Abstract
Background
Atrazine suppression of the LH surge slowly develops over time and peaks after 4 days; sensitivity to atrazine decreases after 8 or 14 days of dosing. Adaptation of the LH response was correlated with increased phase I and phase II liver enzyme activity/expression.
Methods
The effect of atrazine on the LH surge was evaluated in female Sprague-Dawley rats administered 100 mg/kg/day atrazine by gavage for 1, 2, 3, or 4 consecutive days or 6.5, 50, or 100 mg/kg/day atrazine for 4, 8, or 14 days.
Results
No statistically significant effects of atrazine were seen on peak plasma LH or LH area under the curve (AUC) after one, two, or three doses of 100 mg/kg/day. Four daily doses of 50 or 100 mg/kg atrazine significantly reduced peak LH and LH AUCs, whereas 6.5 mg/kg/day had no effect. After 8 or 14 days of treatment, statistically significantly reduced peak LH and LH AUC were observed in the 100 mg/kg/day dose group, but not in the 6.5 or 50 mg/kg/day dose groups, although significantly reduced LH was observed in one sample 9 hr after lights-on in the 50 mg/kg/day dose group on day 14. The number of days of treatment required to achieve a significant suppression of the LH surge is consistent with the repeat-dose pharmacokinetics of the chlorotriazines.
Conclusion
The apparent adaptation to the effect of atrazine on the LH surge after 8 or 14 days may be related to the induction of phase I or, more likely, phase II metabolism observed in this study after 8 days, or to a decreased sensitivity of the hypothalamic-pituitary-adrenal axis or an homeostatic adaption of the effect of atrazine on the LH surge mechanism.
Keywords: LH surge, gavage, atrazine, sensitivity, adaptation, phase I metabolism, phase II metabolism
Introduction
The chlorotriazine herbicide, atrazine (2-chloro-4-[ethylamino]-6-[isopropylamino]-s-triazine; ATZ), is widely used in agriculture to control broadleaf and grassy weeds (Bridges, 2008). ATZ exerts its herbicidal effects by inhibiting the electron transport system necessary for photosynthesis (Good, 1961; Tischer and Strotmann, 1977). When administered by gavage to ovariectomized female Sprague-Dawley (SD) rats, ATZ suppresses the estrogen-induced or the estrogen + progesterone-induced luteinizing hormone (LH) surge (Cooper et al., 2000; McMullin et al., 2004; Foradori et al., 2009a). ATZ also reduces the endogenous LH surge that occurs on the afternoon of proestrus in intact rodents (Foradori et al., 2014). The mechanism of this action is unclear, but it appears that ATZ’s suppression of the gonadotropin-releasing hormone and LH surge is mediated centrally, and not at the level of the anterior pituitary (Cooper et al., 2007).
The effect of ATZ on the LH surge occurs after three or four daily gavage doses of greater than 50 mg/kg/day (Goldman et al., 2013). These effects of ATZ are short-lived and disappear within 4 days of the termination of dosing (Foradori et al., 2009a). The progressive increase in the magnitude of the effect of ATZ on the LH surge observed over 4 days of dosing may in part be explained by the rapid pharmacokinetics of atrazine and its chlorotriazine metabolites (Campbell et al., 2016). Thus, the amount of time needed to achieve 90% of the steady-state plasma concentration of diaminochlorotriazine (DACT) following an ATZ gavage dose of 50 mg/kg/day was 11 hr, and the half-life for clearance of DACT from plasma was 11.5 hr. ATZ and its two mono-dealkylated metabolites (deethylatrazine [DEA] and deisopropylatrazine [DIA]) were cleared within 2 to 4 hr of dosing, and hence never achieved sustained blood levels with once-a-day dosing (Campbell et al., 2016).
To determine if the effect of ATZ on the estradiol-induced LH surge adapts over time, the LH surge was evaluated in young-adult female SD rats administered ATZ by oral gavage at doses of 6.5, 50, and/or 100 mg/kg/day for 1, 2, 3, 4, 8, or 14 days. Since liver enzymes responsible for the oxidative (phase I) and conjugative (phase II) metabolism of ATZ are inducible (Islam et al., 2002), liver samples were collected and analyzed for the expression of hepatic enzymes known to be involved in ATZ metabolism. We found that four daily doses of 50 or 100 mg/kg ATZ significantly reduced peak LH and LH area under the curve (AUC), whereas 8 or 14 days of dosing reduced peak LH and LH AUC in the 100 mg/kg/day dose group, but not in the 6.5 or 50 mg/kg/day dose groups. In the 100 mg/kg/day dose group, group mean body weight (BW) loss was positively correlated with the ATZ dose, but inversely correlated with LH AUC. Individual animal weight loss was not predictive of LH AUC after 4, 8, or 14 days of ATZ treatment. Although several CYP450 enzymes were induced after 8 or 14 days of treatment (most noticeably, CYP2B2B mRNA expression), overall CYP450 enzyme expression was poorly predictive of the effects of ATZ on LH AUC. In contrast, the expression of mRNA for glutathione-s-transferase (GST) isoforms was strongly induced by 8 days of ATZ treatment at 50 mg/kg/day, but not after 4 or 14 days of dosing. The overall expression of glutathione (GSH) and the GST isoenzymes were dose- and time-dependently induced by ATZ, and were predictive of reduced LH AUC after 4, 8, or 14 days of treatment.
Materials and Methods
ANIMALS
Eight- to 9-week-old female SD rats (Crl:CD(SD)) were received in two batches from Charles River Laboratories, Inc. (Raleigh, NC) and maintained in AAALAC-approved animal facilities at WIL Research Laboratories, LLC (Ashland, OH). Animals were housed individually in clean, suspended, wire-mesh cages. The animals were maintained in an environmentally controlled room (22 ± 3°C; 50 ± 20% humidity) on a 14-hr light/10-hr dark photoperiod (lights on at 05:00 hr, off at 19:00 hr) with water and food (Rodent LabDiet, PMI Nutrition International, LLC) available ad libitum. During the first 2 weeks of the 3- to 4-week acclimation period, all animals in experiments 1 and 2 were monitored for estrous cyclicity by taking daily vaginal swabs. Only those animals showing regular 4-day estrous cycles were retained for use in these studies.
Seven days before randomization, regularly cycling females were acclimated to the dosing procedure by administering a daily oral gavage dose of the vehicle (1% methylcellulose in deionized water) at a dose volume of 5 ml/kg. At the end of the acclimation period, 25 animals were randomly assigned to either the vehicle control group or the ATZ-treated group (experiment 1), or to either the vehicle control group or one of three ATZ-treated groups (experiment 2).
ATRAZINE FORMULATION AND ADMINISTRATION
ATZ was supplied by Syngenta Crop Protection, LLC, as an analytically certified, 98.8% pure, white powder that was stable for use during the period of the study. ATZ was prepared approximately weekly as a suspension in 1% methylcellulose and deionized water at concentrations of 1.3, 5, or 20 mg/ml. Suspensions were stored refrigerated (at 2 to 8°C) until use. Sample storage stability and sample homogeneity were established by high-pressure liquid chromatography before study conduct. The concentration of ATZ, which was assessed in every dose suspension by high-pressure liquid chromatography, ranged from 94.7 to 101% of target.
In experiment 1, 10 to 15 animals were randomly assigned to either the control (N = 10) or an ATZ-treated group (N = 15) in each of four cohorts. The cohorts of animals were administered either the vehicle (5 ml/kg 1% aqueous methylcellulose suspension) or ATZ (100 mg/kg/ day) by gavage for either 1, 2, 3, or 4 days. Dosing occurred immediately after lights on at approximately 05:00 hr. In experiment 2, animals were randomly assigned to four separate cohorts of 25 animals each and dosed daily by oral gavage with either the vehicle (0 mg/ kg/day) or one of three doses of ATZ (6.5, 50, or 100 mg/ kg/day) for either 4 days, 8 days, or 14 consecutive days before blood sampling.
SURGICAL PROCEDURES BEFORE BLOOD COLLECTION
Three days before the scheduled day of blood collection, each animal was anesthetized with isoflurane, and a polyurethane catheter was implanted into the femoral vein. The opposite end of the cannula was tunneled subcutaneously to exit the skin in the scapular region. The catheter was attached to an SAI Quick Connect™ with an injection cap and a jacket adaptor. A bilateral ovariectomy was performed, and a 12- to 14-mm-long Silastic capsule (0.062″ internal diameter × 0.125″ outside diameter) containing estradiol benzoate (4 mg/ml in sesame oil) was implanted subcutaneously in the right flank region. This procedure was performed in the morning (06:00 hr–12:00 hr). Implantation of the estradiol capsule produces a synchronized cohort of animals with estradiol blood levels comparable to intact animals in proestrus, resulting in a daily afternoon surge in LH levels (Legan and Karsch, 1975).
BLOOD COLLECTION
Four days following ovariectomy and estradiol capsule implantation, blood samples were collected from each animal at 6, 9, 11, 13, 15, and 18 hr after lights on (11:00, 14:00, 16:00, 18:00, 20:00, and 23:00 hr, respectively), to capture the profile of the LH surge, which in our colony peaks at 11–13 hr after lights on (16:00–18:00 hr). For each rat, blood samples were collected within 10 min of the target collection time by means of the surgically implanted femoral vein catheter. For collection times after lights out (1900 hr), samples were collected under red-light conditions. To minimize stress to the animals during blood collection, noise in the animal room and handling of the animals before blood collection was kept to a minimum.
Blood samples (0.5 ml/animal/time point) were collected into prechilled, uniquely labeled tubes with sodium heparin as the anticoagulant. Blood samples were centrifuged, plasma was aliquoted to two uniquely labeled polypropylene tubes, and then the samples were stored at −70°C. All plasma samples were shipped on dry ice from WIL Research Laboratories to the University of Arizona, College of Medicine, Phoenix, AZ. Upon receipt, the samples were catalogued and stored at −80°C until analysis of LH concentrations by radioimmunoassay.
LIVER SAMPLES COLLECTED AT STUDY TERMINATION
In experiment 1, liver samples collected from 5 rats/group killed on day 1, 2, 3, or 4 were analyzed using real-time polymerase chain reaction analyses for GST mRNA expression. Total GSH levels were evaluated using the Cayman Chemical GSH assay kit (Ann Arbor, MI) as described by Zimmerman et al. (2017). In experiment 2, GST, GSH, and P450 analyses were conducted on liver samples collected from 5 rats/sex/group on day 4, 8, or 14. Each rat was anesthetized, and the abdominal cavity was opened to expose the liver. An approximately 5 mm3 sample of liver was collected from the left and median lobes, and then placed in individual sterile plastic tubes containing 2–5 ml of RNAlater® reagent. The samples were refrigerated overnight at 4°C and then transferred to a freezer at −70°C, until shipment on dry ice to Auburn University for analyses of messenger RNA (real-time polymerase chain reaction), enzyme expression, and total protein levels (Western blots) for the following thirteen GST liver enzymes: GSTA3, GSTA4, GSTK1, GSTM1, GSTM2, GSTM3, GSTO1, GSTO2, GSTP1, GSTT1, GSTT2, MGST1, and MGST2. Overall GST activity was determined in cytosolic (GSTC) and microsomal (GSTM) fractions (Zimmerman et al., 2017).
After liver samples were collected from the left and median lobes, the animal was cardiac-perfused with physiologically buffered saline until it was exsanguinated. The right lobe of the liver was collected, weighed, and flash-frozen in liquid nitrogen, and were then shipped on dry ice to Auburn University for analyses of mRNA levels for the following 29 P450 liver enzymes: CYP17A1, CYP1A2, CYP26A1, CYP26C1, CYP27A1, CYP2B2, CYP2B3, CYP2C22, CYP2C23, CYP2C37, CYP2C6, CYP2C7, CYP2D2, CYP2D4, CYP2E1, CYP2F4, CYP2R1, CYP2T1, CYP3A18, CYP3A23/ 3A1, CYP3A9, CYP4A3, CYP4B1, CYP4F1, CYP4F4, CYP4F6, CYP7A1, CYP7B1, and CYP8B1B. In addition, total GSH levels (nmol GSH/mg protein) were measured and standardized to total protein levels in the supernatant of homogenized, centrifuged liver samples (Zimmerman et al., 2017).
RADIOIMMUNOASSAY FOR LH
Plasma LH concentrations were measured at the University of Arizona, using reagents provided by the National Hormone and Peptide Program (NHPP) (Harbor UCLA Medical Center, Torrance, CA). Rat LH-RP3 was used to create a standard curve (range: 10.0 to 0.0049 ng). Tracer ovine LH (oLH, NHPP) was iodinated using the chloramine-T method by the Colorado State University peptide assay core and then shipped to the University of Arizona within 3 days of iodination. For assay, plasma samples were incubated overnight at 4°C with antiserum (NIDDK-Anti-rLH-SII; diluted 1:300,000). Following incubation, iodinated oLH was added to each tube (~10,000 cpm/tube) and incubated overnight at 4°C. Bound LH was separated from free LH by incubation with goat anti-rabbit γ globin (NHPP, Harbor UCLA Medical Center, 1:1000) in 5% polyethylene glycol (Fisher Biotech; BP233-1), followed by centrifugation in a Beckman J6B centrifuge (2000 × g - 15 min). The mean intra-assay and inter-assay coefficients of variation were 11.5% and 3.0%, respectively, and the average lower limit of quantitation was 0.61 ng/ml.
DATA ANALYSIS
LH concentrations that fell outside the upper and lower limits of the assay (15 and 85% bound, respectively) were diluted appropriately, if sample volume allowed, and reassayed until the sample fell within the analytical range. In the event that a sample was below the lower limit of quantitation, the limit of quantitation value was used so as not to bias the data against the appearance of low values. LH levels were first analyzed using a two-way analysis of variance (ANOVA) to evaluate the effects of dose, time, and the interaction between dose and time. Posthoc analysis was performed using the Bonferroni method (Motulsky, 1995). For peak LH levels and LH AUC, data were analyzed by one-way ANOVA followed by posthoc analysis using the Dunnett’s t test (Dunnett, 1964). Statistical comparisons of group mean LH within dose groups across treatment days were done using a one-sided t test. For all analyses, the level of statistical significance was set at p ≤ 0.05. Numerical values are reported as the mean ± SEM.
Microsoft Excel was used to calculate the correlation, expressed as a coefficient of determination (R2), between individual animal peak LH and LH AUC. Since LH AUC was correlated with changes in BW during dosing, an analysis of covariance was conducted on peak LH and LH AUC, where the covariate was BW change during the treatment period. Regression equations based on the relationship between individual animal weight change during treatment and LH AUC were calculated, and the statistical significance of the correlation (R2), was assessed using a two-sided t test.
Group mean P450 liver mRNA enzyme expression, measured in liver samples collected after 4, 8, or 14 days of ATZ treatment, were calculated, and the statistical significance of the differences between ATZ-treated groups and the control group was evaluated using a two-way analysis of variance, followed by Bonferroni post hoc tests (Motulsky, 1995). Since the expression of mRNA for CYP2B2 was dose-dependently induced by ATZ, individual animal CYP2B2 expressions were correlated with LH AUC data from the same animals. For this analysis, individual LH AUC and P450 enzyme activity values were normalized as percentages of the control group means. The statistical significance of the R2 between LH AUC and CYP enzyme mRNA expression was determined, and linear regression equations were calculated after 4, 8, and 14 days of ATZ treatment. The statistical significance of correlation between these parameters (R2) was assessed using a two-sided t test. A similar analysis was conducted for the isoforms of GST mRNA expression after 4, 8, or 14 days of ATZ treatment (Zimmerman et al., 2017). However, since GST mRNA expression increased in a dose-dependent manner after 4, 8, or 14 days of ATZ treatment for all GST isoforms, the average fold-change in GST mRNA expression was calculated for each individual animal, relative to the control group mean, and correlated with its LH AUC by calculating the R2.
In some graphical presentations, the relationships between group mean LH AUC and group mean BW loss, group mean CYP2B2, and GST enzyme expression were plotted. However, the R2 and the linear regression functions shown in these figures were based on an analysis of individual animal data, not group means.
Linear regressions with LH AUC regressed on multiple independent variables (covariates) were also used to analyze the relative explanatory ability of different combinations of day, dose, BW loss, seven different CYP enzymes, and two GST enzymes. Twelve different mean LH AUC values (three durations [4, 8, and 14 days] and four doses [0, 6.5, 50, and 100 mg/kg/day]) were regressed on different combinations of the mean values of the covariates. Regressions with higher values of the R2 (i.e., fractions of variations explained) were considered to be better predictors of LH AUC.
Results
BW, FOOD CONSUMPTION, AND CLINICAL SIGNS DATA FROM EXPERIMENTS 1 AND 2
Group mean BW reductions were not statistically significant after one, two, or three doses of ATZ doses up to 100 mg/kg/day in experiments 1 and 2. Group mean BW was statistically significantly reduced compared to controls in groups administered ATZ at doses of 50 and 100 mg/kg/ day for 4, 8, or 14 days (Fig. 1, experiment 2; data from experiment 1 not shown). Group mean BW of the 6.5 mg/ kg/day ATZ-treated group was not statistically significantly reduced compared with the control group mean (Fig. 1, experiment 2). In addition to the effect of ATZ treatment on BW, the surgical procedure carried out 3 days prior to blood collection (after 4, 8, or 14 days of treatment) also reduced BWs in the vehicle control and the ATZ-treated groups. The group × day interaction was not statistically significant, indicating that the effect of surgery on BW was similar in the control and the ATZ-treated groups.
FIGURE 1.
Effect of atrazine (0, 6.5, 50, or 100 mg/kg/day) on mean (±SEM) BW (g) after 4, 8, or 14 days of treatment. If the SEM bars are not shown, they are smaller than the symbol used to depict the mean. * p < 0.05 versus the control group on the same day.
Food consumption tended to be less in ATZ-treated groups compared to controls, but it was highly variable, and none of the differences were statistically significant (data not shown). There were no treatment-related clinical symptoms observed in ATZ-treated rats, irrespective of dose level or the number of doses received.
LH SURGE
Experiment 1: Effect of 1, 2, 3, or 4 days of ATZ treatment on the LH surge
The results of the statistical analyses of the LH data collected in experiment 1 (i.e., LH surge, peak LH, and LH AUC) after one, two, three, or four ATZ doses of 100 mg/kg/ day are presented in Table 1. No statistically significant effect of a single dose of ATZ (100 mg/kg/day) was observed for the estradiol-induced LH surge (Fig. 2A), as indicated by an absence of a treatment effect in the ANOVA or by any effect on LH, peak LH, or LH AUC, based on paired statistical comparisons using Bonferroni’s or Student’s t tests (Table 1). Similar results were obtained after two or three daily doses (Fig. 2B and 2C), except that a statistically significant overall treatment effect was indicated by the statistically significant ANOVA, but not by the more conservative Bonferroni t test. Neither peak LH nor LH AUC was statistically significant (Student’s t test) after two or three ATZ doses. In contrast, after four daily doses of 100 mg/kg ATZ, a statistically significant reduction in the LH surge was observed (Table 1; graphical data not shown), as indicated by a significant ANOVA, a significant reduction in LH at 13:00 hr. (Bonferroni t test), and statistically significantly reduced peak LH and LH AUC (Student’s t test).
TABLE 1.
Statistically significant effects of repeated daily doses of atrazine on the LH surge in experiments 1 and 2
| Number of ATZ doses (mg/kg/day) |
LH surge: Two-way ANOVA F statistic, (p -value) |
LH surge: Bonferroni t-test Control × dose group |
t-Test (p -value)1 Treatment group vs. control group |
|||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Time | Treatment × time | Dose (mg/kg/day) |
Sample time (hr) |
p -Value | LH peak amplitude |
LH AUC |
|
| Experiment 1: Effects of 1, 2, 3, or 4 days of atrazine treatment on the LH surge | ||||||||
| 1 × (0, 100) | 0.2 (0.65) | 20.3 (< 0.01) | 0.3 (0.93) | 100 | All time points | NS | 0.48 | 0.37 |
| 2 × (0, 100) | 4.2 (0.04)a | 22.1 (< 0.01) | 0.7 (0.60) | 100 | All time points | NS | 0.08 | 0.22 |
| 3 × (0, 100) | 6.7 (0.01)a | 24.3 (< 0.01) | 0.5 (0.76) | 100 | All time points | NS | 0.17 | 0.08 |
| 4 × (0, 100) | 11.0 (p < 0.01)a | 14.6 (< 0.01) | 2.2 (0.06) | 100 | 1300 | < 0.01 | 0.02 | 0.02 |
| Experiment 2: Effects of 4, 8, or 14 days of atrazine treatment on the LH surge | ||||||||
| 4 × (0, 6.5, 50, 100) | 16.8 (p < 0.01)a | 83.5 (< 0.01) | 3.1 (< 0.01) | 6.5 | 1300 | < 0.05 | NS | NS |
| 50 | 1100, 1300 | < 0.01 | < 0.05 | < 0.01 | ||||
| 100 | 1100, 1300 | < 0.01 | < 0.01 | < 0.01 | ||||
| 8 × (0, 6.5, 50, 100) | 13.4 (p < 0.01) | 137 (< 0.01) | 1.7 (0.04) | 6.5 | All time points | NS | NS | NS |
| 50 | All time points | NS | NS | NS | ||||
| 100 | 0900, 1100, 1300 | < 0.05 | < 0.05 | < 0.05 | ||||
| 14 × (0, 6.5, 50, 100) | 12.6 (p < 0.01) | 73.2 (< 0.01) | 3.1 (< 0.01) | 6.5 | All time points | NS | NS | NS |
| 50 | 1400, 1600 | < 0.01 | NS | NS | ||||
| 100 | 1400, 1600 | < 0.01 | < 0.01 | < 0.01 | ||||
Pairwise statistical comparison was performed using the Student’s t-test in experiment 1 and the Dunnett’s t-test in experiment 2.
Bolded p -values indicate statistically significant differences (p < 0.05 or 0.01) between the designated treated and control groups.
FIGURE 2.
Effect of atrazine (100 mg/kg BW) on the LH surge. Graphs represent LH values across the afternoon and evening following 1 (A), 2 (B), 3 (C), or 4 (D) daily doses of 100 mg/kg/day atrazine, or 4 (D), 8 (E), or 14 (F) daily doses of 0, 6.5, 50, or 100 mg/kg atrazine. Atrazine was administered by gavage at the time of lights on. Blood samples were taken at 6, 9, 11, 13, 15, and 18 hrs. after lights on. Dark bars on abscissa represent time of lights off. Each point represents the mean +/− SEM of 9–15 individuals. If SEM bars are not shown, they are smaller than the symbol used to depict the mean. * p < 0.05 versus controls; ** p < 0.01 versus controls.
Experiment 2: Effects of 4, 8, or 14 days of ATZ dosing on the LH surge
The administration of ATZ daily by gavage at doses of 50 or 100 mg/kg to female SD rats for 4 days statistically significantly reduced the LH surge, as indicated by a statistically significant treatment effect shown by ANOVA, with significant reductions in LH at 11:00 and 13:00 hr, based on the Bonferroni t test, and with statistically significant reduced peak LH and LH AUC, based on the Student’s t test. An overall treatment effect on LH was also indicated in the 6.5 mg/kg/day dose group by a statistically significant Bonferroni t test at 1300 hr, although no differences between the control and the 6.5 mg/kg ATZ treated groups were evident, based upon peak LH or LH AUC (Figs. 2D–F and 3, and Table 1).
FIGURE 3.
Effect of atrazine on the peak LH surge amplitude (A, B, and C) or LH AUC (D, E, F) after 4, 8, or 14 days of atrazine administration by gavage at doses of 0, 6.5, 50, or 100 mg/kg/day. LH peak was calculated as the highest value attained by each individual animal on one of the six sampling occasions. Each bar represents the mean +/− SEM of 10–15 individuals. * p < 0.05 versus controls; ** p < 0.01 versus controls.
In contrast, after 8 days of ATZ administration, the effect of treatment on the LH surge, peak LH, or LH AUC was only observed in the 100 mg/kg/day dose group, but not in either the 6.5 or 50 mg/kg/day dose groups (Figs. 2 and 3). By 14 days, the LH surge was statistically significantly reduced in the 50 and 100 mg/kg/day dose groups at 1400 and 1600 hr; peak LH and LH AUC were reduced in the 100 mg/kg/day group, but not in the 50 mg/kg/day group. There were no statistically significant effects of 14 days of ATZ treatment on any LH parameter in the 6.5 mg/kg/day dose group.
Peak LH was highly-correlated with LH AUC (R2 = 0.94; p < 0.01; Supplementary Fig. S1). The relationship was independent of the dose level or the number of dose days. Based on these results, LH AUC was used as the covariate in all subsequent correlational analyses.
ADAPTATION OF THE EFFECT OF ATRAZINE ON THE LH AUC WITH REPEATED DOSING
The magnitude of the effect of ATZ on the LH surge, as measured by LH AUC, adapted with repeated dosing (Fig. 4). The magnitude of the effect on the LH AUC in the 6.5 mg/kg/day ATZ-treated group was statistically significantly less after 8 and 14 days of treatment than after 4 days (Fig. 4). A similar pattern was present in the 50 mg/kg/day ATZ-treated group, although none of the differences between days was statistically significant. In the high dose group administered 100 mg/kg/day ATZ for 4, 8, or 14 days, LH AUC was significantly less on day 4 compared with day 8, but not when compared with day 14.
FIGURE 4.
Adaptation of the effect of atrazine on the LH AUC after 4, 8, or 14 doses.
CORRELATION BETWEEN LH AUC AND BW
An analysis of individual animal weight change over 4 (Supplementary Fig. S2a), 8 (Supplementary Fig. S2b), or 14 days of treatment (Supplementary Fig. S2c) indicated that BW change was not correlated with LH AUC, irrespective of whether the animals were in the control-, low-, mid-, or high-dose ATZ-treated groups. In an analysis of the three covariates (BW loss during treatment, ATZ dose level, and the number of days of treatment), BW loss alone did not account for significantly more variance in LH AUC than did the ATZ dose level alone. The contribution of the day of treatment to the R2 was minimal (Fig. 5).
FIGURE 5.
R2, which estimated the relative contribution of three covariates (day, dose level, and BW loss during the dosing period), to account for variance in the measured LH AUC in experiment 2.
RELATIONSHIP BETWEEN THE EFFECT OF ATZ ON LIVER P450 ENZYME EXPRESSION AND LH AUC
Although there was variability across assessment occasions, the high dose of ATZ consistently resulted in a greater than twofold increased expression of CYP2C22, CYP2C23, CYP2B3, CYP2E1, and CYP2B2 (Supplementary Table S1), whereas CYP1A2 and CYP7B1 were often significantly less than controls. An assessment of the dose-response indicated that many CYP enzymes were induced on day 8 in the 50 mg/kg/day dose group, only to later fall back to near baseline levels by day 14 (Supplementary Table S1). In the 6.5 mg/kg ATZ treated groups, overall CYP enzyme induction was relatively weak on all assessment occasions, with the exceptions of CYP3A9, CYP2D4, and CYP3A18, which were most noticeably induced on days 4 and/or 8, as well as CYP2B2 on day 14. The progressively stronger induction of CYP2B2 expression as a function of dose over time was indicated by a relationship between CYP2B expression and dose on days 4, 8, and 14 (Fig. 6a, Panel A; Fig. 6b). This relationship was the inverse of the dose-dependent decline in LH AUC (Fig. 6b).
FIGURE 6.
(A) Panel A: Atrazine dose (6.5, 50, or 100 mg/kg/day) and time dependent (day 4, 8, or 14) expression of CYP2B2 mRNA expression (Mean ± SEM fold change relative to controls). Panel B: Group mean plasma LH AUC and fold change in liver CYP2B2 mRNA enzyme expression in female SD rats administered atrazine at dose of 6.5, 50, or 100 mg/kg/day for 4 days (experiment 2). The regression equations were calculated using individual animal data. Control data were from animals sacrificed after 4, 8, or 14 days. Panel C: Group mean plasma LH AUC and fold change in liver CYP2B2 mRNA enzyme expression in female SD rats administered atrazine at dose of 6.5, 50, or 100 mg/kg/day for 8 or 14 days (experiment 2). The regression equations were calculated using individual animal data. (B) Relationships between mean plasma LH AUC and liver CYP2B2 mRNA enzyme expression after 4, 8, or 14 days of atrazine dosing.
RELATIONSHIPS BETWEEN THE EFFECTS OF ATZ ON GSH LEVELS, GST ENZYME ACTIVITY, AND LH AUC
Rats administered 100 mg/kg ATZ over 4 days of treatment displayed a progressive increase in liver concentrations of GSH, cytosolic GST, and mitochondrial GST compared to untreated controls (Supplementary Fig. S4). In the 100 mg/kg/day dose group, mean GST mRNA activity increased progressively after 2 days of treatment, reached a maxima by day 8, and then fell back to control levels by day 14 (Fig. 7a).
FIGURE 7.
(A) Mean (±SEM) GST mRNA expression in the control and 100 mg/kg/day ATZ group after 1, 2, 3, 4, 8, or 14 days. * p < 0.05 versus controls. (B) Mean (±SEM) GST liver enzyme induction compared to LH AUC after 4, 8, or 14 daily atrazine doses.
Isoforms of the GST enzymes also tended to be increased in rats administered 100 mg/kg ATZ for 3, 4, 8, or 14 days when compared with groups of rats administered ATZ for only 1 or 2 days (Supplementary Fig. S5). In contrast to the results for GST mRNA, the majority of the GST isoforms displayed a dose-dependent response to ATZ treatment, which was more evident after 4 and 8 days than after 14 days of treatment (Supplementary Fig. S6). In general, isoforms of specific GST enzymes in the 50 mg/kg/day dose group increased more compared to other doses by day 8 and fell below control levels by day 14 (Supplementary Fig. S6). The time- and dose-dependent induction of GST enzyme expression in experiment 2 was the inverse of the reduction in LH AUC observed in these same groups of rats (Fig. 7b).
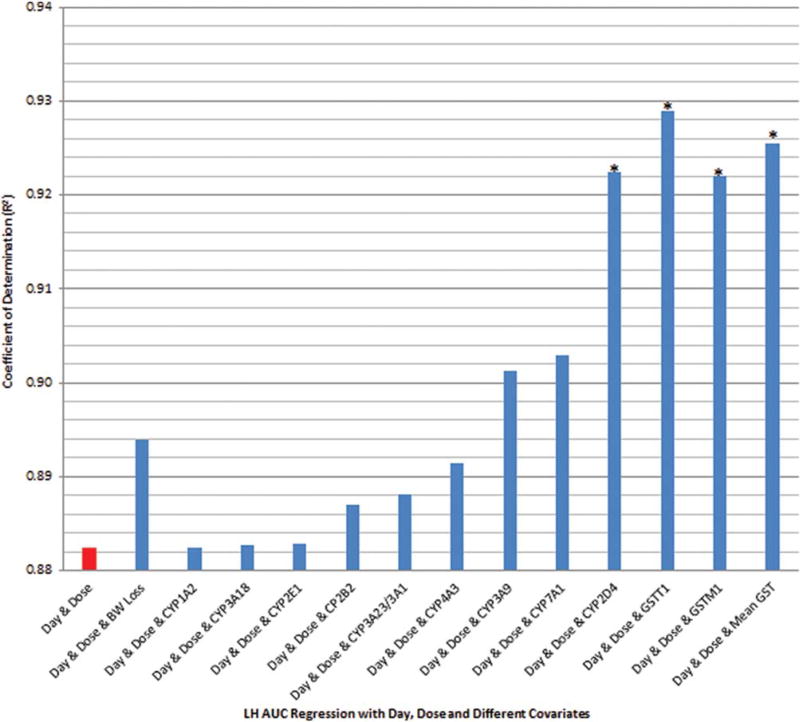
R2 calculated for LH AUC indicated that the proportion of the variance in LH AUC, after taking into account dose and duration of treatment, was strongly affected by the covariates for CYP2D4, GSTT1, GSTM1, and mean GST activity (Fig. 8).
FIGURE 8.
R2, which estimated the ability of the number of dose days, daily dose level, and a third covariate (BW loss, CYP enzyme expression, or GST enzyme expression) to account for variance in the measured LH AUC in experiment 2; * indicates that R2 was statistically significant (p < 0.05).
Discussion
This study was designed to determine if the total number of doses of atrazine administered by gavage to female SD rats would alter the magnitude of the effect of ATZ on the estrogen-induced surge of LH. The results indicate that 100 mg/kg ATZ reduced the LH surge (peak LH, LH AUC) after 2 days of treatment, but these effects were not statistically significantly different from control groups until day 4. In experiment 2, by day 4, LH levels were significantly reduced on at least one sampling occasion in each of the ATZ-treated groups, however, peak LH and LH AUC were significantly reduced in only the 50 and 100 mg/kg/day ATZ-treated groups. Following 8 or 14 days of ATZ treatment, there were no effects on the LH surge in the 6.5 mg/kg/day dose group; peak LH and LH AUC were only statistically significantly reduced in the 100 mg/kg/day ATZ group. This suggests that there is an “adaptation” to the effect of ATZ after multiple daily doses of ATZ at levels near or above a dose that has no effect on the LH surge (i.e., 6.5 mg/kg/day).
These results are consistent with previous studies using SD female rats that have shown that oral gavage administration of ATZ at four daily doses of 50 mg/kg/day or higher can inhibit the spontaneous proestrous LH surge (Foradori et al., 2009a), as well as the estrogen-induced and estrogen-plus-progesterone-induced LH surges (McMullin et al., 2004; Foradori et al., 2009a, 2014). In addition, recent work also indicated that there was no effect of ATZ treatment in female SD rats exposed to ATZ for 14 days in adulthood, in female rats exposed to ATZ prenatally through adulthood (postnatal day 133), or in females exposed from weaning through adulthood (Breckenridge et al., 2015). The results from Breckenridge et al. are consistent with the observations in the current study, where the 50 mg/kg/day dose, although effective in attenuating the LH surge following four daily doses, was ineffective following 8 or 14 days of treatment (with respect to peak LH and AUC).
The timing of the appearance and disappearance of the effect of ATZ on the LH surge are consistent with the observation that the LH surge returns to normal within 4 days of terminating ATZ dosing (Foradori et al., 2009a). This is consistent with the rapid pharmacokinetic clearance of ATZ and its chlorometabolites from plasma (Campbell et al., 2016). However, in the present studies, the effect of ATZ on LH was reduced on day 8, 4 days after reaching its apparent maximum. The mechanism underlying the development and attenuation of ATZ’s ability to inhibit the LH surge is unknown.
It is possible that an interaction between the acute effect of ATZ on the hypothalamic-pituitary-adrenal (HPA) axis and the effect of ATZ on the HPA axis after short-duration, high-dose exposure underlies the LH surge-inhibiting effect of ATZ (Cooper et al., 2000; Foradori et al., 2009a, 2014). It is known that single high doses of ATZ or its mono-dealkylated chlorometabolites, DIA and DEA, result in increased plasma progesterone and corticosterone levels within 15–30 min of dosing in female (Fraites et al., 2009) and/or male (Laws et al., 2009) rats. Foradori et al. (2011) confirmed these findings for ATZ, and showed that increased plasma corticosterone levels persisted for 12 hr following a single ATZ dose of 200 mg/kg.
However, HPA activation did not persist in female rats administered ATZ at a dose of 50 mg/kg/day for 7, 14, or 28 days (Foradori et al., 2017). In contrast, DACT, the final common chlorometabolite of DEA and DIA (Campbell et al., 2016), did not activate the HPA axis (Fraites et al., 2009; Laws et al., 2009). Despite the failure of DACT to activate the HPA axis, in other experiments, DACT suppressed the LH surge in rats after 4 (Foradori et al., 2011; Goldman et al., 2013), 30 (Minnema, 2001), or 180 days (Minnema, 2002) of treatment.
Furthermore, Foradori et al. (2011) showed that adrenalectomy abolished the effect of ATZ on pulsatile LH (Foradori et al., 2009b), but did not alter its effects on the LH surge, suggesting that adrenal corticosterone or progesterone may not be the sole mediators of the inhibitory effect of ATZ on the LH surge. It is well known that progesterone, when delivered at the appropriate time of day, can augment (Kamel and Kubajak, 1987; Gore et al., 2006) or inhibit (Freeman et al., 1976; Kempers and Ryan, 1977; Attardi, 1984) the estrogen-induced LH surge. Depending on the timing and magnitude of ATZ-induced progesterone release from the adrenal gland, ATZ’s inhibition of the LH surge may be modulated by the short-lived spike of plasma progesterone following repeated administration of high doses of ATZ.
To determine what other factors might contribute to the dose- and time-dependent changes in sensitivity of the LH surge to ATZ, we conducted multivariate regression analyses, where the magnitude of the variability in LH AUC accounted for by time- and dose-dependent response parameters (BW loss, phase I, and phase II liver enzyme expression) was assessed. These analyses indicate that dose level and the number of dose days had the largest effects on the R2, followed by a modest contribution of BW loss. However, animals that received an ATZ dose of 50 mg/kg/day sustained a BW gain reduction that was approximately the same as the group of animals that received 100 mg/kg/day ATZ (Fig. 1), yet LH AUC was not significantly different from control animals on day 8 or 14 (Fig. 3). In the 6.5 mg/kg/day ATZ dose group, initial BW gain reductions recovered by days 2 through 4, and LH levels were indistinguishable from controls by day 8. Furthermore, the analysis of the relationship between the LH AUC and changes in BW gain during the dosing interval indicated that these parameters were not correlated, irrespective of dose group.
Cooper et al. (2000) reported that the LH surge of Long-Evans (LE), but not SD rats, was significantly reduced after ATZ daily doses of 50, 100, 200, or 300 mg/ kg/day were administered for 1 or 3 days. BW was unaffected by treatment in either SD or LE rats after one or three doses, although after 21 days, high-dose LE rats displayed significantly reduced BW. Goldman et al. (2013) reported that two doses of ATZ (100 mg/kg/day) had no effect on BW, but a non-statistically significant reduction in LH AUC was observed. After four doses, there was no effect on BW, but LH AUC was now significantly reduced in the ATZ group, when compared to the controls. The authors attributed the non-significant effect of ATZ on LH AUC after 2 days of treatment to individual animal variability in the LH AUC response, with some animals responding with a robust reduction in LH AUC, while other animals were more like controls. The data from Cooper et al. (2000) and Goldman et al. (2013) support the conclusion from our study that the effect of ATZ on the LH surge is not linked to its effect on BW.
Among the CYP450 enzymes evaluated by Zimmerman et al. (2017), mRNA expression of a number of CYP enzymes (CYP1A2, CYP3A18, CYP2E1 CYP2B2, CYP3A23/31, CYP4A3, CYP3A9, and CYP2D4; Supplementary Figs. S1 and S2) displayed large, ATZ-dose-related, increased levels relative to controls. Average CYP2B expression, which displayed the largest increase among all CYP enzymes evaluated (Supplementary Fig. S2), was inversely proportional to LH AUC (see Fig. 7b). However, linear regression analysis conducted using individual animal data indicated that CYP2B2 did not contribute much to the R2 for LH AUC (Fig. 8).
A similar analysis of GSH and GST indicated that both were dose-dependently induced above baseline levels by repeated ATZ administration (Supplementary Figs. S3, S4, and S5). Linear regression analysis indicated that GST enzymes contributed more in predicting the effect of ATZ on LH AUC than did the CYP enzymes (Fig. 8). It is plausible that the ATZ-induced increased synthesis of GSH and expression of GST enzymes contributed to the day 8 decrease in LH AUC in ATZ-treated animals. It is expected that increased levels of GSH and GST in the liver would facilitate the conjugation and elimination of ATZ, DEA, and DACT (Campbell et al., 2016), thereby attenuating the effect of ATZ on the LH surge. This hypothesis is consistent with the results of a sensitivity analysis indicating that of the 84 parameters used in an ATZ-specific PBPK model, the rate of urinary clearance of DACT had up to a 4.5-fold effect on the model-estimated internal dose of DACT (Breckenridge et al., 2016). It is also consistent with the observation that the tolerance of maize to chlorotriazines is linked to higher levels of expression and activity of GST (Timmerman, 1989), and that when weeds develop resistance to ATZ, it has been attributed to increased expression/activity of GST (Anderson and Gronwald, 1991; Evans et al., 2017).
In summary, the results of the current study demonstrate that ATZ, when administered orally by gavage at a dose of 50 or 100 mg/kg/day for 4 consecutive days, is sufficient to impair the estrogen-induced LH surge in ovariectomized female SD rats. The magnitude of this effect appears to develop over the course of several days, since there was no significant effect on LH peak amplitude or LH AUC after 1 to 3 days of treatment. Moreover, the ability of the 50-mg/kg/day dose to reduce the LH surge at 4 days was not completely sustained after 8 or 14 days of treatment, whereas a higher dose of 100 mg/kg/day still retained its ability to suppress the LH surge through 14 days of exposure. Together, these data underscore the importance of using the appropriate time period for testing rodent LH response dynamics to endocrine-active chemicals. It also indicates that shifts in sensitivity to ATZ occur over relatively short periods of time. The mechanism by which this change in sensitivity occurs remains unresolved, although it is plausible that increased expression of phase I, or more likely, phase II enzymes could underlie the observed changes.
Supplementary Material
Acknowledgments
These studies were funded in their entirety by Syngenta Crop Protection, LLC, a registrant and basic manufacturer of ATZ. All studies were conducted by laboratories independent of Syngenta. Conflict of Interest Statement: Dr. Charles B. Breckenridge is an employee of Syngenta Crop Protection, LLC, a basic manufacturer of atrazine. Syngenta paid hourly fees for the time spent by Dr. James W. Simpkins (JSM), who assisted in study design and in the review of this publication, and Dr. Robert L. Sielken, Jr., who performed some of the statistical analysis reported herein. Drs. Robert J. Handa and Chad D. Foradori received research grants from Syngenta Crop Protection, LLC, to support research conducted in their laboratories. WIL Research conducted the in-life phase of the studies under contract to Syngenta Crop Protection LLC. Dr. Pragati Sawhney Coder, who was the designated study director, was an employee of WIL Research at the time the studies were conducted. Patents: There are no patents, products in development, or marketed products to declare. Contributions: Dr. Kun Don Yi provided advice on methods employed for the collection, storage, and shipment of the liver samples. Merrill Tisdel audited the conduct of the in-life phase of the studies at WIL Research Laboratories and audited final reports from WIL and the Handa laboratory.
Footnotes
Additional supporting information may be found in the online version of this article.
References
- Anderson MP, Gronwald JW. Atrazine resistance in a velvet-leaf (Abutilon theophrasti) biotype due to enhanced glutathione s-transferase activity. Plant Physiol. 1991;96:104–109. doi: 10.1104/pp.96.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attardi B. Progesterone modulation of the luteinizing hormone surge: regulation of hypothalamic and pituitary progestin receptors. Endocrinology. 1984;115:2113–2122. doi: 10.1210/endo-115-6-2113. [DOI] [PubMed] [Google Scholar]
- Breckenridge CB, Campbell JL, Clewell HJ, et al. PBPK-based probabilistic risk assessment for total chlorotriazines in drinking water. Toxicol Sci. 2016;150:269–282. doi: 10.1093/toxsci/kfw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckenridge CB, Sawhney Coder P, Tisdel MO, et al. Effect of age, duration of exposure, and dose of atrazine on sexual maturation and the luteinizing hormone surge in the female Sprague-Dawley rat. Birth Defects Res B Dev Reprod Toxicol. 2015;104:204–217. doi: 10.1002/bdrb.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges DC. Benefits of triazine herbicides in corn and sorghum production. In: LeBaron HM, McFarland JE, Burnside OC, editors. The triazine herbicides: 50 years revolutionizing agriculture. San Diego: Elsevier; 2008. pp. 163–174. [Google Scholar]
- Campbell JL, Jr, Andersen ME, Hinderliter PM, et al. PBPK model for atrazine and its chlorotriazine metabolites in rat and human. Toxicol Sci. 2016;150:441–453. doi: 10.1093/toxsci/kfw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RL, Laws SC, Das PC, et al. Atrazine and reproductive function: mode and mechanism of action studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:98–112. doi: 10.1002/bdrb.20110. [DOI] [PubMed] [Google Scholar]
- Cooper RL, Stoker TE, Tyrey L, et al. Atrazine disrupts the hypothalamic control of pituitary-ovarian function. Toxicol Sci. 2000;53:297–307. doi: 10.1093/toxsci/53.2.297. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. New tables for multiple comparisons with a control. Biometrics. 1964;20:482–491. [Google Scholar]
- Evans AF, Jr, O’Brien SR, Ma R, et al. Biochemical characterization of metabolism-based atrazine resistance in Amaranthus tuberculatus and identification of an expressed GST associated with resistance. Plant Biotechnol J. 2017;15:1238–1249. doi: 10.1111/pbi.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foradori C, Zimmerman A, Coder P, et al. Lack of immunotoxic effects of repeated exposure to atrazine associated with the adaptation of adrenal gland activation. Regul Toxicol Pharmacol. 2017;89:200–214. doi: 10.1016/j.yrtph.2017.07.017. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Hinds LR, Hanneman WH, Handa RJ. Effects of atrazine and its withdrawal on gonadotropin-releasing hormone neuroendocrine function in the adult female Wistar rat. Biol Reprod. 2009a;81:1099–1105. doi: 10.1095/biolreprod.109.077453. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Hinds LR, Hanneman WH, et al. Atrazine inhibits pulsatile luteinizing hormone release without altering pituitary sensitivity to a gonadotropin-releasing hormone receptor agonist in female Wistar rats. Biol Reprod. 2009b;81:40–45. doi: 10.1095/biolreprod.108.075713. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Hinds LR, Quihuis AM, et al. The differential effect of atrazine on luteinizing hormone release in adrenalectomized adult female Wistar rats. Biol Reprod. 2011;85:684–689. doi: 10.1095/biolreprod.111.092452. [DOI] [PubMed] [Google Scholar]
- Foradori CD, Sawhney Coder P, Tisdel M, et al. The effect of atrazine administered by gavage or in diet on the LH surge and reproductive performance in intact female Sprague-Dawley and Long Evans rats. Birth Defects Res B Dev Reprod Toxicol. 2014;101:262–275. doi: 10.1002/bdrb.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraites MJ, Cooper RL, Buckalew A, et al. Characterization of the hypothalamic-pituitary-adrenal axis response to atrazine and metabolites in the female rat. Toxicol Sci. 2009;112:88–99. doi: 10.1093/toxsci/kfp194. [DOI] [PubMed] [Google Scholar]
- Freeman MC, Dupke KC, Croteau CM. Extinction of the estrogen-induced daily signal for LH release in the rat: a role for the proestrous surge of progesterone. Endocrinology. 1976;99:223–229. doi: 10.1210/endo-99-1-223. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Davis LK, Murr AS, Cooper RL. Atrazine-induced elevation or attenuation of the LH surge in the ovariectomized, estrogen-primed female rat: role of adrenal progesterone. Reproduction. 2013;146:305–314. doi: 10.1530/REP-13-0011. [DOI] [PubMed] [Google Scholar]
- Good NE. Inhibitors of the Hill reaction. Plant Physiol. 1961;36:788–803. doi: 10.1104/pp.36.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Attardi B, DeFranco DB. Glucocorticoid repression of the reproductive axis: effects on GnRH and gonadotropin sub-unit mRNA levels. Mol Cell Endocrinol. 2006;256:40–48. doi: 10.1016/j.mce.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Islam MO, Hara M, Miyake J. Induction of P-glycoprotein, glutathione-S-transferase and cytochrome P450 in rat liver by atrazine. Environ Toxicol Pharmacol. 2002;12:1–6. doi: 10.1016/s1382-6689(01)00121-1. [DOI] [PubMed] [Google Scholar]
- Kamel F, Kubajak CL. Modulation of gonadotropin secretion by corticosterone: interaction with gonadal steroids and mechanism of action. Endocrinology. 1987;121:561–568. doi: 10.1210/endo-121-2-561. [DOI] [PubMed] [Google Scholar]
- Kempers RD, Ryan RJ. Acute effects of intravenous infusion of 17beta-estradiol and 17alpha-hydroxyprogesterone on gonadotropin release. Fertil Steril. 1977;28:631–637. doi: 10.1016/s0015-0282(16)42614-2. [DOI] [PubMed] [Google Scholar]
- Laws SC, Hotchkiss M, Ferrell J, et al. Chlorotriazine herbicides and metabolites activate an ACTH-dependent release of corticosterone in male Wistar rats. Toxicol Sci. 2009;112:78–87. doi: 10.1093/toxsci/kfp190. [DOI] [PubMed] [Google Scholar]
- Legan SJ, Karsch FJ. A daily signal for the LH surge in the rat. Endocrinology. 1975;96:57–62. doi: 10.1210/endo-96-1-57. [DOI] [PubMed] [Google Scholar]
- McMullin TS, Andersen ME, Nagahara A, et al. Evidence that atrazine and diaminochlorotriazine inhibit the estrogen/progesterone induced surge of luteinizing hormone in female Sprague-Dawley rats without changing estrogen receptor action. Toxicol Sci. 2004;79:278–286. doi: 10.1093/toxsci/kfh127. [DOI] [PubMed] [Google Scholar]
- Minnema DJ. Comparison of the LH surge in female rats administered atrazine, simazine or DACT via oral gavage for one month. Vienna, VA: Covance Laboratories Inc; 2001. p. 544. [Google Scholar]
- Minnema DJ. 52-week toxicity study of simazine, atrazine and DACT administered in the diet to female rats. Vienna, VA: Covance Laboratories Inc.; 2002. p. 1199. [Google Scholar]
- Motulsky H. Intuitive biostatistics. New York: Oxford University Press; 1995. [Google Scholar]
- Timmerman KP. Molecular characterization of corn glutathione S-transferase isozymes involved in herbicide detoxication. Physiol Plant. 1989;77:465–471. [Google Scholar]
- Tischer W, Strotmann H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron transport. Biochim Biophys Acta. 1977;460:113–125. doi: 10.1016/0005-2728(77)90157-8. [DOI] [PubMed] [Google Scholar]
- Zimmerman AD, Breckenridge CB, Yi KD, et al. Effect of the number of atrazine doses on hepatic phase I and phase II enzyme expression and substrate availability in female Sprague-Dawley rats. Xenobiotica. 2017 doi: 10.1080/00498254.2017.1374486. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.