Abstract
Coexistence of multiple chronic conditions (i.e., multimorbidity) is the most common chronic health problem in adults. However, clinical practice guidelines have primarily focused on patients with a single disease, resulting in uncertainty about the care of patients with multimorbidity. The American Thoracic Society convened a workshop with the goal of establishing a strategy to address multimorbidity within clinical practice guidelines. In this Workshop Report, we describe a framework that addresses multimorbidity in each of the key steps of guideline development: topic selection, panel composition, identifying clinical questions, searching for and synthesizing evidence, rating the quality of that evidence, summarizing benefits and harms, formulating recommendations, and rating the strength of the recommendations. For the consideration of multimorbidity in guidelines to be successful and sustainable, the process must be both feasible and pragmatic. It is likely that this will be achieved best by the step-wise addition and refinement of the various components of the framework.
Keywords: guidelines, multimorbidity, comorbidity, chronic conditions
Overview
Multimorbidity (i.e., coexistence of multiple chronic conditions) is a common chronic health problem in adults. It is associated with many undesirable consequences; for example, as the number of chronic conditions increases in individuals, so does the occurrence of poor outcomes, such as lower quality of life, decreased functional capacity, increased hospital readmissions, and both increased and more severe adverse effects of treatments. Clinical practice guidelines have almost exclusively focused on patients with a single condition. Uncertainty, therefore, exists regarding the evidence-based management of patients with multimorbidity. The American Thoracic Society (ATS) convened a workshop with the goal of establishing a strategy to address multimorbidity within clinical practice guidelines. Key conclusions from the workshop included the following:
Clinical practice guidelines should maximize the use of therapies likely to benefit patients with multimorbidity, while minimizing the use of therapies that are either unlikely to benefit or likely to harm such patients. This is best done by applying a patient-centered, rather than a disease-specific, framework.
Guidelines can focus on an index condition and one or more important coexisting conditions or modifying factors. Alternatively, guidelines can address a pair or cluster of conditions, among which no single condition is considered to be primary. Finally, guidelines can address multimorbidity in a more general way, such as guidelines addressing the coordination of care. Figure 1 illustrates these approaches.
Guidelines that address an index condition and either coexisting conditions or modifying factors benefit from having participants with a broad range of experience on the guideline development panel. We suggest that the guideline development group include everyone involved in the care of patients with multimorbidity: physicians (specialists who care for the index condition, specialists who care for coexisting conditions, generalists), nonphysicians (e.g., nurses, physicians assistants, respiratory therapists), patients, caregivers, and other stakeholders.
There are several reasonable approaches to identifying questions that will be addressed in a guideline. One approach is to specify multiple questions, each addressing the management of the index condition and a different coexisting condition. Alternatively, a single question can be developed for the index condition with the intention of addressing the coexisting conditions during the evidence synthesis and formulation of recommendations.
Clinical outcomes related to health benefits, harms, and burdens are used to assess the effects of various management options. Many outcomes are specifically related to the index condition; however, when addressing questions about the management of patients with multimorbidity, a broader spectrum of outcomes that includes those specifically related to common coexisting conditions is warranted. Selection of outcomes should be informed by patients who have multimorbidity and their family members or caregivers.
Confidence in the estimated effects (i.e., quality of evidence) of an intervention is generally lower in the context of multimorbidity than when only a single condition is considered. Randomized trials provide greater confidence in estimated effects than observational studies; however, they are also less likely to include patients with multimorbidity. Therefore, guideline developers often rely upon observational evidence or randomized trial evidence from other populations to inform judgments related to patients with multimorbidity, both of which provide lower confidence in the estimated effects of an intervention.
To inform judgments about whether or not to use an intervention, guideline developers should examine the direction and magnitude of the estimated health benefits and harms, the duration until each outcome is likely to occur, values and preferences, required resources, and the feasibility of implementing the intervention in individuals with multimorbidity.
When making recommendations for patients with multimorbidity, it is important to identify subgroups that are more likely to benefit or be harmed by the recommended action owing to different baseline risk or potential interactions among several interventions that are likely to be used concomitantly.
Recommendations made for patients with multimorbidity are more likely to be conditional (i.e., weak) compared with those targeting individuals who have only the index condition. This stems from the lower confidence in the estimated effects (i.e., the lower quality of evidence), likely higher cost and greater resources needed to deliver certain interventions to patients with multimorbidity, and more variability in values and preferences among patients with multimorbidity. This uncertainty will hopefully help to stimulate and prioritize future studies.
Figure 1.
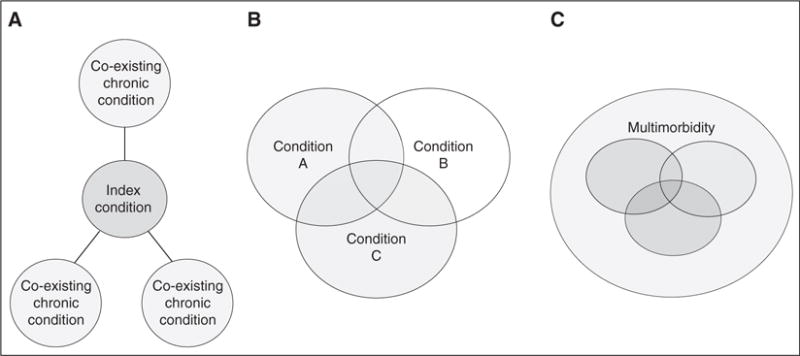
Considering multimorbidity in clinical practice guidelines. There are numerous ways to address multimorbidity in clinical practice guidelines. (A) Guidelines can focus on an index condition and pick one or more important coexisting conditions to address their combined management. (B) Guidelines can address a pair of conditions, in which neither condition is the primary condition. A potential downside to this approach is that it might leave other important combinations unaddressed. (C) Guidelines can address multimorbidity in a non–disease-specific way, such as guidelines addressing the coordination of care or self-management (adapted by permission from Reference 17).
Introduction
Multimorbidity (i.e., coexistence of multiple chronic conditions) has been described as the most common chronic health problem in adults (1). Multiple studies report that more than 25% of all adults and in excess of 65% of Medicare beneficiaries have two or more chronic conditions (2–10). Thus, multimorbidity is the usual patient experience among older adults. As examples, asthma and chronic obstructive pulmonary disease (COPD) are the lone conditions in only 4 and 3% of Medicare beneficiaries, respectively (11). Among those with asthma, 21% have one to two coexisting conditions, 30% have three to four coexisting conditions, and 45% have more than five coexisting conditions (11). Among those with COPD, 18% have one to two coexisting conditions, 30% have three to four coexisting conditions, and 49% have more than five coexisting conditions (11); the pattern of coexisting conditions in patients with COPD also varies according to age (Table 1).
Table 1.
The Top 10 Most Common Co-occurring Chronic Conditions among Medicare Beneficiaries with Chronic Obstructive Pulmonary Disease (n = 3,850,265), 2012
| % | |
|---|---|
| Beneficiaries under 65 yr of age (n = 688,542) | |
| COPD prevalence | 11.0 |
| Top 10 comorbidities | |
| Hypertension | 70.7 |
| Hyperlipidemia | 52.3 |
| Depression | 47.3 |
| Ischemic heart disease | 42.8 |
| Diabetes | 42.5 |
| Arthritis (RA/OA) | 42.3 |
| Anemia | 36.3 |
| Heart Failure | 30.3 |
| Chronic kidney disease | 24.7 |
| Asthma | 23.4 |
| Beneficiaries 65 yr of age and older (n = 3,161,723) | |
| COPD prevalence | 11.3 |
| Top 10 comorbidities | |
| Hypertension | 81.4 |
| Hyperlipidemia | 61.3 |
| Ischemic heart disease | 57.6 |
| Anemia | 45.4 |
| Arthritis (RA/OA) | 44.1 |
| Heart failure | 42.7 |
| Diabetes | 38.6 |
| Chronic kidney disease | 34.3 |
| Depression | 26.6 |
| Alzheimier’s Disease | 20.2 |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; OA = osteoarthritis; RA = rheumatoid arthritis. Prepared by the Centers for Medicare and Medicaid Services (CMS)/Office of Information Products and Data Analytics on October 6, 2014. Data Source: CMS administrative claims data, January 2012–December 2012, from the Chronic Condition Warehouse (https://www.ccwdata.org).
The high prevalence of multimorbidity has many undesirable consequences. Healthcare expenditures and hospital readmissions are directly related to the number of chronic conditions (11). In addition, cumulatively higher numbers of chronic conditions correlate directly with incrementally poorer clinical outcomes, including mortality, poor functional status, hospitalizations, hospital readmissions (Figure 2), and adverse drug events.
Figure 2.
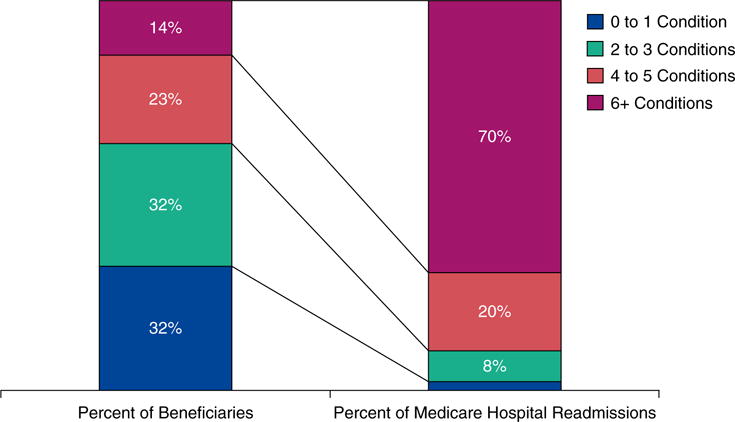
The prevalence of multimorbidity and its association with readmissions among U.S. Medicare beneficiaries (Reproduced by permission from Reference 11).
Recognizing the importance of multimorbidity, the U.S. Department of Health and Human Services (DHHS) created a working group in 2009, which subsequently released a strategic framework on multimorbidity in 2010 (12). One of the goals of the framework was to provide better tools and information to healthcare, public health, and social service workers. Within this domain, the DHHS included an objective and initiated a concerted effort to address multimorbidity in clinical practice guidelines.
Clinical practice guidelines have an important role in guiding how we provide care and share decision making with patients; however, their applicability for real-world care is limited, because they have been largely developed for and emphasize a single-condition perspective (13). As a result, such single-morbidity guidelines seldom account for the uncertain benefit and potential harm of numerous simultaneous treatments, the possibility of exacerbating a particular condition by treating a coexisting one, or the complexity and treatment burden arising from simultaneously trying to apply multiple clinical guidelines for several conditions (1, 14, 15).
The ATS participated in a DHHS/Institute of Medicine meeting on clinical practice guidelines and multimorbidity in May 2012 (16). As follow up to that meeting, the ATS launched its own initiative to address multimorbidity in its clinical practice guidelines. The initial step was to convene a workshop in October 2014 with the goal of establishing a framework to address multimorbidity in ATS clinical practice guidelines for pulmonary disease, critical illness, and sleep medicine. This report describes the proceedings and conclusions from the workshop. The framework will serve as the basis of future steps to consider multimorbidity in its guidelines.
Methods
The ATS Documents Development and Implementation Committee selected co-chairs (M.K.G., J.A.K., and K.C.W.) to lead a workshop with the goal of developing a framework that will be used during the development of future ATS guidelines. Workshop participants included an interdisciplinary group of clinicians, researchers, and guideline methodologists within ATS, and individuals with experience working with other professional societies (C.M.B., R.A.G., and R.M.R.). The workshop proposal was approved and funded by the ATS Board of Directors.
The workshop was convened in Orlando, Florida on October 17, 2014. Speakers (C.M.B., J.L.B., R.A.G., and R.M.R.) outlined the scope of the problem, described a potential framework, discussed potential challenges in applying the framework, and described another professional society’s experience addressing multimorbidity in guidelines. Each talk was followed by discussion among the entire panel of participants. After the talks, the participants were divided into two groups for breakout sessions to discuss the application of the framework using different index conditions as examples.
The initial draft of the Workshop Report was authored by the co-chairs and speakers. The other panel members then reviewed and edited the draft report. The Workshop Report underwent several cycles of external peer review and revisions, followed by review and approval by the ATS Board of Directors.
Addressing Multimorbidity in Guidelines
Framework
The workshop participants used a framework developed by Katrin Uhlig and colleagues (17) as the foundation upon which to inform their judgments. The Uhlig framework was highly regarded by the workshop participants for several reasons. First, it addresses most of the steps of guideline development, including selecting the topic, assembling the panel, choosing and formulating the questions, reviewing and synthesizing the evidence, appraising the quality of evidence, summarizing the benefits and harms, and formulating the recommendations and rating their strength. Second, it shares the belief of ATS that clinical practice guidelines should maximize patient-centered care by incorporating patients’ and caregivers’ perspectives, recognizing their need for information, enhancing prevention and health promotion, and facilitating a continuing relationship between patients and clinicians.
Topic selection
There are numerous ways to address multimorbidity in clinical practice guidelines. Guidelines can focus on an index condition and one or more important coexisting conditions or modifying factors, and then address their combined management. Alternatively, guidelines can address a pair or complex of conditions, among which no one condition is primary. A potential downside to this approach is that it might leave other important combinations unaddressed. Finally, guidelines can address multimorbidity in a non–disease-specific way, such as guidelines addressing the coordination of care or self-management (17). These approaches are illustrated in Figure 1.
Clinical practice guidelines will be most useful if they address combinations of coexisting conditions that are common and the interactions of which are important or uncertain. The latter includes interactions in which the index condition and the coexisting conditions aggravate or ameliorate one another (e.g., COPD and heart failure), treatment of the index condition affects the coexisting conditions (e.g., the impact of long-term steroid therapy for sarcoidosis on diabetes and osteoporosis), and/or interactions between the treatments of the index condition and treatment of the coexisting conditions (i.e., polypharmacy). When deciding which interactions to address, it is helpful to look for interactions that affect outcomes that matter to patients, such as symptoms or functional impairment. Data regarding the frequency of various combinations of coexisting conditions may derive from epidemiological studies and cross-sectional studies of databases of diagnostic codes and administrative claims; data regarding interactions among coexisting conditions are more scarce, but may derive from observational studies that follow individuals with an index condition or a combination of coexisting conditions and measure adverse outcomes attributable to condition–condition, condition–medication, or medication–medication interactions. An appropriate next step for guideline developers who want to address multimorbidity is to operationalize these concepts, a process that will necessarily be iterative to ensure that it is feasible.
Guideline development group composition
It is well accepted that a guideline development group should include clinicians from specialties relevant to the index condition. As an example, a guideline on lung cancer might include a pulmonologist, thoracic surgeon, medical oncologist, radiation oncologist, and palliative care clinician. To address multimorbidity, however, even broader representation may be necessary. This may include specialists who manage each of the coexisting conditions, a generalist (i.e., a geriatrician, internist, or family physician) who frequently coordinates and balances a patient’s care among many specialists, and nonphysicians (17). Examples of relevant nonphysician participants include healthcare professionals (e.g., nurses, physicians’ assistants, and respiratory therapists), patients, caregivers, and others who can provide insights related to values and preferences relevant to decision making (17).
Clinical questions
The clinical questions addressed in guidelines are typically formulated using the population, intervention, comparator, outcomes (PICO) format. One possible approach to formulating questions that incorporate multimorbidity is to ask multiple questions, each addressing the index condition and a different coexisting condition or clusters of chronic conditions. Alternatively, a single question related to the index condition can be asked with the plan to address coexisting conditions as subgroup considerations. The impact of these options on the formulation of recommendations is discussed in greater detail below.
An important part of deriving the PICO questions is selection of the outcomes. Outcomes used in guideline development are generally related to potential health benefits and harms that are specific to the index condition. When addressing patients with multimorbidity, however, consideration of a broader spectrum of outcomes may be warranted (17). Outcomes related to both the index condition and common coexisting conditions should be considered. As an example, consider a question about whether or not to prescribe a medication to a patient who has COPD. A guideline that addresses multimorbidity might consider not only COPD-specific outcomes, such as dyspnea, exercise capacity, and the frequency of COPD exacerbations, but also outcomes, such as the frequency of myocardial infarction, coronary revascularization, sudden cardiac death, and hypercholesterolemia, because ischemic heart disease and hyperlipidemia are conditions that commonly coexist with COPD and may be affected by its treatments.
Outcomes that are specific for neither the index condition nor coexisting conditions should also be included; as an example, functional status is a nonspecific outcome. The timeframe for outcomes to develop should also be considered, as long-term outcomes may be irrelevant for decision making if the population of interest has a short life expectancy. Finally, outcomes should be realistic; return to full-time employment would not be a realistic outcome for those anticipated to have physical or cognitive changes that would make this unrealistic.
Searching and selecting evidence
Evidence syntheses generally prioritize the highest-quality evidence in the form of existing systematic reviews and randomized trials. Ideally, studies would report treatment–comorbidity interactions or supply sufficient data to perform quantitative or qualitative summaries of trends (18). Given the paucity of randomized trial data with sufficient inclusivity of those with multimorbidity (19–24), evidence searches should anticipate including nonrandomized studies that estimate the differential effects and safety among those with comorbidity.
Summarizing the evidence
Explicit delineation of health benefits and harms, values and preferences, implementation issues, and resource requirements (when available) allows guideline development groups to weigh the desirable consequences of an intervention with the undesirable consequences. Guideline developers should ask whether these factors and the resulting balance of desirable and undesirable consequences are the same or different among individuals with multimorbidity compared with the populations from which the estimates were derived (17). In addition to the usually serious indirectness of the evidence, individuals with multimorbidity often have a different baseline risk for certain outcomes. Thus, relative effects derived from studies among patients with single conditions are more likely to be correct among patients with multimorbidity than absolute effects (25, 26). Guideline panels should be cautious about the assumption of fairly constant relative effects, however, because disease and treatment interactions may substantially modify relative risks. Guideline developers should always seek estimates of the baseline risk of outcomes among populations with characteristics closest to that for which a recommendation is being made. This is especially important when making recommendations for patients with multimorbidity. Such information may be found in separate observational studies that provide relevant prognostic information (27).
Guideline developers should estimate the required duration for a particular treatment to achieve the desired benefit. In addition, careful consideration of important trade-offs over time is imperative. One is less likely to recommend a potentially harmful, burdensome, or expensive intervention if the benefit from that intervention is unlikely to be realized during the patient’s lifetime. This latter consideration is not different from similar decisions for patients with single conditions, but is likely to be significantly more challenging to estimate among patients with multimorbidity. As an example, guidelines recommend against low-dose computed tomography screening for lung cancer among individuals with severe comorbidities that limit life expectancy, because the benefit of screening (preventing death from lung cancer) is unlikely to be realized if the patient is likely to die in a short timeframe from another cause.
The values and preferences that patients assign to particular outcomes (e.g., reduction of symptoms versus the risk of side effects) and the interventions themselves (e.g., anxiety, burden, or cultural attitudes toward some health interventions) are likely to be more variable among patients with multimorbidity than among those with single health problems. Acceptance and tolerance of multiple nonspecific symptoms may be more common as a baseline state among patients with multimorbidity. Resources required for implementation of an intervention and the effect of an intervention on health inequities may similarly be more variable among patients with multimorbidity compared with patients with single health problems. Such variability should be summarized when available, because it may substantially influence the acceptability of interventions to various stakeholders (patients, caregivers, clinicians, third party payers, etc.).
Rating the quality of evidence
The quality of evidence (also referred to as certainty of evidence) indicates the confidence that the estimated effect of an intervention is correct. There are many strategies for rating the quality of evidence, but the ATS and many other guideline developers have adopted the Grading of Recommendations, Assessment, Development, and Evaluation approach. With this approach, the quality of the body of evidence for each outcome is determined by the study design, risk of bias, directness of the available evidence, precision of the estimate, consistency of the effects across studies, likelihood of publication bias, magnitude of an effect, presence or absence of a dose–effect relationship, and effect of plausible residual confounding. The quality of evidence rating is often lower when evidence is applied to individuals with multimorbidity than when the same body of evidence is applied to individuals with single conditions. There are many reasons for this, but the two most common are study design and indirectness.
With respect to study design, patients with multimorbidity are less likely to be included in randomized trials and, therefore, the evidence about treatment effects in this population often comes only from observational studies. Although observational studies are generally more inclusive than randomized trials, they are usually at substantially greater risk of bias. The frequent exclusion of patients with multimorbidity from randomized trials was illustrated by a study that reported that more than 30% of COPD trials excluded patients who: were receiving supplemental oxygen or oral steroid therapy; had coronary artery disease, musculoskeletal disease, lung disease other than COPD, or other unspecified serious coexisting diseases; were younger than 40 years or older than 65 years of age; or were unable to exercise for unspecified reasons (19).
With respect to indirectness, exclusion of patients with multimorbidity from randomized trials means that the mix of coexisting conditions in patients who are studied in randomized trials may be different than in patients typically seen in routine clinical practice. This indirectness lowers confidence that the estimated effects used to inform the recommendation reflect the actual effects of the intervention in the population for which the recommendation is intended (28, 29). The possibility that effects differ between the studied populations and the population for which the recommendation is intended stems from the difference in health status (individuals with multiple coexisting health problems tend to have poorer health status than individuals with single conditions) and the difference in the number and type of cointerventions (individuals with single conditions usually receive fewer concomitant treatments than individuals with multiple conditions).
Another source of indirectness is related to the fact that dosing or route of administration of certain interventions may be different between patients with a single health problem compared with patients with multimorbidity (e.g., there may be a need to reduce the preferred dose of a medication or choose an alternative agent to avoid drug–drug interactions). For instance, interactions among several interventions used in those with multiple health problems may reduce or increase their relative effect on certain outcomes compared with when those same interventions are used separately.
Although not as common as risk of bias and indirectness, imprecision (i.e., wide confidence intervals) may also contribute to lower quality of evidence. Outcomes estimated for patients with specific comorbidities frequently derive from subgroup analyses performed within studies that enrolled patients with an index condition. The subgroups are smaller, with fewer events than the entire study population, and, therefore, the estimated effects of the subgroups tend to have wider confidence intervals. Criteria that suggest that a subgroup effect is valid include the following: (1) the effect is statistically significant; (2) the effect is consistent across studies; (3) the subgroup was defined a priori and there is a limited number of subgroups; (4) the effect is biologically plausible; and (5) the effect is based upon a within-study comparison (30).
Formulating recommendations and rating their strength
Guidelines recommend an intervention if its desirable consequences outweigh its undesirable consequences. Panels make this decision by considering the following factors: the balance of health benefits versus harms and burdens; the quality of the available evidence; the values and preferences of those affected; required resources; feasibility; acceptability; and impact on health inequities. In contrast, guidelines recommend against an intervention if the undesirable consequences outweigh the desirable consequences.
When recommendations are made for patients with multimorbidity, there are several reasonable approaches, which are related to the approach to identifying questions described above. If the guideline panel decided to ask multiple questions, each addressing the index condition and a different coexisting condition or clusters of chronic conditions, then the panel may provide a recommendation for each of the questions. Alternatively, if the guideline panel decided to ask a single question related to the index condition with the plan to address coexisting conditions as subgroups considerations, then the panel may either: (1) provide a single recommendation that is applicable to most patients, followed by a description of additional considerations related to coexisting conditions; or (2) provide a single recommendation that is applicable to most patients, followed by additional recommendations for coexisting conditions. The preferred approach will depend upon the context, such as the nature, number, and prevalence of coexisting conditions (17), as well as the clarity of the final presentation of recommendations. Regardless of the approach taken, it is most important to identify subgroups that are more likely to benefit from or be harmed by the recommended action. Patients with multimorbidity may have an increased likelihood of benefiting from certain interventions due to their greater baseline risk of an adverse outcome; however, they may also be more likely to be harmed due to potential interactions among several interventions likely to be used concomitantly.
The strength of a recommendation indicates the degree of certainty among the guideline development group’s members that the desirable consequences of an intervention outweigh the undesirable consequences. When a guideline panel is certain, they make a strong recommendation; when they are uncertain, they make a conditional (i.e., weak) recommendation. In other words, a conditional recommendation implies that the majority of well informed people in such situations would choose the recommended course of action, but a substantial number would not. Recommendations for patients with multimorbidity are more likely to be conditional than recommendations targeting individuals who have only the index condition, because of the uncertainties about the magnitude of effects, directness of the information, and other factors discussed previously. Conditional recommendations may stimulate and prioritize future studies among patients with multiple chronic conditions, including studies employing a comparative effectiveness or implementation science framework.
Illustrative Case Example
Consider the example of clinical practice guidelines that address the evaluation of incidentally identified pulmonary nodules. To guide shared decision making, the guideline panel should consist of those who detect the nodules, those who order the imaging studies and then act upon the results, and those with whom a patient with a newly detected nodule may consult. Hence the guideline development group might include a radiologist, primary care clinician, hospitalist, nurse practitioner, physician’s assistant, pulmonologist, and thoracic surgeon. Inclusion of a patient with multimorbidity, a caregiver, and at least one patient who has been evaluated for incidentally detected pulmonary nodules is also important. The perspective of the patient should be carefully considered; as an example, if the patient is someone whose life was saved by screening and subsequent curative resection of a malignant nodule, a second patient who experienced complications from unnecessary diagnostic testing should also be added to balance the views of the first patient.
Multimorbidity is common among patients with incidentally discovered pulmonary nodules according to the collective clinical experience of the workshop participants. During or before group composition, the most common coexisting conditions and the coexisting conditions most likely to affect the management of pulmonary nodules should be determined. The former may include COPD, heart failure, tobacco dependence, ischemic heart disease, hypertension, and depression, whereas the latter may include atrial fibrillation and venous thromboembolic disease (i.e., patient taking anticoagulants), obesity (i.e., increased technical difficulty of diagnostic procedures), and limited life expectancy due to any cause (i.e., terminal disease, advanced age). To address these coexisting conditions, a geriatrician, cardiologist, and psychiatrist may be added to the guideline development group.
The assembled guideline development group’s first task is to develop the PICO questions that will form the basis of the systematic review and then be answered by a recommendation. Among the possible approaches—(1) developing multiple PICO questions that each address patients with lung nodules and a different coexisting condition, or (2) developing a single PICO question in anticipation of addressing coexisting conditions via either remarks or multiple recommendations—option 2 is the preferred approach for these guidelines, because the number of common coexisting conditions (i.e., COPD, heart failure, tobacco dependence, ischemic heart disease, hypertension, and depression) and coexisting conditions that may influence management (i.e., atrial fibrillation, venous thromboembolic disease, obesity, and old age) is so large that option 1 is impractical.
The evidence synthesis should evaluate the types of patients enrolled in each study selected during the systematic review. Of particular interest is whether the studies excluded patients with the chronic coexisting conditions of interest. This evaluation lends itself to sensitivity analyses (i.e., whether results are different among the studies that excluded patients with a coexisting condition of interest than among the studies that did not exclude patients with that coexisting condition). It also facilitates recognition of whether the population of interest is the same as the population studied (i.e., whether there is indirectness of the population) and whether the results are consistent across studies.
After the evidence synthesis, the guideline development group will begin to formulate its recommendations based upon consideration of the balance of benefits versus harms and burdens, quality of evidence, patient values and preferences, cost, and resource use. The potential benefit of evaluating incidentally detected lung nodules is decreased mortality due to lung cancer, whereas the potential harms include the complications of diagnostic testing (e.g., pneumothorax or bleeding after biopsy), consequences of false-positive results (e.g., unnecessary additional diagnostic testing or treatment), and consequences of false-negative results (i.e., false reassurance, delayed diagnosis). The balance of potential benefits versus harms may be substantially affected by coexisting conditions. As examples, patients with a limited life expectancy have a less favorable benefit-to-harm ratio due to both decreased benefit and increased harm, whereas patients taking anticoagulants or who are obese have a less favorable benefit-to-harm ratio due to increased risk of harms alone. It is preferable to make separate recommendations for each coexisting condition or cluster of conditions, because the different courses of actions will be more apparent to readers.
Other Organizations’ Experiences
The American Academy of Otolaryngology–Head and Neck Surgery (AAO-HNS) has already begun to consider multimorbidity in its professional society guidelines, which provides an opportunity for other guideline developers to learn from their experiences (31). Among the steps taken by the AAO-HNS to address multimorbidity in its guidelines, perhaps the most successful has been routine establishment of guideline development groups in which generalist physicians (e.g., pediatricians, family physicians, and internists), non–otolaryngology specialists (e.g., radiologists, pathologists, and anesthesiologists), and nonphysicians (e.g., nurses, audiologists, speech pathologists, consumers, physician assistants, and researchers) outnumber otolaryngologists. The generalists in particular tend to be helpful in keeping the guideline development group aware of coexisting conditions and modifying factors (e.g., asthma and sinusitis) throughout the development process. Diverse guideline development groups appear to balance biases, build support for the guidelines, and increase the likelihood that all relevant scientific evidence will be located, that practical aspects will be addressed, that debate will be robust, and that group members will learn from one another about different perspectives on multimorbidity.
The AAO-HNS has observed that many clinicians do not consider the common coexisting conditions that may influence treatment choices. To address this, the organization routinely provides statements in its guidelines, such as “clinicians should assess the patient with chronic or recurrent sinusitis for multimorbidity that would modify management, such as asthma, cystic fibrosis, an immunocompromised state, and ciliary dyskinesia” (32). Such statements explicitly alert readers to common coexisting conditions and provide the foundation for subsequent discussions in the text. The statements also set the stage for recommendations that either target patients with such coexisting conditions or are followed by descriptions of caveats associated with coexisting conditions.
Broad peer review of guidelines by many types of stakeholders allows the guidelines to be reviewed from many perspectives. For example, AAO-HNS routinely sends its guidelines to more than 40 external peer reviewers for consideration. This approach is particularly helpful in the context of multimorbidity, because each chronic condition may prompt a unique perspective worthy of consideration (e.g., the perspective of a cardiologist is likely different than that of a pulmonologist for a question addressing the management COPD exacerbations in patients with coexisting ischemic cardiomyopathy) and should include patient and caregiver input.
Although guideline developers, such as the AAO-HNS, have made positive strides in addressing multimorbidity, there are ways in which the process can be improved. As examples, it would be helpful to precede each guideline with research identifying the most common coexisting conditions and to incorporate a process for considering multimorbidity into each step of guideline development. Regardless of a guideline developer’s initial steps, many hope to eventually address multimorbidity as the main focus, rather than something that accompanies an index condition.
The AAO-HNS is not the only professional society that has begun to address multimorbidity in its guidelines. The American Heart Association and American College of Cardiology have developed their own framework for addressing multimorbidity (33), and have begun to include new content related to multimorbidity in guideline updates (34). The American Society of Clinical Oncology has addressed multimorbidity in some of its most recent guidelines (35), and the Global Initiative for Chronic Obstructive Lung Disease (GOLD) Stretegy address multimorbidity. GOLD says that COPD rarely exists alone, coexisting conditions are often neither diagnosed nor treated, frequent coexisting conditions should be actively sought, and coexisting conditions in patients with COPD should be managed the same as in patients who do not have COPD (36). Although we acknowledge that, in many circumstances, management of the coexisting condition is the same whether the patient does or does not have COPD, we believe that the primary reason that guidelines should address multimorbidity is to systematically and rigorously determine whether or not recommendations should vary depending upon the constellation of coexisting conditions.
Challenges and Future Plans
The framework for addressing multimorbidity in clinical practice guidelines described above will require additional effort from guideline developers—additional guideline development group members will need to be recruited and coordinated, evidence syntheses will need to be broader and consider more observational data and outcomes, and decision making (i.e., formulating and grading recommendations) will be more complex. Guideline development bodies should strive to determine which chronic coexisting conditions or combinations of conditions are most important to address, which require measuring the prevalence of various conditions, influence on management, and/or influence on outcomes. More diverse representation in the guideline development group requires that resources must be secured to support a larger and more diverse guideline panel, which requires commitment from organizational leadership and buy-in from clinical experts. Methods need to be established to measure the heterogeneous effects of interventions across patients with different combinations of chronic conditions, the baseline risk of outcomes among patients with various combinations of chronic conditions, and the time horizon for benefits and harms to accrue. Professional societies and federal scientific funding agencies should encourage and fund studies that specifically evaluate therapeutic efficacy in the context of varying strata of prespecified baseline risk. Finally, testing and validation of the various components of the framework by guideline methodologists will be necessary to determine essential steps and demonstrate measurable benefit in effectiveness, implementation, and, ultimately, patient outcomes. We recommend that guideline developers who are beginning to develop a process for addressing multimorbidity in their guidelines adopt a test case to operationalize the concepts in this Workshop Report. This may involve a guideline directed at adult or pediatric patients, because the concepts included in the framework are equally applicable to pediatric patients with multimorbidity.
Traditional guidelines directed at an index condition can play an important role in addressing multimorbidity during the transition to the more multimorbidity-focused process described above. During the evidence synthesis, guideline developers should assess and summarize evidence from subgroups of patients who have the index condition and important coexisting conditions. Then, specific recommendations should be made for each combination of the index condition and a coexisting condition, if appropriate. This can be implemented immediately, providing much-needed guidance for managing patients with multiple coexisting conditions.
Conclusions
For the consideration of multimorbidity in guidelines to be successful and sustained, it must be both feasible and pragmatic. It is likely that this will be best achieved by the step-wise addition, refinement, and codification of the various components of the framework. To facilitate development and testing of this methodology, the ATS Documents Development and Implementation Committee plans to work with ATS leadership to select one or more guideline topics that address interactions between an index condition and its important chronic coexisting conditions. The guideline development groups will be composed of individuals that include specialists in the index condition, specialists in the coexisting conditions, generalists, nurses, other allied health professionals, patients, caregivers, and other stakeholders. The guideline development groups will be reminded to select outcomes of importance to patients, including those that may result from the coexisting conditions. The evidence syntheses will be required to describe the inclusion and exclusion criteria of the selected studies, the effects of multimorbidity on outcomes, and the directness of the evidence. Finally, guideline development will address the effect of chronic coexisting conditions via separate recommendations or in the remarks that follow each recommendation. After the completion of such guidelines, the success in addressing multimorbidity will be evaluated and the steps modified accordingly.
Acknowledgments
Author disclosures
K.C.W., M.K.G., J.A.K., J.L.B., C.R.C., I.S.D., M.J.J., S.L., R.A.M., M.R.P., R.M.R., M.S., C.C.T. and R.S.W. reported no commercial interests relevant to document subject matter. C.M.B. received royalties from UpToDate, Inc. R.A.G. was employed by the U.S. Centers for Disease Control and Prevention. H.S. served as a consultant to Meridian Rx, a pharmacy benefits manager company. C.S. was employed by the U.S. Department of Veterans Affairs. B.S. represented the Cystic Fibrosis Foundation on the American Thoracic Society Patient Advisory Roundtable and is an attorney and partner in Sufian and Passamano, a civil litigation practice whose clients have included patients, physicians, researchers, hospitals, medical device manufacturers, and pharmaceutical companies.
Footnotes
This official Workshop Report of the American Thoracic Society (ATS) was approved by the ATS Board of Directors December 2015.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs (VA), Centers for Disease Control and Prevention, or the U.S. Government. The VA did not have a role in the conduct of the study, in the collection, management, analysis, interpretation of data, or in the preparation of the manuscript.
This Workshop was prepared by the ATS Guideline Methodology Working Group.
Members of the working group are as follows:
Kevin C. Wilson, M.D. (Co-Chair)
Michael K. Gould, M.D., M.S. (Co-Chair)
Jerry A. Krishnan, M.D., Ph.D. (Co-Chair)
Cynthia M. Boyd, M.D., M.P.H.
Jan L. Brozek, M.D., PH.D.
Colin R. Cooke, M.D., M.SC., M.S.
Ivor S. Douglas, M.D.
Richard A. Goodman, M.D., M.P.H.
Min J. Joo, M.D., M.P.H.
Suzanne Lareau, M.S., R.N.
Richard A. Mularski, M.D.
Minal R. Patel, PH.D., M.P.H.
Richard M. Rosenfeld, M.D., M.P.H.
Hasan Shanawani, M.D.
Christopher Slatore, M.D., M.S.
Marianna Sockrider, M.D., DR.P.H.
Beth Sufian, J.D.
Carey C. Thomson, M.D.
Renda Soylemez Wiener, M.D., M.P.H.
References
- 1.Tinetti ME, Fried TR, Boyd CM. Designing health care for the most common chronic condition–multimorbidity. JAMA. 2012;307:2493–2494. doi: 10.1001/jama.2012.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kronick RG, Bella M, Gilmer TB. The faces of Medicaid III: refining the portrait of people with multimorbidity. Center for Health Care Strategies Inc.; 2009. [updated 2009 Oct; accessed 2016 Feb 8]. Available from: http://www.chcs.org/media/Faces_of_Medicaid_III.pdf. [Google Scholar]
- 3.Lee TA, Shields AE, Vogeli C, Gibson TB, Woong-Sohn M, Marder WD, Blumenthal D, Weiss KB. Mortality rate in veterans with multiple chronic conditions. J Gen Intern Med. 2007;22:403–407. doi: 10.1007/s11606-007-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Machlin S, Cohen S, Yu W. Health care access and expenditures among non-elderly adults with multimorbidity: variations by insurance coverage status, 2007–8 (average annual) Rockville, MD: Agency for Healthcare Research and Quality; Apr, 2011. (Statistical brief no. 320). [Google Scholar]
- 5.Vogeli C, Shields AE, Lee TA, Gibson TB, Marder WD, Weiss KB, Blumenthal D. Multiple chronic conditions: prevalence, health consequences, and implications for quality, care management, and costs. J Gen Intern Med. 2007;22:391–395. doi: 10.1007/s11606-007-0322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward BW, Schiller JS. Prevalence of multimorbidity among US adults: estimates from the National Health Interview Survey, 2010. Prev Chronic Dis. 2013;10:120203. doi: 10.5888/pcd10.120203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson G. Chronic care: making the case for ongoing care. Princeton: Robert Wood Johnson Foundation; 2010. [Google Scholar]
- 8.Warshaw G. Introduction: advances and challenges in care of older people with chronic illness. Generations. 2006;30:5–10. [Google Scholar]
- 9.Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162:2269–2276. doi: 10.1001/archinte.162.20.2269. [DOI] [PubMed] [Google Scholar]
- 10.Charts and maps. Centers for Medicare and Medicaid Services. [updated 2016 Jan 5; accessed 2016 Feb 8]. Available from: http://www.cms.gov/research-statistics-data-and-systems/statistics-trends-and-reports/chronic-conditions/chartbook_charts.html.
- 11.Chronic conditions among Medicare beneficiaries, chart book: 2012 edition. Baltimore: Centers for Medicare and Medicaid Services; 2012. [Google Scholar]
- 12.U.S. Department of Health and Human Services. Multimorbidity—a strategic framework: optimum health and quality of life for individuals with multimorbidity. Washington, D.C.: U.S. DHHS; 2010. [Google Scholar]
- 13.Lugtenberg M, Burgers JS, Clancy C, Westert GP, Schneider EC. Current guidelines have limited applicability to patients with comorbid conditions: a systematic analysis of evidence-based guidelines. PLoS One. 2011;6:e25987. doi: 10.1371/journal.pone.0025987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294:716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 15.Fabbri LM, Boyd C, Boschetto P, Rabe KF, Buist AS, Yawn B, Leff B, Kent DM, Schünemann HJ, ATS/ERS Ad Hoc Committee on Integrating and Coordinating Efforts in COPD Guideline Development How to integrate multiple comorbidities in guideline development: article 10 in Integrating and coordinating efforts in COPD guideline development: an official ATS/ERS Workshop Report. Proc Am Thorac Soc. 2012;9:274–281. doi: 10.1513/pats.201208-063ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman RA, Boyd C, Tinetti ME, Von Kohorn I, Parekh AK, McGinnis JM. IOM and DHHS meeting on making clinical practice guidelines appropriate for patients with multiple chronic conditions. Ann Fam Med. 2014;12:256–259. doi: 10.1370/afm.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhlig K, Leff B, Kent D, Dy S, Brunnhuber K, Burgers JS, Greenfield S, Guyatt G, High K, Leipzig R, et al. A framework for crafting clinical practice guidelines that are relevant to the care and management of people with multimorbidity. J Gen Intern Med. 2014;29:670–679. doi: 10.1007/s11606-013-2659-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trikalinos TA, Segal JB, Boyd CM. Addressing multimorbidity in evidence integration and synthesis. J Gen Intern Med. 2014;29:661–669. doi: 10.1007/s11606-013-2661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyd CM, Vollenweider D, Puhan MA. Informing evidence-based decision-making for patients with comorbidity: availability of necessary information in clinical trials for chronic diseases. PLoS One. 2012;7:e41601. doi: 10.1371/journal.pone.0041601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fortin M, Dionne J, Pinho G, Gignac J, Almirall J, Lapointe L. Randomized controlled trials: do they have external validity for patients with multiple comorbidities? Ann Fam Med. 2006;4:104–108. doi: 10.1370/afm.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurwitz JH, Col NF, Avorn J. The exclusion of the elderly and women from clinical trials in acute myocardial infarction. JAMA. 1992;268:1417–1422. [PubMed] [Google Scholar]
- 22.Van Spall HG, Toren A, Kiss A, Fowler RA. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA. 2007;297:1233–1240. doi: 10.1001/jama.297.11.1233. [DOI] [PubMed] [Google Scholar]
- 23.Zulman DM, Sussman JB, Chen X, Cigolle CT, Blaum CS, Hayward RA. Examining the evidence: a systematic review of the inclusion and analysis of older adults in randomized controlled trials. J Gen Intern Med. 2011;26:783–790. doi: 10.1007/s11606-010-1629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jadad AR, To MJ, Emara M, Jones J. Consideration of multiple chronic diseases in randomized controlled trials. JAMA. 2011;306:2670–2672. doi: 10.1001/jama.2011.1886. [DOI] [PubMed] [Google Scholar]
- 25.Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21:1575–1600. doi: 10.1002/sim.1188. [DOI] [PubMed] [Google Scholar]
- 26.Engels EA, Schmid CH, Terrin N, Olkin I, Lau J. Heterogeneity and statistical significance in meta-analysis: an empirical study of 125 meta-analyses. Stat Med. 2000;19:1707–1728. doi: 10.1002/1097-0258(20000715)19:13<1707::aid-sim491>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 27.Yu T, Fain K, Boyd CM, Singh S, Weiss CO, Li T, Varadhan R, Puhan MA. Benefits and harms of roflumilast in moderate to severe COPD. Thorax. 2014;69:616–622. doi: 10.1136/thoraxjnl-2013-204155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prieto-Centurion V, Rolle AJ, Au DH, Carson SS, Henderson AG, Lee TA, Lindenauer PK, McBurnie MA, Mularski RA, Naureckas ET, et al. CONCERT Consortium Multicenter study comparing case definitions used to identify patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014;190:989–995. doi: 10.1164/rccm.201406-1166OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travers J, Marsh S, Caldwell B, Williams M, Aldington S, Weatherall M, Shirtcliffe P, Beasley R. External validity of randomized controlled trials in COPD. Respir Med. 2007;101:1313–1320. doi: 10.1016/j.rmed.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Sun X, Ioannidis JP, Agoritsas T, Alba AC, Guyatt G. How to use a subgroup analysis: users’ guide to the medical literature. JAMA. 2014;311:405–411. doi: 10.1001/jama.2013.285063. [DOI] [PubMed] [Google Scholar]
- 31.Rosenfeld RM, Shiffman RN, Robertson P, Department of Otolaryngology State University of New York Downstate Clinical practice guideline development manual, third edition: a quality-driven approach for translating evidence into action. Otolaryngol Head Neck Surg. 2013;148(suppl 1):S1–S55. doi: 10.1177/0194599812467004. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Kumar KA, Kramper M, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. doi: 10.1177/0194599815572097. In press. [DOI] [PubMed] [Google Scholar]
- 33.Arnett DK, Goodman RA, Halperin JL, Anderson JL, Parekh AK, Zoghbi WA. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and US Department of Health and Human Services. Circulation. 2014;130:1662–1667. doi: 10.1161/CIR.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, et al. Writing Committee Members; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. doi: 10.1161/CIR.0b013e31829e8776. [DOI] [PubMed] [Google Scholar]
- 35.Lyman GH, Temin S, Edge SB, Newman LA, Turner RR, Weaver DL, Benson AB, Bosserman LD, Burstein JH, Cody H, III, et al. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol. 2014;32:1365–1383. doi: 10.1200/JCO.2013.54.1177. [DOI] [PubMed] [Google Scholar]
- 36.The Global Strategy for the Diagnosis, Management, and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2016 doi: 10.3760/cma.j.issn.0376-2491.2016.34.001. [updated 2015 Dec 15; accessed 2016 Feb 8]. Available from: http://www.goldcopd.org/ [DOI] [PubMed]


