Abstract
Background
HIV prevention interventions in the United States have failed to eliminate racial inequities. Here, we evaluate factors associated with racial inequities in HIV prevalence among people who inject drugs using HIV Prevention Trial Network 037 data.
Methods
We measured racial homophily (ie, all members share the same race), being in an HIV+ network (network with ≥1 HIV+ member), and drug and sex risk behaviors. A 2-level logistic regression with a random intercept evaluated the association between being in an HIV+ network and race adjusting for individual-level and network-level factors.
Results
Data from 232 index participants and 464 network members were included in the analysis. Racial homophily was high among blacks (79%) and whites (70%); 27% of all-black, 14% of all-white, and 23% of racially mixed networks included HIV+ members. Sex risk was similar across networks, but needle sharing was significantly lower in all-black (23%) compared with all-white (48%) and racially mixed (46%) networks. All-black [adjusted odds ratio (AOR), 3.6; 95% confidence interval (CI), 1.4 to 9.5] and racially mixed (AOR, 2.0; 95% CI: 1.1 to 3.7) networks were more likely to include HIV+ network members; other factors associated with being in HIV+ network included homelessness (AOR, 2.0; 95%CI, 1.2 to 3.2), recent incarceration (AOR, 0.4; 95%CI, 0.2 to 0.7), and cocaine injection (AOR, 1.7; 95% CI, 1.0 to 2.7). Risk behaviors were not associated with being in an HIV+ network.
Conclusion
Despite having lower drug risk behavior, all-black networks disproportionately included HIV+ members. HIV prevention interventions for people who inject drugs need to go beyond individual risk and consider the composition of risk networks.
Keywords: racial inequities, HIV, sexual networks, drug networks, people who inject drugs
INTRODUCTION
Interventions in the United States have reduced but not eliminated racial disparities with respect to HIV infection among people who inject drugs (PWID).1–3 Trends analysis from National HIV Surveillance System data, between 2008 and 2013, show that HIV incidence has decreased among PWID, and although diminishing, racial inequities persist with a black-to-white PWID diagnosis ratio of 2 to 1.1 Most attempts to explain racial inequities focus on individual-level sex and drug risk behaviors, but these interpretations fail to take into account the social and contextual factors that drive health inequities. In addition, many studies have shown that blacks engage in less high-risk behaviors than whites2,4–10 but remain disproportionately affected by HIV, highlighting the potential significance of one’s peer network and community HIV prevalence. Few intervention strategies have shown promise in reducing racial inequities in network HIV prevalence.
Risk network characteristics and HIV prevalence within networks play a critical role in the spread of HIV.9,11–13 Size, density, and connectivity of networks contribute to disease spread with smaller dynamic networks, contributing to higher HIV transmission.13–15 Racial homophily occurs when all members within a network share the same race. Racial homophily among black networks, where HIV prevalence is high, also contributes to higher rates of HIV spread even in lower-risk individuals.13,15 A better understanding of risk networks is needed to tailor innovative interventions at the network level that can effectively reduce inequities.
Data from the HIV Prevention Trial Network (HPTN) 037 provide a unique opportunity to examine the relationship between risk behaviors, HIV prevalence, and race among a large group of PWID and their risk network members (ie, individuals with whom one injects drugs or has sex). HPTN 037 assessed the efficacy of a network-oriented peer education intervention to reduce HIV risk among PWID and their network members in Philadelphia and Chiang Mai, Thailand.10,16–19 In Philadelphia, the study enrolled 646 participants among 232 networks and provided repeated measures on risk behaviors between 2002 and 2006. Using baseline HPTN 037 data, we aimed to evaluate the association between individual demographics (age, gender, race), socioeconomic/structural factors (education, employment, housing, incarceration), and network-level factors (racial homophily and network-level drug and sex risk behaviors) associated with being in an HIV positive (+) network. We hypothesized that HIV risk is a function of the interaction between individual risk behavior and HIV prevalence within one’s network and that persistence of racial inequities in HIV prevalence can be explained by racial homogeneity among networks where HIV prevalence exists. These analyses are designed to add to our understanding of ecological models of HIV risk by quantifying personal and interpersonal risk behaviors and racial homophily at the network level among a group of individuals at high risk of HIV acquisition.
METHODS
We performed a secondary analysis of HPTN 037 using baseline Philadelphia data of index participants who regularly injected drugs and their drug and sex network members.10,17,19 Data from the Chang Mai site was not used because the primary aim of the study was to investigate factors associated with racial inequities in HIV prevalence in the United States. The data analyzed were collected during the screening process before randomization.
Laboratory Procedures
All participants received voluntary HIV counseling and testing at the baseline visit. Laboratory procedures adhered to standards of good laboratory practice and the HPTN Laboratory Standard Operating Procedures. HIV screening was followed by confirmatory testing.
Procedures for Study Enrollment
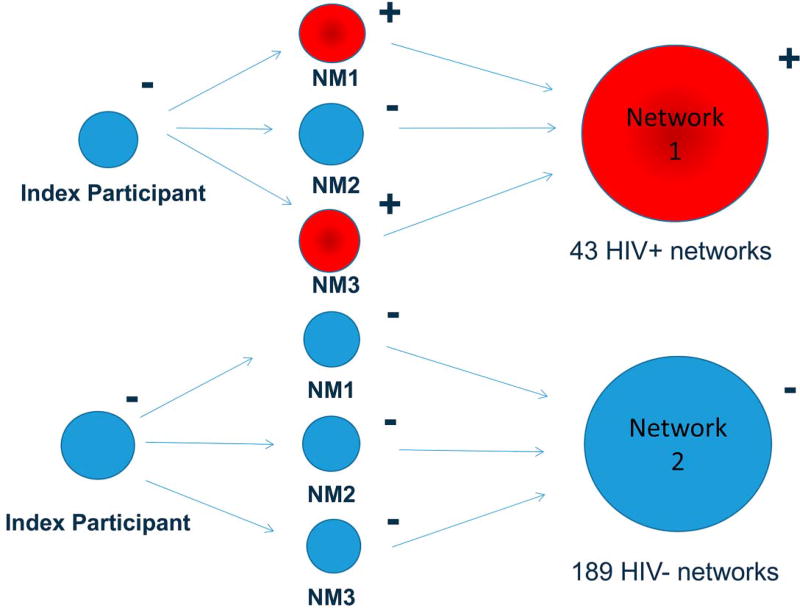
Study participants in Philadelphia were recruited by outreach workers using mobile research facilities in neighborhoods with high concentrations of drug use, drug sales, and HIV/AIDS prevalence. Eligibility criteria for index participants, who were the initial participants recruited and asked to identify and recruit their drug and sex network members, included the following: legal age to provide written informed consent, injected drugs at least 12 times in the prior 3 months, not in methadone maintenance in the previous 3 months, HIV negative (−) antibody test results at the baseline visit, and willingness to identify and attempt to recruit at least 2 network members who were eligible for the study.17 Eligibility requirements for the network members included the following: legal age to provide written informed consent, recruited by an eligible index participant, and injected drugs or had sex with the relevant index participant within the prior 3 months.17 Unlike the index participants who were required to be HIV− at baseline, network members could be either HIV+ or HIV− and therefore networks consisted of members with and without HIV infection (Fig. 1). For the analyses, we grouped the index and their network members into networks. Data for these analyses did not involve the use of personal identifiers, and the Drexel College of Medicine Institutional Review Board exempted the study for review.
FIGURE 1.
Network composition of HPTN 037 study participants, 2002.
Dependent Variable
The dependent variable was being in an HIV+ network, defined as a network where ≥1 member is HIV+. HIV status for index participants and network members was determined by HIV antibody test results.
Independent Variables
Independent variables included both individual-level and network-level characteristics. These variables were collected via individual questionnaires at the baseline visit. Individual-level variables consisted of participant’s age; gender, categorized as male or female; and race, categorized as black non-Hispanic, white non-Hispanic, Hispanic, and other race. Socioeconomic variables included education classified as having at least a high school degree or general education development; employment was classified as fulltime, part-time, or unemployed; homeless defined as living on the street, in a car, in a park, or in an abandoned building at any time during the 6 months preceding the baseline visit; being in residential treatment was defined as having been admitted to a residential drug treatment facility in the past 6 months; having a history of incarceration was defined as having spent time in jail in the past 6 months. Drug risk variables included type of drug injected: heroin (yes/no), heroin mixed with other substances (yes/no), and cocaine (yes/no); needle sharing was assessed using the answer to the following question: “In the past month, did you ever once use a needle or syringe after someone used it?” For sex risk variables among people who had vaginal or anal sex within the last week, unprotected sexual intercourse was defined by the number of unprotected sex events during that week and the number of sex partners equaled the reported number of female and male sex partners in the past month. A total of 3 outliers were removed because they reported having unprotected sex more than 30 times in 1 week or having more than 100 sex partners in the past month.
Network-level characteristics included racial homophily defined as being in a network where all members share the same race. Using this variable, networks were classified as all-black, all-white, all-Hispanic, or racially mixed. Network racial homophily was evaluated among all participants and was not restricted to the index participants. Network drug and sex risk variables were selected based on the factors that have been strongly associated with HIV risk, such as needle sharing, unprotected sexual intercourse, and having multiple sex partners. At the network level, drug risk was calculated using the prevalence of needle sharing among network members and sex risk was calculated using the average of the reported sex risk variables.
Analyses
Univariate analyses were performed to assess data quality and review frequency distributions for each variable. We then described the composition of risk networks and measured the bivariate associations between demographic, socioeconomic, and behavioral characteristics among (1) index participants and network members, (2) index participants belonging to HIV+ and HIV− networks, and (3) network members belonging to HIV+ and HIV− networks. Racial homophily and network HIV prevalence among black, white, Hispanic, and racially mixed networks were evaluated. Drug risk (prevalence of needle sharing) and sex risk (mean condom use and mean number of sex partners) were also evaluated at the network level by race. To account for nesting of individuals within networks, a 2-level (ie, mixed-effects) logistic regression model with a random intercept for network was performed to measure the association of being in an HIV+ network with individual-level and network-level characteristics. The model included all demographic, socioeconomic, and network characteristics described previously regardless of their significance in the bivariate analysis.
RESULTS
Composition of Risk Networks
There were 696 individuals (232 index participants and 464 network members) enrolled in 232 egocentric networks (Table 1). The average network size, excluding index participants, was 3.4 (SD, 1.3; range 1–7). The mean age of study participants was 40.8 years (SD, 9.0); 69% were male, and 31% were female; among those, 36% were female who reported exchanging money or drugs for sex; 48% were black, 44% white, and 8% Hispanic or another race. Overall, there were 43 HIV+ networks (18.5%) and 189 HIV− networks (81.5%) (Fig. 1). The majority of HIV+ networks only included 1 HIV+ individual: of the 43 HIV+ networks, 38 had 1 HIV+ individual, 4 networks had 2 HIV+ individuals, and 1 network had 4 HIV+ individuals (Supplemental Digital Content Table S1, http://links.lww.com/QAI/B70). The majority of networks (50.9%) consisted of drug partners only, 4.7% consisted of sex partners only, and 44.4% included both sex and drug partners.
TABLE 1.
Baseline Demographic Characteristics and Risk Behaviors Among Drug and Sex Networks Enrolled in the HPTN 037, 2002
| Index Participants | Network Members | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| All Index Participants |
All Network Members |
P | HIV-Negative Network |
HIV-Positive Network |
P | HIV-Negative Network |
HIV-Positive Network |
P | |
| Number enrolled | 232 | 464 | 189 | 43 | 360 | 104 | |||
| Demographic characteristics | |||||||||
| Age, average (SD), yr | 40.4 (9.6) | 41.0 (10.1) | 0.46 | 39.9 (9.6) | 42.5 (9.4) | 0.11 | 41.0 (10.0) | 41.2 (10.4) | 0.81 |
| Sex, n (%) | <0.01 | 0.12 | |||||||
| Male | 185 (79.7) | 294 (63.4) | 147 (77.8) | 38 (88.4) | 224 (62.2) | 70 (67.3) | 0.34 | ||
| Female | 47 (20.3) | 170 (36.6) | 42 (22.2) | 5 (11.6) | 136 (37.8) | 34 (32.7) | |||
| Race/ethnicity, n (%) | 0.09 | 0.08 | 0.37 | ||||||
| Black | 101 (43.5) | 230 (49.6) | 76 (40.2) | 25 (58.2) | 173 (48.1) | 57 (54.8) | |||
| White | 116 (50.0) | 193 (41.6) | 99 (52.4) | 17 (39.5) | 156 (43.3) | 37 (35.6) | |||
| Hispanic/Other | 15 (6.5) | 41 (8.8) | 14 (7.4) | 1(2.3) | 31 (8.6) | 10 (9.6) | |||
| Socioeconomic characteristics | |||||||||
| Education, n (%) | 0.69 | 0.14 | 0.43 | ||||||
| <High school graduation/GED | 76 (32.8) | 159 (34.3) | 66 (34.9) | 10 (23.3) | 120 (33.3) | 39 (37.5) | |||
| ≥High school graduation/GED | 156 (67.2) | 305 (65.7) | 123 (65.1) | 33 (76.7) | 240 (66.7) | 65 (62.5) | |||
| Employment | 0.06 | 0.94 | 0.61 | ||||||
| Employed | 53 (22.8) | 79 (17.0) | 43 (22.8) | 10 (23.2) | 63 (17.5) | 16 (15.4) | |||
| Unemployed | 179 (77.2) | 385 (83.0) | 146 (77.2) | 33 (76.8) | 297 (82.5) | 88 (84.6) | |||
| Housing, n (%)* | |||||||||
| Homeless† | 61 (26.3) | 110 (23.8) | 0.46 | 47 (24.9) | 14 (32.6) | 0.30 | 75 (20.9) | 35 (33.6) | <0.01 |
| Residential treatment in the past 6 mo | 30 (13.0) | 88 (19.0) | 0.05 | 23 (12.2) | 7 (16.3) | 0.48 | 67 (18.7) | 21 (20.2) | 0.726 |
| Spent time in jail in past 6 mo | 49 (21.1) | 70 (15.1) | 0.05 | 44 (23.3) | 5 (11.6) | 0.09 | 62 (17.3) | 8 (7.7) | 0.02 |
| Type of drug injected in last month, n (%)* | |||||||||
| Heroin | 217 (94.8) | 386 (94.6) | 0.93 | 177 (94.6) | 40 (95.2) | 0.88 | 305 (95.6) | 81 (91.0) | 0.09 |
| Heroin with other substances | 96 (41.9) | 149 (36.5) | 0.18 | 77 (41.2) | 19 (45.2) | 0.63 | 108 (33.9) | 41 (46.1) | 0.03 |
| Cocaine | 93 (40.6) | 145 (35.5) | 0.20 | 74 (39.6) | 19 (45.2) | 0.50 | 99 (31.0) | 46 (51.7) | <0.01 |
| Risk behaviors | |||||||||
| A. Drug risk | |||||||||
| Any needle sharing in past month, n (%) | 90 (39.3) | 158 (38.7) | 0.89 | 77 (41.2) | 13 (30.9) | 0.22 | 129 (40.4) | 29 (32.6) | 0.18 |
| B. Sex | |||||||||
| No. sexual partners in the past month, average (SD) | 2.8 (6.1) | 2.2 (3.8) | 0.14 | 2.8 (1.8) | 2.4 (3.5) | 0.73 | 2.3 (3.9) | 1.9 (3.6) | 0.43 |
| No. unprotected sex events in the past wk, average (SD) | 2.0 (2.8) | 1.7 (2.7) | 0.18 | 2.1 (2.9) | 1.5 (2.0) | 0.17 | 2.0 (2.6) | 1.8 (3.6) | 0.63 |
Each network included an index participant who was HIV negative at baseline and drug or sex network members who were either HIV positive or HIV negative. The column percentage is displayed for each variable. HIV−positive individuals contributed to the count of individuals in HIV+ networks.
Variables were individually categorized in the study questionnaire.
Homeless was defined as living on the street, in a car, in a park, or in an abandoned building at any time during the 6 months preceding the baseline visit.
GED, general educational development.
Index participants did not differ significantly from their drug and sex network members except by gender and housing status (Table 1): the proportion of women participants was low overall but was lower among index participants (20.3%) compared with network members (36.6%; P < 0.01). A higher proportion of network members had been in residential treatment over the past 6 months (P = 0.05), whereas a larger proportion of index participants had a recent history of incarceration (P = 0.05). Index participants in HIV− networks had similar demographic characteristics and engaged in similar drug and sex risk behaviors as index participants in HIV+ networks. In particular, there were no differences in the type of drug used, needle sharing, number of sex partners, and number of unprotected sex events by index participants’ network HIV status. A nonsignificant trend (P = 0.08) was found by race because the proportion of black index participants in HIV+ networks (58.2%) was higher than that of white index participants in HIV+ networks (39.5%). Network members who belonged to HIV+ networks were more likely to be homeless (P < 0.01), less likely to have been in jail in the past 6 months (P = 0.02), and more likely to inject heroin mixed with other substances (P = 0.03) or to inject cocaine (P < 0.01).
Racial Homophily and Network HIV Prevalence
Racial homophily within risk networks was very high among blacks and whites and lower among Hispanic participants: 79% of blacks were in all-black networks, 70% of whites were in all-white networks, and 31% of Hispanics were in all-Hispanic networks. The remainder of study participants were in racially mixed networks (n = 203). Marked differences in HIV prevalence were observed by race: among enrolled participants, the prevalence of HIV was 10.0% among blacks, 4.2% among whites, and 8.0% among Hispanics (P = 0.02). Considering individuals who had at least 1 HIV+ member in their network, including themselves, 26.9% of them were associated with all-black, 14.3% were associated with all-white, 0% was associated with all-Hispanic networks, and 22.7% were associated with racially mixed networks (Table 2).
TABLE 2.
Demographic, Socioeconomic, Risk Behavior Characteristics Across Racial Networks and Factors Associated With Being in an HIV-Positive Network in a Multivariable Regression Model in HPTN 037
| Individuals in All- Black Networks, n = 260 |
Individuals in All- White Networks, n = 217 |
Individuals in All- Hispanic Networks, n = 16 |
Individuals in Racially Mixed Networks, n = 203 |
P | Being in HIV+ Network, AOR (95% CI) |
P | |
|---|---|---|---|---|---|---|---|
| Demographic characteristics | |||||||
| Age, average (SD), yr | 45.9 (7.9) | 36.8 (10.0) | 34.7 (6.1) | 39.0 (9.6) | <0.01 | 1.0 (0.9 to 1.0) | 0.74 |
| Gender, n (%) | 0.37 | ||||||
| Male | 189 (72.7) | 142 (65.4) | 11 (68.7) | 137 (67.5) | Ref | ||
| Female | 71 (27.3) | 75 (34.6) | 5 (31.2) | 66 (32.5) | 0.8 (0.5 to 1.3) | 0.44 | |
| Race, n (%) | <0.01 | ||||||
| Black | 260 (100.0) | 0 (0.0) | 0 (0.0) | 71 (35.0) | 0.6 (0.2 to 1.3) | 0.20 | |
| White | 0 (0.0) | 217 (100.0) | 0 (0.0) | 92 (45.3) | Ref | ||
| Hispanic | 0 (0.0) | 0 (0.0) | 16 (100.0) | 40 (19.7) | 1.0 (0.4 to 2.6) | 0.89 | |
| Socioeconomic characteristics | |||||||
| Education, n (%) | 0.27 | ||||||
| High school graduation/GED | 175 (67.3) | 137 (63.1) | 8 (50.0) | 141 (69.5) | 1.0 (0.6 to 1.4) | 0.83 | |
| Less than high school graduation/GED | 85 (32.7) | 80 (36.9) | 8 (50.0) | 62 (30.5) | Ref | ||
| Employment, n (%) | 0.37 | ||||||
| Employed | 56 (21.5) | 38 (17.5) | 1 (6.2) | 37 (18.2) | Ref | ||
| Unemployed | 204 (78.5) | 179 (82.5) | 15 (93.8) | 166 (81.8) | 1.3 (0.8 to 2.2) | 0.29 | |
| Housing, n (%) | |||||||
| Homeless | 44 (17.0) | 61 (28.1) | 4 (25.0) | 62 (30.5) | <0.01 | 2.0 (1.2 to 3.2) | <0.01 |
| Residential treatment | 37 (14.3) | 35 (16.2) | 4 (25.0) | 42 (20.7) | 0.25 | 1.1 (0.6 to 1.8) | 0.82 |
| Spent time in jail in past 6 mo | 26 (10.0) | 49 (22.6) | 2 (12.5) | 42 (20.7) | <0.01 | 0.4 (0.2 to 0.7) | <0.01 |
| Type of drug injected in last month, n (%) | |||||||
| Heroin | 209 (80.4) | 201 (92.6) | 13 (81.2) | 180 (88.7) | <0.01 | 0.7 (0.4 to 1.2) | 0.14 |
| Heroin with other substances | 103 (39.6) | 64 (29.5) | 9 (56.2) | 69 (34.0) | 0.036 | 0.9 (0.6 to 1.5) | 0.73 |
| Cocaine | 78 (30.0) | 82 (37.8) | 5 (31.2) | 73 (36.0) | 0.30 | 1.7 (1.0 to 2.7) | 0.03 |
| Network variables | |||||||
| A. Racial homophily | |||||||
| Black | — | — | — | — | 3.6 (1.4 to 9.5) | <0.01 | |
| White | — | — | — | — | Ref | ||
| Hispanic | — | — | — | — | — | ||
| Mixed | — | — | — | — | 2.0 (1.1 to 3.7) | 0.03 | |
| B. Drug risk | |||||||
| Needle sharing in the past month, n (%) | 53 (22.8) | 98 (48.0) | 10 (71.4) | 87 (46.5) | <0.01 | 0.8 (0.4 to 1.4) | 0.41 |
| C. Sexual risk | |||||||
| No. unprotected sex events in the past wk, average (SD) | 1.7 (1.6) | 1.8 (1.9) | 2.4 (1.5) | 1.9 (1.9) | 0.36 | 1.0 (0.8 to 1.1) | 0.61 |
| No. sexual partners in the past month, average (SD) | 2.5 (3.1) | 2.4 (3.6) | 1.5 (0.3) | 2.3 (2.6) | 0.58 | 0.9 (0.9 to 1.0) | 0.20 |
| D. In HIV+ network, n (%) | 70 (26.9) | 31 (14.3) | 0 (0.0) | 46 (22.7) | <0.01 | — | — |
The regression model included all of the variables listed on the table.
AOR, adjusted odds ratio; GED, general educational development.
Drug and Sex Risk Behavior of Individuals Within Race Networks
Reported needle sharing was lower among individuals in all-black networks compared with those in other race networks (Table 2): 22.8% of individuals in all-black networks shared needles in the past month compared with 48.0% for all-white, 71.4% for all-Hispanic, and 46.5% for individuals in racially mixed networks (P < 0.01). Network sexual risks were similar by race: the mean number of unprotected sex events was approximately 2 in the week before the study questionnaire, and the mean number of sex partners in the previous month was approximately 2 across all racial groups.
Network and Individual Factors Associated With Being in an HIV+ Network
Table 2 shows individual and network characteristics associated with individuals in HIV+ networks in the adjusted logistic regression model. Because none of the all-Hispanic networks included HIV+ individuals, this analysis excludes them. Membership in an all-black network was the strongest factor associated with being in an HIV+ network [adjusted odds ratio (AOR), 3.6; 95% confidence interval (CI), 1.4 to 9.5]; being in a racially mixed network was moderately associated with being in an HIV+ network (AOR, 2.0; 95% CI, 1.1 to 3.7) relative to an all-white network. Reflecting the same trends present in the overall cohort, when breaking down the patterns of needle sharing by race among racially mixed networks, we found that needle sharing was highest among whites and lowest among blacks and that HIV prevalence among blacks was more than double that of whites (data not shown). Housing status and cocaine injection were associated with being in an HIV+ network: homeless individuals (AOR, 2.0; 95% CI, 1.2 to 3.2) and cocaine injectors (AOR, 1.7; 95% CI, 1.0 to 2.7) were twice as likely to be in HIV+ networks compared with those who were housed and noncocaine injectors. A recent history of incarceration was negatively associated with being in an HIV+ network (AOR, 0.4; 95% CI, 0.2 to 0.7). A secondary analysis was done at the network level (n = 232) and measured the association between racial homophily, risk behaviors, network size, and being in an HIV+ network (Supplemental Digital Content Table S2, http://links.lww.com/QAI/B70). We found that all-black networks remained twice as likely to be in HIV+ networks compared with all-white networks (AOR, 2.4; 95% CI, 1.0 to 6.0). Network size was also associated with network HIV status: with every unit increase in network size, there was a 40% increase in the odds of being in an HIV+ network (AOR, 1.4; 95% CI, 1.1 to 1.8).
DISCUSSION
In this large cohort of drug and sex networks, we found significant racial differences in HIV prevalence: the prevalence of HIV among blacks was more than double that of whites, and in the multivariable logistic regression models, being in an all-black network was strongly associated with being in an HIV+ network. To date, this is the largest risk network study of PWID done in the United States evaluating the risk behaviors and HIV prevalence among these networks. Recent studies of PWID done in India and the Philippines have evaluated network composition and its relationship with HIV risk and found that larger networks and network clustering were significantly associated with HIV transmission.20,21 Although these studies did not particularly address issues related to racial/ethnic inequities in HIV incidence, they show the importance of considering network composition when evaluating HIV risk and building prevention strategies. In the United States, the majority of network studies have been done among heterosexual and men who have sex with men (MSM) populations and show, as we found in our study, that risk behaviors tend to be lower and racial homophily higher among blacks.22–24 Although HIV incidence has been decreasing in the United States,25,26 the persisting disproportionate rate of HIV infection among blacks calls for renewed efforts to diminish this inequity.
Based on our study findings, the higher prevalence of HIV observed among blacks cannot be explained by higher drug and sex risk behaviors because these behaviors were either lower or similar compared with that of whites but, instead, can be explained by racial mixing patterns within HIV + networks. The disproportionately high prevalence of HIV among black networks and the racial homogeneity for drugs and sex partnerships among these networks create an environment where the risk of HIV transmission is much higher even if black network members engage in minimal risk behaviors. Indeed, needle sharing was twice as low in all-black networks compared with all-white networks, and even after controlling for traditional socioeconomic factors, racial inequities persisted. Our results suggest that interventions only targeting individual drug and sex risk behaviors would yield minimal impact in reducing racial inequities. On the other hand, using multilevel interventions combining biomedical approaches, such as preexposure prophylaxis (PrEP) and treatment as prevention (TasP) and behavioral approaches with continued harm-reduction practices, and changing models of care to deliver medication-assisted drug treatment, mental health services, HIV and other infectious diseases treatment in a nonstigmatizing manner might yield more promising results and be more cost-effective.27–30 Indeed, TasP would result in a significant reduction of network HIV viral load and by such would yield a stronger public health benefit among black networks where HIV prevalence is the highest. In addition, changing social norms regarding disclosure of HIV status within a network, reducing stigma, and addressing barriers regarding access to HIV care, primary care, and drug/mental health treatment are also needed.
Although the prevalence of HIV was lower among whites and all-white networks, they remain a vulnerable group for HIV acquisition because our results show that they were less likely to engage in harm reduction practices, a pattern not limited to urban settings. Lessons learned from the 2015 HIV outbreak in rural Indiana, where 135 individuals acquired HIV from injection of prescription opioid oxymorphone, show that when high-risk sex and drug practices occur in somewhat closed networks, rapid HIV transmission takes place.31 Statistics from the Centers for Disease Control and the 2016 U.S. Surgeon General’s report on addiction show that opioid injection is expanding in the United States to reach an alarming epidemic proportion: during 2002–2013, heroin overdose death rates nearly quadrupled, from 0.7 deaths to 2.7 deaths per 100,000 population.32,33 This rapidly evolving epidemic calls for renewed approaches moving away from knee jerk responses, like mass incarceration, and increasing investment in clinical and health services research to improve access to effective prevention and treatment services. Capitalizing on existing risk networks and the social support within these networks might present an opportunity to diffuse multilevel interventions to avoid or treat overdose but also for HIV testing, treatment, and prevention.
Individuals in racially mixed networks were more likely to be in HIV+ networks and to engage in high-risk drug behavior. These findings point to the importance of evaluating the composition of risk networks when building prevention strategies, in particular, to identify and target network members who represent a high transmission risk and those who represent a high acquisition risk for HIV treatment and prevention interventions. The use of personalized approaches within networks, with secondary needle exchange among HIV− partners with high-risk drug practices combined with PreP and treatment of HIV+ members, for example, need to be tested.
Other factors associated with being in an HIV+ network included homelessness and cocaine injection. Homeless individuals were more likely to be in HIV+ networks. Like many urban cities, people living in Philadelphia are segregated by race and socioeconomic status.34 Housing instability geographically restricts where people live and limits sex and drug partnerships, resulting in an increased risk of HIV infection.35,36 Homeless individuals are also disproportionately affected by serious mental illness37 and are less likely to access drug treatment, antiretroviral therapy, and other needed services.38 There are data suggesting that homeless individuals can reduce their risk behaviors with improvement in housing status39; however, these findings have not been replicated in clinical trials.40 Cocaine injectors were more likely to be in an HIV+ network than noncocaine injectors (P = 0.02). HIV transmission risk is known to be higher among people using cocaine, and in some cases, cocaine injection can be predictive of HIV infection in a dose-dependent fashion.41 Surprisingly, we found that people with a recent history of incarceration were less likely to be in an HIV+ network. Many studies have found that incarceration, which is traditionally disproportionately high among black men, destabilizes relationships and influences drug and sex mixing patterns, resulting in an increased risk of HIV infection.13 In this cohort, a higher percentage of all-white network members had a recent history of incarceration compared with all-black network members, which could have been a reflection of the sample recruited or a result of local policing strategies.42 With the expansion of the opioid epidemic, a stronger public health response bridging health professionals, the criminal justice system, and local communities is urgently needed to develop policies that promote public health and access to supportive services over the criminalization of PWID.43
This study has several limitations. Because HIV incidence was very low (a total of 8 seroconversions took place in the study period), we were unable to prospectively measure the risk of acquiring HIV and instead used being in an HIV+ network as the dependent variable. This measure, although limited, is still proportional to the risk of HIV infection. By design, egocentric networks rely on the index participant to recall who their drug and sex network members are and recruit them into the study. The mean network size was 3, and in the majority of cases, HIV+ networks only included 1 HIV+ individual, suggesting that the actual structure of networks was not captured in a comprehensive manner. Methodologic features invested great amount of energy to minimize the number of indexes who were unable to engage network members; however, despite this effort, a few network members were enrolled in the study. This could have been because of the fact that, as part of the protocol, there was no direct attempt from the study team to obtain locating information on network members. Instead, the index participants were responsible to contact and bring in network members for study enrollment. Because of lack of available data on participants who did not enroll, we are unable to know how this could have biased our results; however, it is possible that network members who enrolled were the ones with tighter connections with index participants. Despite these limitations, this study represents one of the largest network studies of PWID. The study relied on self-report for risk behavior, which could be subject to recall or social desirability bias; however, study participants openly reported very risky behaviors and the questions focused on a short time frame for recall. In addition, our findings are consistent with other studies that report on the racial difference in risk behaviors with black MSM engaging in lower individual-level risk behaviors compared with white MSM and extends these findings to PWID.22 Hispanics comprised a small proportion of the cohort (n = 56); for this reason, any findings related to Hispanics have to be interpreted with caution. Because there was no variability of HIV status among that group, an odds ratio could not be generated in the multivariable logistic regression. Although the data for these analyses are a decade old, there is no indication that racial mixing patterns or type of drug has changed in this setting. Despite these limitations, this study provides detailed information on a large sample of PWID and explores the ecological mechanisms of racial inequity in HIV prevalence in an urban American city.
As racial inequity persists in the United States, we cannot afford to continue using the same approach for HIV prevention. Individual and harm reduction behaviors must be coupled with innovative approaches considering network composition and network HIV prevalence and must incorporate biomedical approaches such as TasP, PrEP, drug treatment, and syringe exchange, to effectively reduce the burden of HIV among ethnic minorities and to prevent HIV outbreaks among whites. It is also necessary to strengthen existing infrastructure to support timely linkage and access to comprehensive HIV, mental health, and medication-assisted treatment and move away from criminalization of PWID. The role of economic and housing opportunities for PWID as structural interventions for HIV prevention remain to be tested. Finally, future novel HIV testing and HIV treatment models have to be evaluated in risk networks where people are often marginalized by the health care infrastructure.
Supplementary Material
Acknowledgments
HPTN Scholar’s Program was funded through a supplement from the National Institute of Allergy and Infectious Diseases and the National Institute on Drug Abuse.
Footnotes
Presented as an oral presentation at the 2016 National HIV Prevention Trial Network Meeting; June 2016; Washington, DC and as a poster at the Center for AIDS Research Social and Behavioral Science Research Network; Thursday October 20, 2016.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.jaids.com).
References
- 1.Mitsch AJ, Hall HI, Babu AS. Trends in HIV infection among persons who inject drugs: United States and Puerto Rico, 2008–2013. Am J Public Health. 2016;106:2194–2201. doi: 10.2105/AJPH.2016.303380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spiller MW, Broz D, Wejnert C, et al. HIV infection and HIV-associated behaviors among persons who inject drugs–20 cities, United States, 2012. MMWR Morb Mortal Wkly Rep. 2015;64:270–275. [PMC free article] [PubMed] [Google Scholar]
- 3.Des Jarlais DC, Bramson HA, Wong C, et al. Racial/ethnic disparities in HIV infection among people who inject drugs: an international systematic review and meta-analysis. Addiction. 2012;107:2087–2095. doi: 10.1111/j.1360-0443.2012.04027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Friedman SR, Young PA, Snyder FR, et al. Racial differences in sexual behaviors related to AIDS in a nineteen-city sample of street-recruited drug injectors. AIDS Educ Prev. 1993;5:196–211. [PubMed] [Google Scholar]
- 5.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, USA: a meta-analysis. Lancet. 2012;380:341–348. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 6.De P, Cox J, Boivin JF, et al. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007;102:1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe TT, Harrison KM, Dean HD. Summary of CDC consultation to address social determinants of health for prevention of disparities in HIV/AIDS, viral hepatitis, sexually transmitted diseases, and tuberculosis. Public Health Rep. 2010;125(suppl 4):11. doi: 10.1177/00333549101250S404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hallfors DD, Iritani BJ, Miller WC, et al. Sexual and drug behavior patterns and HIV and STD racial disparities: the need for new directions. Am J Public Health. 2007;97:125–132. doi: 10.2105/AJPH.2005.075747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kottiri BJ, Friedman SR, Neaigus A, et al. Risk networks and racial/ethnic differences in the prevalence of HIV infection among injection drug users. J Acquir Immune Defic Syndr. 2002;30:95–104. doi: 10.1097/00042560-200205010-00013. [DOI] [PubMed] [Google Scholar]
- 10.Williams C, Eisenberg M, Becher J, et al. Racial disparities in HIV prevalence and risk behaviors among injection drug users and members of their risk networks. J Acquir Immune Defic Syndr. 2013;63:S90–S94. doi: 10.1097/QAI.0b013e3182921506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneider JA, Cornwell B, Ostrow D, et al. Network mixing and network influences most linked to HIV Infection and risk behavior in the HIV epidemic among black men who have sex with men. Am J Public Health. 2013;103:e28–e36. doi: 10.2105/AJPH.2012.301003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty IA, Schoenbach VJ, Adimora AA. Sexual mixing patterns and heterosexual HIV transmission among African Americans in the southeastern United States. J Acquir Immune Defic Syndr. 2009;52:114. doi: 10.1097/QAI.0b013e3181ab5e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. J Infect Dis. 2005;191(suppl 1):S115–S122. doi: 10.1086/425280. [DOI] [PubMed] [Google Scholar]
- 14.Riolo CS, Koopman JS, Chick SE. Methods and measures for the description of epidemiologic contact networks. J Urban Health. 2001;78:446–457. doi: 10.1093/jurban/78.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris M, Kurth AE, Hamilton DT, et al. Concurrent partnerships and HIV prevalence disparities by race: linking science and public health practice. Am J Public Health. 2009;99:1023. doi: 10.2105/AJPH.2008.147835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latkin C, Donnell D, Celentano DD, et al. Relationships between social norms, social network characteristics, and HIV risk behaviors in Thailand and the United States. Health Psychol. 2009;28:323. doi: 10.1037/a0014707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latkin CA, Donnell D, Metzger D, et al. The efficacy of a network intervention to reduce HIV risk behaviors among drug users and risk partners in Chiang Mai, Thailand and Philadelphia, USA. Soc Sci Med. 2009;68:740–748. doi: 10.1016/j.socscimed.2008.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Williams CT, Metzger DS. Race and distance effects on regular syringe exchange program use and injection risks: a geobehavioral analysis. Am J Public Health. 2010;100:1068–1074. doi: 10.2105/AJPH.2008.158337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latkin C, Donnell D, Liu TY, et al. The dynamic relationship between social norms and behaviors: the results of an HIV prevention network intervention for injection drug users. Addiction. 2013;108:934–943. doi: 10.1111/add.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cepeda JA, Solomon SS, Srikrishnan AK, et al. Injection drug network characteristics are important markers of HIV risk behavior and lack of viral suppression. J Acquir Immune Defic Syndr. 2017;75:257–264. doi: 10.1097/QAI.0000000000001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verdery AM, Siripong N, Pence BW. Social network clustering and the spread of HIV/AIDS among persons who inject drugs in two cities in the Philippines. J Acquir Immune Defic Syndr. 2017;76:26–32. doi: 10.1097/QAI.0000000000001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan PS, Peterson J, Rosenberg ES, et al. Understanding racial HIV/STI disparities in black and white men who have sex with men: a multilevel approach. PLoS One. 2014;9:e90514. doi: 10.1371/journal.pone.0090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mustanski B, Birkett M, Kuhns LM, et al. The role of geographic and network factors in racial disparities in HIV among young men who have sex with men: an egocentric network study. AIDS Behav. 2015;19:1037–1047. doi: 10.1007/s10461-014-0955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newcomb ME, Mustanski B. Racial differences in same-race partnering and the effects of sexual partnership characteristics on HIV risk in MSM: a prospective sexual diary study. J Acquir Immune Defic Syndr. 2013;62:329. doi: 10.1097/QAI.0b013e31827e5f8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall HI, Song R, Tang T, et al. HIV Trends in the United States: diagnoses and estimated incidence. JMIR Public Health Surveill. 2017;3:e8. doi: 10.2196/publichealth.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Des Jarlais DC, Arasteh K, McKnight C, et al. Racial/ethnic disparities at the end of an HIV epidemic: persons who inject drugs in New York city, 2011–2015. Am J Public Health. 2017;107:e1–e7. doi: 10.2105/AJPH.2017.303787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uyei J, Fiellin DA, Buchelli M, et al. Effects of naloxone distribution alone or in combination with addiction treatment with or without preexposure prophylaxis for HIV prevention in people who inject drugs: a cost-effectiveness modelling study. Lancet Public Health. 2017;2:e133–e140. doi: 10.1016/S2468-2667(17)30006-3. [DOI] [PubMed] [Google Scholar]
- 28.Parashar S, Collins AB, Montaner JS, et al. Reducing rates of preventable HIV/AIDS-associated mortality among people living with HIV who inject drugs. Curr Opin HIV AIDS. 2016;11:507–513. doi: 10.1097/COH.0000000000000297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gardner LI, Marks G, Strathdee SA, et al. Faster entry into HIV care among HIV-infected drug users who had been in drug-use treatment programs. Drug Alcohol Depend. 2016;165:15–21. doi: 10.1016/j.drugalcdep.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 30.Belani H, Chorba T, Fletcher F, et al. Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: summary guidance from CDC and the US Department of Health and Human Services. MMWR Morb Mortal Wkly Rep. 2012;61:1–43. [PubMed] [Google Scholar]
- 31.Des Jarlais DC, Kerr T, Carrieri P, et al. HIV infection among persons who inject drugs: ending old epidemics and addressing new outbreaks. AIDS. 2016;30:815–826. doi: 10.1097/QAD.0000000000001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones CM, Logan J, Gladden RM, et al. Vital signs: demographic and substance use trends among heroin Users-United States, 2002–2013. MMWR Morb Mortal Wkly Rep. 2015;64:719–725. [PMC free article] [PubMed] [Google Scholar]
- 33.Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: HHS; 2016. Mental, Health Services Administration US, and Office of the Surgeon General. [PubMed] [Google Scholar]
- 34.Massey DS, Tannen J. A research note on trends in Black hypersegregation. Demography. 2015;52:1025–1034. doi: 10.1007/s13524-015-0381-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adimora AA, Schoenbach VJ, Floris-Moore MA. Ending the epidemic of heterosexual HIV transmission among African Americans. Am J Prev Med. 2009;37:468–471. doi: 10.1016/j.amepre.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sypsa V, Paraskevis D, Malliori M, et al. Homelessness and other risk factors for HIV infection in the current outbreak among injection drug users in Athens, Greece. Am J Public Health. 2015;105:196–204. doi: 10.2105/AJPH.2013.301656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hwang SW, Kirst MJ, Chiu S, et al. Multidimensional social support and the health of homeless individuals. J Urban Health. 2009;86:791–803. doi: 10.1007/s11524-009-9388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riley ED, Neilands TB, Moore K, et al. Social, structural and behavioral determinants of overall health status in a cohort of homeless and unstably housed HIV-infected men. PLoS One. 2012;7:e35207. doi: 10.1371/journal.pone.0035207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aidala A, Cross JE, Stall R, et al. Housing status and HIV risk behaviors: implications for prevention and policy. AIDS Behav. 2005;9:251–265. doi: 10.1007/s10461-005-9000-7. [DOI] [PubMed] [Google Scholar]
- 40.Wolitski RJ, Kidder DP, Pals SL, et al. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14:493–503. doi: 10.1007/s10461-009-9643-x. [DOI] [PubMed] [Google Scholar]
- 41.Tyndall MW, Currie S, Spittal P, et al. Intensive injection cocaine use as the primary risk factor in the Vancouver HIV-1 epidemic. AIDS. 2003;17:887–893. doi: 10.1097/00002030-200304110-00014. [DOI] [PubMed] [Google Scholar]
- 42.Davis CS, Burris S, Kraut-Becher J, et al. Effects of an intensive street-level police intervention on syringe exchange program use in Philadelphia, PA. Am J Public Health. 2005;95:233–236. doi: 10.2105/AJPH.2003.033563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strathdee SA, Beletsky L, Kerr T, et al. HIV, drugs and the legal environment. Int J Drug Policy. 2015;26:S27–S32. doi: 10.1016/j.drugpo.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.