Abstract
Evaluating fungal contamination indoors is complicated because of the many different sampling methods utilized. In this study, fungal contamination was evaluated using five sampling methods and four matrices for results. The five sampling methods were a 48 hour indoor air sample collected with a Button™ inhalable aerosol sampler and four types of dust samples: a vacuumed floor dust sample, newly settled dust collected for four weeks onto two types of electrostatic dust cloths (EDCs) in trays, and a wipe sample of dust from above floor surfaces. The samples were obtained in the bedrooms of asthmatic children (n = 14). Quantitative polymerase chain reaction (qPCR) was used to analyze the dust and air samples for the 36 fungal species that make up the Environmental Relative Moldiness Index (ERMI). The results from the samples were compared by four matrices: total concentration of fungal cells, concentration of fungal species associated with indoor environments, concentration of fungal species associated with outdoor environments, and ERMI values (or ERMI-like values for air samples). The ERMI values for the dust samples and the ERMI-like values for the 48 hour air samples were not significantly different. The total cell concentrations of the 36 species obtained with the four dust collection methods correlated significantly (r = 0.64–0.79, p < 0.05), with the exception of the vacuumed floor dust and newly settled dust. In addition, fungal cell concentrations of indoor associated species correlated well between all four dust sampling methods (r = 0.68–0.86, p < 0.01). No correlation was found between the fungal concentrations in the air and dust samples primarily because of differences in concentrations of Cladosporium cladosporioides Type 1 and Epicoccum nigrum. A representative type of dust sample and a 48 hour air sample might both provide useful information about fungal exposures.
1. Introduction
Microbial contamination in the homes and workplaces is a major concern.1–3 Various methods have been used to characterize exposures to viable microorganisms and microbial cell debris. Personal or stationary sampling of airborne dust can be performed to quantify inhalation exposure, but can significantly be impacted by seasonal variation and ventilation.4 Floor dust can be used as a surrogate for inhalation exposure and has the added benefit of being less influenced by short-term variability in indoor activities and ventilation.5,6 Electrostatic wiping cloths can be used to collect settled dust from horizontal surfaces and are similar to vacuuming in that they are less influenced by short-term variability.7 The dust fall collector passively collects newly settled dust on an electrostatic dust cloth (EDC), which allows for a long-term sample and eliminates the unknown accumulation time for vacuum and wipe samples.8 Short term air samples have been compared with EDCs and vacuum samples with respect to culturable fungi and bacteria, endotoxin, and the total inflammatory potential.9,10 However, few method comparison studies have utilized quantitative polymerase chain reaction (qPCR) assays in the analysis.
Quantitative PCR reduces the variability associated with culture-based methods in determining indoor microbial biota.11 Mve et al. compared culture, microscopic cell count, β-N-acetylhexosaminidase assay and qPCR within dust collected form heating, ventilation and air conditioning systems and determined that qPCR yielded the most sensitivity and precision.12 Adams et al.compared different passive, settled dust sampling methods to quantify total bacterial and fungal biomass using qPCR. They determined that the choice of passive settled-dust collector did not strongly alter an investigation as the correlations between sampler types were strong both in compositional and quantitative values.13 However, there is has not been a comprehensive evaluation of the relationship between results for dust sampling methods and long-term air samples.
In this study, four different types of settled dust and a 48 hour air sample were obtained from the homes of children with asthma. Using qPCR, 36 fungal species were evaluated and divided into two groups, in which Group 1 species have shown to be associated with homes with water damage and Group 2 species have been found in homes independent of water damage. These molds generate an Environmental Relative Moldiness Index (ERMI) value, which describes the mold burden in a home with a single numeric value. Our goal was to compare the estimates of mold contamination in homes obtained from air sampling and four dust collection methods: sedimentation onto two types of electrostatic dust cloths (EDCs), a floor vacuum sample, and a surface wipe sample.8,10,11,14
2. Methods and Materials
2.1 Home Selection
The homes (n = 14) utilized for this study were selected from homes recruited for the “HEPA Intervention Study for Asthma” conducted in Cincinnati, OH. The HEPA intervention study is evaluating the impact of HEPA filtration on the air quality and the asthma of children. The samples referenced in this paper were collected during the placebo treatment of the study. Assessment of conditions in the home such as age and type of building, number of occupants, type of ventilation and indications of moisture damage, were recorded by the research team. Nine of the 14 homes in this study were single family homes, and the remaining were apartments or condominiums. The homes were built between 1865 and 2011. Eight homes were heated with a gas furnace, and six were heated with electric furnaces and all but two utilized air conditioning. Seven of the 14 homes had some type of moisture damage observations (moldy odor, visible mold, or visible moisture damage). Home specific observations can be seen in Table S1.† This study required and received approval from the Institutional Review Board of the University of Cincinnati.
2.2 Collection Methods
In the laboratory, two Dutch EDCs (Albert Heijn, Zaandam, Netherlands) (each 20.2 × 26.5 cm) were placed in two aluminum trays (each 26.4 × 32.4 cm) cleaned with 70% ethanol.15 The trays were then placed into a polypropylene bag until arrival at the home. Also in the laboratory, a cardboard box (62 cm × 28 cm) was covered with an aluminum foil sheet and then a Swiffer extra-large cloth (Procter & Gamble, Cincinnati, OH) (45.2 × 25.4 cm). The box was closed and was not opened until arrival in the home. Different types of EDCs have been used in previous studies,13,16 and we compared these EDCs to ensure that they would provide similar results. The trays were placed in the bedroom at a location where they were least likely to be disturbed,e.g., top of a bookshelf or desk. These EDCs were left to collect the settling dust for four weeks. At the end of the four weeks, the cloths were recovered by folding the sides on top of each other and then the folded EDCs were placed in a sealable plastic bag (Ziplock®, SC Johnson, Racine, WI). The samples were placed in a cooler (4 °C) and returned to the laboratory where they were stored at −20 °C until analyzed.
During the last two days of the four-week period, airborne inhalable particles were collected onto 25 mm diameter, 1 μm pore-size polytetrafluoroethylene (PTFE) filter (Merck Millipore, Billerica, MA) using a Button™ sampler (SKC, Inc., Eighty-four, PA) at 4 l min−1 for 48 hours. Filters collected with the Button™ samplers were placed into a glass-bead filled tube and stored at −20 °C before analysis.
After the sedimentation and air samples were collected, a floor dust sample was obtained in the child’s bedroom by vacuuming 1 m2 of a rug for 4 min m−2 (2 min horizontally and 2 min vertically). For a non-carpeted floor (wood, linoleum, or tile), the sample was collected from the entire room at a rate of 1 min m−2, as previously described.17 The vacuuming was performed using a Filter Queen Majestic® (HMI Industries Inc., Seven Hills, Ohio) vacuum cleaner with a high-efficiency particle (HEPA) filter trap (Midwest Filtration, Cincinnati, OH).18 These traps were recovered and placed in a sealable plastic Ziploc® bag, placed in a cooler (4 °C) and returned to the laboratory where the samples were stored at −20 °C until analyzed.
Immediately after the collection of the vacuum dust sample, a wipe dust sample was collected using a regular-sized dry, Swiffer cloth (26.5 × 20.3 cm) in the child’s bedroom where dust collects, e.g., door frames, bookshelves and window sills. Once the cloth was gray with dust, it was placed in a Ziploc® plastic bag, placed in a cooler (4 °C) and returned to the laboratory where the samples were stored at −20 °C until analyzed.
Temperature and humidity were recorded (HOBO Humidity Data Logger, Onset, Borne, MA) for the entire one-month duration of the EDC sampling.
2.3 Quality Control
A minimum of three blanks of each method, e.g., vacuum filter traps, Swiffer wipe cloths, sedimentation Swiffer EDCs, and sedimentation Dutch EDCs, were collected and placed in a Ziploc® plastic bag and stored at −20 °C before analysis. Blank Button™ filters were also placed in glass bead tubes and stored at −20 °C before analysis. Control outdoor air samples were collected using Button™ samplers outside each home during the same time as the indoor air samples to assess outdoor species.
2.4 Fungal Analysis
Dust was recovered from the surface wipe sample cloths and passive collection EDCs using a laboratory paddle blender (Seward 80 Stomacher® Lab Blender, Seward Limited, Worthing, UK).13 Each surface wipe and EDC was placed into a sterile Stomacher® roll-bag. Two extractions of 30 ml sterile water plus 0.05% Tween 20 were used. The roll-bags were placed in the Stomacher® for 10 min per extraction. The two extracts (total 60 ml) were pelleted by centrifugation (6000 × g, 15 min, 4 °C) and the pellets dried at 30 °C.
Five mg of sieved (300 μm pore size) vacuum dust, 5 mg of surface wipe pellet, 5 mg of sedimentation EDC pellet, or the entire air sampling PTFE filter was added to a 2 ml extraction tube containing 0.3 g of glass beads, as previously described.13 Each sample (vacuum, wipe, Dutch, Swiffer and Button) was spiked with 1 × 106 conidia of Geotrichum candidum at the time of extraction as an internal reference to ensure that the extraction and purification were performed correctly.19 A bead beater (Biospec Products, Bartlesville, OK) was used to shake each extraction tube at 5000 rpm for one minute to release the DNA from the cells. The DNA was then purified using the DNA-EZ extraction kit (GeneRite, Monmouth Junction, NJ), following the manufacturer’s instructions.
The fungal species that form the ERMI20–22 were quantified with qPCR assays described earlier.23 The standard qPCR assay contained 1 μl of a mixture of forward and reverse primers at 25 μM each, 12.5 μl of “Universal Master Mix” (Applied Biosystems Inc., Foster City, CA), 2.5 μl of 2 mg ml−1 fraction V bovine serum albumin (Sigma Chemical, St. Louis, MO), 2.5 μl of a 400 nM TaqMan probe (Applied Biosystems Inc., Foster City, CA) and 2.5 μl of DNA free water (Cepheid, Sunnyvale, CA). Five μl of the DNA extract from the sample and this mix were combined. Reactions were performed with thermal cycling conditions consisting of 2 min at 50 °C, 10 minutes at 95 °C, followed by 40 cycles of 15 seconds at 95 °C for template denaturation and 1 minute at 60 °C for probe and primer annealing and primer extension. The ERMI value for each sample was then calculated, as described previously.20,24
The ERMI metric classifies the 36 indicator mold species into two groups (Table S2†). Group 1 includes the 26 species indicating water damage, and Group 2 includes 10 species which come primarily from outdoors and are commonly found in homes, even without water damage, across the United States.20 The ERMI calculation mathematically converts the results from the concentrations (cells per mg dust) of each of 36 molds into a single number as shown in eqn (1).
Since air samples are in different units (cells per m3) than the dust samples (cells per mg), the term “ERMI-like” was used instead of ERMI for air samples.25
2.5 Statistical Analysis
Statistical analysis in R (version 3.1.1) was used to compare the methods in terms of ERMI/ERMI-like values and the total cell concentrations (sum of the cell concentrations of 36 species; cells per mg for Swiffer EDC, Dutch EDC, vacuum and wipe; cells per m3 for Button™ air samples), as well as concentrations of Group 1 species and Group 2 species (sum of the cell concentrations of species in Group 1 and Group 2, respectively). Kruskal–Wallis test by ranks was performed to determine if there was a difference in cell concentrations and ERMI/ERMI-like values between the methods. Spearman’s correlation was performed to determine if there was a correlation between the results obtained with the tested methods. When comparing the species-specific data, the concentration results were normalized to account for the differing units between the air and dust samples. Each sample was normalized to a percentage so that the concentration of each species was divided by the total cell concentration of the 36 species in that sample and multiplied by 100. A multidimensional scaling (MDS) ordination plot was created from normalized sample data to visually demonstrate the relationships within fungal species across the various methods.26 Multidimensional scaling is a method for visualizing proximities or similarities of individual points in the multidimensional data. The idea of MDS is to place each multidimensional data point into two dimensional space in such a way that the distances between points are preserved as well as possible. The corresponding two dimensional points are then easily visualized using two dimensional scatterplots, as opposed to multidimensional plots that are hard to interpret.
Principal component analysis (PCA) was also performed to represent the normalized species data.27 While principle component analysis (PCA) is also a multivariate data analysis method similar to MDS, the main idea of PCA is to reduce the dimensionality of the data by considering a smaller number of important variables (usually two or three), called principle components. In PCA, these principal components are constructed as linear combinations of the original variables in the data in such a way that (1) they are orthogonal or uncorrelated to each other, and (2) they account for as much of the variability in the observed data as possible. These lower dimensional principal components are then visualized in two or three dimensional plots. Finally, also utilizing the normalized data, multivariate analysis of variance (MANOVA) followed by analysis of variance (ANOVA) was performed to determine if there were differences in the species specific data obtained with the five sampling methods. If the multivariate test, MANOVA, found the species within the test were significantly different, ANOVA was performed to determine which species were causing this difference. P-Values less than 0.05 were considered significant for all tests with the exception of the ANOVA. Bonferroni adjustment for multiple comparisons was performed on the ANOVA p-values to determine significance.
3. Results
3.1 Differences within and correlation between sampling results
The median of the total cell concentrations in the dust samples ranged from 3135 to 8427 cells per mg, with both passive collections yielding the highest concentrations. The median for the Button™ air samples was 44 cells per m3 (Fig. 1). There were no significant differences found in the total cell concentrations (cells per mg) collected with any of the four dust sampling methods. However, there was a significant difference in the total concentration of fungal cells collected in the air samples and four dust sampling methods (Fig. 1, right y-axis). The median ERMI values for the dust collection methods ranged from −0.02 to 1.3, and the median ERMI-like value for the Button™ air sample was 0.6 (Fig. 2). There was no significant difference between the ERMI values calculated for each of the four dust sampling methods or the ERMI-like values calculated from the results of the air samples.
Fig. 1.
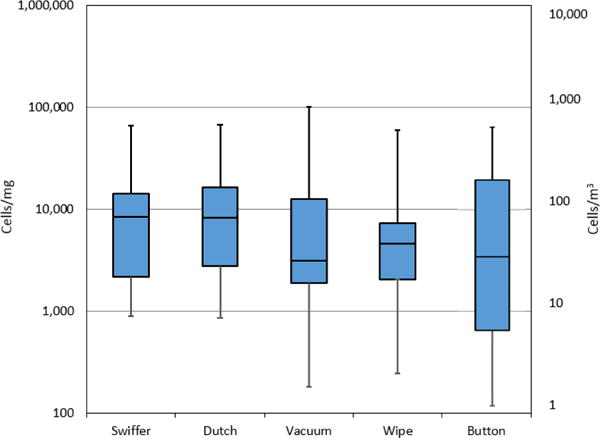
Cell concentrations obtained with five tested methods. Horizontal lines in the box plot represent the minimum, 25%, 50%, 75% percentiles and the maximum. Swiffer, Dutch, vacuum and wipe are in cells per mg and Button™ air samples in cells per m3 (right y-axis).
Fig. 2.
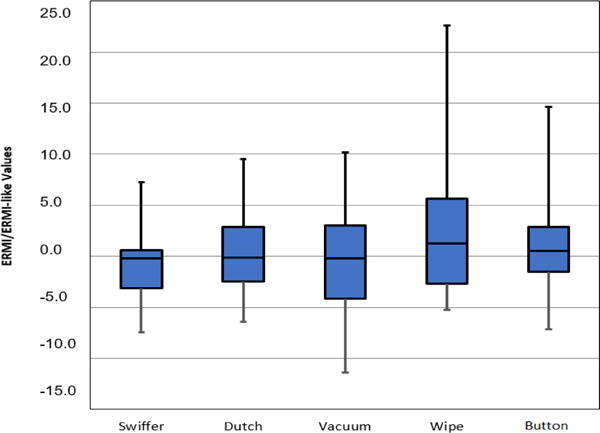
ERMI (Swiffer, Dutch, vacuum and wipe) and ERMI-like values (Button™ air samples) obtained with the five tested methods. The horizontal lines in the box plot from the bottom represent the minimum, 25%, 50%, 75% percentiles, and the maximum.
The total cell concentrations obtained with three of the dust sampling methods, Swiffer EDC, Dutch EDC and wipe values, were significantly correlated (range of r = 0.64–0.79, p < 0.05). The strongest correlation for the total cell concentrations was found between the sedimentation Dutch EDCs and wipe sample results (r = 0.79, p < 0.01) (Table 1). The total cell concentrations obtained with vacuum and wipe methods had a significant correlation (r = 0.67, p < 0.05), but a significant correlation was not found between the concentrations of the vacuum and the passive dust collection methods (Dutch EDC or Swiffer EDC).
Table 1.
Correlation coefficients obtained with the five tested methods for total, Group 1, and Group 2 concentrations, as well as ERMI and ERMI-like valuesa
| Method | Comparison Method | Total Concentration r | Group 1 Concentration r | Group 2 Concentration r | ERMI & ERMI-like r |
|---|---|---|---|---|---|
| Swiffer EDC | Dutch EDC | 0.71** | 0.86*** | 0.69** | 0.78** |
| Vacuum | 0.42 | 0.80** | 0.28 | 0.60* | |
| Wipe | 0.64* | 0.68** | 0.62* | 0.93*** | |
| Button | 0.17 | 0.10 | 0.40 | 0.52 (p=0.06) | |
|
|
|||||
| Dutch EDC | Swiffer EDC | 0.71** | 0.86*** | 0.69** | 0.78** |
|
|
|||||
| Vacuum | 0.53 (p=0.06) | 0.75** | 0.34 | 0.80** | |
| Wipe | 0.79** | 0.77** | 0.70** | 0.90*** | |
| Button | 0.12 | 0.22 | 0.29 | 0.38 | |
|
|
|||||
| Vacuum | Swiffer EDC | 0.42 | 0.80** | 0.28 | 0.60* |
| Dutch EDC | 0.53 (p=0.06) | 0.75** | 0.34 | 0.80** | |
|
|
|||||
| Wipe | 0.67* | 0.76** | 0.55* | 0.76** | |
| Button | 0.06 | 0.39 | −0.09 | 0.33 | |
|
|
|||||
| Wipe | Swiffer EDC | 0.64* | 0.68** | 0.62* | 0.93*** |
| Dutch EDC | 0.79** | 0.77** | 0.70** | 0.90*** | |
| Vacuum | 0.67* | 0.76** | 0.55* | 0.76** | |
|
|
|||||
| Button | 0.07 | 0.12 | 0.12 | 0.42 | |
|
|
|||||
| Button | Swiffer EDC | 0.17 | 0.10 | 0.40 | 0.52 (p=0.06) |
| Dutch EDC | 0.12 | 0.22 | 0.29 | 0.38 | |
| Vacuum | 0.06 | 0.39 | −0.09 | 0.33 | |
| Wipe | 0.07 | 0.12 | 0.12 | 0.42 | |
Swiffer, Dutch, vacuum and wipe results are in concentration (cells/mg) and ERMI values; Button air sample results are in concentration (cells/m3) and ERMI-like values. Repeated information is not shaded.
p<0.50,
p<0.01,
p<0.001
The ERMI values obtained with all four dust collection methods were significantly correlated (range of r = 0.60–0.93, p < 0.01, Table 1). When evaluating the total cell concentrations of just the 26 Group 1 species, the dust samples all correlated significantly (range of r = 0.68–0.86, p < 0.01, Table 1). When only the 10 Group 2 species were compared, the total concentrations in vacuumed-floor samples were significantly correlated with the wipe samples (r = 0.55, p < 0.05) and the two newly-settled dust samples (Dutch and Swiffer EDC) and wipe samples were also significantly correlated (range of r = 0.62–0.70, p < 0.05). However, Group 2 concentrations were not correlated between the vacuumed-floor dust samples and the newly-settled dust samples (Table 1).
None of the correlations between air and dust results were significant. However, a borderline significant correlation was demonstrated between the ERMI-like Button™ air sampling results and the ERMI Swiffer EDC results (r = 0.52, p = 0.06, Table 1).
3.2 Multidimensional scaling ordination plot
Fig. S1† shows each normalized sample taken within all five tested methods plotted separately on a MDS ordination plot. This plot reflects the species similarities between samples and allows for the visualization of the relationships between the different sampling methods by clustering. As expected from the analysis described in Table 1, the dust sample results clustered together regardless of sampling method. However, the air sampling results clustered loosely on the right side of the plot (Fig. S1†). Principal component analysis orthogonal plot shows the same trends as the MDS ordination plot with the dust collection methods and air samples clustering separately (Fig. S2†).
3.3 Species abundance, multivariate analysis of variance (MANOVA) and analysis of variance (ANOVA)
Across all methods, the 10 most abundant species accounted for 90% of the 36 species sampled based on the normalized data. Within each method, the most abundant 10 species accounted for 94% in Swiffer and Dutch results, 92% in vacuum results, 88% in wipe results and 83% in Button™ air sampling results. The concentrations of each of the 10 species for each method can be seen in Table S3.† The normalized data was averaged by method and the percentage of each of the 10 species within each method can be seen in Fig. S2.†
MANOVA followed by ANOVA was performed on the normalized data to study differences in species-specific results between the methods (Table 2). MANOVA analysis showed that there was no significant difference when including the results of all of the 36 species from the dust collection methods. However, the results for the dust sampling and air sampling methods combined were borderline significantly different (p = 0.08, Table 2). Further ANOVA analysis of the results of the 36 species from the dust sampling methods and the air samples revealed Cladosporium cladosporioides Type 1 and Epicoccum nigrum percentages were significantly different between the methods. MANOVA analysis of the 26 Group 1 species yielded no difference between the dust methods with or without air sampling. However, MANOVA analysis showed that the composition of the 10 Group 2 species was significantly different between the sampling methods. Further ANOVA analysis within the dust collection methods revealed that Cladosporium cladosporioides Type 1 was the only species in Group 2 with a significant difference. Further ANOVA analysis within the air and dust sample collection methods revealed Cladosporium cladosporioides Type 1 and Epicoccum nigrum were significantly different between the methods.
Table 2.
MANOVA and ANOVA results on differences in the species-specific data within all collection methods
| Data | Statistical Test | Dust Methods | Air and Dust Methods |
|---|---|---|---|
| p-value | p-value | ||
| 36 Fungal Species | MANOVA | NS | p=0.08 |
| ANOVA | NA | p<0.0011,2 | |
| Group 1 Species | MANOVA | NS | NS |
| ANOVA | NA | NA | |
| Group 2 Species | MANOVA | p<0.05 | p<0.001 |
| ANOVA | p=0.0071 | p<0.0011,2 |
Cladosporium cladosporioides 1,
Epicoccum nigrum, NS – Not significant, NA – Not applicable, Significant p-values are bolded
The two most abundant and statistically different species were Cladosporium cladosporioides Type 1 and Epicoccum nigrum. Air samples consisted of 27% C. cladosporioides and 13% E. nigrum, and dust samples consisted of 6% to 17% C. cladosporioidesand 45% to 64% E. nigrum (Fig. S3†). For the dust sampling methods, the medians of C. cladosporioides concentrations ranged from 290 to 490 cells per mg and the median in air samples was 4 cells per m3. For Epicoccum nigrum, the median concentrations in the dust samples ranged from 1100 to 2450 cells per mg, and the median value in air samples was 8 cells per m3. Cladosporium cladosporioides and E. nigrum are represented in boxplots for each of the methods in Fig. 3.
Fig. 3.
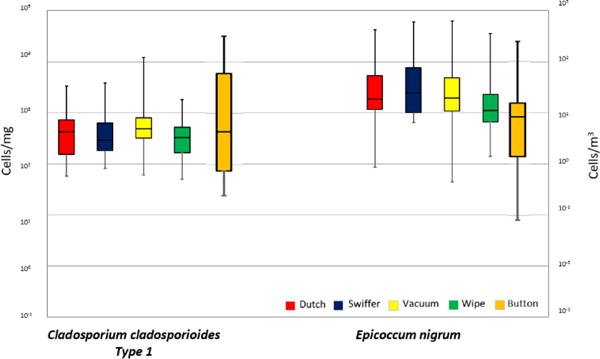
Cell concentrations of the two species that were found to cause differences in the MANOVA/ANOVA analysis between methods. The horizontal lines in the box plot from the bottom represent the minimum, 25%, 50%, 75% percentiles, and the maximum. Swiffer, Dutch, vacuum and wipe are in cells per mg and Button™ air samples in cells per m3 (right y-axis).
3.4 Quality control and environmental conditions
The concentrations in the blank Button™ samples ranged 1–25 cells per m3, which comprised ≤1.5% of the averaged Button™ samples (1660 cells per m3). The concentrations in the vacuum blanks ranged from 0–51 cells per mg, which consisted of ≤0.3% of the averaged vacuum samples (14 619 cells per mg), and the respective values of the wipe blanks ranged from 2–9 cells per mg, which consisted of ≤0.1% of the averaged wipe samples (8542 cells per mg). The concentrations Swiffer blanks ranged from 10–284 cells per mg and consisted of ≤2% of the averaged Swiffer samples (13 121 cells per mg), and the values in Dutch blanks ranged from 2–27 cells per mg, which constituted ≤0.2% of the averaged Dutch samples (13 266 cells per mg). All blanks were under 51 cells per mg with the exception of one Swiffer EDC cloth (284 cells per mg) where Epicoccum nigrum and Aspergillus versicolor were the primary contributors to the increased cell count. Outdoor air samples consisted of 33% C. cladosporioidesand 31% E. nigrum. The outdoor Button™ validated the species associated with outdoor sources and showed consistency with the indoor air samples (Fig. S3†). The temperature in the homes collected for the entire month of passive Swiffer and Dutch sample collection ranged from 16 °C to 36 °C with an average of 23 °C and a relative humidity that ranged from 15% to 98% with an average of 45%. Sampling occurred in the span of one year and all seasons were included. Home specific temperature and humidity data can be seen in Table S4.†
4. Discussion
We found a strong correlation between floor dust and wipe sample estimates of mold contamination, based on qPCR data, both for total cell concentrations and ERMI values. A previous study reported a strong correlation between floor dust and wipe sample estimates of mold contamination based on culture data.9 The ERMI metric was created from the analysis of vacuumed, floor dust samples collected during the American Healthy Homes Survey.20 Obtaining a floor dust sample using a vacuum cleaner can be cumbersome, time-consuming and often inconvenient. Our results show that the results from the qPCR analysis of total cells or after calculation of the ERMI will provide comparable results for dust collected by vacuuming the floor or by collecting dust by wiping above floor surfaces.
Cell concentrations in wipe samples were also consistent with the concentrations in newly settled-dust, i.e. settled during a four-week period and collected in a tray containing either the Dutch or Swiffer EDC. However, the total cell concentrations in newly-settled dust samples were different from the floor dust concentrations. This difference was not associated with the concentrations of Group 1 molds, which were all correlated, but with Group 2 mold concentrations, which were not correlated. Group 2 molds are associated with outdoor sources and are more likely to be influenced by seasonal variations. Since newly settled dust sample types represent different sampling periods, they may be affected by the seasonal variation of outdoor concentrations by different degrees. If sampling occurred in the spring or fall, during higher mold trends, the outdoor concentrations in Group 2 could also be increased.
We found that either the Dutch or Swiffer EDC provided similar results in assessing total cells or ERMI values. Adams et al.13 also tested different materials for passive dust collection. They found little difference in the recovery of fungal biomass, analyzed using qPCR, from any of the passive collectors tested, including EDC. However, Adams et al.13 did not compare their results with other dust collection methods, such as floor vacuuming or above-floor wipes.
The total cell concentrations in air and dust samples cannot be directly compared due to different units. However, when comparing the ERMI values obtained with dust sampling and the ERMI-like values obtained with the air sampling, no significant difference was found. Our results also showed a borderline correlation of ERMI-like values for the air samples and ERMI values for the dust collected with the passive Swiffer EDC. This result is consistent with Frankel et al.9 who demonstrated that two types of air sampling measurements (5 hour impinger and 6 hour filter collection) correlated significantly with passive dust fall collection and vacuum sampling results for the estimation of mold exposure.9 Thus, the ERMI-like index calculated for air samples could also provide comparable results to the ERMI obtained with dust collection methods.
The MANOVA analyses demonstrated that the species composition between the four types of dust samples were not significantly different from each other. However, a significant difference was found between the results for the four dust sampling methods and air sampling method in the composition of the mold species. The ANOVA analysis showed that only one species, C. cladosporioides, of the Group 2 molds was different in the samples from the four dust sampling methods, and two species, Cladosporium cladosporioides and Epicoccum nigrum, of the Group 2 molds were different when considering both the air and dust sampling methods. The differences in the species composition results between dust and air samples can also be seen in the multidimensional scaling ordination plot where dust samples were clustered on the left of the plot, separate from the air samples on the right.
Cladosporium cladosporioides was the most common species in air samples whereas Epicoccum nigrum was the most common in dust samples. While both E. nigrum and C. cladosporioides are considered to be mostly outdoor saprophytic fungi, the differences in size and shape could determine the prevalence of each of the species in dust and air samples. The conidia of E. nigrum are roughly spherical to pyriform shape most measuring 15–25 μm physical diameter and 11.8 μm aerodynamic diameter,28–30 and the conidia of C. cladosporioides are ellipsoidal to lemon-shaped most measuring 3.6–4 μm physical diameter and 2.8–5.5 μm aerodynamic diameter.30–32 Due to the larger size of E. nigrum spores, they are likely to settle down from the air at a higher rate and be more associated with dust samples, which was confirmed as E. nigrum was 45–64% of the total fungal concentrations in dust samples. Alternatively, due to the smaller size of C. cladosporioides spores, they remain airborne longer allowing them to be captured by air sampling, which was also confirmed as C. cladosporioides was 27% of the total fungal concentration in air samples. The ventilation and air circulation in each home could have varied, which would have impacted the rate at which the spores stay airborne. However, all homes were using a placebo air purifier at the same setting with the room sizes between 59 and 201 ft2. Activities in the sampling room, such as cleaning, could also affect the air and dust samples, however, in an effort to minimalize these effects the participants were asked not to clean and to keep other activities consistent.
One limitation of our study was the relatively small number of homes studied. This could have caused the low statistical significance of the correlations between some dust and air samples. While only one type of air sampler was selected for this study, the selection of a suitable bioaerosol sampler was based on long-term (48 hours) air sampling to be utilized for DNA analysis.33 Media such as agar or liquid impinger could not have withstood the time duration and Button™ samplers have been shown to be efficient at collecting a wide range of particle sizes.34 In addition, our longer 48 hour inhalable air samples provided a more detailed assessment to better compare with dust samples than previous experiments with air sampling. While this study only focused on a limited number of species, the 36 molds quantified can be representative of the species that can originate from both inside and outside the home.
5. Conclusions
All of the dust collection methods provided fungal assessments consistent with each other. Among the four tested result matrices, the cell concentrations of Group 1 species had the strongest correlations between the results obtained with the four dust collection methods. However, the air samples results were significantly different and showed little correlation with the results from the four tested dust collection methods. The results from air and dust samples were comparable only when using the ERMI and ERMI-like indices. A representative type of dust sample and a 48 hour air sample might both provide useful information about mold exposures.
Supplementary Material
Environmental Significance.
Many different sampling and analytical methods have been used to quantify fungal contamination in homes and other buildings. The sampling methods have generally been in the form of short air samples, usually 5 to 10 min, or the collection of dust. Dust sampling methods and long-term air samples have not been previously compared. In this study, we analysed concentrations of 36 common fungal species, using qPCR, based on 5 sampling methods demonstrating a 48 hour air sample and a representative type of dust provide complementary information about fungal exposures.
Acknowledgments
This work was supported by the Department of Housing and Urban Development (Healthy Homes Technical Studies Grant No. OHHHU0027-14) and the United States Environmental Protection Agency. JC was partially funded by the University of Cincinnati Education and Research Center funded by the National Institute for Occupational Safety and Health (Grant No. T42-OH008432).
The U.S. Environmental Protection Agency (EPA) through its Office of Research and Development collaborated in the research described here. It has been subjected to the Agency’s peer review and has been approved as an EPA publication. Mention of trade names or commercial products does not constitute endorsement or recommendation by the EPA for use. The findings and the conclusions in this report are those of the authors and do not necessarily represent the views of the US EPA.
Footnotes
Conflicts of Interest
There are no additional conflicts to declare.
References
- 1.Nevalainen A, Täubel M, Hyvärinen A. Indoor Air. 2015;25:125–156. doi: 10.1111/ina.12182. [DOI] [PubMed] [Google Scholar]
- 2.Kettleson EM, Adhikari A, Vesper S, Coombs K, Indugula R, Reponen T. Environmental Research. 2015;138:130–135. doi: 10.1016/j.envres.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Górny RL, Reponen T, Willeke K, Schmechel D, Robine E, Boissier M, Grinshpun SA. Applied and Environmental Microbiology. 2002;68:3522–3531. doi: 10.1128/AEM.68.7.3522-3531.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flannigan B. Journal of Aerosol Science. 1997;28:381–392. [Google Scholar]
- 5.Wickman M, Gravesen S, Nordvall SL, Pershagen G, Sundell J. Journal of Allergy and Clinical Immunology. 1992;89:752–759. doi: 10.1016/0091-6749(92)90384-e. [DOI] [PubMed] [Google Scholar]
- 6.Meyer HW, Würtz H, Suadicani P, Valbjørn O, Sigsgaard T, Gyntelberg F. Indoor Air. 2004;14:65–72. doi: 10.1046/j.1600-0668.2003.00213.x. [DOI] [PubMed] [Google Scholar]
- 7.Thorne PS, Metwali N, Avol E, McConnell RS. Ann Occup Hyg. 2005;49:401–406. doi: 10.1093/annhyg/mei002. [DOI] [PubMed] [Google Scholar]
- 8.Würtz H, Sigsgaard T, Valbjørn O, Doekes G, Meyer HW. Indoor Air. 2005;15:33–40. doi: 10.1111/j.1600-0668.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 9.Frankel M, Timm M, Hansen E, Madsen A. Indoor Air. 2012;22:405–414. doi: 10.1111/j.1600-0668.2012.00770.x. [DOI] [PubMed] [Google Scholar]
- 10.Noss I, Wouters IM, Visser M, Heederik DJ, Thorne PS, Brunekreef B, Doekes G. Appl Environ Microbiol. 2008;74:5621–5627. doi: 10.1128/AEM.00619-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shorter C, Täubel M, Pierse N, Douwes J, Howden-Chapman P, Hyvärinen A, Crane J. J Allergy Clin Immunol. 2016;137:622–624. doi: 10.1016/j.jaci.2015.06.039. [DOI] [PubMed] [Google Scholar]
- 12.Mve MJBB, Cloutier Y, Lacombe N, Lavoie J, Debia M, Marchand G. Environ Monit Assess. 2016;189:8. doi: 10.1007/s10661-016-5682-8. [DOI] [PubMed] [Google Scholar]
- 13.Adams RI, Tian Y, Taylor JW, Bruns TD, Hyvärinen A, Täubel M. Microbiome. 2015;3:1. doi: 10.1186/s40168-015-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Täubel M, Rintala H, Pitkäranta M, Paulin L, Laitinen S, Pekkanen J, Hyvärinen A, Nevalainen A. J Allergy Clin Immunol. 2009;124:834–840. doi: 10.1016/j.jaci.2009.07.045. [DOI] [PubMed] [Google Scholar]
- 15.Kilburg-Basnyat B, Metwali N, Thorne PS. J Occup Environ Hyg. 2016;13:85–93. doi: 10.1080/15459624.2015.1078468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noss I, Doekes G, Sander I, Heederik DJ, Thorne PS, Wouters IM. Ann Occup Hyg. 2010;54:651–658. doi: 10.1093/annhyg/meq026. [DOI] [PubMed] [Google Scholar]
- 17.Appatova AS, Ryan PH, LeMasters GK, Grinshpun SA. J Environ Plann Manag. 2008;51:631–646. [Google Scholar]
- 18.Cho SH, Reponen T, Bernstein DI, Olds R, Levin L, Liu X, Wilson K, LeMasters G. Sci Total Environ. 2006;371:31–43. doi: 10.1016/j.scitotenv.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haugland RA, Brinkman N, Vesper SJ. J Microbiol Methods. 2002;50:319–323. doi: 10.1016/s0167-7012(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 20.Vesper S, McKinstry C, Haugland R, Wymer L, Bradham K, Ashley P, Cox D, Dewalt G, Friedman W. J Occup Environ Med. 2007;49:829–833. doi: 10.1097/JOM.0b013e3181255e98. [DOI] [PubMed] [Google Scholar]
- 21.Täubel M, Karvonen AM, Reponen T, Hyvärinen A, Vesper S, Pekkanen J. Appl Environ Microbiol. 2016;82:578–584. doi: 10.1128/AEM.02785-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adhikari A, Kettleson EM, Vesper S, Kumar S, Popham DL, Schaffer C, Indugula R, Chatterjee K, Allam KK, Grinshpun SA, Reponen T. Sci Total Environ. 2014;482–483:92–99. doi: 10.1016/j.scitotenv.2014.02.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Microbiological and Chemical Exposure Assessment EPA Technology for Mold Identification and Enumeration, https://irp-cdn.multiscreensite.com/c4e267ab/files/uploaded/gCQnkBNWQuSD96fPIikY_EPA_Technology%20for%20Mold%20Identification%20and%20Enumeration.pdf, accessed 2/23, 2017.
- 24.Vesper S, McKinstry C, Cox D, Dewalt G. Bull N Y Acad Med. 2009;86:850–860. doi: 10.1007/s11524-009-9384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Méheust D, Gangneux JP, Reponen T, Wymer L, Vesper S, Le Cann P. Sci Total Environ. 2012;438:319–324. doi: 10.1016/j.scitotenv.2012.08.085. [DOI] [PubMed] [Google Scholar]
- 26.Borg I, Groenen PJ. Modern Multidimensional Scaling: Theory and Applications. Springer Science & Business Media; 2005. [Google Scholar]
- 27.Bro R, Smilde AK. Anal Methods. 2014;6:2812–2831. [Google Scholar]
- 28.Mims CW, Richardson EA. Can J Bot. 2005;83:1354–1363. [Google Scholar]
- 29.Yamamoto N, Schmechel D, Chen BT, Lindsley WG, Peccia J. J Aerosol Sci. 2011;42:499–507. [Google Scholar]
- 30.Yamamoto N, Nazaroff WW, Peccia J. J Aerosol Sci. 2014;78:1–10. [Google Scholar]
- 31.Reponen T, Grinshpun S, Conwell K, Wiest J, Anderson M. Grana. 2001;40:119–125. [Google Scholar]
- 32.Cole GT, Samson RA. In: Mould Allergy. Al-Doory Y, Domson JF, editors. Lea & Febiger; Philadelphia, PA: 1984. pp. 66–104. [Google Scholar]
- 33.Kulkarni P, Baron PA, Willeke K. Aerosol measurement: principles, techniques, and applications. John Wiley & Sons; 2011. [Google Scholar]
- 34.Aizenberg V, Reponen T, Grinshpun S, Willeke K. AIHAJ. 2000;61:855–864. doi: 10.1080/15298660008984598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


