) for categorical variables.
Current depressive symptoms were associated with lower HRV (Table 2). A graded effect was noted, with increasingly higher BDI scores being associated with progressively lower HRV. Power in each HRV frequency band was 29% to 36% lower (nontransformed units) in the highest compared with the lowest BDI score category. The BDI score as a continuous variable was also associated with HRV (p < .01 for all frequency bands except HF). After adjusting for potential confounding factors, the association between BDI and HRV was slightly attenuated but remained statistically significant for TPow, ULF, and VLF (Table 2). HRV was 12% to 21% lower in twins with a history of MDD than those without (p < .05 for all spectra except HF). However, none of the HRV spectra remained significantly associated with a lifetime history of MDD in multivariable analysis.
TABLE 2.
Unadjusted and Adjusted HRV According to Current Depressive Symptom Level
| Unadjusted
|
Adjusted*
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ln TPow, ms2 | LnULF, ms2 | LnVLF, ms2 | LnLF, ms2 | LnHF, ms2 | Ln TPow, ms2 | LnULF, ms2 | LnVLF, ms2 | LnLF, ms2 | LnHF, ms2 | |
| BDI <10 (n = 239) | 9.50 ± 0.04 | 9.19 ± 0.04 | 7.66 ± 0.04 | 6.71 ± 0.06 | 5.42 ± 0.06 | 9.48 ± 0.04 | 9.17 ± 0.04 | 7.63 ± 0.04 | 6.68 ± 0.05 | 5.42 ± 0.07 |
| BDI 10–13 (n = 17) | 9.27 ± 0.12 | 8.97 ± 0.13 | 7.47 ± 0.13 | 6.31 ± 0.17 | 5.29 ± 0.19 | 9.35 ± 0.12 | 9.06 ± 0.13 | 7.54 ± 0.13 | 6.40 ± 0.18 | 5.29 ± 0.20 |
| BDI 14–19 (n = 18) | 9.22 ± 0.11 | 8.90 ± 0.12 | 7.24 ± 0.13 | 6.33 ± 0.17 | 5.13 ± 0.19 | 9.28 ± 0.11 | 8.96 ± 0.12 | 7.35 ± 0.13 | 6.44 ± 0.17 | 5.14 ± 0.20 |
| BDI ≥20 (n = 14) | 9.06 ± 0.13 | 8.74 ± 0.14 | 7.25 ± 0.15 | 6.36 ± 0.20 | 5.04 ± 0.22 | 9.19 ± 0.14 | 8.86 ± 0.15 | 7.45 ± 0.15 | 6.60 ± 0.20 | 5.10 ± 0.23 |
| % difference, highest versus lowest category | −35.71 | −36.43 | −33.79 | −29.19 | −31.17 | −25.23 | −26.86 | −16.63 | −8.06 | −26.68 |
| % difference, 1 -category incrementa | −14.08 | −14.26 | −15.21 | −14.90 | −12.37 | −9.56 | −9.99 | −8.50 | −7.22 | −10.96 |
| Test for trend | p = <.0001 | p = .0001 | p = <.0001 | p = .002 | p = .03 | p = .009 | p = .01 | p = .04 | p = .18 | p = .07 |
BDI = Beck Depression Inventory; HRV = heart rate variability; TPow = total power; ULF = ultra low frequency; VLF = very low frequency; LF = low frequency; HF = high frequency.
Mean ± standard error; percent differences refer to HRV in nontransformed units.
Adjusted for waist-hip ratio, current cigarette smoking, physical activity score, history of diabetes and history of coronary heart disease. Further adjustment for age, marital status, low-density lipoprotein cholesterol, number of caffeinated beverages per day, heart rate over 24 hours, current use of antidepressants. Current use of β blockers did not substantially affect the study estimates.
Difference in HRV per one-category difference in BDI between co-twins in a pair.
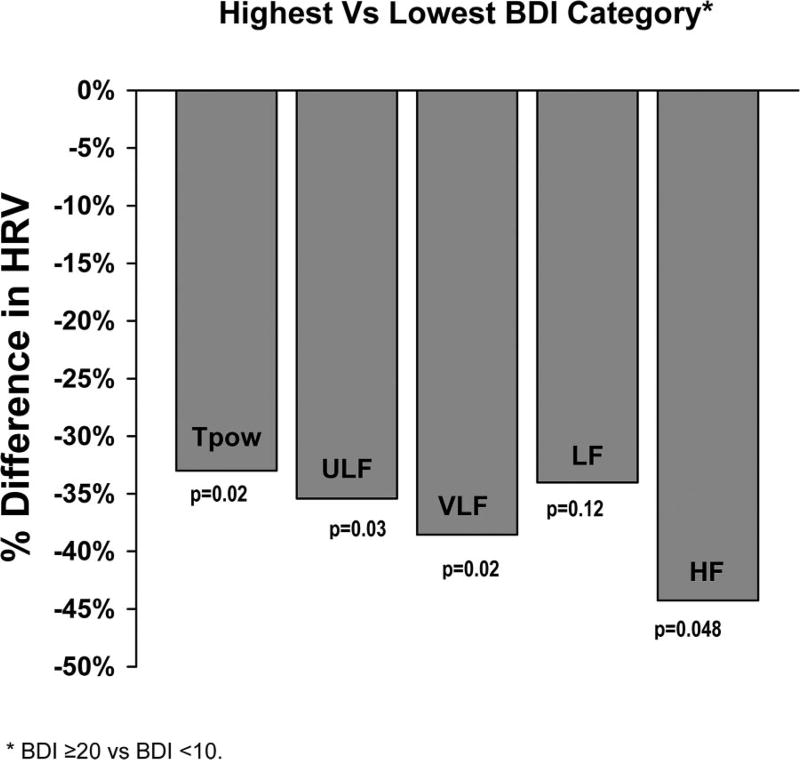
Of the 115 complete twin pairs, 36 pairs (18 MZ and 18 DZ) were discordant for BDI category. Results of within-pair analyses in MZ and DZ combined confirmed a robust association between depressive symptoms and HRV (Figure 1). These results indicate that the association is not due to environmental factors that are matched between twin brothers, such as activity level during examination and family background, although it could be due to other environmental exposures unique to each twin.
Figure 1.
Within-pair differences in HRV based on current depressive symptoms. BDI = Beck Depression Inventory; HRV = heart rate variability; Tpow = total power; ULF = ultra low frequency; VLF = very low frequency; LF = low frequency; HF = high frequency.
To explore the role of genetic factors in the association between depressive symptoms and HRV, we separated within-and between-pair differences in HRV according to BDI level, and compared within-pair differences in HRV between MZ and DZ twin pairs, using a twin modeling approach (49). All twins were included in the models; however, only the twin pairs discordant for BDI level contributed to the within-pair results. This analysis revealed a highly significant within-pair association between BDI score and HRV spectra in the DZ but not in the MZ twins, with a significant interaction between BDI and zygosity in all spectra except HF (Table 3). These results were not substantially changed after multivariable analysis, where the interaction effects remained significant. These data are consistent with a shared genetic effect between depressive symptoms and HRV for all HRV bands except HF. The LF/HF ratio was also lower in DZ twins with higher BDI than their brothers with lower BDI (2.98 versus 4.25, p = .01), whereas no significant difference was noted within DZ pairs (p = .02 for the interaction between ordinal BDI and zygosity).
TABLE 3.
Unadjusted and Adjusted Within-Pair Differences in Heart Rate Variability According to Current Depressive Symptom Level Comparing Monozygotic (n = 18 Pairs) and Dizygotic (n = 18 Pairs) Twin Pairs Discordant for BDI Category
| Ln TPow, ms2
|
LnULF, ms2
|
LnVLF, ms2
|
LnLF, ms2
|
LnHF, ms2
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | MZ | DZ | |
| Unadjusted | ||||||||||
| HRV in brother with higher BDI category (mean ± SE) | 9.39 ± 0.13 | 8.68 ± 0.17 | 9.07 ± 0.14 | 8.41 ± 0.18 | 7.55 ± 0.15 | 6.76 ± 0.20 | 6.65 ± 0.18 | 5.64 ± 0.28 | 5.25 ± 0.22 | 4.58 ± 0.31 |
| HRV in brother with lower BDI category (mean ± SE) | 9.37 ± 0.13 | 9.21 ± 0.17 | 9.01 ± 0.14 | 8.94 ± 0.17 | 7.68 ± 0.15 | 7.26 ± 0.20 | 6.73 ± 0.19 | 6.37 ± 0.27 | 5.61 ± 0.22 | 5.01 ± 0.31 |
| % difference, higher versus lower BDI category | 1.60 | −41.30 | 6.88 | −41.60 | −12.23 | −39.17 | −10.25 | −51.64 | −30.12 | −34.68 |
| % difference, 1-category incrementa | 0.25 | −25.02 | 2.36 | −24.99 | −6.11 | −25.00 | −4.24 | −30.27 | −15.99 | −22.77 |
| Test for trend | p = .96 | p = <.0001 | p = .68 | p = .002 | p = .30 | p = .0002 | p = .61 | p = .0003 | p = .08 | p = .01 |
| BDI × Zygosity interaction | p = .0008 | p = .0009 | p = .02 | p = .01 | p = .59 | |||||
| Adjusteda | ||||||||||
| HRV in brother with higher BDI category (mean ± SE) | 9.39 ± 0.12 | 8.68 ± 0.17 | 9.08 ± 0.13 | 8.41 ± 0.18 | 7.64 ± 0.14 | 6.74 ± 0.20 | 6.64 ± 0.17 | 5.64 ± 0.27 | 5.22 ± 0.21 | 4.55 ± 0.31 |
| HRV in brother with lower BDI category (mean ± SE) | 9.35 ± 0.13 | 9.08 ± 0.17 | 8.99 ± 0.14 | 8.84 ± 0.17 | 7.57 ± 0.14 | 7.06 ± 0.20 | 6.73 ± 0.17 | 6.10 ± 0.27 | 5.63 ± 0.22 | 4.80 ± 0.31 |
| % difference, higher versus lower BDI category | 3.60 | −33.38 | 9.77 | −35.01 | −7.18 | −27.19 | −8.49 | −36.71 | −33.74 | −22.49 |
| % difference, 1-category incrementb | 1.59 | −22.55 | 1.04 | −23.62 | −2.88 | −19.97 | −3.64 | −21.57 | −19.19 | −16.36 |
| Test for trend | p = .76 | p = .0008 | p = .50 | p = .0009 | p = .62 | p = .008 | p = .68 | p = .02 | p = .04 | p = .11 |
| BDI × zygosity interaction | p = .002 | p = .002 | p = .038 | p = .04 | p = .69 | |||||
HRV = heart rate variability; MZ = monozygotic; DZ = dizygotic; BDI = Beck Depression Inventory.
BDI categories = BDI <10; 10–13, 14–19, ≥20. SE = standard error. Percent differences refer to HRV in nontransformed units.
Adjusted for waist-hip ratio, current cigarette smoking, physical activity score, history of diabetes, and history of coronary heart disease. Further adjustment for age, marital status, low-density lipoprotein cholesterol, number of caffeinated beverages per day, heart rate over 24 hours, current use of antidepressants, current use of β blockers, history of alcohol abuse, history of drug abuse, and history of posttraumatic stress disorder, did not substantially affect the study estimates.
Difference in HRV per one-category difference in BDI between co-twins in a pair.
Similar trends were found when within-pair differences in HRV by zygosity were examined with respect to a history of MDD. The coefficient for MDD was at least twice as large in the DZ twins as in the MZ twins, except for HF. However, the association between MDD and HRV remained weaker than for depressive symptoms and none of the associations, or their interaction with zygosity, were statistically significant in multivariable analysis (data not shown). Because of the weak association between history of MDD and HRV in our sample, a shared genetic effect cannot be confirmed or excluded for MDD and HRV.
Heritability calculation revealed overall strong genetic influences on HRV, with heritability decreasing progressively from TPow to HF. Heritability for TPow, ULF, VLF, LF, and HF was, respectively, 0.91, 0.91, 0.62, 0.35, and 0.25. Thus, whereas genetic background explained most of the variance of TPow, ULF, and VLF, environmental factors—rather than genes—explained a larger portion of the variance of LF, especially HF.
DISCUSSION
In a sample of predominantly healthy individuals, we found a robust association between depressive symptoms and HRV: the higher the depressive symptoms, the lower the HRV. This finding persisted using a highly controlled design in which each twin was compared with his brother. The difference in HRV between the lowest and the highest depressive symptom group was substantial and at least as large as that previously found to affect cardiovascular risk (11–13). A history of MDD was a weaker correlate of HRV; however, MDD was mainly in remission in our sample. We also found that the association between depressive symptoms and HRV is, in part, due to a common genetic substrate, indicating that these two phenotypes share a common biological mechanism. Overall, our results suggest that depressive mood and reduced HRV are both the expression of a generalized neurobiological perturbation, which is under genetic control.
Depression and HRV in Noncardiac Populations
Depression has been repeatedly associated with lower HRV in patients with CAD (14–17), but in noncardiac samples, this relationship is much less clear (19–25). In a group of medically healthy participants, Yeragani and colleagues found no difference in HRV in those with major depression versus those without (23). In a sample of healthy women, depressive symptoms were not related to VLF, LF, and HF power and were related only to the LF/HF ratio (19). In contrast, in the Women’s Health Initiative Observational Study, depressive symptoms were associated with lower HRV (20). Similarly, in a study of elderly primary care outpatients, MDD was significantly associated with lower HRV (25). These discrepant findings may be due to differences in the assessment of depression, in the techniques of HRV analysis, or in the ability to control for confounding factors. Unlike the current study, community studies have not controlled for physical activity, which was shown to influence ULF and VLF (27,28). Our study overcomes many limitations of previous studies by assessing twin pairs and employing a tight control of activity levels and other environmental factors during HRV recording. In our study, the strongest associations between depression and HRV were seen in the lower-frequency bands, ULF, and VLF, which are also the most powerful predictors of mortality in many studies (6,51–53).
HRV in the VLF, LF, and HF band are all influenced, in large part, by parasympathetic activity (4,5,54). However, VLF may also be influenced by other neurohormonal influences, such as the renin-angiotensin-aldosterone system, and LF may be influenced by sympathetic influences (28,54). ULF may be affected by day-night changes in heart period, especially when activity is controlled (27,55); these changes, in turn, may be influenced by autonomic activity. Although sympathetic activity is not well measured by HRV (56), our findings suggest that disordered parasympathetic regulation and depression may be linked.
Our findings are similar to previous studies in which associations between HRV and depression were less robust for HF than for other frequency domains (16), a finding reported also for other psychosocial variables (31). It is possible that 24-hour HF is less reflective of long-term underlying stress, such as that due to depression, or alternatively, that HF reflects processes less influenced by genetic factors. LF/HF was also lower in twins with higher BDI scores than their brothers. Whereas the physiological underpinnings of LF/HF are controversial (56), lower LF/HF has been associated with worsened survival post myocardial infarction (57), further suggesting that autonomic dysregulation may mediate poor outcomes in depressed individuals.
Depressive Symptoms Versus History of MDD
We found that the association with HRV was more robust for current depressive symptoms than for a lifetime history of MDD. At first surprising, this finding can be explained by the fact that MDD was, for the most part, in remission. Only seven individuals met the criteria for a current major depressive episode and the majority of those with MDD history reported only one remote depressive episode. Thus, the BDI score in our study may be a better measure to capture cognitive or somatic/neurovegetative symptoms of depression that may be linked to autonomic function as measured by HRV. Our data suggest that active depression is important in the relationship with HRV. This is also supported by the dose-response relationship we found between depressive symptom severity and lower HRV. Our results are consistent with previous literature describing an association between depression and HRV, because these studies have either examined current major depressive episode, or increased depressive symptoms (14–17,20,22,25). We are not aware of studies reporting an association between remitted depression and lower HRV.
Genetic Influences
We found a significant within-pair association between depressive symptoms and HRV in DZ but not in MZ twins. This was true for all HRV spectra, except HF. Thus, the link between disordered autonomic function as measured by HRV and depression is, at least in part, under genetic control, suggesting that there are shared genes between depression and HRV. These results are supported by the large heritability estimates we found for most HRV spectra. Of all, HF showed the least heritability and was mostly influenced by environmental factors. This may explain why HF did not show a shared genetic effect with depressive symptoms.
Previous twin and family studies have shown an important role of genetic factors in the liability to depression (32) and in the regulation of cardiac autonomic activity (33,34); however, depression and HRV were not studied jointly as in our study. Our results suggest that there is a common genetic substrate leading to both depression and autonomic dysfunction; thus, these two traits could each be a separate product of genes regulating key neurobiological pathways. Genetic pleiotropy has recently been suggested in the association between depression and CAD (58,59). Common genes that may influence both depression and autonomic function include, for example, those involved in the hypothalamus-pituitary-adrenal (HPA) axis, or in the sympathetic, parasympathetic, and serotonin pathways, which have been related to depression and may be relevant for autonomic regulation (32,60). For example, a single nucleotide polymorphism in the 3′UTR of the choline transporter gene has been associated with HF, LF, and LF/HF ratios (61). The same genetic variation was linked to regional brain reactivity relevant to autonomic function as well as behavioral and physiologic arousal, suggesting that it may affect cognition, mood, and autonomic cardiac function at the same time (62). Similarly, HPA axis function is implicated in both mood disorders and autonomic function, and it is likely that these effects are modulated by the same genes (63,64). Genetic polymorphisms in serotoninergic genes, such as serotonin transporter (5-HT), serotonin receptors, and monoamine oxidase A (MAO-A), have been associated with depression (32). Although the relationship of these polymorphisms with autonomic dysfunction is not known, there are suggestions that impaired serotoninergic function may affect the variability of heart rate (65). Furthermore, mice deficient in MAO-A (MAO-A knockout), in addition to increased brain levels of serotonin and norepinephrine, show altered heart rate dynamics during a behavioral challenge (66).
Limitations
Our study is cross-sectional, thus limited in the ability to discern the temporal order between depression and HRV. However, because of the twin study design, we were able to control for numerous measured and unmeasured possible confounding factors. Also, few twins met the criteria for a current major depressive episode, and thus we were unable to determine the role of a current depressive episode on HRV and whether it differed from depressive symptoms in the absence of MDD. As in all studies of depression, and particularly in ours that required twins to travel a long way and undergo a lengthy protocol, it can be expected that the most depressed subjects did not choose to participate. However, the graded effect seen with increasing depressive symptoms suggests that current depression would likely be associated with HRV. Furthermore, because our twins were all middle-aged male military veterans, our results may not be generalizable to women or older individuals.
CONCLUSION
Autonomic dysfunction, as reflected by reduced HRV, is an important comorbid condition to depression largely because of a common genetic substrate. Future research should elucidate the specific genetic pathways involved. Ultimately, these data may help identify depressed persons who are at highest risk of autonomic dysregulation, and who may be the best target of interventions to ameliorate the adverse cardiac consequences of depression.
Acknowledgments
Financial disclosure: The Holter scanning software was a gift from GE Medical.
This study was supported by Grants K24HL077506, R01 HL68630, and R01 AG026255 from the National Institutes of Health; Grant MO1-RR00039 from the Emory University General Clinical Research Center; and Grant 0245115N from the American Heart Association. The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Institutes of Health; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University.
We gratefully acknowledge the continued cooperation and participation of the members of the Vietnam Era Twin Registry. Without their contribution, this research would not have been possible.
Glossary
- BDI
Beck Depression Inventory
- CAD
coronary artery disease
- DZ
dizygotic
- HRV
heart rate variability
- MDD
major depressive disorder
- MZ
monozygotic
- PTSD
posttraumatic stress disorder
References
- 1.Barth J, Schumacher M, Herrmann-Lingen C. Depression as a risk factor for mortality in patients with coronary heart disease: a meta-analysis. Psychosom Med. 2004;66:802–13. doi: 10.1097/01.psy.0000146332.53619.b2. [DOI] [PubMed] [Google Scholar]
- 2.Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65:201–10. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- 3.Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med. 2005;67:S29–S33. doi: 10.1097/01.psy.0000162254.61556.d5. [DOI] [PubMed] [Google Scholar]
- 4.Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympathovagal interaction in man and conscious dog. Circ Res. 1986;59:178–93. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- 5.Akselrod S, Gordon D, Ubel FA, Shannon DC, Barger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–2. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- 6.Bigger J, Fleiss J, Steinman R, Rolnitsky L, Kleiger R, Rottman J. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–71. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- 7.Zuanetti G, Neilson JMMM, Latini R, Santoro E, Maggioni AP, Ewing DJ. Prognostic significance of heart rate variability in post-myocardial infarction patients in the fibrinolytic era. Circulation. 1996;94:432–6. doi: 10.1161/01.cir.94.3.432. [DOI] [PubMed] [Google Scholar]
- 8.Lampert R, Ickovics JR, Viscoli CJ, Horwitz RI, Lee FA. Effects of propranolol on recovery of heart rate variability following acute myocar-dial infarction and relation to outcome in the beta-blocker heart attack trial. Am J Cardiol. 2003;91:137–42. doi: 10.1016/s0002-9149(02)03098-9. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji H, Larson MG, Venditti FJ, Manders ES, Evans JC, Feldman CL, Levy D. Impact of reduced heart rate variability on risk for cardiac events: the Framingham heart study. Circulation. 1996;94:2850–5. doi: 10.1161/01.cir.94.11.2850. [DOI] [PubMed] [Google Scholar]
- 10.Dekker JM, Schouten EG, Klootwijk P, Pool J, Swenne CA, Kromhout D. Heart rate variability from short electrocardiographic recordings predicts mortality from all causes in middle-aged and elderly men. The Zutphen study. Am J Epidemiol. 1997;145:899–908. doi: 10.1093/oxfordjournals.aje.a009049. [DOI] [PubMed] [Google Scholar]
- 11.Dekker JM, Crow RS, Folsom AR, Hannan PJ, Liao D, Swenne CA, Schouten EG. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: the ARIC study. Circulation. 2000;102:1239–44. doi: 10.1161/01.cir.102.11.1239. [DOI] [PubMed] [Google Scholar]
- 12.Huikuri HV, Makikallio TH, Airaksinen J, Seppanen T, Puuka P, Raiha IJ, Sourander LB. Power-law relationship of heart rate variability as a predictor of mortality in the elderly. Circulation. 1998;97:2031–6. doi: 10.1161/01.cir.97.20.2031. [DOI] [PubMed] [Google Scholar]
- 13.Liao D, Cai J, Rosamond WD, Barnes RW, Hutchinson RG, Whitsel EA, Rautaharju P, Heiss G. Cardiac autonomic function and incident coronary heart disease: a population-based case-cohort study. The ARIC study. Atherosclerosis risk in communities study. Am J Epidemiol. 1997;145:696–706. doi: 10.1093/aje/145.8.696. [DOI] [PubMed] [Google Scholar]
- 14.Krittayaphong R, Cascio W, Light K, Sheffield D, Golden R, Finkel J, Glekas G, Koch G, Sheps D. Heart rate variability in patients with coronary artery disease: differences in patients with higher and lower depression scores. Psychosom Med. 1997;59:231–5. doi: 10.1097/00006842-199705000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Carney R, Saunders R, Freedland K, Stein P, Rich M, Jaffe A. Association of depression with reduced heart rate variability in coronary artery disease. Am J Cardiol. 1995;76:562–4. doi: 10.1016/s0002-9149(99)80155-6. [DOI] [PubMed] [Google Scholar]
- 16.Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–8. doi: 10.1161/hc4201.097834. comment in Circulation 2002;105:e83. [DOI] [PubMed] [Google Scholar]
- 17.Stein PK, Carney RM, Freedland KE, Skala JA, Jaffe AS, Kleiger RE, Rottman JN. Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. J Psychosom Res. 2000;48:493–500. doi: 10.1016/s0022-3999(99)00085-9. [DOI] [PubMed] [Google Scholar]
- 18.Gehi A, Mangano D, Pipkin S, Browner WS, Whooley MA. Depression and heart rate variability in patients with stable coronary heart disease: findings from the heart and soul study. Arch Gen Psychiatry. 2005;62:661–6. doi: 10.1001/archpsyc.62.6.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hordsten M, Ericson M, Perski A, Wamala S, Schenk-Gustafsson K, Orth-Gomer K. Psychosocial factors and heart rate variability in healthy women. Psychosom Med. 1999;61:49–57. doi: 10.1097/00006842-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Kim CK, McGorray SP, Bartholomew BA, Marsh M, Dicken T, Wassertheil-Smoller S, Curb JD, Oberman A, Hsia J, Gardin J, Wong ND, Barton B, McMahon RP, Sheps DS. Depressive symptoms and heart rate variability in postmenopausal women. Arch Intern Med. 2005;165:1239–44. doi: 10.1001/archinte.165.11.1239. [DOI] [PubMed] [Google Scholar]
- 21.Thayer JF, Smith M, Rossy LA, Sollers JJ, Friedman BH. Heart period variability and depressive symptoms: gender differences. Biol Psychiatry. 1998;44:304–6. doi: 10.1016/s0006-3223(98)00008-0. [DOI] [PubMed] [Google Scholar]
- 22.Rechlin T, Weis M, Spitzer A, Kaschka WP. Are affective disorders associated with alterations of heart rate variability? J Affect Disord. 1994;32:271–5. doi: 10.1016/0165-0327(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 23.Yeragani VK, Pohl R, Balon R, Ramesh C, Glitz D, Jung I, Sherwood P. Heart rate variability in patients with major depression. Psychiatry Res. 1991;37:35–46. doi: 10.1016/0165-1781(91)90104-w. [DOI] [PubMed] [Google Scholar]
- 24.Udupa K, Sathyaprabha TN, Thirthalli J, Kishore KR, Lavekar GS, Raju TR, Gangadhar BN. Alteration of cardiac autonomic functions in patients with major depression: a study using heart rate variability measures. J Affect Disord. 2006 doi: 10.1016/j.jad.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 25.van der Kooy KG, van Hout HP, van Marwijk HW, de Haan M, Stehouwer CD, Beekman AT. Differences in heart rate variability between depressed and non-depressed elderly. Int J Geriatr Psychiatry. 2006;21:147–50. doi: 10.1002/gps.1439. [DOI] [PubMed] [Google Scholar]
- 26.Bernardi L, Wdowczyk-Szulc J, Valenti C, Castoldi S, Passino C, Spadacini G, Sleight P. Effects of controlled breathing, mental activity and mental stress with or without verbalization on heart rate variability. J Am Coll Cardiol. 2000;35:1462–9. doi: 10.1016/s0735-1097(00)00595-7. [DOI] [PubMed] [Google Scholar]
- 27.Roach D, Wilson W, Ritchie D, Sheldon R. Dissection of long-range heart rate variability: controlled induction of prognostic measures by activity in the laboratory. J Am Coll Cardiol. 2004;43:2271–7. doi: 10.1016/j.jacc.2004.01.050. [DOI] [PubMed] [Google Scholar]
- 28.Anonymous. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 29.Lorant V, Deliege D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157:98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 30.Hemingway H, Shipley M, Brunner E, Britton A, Malik M, Marmot M. Does autonomic function link social position to coronary risk? The Whitehall II study. Circulation. 2005;111:3071–7. doi: 10.1161/CIRCULATIONAHA.104.497347. [DOI] [PubMed] [Google Scholar]
- 31.Lampert R, Ickovics J, Horwitz R, Lee F. Depressed autonomic nervous system function in African Americans and individuals of lower social class: a potential mechanism of race- and class-related disparities in health outcomes. Am Heart J. 2005;150:153–60. doi: 10.1016/j.ahj.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 32.Levinson DF. The genetics of depression: a review. Biol Psychiatry. 2006;60:84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 33.Kupper NHM, Willemsen G, van den Berg M, de Boer D, Posthuma D, Boomsma DI, de Geus EJC. Heritability of ambulatory heart rate variability. Circulation. 2004;110:2792–6. doi: 10.1161/01.CIR.0000146334.96820.6E. [DOI] [PubMed] [Google Scholar]
- 34.Singh JP, Larson MG, O’Donnell CJ, Tsuji H, Evans JC, Levy D. Heritability of heart rate variability: the Framingham heart study. Circulation. 1999;99:2251–4. doi: 10.1161/01.cir.99.17.2251. [DOI] [PubMed] [Google Scholar]
- 35.Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L, Buckham R, Murrah NV, Veledar E, Wilson PW, Vaccarino V. Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men. A twin study. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.107.710699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao J, Cheema FA, Reddy U, Bremner JD, Su S, Goldberg J, Snieder H, Vaccarino V. Heritability of flow-mediated dilation: a twin study. J Thromb Haemost. 2007;5:2386–92. doi: 10.1111/j.1538-7836.2007.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao J, Cheema FA, Bremner JD, Goldberg J, Su S, Snieder H, Maisano C, Jones L, Javed F, Murrah N, Le NA, Vaccarino V. Heritability of carotid intima-media thickness: a twin study. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldberg J, Curran B, Vitek ME, Henderson WG, Boyko EJ. The Vietnam era twin registry. Twin Res. 2002;5:476–81. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 39.Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam era twin registry: a resource for medical research. Public Health Rep. 1990;105:368–73. [PMC free article] [PubMed] [Google Scholar]
- 40.Scherrer JF, Xian H, Bucholz KK, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosom Med. 2003;65:548–57. doi: 10.1097/01.psy.0000077507.29863.cb. [DOI] [PubMed] [Google Scholar]
- 41.Robins LM, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–9. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- 42.Beck AT, Steer RA, Brown GK. Beck Depression Inventory, 2nd Edition (BDI-II) San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 43.Osman A, Kopper BA, Barrios F, Gutierrez PM, Bagge CL. Reliability and validity of the Beck depression inventory-II with adolescent psychiatric inpatients. Psychol Assess. 2004;16:120–32. doi: 10.1037/1040-3590.16.2.120. [DOI] [PubMed] [Google Scholar]
- 44.Di Benedetto M, Lindner H, Hare DL, Kent S. Depression following acute coronary syndromes: a comparison between the cardiac depression scale and the Beck depression inventory II. J Psychosom Res. 2006;60:13–20. doi: 10.1016/j.jpsychores.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 45.First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM IV-Patient Edition (SCID-P) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 46.Berger RD, Akselrod S, Gordon D, Cohen RJ. An efficient algorithm for spectral analysis of heart rate variability. IEEE Trans Biomed Eng. 1986;33:900–4. doi: 10.1109/TBME.1986.325789. [DOI] [PubMed] [Google Scholar]
- 47.Hamming R. Numerical Methods for Scientists and Engineers. 2. New York: Dover Publications, Inc; 1973. [Google Scholar]
- 48.Richardson MT, Ainsworth BE, Wu H, Jacobs DR, Leon AS. Ability of the Atherosclerosis risk in communities (ARIC)/Baecke questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–93. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 49.Carlin JB, Gurrin LC, Sterne JAC, Morley R, Dwyer T. Regression models for twin studies: a critical review. Int J Epidemiol. 2005;34:1089–99. doi: 10.1093/ije/dyi153. [DOI] [PubMed] [Google Scholar]
- 50.Division of Statistics and Scientific Consulting. Multilevel model ICC using PROC MIXED. University of Texas; Austin, Texas: Available at http://ssc.utexas.edu/consulting/answers/sas/sas97.html. [Google Scholar]
- 51.Carney RM, Blumenthal JA, Freedland KE, Stein PK, Howells WB, Berkman LF, Watkins LL, Czajkowski SM, Hayano J, Domitrovich PP, Jaffe AS. Low heart rate variability and the effect of depression on post-myocardial infarction mortality. Arch Intern Med. 2005;165:1486–91. doi: 10.1001/archinte.165.13.1486. [DOI] [PubMed] [Google Scholar]
- 52.Algra A, Tijssen JG, Roelandt JR, Pool J, Lubsen J. Heart rate variability from 24-hour electrocardiography and the 2-year risk for sudden death. Circulation. 1993;88:180–5. doi: 10.1161/01.cir.88.1.180. [DOI] [PubMed] [Google Scholar]
- 53.Rich MW, Saini JS, Kleiger RE, Carney RM, teVelde A, Freedland KE. Correlation of heart rate variability with clinical and angiographic variables and late mortality after coronary angiography. Am J Cardiol. 1988;62:714–7. doi: 10.1016/0002-9149(88)91208-8. [DOI] [PubMed] [Google Scholar]
- 54.Taylor JA, Carr DL, Myers CW, Eckberg DL. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation. 1998;98:547–55. doi: 10.1161/01.cir.98.6.547. [DOI] [PubMed] [Google Scholar]
- 55.Roach D, Sheldon A, Wilson W, Sheldon R. Temporally localized contributions to measures of large-scale heart rate variability. Am J Physiol. 1998;274:H1465–H1471. doi: 10.1152/ajpheart.1998.274.5.H1465. [DOI] [PubMed] [Google Scholar]
- 56.Eckberg DL. Sympathovagal balance: a critical appraisal. Circulation. 1997;96:3224–32. doi: 10.1161/01.cir.96.9.3224. [DOI] [PubMed] [Google Scholar]
- 57.Singh N, Mironov D, Armstrong PW, Ross AM, Langer A. Heart rate variability assessment early after acute myocardial infarction. Pathophysiological and prognostic correlates. GUSTO ECG Substudy Investigators. Global Utilization of Streptokinase and TPA for Occluded Arteries. Circulation. 1996;93:1388–95. doi: 10.1161/01.cir.93.7.1388. comment in Circulation 1997;96:1043–4. [DOI] [PubMed] [Google Scholar]
- 58.de Geus EJ. Genetic pleiotropy in depression and coronary artery disease. Psychosom Med. 2006;68:185–6. doi: 10.1097/01.psy.0000208628.90274.bc. [DOI] [PubMed] [Google Scholar]
- 59.McCaffery JM, Frasure-Smith N, Dube MP, Theroux P, Rouleau GA, Duan Q, Lesperance F. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68:187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- 60.Mann JJ, Currier D. Effects of genes and stress on the neurobiology of depression. Int Rev Neurobiol. 2006;73:153–89. doi: 10.1016/S0074-7742(06)73005-7. [DOI] [PubMed] [Google Scholar]
- 61.Neumann SA, Lawrence EC, Jennings JR, Ferrell RE, Manuck SB. Heart rate variability is associated with polymorphic variation in the choline transporter gene. Psychosom Med. 2005;67:168–71. doi: 10.1097/01.psy.0000155671.90861.c2. [DOI] [PubMed] [Google Scholar]
- 62.Neumann SA, Brown SM, Ferrell RE, Flory JD, Manuck SB, Hariri AR. Human choline transporter gene variation is associated with corticolimbic reactivity and autonomic-cholinergic function. Biol Psychiatry. 2006;60:1155–62. doi: 10.1016/j.biopsych.2006.03.059. [DOI] [PubMed] [Google Scholar]
- 63.Chrousos GP, Gold PW. The concepts of stress and stress system disorders: overview of physical and behavioral homeostasis. JAMA. 1992;267:1244–52. [PubMed] [Google Scholar]
- 64.Aihara M, Ida I, Yuuki N, Oshima A, Kumano H, Takahashi K, Fukuda M, Oriuchi N, Endo K, Matsuda H, Mikuni M. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry Res. 2007;155:245–56. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 65.Hildreth CM, Padley JR, Pilowsky PM, Goodchild AK. Impaired serotonergic regulation of heart rate may underlie reduced baroreflex sensitivity in an animal model of depression. Am J Physiol Heart Circ Physiol. 2008;294:H474–H480. doi: 10.1152/ajpheart.01009.2007. [DOI] [PubMed] [Google Scholar]
- 66.Holschneider DP, Scremin OU, Chialvo DR, Chen K, Shih JC. Heart rate dynamics in monoamine oxidase-A- and -B-deficient mice. Am J Physiol Heart Circ Physiol. 2002;282:H1751–H1759. doi: 10.1152/ajpheart.00600.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]