Abstract
Exposure to the toxins methylene cyclopropyl acetic acid (MCPA) and methylene cyclopropyl glycine (MCPG) of unripe ackee and litchi fruit can lead to hypoglycemia and death; however, the molecular mechanisms by which MCPA and MCPG cause hypoglycemia have not been established in vivo. To determine the in vivo mechanisms of action of these toxins we infused them into conscious rodents and assessed rates of hepatic gluconeogenesis and ketogenesis, hepatic acyl-CoA and hepatic acetyl-CoA content, and hepatocellular energy charge. MCPG suppressed rates of hepatic β-oxidation as reflected by reductions in hepatic ketogenesis, reducing both short- and medium-chain hepatic acyl-CoA concentrations. Hepatic acetyl-CoA content decreased, and hepatic glucose production was inhibited. MCPA also suppressed β-oxidation of short chain acyl-CoAs, rapidly inhibiting hepatic ketogenesis and hepatic glucose production, depleting hepatic acetyl-CoA content and ATP content, while increasing other short chain acyl CoAs. Utilizing a recently-developed positional isotopomer NMR tracer analysis (PINTA) method we demonstrated that MCPA-induced reductions in hepatic acetyl-CoA content were associated with a marked reduction of hepatic pyruvate carboxylase flux. Taken together, these data reveal the in vivo mechanisms of action of MCPA and MCPG: the hypoglycemia associated with ingestion of these toxins can be ascribed mostly to MCPA- or MCPG- induced reductions in hepatic pyruvate carboxylase flux due to inhibition of β-oxidation of short chain acyl-CoAs by MCPA or inhibition of both short and medium chain acyl-CoAs by MCPG with resultant reductions in hepatic acetyl-CoA content, with an additional contribution to hypoglycemia through reduced hepatic ATP stores by MCPA.
INTRODUCTION
Ingestion of toxin-containing fruit such as the unripe ackee and litchi fruits causes potentially fatal hypoglycemia. Outbreaks of hypoglycemia, diffuse encephalopathy, seizures, and cerebral edema in children in litchi-growing regions of Asia and India have been reported during the litchi harvest, resulting in numerous deaths annually and occurrences are increasing due to the expansion of litchi fruit production [1, 2]. Similarly, ingestion of unripe ackee fruit can lead to protracted vomiting followed by hypoglycemia and CNS depression, a syndrome known as the Jamaican Vomiting Sickness [3–6]. While numerous factors may potentiate these syndromes, such as exposure to banned pesticides [7], both hypoglycemic syndromes require exposure to toxins. Structurally similar toxins that have been implicated in causing these syndromes have been isolated from both ackee and litchi fruits. Hypoglycin A (L-2-amino-3-methylenecyclopropylpropionic acid) is the hypoglycemia-inducing toxin of unripe ackee fruit, and methylene cyclopropyl acetic acid (MCPA) is the hypoglycemia-inducing metabolite of hypoglycin A [8, 9]. In addition to hypoglycin A, methylene cyclopropyl glycine (MCPG) has been isolated from litchi fruit [10], and administration of MCPG to rodents also causes hypoglycemia [11, 12].
Previous in vitro and in vivo studies have found that MCPA and MCPG can inhibit β-oxidation [11–17], suggesting that these toxins may inhibit hepatic gluconeogenesis by several mechanisms: 1) depletion of the hepatocellular energy charge (ATP/ADP), 2) depletion of acetyl-CoA, which is an allosteric activator of pyruvate carboxylase, leading to reduced activity of pyruvate carboxylase activity and/or 3) increases in butyryl-CoA, which is an allosteric inhibitor of pyruvate carboxylase [12, 14, 18–20]. However, the molecular mechanism by which MCPA and MCPG cause hypoglycemia in vivo has not been established due to technical limitations assessing in vivo rates of hepatic pyruvate carboxylase flux. Recently, our group developed a Positional Isotopomer NMR Tracer Analysis (PINTA) enabling measurement of pyruvate carboxylase flux in awake animals utilizing Mass Isotopomer Distribution Analysis (MIDA) as well as a sensitive LC-MS/MS method to assess hepatic acetyl-CoA content in vivo [21, 22]. Therefore, in the current study we applied these methods to assess the mechanism by which MCPA and MCPG induce metabolic dysfunction in awake rodents. Furthermore, ex vivo measurements of hepatic acyl-CoAs by LC-MS/MS allowed us to identify the specific step in hepatic β-oxidation that these toxins inhibit in vivo resulting in reductions in hepatic gluconeogenesis and hypoglycemia.
EXPERIMENTAL PROCEDURES
Animals
All animal protocols were approved by the Yale University Animal Care and Use Committee. Male Sprague-Dawley/CD (250–400 grams) rats were obtained from Charles River Laboratories and male C57BL/6 mice (11–13 weeks of age) were obtained from Jackson Laboratory. Rodents acclimated for at least 3 days after arrival and were randomly assigned to treatment groups before studies commenced. Rodents were housed in accordance with the Guide for the Care and Use of Laboratory Animals and standard operating procedures of the Yale Animal Resource Center. Catheters were surgically implanted under general anesthesia in the carotid artery and jugular vein for rat infusion studies, and in the jugular vein for mouse infusion studies. At least six days were allowed for recovery from surgery before infusions were performed. Rodents were maintained on standard regular chow (Envigo 2018S: 24% protein/ 58% carbohydrate/ 18% fat calories).
Infusion studies
Methylene cyclopropyl glycine (MCPG) studies were performed in conscious mice. Mice were fasted overnight (12–16 hours) prior to study. A bolus of 40 mg-kg−1 MCPG (Akos) or vehicle (saline) was administered intravenously at the start of the experiment. For the initial study (Supplement), [3–3H] glucose tracer (Perkin Elmer) was infused at a rate of 0.05 µCi/min to assess endogenous glucose production, blood was sampled every 15–30 minutes for measurement of plasma glucose concentrations, and mice were euthanized with intravenous pentobarbital at 120 minutes, after which livers were immediately freeze clamped in liquid N2. For the second study, [1,2,3,4,5,6,6-2H7] glucose (Cambridge Isotope Laboratories) tracer was infused at 0.1 mg/(kg-min) for 4 hours. Without any natural abundance enrichment/specific activity, these tracers are expected to provide optimum sensitivity to detect small differences in glucose production. Blood samples were taken at 2 hours and 4 hours. At 4 hours, mice were sacrificed with intravenous pentobarbital, and livers were immediately freeze-clamped in liquid N2.
Methylene cyclopropyl acetic acid (MCPA) studies were performed in awake, unrestrained rats. Rats were fasted for 18–20 hours prior to study. A bolus of 80 mg/kg MCPA (Fluka) in 0.9% saline or vehicle (saline) was administered intravenously at the initiation of the experiment. Rats underwent a 120-minute primed-continuous arterial infusion with [3-13C] lactate (Sigma), [1,2,3,4,5,6,6-2H7] glucose, and [U-13C4] sodium β-hydroxybutyrate (Cambridge Isotope Laboratories). The prime consisted of 3× continuous rate for five minutes, and continuous infusion rates were as follows: 10 µmol/(kg-min) lactate, 0.3 mg/(kg-min) glucose, 0.1 mg/(kg-min) β-hydroxybutyrate (β-OHB). Blood samples were taken every 10–15 minutes throughout the experiment. Euglycemia was maintained in the MCPA group with a variable infusion of 5% dextrose. At 2 hours, rats were sacrificed with intravenous pentobarbital, and livers were immediately freeze-clamped in liquid N2, and stored at −80°C for subsequent analysis.
Metabolites
Glucose concentrations were determined using a YSI (2700 Select). β-OHB concentrations were measured by a spectrophotometric assay (Stanbio). Insulin concentrations were determined by radioimmunoassay (EMD Millipore). Plasma stable isotope enrichments were measured by PCI gas chromatography-mass spectrometry (Agilent) [23]. Radioisotope specific activities were assessed by liquid scintillation counting (Perkin Elmer).
Flux calculations
We applied a Positional Isotopomer NMR Tracer Analysis (PINTA) method [21] for the assessment of hepatic pyruvate carboxylase flux in conscious rats infused with [3-13C]lactate and [1,2,3,4,5,6,6-2H7]glucose at the rates listed above. Positional enrichment of each carbon of glucose was determined by NMR in combination with GC-MS measurements of total and C4C5C6 [m+1] and [m+2] glucose enrichment [21]. The glucose isotopomer data were used to calculate the ratio of pyruvate carboxylase flux to endogenous glucose production (VPC/VEGP) and the absolute VEGP rate. Briefly, VEGP was calculated using the equation , where APE denotes the measured 13C atom percent enrichment in the plasma and the glucose infusate, and VPC/VEGP was calculated independently of pyruvate cycling, malic enzyme flux, or pyruvate dehydrogenase flux by Mass Isotopomer Distribution Analysis [21, 24] as the ratio VPC/VEGP = G2/XFE2, where G2 denotes the m+2 glucose enrichment corrected to account for m+2 glucose that arises from the condensation of two 13C trioses: Corrected [m+2]glucose = Measured [m+2]glucose-2 * C4C5C6 [m+2]glucose, and where m+2 glucose is corrected as described above. Detailed derivations of these equations can be found in our previous report [21]. The absolute VPC flux was calculated by multiplying the VPC/VEGP ratio by the measured VEGP.
Tissue Analyses
Hepatic short chain and medium chain CoAs were extracted from 50–100 mg liver as previously described [22]. Hepatic long chain CoAs were extracted from 50–100 mg liver as previously described [25]. Analysis was performed by LC-MS/MS (Shimazu UFLCXR, Sciex 6500 QTRAP) by negative electrospray ionization. Parent-daughter ion pairs were monitored and quantified using internal standards [13C2]acetyl-CoA for short chain CoAs and hepatdecanoyl-CoA for long chain CoAs. Of note, the CoA adducts of MCPA and MCPG are MCP-acetyl-CoA and MCP-formyl-CoA, respectively. For these toxin-CoAs, parent-daughter ion pairs resulting from the loss of 3’-phosphoadenosine-diphosphate are as follows: MCP-acetyl-CoA, 862/355; MCP-formyl-CoA, 848/341.
Hepatic ATP, ADP, and AMP were extracted from tissue homogenized in 50% acetonitrile, 10 µM EDTA, 12 mM ammonium acetate, and 10 mM spermine. Debris was pelleted by centrifugation; the supernatant was filtered with a 0.22 µm filter. The flow-through was injected with a Shimadzu HPLC system into a Hypercarb HPLC column 100 × 2.1 mm with a particle size of 5 u (Thermo), where the nucleotides were separated with a gradient from Buffer A (12 mM ammonium acetate and 10 uM EDTA) and buffer B (acetonitrile) over a time course of 35 mins and analyzed by a 6500 Qtrap LC-MS/MS system (Sciex).
Statistical Analyses
Statistical analyses were performed using GraphPad Prism 7. Comparisons between two groups were performed by the two-tailed unpaired Student’s t test. P-values less than 0.05 were considered significant. Data are presented as the mean ± S.E.M. of the numbers indicated in the figure legends.
RESULTS
Methylene cyclopropyl glycine (MCPG) inhibits β-oxidation and hepatic glucose production
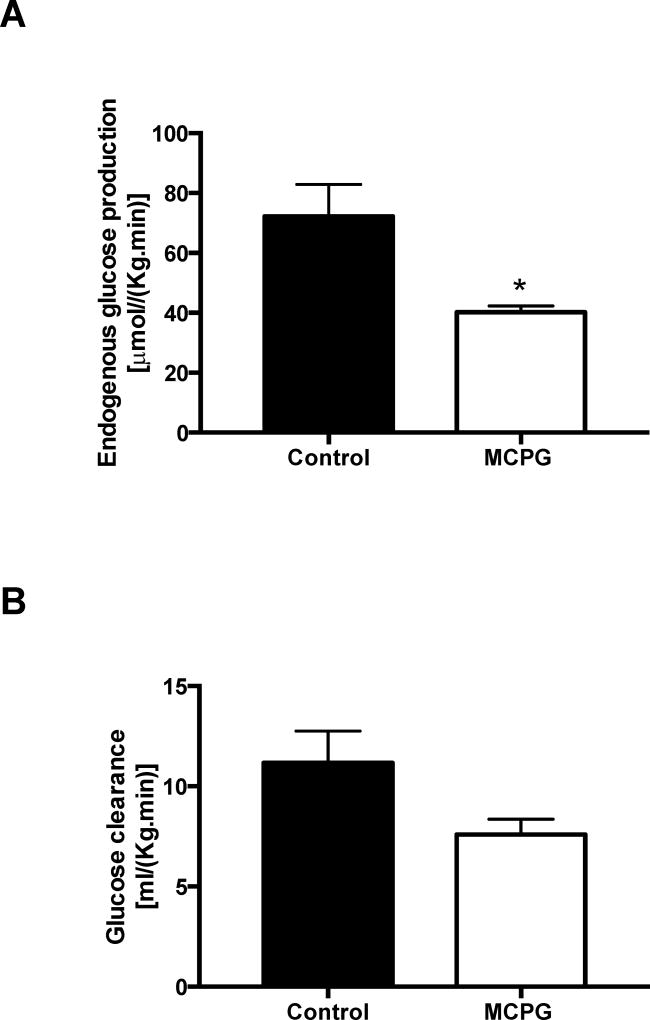
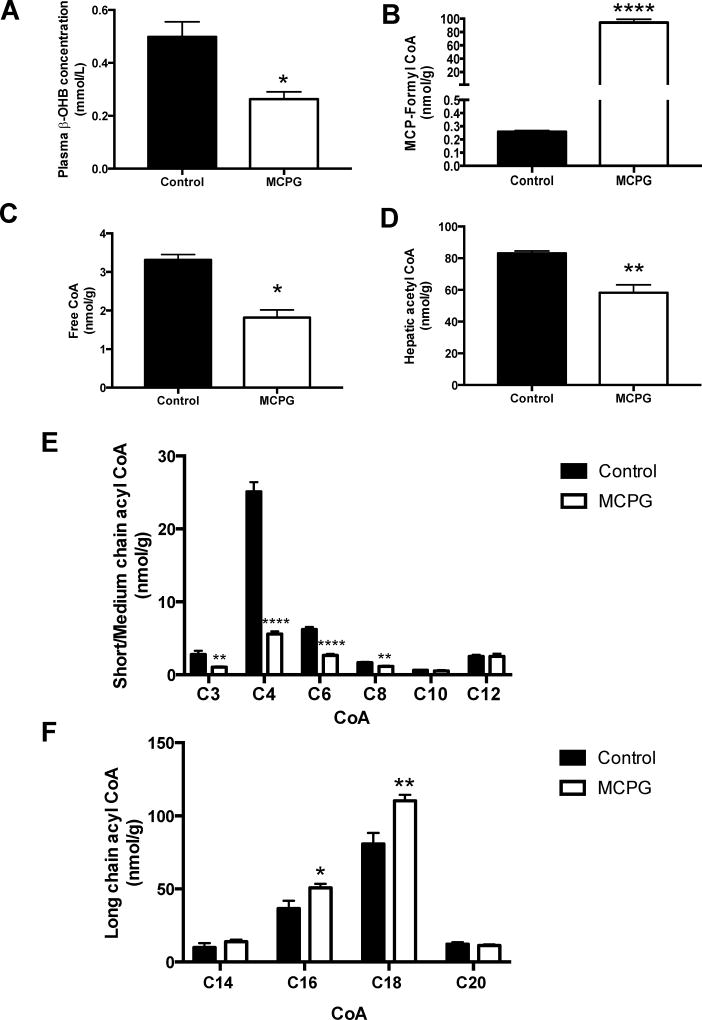
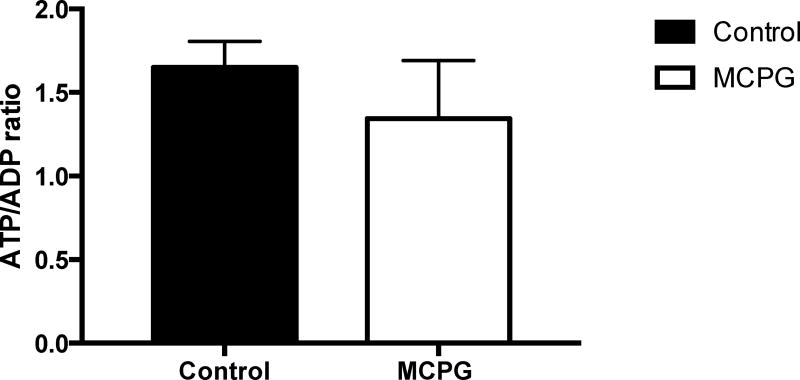
Two hours after MCPG administration in overnight fasted mice, we observed no change in plasma glucose or endogenous glucose production or glucose clearance (Figure S1). Consistent with the role of MCPG as an inhibitor of β-oxidation, plasma β-hydroxybutyrate was reduced by 48% (Figure S2A). Notably, we identified the MCPG-CoA adduct in the liver, MCP-formyl CoA (MCPG CoA, Figure S2B). Divergent effects were seen on short chain vs. long chain acyl CoAs (Figure S2C-F). The short chain acyl CoAs propionyl, butyryl, and hexaryl CoAs were reduced by MCPG treatment, although acetyl-CoA was unchanged. In contrast, C16, C18, and C20 acyl CoAs were all increased in the livers of treated animals. Hepatocellular energy charge was unchanged (Figure S3). With unchanged rates of hepatic glucose production in MCPG treated mice, but marked changes in acyl CoAs, we decided to repeat the experiment with a longer (four hour) time course. At four hours, we observed a significant 44% decline in hepatic glucose production in MCPG treated mice (Figure 1A) without a significant change in glucose clearance (Figure 1B), and a ~50% reduction in plasma β-hydroxybutyrate concentrations (Figure 2A). The hepatic MCP-formyl CoA was again observed following MCPG treatment (Figure 2B). In this experiment, free CoA and short chain acyl CoAs through C8 were all decreased, including acetyl-CoA (Figure 2C-E), while long chain acyl CoAs C16 and C18 increased (Figure 2F). No significant change in hepatocellular energy charge was observed, as reflected by an unchanged ATP/ADP ratio (Figure 3). In sum, administration of MCPG to mice inhibited β-oxidation, which was associated with the depletion of hepatic short chain acyl CoAs and the accumulation of hepatic long chain acyl CoAs.
Figure 1. Glucose turnover after MCPG treatment.
(A) Endogenous glucose production and (B) glucose clearance were assessed four hours after infusion of vehicle (saline) or MCPG. Data are mean ± S.E.M.. (Control group n = 5; MCPG group n = 6), *p< 0.05.
Figure 2. Effect of MCPG on hepatic β-oxidation.
Assessed four hours after vehicle vs. MCPG treatment. (A) Plasma β-OHB concentration. (B) Hepatic MCP-formyl CoA (MCPG-CoA conjugate) concentration. (C) Hepatic free CoA concentration. (D) Hepatic acetyl-CoA concentration. (E) Hepatic short and medium chain acyl CoA concentrations. (F) Hepatic long chain acyl CoA concentrations. In all panels, data are mean ± S.E.M.. (Control group n = 5; MCPG group n = 6), *p< 0.05, **p< 0.01, ****p< 0.0001.
Figure 3. Hepatic ATP:ADP ratios in MCPG treated mice.
Assessed four hours after treatment. Data are mean ± S.E.M. (Control group n = 5; MCPG group n = 6).
Methylene cyclopropyl acetic acid (MCPA) rapidly induces hypoglycemia
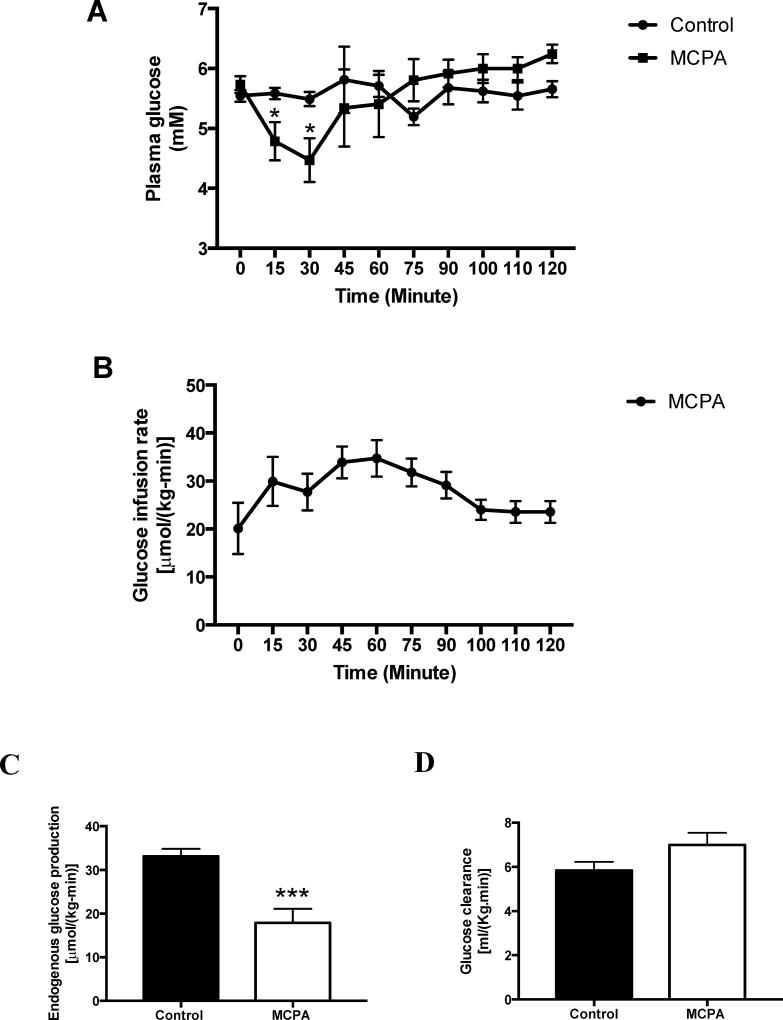
In rats fasted for one day, MCPA infusion rapidly lowered plasma glucose concentrations, such that infusion of dextrose was required to maintain euglycemia (Figure 4A-B). EGP was assessed at 90–120 minutes after MCPA administration, and was reduced by half (Figure 4C). Glucose clearance, however, was unchanged (Figure 4D).
Figure 4. Glucose turnover after MCPA treatment.
(A) Plasma glucose concentration vs. time. (Control group n = 8; MCPA group n = 10) (B) Glucose infusion rate. (MCPA group n = 10) (C) Endogenous glucose production. (Control group n = 11; MCPA group n = 7) (D) Glucose clearance. (Control group n = 11; MCPA group n = 7) In all panels, data are mean ± S.E.M.. * P<0.05, ***p< 0.001,§p< 0.0001.
MCPA inhibits β-oxidation in vivo
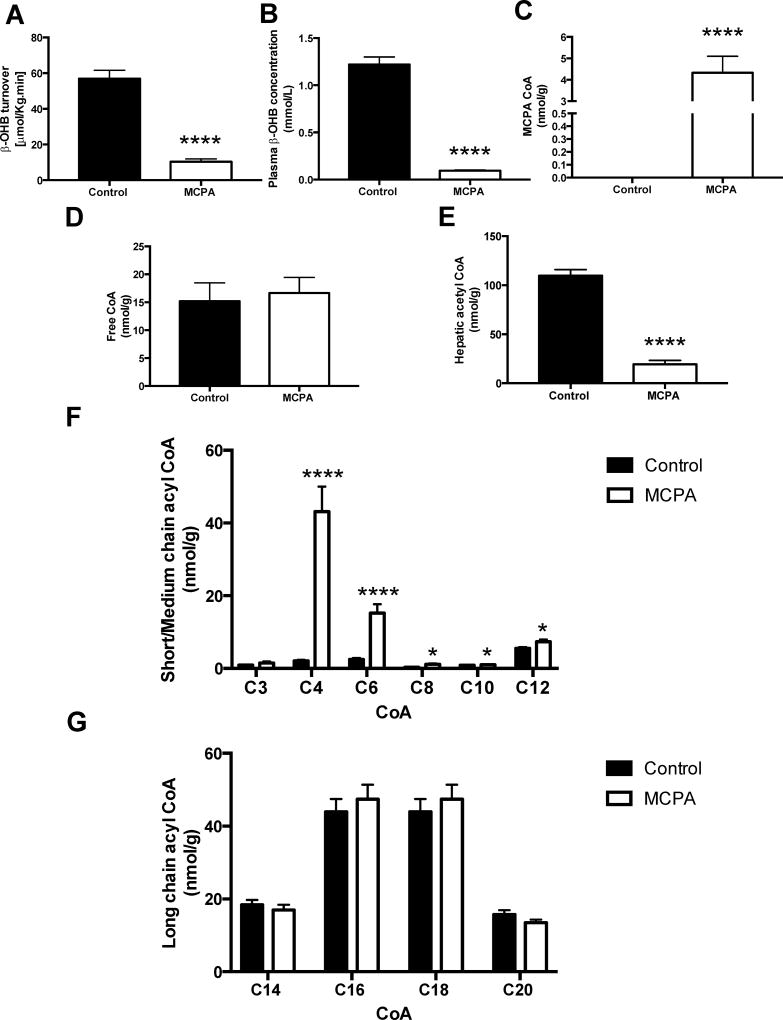
After administration of MCPA, β-hydroxybutyrate turnover decreased by 82% and plasma β-hydroxybutyrate concentration fell by 92%, reflecting a near-ablation of ketone body production (Figure 5A-B), attributed to inhibition of β-oxidation. We identified the MCPA-CoA adduct by tandem LC-mass spectrometry in MCPA treated rat liver (Figure 5C). Hepatic acetyl-CoA was reduced by 84% (Figure 5E), while other short chain CoAs, including butyryl CoA and hexaryl CoA, were markedly increased (Figure 5F). Longer chain acyl CoAs, other than C12 CoA, were unchanged in the livers of MCPA treated rats (Figure 5G). Thus, MCPA inhibits β-oxidation, particularly the oxidation of short chain CoAs, thereby depleting acetyl-CoA.
Figure 5. Effect of MCPA on hepatic β-oxidation.
(A) Whole-body β-OHB turnover. (Control group n = 8; MCPA group n = 6) (B) Plasma β-OHB concentration. (Control group n = 12; MCPA group n = 10) (C) MCPA CoA conjugate content. (Control group n = 9; MCPA group n = 8) (D) Free CoA content. (Control group n = 9; MCPA group n = 9) (E) Hepatic acetyl-CoA concentration. (Control group n = 9; MCPA group n = 9) (F) Short and medium chain acyl CoA content. (Control group n = 9; MCPA group n = 9) (G) Long chain acyl-CoA content. (Control group n = 10; MCPA group n = 10), *p< 0.05, ****p< 0.0001.
Pyruvate carboxylase flux is reduced by MCPA in vivo
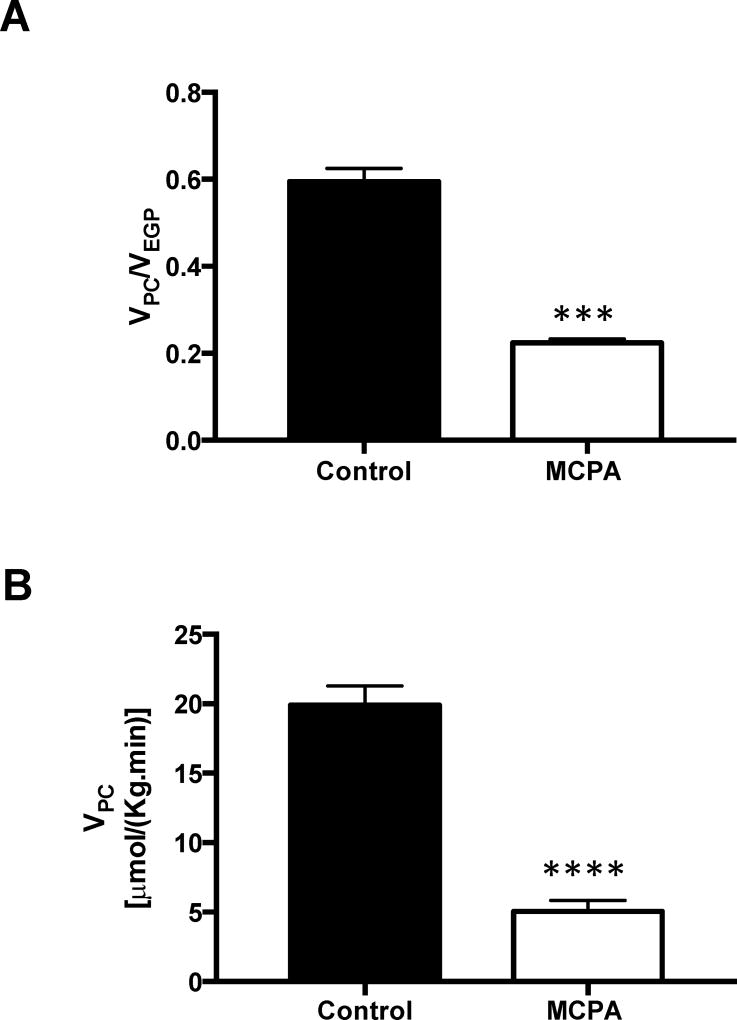
To test the hypothesis that pyruvate carboxylase (PC) flux is inhibited by MCPA administration, we assessed PC flux in MCPA treated rats, using a Positional Isotopomer NMR Tracer Analysis (PINTA) method recently developed in our laboratory. Hepatic metabolite isotope enrichments following 2-hour [3-13C] lactate infusion are reported in Table S1. PC flux as a fraction of total EGP decreased by 62% in MCPA treated rats (Figure 6A). Accounting for the reduction we observed in EGP, absolute PC flux was reduced by 75% in MCPA treated rats (Figure 6B).
Figure 6. Pyruvate carboxylase flux as assessed by PINTA.
(A) Ratio of pyruvate carboxylase flux to EGP. (B) Pyruvate carboxylase flux. (Control group n = 7; MCPA group n = 5) In all panels, data are mean ± S.E.M., ***p< 0.001, ****p< 0.0001.
Hepatic ATP stores decline after treatment with MCPA
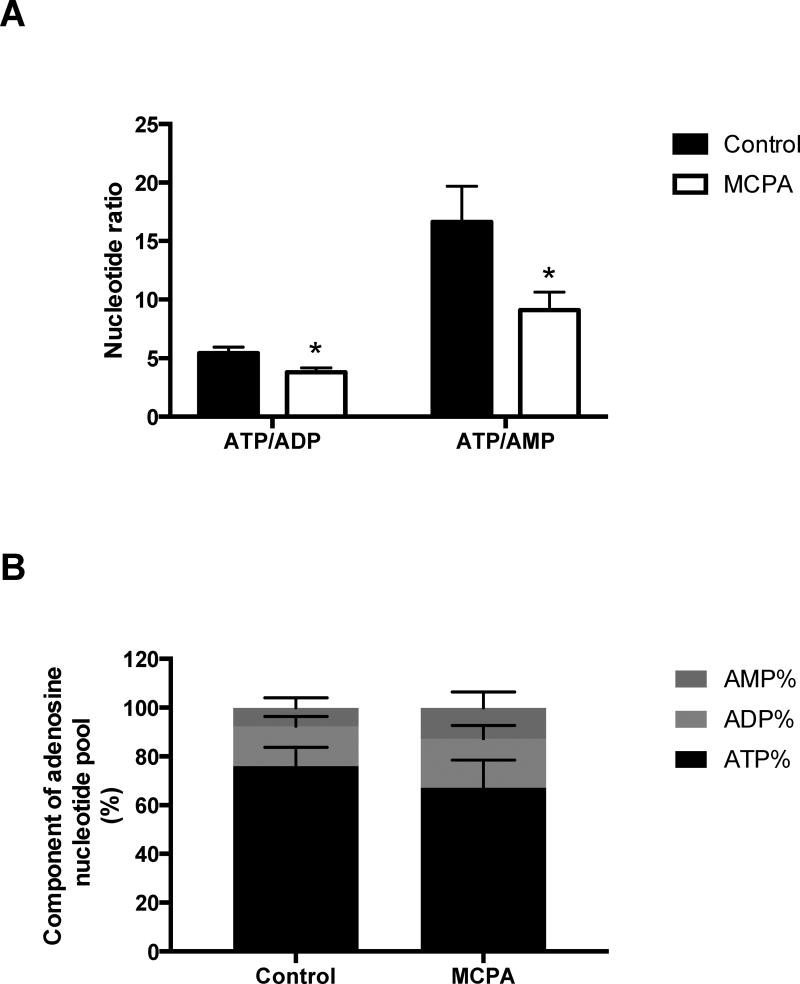
Because β-oxidation is an important source of reducing equivalents for use in oxidative phosphorylation, we measured the ratio of ATP:ADP:AMP by tandem LC-mass spectrometry. ATP stores were significantly reduced in the livers of MCPA treated rats (Figure 7). The ATP/ADP and ATP/AMP ratios decreased by 30% and 45% respectively. Most remarkably, the percentage of the adenosine phosphate pool made up by AMP increased by 62%.
Figure 7. Hepatic ATP, ADP, and AMP content in MCPA treated rats.
(A) ATP/ADP and ATP/AMP ratios. (B) ATP%, ADP% and AMP%. In all panels, data are mean ± S.E.M.. (n =10 per group), *p< 0.05.
DISCUSSION
To determine the mechanisms by which MCPG and MCPA promote hypoglycemia in vivo we infused MCPG and MCPA into conscious rodents and assessed rates of hepatic gluconeogenesis and hepatic ketogenesis in conjunction with hepatic acyl CoAs, hepatic acetyl-CoA content and hepatocellular energy charge (ATP/ADP). Employing the PINTA method, which employs MIDA analysis of plasma to determine substrate contributions to gluconeogenesis, this study demonstrates the impact of these toxins on hepatic pyruvate carboxylase flux in vivo for the first time. Using this approach, we link the formation of the toxin-CoA conjugate to inhibition of β-oxidation, to suppression of hepatic acetyl-CoA content and pyruvate carboxylase flux and reduction of hepatic glucose production in awake rodents. Furthermore, we demonstrate the depletion of energy charge in MCPA treated rats, providing two mechanisms by which gluconeogenesis is inhibited, thereby driving the toxin-mediated hypoglycemic syndrome.
Saturated fatty acyl-CoAs are broken down two carbons at a time by the four sequential reactions of β-oxidation: 1. an acyl-CoA dehydrogenase oxidizes the acyl-CoA resulting in an enoyl-CoA with a double bond between C2 and C3; 2. an enoyl-CoA hydratase hydrates the new double bond to produce an L-hydroxyacyl-CoA; 3. a hydroxyacyl-CoA dehydrogenase catalyzes the oxidation of the L-hydroxyacyl-CoA to produce a ketoacyl-CoA; 4. a thiolase cleaves the ketoacyl-CoA to produce one acetyl-CoA and an acyl-CoA shortened by two carbons [26, 27]. While we do not have an in vivo method to probe flux through the individual steps of β-oxidation, we are able to infer the step in β-oxidation targeted by the toxin-CoA conjugates by comparing the profile of acyl-CoAs with information from prior in vitro studies of these toxins, and with the published substrate specificities of the individual enzymes of β-oxidation.
Thus, to probe the step in β-oxidation targeted by the MCPA and MCPG toxins in vivo, we applied an LC-MS/MS method to directly measure the full hepatic acyl CoA profile and acetyl-CoA content in MCPA and MCPG-treated rodents. Of note, while MCPA-CoA has been monitored by change in NMR chemical shift in vitro [16], and MCPF-CoA has been assessed by HPLC in hepatic mitochondrial preparations [12], to our knowledge the mass spectrometry analysis presented here is the first time these toxin-CoA conjugates have been identified ex vivo. The chemical mechanism of action of MCPA-CoA is thought to be primarily through suicide-inhibition of Short Chain Acyl CoA Dehydrogenase (SCAD), and to a lesser degree through the inhibition of Medium Chain Acyl CoA Dehydrogenase (MCAD) [13, 16, 17, 20, 28, 29]. MCPA caused a large decrease in ex vivo hepatic acetyl-CoA concentration and resulted in a marked rise in hepatic butyryl CoA with smaller increases in other short- and medium-chain CoAs. Considering SCAD’s substrate specificity for C4 and C6 CoAs [30, 31], this pattern is most consistent with MCPA’s function as a SCAD inhibitor.
The proposed mechanism of action of MCPG is analogous to the mechanism of action of MCPA, insofar as the MCPG CoA adduct MCP-formyl CoA inhibits β-oxidation. We observed that MCPG administration led to a rapid decline in hepatic short chain CoAs C4-C8 with a rise in long chain CoAs C16-C20. Four hours after treatment, we observed a decrease in hepatic acetyl-CoA concentration as well. A reduction in short chain acyl CoAs and an increase in long chain acyl CoAs is consistent with a block in oxidation of short and medium chain acyl CoAs. These data would be consistent with inhibition of MCAD, a dehydrogenase most active against C8 CoA, with a range of activity against C4-C12 CoAs [30, 31]. However, the MCPG CoA adduct does not inhibit MCAD in vitro [11, 12]; and instead inhibits the thiolase reaction [12] and the enoyl CoA hydratase reaction [32–34]. Our in vivo results are not consistent with thiolase inhibition, as the thiolases have quite broad substrate specificities [35]. Rather, our data suggest that MCPG inhibits β-oxidation at both the short chain enoyl CoA hydratase step in the mitochondria and the enoyl CoA hydratase step in the bifunctional protein in the peroxisome [35, 36], with sparing of the long chain enoyl CoA hydratase in the trifunctional protein in the mitochondria.
Next, we sought to determine the mechanism by which inhibition of β-oxidation at the steps demonstrated above leads to hypoglycemia following treatment with MCPA and MCPG in vivo. We observed a reduction in hepatic glucose production with both MCPA and MCPG treatment, while glucose clearance was not significantly changed. These observations are consistent with previous studies performed in anesthetized animals showing that these toxins promote hypoglycemia through reduced glucose production rather than increased peripheral glucose disposal [18, 19]. These findings allowed us to focus our investigation on the effects of MCPA and MCPG on hepatic glucose production.
Treatment with MCPG modestly reduced EGP, an effect which was associated with inhibition of β-oxidation and suppression of hepatic acetyl-CoA content. In the parallel MCPA experiment, we observed rapid reductions in EGP leading to decreased plasma glucose concentrations sufficiently dramatic as to require intravenous dextrose infusion to maintain euglycemia. Ketone body (β-OHB) turnover was dramatically reduced, as was hepatic acetyl-CoA content. In contrast with MCPG treatment, other short chain CoA concentrations increased after MCPA treatment. The disruptions in hepatic acyl CoA content have the potential to alter flux through PC: acetyl-CoA is a potent allosteric activator of PC both in vitro [37–41] and in vivo [22], while several short chain CoAs (C4 and C6 acyl-CoAs) are known to inhibit pyruvate carboxylase flux [42, 43]. We therefore tested the hypothesis that toxin-mediated hypoglycemia is downstream of suppression of β-oxidation with resultant alterations in the hepatic acyl CoA profile leading to impairment of hepatic pyruvate carboxylase flux. We employed the PINTA method to assess contributions of pyruvate carboxylase flux to in vivo glucose turnover in awake rats treated with MCPA. Hepatic pyruvate carboxylase flux was inhibited by 75% after MCPA treatment, consistent with MPCA-mediated alterations in hepatic acyl CoA content as a significant mechanism responsible for MPCA-induced hypoglycemia.
In addition to the effects on hepatic pyruvate carboxylase flux through reductions in stimulatory hepatic acetyl-CoA and/or increases in inhibitory C4 and C6 acyl-CoAs, inhibitors of β-oxidation like MCPA or MCPG may also impair the ability of the cell to produce sufficient ATP to keep up with hepatocellular energy demands. Depleted energy charge then could also impair the capacity of the liver to produce glucose via gluconeogenesis. While the more modestly effective toxin, MCPG, did not cause any measurable change to ATP stores, the more potent toxin, MCPA, caused a 13% reduction in hepatic ATP content and a 65% increase in hepatic AMP content. Thus, depletion of energy stores is another potential mechanism by which MCPA exerts an effect to reduce hepatic gluconeogenesis in vivo.
An alternate hypothesis for the hypoglycemic effects of MCPA and MCPG is that free CoA is sequestered as the non-degradable MCPA-CoA and MCPF-CoA adducts, preventing CoA-dependent metabolic reactions by depletion of CoA substrate [44]. In the present study, hepatocellular CoA levels were decreased by less than 50% in MCPG treated mice and hepatocellular CoA was unchanged in MCPA treated rats. Thus, CoA sequestration is unlikely to significantly contribute to the inhibition of beta oxidation and gluconeogenesis seen in MCPA and MCPG induced hypoglycemia.
In summary, both MCPA and MCPG inhibit β-oxidation, with MCPA targeting SCAD, while MCPG appears to inhibit several short chain enoyl CoA hydratases. Disruption of β-oxidation with MCPA depletes hepatic ATP stores, reducing the energy charge available for gluconeogenesis. Both toxins deplete acetyl-CoA content, a critical allosteric activator of PC, while MCPA also increased C4 and C6 acyl-CoAs, allosteric inhibitors of PC activity. As a result, MCPA reduces PC flux, thereby impinging on gluconeogenic flux.
Supplementary Material
Acknowledgments
The authors thank Jianying Dong, Gina Butrico, Xiaoxian Ma and Maria Batsu, Codruta Todeasa and the Yale Diabetes Research Center core facility (Yale University School of Medicine) for their invaluable technical assistance.
Funding information: These studies were supported by grants from the National Institutes of Health/NIDDK (K23 DK-10287, R01 DK-40936, U24 DK-059635, P30 DK-045735, K99 CA-215315), and the National Natural Science Foundation of China (81170779).
Footnotes
Declarations of interest: The authors declare that they have no conflicts of interest with the contents of this article.
Author contribution statement: G.I.S. and D.F.V. conceived the study. Y.Q., J.G.C., X.Z., M.K., and D.F.V. performed experiments. R.J.P., M.K., G.W.C, and G.I.S. developed methodology. Y.Q., R.J.P., and D.F.V. wrote the original draft. Y.Q., R.J.P., G.W.C., G.I.S., and D.F.V. edited the manuscript. All authors analyzed data, reviewed results, and approved the final version of the manuscript.
References
- 1.Shrivastava A, Srikantiah P, Kumar A, Bhushan G, Goel K, Kumar S, Kumar T, Mohankumar R, Pandey R, Pathan P, Tulsian Y, Pappanna M, Pasi A, Pradhan A, Singh P, Somashekar D, Velayudhan A, Yadav R, Chhabra M, Mittal V, Khare S, Sejvar JJ, Dwivedi M, Laserson K, Earhart KC, Sivaperumal P, Kumar AR, Chakrabarti A, Thomas J, Schier J, Singh R, Singh RS, Dhariwal AC, Chauhan LS. Outbreaks of Unexplained Neurologic Illness - Muzaffarpur, India, 2013–2014. Mmwr-Morbid Mortal W. 2015;64:49–53. [PMC free article] [PubMed] [Google Scholar]
- 2.Spencer PS, Palmer VS. The enigma of litchi toxicity: an emerging health concern in southern Asia. Lancet Glob Health. 2017;5:e383–e384. doi: 10.1016/S2214-109X(17)30046-3. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka K, Kean EA, Johnson B. Jamaican vomiting sickness. Biochemical investigation of two cases. N Engl J Med. 1976;295:461–467. doi: 10.1056/NEJM197608262950901. [DOI] [PubMed] [Google Scholar]
- 4.Hill KR, Bras G, Clearkin KP. Acute toxic hypoglycaemia occurring in the vomiting sickness of Jamaica; morbid anatomical aspects. West Indian Med J. 1955;4:91–104. [PubMed] [Google Scholar]
- 5.Surmaitis R, Hamilton R. Toxicity, Ackee Fruit. StatPearls; Treasure Island (FL): 2017. [PubMed] [Google Scholar]
- 6.Jelliffe DB, Stuart KL. Acute toxic hypoglycaemia in the vomiting sickness of Jamaica. Br Med J. 1954;1:75–77. doi: 10.1136/bmj.1.4853.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam MS, Sharif AR, Sazzad HMS, Khan A, Hasan M, Akter S, Rahman M, Luby SP, Heffelfinger JD, Gurley ES. Outbreak of Sudden Death with Acute Encephalitis Syndrome Among Children Associated with Exposure to Lychee Orchards in Northern Bangladesh, 2012. Am J Trop Med Hyg. 2017;97:949–957. doi: 10.4269/ajtmh.16-0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka K. On the mode of action of hypoglycin A. 3. Isolation and identification of cis-4-decene-1,10-dioic, cis, cis-4,7-decadiene-1,10-dioic, cis-4-octene-1,8-dioic, glutaric, and adipic acids, N-(methylenecyclopropyl)acetylglycine, and N-isovalerylglycine from urine of hypoglycin A-treated rats. The Journal of biological chemistry. 1972;247:7465–7478. [PubMed] [Google Scholar]
- 9.Von Holt C, Von Holt M, Bohm H. Metabolic effects of hypoglycin and methylenecyclopropaneacetic acid. Biochim Biophys Acta. 1966;125:11–21. doi: 10.1016/0005-2760(66)90139-1. [DOI] [PubMed] [Google Scholar]
- 10.Gray DO, Fowden L. alpha-(Methylenecyclopropyl)glycine from Litchi seeds. The Biochemical journal. 1962;82:385–389. doi: 10.1042/bj0820385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Melde K, Buettner H, Boschert W, Wolf HP, Ghisla S. Mechanism of hypoglycaemic action of methylenecyclopropylglycine. The Biochemical journal. 1989;259:921–924. doi: 10.1042/bj2590921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melde K, Jackson S, Bartlett K, Sherratt HS, Ghisla S. Metabolic consequences of methylenecyclopropylglycine poisoning in rats. The Biochemical journal. 1991;274(Pt 2):395–400. doi: 10.1042/bj2740395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osmundsen H, Sherratt HS. A novel mechanism for inhibition of beta-oxidation by methylenecyclopropylacetyl-CoA, a metabolite of hypoglycin. FEBS letters. 1975;55:38–41. doi: 10.1016/0014-5793(75)80951-3. [DOI] [PubMed] [Google Scholar]
- 14.Stanley H, Sherratt A, Osmundsen H. On the mechanisms of some pharmacological actions of the hypoglycaemic toxins hypoglycin and pent-4-enoic acid. A way out of the present confusion. Biochem Pharmacol. 1976;25:743–750. doi: 10.1016/0006-2952(76)90139-8. [DOI] [PubMed] [Google Scholar]
- 15.Billington D, Osmundsen H, Sherrat HS. The time-course of the changes in the concentrations of some volatile fatty acids after administration of hypoglycin to rats. Biochemical Society transactions. 1976;4:102–105. doi: 10.1042/bst0040102. [DOI] [PubMed] [Google Scholar]
- 16.Wenz A, Thorpe C, Ghisla S. Inactivation of general acyl-CoA dehydrogenase from pig kidney by a metabolite of hypoglycin A. The Journal of biological chemistry. 1981;256:9809–9812. [PubMed] [Google Scholar]
- 17.Lieu YK, Hsu BY, Price WA, Corkey BE, Stanley CA. Carnitine effects on coenzyme A profiles in rat liver with hypoglycin inhibition of multiple dehydrogenases. The American journal of physiology. 1997;272:E359–366. doi: 10.1152/ajpendo.1997.272.3.E359. [DOI] [PubMed] [Google Scholar]
- 18.Billington D, Osmundsen H, Taylor JR, Sherratt HS. The effect of hypoglycin on some parameters of glucose metabolism in the rat. Biochemical Society transactions. 1976;4:1035–1038. doi: 10.1042/bst0041035. [DOI] [PubMed] [Google Scholar]
- 19.Osmundsen H, Billington D, Taylor JR, Sherratt HS. The effects of hypoglycin on glucose metabolism in the rat. A kinetic study in vivo and [U-14C,2-3H]glucose. The Biochemical journal. 1978;170:337–342. doi: 10.1042/bj1700337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kean EA, Pogson CI. Inhibition of gluconeogenesis in isolated rat liver cells by methylenecyclopropylpyruvate (ketohypoglycin) The Biochemical journal. 1979;182:789–796. doi: 10.1042/bj1820789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perry RJ, Peng L, Cline GW, Butrico GM, Wang Y, Zhang XM, Rothman DL, Petersen KF, Shulman GI. Non-invasive assessment of hepatic mitochondrial metabolism by positional isotopomer NMR tracer analysis (PINTA) Nat Commun. 2017;8:798. doi: 10.1038/s41467-017-01143-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perry RJ, Camporez JP, Kursawe R, Titchenell PM, Zhang D, Perry CJ, Jurczak MJ, Abudukadier A, Han MS, Zhang XM, Ruan HB, Yang X, Caprio S, Kaech SM, Sul HS, Birnbaum MJ, Davis RJ, Cline GW, Petersen KF, Shulman GI. Hepatic Acetyl CoA Links Adipose Tissue Inflammation to Hepatic Insulin Resistance and Type 2 Diabetes. Cell. 2015;160:745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perry RJ, Peng L, Abulizi A, Kennedy L, Cline GW, Shulman GI. Mechanism for leptin's acute insulin-independent effect to reverse diabetic ketoacidosis. The Journal of clinical investigation. 2017;127:657–669. doi: 10.1172/JCI88477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellerstein MK, Neese RA. Mass isotopomer distribution analysis: a technique for measuring biosynthesis and turnover of polymers. The American journal of physiology. 1992;263:E988–1001. doi: 10.1152/ajpendo.1992.263.5.E988. [DOI] [PubMed] [Google Scholar]
- 25.Neschen S, Morino K, Hammond LE, Zhang D, Liu ZX, Romanelli AJ, Cline GW, Pongratz RL, Zhang XM, Choi CS, Coleman RA, Shulman GI. Prevention of hepatic steatosis and hepatic insulin resistance in mitochondrial acyl-CoA:glycerol-sn-3-phosphate acyltransferase 1 knockout mice. Cell metabolism. 2005;2:55–65. doi: 10.1016/j.cmet.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Voet D, Voet JG. Biochemistry J. Wiley & Sons; New York: 1995. [Google Scholar]
- 27.Stryer L. Biochemistry. W.H. Freeman; New York: 1988. [Google Scholar]
- 28.Tserng KY, Jin SJ, Hoppel CL. Spiropentaneacetic acid as a specific inhibitor of medium-chain acyl-CoA dehydrogenase. Biochemistry. 1991;30:10755–10760. doi: 10.1021/bi00108a021. [DOI] [PubMed] [Google Scholar]
- 29.Kean EA. Selective inhibition of acyl-CoA dehydrogenases by a metabolite of hypoglycin. Biochim Biophys Acta. 1976;422:8–14. doi: 10.1016/0005-2744(76)90003-6. [DOI] [PubMed] [Google Scholar]
- 30.Ikeda Y, Okamura-Ikeda K, Tanaka K. Purification and characterization of short-chain, medium-chain, and long-chain acyl-CoA dehydrogenases from rat liver mitochondria. Isolation of the holo- and apoenzymes and conversion of the apoenzyme to the holoenzyme. The Journal of biological chemistry. 1985;260:1311–1325. [PubMed] [Google Scholar]
- 31.Shen YQ, Lang BF, Burger G. Diversity and dispersal of a ubiquitous protein family: acyl-CoA dehydrogenases. Nucleic acids research. 2009;37:5619–5631. doi: 10.1093/nar/gkp566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agnihotri G, He S, Hong L, Dakoji S, Withers SG, Liu HW. A revised mechanism for the inactivation of bovine liver enoyl-CoA hydratase by (methylenecyclopropyl)formyl-CoA based on unexpected results with the C114A mutant. Biochemistry. 2002;41:1843–1852. doi: 10.1021/bi0119363. [DOI] [PubMed] [Google Scholar]
- 33.Wu L, Lin S, Li D. Comparative inhibition studies of enoyl-CoA hydratase 1 and enoyl-CoA hydratase 2 in long-chain fatty acid oxidation. Org Lett. 2008;10:3355–3358. doi: 10.1021/ol801267e. [DOI] [PubMed] [Google Scholar]
- 34.Agnihotri G, Liu HW. Enoyl-CoA hydratase. reaction, mechanism, and inhibition. Bioorg Med Chem. 2003;11:9–20. doi: 10.1016/s0968-0896(02)00333-4. [DOI] [PubMed] [Google Scholar]
- 35.Uchida Y, Izai K, Orii T, Hashimoto T. Novel fatty acid beta-oxidation enzymes in rat liver mitochondria. II. Purification and properties of enoyl-coenzyme A (CoA) hydratase/3-hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase trifunctional protein. The Journal of biological chemistry. 1992;267:1034–1041. [PubMed] [Google Scholar]
- 36.Jiang LL, Miyazawa S, Hashimoto T. Purification and properties of rat D-3-hydroxyacyl-CoA dehydratase: D-3-hydroxyacyl-CoA dehydratase/D-3-hydroxyacyl-CoA dehydrogenase bifunctional protein. J Biochem. 1996;120:633–641. doi: 10.1093/oxfordjournals.jbchem.a021459. [DOI] [PubMed] [Google Scholar]
- 37.Keech DB, Utter MF. Pyruvate Carboxylase. Ii. Properties. The Journal of biological chemistry. 1963;238:2609–2614. [PubMed] [Google Scholar]
- 38.Krebs HA, Speake RN, Hems R. Acceleration of Renal Gluconeogenesis by Ketone Bodies and Fatty Acids. The Biochemical journal. 1965;94:712–720. doi: 10.1042/bj0940712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williamson JR, Kreisberg RA, Felts PW. Mechanism for the stimulation of gluconeogenesis by fatty acids in perfused rat liver. Proceedings of the National Academy of Sciences of the United States of America. 1966;56:247–254. doi: 10.1073/pnas.56.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barritt GJ, Keech DB, Ling AM. Apparent co-operative effect of acetyl-CoA on sheep kidney pyruvate carboxylase. Biochemical and biophysical research communications. 1966;24:476–481. doi: 10.1016/0006-291x(66)90186-0. [DOI] [PubMed] [Google Scholar]
- 41.Cooper TG, Benedict CR. The participation of acetyl-CoA in pyruvate carboxylase. Biochemical and biophysical research communications. 1966;22:285–290. doi: 10.1016/0006-291x(66)90479-7. [DOI] [PubMed] [Google Scholar]
- 42.Scrutton MC. Pyruvate carboxylase. Studies of activator-independent catalysis and of the specificity of activation by acyl derivatives of coenzyme A for the enzyme from rat liver. The Journal of biological chemistry. 1974;249:7057–7067. [PubMed] [Google Scholar]
- 43.Billington D, Osmundsen H, Sherratt HS. Mechanisms of the metabolic disturbances caused by hypoglycin and by pent-4-enoic acid. In vitro studies. Biochem Pharmacol. 1978;27:2879–2890. doi: 10.1016/0006-2952(78)90204-6. [DOI] [PubMed] [Google Scholar]
- 44.Mitchell GA, Gauthier N, Lesimple A, Wang SP, Mamer O, Qureshi I. Hereditary and acquired diseases of acyl-coenzyme A metabolism. Molecular genetics and metabolism. 2008;94:4–15. doi: 10.1016/j.ymgme.2007.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.