Abstract
OBJECTIVES
Completion rates for the human papillomavirus vaccine (HPV) series among adolescents remain low. Effectiveness of recall with parents choosing the method (preference-based recall) for increasing HPV series completion is unstudied. Within a cluster-randomized trial, we examined effectiveness of preference-based recall compared with usual care for increasing series completion and the association of recall choices with completion.
METHODS
All Kaiser Permanente Colorado pediatric practices (n = 7) were randomized to intervention (n = 4) or control (n = 3) by using covariate-constrained randomization. From January to June 2013, parents at intervention practices whose adolescents received HPV 1 were asked the recall method they preferred for subsequent doses and if they also wanted their child reminded. Completion rates were assessed 1 year after HPV 1.
RESULTS
At intervention practices, 374 (43%) of 867 patients were enrolled; 39% preferred text, 18% e-mail, 9% auto-dialer, and 34% 2-methods; 19% chose to have adolescent also recalled. Intervention adolescents were more likely to complete (63% vs 38%) than were controls (adjusted risk ratio 1.47 [1.38–1.57]) and less likely to be late in completing the series (45% vs 57%, P = .02). Rates of completion were similar between different recall methods, but significantly higher for those preferring e-mail and phone compared withother methods (90% vs 60%. P = .008). Completion rates were similar for adolescents who also received recalls (62%) versus those who did not (63%).
CONCLUSIONS
Preference-based recall was effective in increasing HPV series completion rates, with point estimates substantially higher than for most published studies of reminder/recall.
Human papillomavirus (HPV) vaccines present an enormous opportunity to decrease the burden of cervical cancer precursors, cervical cancer, other anogenital cancer precursors and cancers, genital warts, and HPV-attributable oropharyngeal cancers.1 In the United States, 263000 new cancers are attributable to HPV and >4000 women die of cervical cancer annually.2,3 Modeling studies predict marked reduction in HPV-associated cancers if high HPV vaccination rates can be achieved.4–7 Therefore, vaccination against HPV has been routinely recommended by the Advisory Committee on Immunization Practices for adolescent girls since 2007 and for adolescent boys since 2011.6,8 Each of the 3 HPV vaccines licensed are recommended as a 3-dose series, with the second dose administered 1 to 2 months after the first dose and the third dose 6 months after the first dose.1
Despite the promise of HPV vaccines, rates of completion of the series remain disappointingly low. Among girls 13 to 17 years in 2013, 57.3% initiated the series and 37.6% completed it.9 Among boys of the same age, 34.6% initiated and 13.9% completed.9 Numerous barriers to vaccination have been identified.10–16 One of the major obstacles to series completion among adolescents is the need to have 3 visits within 6 months within a population that historically has low rates of health care visits.16–20 Patient reminder/recall (R/R), messages to parents and patients about needed upcoming (reminders) or overdue vaccinations (recalls), may be a particularly important tool for assisting in completion of the HPV series.
The effectiveness of R/R for increasing vaccination rates has been demonstrated in numerous studies for childhood, adolescent, and adult vaccines,21–31 but there has been limited examination of the effectiveness of R/R for increasing HPV series completion. In addition, preference-based R/R, in which parents choose the method of reminder or recall they prefer, has not, to our knowledge, been evaluated in a randomized controlled trial. The objectives of the current study were (1) to describe parental preferences for HPV recall; (2) to assess the effectiveness of preference-based recalls compared with usual care for increasing HPV series completion (primary outcome) and timeliness of vaccination; and (3) within the intervention group, to assess the association of different recall choices with series completion.
METHODS
The study was approved by the Kaiser Permanente Colorado (KPCO) and the Colorado Multi-Institutional Review Boards as requiring verbal parental consent only.
Study Setting and Population
This was a cluster randomized pragmatic trial, with randomization at the level of the practice, involving all pediatric practices (n = 7) within a large integrated health care system, KPCO. Enrollment was active in the intervention group, whereas all members of the control group were passively enrolled. Practices were randomized to intervention (n = 4) or control (n = 3) by using covariate constrained randomization to balance study arms on available baseline characteristics.32–36 Covariates included in the randomization were the number of active patients ages 11 to 17, the proportion of patients self-reporting as African American and Hispanic, the proportion of adolescents at baseline with ≥1 doses of HPV, and the proportion of patients with Medicaid insurance. Eligible patients in both the intervention and control arms included adolescents between ages 11 and 17 who were enrolled at KPCO within the past 2 years and who received their first HPV dose between January and June 2013.
Descriptions of Intervention and Usual Care
To minimize confusion for families who were already planning to return, the intervention was focused on recalling adolescents who were late for HPV doses. Because KPCO was interested in the sustainability of the intervention, only the usual clinic staff, including medical assistants or nurses, were involved with enrollment. Although it was encouraged that all parents be asked about participation, if patient volumes were extremely high, the clinic was understaffed, or there were substitute staff, parents may not have been approached about enrollment. In addition, KPCO determined it was not feasible for clinic personnel to collect reasons for nonenrollment.
Parents of eligible adolescents receiving their first HPV vaccine at intervention practices were told by the medical assistant or nurse giving the vaccine that KPCO was doing a study to see how best to remind parents and adolescents about getting future HPV doses and asked if they wished to be recalled for future doses. Adolescents who were not accompanied by a parent were not asked to participate. Parents who wanted to receive reminders were given a short check-off form clarifying (1) which recall method they preferred (text, e-mail, automated telephone message), (2) if they also wanted a recall sent to their child, and (3) the contact information for their preferred method. Parents were told they could select up to 2 methods and that, if they wanted to have their adolescent reminded, they had to pick the same method for both. The number of recalls parents would receive was not specified.
For recalls, KPCO used an Interactive Voice Response (IVR) system, which is capable of producing multiple automated recall messages. If a single recall method was chosen, a recall was sent on alternating weeks, for up to 3 recalls per 6 weeks. If 2 methods were chosen, 6 recalls were sent, 1 each week, alternating between the 2 preferred recall methods, for up to 6 weeks. Recalls for dose 2 began 9 weeks after dose 1 and for dose 3, 18 weeks after dose 2. Health clinics randomized to the usual care arm did not implement reminders or recalls for HPV vaccine.
Outcomes
The primary study outcome was a comparison of series completion rates among adolescents age 11 to 17 in the intervention and usual care arms 1 year after the first HPV dose was received. Analyses were intention-to-treat for all enrolled patients, regardless of whether the intervention was received. Completion rates for HPV 2 dose were also assessed. Immunization rates were assessed by using KPCO administrative electronic medical record data and data from the Colorado Immunization Information System for both study arms. Patients were considered late for each dose if they exceeded the minimum recommended interval by 2 months.37 For the intervention group only, we also assessed differences in HPV completion rates by IVR recall method, contact person (parent only versus parent/child), age group, gender, and race/ethnicity.
Analysis
Generalized linear mixed models (SAS GLIMMIX procedure) were used to assess differences in HPV dose 2 and dose 3 completion rates. Models were adjusted for gender, age as a continuous variable, and race/ethnicity and the random effect of clinic site. Relative risks were generated by using a log link with a binomial distribution in the regression model.38 Secondary analyses were performed to examine differences in HPV completion rates and recall and contact preferences among intervention participants. A χ2 test of proportions was used for comparisons between categorical/dichotomous variables. All analyses were conducted by using SAS (SAS 9.3; SAS Institute, Inc, Cary, NC).
RESULTS
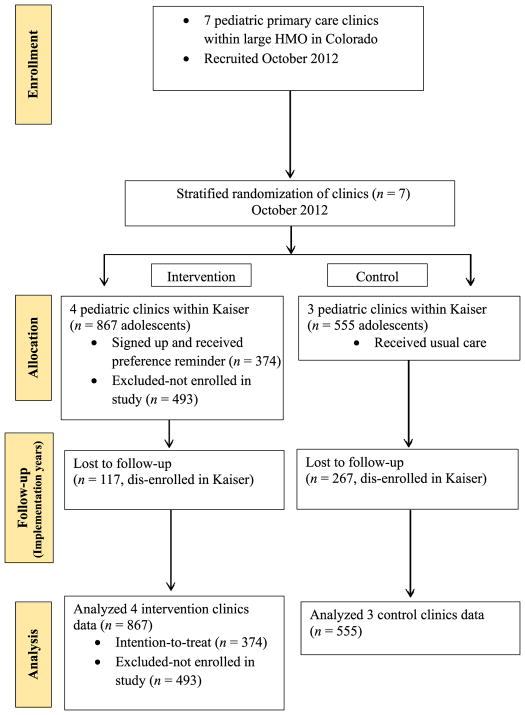
As shown in Fig 1 (consort diagram), parents of 43% (374/867) of eligible adolescents were enrolled at the 4 intervention sites and data from 555 eligible adolescents were assessed at the 3 control sites. Table 1 compares characteristics of the intervention group enrolled with adolescents at intervention sites who were not enrolled and with adolescents at the control sites. Adolescents from intervention sites who were not enrolled were slightly older, largely because older adolescents were more likely to come without a parent and, therefore, would not be eligible for enrollment. Nonenrolled adolescents from intervention sites were also more likely to have unknown race/ethnicity, because this information was not checked at enrollment. The intervention enrolled and control groups were well matched with respect to gender and age, but there were statistically significant, although relatively minor, differences in race and ethnicity between the groups. Baseline rates at the time of study initiation for HPV dose 1 were 18% in the intervention sites (total n = 7577) and 20% in the control sites (total n = 103875, P < .01); series completion rates at baseline were 6% and 7%, respectively (P < .01).
FIGURE 1.
Consort diagram.
TABLE 1.
Study Populations
| Measure | Level | Intervention, Enrolled, n = 374 | Intervention, Not Enrolled, n = 493 | Enrolled Versus Not Enrolled Pa | Control, n = 555 | Enrolled Versus Control Pa |
|---|---|---|---|---|---|---|
| Gender, % | Male | 67 | 65 | .51 | 64 | .43 |
| Female | 33 | 35 | 36 | |||
| Age | Mean (SD) | 13.0 (2.2) | 13.4 (2.4) | .01 | 13.0 (2.2) | .97 |
| 11–12 | 47 | 43 | .06 | 48 | .76 | |
| 13–15 | 35 | 32 | 33 | |||
| 16–17 | 18 | 25 | 19 | |||
| Race, % | White | 48 | 47 | .03 | 53 | <.01 |
| Black | 14 | 9 | 8 | |||
| Other | 18 | 17 | 13 | |||
| Unknown | 20 | 27 | 25 | |||
| Hispanic, % | Yes | 22 | 23 | .01 | 26 | .08 |
| No | 70 | 62 | 63 | |||
| Unknown | 9 | 15 | 12 |
χ2 test of proportions used for comparisons between categories; Wilcoxon signed-rank test used for comparison between means.
Parental Preferences for Recall Method
Text alone was requested by 39% of parents, followed by text and e-mail (19%), e-mail only (18%), text and phone (9%), phone only (9%), or phone and e-mail (6%). As shown in Table 2, parents who chose text messaging were more likely to report their adolescents to be of Hispanic ethnicity (27%) versus all other methods (12%). Parents of white adolescents were more likely to prefer methods other than text messaging. Nineteen percent of parents preferred their adolescent to be reminded in addition to them. Parents of older versus younger adolescents were more likely to ask for their adolescent to be reminded in addition to themselves. Parents who requested that their child be reminded were more likely to choose a method involving text (80%), compared with parents who chose not to remind their child (64%, P = .01).
TABLE 2.
Factors Associated With Reminder Preferences
| Characteristics of Adolescents | Parent Only, n = 303 | Parent and Adolescent, n = 71 | P | Any Text Method, n = 252a | Nontext Methods, n = 122b | P |
|---|---|---|---|---|---|---|
| Age, mean (SD) | 12.7 (2.1) | 14.4 (2.3) | <.001 | 13.1 (2.2) | 12.8 (2.2) | .11 |
| Boys, % | 66 | 69 | .67 | 68 | 65 | .55 |
| Race, % | ||||||
| White | 48 | 51 | .96 | 42 | 61 | .01 |
| Black | 14 | 14 | 15 | 10 | ||
| Asian/Others | 19 | 17 | 21 | 14 | ||
| Unknown | 20 | 18 | 22 | 15 | ||
| Ethnicity, % | ||||||
| Hispanic | 21 | 24 | .80 | 27 | 12 | .003 |
| Non-Hispanic | 70 | 66 | 64 | 80 | ||
| Unknown | 9 | 10 | 9 | 8 | ||
Includes text only, text + e-mail, and text + phone call.
Includes e-mail only, phone call only, and e-mail + phone call.
Effectiveness of Preference-Based Recall Compared With Usual Care on HPV Series Completion
Table 3 compares the percentage receiving doses 2 and 3 for intervention versus control groups, as well as adjusted relative risks for these comparisons. Rates of completion for adolescents at intervention sites that were not enrolled (33%) were similar to the control group (38%) and the adjusted relative risk for HPV completion for the intervention enrolled group compared with the intervention not enrolled group was 1.82 (P < .001). Because of the possibility of selection bias in the intervention groups related to lack of uniform enrollment, we also assessed differences in HPV series completion rates for all adolescents at the intervention sites, both enrolled and unenrolled, compared with control sites. Completion rates were 46% versus 38% with an adjusted risk ratio of 1.22 (P < .01).
TABLE 3.
HPV 2 and 3 Completion
| HPV | Intervention, Enrolled, n = 374, % | Control, n = 555, % | Adjusteda Relative Risk (95% CI) | P |
|---|---|---|---|---|
| Received dose 2 | 83 | 71 | 1.14 (1.07–1.22) | <.001 |
| Received dose 3 | 63 | 38 | 1.59 (1.39–1.83) | <.001 |
CI, confidence interval.
Adjusted for age, gender, race, and ethnicity.
Timeliness of Vaccination
As shown in Table 4, adolescents in the intervention group were more likely to receive vaccines within the recommended dosing intervals for doses 1 to 2 (P < .001), doses 2 to 3 (P = .02), and doses 1 to 3 (P = .01).
TABLE 4.
Timeliness of HPV Vaccination
| Dosing Interval | Definition of Adherence, moa | Percent Late for Vaccine Intervention | Control, % | Pb |
|---|---|---|---|---|
| Doses 1–2 | 1–3 | 47 | 65 | <.001 |
| Doses 2–3 | 3–5 | 48 | 58 | .02 |
| Doses 1–3 | 6–8 | 45 | 57 | .01 |
One month equivalent to 30.5 days.
Adjusted for age, gender, race and ethnicity.
Association of Different Recall Choices With Series Completion
As shown in Table 5, the only recall method that was associated with higher series completion rates was the combination of phone and e-mail, which did differ significantly when compared with all other groups (P < .01). Rates of completion were higher for younger adolescents (P < .01) but did not differ significantly by other factors examined.
TABLE 5.
Completion Rates by Method, Patient Characteristic, and Preference (Intervention Only)
| Characteristic | Level | HPV Dose 2 Completed, n = 312 | HPV Dose 3 Completed, n = 223 |
|---|---|---|---|
|
| |||
| % (n) | % (n) | ||
| Contact preference | Parent and child | 85.9 (61) | 62.1 (41) |
| Parent only | 82.8 (251) | 62.5 (182) | |
| Age, ya | 11–12 | 86.4 (152) | 70.8 (119) |
| 13–15 | 81.7 (107) | 58.9 (73) | |
| 16–17 | 79.1 (53) | 47.7 (31) | |
| Gender | Boys | 81.2 (203) | 62.0 (147) |
| Girls | 87.9 (109) | 63.3 (76) | |
| Race | White | 86.2 (156) | 64.5 (111) |
| Black | 86.3 (44) | 57.4 (27) | |
| Other | 81.2 (56) | 67.6 (46) | |
| Unknown | 76.7 (56) | 55.7 (39) | |
| Hispanic | Yes | 77.8 (63) | 60.8 (48) |
| No | 85.8 (223) | 65.2 (161) | |
| Unknown | 78.8 (26) | 45.2 (14) | |
| Type of contact | One method | 82.5 (203) | 59.1 (137) |
| Two methods | 85.2 (109) | 68.8 (86) | |
| Contact methodb | Text only | 82.1 (119) | 56.2 (77) |
| E-mail only | 85.1 (57) | 60.7 (37) | |
| Phone only | 79.4 (27) | 67.6 (23) | |
| Text and e-mail | 81.9 (59) | 64.3 (45) | |
| Text and phone | 85.7 (30) | 65.7 (23) | |
| Phone and e-mail | 95.2 (20) | 90.0 (18) | |
P < .01, distribution of age statistically different between completed and not completed for HPV dose 3.
P = .008 for phone and e-mail versus all other contact methods for dose 3 completion.
DISCUSSION
For the promise of the HPV vaccine to be realized, rates of vaccine initiation and series completion must be markedly increased. The current study demonstrates that a preference-based recall intervention, including options for text, e-mail, or automatic telephone messages, instituted at the time of initiation of the HPV series, resulted in increases in series completion that were higher than those achieved by most published R/R studies for other vaccines. In addition, the intervention resulted in more timely completion of the series. The intervention was most effective for younger adolescents, and reminding the adolescent in addition to the parent did not increase effectiveness.
Numerous studies have demonstrated the effectiveness of R/R in increasing immunization rates for a variety of vaccines; however, there are limited data about its utility in achieving HPV series completion. Studies conducted in the first few years after HPV was first recommended by using text or a mixture of mail and telephone reminders, showed either no or small effects.23,39,40 A more recent study, conducted in 2013–2014, within a large managed care population demonstrated a 10% increase in completion rate for HPV vaccine among those needing either 1 or 2 doses of the series.41 Similar to our findings, this study also observed a higher rate of effectiveness among younger adolescents.
Our study showed substantially higher effect sizes than most previous R/R trials for any vaccine, with an absolute increase of 25 percentage points for HPV completion among adolescents in the intervention group. There are a number of possible reasons for this large effect size. To our knowledge, this is the first randomized trial to study a preference-based recall model in which parents were allowed to choose the method of contact, although 1 previous study examined the effectiveness of methods chosen by parents in a nonrandomized fashion.42 Allowing parents to choose the R/R method may increase the likelihood that they actually hear or see the message, as well as their receptivity to responding. In addition, the population being studied had initiated the series and were, therefore, potentially more receptive to recalls than populations who had never previously received a particular vaccine. This trial was also conducted within a health care delivery system with a high level of experience with the use of IVR systems and e-mails to patients, which may have contributed to parental receptivity. In addition, characteristics of the population enrolled in KPCO may have played a role, although the direction of potential influences of sociodemographic factors based on previous literature could be postulated to either increase or decrease the effect size. The study population was insured and lived in census tracts with a median family income of $743900 (SD $323420), as assessed by using geocoding to estimate area-based socioeconomic measures.43,44 Historically, studies comparing mail and telephone R/R interventions have shown higher effectiveness in high-income versus lower income populations.25,45–47 However, the present trial used mobile health technologies as the predominant R/R methods, which have been shown to be used at a higher rate in low-income and younger populations.48–51 In particular, text messaging has been shown to be especially effective in low-income minority populations who are more likely to be cell-phone–only users and to use text messaging compared with high-income nonminority populations.52–54 Therefore, it is reasonable to think that our data may be generalizable to low-income and minority populations.
Our data regarding parental preferences for recall method must be examined in the context of previous survey data assessing theoretical choices about reminder or recall methods. In a cross-sectional national survey of parents of 0- to 17-year-olds conducted in 2010, roughly one-third preferred mail, one-third telephone, 16% e-mail, and only 3% text; 44% were unwilling to register their cell phone numbers and, among these, cost of minutes was a prominent concern.55 In a 2011 survey in 7 Colorado counties of parents of children 19 to 35 months 58% preferred mail, 17% telephone, 13% e-mail, and 11% text, although 60% were “okay with” being contacted by e-mail and 46% by text on their cell phones.56 There were significant differences between urban and rural parents, with urban parents being more likely to prefer text or e-mail option. In a third survey conducted in 2011 in an urban setting with a primarily Latino and low-income patient population, most parents reported owning a cell phone with text messaging capabilities (89%), had unlimited messaging plans (85%), and reported being comfortable receiving text messages (88%) or e-mailing with a provider (84%) about health-related issues.57 Among parents in our study, there was a clear preference for text messages and, less commonly, e-mail messages, with phone being a distant third. Taken together, these cumulative data underline the fact that context and patient characteristics are extremely important in determining the optimal methods for R/R. In addition, technology is changing quickly, with populations in some parts of the country routinely having no limitations in their texting plans, whereas for others this is still a limitation.
This study has important limitations. Because a moderate proportion of patients in the intervention clinics were not recruited to participate, it is possible that selection bias contributed to our findings. Similarly, being unable to recruit adolescents seen alone could also produce selection bias. However, when including all patients from intervention clinics (whether successfully recruited or not) in analyses, which would effectively eliminate the risk of selection bias, a significant intervention effect was still seen. In addition, we were able to show that outcomes in the unenrolled eligible groups resembled the control group and that comparisons between the enrolled and unenrolled resembled those between the enrolled and control groups, suggesting that selection bias was not a large factor. It is also possible that randomization at the level of the clinic did not distribute known and unknown confounders equally between intervention and control arms. However, baseline HPV vaccination rates were in fact slightly higher in control clinics, which would, if anything, bias results toward the null.
In addition, the patient populations and setting of our study may not be generalizable to other settings. We did not examine preference-based recall with respect to initiation of the HPV series, only its effect on series completion. Our intervention focused on recalling adolescents overdue for HPV doses rather than reminding them of upcoming doses; it is possible that reminders combined with recall notices would have increased the timeliness of series completion. Although we did not show a difference in success of recall based on whether the adolescent was recalled in addition to the parent, only one-fifth of parents agreed to the recalling of the adolescent directly. It is possible that the addition of the adolescent could make a difference if broadly applied across populations. Finally, this intervention relied on the use of an existing IVR system, which facilitated tailoring methods of R/R and personalizing messages. Although our study did not examine cost, a previous IVR-based reminder effort at KPCO using the same system estimated cost per reminder ranged from $0.05 to $1.23, depending on the volume of calls being generated.58 IVR services are also available from IVR vendors, without the need to purchase an entire IVR system, but practices may not be willing or able to pay for this type of service. In the absence of IVR technology, practices would need to use office personnel to implement preference-based R/R, which could limit widespread adoption.
Results of this study demonstrate that preference-based recall could have a major impact on increasing HPV series completion rates and in increasing the timeliness of full vaccination. Whether this method could also increase initiation of the series also should be examined, as barriers to initiation and to completion have been shown to differ.10,59–61 Preference-based R/R may have much broader applicability in primary care. If preferences were collected and electronically captured at the time of new patient enrollment, preference-based R/R could be broadly applied for both preventive and follow-up reminders or recalls for many patient populations. This aligns well with the Centers for Medicare and Medicaid Services financial incentives for practices that meet Meaningful Use criteria, as 1 criterion is the implementation of reminders or recalls to patients for preventive care and follow-up care according to their method of preference.62 With almost universal access to mobile phones and increasing use of smart phones among most patient populations, increased stability of mobile phone numbers over landline numbers or addresses and availability of low-cost texting programs,55 such methods may become a mainstay for increasing delivery of preventive services.
Acknowledgments
FUNDING SOURCE: This investigation was supported by the Centers for Disease Control and Prevention (grant 5U01IP000310–02). The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
ABBREVIATIONS
- HPV
human papillomavirus vaccine
- IVR
interactive voice response system
- KPCO
Kaiser Permanente Colorado
- R/R
reminder/recall
Footnotes
Portions of this article were presented at the Pediatric Academic Societies’ Annual Meeting Academic Pediatric Association Presidential Plenary; San Diego, CA; May 2015.
Dr Kempe conceptualized and designed the study, contributed to the data collection instrument design, and drafted the initial and final manuscript; Drs O’Leary and Daley, Ms Shoup, and Ms Stokley assisted in study design and creation of the data collection instrument and reviewed and revised the manuscript; Dr Dickinson and Ms Furniss contributed to the study design, carried out the initial and further analyses, and reviewed and revised the manuscript; Mr Lockhart and Ms Barnard contributed to the study design and data collection instrument design, coordinated and supervised all data collection, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01577979).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Markowitz LE, Dunne EF, Saraiya M, et al. Centers for Disease Control and Prevention (CDC) Human papillomavirus vaccination: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2014;63(RR-05):1–30. [PubMed] [Google Scholar]
- 2.US Cancer Statistics Working Group. [Accessed June 29, 2015];United States cancer statistics: 1999–2011 incidence and mortality Web-based report. 2014 Available at: www.cdc.gov/uscs.
- 3.Benard VB, Watson M, Castle PE, Saraiya M. Cervical carcinoma rates among young females in the United States. Obstet Gynecol. 2012;120(5):1117–1123. doi: 10.1097/aog.0b013e31826e4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chesson HW, Ekwueme DU, Saraiya M, Markowitz LE. Cost-effectiveness of human papillomavirus vaccination in the United States. Emerg Infect Dis. 2008;14(2):244–251. doi: 10.3201/eid1402.070499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER Centers for Disease Control and Prevention (CDC); Advisory Committee on Immunization Practices (ACIP) Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56(RR-2):1–24. [PubMed] [Google Scholar]
- 7.Steinbrook R. The potential of human papillomavirus vaccines. N Engl J Med. 2006;354(11):1109–1112. doi: 10.1056/NEJMp058305. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Recommendations on the use of quadrivalent human papillomavirus vaccine in males—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60(50):1705–1708. [PubMed] [Google Scholar]
- 9.Stokley S, Jeyarajah J, Yankey D, et al. Immunization Services Division, National Center for Immunization and Respiratory Diseases, CDC; Centers for Disease Control and Prevention (CDC) Human papillomavirus vaccination coverage among adolescents, 2007–2013, and postlicensure vaccine safety monitoring, 2006–2014—United States. MMWR Morb Mortal Wkly Rep. 2014;63(29):620–624. [PMC free article] [PubMed] [Google Scholar]
- 10.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76–82. doi: 10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) Human papillomavirus vaccination coverage among adolescent girls, 2007–2012, and postlicensure vaccine safety monitoring, 2006–2013—United States. MMWR Morb Mortal Wkly Rep. 2013;62(29):591–595. [PMC free article] [PubMed] [Google Scholar]
- 12.Opel DJ, Robinson JD, Heritage J, Korfiatis C, Taylor JA, Mangione-Smith R. Characterizing providers’ immunization communication practices during health supervision visits with vaccine-hesitant parents: a pilot study. Vaccine. 2012;30(7):1269–1275. doi: 10.1016/j.vaccine.2011.12.129. [DOI] [PubMed] [Google Scholar]
- 13.Vannice KS, Salmon DA, Shui I, et al. Attitudes and beliefs of parents concerned about vaccines: impact of timing of immunization information. Pediatrics. 2011;127(suppl 1):S120–S126. doi: 10.1542/peds.2010-1722R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Daley MF, Crane LA, Markowitz LE, et al. Human papillomavirus vaccination practices: a survey of US physicians 18 months after licensure. Pediatrics. 2010;126(3):425–433. doi: 10.1542/peds.2009-3500. [DOI] [PubMed] [Google Scholar]
- 15.Downs LS, Jr, Scarinci I, Einstein MH, Collins Y, Flowers L. Overcoming the barriers to HPV vaccination in high-risk populations in the US. Gynecol Oncol. 2010;117(3):486–490. doi: 10.1016/j.ygyno.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 16.Szilagyi PG, Rand CM, McLaurin J, et al. Working Group on Adolescent Vaccination in the Medical Home. Delivering adolescent vaccinations in the medical home: a new era? Pediatrics. 2008;121(suppl 1):S15–S24. doi: 10.1542/peds.2007-1115C. [DOI] [PubMed] [Google Scholar]
- 17.Tsai Y, Zhou F, Wortley P, Shefer A, Stokley S. Trends and characteristics of preventive care visits among commercially insured adolescents, 2003–2010. J Pediatr. 2014;164(3):625–630. doi: 10.1016/j.jpeds.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broder KR, Cohn AC, Schwartz B, et al. Working Group on Adolescent Prevention Priorities. Adolescent immunizations and other clinical preventive services: a needle and a hook? Pediatrics. 2008;121(suppl 1):S25–S34. doi: 10.1542/peds.2007-1115D. [DOI] [PubMed] [Google Scholar]
- 19.Smith PJ, Kennedy AM, Wooten K, Gust DA, Pickering LK. Association between health care providers’ influence on parents who have concerns about vaccine safety and vaccination coverage. Pediatrics. 2006;118(5) doi: 10.1542/peds.2006-0923. Available at: www.pediatrics.org/cgi/content/full/118/5/e1287. [DOI] [PubMed] [Google Scholar]
- 20.Dombkowski KJ, Lantz PM, Freed GL. Role of health insurance and a usual source of medical care in age-appropriate vaccination. Am J Public Health. 2004;94(6):960–966. doi: 10.2105/ajph.94.6.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kempe A, Saville AW, Dickinson LM, et al. Collaborative centralized reminder/recall notification to increase immunization rates among young children: a comparative effectiveness trial. JAMA Pediatr. 2015;169(4):365–373. doi: 10.1001/jamapediatrics.2014.3670. [DOI] [PubMed] [Google Scholar]
- 22.Kempe A, Saville A, Dickinson LM, et al. Population-based versus practice-based recall for childhood immunizations: a randomized controlled comparative effectiveness trial. Am J Public Health. 2013;103(6):1116–1123. doi: 10.2105/AJPH.2012.301035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suh CA, Saville A, Daley MF, et al. Effectiveness and net cost of reminder/recall for adolescent immunizations. Pediatrics. 2012;129(6) doi: 10.1542/peds.2011-1714. Available at: www.pediatrics.org/cgi/content/full/129/6/e1437. [DOI] [PubMed] [Google Scholar]
- 24.Kempe A, Barrow J, Stokley S, et al. Effectiveness and cost of immunization recall at school-based health centers. Pediatrics. 2012;129(6) doi: 10.1542/peds.2011-2921. Available at: www.pediatrics.org/cgi/content/full/129/6/e1446. [DOI] [PubMed] [Google Scholar]
- 25.Szilagyi PG, Schaffer S, Barth R, et al. Effect of telephone reminder/recall on adolescent immunization and preventive visits: results from a randomized clinical trial. Arch Pediatr Adolesc Med. 2006;160(2):157–163. doi: 10.1001/archpedi.160.2.157. [DOI] [PubMed] [Google Scholar]
- 26.Jacobson Vann JC, Szilagyi P. Patient reminder and patient recall systems to improve immunization rates. Cochrane Database Syst Rev. 2005;(3):CD003941. doi: 10.1002/14651858.CD003941.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daley MF, Barrow J, Pearson K, et al. Identification and recall of children with chronic medical conditions for influenza vaccination. Pediatrics. 2004;113(1 pt 1) doi: 10.1542/peds.113.1.e26. Available at: www.pediatrics.org/cgi/content/full/113/1pt1/e26. [DOI] [PubMed] [Google Scholar]
- 28.Tierney CD, Yusuf H, McMahon SR, et al. Adoption of reminder and recall messages for immunizations by pediatricians and public health clinics. Pediatrics. 2003;112(5):1076–1082. doi: 10.1542/peds.112.5.1076. [DOI] [PubMed] [Google Scholar]
- 29.Szilagyi PG, Schaffer S, Shone L, et al. Reducing geographic, racial, and ethnic disparities in childhood immunization rates by using reminder/recall interventions in urban primary care practices. Pediatrics. 2002;110(5) doi: 10.1542/peds.110.5.e58. Available at: www.pediatrics.org/cgi/content/full/110/5/e58. [DOI] [PubMed] [Google Scholar]
- 30.Szilagyi PG, Bordley C, Vann JC, et al. Effect of patient reminder/recall interventions on immunization rates: A review. JAMA. 2000;284(14):1820–1827. doi: 10.1001/jama.284.14.1820. [DOI] [PubMed] [Google Scholar]
- 31.Szilagyi PG, Rodewald LE, Savageau J, Yoos L, Doane C. Improving influenza vaccination rates in children with asthma: a test of a computerized reminder system and an analysis of factors predicting vaccination compliance. Pediatrics. 1992;90(6):871–875. [PubMed] [Google Scholar]
- 32.Dickinson LM, Beaty B, Fox C, et al. Achieving balanced study arms in pragmatic cluster randomized trials using covariate constrained randomization. J Am Board Fam Pract. 2015;28(5):663–672. doi: 10.3122/jabfm.2015.05.150001. [DOI] [PubMed] [Google Scholar]
- 33.Kraschnewski JL, Keyserling TC, Bangdiwala SI, et al. Optimized probability sampling of study sites to improve generalizability in a multisite intervention trial. Prev Chronic Dis. 2010;7(1):A10. [PMC free article] [PubMed] [Google Scholar]
- 34.Moulton LH, Golub JE, Durovni B, et al. Statistical design of THRio: a phased implementation clinic-randomized study of a tuberculosis preventive therapy intervention. Clin Trials. 2007;4(2):190–199. doi: 10.1177/1740774507076937. [DOI] [PubMed] [Google Scholar]
- 35.Chaudhary MA, Moulton LH. A SAS macro for constrained randomization of group-randomized designs. Comput Methods Programs Biomed. 2006;83(3):205–210. doi: 10.1016/j.cmpb.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 36.Raab GM, Butcher I. Balance in cluster randomized trials. Stat Med. 2001;20(3):351–365. doi: 10.1002/1097-0258(20010215)20:3<351::aid-sim797>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 37.Dorell CG, Stokley S, Yankey D, Markowitz LE. Compliance with recommended dosing intervals for HPV vaccination among females, 13–17 years, National Immunization Survey-Teen, 2008–2009. Vaccine. 2012;30(3):503–505. doi: 10.1016/j.vaccine.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 38.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 39.Szilagyi PG, Albertin C, Humiston SG, et al. A randomized trial of the effect of centralized reminder/recall on immunizations and preventive care visits for adolescents. Acad Pediatr. 2013;13(3):204–213. doi: 10.1016/j.acap.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kharbanda EO, Stockwell MS, Fox HW, Andres R, Lara M, Rickert VI. Text message reminders to promote human papillomavirus vaccination. Vaccine. 2011;29(14):2537–2541. doi: 10.1016/j.vaccine.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 41.Chao C, Preciado M, Slezak J, Xu L. A randomized intervention of reminder letter for human papillomavirus vaccine series completion. J Adolesc Health. 2015;56(1):85–90. doi: 10.1016/j.jadohealth.2014.08.014. [DOI] [PubMed] [Google Scholar]
- 42.Morris J, Wang W, Wang L, Peddecord KM, Sawyer MH. Comparison of reminder methods in selected adolescents with records in an immunization registry. J Adolesc Health. 2015;56(suppl 5):S27–S32. doi: 10.1016/j.jadohealth.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 43.Berkowitz SA, Traore CY, Singer DE, Atlas SJ. Evaluating area-based socioeconomic status indicators for monitoring disparities within health care systems: results from a primary care network. Health Serv Res. 2015;50(2):398–417. doi: 10.1111/1475-6773.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the public health disparities geocoding project. Am J Public Health. 2003;93(10):1655–1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irigoyen MM, Findley S, Wang D, et al. Challenges and successes of immunization registry reminders at inner-city practices. Ambul Pediatr. 2006;6(2):100–104. doi: 10.1016/j.ambp.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 46.LeBaron CW, Starnes DM, Rask KJ. The impact of reminder-recall interventions on low vaccination coverage in an inner-city population. Arch Pediatr Adolesc Med. 2004;158(3):255–261. doi: 10.1001/archpedi.158.3.255. [DOI] [PubMed] [Google Scholar]
- 47.Daley MF, Steiner JF, Brayden RM, Xu S, Morrison S, Kempe A. Immunization registry-based recall for a new vaccine. Ambul Pediatr. 2002;2(6):438–443. doi: 10.1367/1539-4409(2002)002<0438:irbrfa>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 48.Ahlers-Schmidt CR, Chesser A, Hart T, Paschal A, Nguyen T, Wittler RR. Text messaging immunization reminders: feasibility of implementation with low-income parents. Prev Med. 2010;50(5–6):306–307. doi: 10.1016/j.ypmed.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 49.Blumberg SJ, Luke JV. Reevaluating the need for concern regarding noncoverage bias in landline surveys. Am J Public Health. 2009;99(10):1806–1810. doi: 10.2105/AJPH.2008.152835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blumberg SJ, Luke JV, Cynamon ML. Telephone coverage and health survey estimates: evaluating the need for concern about wireless substitution. Am J Public Health. 2006;96(5):926–931. doi: 10.2105/AJPH.2004.057885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vilella A, Bayas JM, Diaz MT, et al. The role of mobile phones in improving vaccination rates in travelers. Prev Med. 2004;38(4):503–509. doi: 10.1016/j.ypmed.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 52.Lee S, Elkasabi M, Streja L. Increasing cell phone usage among Hispanics: implications for telephone surveys. Am J Public Health. 2012;102(6):e19–e24. doi: 10.2105/AJPH.2012.300681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zickuhr K, Smith A. [Accessed June 29, 2015];Digital differences. 2012 Available at: www.pewinternet.org/2012/04/13/digital-differences/
- 54.Smith A. [Accessed June 29, 2015];Mobile access. 2010 Available at: www.pewinternet.org/2010/07/07/mobile-access-2010/
- 55.Clark SJ, Butchart A, Kennedy A, Dombkowski KJ. Parents’ experiences with and preferences for immunization reminder/recall technologies. Pediatrics. 2011;128(5) doi: 10.1542/peds.2011-0270. Available at: www.pediatrics.org/cgi/content/full/128/5/e1100. [DOI] [PubMed] [Google Scholar]
- 56.Saville AW, Beaty B, Dickinson LM, Lockhart S, Kempe A. Novel immunization reminder/recall approaches: rural and urban differences in parent perceptions. Acad Pediatr. 2014;14(3):249–255. doi: 10.1016/j.acap.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hofstetter AM, Vargas CY, Kennedy A, Kitayama K, Stockwell MS. Parental and provider preferences and concerns regarding text message reminder/recall for early childhood vaccinations. Prev Med. 2013;57(2):75–80. doi: 10.1016/j.ypmed.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Shoup JA, Madrid C, Koehler C, et al. Effectiveness and cost of influenza vaccine reminders for adults with asthma or chronic obstructive pulmonary disease. Am J Manag Care. 2015;21(7):e405–e413. [PubMed] [Google Scholar]
- 59.Bednarczyk RA, Birkhead GS, Morse DL, Doleyres H, McNutt LA. Human papillomavirus vaccine uptake and barriers: association with perceived risk, actual risk and race/ethnicity among female students at a New York State university, 2010. Vaccine. 2011;29(17):3138–3143. doi: 10.1016/j.vaccine.2011.02.045. [DOI] [PubMed] [Google Scholar]
- 60.Cook RL, Zhang J, Mullins J, et al. Factors associated with initiation and completion of human papillomavirus vaccine series among young women enrolled in Medicaid. J Adolesc Health. 2010;47(6):596–599. doi: 10.1016/j.jadohealth.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 61.Dempsey A, Cohn L, Dalton V, Ruffin M. Patient and clinic factors associated with adolescent human papillomavirus vaccine utilization within a university-based health system. Vaccine. 2010;28(4):989–995. doi: 10.1016/j.vaccine.2009.10.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Centers for Medicare and Medicaid Services. 2014 Definition Stage 1 of Meaningful Use. [Accessed June 29, 2015];EHR Incentive Programs. 2014 Available at: www.cms.gov/Regulations-and-Guidance/Legislation/EHRIncentivePrograms/Meaningful_Use.html.