Abstract
DJ-1 is a protein expressed in many tissues including the brain where it has been extensively studied due to its association with Parkinson’s Disease (PD). DJ-1 was reported to function as an antioxidant, redox-sensitive molecular chaperone, and transcription regulator, which protected cells from oxidative stress by modifying signaling pathways that regulate cell survival. Here we discuss our progress toward characterization of the DJ-1 function in the protection of RPE to oxidative stress.
Keywords: Retinal pigment epithelium, Oxidative stress, Reactive oxygen species, DJ-1, Histology
81.1 Introduction
DJ-1 gene encodes a highly conserved protein with 189 amino acids and a molecular weight of ~ 20 kDa, which belongs to the ThiJ/PfpI protein superfamily [1]. The function of ThiJ is not fully known, but it may be related to the biosynthesis of thiamins while PfpI is an intracellular protease, responsive to growth conditions and present in most bacteria and archea [2, 3, 4]. Therefore, both DJ-1 sequence and structure suggested that this protein would be involved in cellular viability.
Initially, DJ-1 was reported as a novel oncogene showing a transforming activity when expressed together with H-ras [5]. Later, it was reported that the gene for human DJ-1 (PARK7) is mutated in rare forms of recessively inherited PD [6].
Recently, we have identified DJ-1 peptides in both young and aged retinal pigment epithelium (RPE) lysates starting with a proteomic approach. We also reported immunolocalization of DJ-1 protein in both young and aged rat RPE cells [7]. Here we extend our initial observations of DJ-1 expression and further discuss DJ-1 function in the RPE cells.
81.2 DJ-1 Function and Oxidative Stress Protection
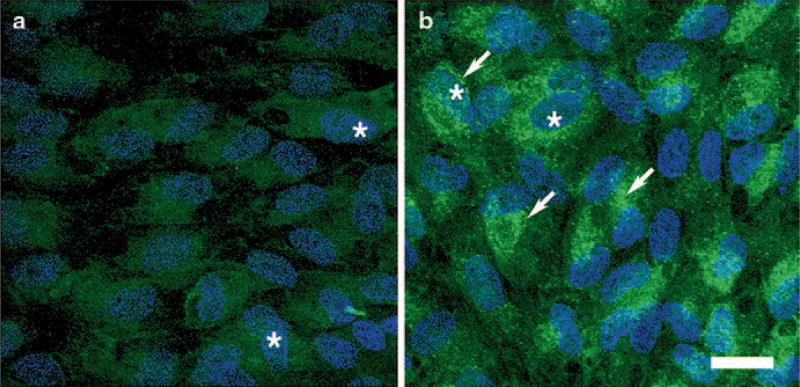
At the subcellular level, under basal conditions, DJ-1 is found mostly in the cytoplasm and to a lesser extent in the mitochondria [6, 8, 9] and nucleus [9]. Under conditions of oxidative stress, more DJ-1 redistributes to mitochondria and later to the nucleus, and this cellular redistribution correlates with the ability of DJ-1 to confer neuroprotection [8, 10, 11]. As previously described in several cell types, under baseline conditions, DJ-1 displays a diffuse cytoplasmic and nuclear staining of all the RPE cell lines analyzed (Fig. 81.1a). Oxidative stress induced by incubation of RPE cultures with H2O2 leads to a visible increase in immunocytochemical staining for DJ-1 (Fig. 81.1b). In addition, these RPE cultures displayed an induced intracellular redistribution of DJ-1 to a perinuclear localization (mitochondria, data not shown). Confirmation of the increased DJ-1 protein levels in RPE cells under oxidative stress was also demonstrated by Western blot analysis (data not shown).
Fig. 81.1.
Immunocytochemical analysis of intracellular distribution of DJ-1 on RPE monolayers exposed to oxidative stress. Immunocytochemical staining of ARPE-19 cells for DJ-1 demonstrates that at basal conditions a DJ-1 is diffused in the cytoplasm and nuclei (*) of the cells. With 1 h of exposure to 800 µM H2O2, b a pronounced aggregated perinuclear staining (arrows) for DJ-1 is apparent. Bar = 20 µm
Several reports suggest that DJ-1 robustly protects cells from oxidative stress [8, 12–18]. First, reports showed that flies and mice deficient in the gene encoding DJ-1 are indeed more susceptible to oxidative toxins [13, 15, 19–21]. Second, it was reported that DJ-1 can eliminate H2O2 in vitro by becoming oxidized itself and thus functioning as a scavenger of reactive oxygen species (ROS) [17, 22]. It was also established that cysteines at residues 46, 53, and 106 become oxidized upon oxidative stress, resulting in scavenging of ROS, enhancing DJ-1 association with mitochondria and allowing DJ-1 to protect cells from oxidative stress [8, 14, 23].
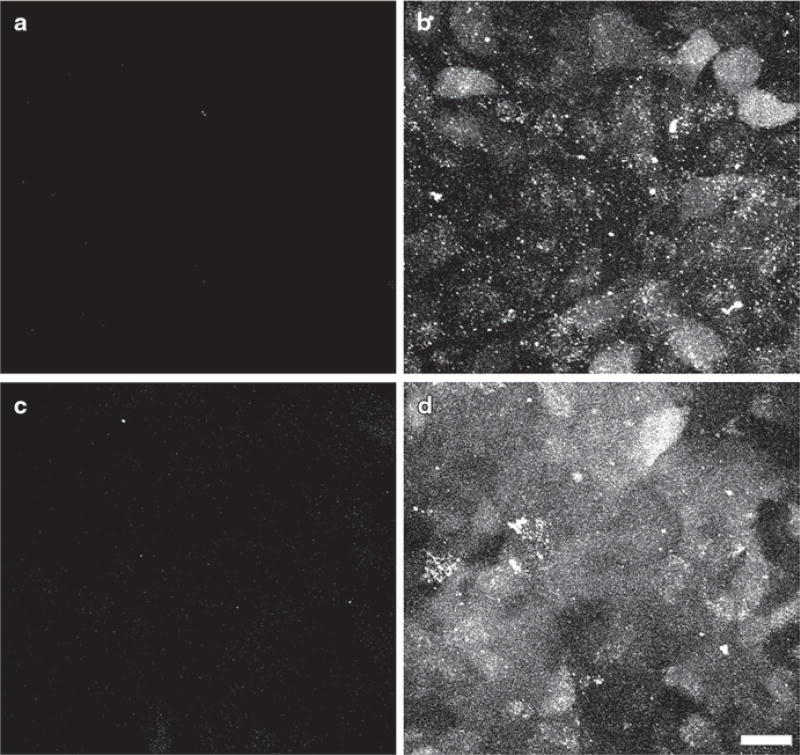
To explore the functional consequences of DJ-1 mutation, adenoviruses carrying both DJ-1 full length and DJ-1 with the three cysteine at residues 46, 53, and 106 mutated to serine were generated and used as agents to induce overexpression of exogenous DJ-1. Control and RPE cultures overexpressing both types of exogenous DJ-1 were exposed to oxidative stress through incubation with H2O2 followed by incubation with the fluorescent indicator used to assess ROS generation 5-(and-6)-carboxy-2’,7’-difluorodihydrofluorescein diacetate (carboxy-DCFDA) and confocal microscopy (Fig. 81.2). Interestingly, no significant amount of ROS was detected in ARPE-19 over-expressing exogenous full-length DJ-1 and exposed to oxidative stress (Fig. 81.2c) and in ARPE cultures at basal conditions (Fig. 81.2a). In contrast, a significant generation of ROS was observed when ARPE-19 cultures were exposed to oxidative stress (Fig. 81.2b) and when RPE cultures overexpressing the mutated DJ-1 were exposed to oxidative stress (Fig. 81.2d). This data suggested that high levels of full-length DJ-1 expression resulted in significant decrease in ROS generation in RPE cells exposed to oxidative stress.
Fig. 81.2.
Staining of ROS generation on ARPE-19 monolayers expressing endogenous and exogenous DJ-1 and exposed to oxidative stress by exposure to 800 µM H2O2 for 1 h. Incubation of RPE monolayers with carboxy-DCFDA indicates that no ROS is generated by the RPE cells at basal conditions (a). While upon oxidative stress incubation, appreciable ROS generation throughout the entire cell body of cells is observed (b). RPE cultures transduced with an adenovirus carrying the full-length hDJ-1 DNA did not display ROS generation when exposed to oxidative stress (c). However, RPE cultures transduced with an adenovirus carrying DJ-1 with the three cysteine at residues 46, 53, and 106 mutated to serine (d). Displayed appreciable ROS generation when cells were exposed to oxidative stress. Bar = 20 µm
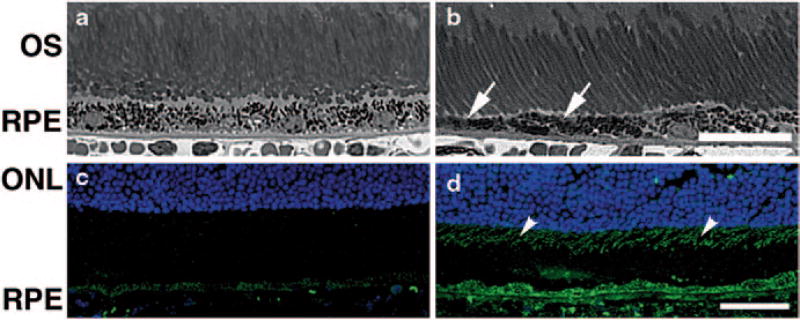
We have also analyzed histologically the eyes of young adult DJ-1 KO mice and noted that the RPE in these mice is characterized by patchy thinning (Fig. 81.3b, arrows) when compared to the control RPE (Fig. 81.3a). Our data suggested that normal RPE structure requires DJ-1 expression. To further relate RPE DJ-1 expression with oxidative stress regulation, we analyzed the expression of 7,8-dihydro-8-oxoguanine (8-oxoG), the most abundant oxidized base generated in vivo by various types of ROS, in control and DJ-1 KO RPE with antibodies specific to this DNA oxidation (Fig. 81.3c, d). Immunocytochemical staining of 8-oxoG, was significantly elevated in the DJ-1 KO RPE (Fig. 81.3d) and photoreceptor inner segments (Fig. 81.3d, arrowheads) when compared to control RPE (Fig. 81.3c). These data indicated that lack of DJ-1 expression leads to increased oxidative stress in RPE in vivo.
Fig. 81.3.
Degeneration in the neural retina of young adult DJ-1 KO mouse. Plastic sections of both control (a) and DJ-1 KO (b) retinas stained with toluidine blue highlighted the RPE and photoreceptor cells. The RPE of the DJ-1 KO displayed thinning (b, arrows). Cryosections of retinas were also labeled with 8-oxoG (c, d) to detect DNA oxidation. Analysis showed that 8-oxoG immunoreactivity is significantly increased in the photoreceptor inner segments (arrowheads) and the RPE cells of DJ-1 KO (d) when compared to the control (c) retinas. ONL outer nuclear layer, OS photoreceptor outer segments, RPE retinal pigment epithelium, Bar a, b = 25 µm and c, d = 40 µm
81.3 Conclusions
Age-related macular degeneration (AMD) is the most common cause of irreversible blindness in the elderly population in industrialized countries. Studies carried out in man, AMD animal models, and RPE cell lines point toward an important role of oxidative stress in the development of AMD. Due to the evidence presented here, connecting DJ-1 to protection against oxidative stress, it is conceivable that DJ-1 function may also be related to oxidative stress implicated in AMD pathology. The years to come will bring further definition of key proteins and pathways involved in the regulation of oxidative stress by DJ-1 in RPE cells as well as a better understanding of its function in AMD.
Acknowledgments
Supported by Research Center grants from the Foundation Fighting Blindness and Research to Prevent Blindness and funds from the Cleveland Clinic Foundation and partially by the intramural research program of National Institute on Aging (HC).
Abbreviations
- PD
Parkinson’s Disease
- RPE
Retinal pigment epithelium
- ROS
Reactive oxygen species
- Carboxy-DCFDA
5-(and-6)-carboxy-2’, 7’-difluorodihydrofluorescein diacetate
- 8-oxoG
7, 8-dihydro-8-oxoguanine
Contributor Information
Vera L. Bonilha, Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine, The Cole Eye Institute, i31, 9500 Euclid Avenue, Cleveland, OH 44195, USA
Mary E. Rayborn, Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine, The Cole Eye Institute, i31, 9500 Euclid Avenue, Cleveland, OH 44195, USA
Xiaoping Yang, Department of Ophthalmology, Cleveland Clinic Lerner College of Medicine, The Cole Eye Institute, i31, 9500 Euclid Avenue, Cleveland, OH 44195, USA.
Chengsong Xie, Laboratory of Neurogenetics, National Institute of Aging, NIH, Bethesda, MD 20892, USA.
Huaibin Cai, Laboratory of Neurogenetics, National Institute of Aging, NIH, Bethesda, MD 20892, USA.
References
- 1.Tao X, Tong L. Crystal structure of human DJ-1, a protein associated with early onset Parkinson’s disease. J Biol Chem. 2003;278:31372–31379. doi: 10.1074/jbc.M304221200. [DOI] [PubMed] [Google Scholar]
- 2.Halio SB, Blumentals II, Short SA, Merrill BM, Kelly RM. Sequence, expression in Escherichia coli, and analysis of the gene encoding a novel intracellular protease (PfpI) from the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol. 1996;178:2605–2612. doi: 10.1128/jb.178.9.2605-2612.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taylor SV, Kelleher NL, Kinsland C, Chiu HJ, Costello CA, et al. Thiamin biosynthesis in Escherichia coli. Identification of ThiS thiocarboxylate as the immediate sulfur donor in the thiazole formation. J Biol Chem. 1998;273:16555–16560. doi: 10.1074/jbc.273.26.16555. [DOI] [PubMed] [Google Scholar]
- 4.Mizote T, Tsuda M, Smith DD, Nakayama H, Nakazawa T. Cloning and characterization of the thiD/J gene of Escherichia coli encoding a thiamin-synthesizing bifunctional enzyme, hydroxymethylpyrimidine kinase/phosphomethylpyrimidine kinase. Microbiology. 1998;145(Pt 2):495–501. doi: 10.1099/13500872-145-2-495. [DOI] [PubMed] [Google Scholar]
- 5.Nagakubo D, Taira T, Kitaura H, Ikeda M, Tamai K, et al. DJ-1, a novel oncogene which transforms mouse NIH3T3 cells in cooperation with ras. Biochem Biophys Res Commun. 1997;231:509–513. doi: 10.1006/bbrc.1997.6132. [DOI] [PubMed] [Google Scholar]
- 6.Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- 7.Gu X, Neric NJ, Crabb JS, Crabb JW, Bhattacharya SK, et al. Age-related changes in the retinal pigment epithelium (RPE) PLoS One. 2012;7:e38673. doi: 10.1371/journal.pone.0038673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, et al. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L, Shimoji M, Thomas B, Moore DJ, Yu SW, et al. Mitochondrial localization of the Parkinson’s disease related protein DJ-1: implications for pathogenesis. Hum Mol Genet. 2005;14:2063–2073. doi: 10.1093/hmg/ddi211. [DOI] [PubMed] [Google Scholar]
- 10.Ashley AK, Hanneman WH, Katoh T, Moreno JA, Pollack A, et al. Analysis of targeted mutation in DJ-1 on cellular function in primary astrocytes. Toxicol Lett. 2009;184:186–191. doi: 10.1016/j.toxlet.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Junn E, Jang WH, Zhao X, Jeong BS, Mouradian MM. Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J Neurosci Res. 2009;87:123–129. doi: 10.1002/jnr.21831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li HM, Taira T, Maita C, Ariga H, Iguchi-Ariga SM. Protection against nonylphenol-induced cell death by DJ-1 in cultured Japanese medaka (Oryzias latipes) cells. Toxicology. 2006;228:229–238. doi: 10.1016/j.tox.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 13.Meulener MC, Xu K, Thomson L, Ischiropoulos H, Bonini NM. Mutational analysis of DJ-1 in Drosophila implicates functional inactivation by oxidative damage and aging. Proc Natl Acad Sci U S A. 2006;103:12517–12522. doi: 10.1073/pnas.0601891103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsumoto A, Nakagawa Y. DJ-1 is an indicator for endogenous reactive oxygen species elicited by endotoxin. Free Radic Res. 2001;35:885–893. doi: 10.1080/10715760100301381. [DOI] [PubMed] [Google Scholar]
- 15.Park J, Kim SY, Cha GH, Lee SB, Kim S, et al. Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene. 2005;361:133–139. doi: 10.1016/j.gene.2005.06.040. [DOI] [PubMed] [Google Scholar]
- 16.Sekito A, Koide-Yoshida S, Niki T, Taira T, Iguchi-Ariga SM, et al. DJ-1 interacts with HIPK1 and affects H2O2-induced cell death. Free Radic Res. 2006;40:155–165. doi: 10.1080/10715760500456847. [DOI] [PubMed] [Google Scholar]
- 17.Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, et al. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahle PJ, Waak J, Gasser T. DJ-1 and prevention of oxidative stress in Parkinson’s disease and other age-related disorders. Free Radic Biol Med. 2009;47:1354–1361. doi: 10.1016/j.freeradbiomed.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Kim RH, Smith PD, Aleyasin H, Hayley S, Mount MP, et al. Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc Natl Acad Sci U S A. 2005;102:5215–5220. doi: 10.1073/pnas.0501282102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Menzies FM, Yenisetti SC, Min KT. Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr Biol. 2005;15:1578–1582. doi: 10.1016/j.cub.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 21.Yang Y, Gehrke S, Haque ME, Imai Y, Kosek J, et al. Inactivation of Drosophila DJ-1 leads to impairments of oxidative stress response and phosphatidylinositol 3-kinase/Akt signaling. Proc Natl Acad Sci U S A. 2005;102:13670–13675. doi: 10.1073/pnas.0504610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blackinton J, Lakshminarasimhan M, Thomas KJ, Ahmad R, Greggio E, et al. Formation of a stabilized cysteine sulfinic acid is critical for the mitochondrial function of the parkinsonism protein DJ-1. J Biol Chem. 2009;284:6476–6485. doi: 10.1074/jbc.M806599200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilson MA. The role of cysteine oxidation in DJ-1 function and dysfunction. Antioxid Redox Signal. 2011;15:111–122. doi: 10.1089/ars.2010.3481. [DOI] [PMC free article] [PubMed] [Google Scholar]