ABSTRACT
We have previously shown that the transcript levels of Vegfc and its receptor Vegfr3 were high in spermatogonia and extremely low in spermatocytes and spermatids. However, it remains unknown about the functions and the mechanisms of VEGFC/VEGFR3 signaling in regulating the fate determinations of spermatogonia. To this end, here we explored the role and signaling pathways of VEGFC/VEGFR3 by using a cell line derived from immortalized mouse spermatogonia retaining markers of mitotic germ cells, namely GC-1 cells. VEGFR3 was expressed in mouse primary spermatogonia and GC-1 cells. VEGFC stimulated the proliferation and DNA synthesis of GC-1 cells and enhanced the phosphorylation of PI3K-AKT and MAPK, whereas LY294002 (an inhibitor for AKT) and CI-1040 (an inhibitor for MAPK) blocked the effect of VEGFC on GC-1 cell proliferation. Furthermore, VEGFC increased the transcripts of c-fos and Egr1 and protein levels of cyclin D1, PCNA and Bcl-2. Conversely, the blocking of VEGFC/VEGFR3 signaling by VEGFR3 knockdown reduced the phosphorylation of AKT/MAPK and decreased the levels of cyclin D1 and PCNA. Additionally, VEGFR3 knockdown not only resulted in more apoptosis of GC-1 cells but also led to a decrease of Bcl-2 and promoted the cleavage of Caspase-3/9 and PARP. Collectively, these data suggested that VEGFC/VEGFR3 signaling promotes the proliferation of GC-1 cells via the AKT /MAPK and cyclin D1 pathway and it inhibits the cell apoptosis through Caspase-3/9, PARP and Bcl-2. Thus, this study sheds a novel insight to the molecular mechanisms underlying the fate decisions of mammalian spermatogonia.
KEYWORDS: VEGFC/VEGFR3, GC-1 cells, proliferation, apoptosis, AKT, MAPK
Introduction
Spermatogenesis comprises the mitosis of spermatogonia, meiosis of spermatocytes and spermiogenesis from round spermatids to spermatozoa, a complex multi-phase process of continuous proliferation, differentiation and apoptosis of male germ cells. For successful fertilization, males have to produce billions of sperm per day. Nevertheless, spermatogonial stem cells (SSCs) are rare in the adult rodent testis, comprising only 0.03% of the total germ cell population [1]. In rodents, almost 75% of spermatogenic cells eventually undergo apoptosis in order to maintain a proper proportion among different cells in seminiferous tubule [2]. Therefore, the balance between proliferation and apoptosis is crucial for normal spermatogenesis. During the mitotic phase, spermatogonia undergo a number of divisions and give rise to primary spermatocytes [3]. It has been reported that exogenous and endogenous signaling molecules, e.g., Nodal [4], Glial cell line-derived neurotrophic factor (GDNF) [5,6], and bone morphogenetic protein 4 (BMP4) [7] can regulate the self-renewal and/or differentiation of SSCs. However, the molecular mechanisms controlling proliferation and apoptosis of spermatogonia, especially the differentiated spermatogonia, remain largely unclear.
Vascular endothelial growth factor (VEGF) is a group of signaling proteins that play crucial roles in stimulating the growth of vascular and neural cells [8,9]. Accumulating evidence has shown that some subtypes of VEGFs, e.g. VEGFA, are closely involved in germ cell survival, spermatogenesis and testicular regeneration [10,11]. We have recently revealed that during the process of spermatogenesis, the transcript of Vegfc is highly expressed in spermatogonia, while decline to an extremely low level once meiosis starts [12]. This phenomenon indicates that VEGFC is associated with the regulation of spermatogonia. To explore the function and mechanisms of VEGFC in mouse germ cells, we used the GC-1 cells, a mouse spermatogonial cell line, which was assumed as phenotypic features of mouse type B spermatogonia and early spermatocytes, as a research model system [13].
VEGFC acts via binding VEGFR2 and VEGFR3 that are expressed predominantly in vascular and lymphatic endothelial cells, respectively [14,15]. VEGFC/VEGFR3 signaling could mediate intracellular activation of PI3K-AKT and MAPK (ERK1/2) pathways that control the fate determinations of neural stem cells (NSCs) [8]. Nevertheless, the mechanisms of VEGFC/VEGFR3 signaling in regulating mouse germ cells remain to be clarified. Here we found that VEGFC was expressed in mouse primary spermatogonia and GC-1 cells, and revealed the function of VEGFC/VEGFR3 in fate determinations of GC-1 cells. Mechanistic study indicated that VEGFC/VEGFR3 signaling modulates the proliferation through the activation of AKT and MAPK pathway and the enhancement of cyclin D1. On the other hand, it suppressed the apoptosis of GC-1 cells via the inactivation of Caspase-3/9 and increase of Bcl-2.
Results
VEGFC and VEGFR3 were expressed in mouse spermatogonia and GC-1 cells
We first examined the expression of VEGFC and VEGFR3 in mouse spermatogonia. RT-PCR showed that Vegfc, Vegfr2 and Vegfr3 transcripts were expressed in mouse primary spermatogonia and GC-1 cells (Fig. 1A). Western blotting revealed that VEGFR3 protein was detected in mouse spermatogonia and GC-1 cells (Fig. 1B). The expression level of Actb was utilized as an internal control, whereas RNA sample without RT but amplified directly with PCR using Actb primer served as a negative control.
Figure 1.
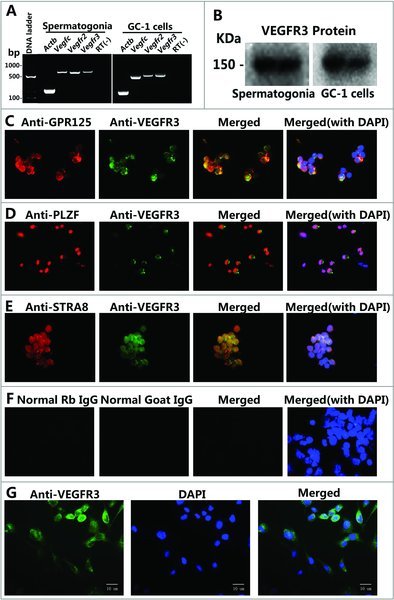
Expression of VEGFC and VEGFR3 in mouse spermatogonia and GC-1 cells. (A) The transcripts of Vegfc and its receptors Vegfr2 and Vegfr3 in GC-1 cells and spermatogonia from 8-day-old mice by RT-PCR. DNase I was added to eliminate the potential contamination of genomic DNA in total RNA. RNA samples, which underwent PCR directly and amplified by Actb primer, were served as negative controls. (B) Western blotting showed the expression of VEGFR3 protein in GC-1 cells and spermatogonia from 8-day-old mice. (C-G) Immunocytochemistry revealed the co-expression of VEGFR3 and GPR125 (C), VEGFR3 and PLZF (D), VEGFR3 and STRA8 (E) in spermatogonia from 8-day-old mice. (G) VEGFR3 protein was also expressed in GC-1 cells. (F) Normal rabbit IgG and normal goat IgG were used as negative controls. Scale bars in (A-G) = 10 μm.
Immunocytochemistry demonstrated that VEGFR3 (green) was co-expressed with GPR125 (Fig. 1C), PLZF (Fig. 1D), STRA8 (Fig. 1E) in freshly isolated germ cells from 8-days-old mice. Approximately 50% of the PLZF-positive and GFRA1-positive cells and almost all STRA8-positive and GPR125-positive cells expressed VEGFR3 protein. VEGFR3 was also expressed in GC-1 cells (Fig. 1G). Replacement of primary antibodies with normal goat and rabbit IgGs were used as negative controls, and no positive staining was observed in the cells mentioned above (Fig. 1F), thus verifying specific staining of the proteins in these cells.
VEGFC promoted the GC-1 Cell proliferation and DNA synthesis whereas MAZ51 and VEGFR3 shRNA inhibited the growth of GC-1 Cells
To explore whether VEGFC/VEGFR3 signaling is related to the proliferation and mitosis of GC-1 cells, CCK-8 and EDU assays were conducted to determine the effects of VEGFC treatment and VEGFR3 knockdown. First, CCK-8 assay was performed to identify the best concentration of VEGFC for inducing the proliferation of GC-1 cells. As expected, the effects of VEGFC from 0 ng/ml to 100 ng/ml resulted in an increase of proliferation in GC-1 cells (Fig. 2A); however, when the concentrations of VEGFC reached above 100 ng/ml, the effects of proliferation for GC-1 cells was decreased (Fig. 2A). Therefore, 100 ng/ml VEGFC was chosen for the proliferation assay of GC-1 cells. We found that cell proliferation was significantly increased in GC-1 cells treated with VEGFC on the 3rd and 4th day comparing with the controls without VEGFC (Fig. 2C). Then, we used VEGFR3 shRNA to explore the function of VEGFR3 on the proliferation of GC-1 cells. Western blotting revealed that the VEGFR3 was knocked down at a rate of 72.4 ± 7.7% after VEGFR3 shRNA transfected in GC-1 cells (Fig. 2B). CCK-8 assay revealed that the proliferation of GC-1 cells was significantly decreased by VEGFR3 shRNA on the 4th and 5th day (Fig. 2D). To confirm this result, GC-1 cells were pretreated with different concentrations of MAZ51, a specific inhibitor for VEGFR3 [16], along with or without 100 ng/ml VEGFC for 4 days. Then all cells collected from one 96-well plate in each group were counted. MAZ51 inhibited the proliferation of GC-1 cells in a dose- dependent manner (Fig. 2F).
Figure 2.
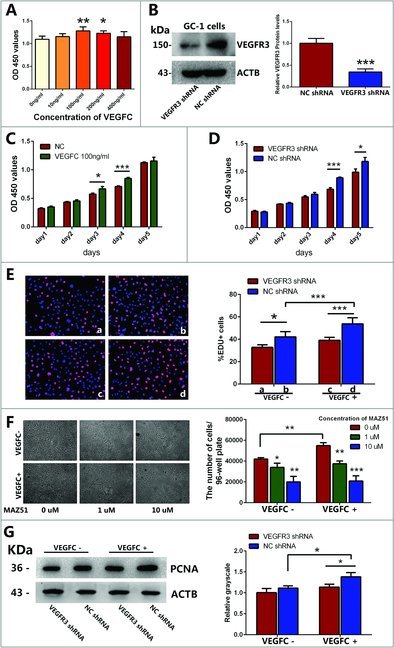
Effects of VEGFC and its receptor VEGFR3 on the proliferation and DNA synthesis of GC-1 cell line. (A) CCK-8 assay showed the growth of GC-1 cells treated with various kinds of concentrations of VEGFC for 3 days. (B) Western blotting showed the expression levels of VEGFR3 protein in GC-1 cells treated with VEGFR3 shRNA or negative control shRNA. ACTB was used as an internal reference of proteins. (C) The growth of GC-1 cells treated with or without VEGFC for 5 days in CCK-8 assay. (D) The growth of GC-1 cells that were stably transfected with VEGFR3 shRNA or negative control shRNA for 5 days in CCK-8 assay. (E) Representative photomicrographs of EDU-positive cells in GC-1 cells treated with or without 100 ng/ml VEGFC, VEGFR3 shRNA or negative control shRNA. Nuclei were counterstained with DAPI (blue) as shown in the left panel. Statistically significant differences among the percentages of EDU-positive cells were indicated by asterisk as shown in the right columns. The data were presented as mean ± SD of the percentages of EDU-positive cells out of per field (200 ×) in five independent wells. (F) Morphology of GC-1 cells transfected with VEGFR3 shRNA or negative shRNA followed by treatments with different concentrations of MAZ51 under the bright field (100 ×) of microscope. The data were presented as mean ± SD of the number of GC-1 cells collected from five independent wells of 96-wells plate respectively. Scale bar = 20 μm. (G) Western blotting demonstrated the level of PCNA protein in the GC-1 cells transfected with VEGFR3 shRNA or negative shRNA after being treated with or without VEGFC. The right panels displayed the quantification of proteins normalized with ACTB. The data shown in (A-G) were representatives of three independent experiments. (*P<0.05, **P<0.01, ***P<0.001).
DNA synthesis of GC-1 cells induced by VEGFC was assessed by EDU incorporation assay. The percentage of EDU-positive cells was increased after being treated with 100 ng/ml VEGFC compared to the control (Fig. 2E). In contrast, EDU-positive cells were decreased by VEGFR3 shRNA in comparison with that by NC shRNA (Fig. 2E). Significantly, the number of EDU-positive cells was reduced by VEGFC deprivation and VEGFR3 knockdown (Fig. 2E). Furthermore, Western blotting showed that VEGFC/VEGFR3 signaling led to an up-regulation of proliferating cell nuclear antigen (PCNA) protein level (Fig. 2G), which is required for the coordinated synthesis of DNA at the replication fork during the S phase, indicating that VEGFC/VEGFR3 signaling promotes DNA synthesis of GC-1 cells.
VEGFC/ VEGFR3 signaling promoted the proliferation of GC-1 cells by activating PI3K-AKT and MAPK pathways
To further illuminate the molecular mechanisms underlying proliferation of mouse spermatogonia by VEGFC/VEGFR3 signaling, the activations of two downstream pathways, AKT and MAPK pathway, were tested. Western blotting clearly showed that phosphorylation (phosphor-) of PI3K-AKT (Fig. 3A,B) and MAPK (Fig. 3C) was increased in GC-1 cells when stimulated by 100 ng/ml VEGFC and reached its peak values by 15 min, and then decreased to normal levels after 1 h. Conversely, phosphorylation of PI3K-AKT (Fig. 3A, B) and MAPK (Fig. 3C) was reduced in the GC-1 cells transfected with VEGFR3 shRNA compared to NC shRNA.
Figure 3.
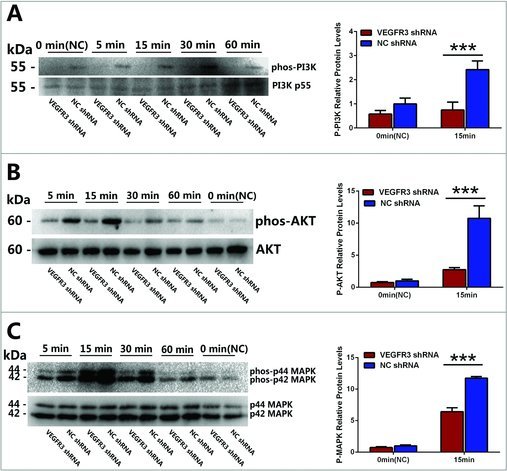
The phosphorylation of PI3K-AKT and MAPK were activated by VEGFC/VEGFR3 signaling. (A-C) Western blotting showed the phosphorylation of PI3K (A), AKT (B) and p44/P42 MAPK (ERK) (C) activated by VEGFC treatment (upper panel). The expression levels of PI3K (A), AKT (B) and p44/P42 MAPK (MAPK) (C) were used as a loading reference for total proteins (lower panel). The ratios of phosphorylation for these proteins were showed in the form of histogram on the right. Values in (A-C) were represented as mean ± SD from three independent experiments. (*P<0.05, **P<0.01, ***P<0.001).
To further explore the effect of PI3K-AKT and MAPK pathways which activated by VEGFC on the proliferation of GC-1 cells from the negative aspect, GC-1 cells were pretreated with LY294002 (an inhibitor for PI3K) or CI-1040 (an inhibitor for MAPK) [17,18], Western blotting showed that 10 μM of LY294002 and CI-1040 could completely inhibit the phosphorylation of PI3K-AKT and MAPK respectively (Fig. 4A, B). Then GC-1 cells were incubated with VEGFC for 4 days in the condition of 10μM LY294002 or CI-1040 treatment. CCK-8 assays showed that the proliferation was blocked in GC-1 cells when treated with LY294002 and CI-1040 compared to NC group, respectively (Fig. 4C), and VEGFC treatment could stimulate the proliferation of GC-1 cells in the presence of LY294002 or CI-1040 (Fig. 4C). This result indicated that both PI3K-AKT and MAPK pathways were included in the downstream signal pathways associated with GC-1 cell proliferation stimulated by VEGFC/VEGFR3 signaling.
Figure 4.
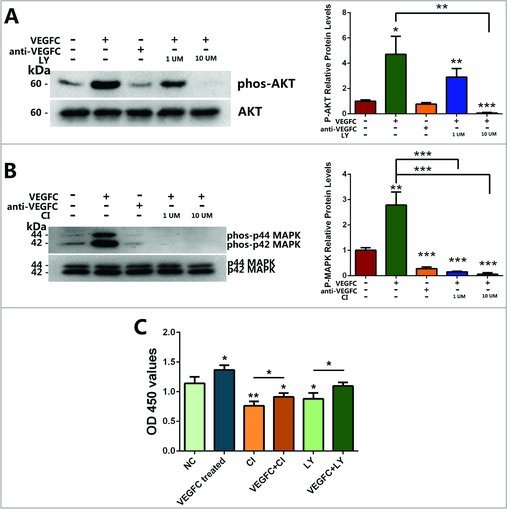
The effects of inhibitor LY294002 and inhibitor CI-1040 on the phosphorylation of AKT and MAPK and on VEGFC-induced proliferation of GC-1 cells. GC-1 cells were pretreated with or without LY294002 and CI-1040 for 30 min, and then VEGFC was added to the culture medium as described in the Materials and Methods. (A, B) Western blotting showed the phosphorylation of p44/p42 MAPK (MAPK) (A) and AKT (B) in GC-1 cells treated with or without VEGFC, LY294002 and CI-1040 compared with the negative control (upper panel), p44/p42 MAPK (MAPK) (A) and AKT(B) was used as a loading control for total proteins (lower panel). The right panels showed the ratio between phosphorylated AKT and MAPK with total AKT and MAPK protein. (C) CCK-8 assay revealed the quantification of the GC-1 cells treated with VEGFC, LY294002, or CI-1040 for 4 days. (*P<0.05, **P<0.01, ***P<0.001).
VEGFC/ VEGFR3 signaling enhanced the expression of Cyclin D1 and up-regulate the transcription of c-fos and Egr1 in GC-1 cells
Since the PI3K-AKT and MAPK pathway were activated by VEGFC/VEGFR3 signaling, we screened the change of some common transcription factor associated with proliferation and also belong to the downstream of PI3K-AKT and MAPK pathways (data from http://www.kegg.jp/) in GC-1 cells. Genes whose expression level changed over 2 folds in GC-1 cells after treated with VEGFC for 15 min were identified to be the differentially-expressed genes. Real-time PCR revealed that the transcription of Early Growth Response gene-1 (Egr1) and Proto-Oncogene Proteins c-fos (c-fos) were increased 7.8 ± 0.7 folds and 6.3 ± 0.6 folds (Fig. 5A, B) respectively, and other transcription factors which showed no significantly different were list in Fig. S1. Interestingly, no significant change was detected in the VEGFR3 shRNA transfected cells even though were treated with VEGFC (Fig. 5A, B). What's more, VEGFR3 knockdown could down-regulate the transcription of c-fos and Egr1 in GC-1 cells (Fig. 5A, B).
Figure 5.
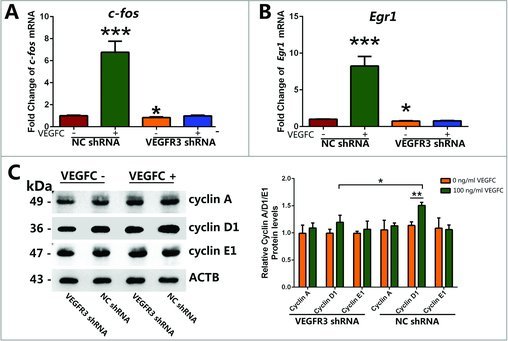
VEGFC/VEGFR3 signaling stimulated the transcript of c-fos and Egr1 and increased the expression level of Cyclin D1. (A, B) Real-time PCR showed that the change of c-fos and Egr1 transcript after VEGFC treatment or VEGFR3 knockdown in GC-1 cells. (C) Western blotting showed the levels of cyclin A, cyclin D1 and cyclin E1 proteins in GC-1 cells transfected with VEGFR3 shRNA or negative shRNA followed by being treated with or without VEGFC. The right panels displayed the amount of proteins normalized with ACTB. Values in (A-C) were represented as mean ± SD from three independent experiments. (*P<0.05, **P<0.01).
To better understand how VEGFC affected DNA synthesis and cell cycle progression of GC-1 cells, we further examined expression changes of cell cycle regulators, including cyclin A, cyclin D1, and cyclin E. Western blotting showed that VEGFC promoted the expression of cyclin D1, while no significant change of cyclin A and cyclin E protein was observed (Fig. 5C). The protein level of cyclin D1 was significantly reduced in GC-1 cells after transfected with VEGFR3 shRNA compared to NC shRNA under both VEGFC addition and vacancy conditions. Taken together, these results indicated that VEGFC/VEGFR3 regulated cell cycle of GC-1 cells via cyclin D1.
VEGFR3 Knockdown Resulted in an Increase of Apoptosis in GC-1 cells
To determine whether the VEGFC/VEGFR3 signaling affected the apoptosis of GC-1 cells, Annexin V/PI apoptosis assay was performed with flow cytometry. Cell populations in Q2 (upper right) and Q3 (lower right) quadrant of flow cytometry scatterplot were counted and expressed as percentages of GC-1 cells in late and early apoptosis phases, respectively. Early apoptosis cells included cell population that was Annexin V positive and PI negative (Q3). Late apoptotic cells included cell population that was both Annexin V and PI positive (Q2) (Fig. 6A). Apoptosis assay showed that VEGFR3 shRNA transfection increased the percentage of GC-1 cells in both early and late apoptosis phase. While the percentage of apoptosis GC-1 cells showed no significant change after VEGFC treatment (Fig. 6B), it indicated that the exogenous VEGFC was not necessary for GC-1 cell survival under the normal culture condition in vitro.
Figure 6.
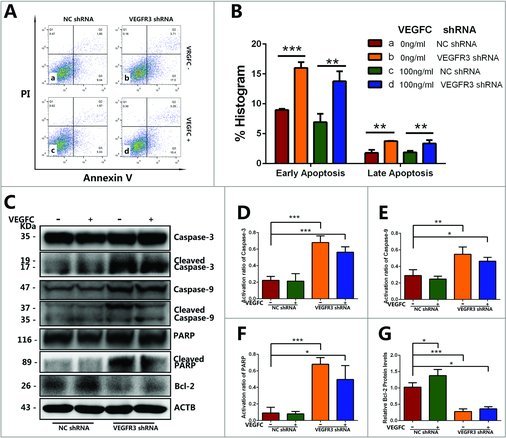
The effects of VEGFC/VEGFR3 on the early and late apoptosis of GC-1 cells. (A) Flow cytometry demonstrated the influence of VEGFR3 knockdown and VEGFC treatment on the early apoptosis (Q3) and late apoptosis (Q2) of GC-1 cells. The existence of Annexin V (X-axis) and the nuclear staining of PI (Y-axis) by flow cytometry were shown. (B) Annexin V/PI flow cytometry analysis showed that VEGFR3 knockdown led to increases in apoptosis in GC-1 cells compared to the negative control shRNA. (C-G) Western blotting showed the transcript levels of apoptosis protein in GC-1 cells treated with VEGFR3 shRNA or negative control shRNA. The activation ratio of Caspase-3 protein (D), Caspase-9 protein (E), and PARP (F) and Bcl-2 protein (G) was showed in the form of histogram. Values in (A-G) were represented as mean ± SD from three independent experiments. (*P<0.05, **P<0.01, ***P<0.001).
To better understand the mechanism by which VEGFC/VEGFR3 signaling affects GC-1 cells apoptosis, we further examined the expression changes of protein involved in the apoptotic pathway, including Bcl-2, Caspase-3, Caspase-9 and PARP (Fig. 6C). The expression level of Bcl-2 was standardized by ACTB protein and the activation of Caspase-3, Caspase-9 and PARP was quantified with the ratio between the cleaved protein and the full-length of protein. VEGFC treatment promoted the expression of Bcl-2 protein but didn't affect caspase protein. Nevertheless, VEGFR3 knockdown down-regulated the expression level of Bcl-2 (Fig. 6G) and triggered the activation of Caspase-3 (Fig. 6D), Caspase-9 (Fig. 6E) and PARP (Fig. 6F) in GC-1 cells.
VEGFC enhanced the Phosphorylation of AKT and MAPK mainly via VEGFR3 but not VEGFR2
To identify the function of VEGFR2, another potential receptor for VEGFC, in the activation of AKT and MAPK pathway in GC-1 cells and compare its effect with VEGFR3, GC-1 cells were pretreated with anti-VEGFR2 or anti-VEGFR3 antibody for 30min and then incubated with VEGFC for 30min. As showed in Fig. 7, with the incubation of anti-VEGFR2 or anti-VEGFR3 antibody the phosphorylation of PI3K-AKT level were declined 27.1 ± 2.6% and 48.6 ± 2.8%, similarly, the phosphorylation of MAPK level were declined 29.7 ± 3.1% and 61.3 ± 2.8%, indicated that VEGFR2 had relatively less effect on the phosphorylation levels of AKT or MAPK in GC-1 cells compared with VEGFR3 (Fig. 7B).
Figure 7.

VEGFR3 was the primary receptor but not VEGFR2 for VEGFC to activate the phosphorylation of PI3K-AKT and MAPK. GC-1 cells were pretreated with anti-VEGFR2 or anti-VEGFR3 antibody for 30 min, and then VEGFC was added to the culture medium as described in the Materials and Methods. (A) Western blotting showed the phosphorylation of AKT and p44/P42 MAPK activated by VEGFC treatment after blocking VEGFR2 or VEGFR3 in GC-1 cells (upper panel). The expression levels of AKT and p44/P42 MAPK (MAPK) were used as a loading reference for total proteins (lower panel). (B) The ratios of phosphorylation for these proteins were showed in the form of histogram on the right. Values in (B) were represented as mean ± SD from three independent experiments. (*P<0.05, **P<0.01, ***P<0.001).
Discussion
Spermatogenesis is a unique cellular developmental process, which includes the proliferation of spermatogonia, meiosis of spermatocytes, and the morphological transformation of spermatids into spermatozoa [19]. The proliferation phase provides enough amounts of spermatogonia to reserve fertilization capacity, and normal male produce about 4 million sperms per hour [20]. Disruption of spermatogenesis often diminishes semen quality, even causes azoospermia sometimes. It is necessary to explore the complex signal networks involved different growth factors, cytokines, micromolecule and other potential regulators during spermatogenesis. Here, we have for the first time demonstrated that VEGFC/VEGFR3 signaling may be involved in the regulation of the proliferation and apoptosis of mouse spermotogonia and illustrated the associated molecular signaling pathway.
Members of VEGF family are vital regulators of many important physiological processes and they are classified as cysteine knot growth factors [21]. The VEGF family consists of numerous members, including VEGFA/B/C/D/E/F, and placental growth factor (PGF). VEGFC is distinguished from other VEGF subtypes due to its pro-peptides at both the N- and C-terminal of the conserved VEGF homology domain. The VEGFs exert their biologic effects through interacting with their receptors, e.g. VEGFR1 (Flt1), VEGFR2 (KDR), VEGFR3 (Flt4) and NRP [21–23]. VEGFR3 was distinct from VEGFR1 and VEGFR2 in that it is depends on two special disulfide linked polypeptides located in the extracellular domain [24]. In our previous work on human spermatogenesis transcriptome, we have found the high expression of VEGFC/VEGFR3 transcript in spermatogonia was reversed by onset of meiosis and drops markedly to a lower level [12]. The identifications of VEGFC/VEGFR3 transcripts and proteins in mouse spermatogonia and GC-1 cells (spermatogonial cell line) suggest that VEGFC may play a role in the maintenance or/and self-renew of spermatogonia in physiological condition.
In rodents, spermatogonia are generally classified as undifferentiated spermatogonia (Asingle, Apaired, and Aaligned) and differentiated spermatogonia (A1-A4, intermediate, and type B) based upon their differences in morphology and phenotype. It is believed that the Asingle spermatogonia are the actual spermatogonial stem cell (SSC). Since male germ cells from 8-day-old ICR mice contain SSCs, undifferentiated spermatogonia and differentiated spermatogonia (A1-A4 spermatogonia) [25]. To confirm the spatio-temporal expression of VEGFR3 in spermatogenesis before spermatocyte stage, double immunofluorescent staining was performed to detect VEGFR3 along with GPR125, PLZF and STRA8. Among these proteins, promyelocytic leukemia zinc finger (PLZF; also known as ZBTB16) is a zinc-finger-containing transcription factor expressing in nearly 80% of undifferentiated spermatogonia of P8 mice including Asingle SSCs and Apaired-Aaligned chains of progenitor spermatogonia [26,27].; Stimulated by Retinoic Acid Gene 8 Protein (STRA8) was used as a differentiation hallmark that was associated with spermatogonial differentiation and meiotic initiation [28]. Since the PLZF was located in the cell nucleus, G protein-coupled receptor 125 (GPR125), a cell membrane marker for mouse undifferentiated spermatogonia and their progenitors [29], was utilized as another marker to confirm the findings. The observations that VEGFR3 expressed in both undifferentiated spermatogonia and differentiated spermatogonia indicate that the effects of VEGFC/VEGFR3 signaling might cover the whole differentiating phase from SSCs to differentiated spermatogonia.
VEGFC is well-known as a mitogen for lymphatic endothelial cells and mediates downstream signaling through the activation of MAPK and AKT [30]. It has been recently shown that VEGFC can convert neural stem cells (NSCs) into progenitor cells and regulates mammary tumor cell survival and proliferation [8,10]. Notably, we found that VEGFC could promote the proliferation and DNA synthesis in GC-1 cells, as evidenced by our CCK-8 assay, PCNA expression detection, and EDU incorporation assay. Our results suggest that VEGFC may also play a role in the regulation of spermatogenesis. To eliminate the interference of VEGFR2, another receptor of VEGFC, which is also the receptor of VEGFA and VEGFD, anti-VEGFR2 or anti-VEGFR3 antibody was used to block VEGFR2 or VEGFR3 signaling before GC-1 cell was treated with VEGFC. The Western blotting results showed that VEGFC/VEGFR2 signaling did promote the activation and AKT and MAPK, but only to a weak extent compared to VEGFC-VEGFR3 signaling. This result was similar to Vladimir Joukov's observation that VEGFC had higher affinity to VEGFR3 (KD = 135 pM) than to VEGFR2 (KD = 410 pM) in activating these receptors [31]. AKT and MAPK signaling pathways are involved in multiple biological functions, including DNA synthesis, cell cycle and cell apoptosis [32–34]. And in testis, they play an essential role in controlling SSC self-renewal and spermatogonial proliferation [35–37]. Signaling by VEGFR3 involves the CrkII, PI-3 kinase (PI3K), and Grb2 pathways, leading to activation of downstream signaling molecules such as AKT and MAPK pathways in endothelial cells[38,39]. MAPK is indispensable for the proliferation of c-kit expressing cells (type A1-A4 spermatogonia) by stem cell factor (SCF) and the self-renewal of mouse SSCs by GDNF [40,41]. It has been reported that conditional activation of the AKT could induce logarithmic proliferation of GS cells in the absence of GDNF for at least 5 months [42]. Our result showed that AKT could also be activated by VEGFC/VEGFR3 signaling in spermatogonial GC-1 cells, indicating that VEGFC could be a potential supplement and compensation for the previously known regulators. To further explore the roles of AKT and MAPK pathways in mediating the effects of VEGFC/VGFR3 on the proliferation in GC-1 cells, LY294002 (an inhibitor for AKT) and CI-1040 (an inhibitor for MAPK) were used to treat GC-1 cell before incubating with VEGFC. Results indicated that both inhibitors could slow down the proliferation of GC-1 cell but VEGFC incubation could stimulate the cell growth even when LY294002 or CI-1040 exists. These results implicated that VEGFC could promote the proliferation of mouse spermatogonia by activating both AKT and MAPK pathways. When one of them was inhibited, another pathway could still be activated by VEGFC and compensate the function in promoting the proliferation.
Furthermore, we detected the expression of PCNA protein, a proliferation marker in the nucleus of cells in the S phase of the cellular cycle [43] and found a significant increase in GC-1 cells after being treated with VEGFC. Our results are consistent with the observation that VEGFC/VEGFR3 signaling increases PCNA expression level in other tissues such as primary breast cancer cells and rat cholangiocytes [9,44]. The level of PCNA was up-regulated in the GC-1 cells stimulated by VEGFC and down-regulated in the GC-1 cells transfected with VEGFR3 shRNA. To further detect how VEGFC/VEGFR3 signaling regulates cell cycle to stimulate the proliferation in GC-1 cells, expressional changes of cyclin A, cyclin D1, and cyclin E were exanimated. Cyclin D1 has been shown to play a specific role during the G1/S phase transition in adult mouse spermatogonia cells and GC-1 cells [45,46]. Overall, these data indicated that VEGFC/VEGFR3 signaling motivates GC-1 cells going through G1/S checkpoint primarily by affecting cyclin D1. On the other hand, levels of cyclin A and cyclin E showed no significant difference between VEGFR3 shRNA and negative control shRNA, although cyclin A and cyclin E also participated in the S-phase of the cell cycle regulation [47,48]. Considered together, these data indicated that VEGFC/VEGFR3 signaling motivates GC-1 cells go through G1/S checkpoint by provoking cyclin D1.
Since the PI3K-AKT and the MAPK pathway could be activated by VEGFC/VEGFR3 signal, we screened and evaluated some transcription factors which were regulated by PI3K-AKT and MAPK pathway. The real-time PCR show c-fos and Egr1 mRNA was markedly increased after VEGFC stimulation. Some mechanistic studies indicated c-fos stimulates cell proliferation by the regulation of cell cycle genes, such as cyclin D1 and cyclin E, which contains AP-1 sites within the promoter region [6,49]. In GC-1 cells, the activation of the rapid MAPK signaling cascade in turn induces the transcription of c-fos and promoting proliferation [46]. What is more, c-fos is also in part mediated by the PI3-kinase signaling pathway in the HaCaT cell line [50]. In similar with c-fos, Egr-1 could also bind with the Egr-1/Sp1 motif of the cyclin D1 promoter through the activation of MAPK pathway to promote proliferation of prostate cancer cells [51]. Since there was no report about the association between Egr1 and the proliferation of mouse spermatogonia, whether Egr1 stimulated by VEGFC could bind the cyclin D1 promoter in GC-1 cells still require further research.
Germ cell apoptosis in early stage of spermatogenesis is a critical requirement in maintaining balance in the ratio of germ cells to Sertoli cells during testis development [52,53]. The proper balance between apoptosis and proliferation of spermatogonia is indispensable for proper numbers of sperms generated and abnormal apoptosis can largely disturb spermatogenesis, and eventually become a culprit of male infertility [54]. In rat, the apoptosis of A2, A3, and A4 spermatogonia reduces the number of the whole progressively differentiated A1 spermatogonia to ∼25% [55]. Nevertheless, there is no report about the association between VEGFC and the apoptosis of germ cell. Yet in other tissue, VEGFC activated PI3K-AKT and MAPK signaling pathway and thus stimulate the expression of Bcl-2 and translocation of mitochondrial membrane, which protected cardiomyocytes by blocking the intrinsic apoptotic pathway and the production of caspases [56,57]. To illuminate whether VEGFC/VEGFR3 signaling has similar effect on spermatogonia, GC-1 cell was transfected with VEGFR3 shRNA or NC shRNA, followed by VEGFC treatment. The results showed there was no significant change of apoptosis after VEGFC treatment but a significant increase of both early and late apoptosis in VEGFR3 knockdown GC-1 cells was detected. Western blotting showed that VEGFC treatment promoted the expression of Bcl-2 in GC-1 cells. Bcl-2 was first discovered in follicular lymphoma as a result of chromosome translocation and was shown to be capable to prevent mitochondrial outer membrane permeabilization [58]. In vivo, overexpression of Bcl-2 inhibited normal spermatogonial apoptosis, leading to accumulation of spermatogonia by 4 weeks [59]. Our results showed that VEGFR3 knockdown resulted in a greatly down-regulation of Bcl-2, indicating that the loss of VEGFC/VEGFR3 signaling may be associated with mitochondrial membrane damage. During apoptosis, mitochondrial cytochrome C release correlated with mitochondrial membrane damage led to the cleavage at Asp315 and Asp330 in Caspase-9 protein and produced p35 and p37 subunit and further processed Caspase-3 [60,61]. So we examined Caspase-3 and Caspase-9 in GC-1 cells with VEGFC treatment or VEGFR3 knockdown. In serum-free medium, neither the total expression of caspase zymogen nor their cleaved forms was changed after VEGFC treatment, which was accordance with the Annexin V/PI cytometry results. This result that indicated exogenous VEGFC was not essential for GC-1 cells survive under common culture conditions. Nevertheless, a recent study revealed that hypoxia significantly enhanced cleaved Caspase 3, 8, and 9 in neonatal cardiomyocytes, which could be abolished by VEGFC treatment [57]. Thus, the function of VEGFC treatment for spermatogonia in pathological conditions deserves further research. Furthermore, we found the activation of caspase-9 and caspase-3 was obviously enhanced by VEGFR3 knockdown. As an important apoptosis regulator, Caspase-3 was highly expressed in germ cells of maturation arrest patients [62]. Caspase-3 was processed by cleaved Caspase-9 and hydrolyzed into activated fragment which were responsible for the proteolytic cleavage of the nuclear enzyme poly (ADP-ribose) polymerase (PARP) [63]. PARP was activated by caspases to repair the damaged DNA [64]. In our study, higher level of cleaved PARP is observed in VEGFR3 knockdown group, similar to that of caspase proteins. Notably, our unpublished results showed that PI3K-AKT and MAPK pathways were also involved in regulating the GC-1 cell apoptosis, so it still requires further study to explain the mechanism about how VEGFC/VEGFR3 signaling suppresses the apoptosis of GC-1 cells via these two pathways. Together, these results indicated that VEGFC/VEGFR3 signaling was essential for GC-1 cells survival and the loss of VEGFC/VEGFR3 signaling would cause excessive apoptosis in GC-1 cells via Bcl-2 down-regulating and Caspase-3/9 activation.
In summary, we have demonstrated that VEGFC and VEGFR3 are expressed in spermatogonia and GC-1 cells. VEGFC/VEGFR3 plays an important role in promoting the proliferation and suppressing apoptosis of GC-1 cells in vitro. Specifically, we revealed that VEGFC activates the PI3K-AKT and MAPK pathways, up-regulates the transcription of c-fos and Egr1, and promotes the expressions of cyclin D1 and Bcl-2 in GC-1 cells (Fig. 8 ). This study thus sheds a light on potential effects of VEGFC/VEGFR3 signaling in the spermatogenesis in vitro and provides another piece of information for a better understanding of spermatogenesis.
Figure 8.
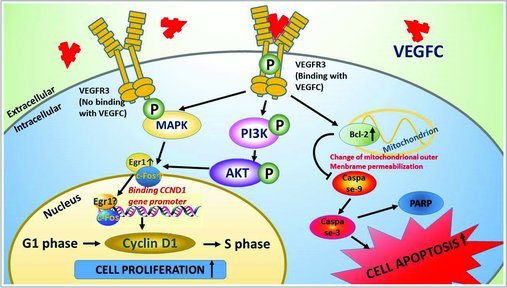
The schematic diagram demonstrates the proliferation and apoptosis pathways including the upstream and downstream cascades activated by VEGFC in the GC-1 cells. VEGFC/VEGFR3 signaling plays a dual role of the fate determinations of GC-1 cells by activating PI3K-AKT/MAPK/Egr1/c-Fos/Cyclin D1 pathway in stimulating proliferation and BCL-2/CASPASE3/9 pathway in regulating apoptosis. “⋌” indicates “down-regulate or inactivation”, “↑” indicates “up-regulate or activation” and “P” indicates “phosphorylate”.
Materials and methods
Experimental animals
Male ICR mice of 8-day-old were purchased from the Shanghai Laboratory Animal Center of the Chinese Academy of Sciences. Mice were maintained in a controlled environment under a 12-hour light-dark cycle. The mice had free access to the purified water and animal food. All the experiments with mice were performed in accordance with the guideline and regulation for the care and use of laboratory animals and were approved by the Shanghai general hospital (License No. SYXK 2011-0121).
Cell isolation
Sertoli cells and spermatogonia were isolated from the testis of 8-day-old mice by two-step enzymatic digestion according to the method [13]. All the testicular cells were cultured for 24 h, because the meiosis of mice initiates from the 14th day after birth, so the suspension cells are predominantly consistent of spermatogonia. Then spermatogonia were kept and adherent cells include Sertoli, Leydig and peritubular myoid cells were discarded [65]. Cell purity was identified by immunocytochemistry using hallmarks (GPR125, PLZF and STRA8) for these cells, and more details can be found in Results part.
Cell culture and treatment
GC-1 cells, a well-established cell line of mouse type B spermatogonia, were obtained from ATCC Company (ATCC, CRL-2053). The GC-1 cells were cultured in DMEM/F-12 medium (Gibco, 11320082) supplemented with 10% fetal bovine serum (FBS) (Gibco, 10099141) and 1% penicillin/streptomycin (Gibco, 15070063).
After the cells reached 80% of confluence, they were starved with FBS-free DMEM/F-12 medium for 16 h and treated with or without recombinant human VEGFC (Peprotech, 100–20C), MAZ51 (Santa Cruz, sc-202703), LY294002 (Selleck, S1105), CI-1040 (Selleck, S1020), VEGFR2 antibody (Thermo Fisher, RB2153219), VEGFR3 antibody (R&D, AF743), and VEGFR3 shRNA (GenePharma). The incubation time was according to the experimental objective.
Short hairpin RNA (shRNA) synthesis and transfection
shRNA targeting sequences against VEGFR3 (sense primer: 5’-CACCGCATCCTGACCATCCACAATGTTCAAGAGACATTGTGGATGGTCAGGATGCTTTTTTG-3’; antisense primer: 5’-GATCCAAAAAAGCATCCTGACCATCCACAATGTCTCTTGAACATTGTGGATGGTCAGGATGC-3’) and negative control shRNA (sense primer 5’-CACCGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTG -3'; anti-sense 5’- GATCCAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAC -3') were designated. All shRNAs were synthesized with an anti-puromycin resistance and GFP, and were purchased from Shanghai GenePharma Limited Co., Shanghai, China. 293T cells were co-transfected with the VEGFR3 shRNA plasmid and three packaging plasmids (GenePharma) using Lipofectamine_3000 (ThermoFisher, L3000001) to generate lentivirus. GC-1 cells were cultured in 6-well plates to a confluence of 80% with complete medium and infected with above lentivirus solutions, which had been five-fold diluted with DMEM/F-12 (2.5% FBS) containing 5 μg/ml polybrene. After 24h transfection, VEGFR3 shRNA lentivirus medium was replaced by fresh complete medium and the interference efficiency was verified by Western blotting.
RNA extraction and reverse transcription polymerase chain reaction (RT-PCR) and quantitative real-time PCR
Total RNA was extracted from mouse spermatogonia and GC-1 cells, using RNAiso Plus Kit (Takara, #9109) and synthesized into cDNA using First Strand cDNA Synthesis Kit (Thermo Fisher, K1651), following the protocol as described previously [66]. The concentrations of total RNA were measured by Nanodrop 2000 (Thermo Fisher), and ratios of A260/A280 were set in 1.8-2.0 to ensure the quality of RNA. The primers sequences of the chosen genes were designed and listed in Table 1. PCR reaction with the cDNA and primers of target genes was carried out for 35 cycles. PCR production of cDNA and Actb primer served as positive controls and PCR production of which RNA was amplified with Actb primer served as negative. The following PCR reactions and products separation by electrophoresis were conducted complying with conditions reported previously [4].
Table 1.
Primer sequences of genes used for RT-PCR.
| Gene | Forward primers(5'-3') | Reverse primers(5'-3') |
|---|---|---|
| Vegfc | CCCAAACCAGTCACAATCAG | TACATTCACAGGCACATTTCC |
| Vegfr2 | ACATAGCCTCCACTGTTTATG | CACATCCCGGTTTACAATCT |
| Vegfr3 | CCCGAGAGCATCTTTGATAAG | GAAGAGCCTGGAGTCTTAGT |
| Actb | GTGACGTTGACATCCGTAAAGA | GCCGGACTCATCGTACTCC |
| Pla1a | TATGGCTCAGCATTGGAAGTTC | AAACTGCACCTTGAGGTTGGT |
| Elk | CAGCCGGAATGAATACATGCG | AGGCTTCTAAGGGGTTTGGAC |
| Etv5 | CAAGTCCCTTTTATGGTCCCAG | ACTCTTCAGAATCGTGAGCCA |
| Egr1 | AGACGAGTTATCCCAGCCAAA | GGTCGGAGGATTGGTCATGC |
| Rbl2 | TTCCCCATGATTAGCGATGATCT | GGGTTCACAAGTTCTTTACGGTT |
| Fasl | TCCGTGAGTTCACCAACCAAA | GGGGGTTCCCTGTTAAATGGG |
| c-fos | CGGGTTTCAACGCCGACTA | TTGGCACTAGAGACGGACAGA |
| c-Jun | CCTTCTACGACGATGCCCTC | GGTTCAAGGTCATGCTCTGTTT |
| C/ebpa | CAAGAACAGCAACGAGTACCG | GTCACTGGTCAACTCCAGCAC |
| Atf2 | CATCAGCTATCGTTCGTCCAG | GTTGGTGAAGGTACTGCTTGT |
| Stat5a | CGCCAGATGCAAGTGTTGTAT | TCCTGGGGATTATCCAAGTCAAT |
| Pou2f1 | AGCTCTTGCTTCTAGTGGCTC | CTGGCTGTAGGTGCAGAGTTC |
Quantitative real-time PCR reactions were performed using Power SYBR® Green PCR Master Mix (Applied Biosystems, 4367659) and a 7500 Fast Real-Time PCR System (Applied Biosystems, 4484643). The primers pair of detected genes were listed in Table 1. PCR products was qualified by the comparative Ct (threshold cycle) method that have been described previously [67] and the transcription of target mRNA were normalized to the Actb mRNA.
Immunocytochemistry
The GC-1 cells have the ability of adherence growth were cultured in 24-well plates, and mouse spermatogonia isolated from 8-day-old mice were spun on the slides by centrifuging. Then cells were washed with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA). The cells were blocked with 5% bull serum albumin (BSA) for 1 h and incubated with relevant primary antibodies, including anti-VEGFR3 antibody (R&D, AF743, dilution: 1:200), anti-PLZF antibody (Santa Cruz, sc-22839, dilution: 1:200), anti-GRP125 antibody (abcam, ab51705, dilution: 1: 200), anti-STRA8 antibody (abcam, ab49602, dilution: 1: 200), normal rabbit IgG (CST, #2729, dilution: 1:200) and normal goat IgG (Santa Cruz, sc-2028, dilution: 1:200) overnight at 4 ℃. These cells were further incubated with anti-goat or anti-rabbit secondary antibody (Invitrogen, USA) for 2 h. Cell nuclei were labeled by DAPI. Replacement of primary antibodies with normal goat or rabbit IgGs were used as negative controls.
Protein extraction and western blotting
Briefly, GC-1 cells treated or non-treated were collected from 6-well plates and lysed with RIPA lysis buffer (Dingguo, WB-0071) on ice. Cell lysates were cleared by centrifugation at 12,000 g at 4℃ for 15 min, and the protein concentrations were measured by the Bio-Rad Bradford Assay. Protein extracts were heated at 95℃ with 5 × SDS-PAGE Loading Buffer (Dingguo, WB-0091) for 10 min. 30 μg proteins were electrophoresed on SDS-PAGE and transferred to polyvinylidene difluoride membranes according to the procedure previously described [4]. After blocking with 5% skim milk, membranes were incubated with VEGFR3 goat polyclonal antibody (R&D, AF743, dilution: 1:500), phosphor-AKT antibody (CST, #4060, dilution: 1:2000), AKT antibody (CST, #4691, dilution: 1:1000), phospho-p44/42 MAPK (CST, #4377, dilution: 1:2000), p44/42 MAPK (CST, #695, dilution: 1:1000), Phospho-PI3 Kinase (CST, #4228, dilution: 1:2000), PI3 Kinase p55 (CST, #11889, dilution: 1:2000),Caspase-3 (Novus, IMG-144A, dilution: 1:1000), Caspase-9 (Novus, IMG-5705, dilution: 1:1000), PARP (CST, #9532, dilution: 1:2000) and Cyclin A (proteintech, 18202, dilution: 1:2000), Cyclin D1(proteintech, 60186, dilution: 1:2000), Cyclin E (proteintech, 11554, dilution: 1:1000) ACTB (Santa Cruz, sc-47778, dilution: 1:500) at 4℃ overnight. After being washed three times with TBS-T, the gradations of blots were detected by chemiluminescence (Chemi-Doc XRS, USA) and analysis with Adobe Photoshop CS6.
Proliferation assay
GC-1 cell proliferation was determined using a Cell Counting Kit (Dojindo, CK04-01) and Cell-Light EDU DNA Cell Proliferation Kit (RiboBio, C10812-1). For the CCK-8 assay, 5 × 103 GC-1 cells transfected with NC shRNA or VEGFR3 shRNA were seeded in 96-well plates and starved for 16 h, and treated with VEGFC. CCK-8 solution was added to the cells and incubated for 3 h. The absorbance at 450 nm (A450 nm) was measured with a Sunrise microplate reader (TECAN). For the EDU incorporation assay, 5 × 103 GC-1 cells were seeded in 96-well plates and starved for 16 h and treated with reagents as described above. After 30 min staining with Hoechst 33342, the percentages of EDU-positive cells were counted from 500 cells and analyzed according to the methods [68]. The staining images were captured with Nikon Eclipse Ti-S fluorescence microscope (Nikon).
Annexin V/ Propidium Iodide (PI) staining and flow Cytometry analysis
To investigate the effect of VEGFC/VEGFR3 signal and its downstream signaling pathway (AKT and MAPK pathway) on the cell survival, apoptosis analysis of GC-1 cells from different treatments was conducted with APC Annexin V/ PI Apoptosis Detection Kit (BioLegend, UK), according to the manufacturer's instructions. GC-1 cells apoptosis analysis was performed on the Beckman Couter Accuri Cytometers C6 flow cytometer (BD), and analyzed using the Accuri CFlow software.
Statistical analysis
All data were analyzed using GraphPad Prism 6.0 (GraphPad Software) and results were presented as the mean ± standard deviation (Mean ± SD) [69]. The Student's t-test and analysis of variance (ANOVA) were used to analyze the differences between the controls, VEGFC-treated or VEGFR3-knockdown groups. All experiments were performed independently at least three times. All statistical tests were two-tailed and P-value<0.05 was considered as a statistical significant difference.
Funding Statement
This work was supported by grants from National Natural Science Foundation of China (81671512); The national high-tech research and development program (863) of China (2015AA020404); National Natural Science Foundation of China (31230048 and 31671550); The frontier technology project of Shanghai (SHDC12015122).
Acknowledgments
This work was supported by grants from National Nature Science Foundation of China (81671512, 31230048, and 31671550), The National High-tech research and development program (863) of China (2015AA020404), The frontier technology project of Shanghai (SHDC12015122), Shanghai Hospital Development Center (SHDC12015122), and Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning (2012.53), and Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20152511).
Disclosure of Potential Conflicts of Interest
The authors declare no competing interests.
References
- [1].Tegelenbosch RA, de Rooij DG. A quantitative study of spermatogonial multiplication and stem cell renewal in the C3H/101 F1 hybrid mouse. Mutat Res. 1993;290:193–200. doi:doi.org/ 10.1016/0027-5107(93)90159-D. PMID:7694110. [DOI] [PubMed] [Google Scholar]
- [2].O'Shaughnessy PJ. Hormonal control of germ cell development and spermatogenesis. Semin Cell Dev Biol. 2014;29:55–65. doi: 10.1016/j.semcdb.2014.02.010. PMID:. [DOI] [PubMed] [Google Scholar]
- [3].Shuttlesworth GA, de Rooij DG, Huhtaniemi I, et al. . Enhancement of A spermatogonial proliferation and differentiation in irradiated rats by gonadotropin-releasing hormone antagonist administration. Endocrinology. 2000;141:37–49. doi: 10.1210/endo.141.1.7272. PMID:. [DOI] [PubMed] [Google Scholar]
- [4].He Z, Jiang J, Kokkinaki M, et al. . Nodal signaling via an autocrine pathway promotes proliferation of mouse spermatogonial stem/progenitor cells through Smad2/3 and Oct-4 activation. Stem Cells (Dayton, Ohio). 2009;27:2580–2590. doi: 10.1002/stem.198. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Meng X, Lindahl M, Hyvonen ME, et al. . Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science (New York, NY). 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. PMID:. [DOI] [PubMed] [Google Scholar]
- [6].He Z, Jiang J, Kokkinaki M, et al. . Gdnf upregulates c-Fos transcription via the Ras/Erk1/2 pathway to promote mouse spermatogonial stem cell proliferation. Stem Cells (Dayton, Ohio). 2008;26:266–278. doi: 10.1634/stemcells.2007-0436. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pellegrini M, Grimaldi P, Rossi P, et al. . Developmental expression of BMP4/ALK3/SMAD5 signaling pathway in the mouse testis: a potential role of BMP4 in spermatogonia differentiation. J Cell Sci. 2003;116:3363–3372. doi: 10.1242/jcs.00650. PMID:. [DOI] [PubMed] [Google Scholar]
- [8].Han J, Calvo CF, Kang TH, et al. . Vascular endothelial growth factor receptor 3 controls neural stem cell activation in mice and humans. Cell Rep. 2015;10:1158–1172. doi: 10.1016/j.celrep.2015.01.049. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Gaudio E, Barbaro B, Alvaro D, et al. . Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. PMID:. [DOI] [PubMed] [Google Scholar]
- [10].Caires KC, de Avila J, McLean DJ. Vascular endothelial growth factor regulates germ cell survival during establishment of spermatogenesis in the bovine testis. Reproduction (Cambridge, England). 2009;138:667–677. doi: 10.1530/rep-09-0020. PMID:. [DOI] [PubMed] [Google Scholar]
- [11].Tian R, Yang S, Zhu Y, et al. . VEGF/VEGFR2 signaling regulates germ cell proliferation in vitro and promotes mouse testicular regeneration in vivo. Cells, Tissues, Organs. 2016;201:1–13. doi: 10.1159/000440949. PMID:. [DOI] [PubMed] [Google Scholar]
- [12].Zhu Z, Li C, Yang S, et al. . Dynamics of the Transcriptome during human spermatogenesis: predicting the potential key genes regulating male gametes generation. Sci Rep. 2016;6:19069. doi: 10.1038/srep19069. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bellve AR, Cavicchia JC, Millette CF, et al. . Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol. 1977;74:68–85. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].DiMaio TA, Wentz BL, Lagunoff M. Isolation and characterization of circulating lymphatic endothelial colony forming cells. Exp Cell Res. 2016;340:159–169. doi: 10.1016/j.yexcr.2015.11.015. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wong HL, Jin G, Cao R, et al. . MT1-MMP sheds LYVE-1 on lymphatic endothelial cells and suppresses VEGF-C production to inhibit lymphangiogenesis. Nat Commun. 2016;7:10824. doi: 10.1038/ncomms10824. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Kirkin V, Thiele W, Baumann P, et al. . MAZ51, an indolinone that inhibits endothelial cell and tumor cell growth in vitro, suppresses tumor growth in vivo. Int J Cancer. 2004;112:986–993. doi: 10.1002/ijc.20509. PMID:. [DOI] [PubMed] [Google Scholar]
- [17].Vlahos CJ, Matter WF, Hui KY, et al. . A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem. 1994;269:5241–5248. PMID:. [PubMed] [Google Scholar]
- [18].Duesbery NS, Webb CP, Vande Woude GF. MEK wars, a new front in the battle against cancer. Nat Med. 1999;5:736–737. doi: 10.1038/10457. PMID:. [DOI] [PubMed] [Google Scholar]
- [19].Griswold MD. Spermatogenesis: The commitment to meiosis. Physiol Rev. 2016;96:1–17. doi: 10.1152/physrev.00013.2015. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Johnson L, Petty CS, Neaves WB. A comparative study of daily sperm production and testicular composition in humans and rats. Biol Reprod. 1980;22:1233–1243. doi: https://doi.org/ 10.1093/biolreprod/22.5.1233. PMID:. [DOI] [PubMed] [Google Scholar]
- [21].Yamazaki Y, Morita T. Molecular and functional diversity of vascular endothelial growth factors. Mol Divers. 2006;10:515–527. doi: 10.1007/s11030-006-9027-3. PMID:. [DOI] [PubMed] [Google Scholar]
- [22].Pepper MS. Lymphangiogenesis and tumor metastasis: myth or reality? Clin Cancer Res: An Official J American Assoc Cancer Res. 2001;7:462–468. PMID:. [PubMed] [Google Scholar]
- [23].Girling JE, Rogers PA. Regulation of endometrial vascular remodelling: role of the vascular endothelial growth factor family and the angiopoietin-TIE signalling system. Reproduction (Cambridge, England). 2009;138:883–893. doi: 10.1530/rep-09-0147. PMID:. [DOI] [PubMed] [Google Scholar]
- [24].Pajusola K, Aprelikova O, Korhonen J, et al. . FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992;52:5738–5743. PMID:. [PubMed] [Google Scholar]
- [25].Niedenberger BA, Busada JT, Geyer CB. Marker expression reveals heterogeneity of spermatogonia in the neonatal mouse testis. Reproduction (Cambridge, England). 2015;149:329–338. doi: 10.1530/rep-14-0653. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Buaas FW, Kirsh AL, Sharma M, et al. . Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. PMID:. [DOI] [PubMed] [Google Scholar]
- [27].Gassei K, Orwig KE. SALL4 expression in gonocytes and spermatogonial clones of postnatal mouse testes. PloS One. 2013;8:e53976. doi: 10.1371/journal.pone.0053976. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Endo T, Romer KA, Anderson EL, et al. . Periodic retinoic acid-STRA8 signaling intersects with periodic germ-cell competencies to regulate spermatogenesis. Proc Nat Acad Sci United States America. 2015;112:E2347–E2356. doi: 10.1073/pnas.1505683112. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Seandel M, James D, Shmelkov SV, et al. . Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Koch S, Claesson-Welsh L. Signal transduction by vascular endothelial growth factor receptors. Cold Spring Harbor Perspect Med. 2012;2:a006502. doi: 10.1101/cshperspect.a006502. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Joukov V, Sorsa T, Kumar V, et al. . Proteolytic processing regulates receptor specificity and activity of VEGF-C. EMBO J. 1997;16:3898–3911. doi: 10.1093/emboj/16.13.3898. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Coffer PJ, Geijsen N, M'Rabet L, et al. . Comparison of the roles of mitogen-activated protein kinase kinase and phosphatidylinositol 3-kinase signal transduction in neutrophil effector function. Biochem J. 1998;329(Pt 1):121–130. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Vogiatzi P, Giordano A. Following the tracks of AKT1 gene. Cancer Biol Ther. 2007;6:1521–1524. doi:dx.doi.org/ 10.4161/cbt.6.10.4834. PMID: [DOI] [PubMed] [Google Scholar]
- [34].Lu X, Zhao J, Li T, et al. . 5,7-Dihydroxy-4'-methoxyisoflavone induces apoptosis by inhibiting the ERK and Akt pathways in human osteosarcoma cells. Connect Tissue Res. 2015;56:59–64. doi: 10.3109/03008207.2014.984064. PMID:. [DOI] [PubMed] [Google Scholar]
- [35].Bruscoli S, Velardi E, Di Sante M, et al. . Long glucocorticoid-induced leucine zipper (L-GILZ) protein interacts with ras protein pathway and contributes to spermatogenesis control. J Biol Chem. 2012;287:1242–1251. doi: 10.1074/jbc.M111.316372. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Liao HF, Chen WS, Chen YH, et al. . DNMT3L promotes quiescence in postnatal spermatogonial progenitor cells. Development (Cambridge, England). 2014;141:2402–2413. doi: 10.1242/dev.105130. PMID:. [DOI] [PubMed] [Google Scholar]
- [37].Tassinari V, Campolo F, Cesarini V, et al. . Fgf9 inhibition of meiotic differentiation in spermatogonia is mediated by Erk-dependent activation of Nodal-Smad2/3 signaling and is antagonized by Kit Ligand. Cell Death Dis. 2015;6:e1688. doi: 10.1038/cddis.2015.56. PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hasegawa K, Namekawa SH, Saga Y. MEK/ERK signaling directly and indirectly contributes to the cyclical self-renewal of spermatogonial stem cells. Stem Cells (Dayton, Ohio). 2013;31:2517–2527. doi: 10.1002/stem.1486. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Salameh A, Galvagni F, Bardelli M, et al. . Direct recruitment of CRK and GRB2 to VEGFR-3 induces proliferation, migration, and survival of endothelial cells through the activation of ERK, AKT, and JNK pathways. Blood. 2005;106:3423–3431. doi: 10.1182/blood-2005-04-1388. PMID:. [DOI] [PubMed] [Google Scholar]
- [40].Dolci S, Pellegrini M, Di Agostino S, et al. . Signaling through extracellular signal-regulated kinase is required for spermatogonial proliferative response to stem cell factor. J Biol Chem. 2001;276:40225–40233. doi: 10.1074/jbc.M105143200. PMID:. [DOI] [PubMed] [Google Scholar]
- [41].Takashima S, Kanatsu-Shinohara M, Tanaka T, et al. . Functional differences between GDNF-dependent and FGF2-dependent mouse spermatogonial stem cell self-renewal. Stem Cell Rep. 2015;4:489–502. doi: 10.1016/j.stemcr.2015.01.010. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lee J, Kanatsu-Shinohara M, Inoue K, et al. . Akt mediates self-renewal division of mouse spermatogonial stem cells. Development (Cambridge, England). 2007;134:1853–1859. doi: 10.1242/dev.003004. PMID:. [DOI] [PubMed] [Google Scholar]
- [43].Merola E, Mattioli E, Minimo C, et al. . Immunohistochemical evaluation of pRb2/p130, VEGF, EZH2, p53, p16, p21waf-1, p27, and PCNA in Barrett's esophagus. J Cell Physiol. 2006;207:512–519. doi: 10.1002/jcp.20590. PMID:. [DOI] [PubMed] [Google Scholar]
- [44].Enholm B, Karpanen T, Jeltsch M, et al. . Adenoviral expression of vascular endothelial growth factor-C induces lymphangiogenesis in the skin. Circ Res. 2001;88:623–629. doi:doi.org/ 10.1161/01.RES.88.6.623. PMID:. [DOI] [PubMed] [Google Scholar]
- [45].Beumer TL, Roepers-Gajadien HL, Gademan IS, et al. . Involvement of the D-type cyclins in germ cell proliferation and differentiation in the mouse. Biol Reprod. 2000;63:1893–1898. doi:doi.org/ 10.1095/biolreprod63.6.1893. PMID:. [DOI] [PubMed] [Google Scholar]
- [46].Sirianni R, Chimento A, Ruggiero C, et al. . The novel estrogen receptor, G protein-coupled receptor 30, mediates the proliferative effects induced by 17beta-estradiol on mouse spermatogonial GC-1 cell line. Endocrinology. 2008;149:5043–5051. doi: 10.1210/en.2007-1593. PMID:. [DOI] [PubMed] [Google Scholar]
- [47].Pal HC, Katiyar SK. Cryptolepine, a plant alkaloid, inhibits the growth of non-melanoma skin cancer cells through inhibition of topoisomerase and induction of DNA damage. Molecules (Basel, Switzerland). 2016;21(12):1758. doi: 10.3390/molecules21121758. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lee JI, Kim IH, Nam TJ. Crude extract and solvent fractions of Calystegia soldanella induce G1 and S phase arrest of the cell cycle in HepG2 cells. Int J Oncol. 2017;50:414–420. doi: 10.3892/ijo.2017.3836. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Pestell RG, Albanese C, Reutens AT, et al. . The cyclins and cyclin-dependent kinase inhibitors in hormonal regulation of proliferation and differentiation. Endocr Rev. 1999;20:501–534. doi: 10.1210/edrv.20.4.0373. PMID:. [DOI] [PubMed] [Google Scholar]
- [50].Gonzales M, Bowden GT. The role of PI 3-kinase in the UVB-induced expression of c-fos. Oncogene. 2002;21:2721–2728. doi: 10.1038/sj.onc.1205366. PMID:. [DOI] [PubMed] [Google Scholar]
- [51].Xiao D, Chinnappan D, Pestell R, et al. . Bombesin regulates cyclin D1 expression through the early growth response protein Egr-1 in prostate cancer cells. Cancer Res. 2005;65:9934–9942. doi: 10.1158/0008-5472.can-05-1830. PMID:. [DOI] [PubMed] [Google Scholar]
- [52].Billig H, Furuta I, Rivier C, et al. . Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology. 1995;136:5–12. doi: 10.1210/endo.136.1.7828558. PMID:. [DOI] [PubMed] [Google Scholar]
- [53].Mizuno K, Shiratsuchi A, Masamune Y, et al. . The role of Sertoli cells in the differentiation and exclusion of rat testicular germ cells in primary culture. Cell Death Differ. 1996;3:119–123. PMID:. [PubMed] [Google Scholar]
- [54].Manku G, Culty M. Mammalian gonocyte and spermatogonia differentiation: recent advances and remaining challenges. Reproduction (Cambridge, England). 2015;149:R139–R157. doi: 10.1530/rep-14-0431. PMID:. [DOI] [PubMed] [Google Scholar]
- [55].Allan DJ, Harmon BV, Roberts SA. Spermatogonial apoptosis has three morphologically recognizable phases and shows no circadian rhythm during normal spermatogenesis in the rat. Cell Proliferat. 1992;25:241–250. PMID:. [DOI] [PubMed] [Google Scholar]
- [56].Chen XG, Lv YX, Zhao D, et al. . Vascular endothelial growth factor-C protects heart from ischemia/reperfusion injury by inhibiting cardiomyocyte apoptosis. Mol Cell Biochem. 2016;413:9–23. doi: 10.1007/s11010-015-2622-9. PMID:. [DOI] [PubMed] [Google Scholar]
- [57].Zhao T, Zhao W, Meng W, et al. . VEGF-C/VEGFR-3 pathway promotes myocyte hypertrophy and survival in the infarcted myocardium. American J Trans Res. 2015;7:697–709. PMID:. [PMC free article] [PubMed] [Google Scholar]
- [58].Maundrell K, Antonsson B, Magnenat E, et al. . Bcl-2 undergoes phosphorylation by c-Jun N-terminal kinase/stress-activated protein kinases in the presence of the constitutively active GTP-binding protein Rac1. J Biol Chem. 1997;272:25238–25242. PMID:. [DOI] [PubMed] [Google Scholar]
- [59].Furuchi T, Masuko K, Nishimune Y, et al. . Inhibition of testicular germ cell apoptosis and differentiation in mice misexpressing Bcl-2 in spermatogonia. Development (Cambridge, England). 1996;122:1703–1709. PMID:. [DOI] [PubMed] [Google Scholar]
- [60].Forand A, Bernardino-Sgherri J. A critical role of PUMA in maintenance of genomic integrity of murine spermatogonial stem cell precursors after genotoxic stress. Cell Res. 2009;19:1018–1030. doi: 10.1038/cr.2009.50. PMID:. [DOI] [PubMed] [Google Scholar]
- [61].Cheng YX, Liu R, Wang Q, et al. . Realgar-induced apoptosis of cervical cancer cell line Siha via cytochrome c release and caspase-3 and caspase-9 activation. Chinese J Integr Med. 2012;18:359–365. doi: 10.1007/s11655-011-0697-z. PMID:. [DOI] [PubMed] [Google Scholar]
- [62].Kilic S, Lortlar N, Bardakci Y, et al. . Caspase-3 and VEGF immunopositivity in seminiferous tubule germ cells in cases of obstructive and non-obstructive azoospermia in smokers versus non-smokers. J Assist Reprod Gen. 2009;26:57–63. doi: 10.1007/s10815-008-9286-2. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nicholson DW, Ali A, Thornberry NA, et al. . Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. PMID:. [DOI] [PubMed] [Google Scholar]
- [64].Oliver FJ, de la Rubia G, Rolli V, et al. . Importance of poly(ADP-ribose) polymerase and its cleavage in apoptosis. Lesson Uncleavable mutant. J Biol Chem. 1998;273:33533–33539. doi: 10.1074/jbc.273.50.33533. PMID: [DOI] [PubMed] [Google Scholar]
- [65].Dirami G, Ravindranath N, Pursel V, et al. . Effects of stem cell factor and granulocyte macrophage-colony stimulating factor on survival of porcine type A spermatogonia cultured in KSOM. Biol Reprod. 1999;61:225–230. doi:doi.org/ 10.1095/biolreprod61.1.225. PMID:. [DOI] [PubMed] [Google Scholar]
- [66].Liu Y, Niu M, Yao C, et al. . Fractionation of human spermatogenic cells using STA-PUT gravity sedimentation and their miRNA profiling. Sci Rep. 2015;5:8084. doi: 10.1038/srep08084. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].He Z, Jiang J, Hofmann MC, et al. . Gfra1 silencing in mouse spermatogonial stem cells results in their differentiation via the inactivation of RET tyrosine kinase. Biol Reprod. 2007;77:723–733. doi: 10.1095/biolreprod.107.062513. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Reddy MN, Verma S. Lesions of the seminal vesicles and their MRI characteristics. J Clin Imag Sci. 2014;4:61. doi: 10.4103/2156-7514.143734. PMID:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Altintas R, Ediz C, Celik H, et al. . The effect of varicocoelectomy on the relationship of oxidative stress in peripheral and internal spermatic vein with semen parameters. Andrology. 2016;4:442–446. doi: 10.1111/andr.12172. PMID:. [DOI] [PubMed] [Google Scholar]


