Abstract
Aims
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors considerably alter the lipid profile. We sought to examine the rates of ischaemic stroke and neurocognitive deficits in patients treated with and without PCSK9 inhibitors.
Methods and results
Randomized controlled trials (RCTs) reporting rates of ischaemic stroke and neurocognitive deficits in patients using PCSK9 inhibitors were identified. Standard meta-analysis techniques were used to compare these outcomes among patients treated with and without PCSK9 inhibitors and the two US Food and Drug Administration-approved PCSK9 inhibitors, evolocumab and alirocumab. The results were presented in terms of risk ratio (RR) with 95% confidence intervals (CIs). Sixteen RCTs with 39 104 patients were included. Evolocumab was used in six RCTs with 33 450 patients, whereas alirocumab was used in 10 RCTs with 5654 patients. We observed a significantly lower risk of ischaemic stroke among those treated with PCSK9 inhibitors (RR 0.77, 95% CI 0.64–0.93) when compared with those without. We did not observe any difference in the risk of neurocognitive deficits between the aforementioned groups (RR 1.11, 95% CI 0.93–1.32). The lower stroke risk in the PCSK9 inhibitors group was driven by evolocumab studies. We observed no difference in the risk of neurocognitive deficits among evolocumab and alirocumab when compared with no PCSK9 inhibitors group.
Conclusion
Treatment with PCSK9 inhibitors significantly lowers the risk of ischaemic stroke, without any increased risk of neurocognitive deficits. PCSK9 inhibitors are neuroprotective due to the decrease in ischaemic-mediated neurovascular events and should be considered cognitively innocuous medications.
Keywords: PCSK9 , Cognition , Stroke , Meta-analysis
Introduction
Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors have recently been approved for the treatment of hypercholesterolaemia.1,2 PCSK9 inhibitors dramatically lower LDL cholesterol levels by up to 62% compared with placebo within 6–12 weeks.3–5 Lower LDL cholesterol levels are associated with a lower incidence of cardiovascular events,6 including ischaemic stroke,6,7 which is the crux of the ‘cholesterol hypothesis’. However, only one recently conducted randomized controlled trial (RCT) has shown lower incidence of ischaemic stroke in patients treated with PCSK9 inhibitors.8 Furthermore, one RCT has reported a higher incidence of neurocognitive deficits in patients receiving PCSK9 inhibitors when compared with standard therapy,9 whereas other RCTs did not show any increase in neurocognitive deficits in patients receiving these agents.5,8,10–17 Prior meta-analyses have contributed to the notion that PCSK9 inhibitors are ‘neurologically unsafe’ due to the increase in the incidence of neurocognitive adverse events with use of these inhibitors when compared with the controls.18,19
Given the variability in the reported rates of neurological outcomes of PCSK9 inhibitors, we conducted this investigation to assess the effect of these agents on ischaemic stroke and neurocognitive deficits. Due to lack of comparative data between the effects of alirocumab and evolocumab on neurocognition, we conducted post hoc subgroup meta-regression analyses to explore the association of class of antibody with the observed treatment effect on neurocognitive deficits.
Methods
Study inclusion and exclusion criteria
All RCTs published between January 1966 and March 2017 reporting any neurological effects in patients receiving the US Food and Drug Administration (US-FDA)-approved PCSK9 inhibitors were eligible for inclusion.1,2 The research study was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension statement for meta-analyses (see Supplementary material online, Table S1).20 The primary outcome of interest for our study was ischaemic stroke during follow-up. The secondary outcomes of interest were neurocognitive deficits. The definitions of these outcomes in the RCTs included in our analyses are presented in Supplementary material online, Tables S2 and S3.
Search strategy and quality assessment
Two authors (A.V. and N.P.) conducted an electronic literature search in SCOPUS. SCOPUS includes Medline, Embase, Compendex, World Textile Index, Fluidex, Geobase, and Biobase.21 The keywords and search strategy are listed in see Supplementary material online, Section S1. One author (N.S.B.) verified the search results. We included all English language human studies. The abstracts were reviewed, and potential studies were identified. Full-text publications of these relevant studies were retrieved for detailed review. In the event of multiple publications from the same population and/or author, the study with the most complete data set was included. We reviewed the reference lists of relevant studies, in addition to clinicaltrials.org to identify other eligible studies. The Phase II and Phase III trials that contributed to larger Phase III trials (OSLER programme) were excluded. The trials reporting neither ischaemic stroke nor neurocognitive deficits were excluded. One author (N.P.) assessed the data quality of the included studies using the Jadad Score. Another author (R.K.) then independently assessed study scoring. The Jadad score is a tool that can be used to determine the quality of a study through examination of the methods of randomization, double blinding, and the number of dropouts.22 A higher score indicates a higher quality study.22 All inconsistencies were resolved by consensus among authors in weekly meetings.
Statistical analysis
Statistical analyses were performed using Stata 14.1 MP (Stata Corp., College Station, TX, USA) and CMA. A random-effects model was used to estimate the summary measures of treatment effects. Results were presented as risk ratios (RRs) with 95% confidence intervals (CIs). The I2 statistic for heterogeneity, as proposed by Higgins and Thompson, was used to estimate heterogeneity among treatment effects in traditional meta-analyses.23 Publication bias was assessed using Egger’s regression intercept for the primary outcome comparison.24 Subgroup analyses were conducted by the PCSK9 inhibitors type, i.e. evolocumab or alirocumab compared with no PCSK9 inhibitors. Exploratory meta-regression analysis using mixed-effects regression model (unrestricted maximum likelihood model) was used to assess the effect of duration of trial, baseline LDL levels, and change in LDL levels in PCSK9 inhibitors on the treatment effect.
Results
Randomized controlled trials stratified by phases (Phases I, II, and III) and antibody class (evolocumab, alirocumab, and bococizumab) published till date are represented in Figure 1. Of these publications, we found 16 RCTs8–17,25–27 with 39 104 patients eligible for the analyses (Figure 2 and Table 1). All RCTs included in our final analyses were Phase III trials.8–17,25–27 Baseline characteristics of included studies and patients are presented in Table 1. The mean/median age of patients of RCTs included in our analysis ranged from 31 years to 63 years. We compared two groups: the PCSK9 inhibitors group (both evolocumab and alirocumab) with the no PCSK9 inhibitors group (any of the four treatment strategies: placebo, placebo and ezetimibe, ezetimibe, and standard therapy), the details of which are listed in Table 1. All included studies were categorized as good to excellent, based on the Jadad scale (see Supplementary material online, Table S4).
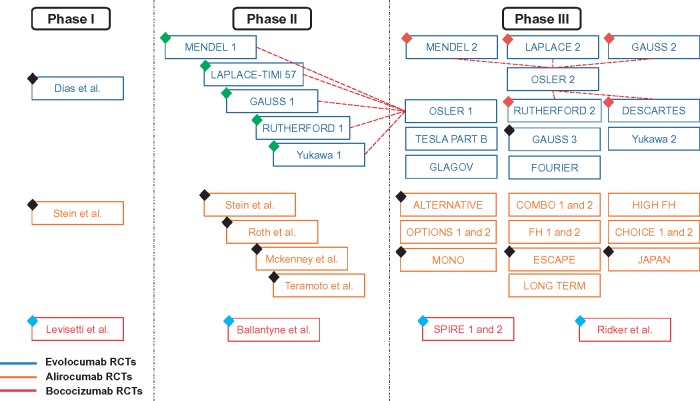
Figure 1.
Randomized controlled trials on PCSK9 inhibitors. Black diamond represents studies lacking desired outcomes; blue diamond represents studies using non-US-FDA-approved PCSK9 inhibitors; green diamond represents studies with overlapping population with OSLER 1 study; and maroon diamond represents studies with overlapping population with OSLER 2 study.
Figure 2.
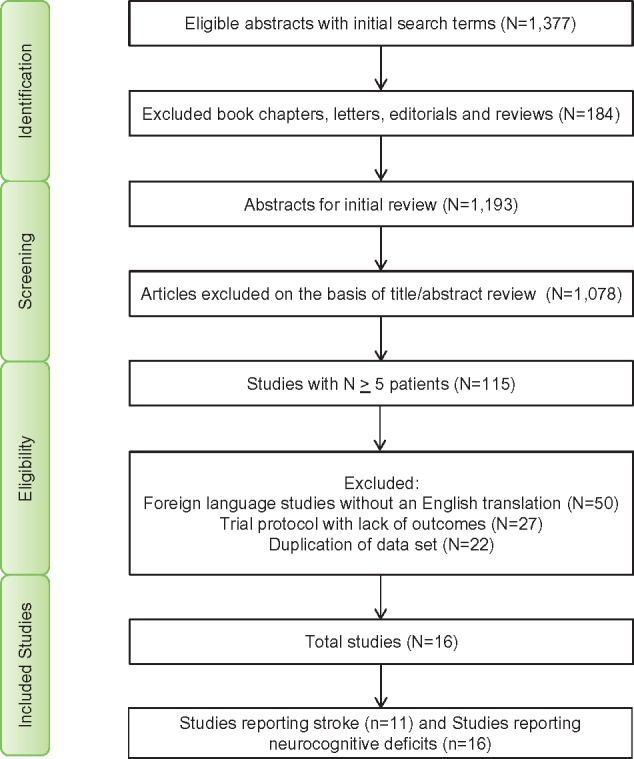
Flow diagram for study selection.
Table 1.
Baseline characteristics of included trials
| Study name/first author and year of publication | Type of PCSK9 inhibitor | Treatment in no PCSK9 inhibitor | PCSK9 inhibitor (n) | No PCSK9 inhibitor (n) | Agea (years) | Male (n) | Hypertension (percentage of patients) | Diabetes mellitus (percentage of patients) | Median follow-up duration (weeks) | Baseline statin usage (percentage of patients) |
|---|---|---|---|---|---|---|---|---|---|---|
| FOURIER/Sabatine et al.8/2017 | Evolocumab 140 mg Q2W; 420 mg Q4W + optimized lipid-lowering regimen | SC placebo Q2W or Q4W + optimized lipid-lowering regimen | 13 784 | 13 780 | 63 ± 9 | 20 795 | 80 | 36 | 114 | 100 |
| GLAGOV/Nicholls et al.15/2016 | Evolocumab 420 mg Q4W | SC placebo Q4W | 484 | 484 | 60 ± 9 | 698 | 82 | 21 | 78 | 99 |
| ODYSSEY CHOICE 1/Roth et al.16/2016 | Alirocumab 75 mg Q2W; 300 mg Q4W ± statin | SC Placebo Q2W and Q4W ± statin | 573 | 230 | 61 ± 10 | 462 | NR | 27 | 48 | 68 |
| ODYSSEY CHOICE 2/Stroes et al.17/2016 | Alirocumab 75 mg Q2W; 150 mg Q4W ± statin | SC placebo Q2W | 175 | 58 | 63 ± 10 | 130 | 61 | 16 | 24 | 0 |
| ODYSSEY COMBO 1/Kereiakes et al.14/2015 | Alirocumab 75 mg/150 mg Q2W + Max. tolerated statin + other lipid lowering therapy | SC Placebo Q2W + Max. tolerated statin + other lipid-lowering therapy | 209 | 107 | 63 ± 9 | 208 | NR | 43 | 52 | 100 |
| ODYSSEY COMBO 2/Cannon et al.10/2015 | Alirocumab 75 mg Q2W + oral placebo + statin | Ezetimibe + SC placebo Q2W + statin | 479 | 241 | 62 ± 9 | 530 | NR | 31 | 52 | 100 |
| ODYSSEY FH 1/Kastelein et al.13/2015 | Alirocumab 75 mg/150 mg Q2W + Max. tolerated lipid lowering therapy | SC placebo Q2W + lipid-lowering therapy | 323 | 163 | 52 ± 13 | 274 | 43 | 12 | 78 | 100 |
| ODYSSEY FH 2/Kastelein et al.13/2015 | Alirocumab 75 mg/150 mg Q2W + Max. tolerated lipid lowering therapy | SC placebo Q2W + lipid-lowering therapy | 167 | 82 | 53 ± 13 | 131 | 33 | 4 | 78 | 100 |
| ODYSSEY HIGH FH/Ginsberg et al.12/2016 | Alirocumab 150mg Q2W + Max. tolerated lipid-lowering therapy | SC Placebo Q2W + lipid-lowering therapy | 72 | 35 | 51 ± 13 | 57 | 57 | 14 | 78 | 100 |
| ODYSSEY LONG TERM/Robinson et al.5/2015 | Alirocumab 150 mg Q2W + statin ± other lipid-lowering therapy | SC Placebo Q2W + statin ± other lipid-lowering therapy | 1553 | 788 | 61 ± 10 | 1457 | NR | 35 | 78 | 100 |
| ODYSSEY OPTIONS 1/Bays et al.25/2015 | Alirocumab 75/150 mg Q2W + atorvastatin | Ezetimibe + atorvastatin | 104 | 102 | 63 ± 10 | 231 | 79 | 50 | 32 | 100 |
| ODYSSEY OPTIONS 2/Farnier et al.11/2015 | Alirocumab 75 mg/150 mg Q2W + rosuvastatin | Ezetimibe + rosuvastatin + SC placebo Q2W | 103 | 101 | 61 ± 10 | 187 | 71 | 39 | 32 | 79 |
| OSLER 1 and OSLER 2/Sabatine et al.9/2015 | Evolocumab 140 mg Q2W; 420 mg Q4W + standard therapy | Standard therapy | 2976 | 1489 | 58 ± 11 | 2255 | 52 | 13 | 48 | 70 |
| TESLA/Raal et al.27/2014 | Evolocumab 420 mg Q4W | SC placebo Q4W | 33 | 16 | 31 ± 13 | 25 | 10 | 6 | 12 | 100 |
| Yukawa 2/Kiyosue et al.26/2016 | Evolocumab 140 mg Q2W; 420 mg Q4W + atorvastatin therapy | Placebo Q2W; Q4W + atorvastatin | 202 | 202 | 62 ± 11 | 244 | 74 | 49 | 12 | 100 |
Max., maximum; n, number of patients; NR, not reported; PCSK9, proprotein convertase subtilisin/kexin type 9; Q1–Q3, interquartile range; Q2W, every 2 weeks; Q4W, every 4 weeks; SD, standard deviation; SC, subcutaneous.
aValues are mean ± SD; median (Q1–Q3).
Comparison of neurological outcomes among those treated with PCSK9 inhibitors vs. no PCSK9 inhibitors
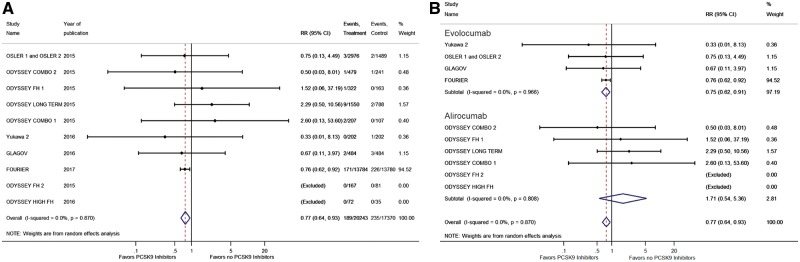
The incidence of ischaemic stroke was reported in 11 RCTs with 37 613 patients.5,8–10,12–15,26 We observed a significantly lower risk of ischaemic stroke among those treated with PCSK9 inhibitors when compared with those without (RR 0.77, 95% CI 0.64–0.93; Figure 3A). This effect was mainly driven by the recently reported FOURIER Trial.8
Figure 3.
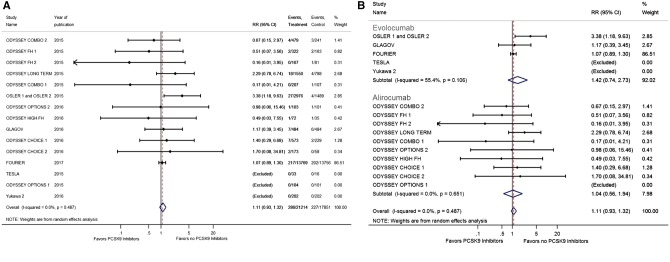
(A) Forest plot comparing the incidence of ischaemic stroke between PCSK9 inhibitor and the no PCSK9 inhibitor groups. (B) Forest plot comparing the incidence of ischaemic stroke between PCSK9 inhibitor and the no PCSK9 inhibitor groups in subgroups. The blue diamond is the point estimate representing the 95% confidence interval. The red dotted lines represent the random effects generated overall estimate. Data are presented with risk ratios and 95% confidence intervals. RR: risk ratio, PCSK9: proprotein convertase subtilisin/kexin type 9.
The incidence of neurocognitive deficits was reported in 16 RCTs with 39 065 patients.8–17,25–27 We did not observe any difference in the incidence of neurocognitive deficits when we compared the PCSK9 inhibitors and the no PCSK9 inhibitors groups (RR 1.11, 95% CI 0.93–1.32; Figure 4A).
Figure 4.
(A) Forest plot comparing the incidence of neurocognitive deficits between PCSK9 inhibitor and the no PCSK9 inhibitor groups. (B) Forest plot comparing the incidence of neurocognitive deficits between PCSK9 inhibitor and the no PCSK9 inhibitor groups in subgroups. The blue diamond is the point estimate representing the 95% confidence interval. The red dotted lines represent the random effects generated overall estimate. Data are presented with risk ratios and 95% confidence intervals. RR: risk ratio, PCSK9: proprotein convertase subtilisin/kexin type 9.
Subgroup comparison of evolocumab and alirocumab vs. no PCSK9 inhibitors
Evolocumab was used in six RCTs with a total of 33 450 enrolled patients,8,9,15,26,27 whereas alirocumab was used in 10 RCTs with a total of 5654 enrolled patients.5,10–14,16,17,25 We observed that evolucumab had lower risk of stroke when compared with no PCSK9 inhibitors (RR 0.75, 95% CI 0.62–0.91), and we did not observe any difference in the risk of ischaemic stroke between the alirocumab and no PCSK9 inhibitors (RR 1.71, 95% CI 0.64–5.36) (Figure 3B). No difference was observed in the risk of neurocognitive deficits between evolocumab (RR 1.42, 95% CI 0.74–2.73) and alirocumab (RR 1.04, 95% CI 0.56–1.94) compared with no PCKS9 inhibitors (Figure 4B).
Publication bias assessment
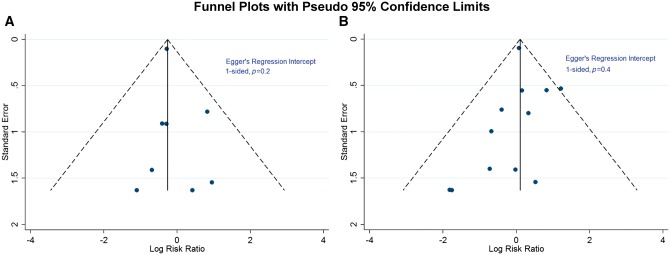
We constructed a funnel plot of RCTs reporting ischaemic stroke and neurocognitive deficits (Figure 5A and B) to assess for any publication bias for either outcome. The studies were distributed symmetrically around the estimated pooled effect, with Egger’s weighted regression statistic (one sided, P = 0.2 for ischaemic stroke and P = 0.4 for neurocognitive deficits), indicating no significant publication bias for either outcome.
Figure 5.
The funnel plot of studies reporting ischaemic stroke (A) and neurocognitive deficits (B). The solid line represents the summary effect line. The dashed lines represent the expected 95% confidence interval around summary estimate. The dark blue circles represent individual studies.
Meta-regression
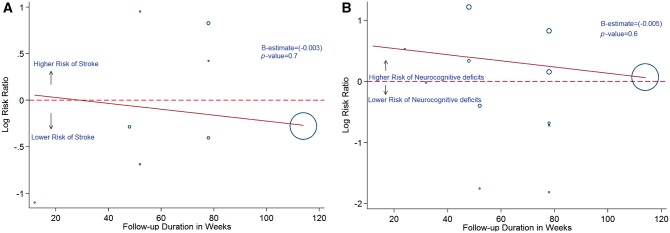
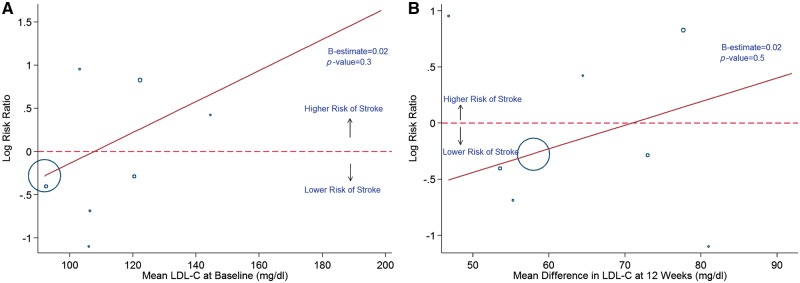
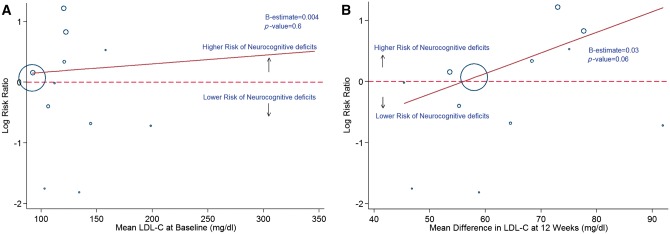
In a meta-regression analyses, we found that the duration of RCT (Figure 6A and B), baseline LDL (Figure 7A and B), and change in LDL (Figure 8A and B) did not have a statistically significant association with the observed effects on ischaemic stroke and neurocognitive deficits, with P > 0.05 for all.
Figure 6.
Meta-regression analysis showing the relationship between incidence of stroke (A) and neurocognitive deficits (B) with duration in weeks. Blue hollow circles represents individual study. Solid red line represents trend line.
Figure 7.
Meta-regression analysis showing the relationship between incidence of stroke with baseline (A) and 12 weeks (B) LDL-C levels. Blue hollow circles represents individual study. Solid red line represents trend line.
Figure 8.
Meta-regression analysis showing the relationship between incidence of neurocognitive deficits with baseline (A) and 12 weeks (B) LDL-C levels. Blue hollow circles represent individual study. Solid red line represents trend line.
Discussion
Our analyses highlight the neurological efficacy and safety of the US-FDA-approved PCSK9 inhibitors.1,2 We observed that the rates of ischaemic stroke were lower in patients treated with PCSK9 inhibitors. However, the rates of neurocognitive deficits were similar and not increased in patients treated with PCSK9 inhibitors. We also observed no effect of duration of trial, baseline LDL levels, and change in LDL levels with PCSK9 inhibitors on the neurological outcomes. Despite previous reports from epidemiological associations that lower LDL cholesterol levels led to a worse neurocognitive status,28 our findings demonstrate no increase in neurocognitive deficits with the use of PCSK9 inhibitors. To the best of our knowledge, our analysis is the first and the largest to systematically examine the literature for the use of PCSK9 inhibitors and present comparisons between the two PCSK9 inhibitors, with a primary focus on neurological outcomes.
There may be several mechanistic explanations for the ischaemic stroke reduction witnessed in our investigation. As LDL has been known to play a causal role in atherosclerotic events, it follows that aggressive reduction of LDL levels reduces incidence of cardiovascular events.29 This seems to be the most direct explanation and well-accepted reasoning for the reduction of ischaemic stroke as well. This LDL reduction effect is undoubtedly magnified by the PSKC9 inhibition to counter the PCSK9 gene up-regulation that statins are known to cause.30 Treatment with PCSK9 inhibitors also seems to offer additional notable improvements in other components of the lipid profile. This includes improvement in HDL and reductions in apolipoprotein B and lipoprotein A to unprecedented levels. Given the emerging data linking lipoprotein A to the increased development of atherosclerotic disease,31,32 reductions in these other lipids may be an important additional mechanism through which PCSK9 inhibitors reduce the incidence of atherosclerotic disease. We also acknowledge that the stroke reduction was largely driven by the results of the FOURIER trial.8
The absence of differences in neurocognitive deficits in our meta-analyses can be explained by the following observations. Only two studies in our meta-analysis, OSLER 1 and 2 and ODYSSEY LONG TERM,9,14 noted three-fold and two-fold higher risk of neurocognitive deficits, respectively, among those treated with PCSK9 inhibitors. All other studies either showed reduction or neutral effects of PCSK9 inhibitors on neurocognitive deficits.8,10–17 One potential explanation is the lack of standardized cognitive testing and reliance on patient’s self-reporting of what constituted as a neurocognitive deficit. This may have led to a reporting bias contributing to the increase in neurocognitive deficits noted in the earlier studies especially in the OSLER studies which were an open-label trial.9 In comparison, the FOURIER trial did not notice any increase in neurocognitive events.8 Concordant to our pooled results were the null observations from the EBBINGHAUS33 (NCT02207634), a sub-study of the FOURIER trial that had formal assessment of neurocognition using a cognitive assessment tool followed by an independent adjudication of all neurocognitive outcomes.8 The results of our meta-analysis are also consistent with the genetic studies of patients with loss of function of PCSK9, with no increase in the incidence of cognitive events.34,35 Having said that, we would like to acknowledge the observation that the antibodies produced against alirocumab as noticed in ODYSSEY LONG TERM trial (which noted two-fold higher neurological deficits) are thought to play a ‘functional’ role in causing the adverse neurological outcomes with alirocumab usage.14 If there is a physiological explanation, we posit that this may be a legitimate theory. This is particularly relevant in view of the recent discontinuation of bococizumab drug development due to the same issue.36 Other mechanisms have also been hypothesized such as detection bias rather than a causal association.37 This area clearly needs to be examined further before additional causal explanations can be offered.
Our investigation offers an interesting contrast to the prior investigations pertaining to stroke reduction with PCSK9 inhibitors. Milionis et al.38 have published a meta-analysis article evaluating the rates of ischaemic stroke, by pooling two studies, ODYSSEY LONG TERM and OSLER.9,14 Their investigation concludes that the incidence of ischaemic strokes was similar in patients who were treated with PCSK9 inhibitors and patients who were not treated with PCSK9 inhibitors.38 This investigation is limited by the lack of a systematic search strategy.38 Consequently, there are several missing studies, and the Milionis et al.38 investigation does not capture the totality of the published literature, making their investigation prone to significant publication bias. Khan et al.18 also published a meta-analysis evaluating the rates of ischaemic stroke with the use of PCSK9 inhibitors. The authors have divided the studies into two groups, early Phase II–III studies and long-term studies, and concluded that the incidence of ischaemic strokes were similar.18 Both of these investigations also lack the cohorts included in the FOURIER/EBBINGHAUS and GLAGOV trials.8,15
Our findings also add to the existing literature on neurocognitive deficits associated with PCSK9 inhibitors. Lipinski et al.,19 Robinson et al.,39 and Khan et al.18 published the meta-analyses that estimated the rates of neurocognitive deficits with the use of PCSK9 inhibitors, albeit in a subgroup fashion. Lipinski et al. demonstrated a significant increase in neurocognitive deficits with the use of PCSK9 inhibitors. The discrepancy in findings between our study and Lipinksi et al.19 relates primarily to the number of studies evaluated. Lipinski et al.19 evaluated six studies with 9581 patients,4,9,14,40,41 whereas ours included 13 additional studies (excluding studies with overlapping population) with a total number of 31 948 patients.8,10–13,15–17,25–27 The point estimate of their pooled results19 were primarily driven by the ODYSSEY LONG TERM and OSLER studies.9,14 However, with the data available from the additional RCTs that we included in our meta-analyses,8,10–13,15–17,25–27 we found that there is no increase in the pooled rates of neurocognitive deficits with the use of PCSK9 inhibitors. Khan et al.18 divided the available studies into two groups, early Phase II and Phase III studies and outcome studies, and concluded that there is an increase in neurocognitive deficits with larger sample size and longer follow-up. However, their findings were also primarily driven by the ODYSSEY LONG TERM and OSLER studies.9,14 In a recent article, patient-level pooled data were reported from 14 RCTs included in the ODYSSEY programme.39 Concordant to our findings, the authors reported no differences in the rates of neurocognitive effects between the alirocumab and the control groups. Furthermore, no increase in neurocognitive disorders were reported even at LDL levels <25 mg/dL.39 Having said that, none of the previously published meta-analyses used the two US-FDA-approved antibodies alirocumab and evolocumab in subgroups, to evaluate their neurological efficacy and safety, as we did.18,19,38,39 Once again, our investigation also includes the contemporary literature.8,15
The results of our investigation also have notable implications for clinical practice, public health, and future research. The absence of increased neurocognitive deficits with the use of PCSK9 inhibitors may aid clinicians in making therapeutic decisions while treating patients who require PCSK9 inhibitors from a cardiovascular standpoint. Our investigation also highlights the importance of conducting larger studies to directly assess the comparative efficacy and safety of alirocumab and evolocumab. We anticipate that the results of the ODYSSEY OUTCOMES trial (ClinicalTrials.gov, NCT01663402), which aims to address the long-term effects of alirocumab on cardiovascular outcomes and ischaemic stroke risk in a cohort of 18 000 patients over the course of 5 years, will form a greater evidence base to evaluate the neurological efficacy and safety profiles of PCSK9 inhibitors. Finally, the health care costs associated with PCSK9 inhibitors are difficult to ignore, despite their remarkable efficacy. The PCSK9 inhibitors were evaluated through a thorough cost–benefit analysis and that needs to be weighed in prior to using them in clinical practice.42
Our study has several limitations. Limitations of meta-analyses are, in general, well recognized.43 We note that our findings were only valid in the relatively younger population included in these RCTs and need to be corroborated in elderly individuals prior to the widespread use of these drugs. Further limitations of our analysis include the pooling of different drugs, dosages, and duration of treatment. However, variability in efficacy and safety outcomes was not apparent among individual studies, despite different doses and durations, suggesting that these variations are less likely to affect the validity of our results. Although our meta-regression analyses did not demonstrate any effect of duration on neurological outcomes, the included patients and the study characteristics among the included RCTs were different. We attempted to temporize this by utilizing random-effects modelling rather than fixed-effects modelling to ensure conservative effect estimates. Finally, many of the studies included in these meta-analyses were not primarily designed to evaluate neurological outcomes. Hence, the results of our meta-analyses should be seen as hypothesis generating.
Conclusions
In summary, PCSK9 inhibitors are a novel class of drugs that result in substantial alterations in lipid profiles. In our meta-analyses, we found that they reduce the incidence of ischaemic stroke. We have not found any evidence of increased neurocognitive deficits with the use of PCSK9 inhibitors, as previously reported. Ongoing studies will help to elucidate the long-term neurological efficacy and safety outcomes of these drugs.
Supplementary material
Supplementary material is available at European Heart Journal—Quality of Care and Clinical Outcomes online.
Funding
Walter B. Frommeyer, Junior Fellowship in Investigative Medicine by the University of Alabama at Birmingham to P.A.; and National Institutes of Health (NIH) grant (5T32HL094301-07) to N.S. B. National Institutes of Health (NIH) grant (1T32HL129948-01A1) to N.P.
Conflict of interest: P.A. has served as an institutional co-investigator on FOURIER and ODYSSEY trials, but has not received any grant or assay support from the sponsor of these trials. All other authors have no conflicts of interest or financial disclosure to disclose.
Supplementary Material
References
- 1. FDA approves Repatha to treat certain patients with high cholesterol. U.S. Food and Drug Administration. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm460082.htm (27 March 2017).
- 2. FDA approves Praluent to treat certain patients with high cholesterol. U.S. Food and Drug Administration. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm455883.htm (27 March 2017).
- 3. Everett BM, Smith RJ, Hiatt WR.. Reducing LDL with PCSK9 inhibitors—the clinical benefit of lipid drugs. N Engl J Med 2015;373:1588–1591. [DOI] [PubMed] [Google Scholar]
- 4. Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, Hovingh GK, Cariou B, Gouni-Berthold I, Somaratne R, Bridges I, Scott R, Wasserman SM, Gaudet D.. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD-2): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:331–340. [DOI] [PubMed] [Google Scholar]
- 5. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ.. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 6. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM.. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 7. Amarenco P, Labreuche J.. Lipid management in the prevention of stroke: review and updated meta-analysis of statins for stroke prevention. Lancet Neurol 2009;8:453–463. [DOI] [PubMed] [Google Scholar]
- 8. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR.. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 9. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA.. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 10. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM.. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J 2015;36:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farnier M, Jones P, Severance R, Averna M, Steinhagen-Thiessen E, Colhoun HM, Du Y, Hanotin C, Donahue S.. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular-risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis 2016;244:138–146. [DOI] [PubMed] [Google Scholar]
- 12. Ginsberg HN, Rader DJ, Raal FJ, Guyton JR, Baccara-Dinet MT, Lorenzato C, Pordy R, Stroes E.. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL-C of 160 mg/dl or higher. Cardiovasc Drugs Ther 2016;30:473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, Blom D, Civeira F, Krempf M, Lorenzato C, Zhao J, Pordy R, Baccara-Dinet MT, Gipe DA, Geiger MJ, Farnier M.. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015;36:2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM.. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J 2015;169:906–915.e13. [DOI] [PubMed] [Google Scholar]
- 15. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE.. Effect of evolocumab on progression of coronary disease in statin-treated patients: the glagov randomized clinical trial. JAMA 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 16. Roth EM, Moriarty PM, Bergeron J, Langslet G, Manvelian G, Zhao J, Baccara-Dinet MT, Rader DJ.. A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add-on to statin: ODYSSEY CHOICE I. Atherosclerosis 2016;254:254–262. [DOI] [PubMed] [Google Scholar]
- 17. Stroes E, Guyton JR, Lepor N, Civeira F, Gaudet D, Watts GF, Baccara-Dinet MT, Lecorps G, Manvelian G, Farnier M.. Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II study. J Am Heart Assoc 2016;5:e003421.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khan AR, Bavishi C, Riaz H, Farid TA, Khan S, Atlas M, Hirsch G, Ikram S, Bolli R.. Increased risk of adverse neurocognitive outcomes with proprotein convertase subtilisin-kexin Type 9 inhibitors. Circ Cardiovasc Qual Outcomes 2017;10:e003153.. [DOI] [PubMed] [Google Scholar]
- 19. Lipinski MJ, Benedetto U, Escarcega RO, Biondi-Zoccai G, Lhermusier T, Baker NC, Torguson R, Brewer HB Jr, Waksman R.. The impact of proprotein convertase subtilisin-kexin type 9 serine protease inhibitors on lipid levels and outcomes in patients with primary hypercholesterolaemia: a network meta-analysis. Eur Heart J 2016;37:536–545. [DOI] [PubMed] [Google Scholar]
- 20. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D.. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med 2009;6:e1000100.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Falagas ME, Pitsouni EI, Malietzis GA, Pappas G.. Comparison of PubMed, Scopus, Web of Science, and Google Scholar: strengths and weaknesses. FASEB J 2007;22:338–342. [DOI] [PubMed] [Google Scholar]
- 22. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ.. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JP, Thompson SG.. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 24. Egger M, Davey Smith G, Schneider M, Minder C.. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni-Berthold I, Robinson J, Zhao J, Hanotin C, Donahue S.. Alirocumab as add-on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab 2015;100:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kiyosue A, Honarpour N, Kurtz C, Xue A, Wasserman SM, Hirayama A.. A phase 3 study of evolocumab (AMG 145) in statin-treated japanese patients at high cardiovascular risk. Am J Cardiol 2016;117:40–47. [DOI] [PubMed] [Google Scholar]
- 27. Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA.. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:341–350. [DOI] [PubMed] [Google Scholar]
- 28. Elias PK, Elias MF, D’Agostino RB, Sullivan LM, Wolf PA.. Serum cholesterol and cognitive performance in the Framingham Heart Study. Psychosom Med 2005;67:24–30. [DOI] [PubMed] [Google Scholar]
- 29. Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ.. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227–239. [DOI] [PubMed] [Google Scholar]
- 30. Dubuc G, Chamberland A, Wassef H, Davignon J, Seidah NG, Bernier L, Prat A.. Statins upregulate PCSK9, the gene encoding the proprotein convertase neural apoptosis-regulated convertase-1 implicated in familial hypercholesterolemia. Arterioscler Thromb Vasc Biol 2004;24:1454–1459. [DOI] [PubMed] [Google Scholar]
- 31. Ohira T, Schreiner PJ, Morrisett JD, Chambless LE, Rosamond WD, Folsom AR.. Lipoprotein(a) and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke 2006;37:1407–1412. [DOI] [PubMed] [Google Scholar]
- 32. Price JF, Lee AJ, Rumley A, Lowe GD, Fowkes FG.. Lipoprotein (a) and development of intermittent claudication and major cardiovascular events in men and women: the Edinburgh Artery Study. Atherosclerosis 2001;157:241–249. [DOI] [PubMed] [Google Scholar]
- 33. Giugliano RP, Mach F, Zavitz K, Kurtz C, Im K, Kanevsky E, Schneider H, Keech A, Pedersen TR, Sabatine MS, Sever PS, Robinson JG, Honarpour N, Wasserman SM, Ott BR.. Cognitive Function in a Randomized Trial of Evolocumab. N Engl J Med 2013;377:633–643. [DOI] [PubMed] [Google Scholar]
- 34. Postmus I, Trompet S, de Craen AJ, Buckley BM, Ford I, Stott DJ, Sattar N, Slagboom PE, Westendorp RG, Jukema JW.. PCSK9 SNP rs11591147 is associated with low cholesterol levels but not with cognitive performance or noncardiovascular clinical events in an elderly population. J Lipid Res 2013;54:561–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Swiger KJ, Martin SS.. PCSK9 inhibitors and neurocognitive adverse events: exploring the FDA directive and a proposal for N-of-1 trials. Drug Saf 2015;38:519–526. [DOI] [PubMed] [Google Scholar]
- 36. Roth EM, Goldberg AC, Catapano AL, Torri A, Yancopoulos GD, Stahl N, Brunet A, Lecorps G, Colhoun HM.. Antidrug antibodies in patients treated with alirocumab. N Engl J Med 2017;376:1589–1590. [DOI] [PubMed] [Google Scholar]
- 37. Banach M, Rizzo M, Nikolic D, Howard G, Howard V, Mikhailidis D.. Intensive LDL-cholesterol lowering therapy and neurocognitive function. Pharmacol Ther 2017;170:181–191. [DOI] [PubMed] [Google Scholar]
- 38. Milionis H, Barkas F, Ntaios G, Papavasileiou V, Vemmos K, Michel P, Elisaf M.. Proprotein convertase subtilisin kexin 9 (PCSK9) inhibitors to treat hypercholesterolemia: effect on stroke risk. European Journal of Internal Medicine 2016;34:54–57. [DOI] [PubMed] [Google Scholar]
- 39. Robinson JG, Rosenson RS, Farnier M, Chaudhari U, Sasiela WJ, Merlet L, Miller K, Kastelein JJ.. Safety of very low low-density lipoprotein cholesterol levels with alirocumab: pooled data from randomized trials. J Am Coll Cardiol 2017;69:471–482. [DOI] [PubMed] [Google Scholar]
- 40. Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, Kim JB, Scott R, Wasserman SM, Bays H.. Anti-PCSK9 monotherapy for hypercholesterolemia: the MENDEL-2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol 2014;63:2531–2540. [DOI] [PubMed] [Google Scholar]
- 41. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R.. Effect of evolocumab or ezetimibe added to moderate- or high-intensity statin therapy on LDL-C lowering in patients with hypercholesterolemia: the LAPLACE-2 randomized clinical trial. JAMA 2014;311:1870–1882. [DOI] [PubMed] [Google Scholar]
- 42. Kazi DS, Moran AE, Coxson PG, Penko J, Ollendorf DA, Pearson SD, Tice JA, Guzman D, Bibbins-Domingo K.. Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA 2016;316:743–753. [DOI] [PubMed] [Google Scholar]
- 43. Kalra R, Arora P, Morgan C, Hage FG, Iskandrian AE, Bajaj NS.. Conducting and interpreting high-quality systematic reviews and meta-analyses. J Nucl Cardiol 2016;1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.