Summary
Background
Retention of patients in HIV care is crucial to ensure timely treatment initiation, viral suppression, and to avert AIDS-related deaths. We did a randomised trial to determine whether a text-messaging intervention improved retention during the first year of HIV care.
Methods
This unmasked, randomised parallel-group study was done at two clinics in informal settlements in Nairobi, Kenya. Eligible participants were aged 18 years or older, HIV-positive, had their own mobile phone or access to one, and were able to use simple text messaging (or have somebody who could text message on their behalf). Participants were randomly assigned (1:1), with random block sizes of 2, 4, and 6, to the intervention or control group. Participants in the intervention group received a weekly text message from the automated WelTel service for 1 year and were asked to respond within 48 h. Participants in the control group did not receive text messages. Participants in both groups received usual care, which comprised psychosocial support and counselling; patient education; CD4 cell count; treatment; screening for tuberculosis, opportunistic infections, and sexually transmitted infections; prevention of mother-to-child transmission and family planning services; and up to two telephone calls for missed appointments. The primary outcome was retention in care at 12 months (ie, clinic attendance 10–14 months after the first visit). Participants who did not attend this 12-month appointment were traced, and we considered as retained those who were confirmed to be active in care elsewhere. The data analyst and clinic staff were masked to the group assignment, whereas participants and research nurses were not. We analysed the intention-to-treat population. This trial is registered with ClinicalTrials.gov, number NCT01630304.
Findings
Between April 4, 2013, and June 4, 2015, we screened 1068 individuals, of whom 700 were recruited. 349 people were allocated to the intervention group and 351 to the control group. Participants were followed up for a median of 55 weeks (IQR 51–60). At 12 months, 277 (79%) of 349 participants in the intervention group were retained, compared with 285 (81%) of 351 participants in the control group (risk ratio 0·98, 95% CI 0·91–1·05; p=0·54). There was one mild adverse event related to the intervention, a domestic dispute that occurred when a participant’s partner became suspicious of the weekly messages and follow-up calls.
Interpretation
This weekly text-messaging service did not improve retention of people in early HIV care. The intervention might have a modest role in improving self-perceived health-related quality of life in individuals in HIV care in similar settings.
Introduction
Retention of individuals with HIV in care is crucial to meet the UNAIDS 90-90-90 targets: that is, by 2020, 90% of people living with HIV will know their status, 90% of those who know their status will be on antiretroviral therapy (ART), and 90% of those on ART will have undetectable levels of HIV.1 When we conceived this study, CD4 cell count determined ART eligibility, and retention in care was worse among those not yet eligible for ART than for those being treated.2 We hypothesised that the WelTel text-messaging intervention, previously found to improve ART adherence and viral suppression,3 might improve retention earlier in the continuum of care. In 2013, during the course of the study, new ART guidelines from WHO increased the lower CD4 limit for treatment eligibility to 500 cells per μL,4 and in 2016 WHO again revised its ART guidelines to recommend that all individuals initiate ART, irrespective of CD4 cell count.5 Retention in care among individuals with HIV, regardless of their position along a changing continuum of care, is imperative to prevent transmission of the virus, reduce morbidity, and improve survival.
The global expansion in mobile phone use, with high uptake in Africa, has presented new opportunities to use mobile phones to engage patients in care. In 2016, the penetration of mobile phone use in Kenya reached 88% and is projected to grow.6 Shared phone use is common in the region and further increases the proportion of Kenyans who have mobile phone access.7 Results from one of the first mobile health (mHealth) trials in Africa, WelTel Kenya1, showed that patient–clinician text messaging significantly improved treatment adherence and viral suppression among individuals who had initiated ART.3 The WelTel intervention involves sending weekly interactive text messages, which are followed up by telephone calls if a patient indicates that they have an issue. Findings from meta-analyses corroborate that weekly, bi-directional text messages are an effective adherence support tool;8,9 however, it was unknown whether regular mobile phone communication could improve retention in care.
With the expansion of HIV care in Kenya, individuals have more options as to where they seek their care, which could make retention in one particular clinic a poor proxy measure for retention in care generally. In addition to clinic visits, we examined retention using telephone and community tracing to determine if participants were retained in care. In this study, we aimed to assess the effect of a weekly, interactive text-message service (WelTel) on retention during the first year of HIV care.
Research in context.
Evidence before this study
Retention in care after a positive HIV test promotes the timely initiation of antiretroviral therapy (ART), and reduces the risk of morbidity and mortality. We searched PubMed, the Cochrane Library, and Google Scholar using the terms “retention”, “HIV”, “engagement in care”, and “mobile phones” for articles published between Aug 2, 2011, and June 28, 2017. We did not use language restrictions. When we conceived this study, in 2011, few interventions had been specifically tested or implemented to retain patients in the early stages of HIV care. At the same time, increasing mobile phone use in limited-resource settings provided new opportunities to engage patients in care. Evidence arose that weekly, interactive text messaging improved ART adherence, but no such studies had been done at the beginning of our literature search to determine whether a similar text messaging service could be used to improve retention in HIV care. During the course of our trial, studies were published showing that mobile phone interventions improved retention in care in prevention of mother-to-child transmission programmes. Several studies of text-message appointment reminders in general HIV populations also emerged, most of which were in higher-resourced settings, but these studies did not show an effect. The studies in adult populations might have been limited by the observation of those with HIV as a subgroup, selection bias, and a before-and-after study design. Additionally, the studies on adult populations published during our trial examined text messages as appointment reminders, rather than as a way to promote patient engagement in care through interactive, weekly communication.
Added value of this study
To the best of our knowledge, this was the first study to examine whether weekly, interactive text messaging had an effect on patient retention during the first year of HIV care. This study measured the effect of the intervention on short-term outcomes, such as whether participants who received the intervention were more likely than those who had usual care only to return to the clinic within 3 weeks to receive their first CD4 cell count test results or to initiate ART sooner, as well as the effect of the intervention on longer-term 12-month retention in care. Since individuals with HIV in this setting have several options as to where they seek their care, we used telephone or community tracing to identify participants who did not return to the study clinics at 12 months. Those active in care elsewhere were considered to be retained. By measuring retention in care (active in care at any clinic) rather than retention in clinic (active in care at the clinic of enrolment), we were able to obtain a more accurate measurement of 12-month retention in care than if we had used retention in clinic as a proxy measure. Although this particular text-messaging intervention did not have an effect on the short-term or longer-term retention outcomes measured in this study, it did, however, have an effect on self-perceived health-related quality-of-life scores.
Implications of all the available evidence
Despite existing evidence that interactive text-messaging between health-care providers and individuals with HIV improves ART adherence, we found no effect on retention during the first year of HIV care. The modest improvement in quality-of-life scores observed in this study requires further evaluation. For optimal success of test and treat strategies, interventions that improve both retention in care and medication adherence from the time of a positive HIV test are required.
Methods
Study design
WelTel Retain10 is an unmasked, randomised, parallel-group study done at two clinics in Nairobi, Kenya.
The original study protocol and the information and consent form were approved by the University of British Columbia Clinical Research Ethics Board (H12-00563) and the African Medical and Research Foundation Ethics and Scientific Review Committee (P40/12). Ethics approval was renewed on an annual basis. The study protocol has been published elsewhere.10
The trial was designed as a single-site study; however, a nationwide shortage of HIV test kits led to slower-than-expected recruitment. To compensate, after the protocol was published we added a second site, which was similar to the original site in that it was a comprehensive care centre located in an informal settlement in Nairobi.
Between April 4, 2013, and June 4, 2015, participants were recruited from the Kibera Community Health Centre, located in a large informal settlement in Nairobi. This clinic is operated by Amref Health Africa, the largest non-governmental health organisation based in Africa. The population the clinic serves either has minimal or no access to services such as education, water, sanitation, and other public services. On Feb 26, 2014, recruitment began at a second clinic, the Baba Dogo Health Centre, situated in another large informal settlement in Nairobi and operated by Partners for Health and Development in Africa, a non-profit organisation registered in Kenya and affiliated with the University of Manitoba. Recruitment ended at this site on May 27, 2015.
Participants
Individuals who tested HIV-positive were referred to a research nurse, who assessed study eligibility using a checklist. To be eligible, patients had to be at least 18 years old, own or have access to a mobile phone, and be able to use simple text messaging (or have somebody who could text message on their behalf). Exclusion criteria were previous assessment for ART eligibility, previous or current exposure to ART, and pregnancy. Written informed consent was obtained from all participants, except for illiterate individuals, who provided consent with a thumbprint in the presence of a literate witness.
Randomisation and masking
Participants were randomly assigned to the intervention or control groups with a 1:1 ratio and random block sizes of 2, 4, and 6. Block sizes were not disclosed. An investigator was responsible for computerised sequence generation, and a research assistant for allocation concealment. Research nurses enrolled participants and implemented group assignments. Allocations were sealed in individual, sequentially numbered opaque envelopes. After meeting inclusion criteria, consenting to participate, and completing baseline assessments, participants were assigned to a study group by the research nurse who opened one of the numbered envelopes to determine allocation.
The research nurses and participants were not masked to study group assignment because the intervention required overt participation; however, the data analyst and clinic staff (who collected data on primary and clinical outcomes), including lab technicians and community health workers who did the community tracing, were masked. We did not investigate the success of masking.
Procedures
Participants were randomly assigned to receive the intervention (WelTel) in addition to usual care, or to usual care only. The WelTel service consisted of weekly text messages to check how patients were doing and provide them with the opportunity to identify whether assistance was required (appendix). Every Monday morning, a short message service (SMS) gateway sent text messages to participants in the intervention group, asking “Mambo?” (Swahili for “How are you?”). Participants were instructed to respond within 48 h of receiving the message, stating either that they were well (eg, Sawa—Swahili for “okay”) or that they were having difficulties (eg, “Shida”—Swahili for “difficulty”). The research nurses telephoned all participants who reported a problem or did not respond and recorded participants’ issues and reasons for non-response. Participants in the intervention group received the intervention until death, study withdrawal, or study exit (after natural completion of the study), whichever came first.
Participants in the control group did not receive text messages from the automated WelTel service. Participants in both the control and intervention groups received usual care and were followed up according to clinic protocol (appendix). Baseline laboratory testing included two rapid HIV tests: Alere Determine HIV-1/2 (Alere, Chiba-ken, Japan) and Uni-Gold (Trinity Biotech, Wicklow, Ireland), which was used as a confirmatory test. CD4 cell counts were measured at the baseline visit. Among other services, usual care included psychosocial support and counselling, patient education, and treatment (appendix). Patients who did not attend a clinic appointment were called 1 day after a missed appointment, and, if necessary, they were called a second time 3 days later.
Outcomes
The primary outcome was 12-month retention in care, measured by whether the participant attended a follow-up appointment 10–14 months after their first clinic visit. We considered as retained participants who were confirmed as actively in care elsewhere. To determine whether a participant attended a follow-up appointment 10–14 months after their first visit, clinicians recorded clinic visit dates on study-specific data collection forms. If a participant did not attend a visit within the 10–14 month timeframe, participants were telephoned to determine if they were active in care elsewhere. If they could not be reached over the phone, community health workers traced the participant in the community to assess their retention-in-care status.
The key secondary outcome was retention in stage 1 HIV care,11 defined as the proportion of participants who returned to the clinic within 3 weeks to complete their first ART eligibility assessment (ie, to receive their first CD4 cell count result).8 Additional secondary outcomes were proportion of participants who were ART-eligible at baseline who initiated ART within 3 months; time to ART initiation; 6-month retention in clinic (attendance at the 6-month appointment within 5–7 months of the first visit); mean proportion of scheduled appointments kept; level of engagement (the proportion of participants who were non-engagers [participants who did not return after their initial visit], sporadic users [participants who attended up to 70% of their scheduled appointments], and regular users [participants who attended between 70% and 100% of their appointments]); level of social support (rated using a five-level Likert-type scale); satisfaction with care (rated using a seven-level Likert-type scale); health-related quality of life (HRQoL; mental and physical composite scores using an adapted version of the 12-Item Short Form Survey); adverse events; and all-cause mortality.
Visit and clinical data were collected at each visit using study-specific forms. Clinic electronic medical records were also a source of data (eg, death data). Data on HRQoL, satisfaction with care, social support, and participants’ perceptions of the intervention were collected using interviewer-administered questionnaires at the baseline, 6-month, and 12-month visits.
The following changes were made to the study outcomes after the study protocol was published, but before we did the analyses. To compare the effect of the intervention on 6-month and 12-month retention in clinic, we included 12-month retention in clinic as an outcome. We defined retention in clinic as attendance at a clinic visit at the site of enrolment during the specified timeframe (5–7 months for 6-month retention in clinic and 10–14 months for 12-month retention in clinic). In the protocol, a timeframe was not specified for the two secondary outcomes involving appointment attended (ie, proportion of scheduled appointments kept and level of engagement).10 Before analysing the data, we established that a participant would be considered to have attended a scheduled appointment if they came to the clinic within 1 week of the appointment date. Although participants were recruited at the time of a positive HIV test, they might have been previously diagnosed with HIV. We collected information on previous diagnoses and report the proportion of participants who had been previously diagnosed. We included newly diagnosed versus previously diagnosed participants as a subgroup. We also included each clinic as a subgroup.
Statistical analysis
Sample size was based on the primary outcome, retention in care at 12 months. Our estimate, based on previously obtained clinic data, assumed 12-month retention in 65% of participants in the control group. We conservatively estimated that 75% of patients would be retained in the intervention group. On the basis of a 1:1 allocation ratio, a two-sided α of 0·05 and 80% power, we required approximately 343 participants in each study group.
An independent data and safety monitoring board periodically reviewed the progress of the trial and its safety. We did a planned single interim analysis when half of the participants reached the primary endpoint. Stopping guidelines are outlined in the protocol.10
Analyses were by intention to treat; therefore, we included all participants according to the study group to which they were originally allocated, regardless of the intervention received, or if they were subsequently deemed ineligible. For the primary and key secondary endpoints, we used a χ2 test to determine if the proportions of participants retained in care differed between the study groups. Secondary binary outcomes were similarly analysed. For other secondary outcomes, we used t-tests for continuous variables and Kruskal–Wallis or Mann–Whitney U tests for non-normally distributed variables. For time-to-event outcomes, we used a Kaplan–Meier analysis and estimated the hazard ratio. We also assessed the proportional hazards assumption. In subgroup analyses, we assessed whether the intervention effect was homogeneous by including an interaction term between the intervention allocation and subgroup-defining variable. We report p values for the interaction tests, rather than the treatment effect within groups. We used Stata (version 12) to analyse the data.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. MLvdK and MAB had access to all the data in the study, and MLvdK had final responsibility for the decision to submit for publication.
Results
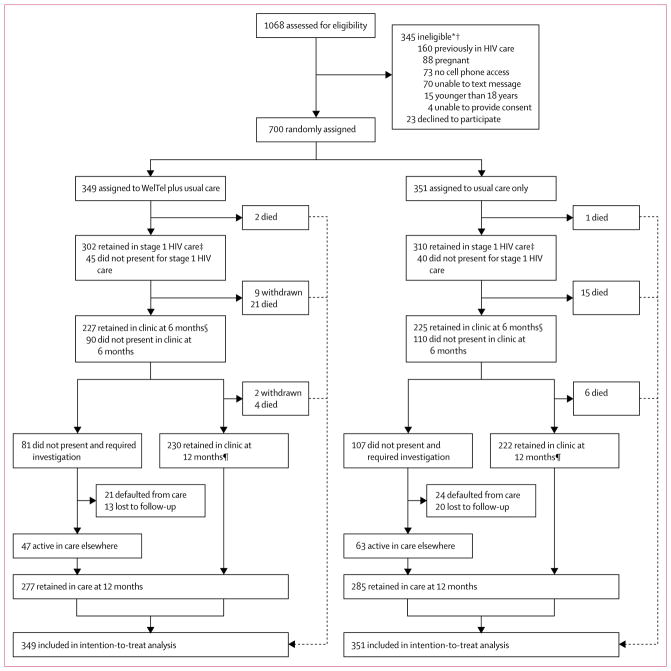
Between April 4, 2013, and June 4, 2015, we screened 1068 individuals, of whom 700 were recruited. 23 (2%) eligible patients declined participation (figure 1). Baseline characteristics are presented in table 1. After allocation, nine participants withdrew from the study, all of whom were in the intervention group. Additionally, study staff withdrew two participants from the intervention group after it was found that they had been previously enrolled in HIV care. These participants were included in the final analysis, as per their assigned study group. Participants were followed up for a median of 55 weeks (IQR 51–60). We investigated the outcomes of 248 participants who did not return to the clinic 10–14 months after their first visit. When clinical and study records did not reveal the participant’s outcome (n=97), participant status was successfully determined by telephone tracing (n=136) and community tracing (n=15; figure 1). Follow-up concluded on Sept 22, 2016.
Figure 1. Study profile.
*Reasons are not mutually exclusive. †One patient received the text messages but was analysed as per their assigned randomisation group. ‡Number of participants who returned to the clinic to receive CD4 cell count results within 3 weeks. §Number of participants who returned to the clinic 5–7 months after their first visit.
¶Number of participants who returned to the clinic 10–14 months after their first visit.
Table 1.
Baseline characteristics
| Control (n=351) | SMS intervention (n=349) | |
|---|---|---|
| Sex | ||
| Female | 213 (61%) | 206 (59%) |
| Male | 138 (39%) | 143 (41%) |
|
| ||
| Age (years) | 33·46 (9·44) | 33·99 (10·07) |
|
| ||
| Education | ||
| No formal education | 9 (3%) | 12 (3%) |
| Primary | 223 (64%) | 210 (60%) |
| Secondary | 119 (34%) | 127 (36%) |
|
| ||
| Marital status | ||
| Single | 54 (15%) | 56 (16%) |
| Married | 206 (59%) | 193 (55%) |
| Widowed or divorced | 91 (26%) | 100 (29%) |
|
| ||
| Clinic | ||
| Kibera | 250 (71%) | 251 (72%) |
| Babadogo | 101 (29%) | 98 (28%) |
|
| ||
| Previously diagnosed with HIV | 199 (57%) | 212 (61%) |
|
| ||
| WHO HIV stage | ||
| 1 | 226 (64%) | 206 (59%) |
| 2 | 47 (13%) | 54 (15%) |
| 3 | 61 (17%) | 62 (18%) |
| 4 | 1 (<1%) | 7 (2%) |
| Missing data | 16 (5%) | 20 (6%) |
|
| ||
| CD4 cell count (per μL) | 307 (148–468) | 289 (143–449) |
| Missing data | 8 (2%) | 10 (3%) |
|
| ||
| ART eligible | 237 (68%) | 251 (72%) |
|
| ||
| Quality of life (physical composite score) | 42·59 (12·10) | 43·12 (11·52) |
|
| ||
| Quality of life (mental composite score) | 52·13 (10·88) | 53·23 (11·04) |
|
| ||
| Travel time to clinic | ||
| <30 min | 172 (49%) | 175 (50%) |
| 30–59 min | 127 (36%) | 112 (32%) |
| ≥60 min | 49 (14%) | 59 (17%) |
| Missing data | 3 (1%) | 3 (1%) |
|
| ||
| Own vs shared mobile phone | 326 (93%) | 337 (97%) |
Data are n (%), mean (SD), or median (IQR), unless otherwise specified.
SMS=short message service. ART=antiretroviral therapy.
All 349 patients allocated to the intervention group received the text messages, in addition to one control group participant. Participants in the intervention group received the intervention for a median of 54·3 weeks (IQR 50·9–60·0). Overall, 17 422 outgoing text messages were sent. Fewer than 1% (n=155) of messages were not sent because of system error. There were 9303 (53%) “OK” responses, 401 (2%) “not OK” responses, and 7718 (44%) instances of non-response. Most problems reported were health-related, including general malaise (n=80 [20%]), gastrointestinal illness (n=69 [17%]), and respiratory issues (n=52 [13%]). Reasons that participants did not respond to the message included problems with their mobile phone (n=1905 [25%]), such as insufficient credit or phone not working; and participant factors (n=807 [10%]), including forgetting to respond or being too busy. In 652 (8%) instances of non-response, participants reported that they had replied, but these responses were not captured by the platform.
In the final analysis (n=700), the intervention had no effect on retention in care at 12 months (risk ratio [RR] 0·98, 95% CI 0·91–1·05; p=0·54), nor did it have an effect on the key secondary outcome, retention in stage 1 HIV care (RR 0·98, 95% CI 0·93–1·04; p=0·48; table 2). Similarly, the intervention had no effect on other retention or treatment outcomes, such as the proportion of those who initiated ART within 3 months, or retention in clinic at 6 months (table 2).
Table 2.
Clinical outcomes
| Intervention (n=349) | Control (n=351) | Risk ratio (95% CI); p value | Risk difference (95% CI) | |
|---|---|---|---|---|
| Primary outcome | ||||
|
| ||||
| Retained in care at 12 months | 277 (79%) | 285 (81%) | 0·98 (0·91 to 1·05); p=0·54 | −0·02 (−0·08 to 0·04) |
|
| ||||
| Key secondary outcome | ||||
|
| ||||
| Retained in stage 1* care | 302 (87%) | 310 (88%) | 0·98 (0·93 to 1·04); p=0·48 | −0·02 (−0·07 to 0·03) |
|
| ||||
| Other secondary outcomes | ||||
|
| ||||
| Initiated ART by month 3 | 207/251 (82%) | 186/237 (78%) | 1·05 (0·96 to 1·15); p=0·27 | 0·04 (−0·03 to 0·11) |
| Retained in clinic at 6 months | 227 (65%) | 225 (64%) | 1·02 (0·91 to 1·13); p=0·80 | 0·01 (−0·06 to 0·08) |
| Retained in clinic at 12 months | 230 (66%) | 222 (63%) | 1·04 (0·93 to 1·16); p=0·46 | 0·03 (−0·04 to 0·10) |
| Death (all causes) | 27 (8%) | 22 (6%) | 1·23 (0·72 to 2·12); p=0·45 | 0·02 (−0·02 to 0·05) |
Data are n (%) or n/N (%), unless otherwise indicated. ART=antiretroviral therapy.
Participant returns to the clinic to receive the first CD4 cell count results.
The proportion of participants who attended scheduled appointments was similar for the two groups. In the intervention group, the median proportion of scheduled visits attended was 89 (IQR 75–100), and in the control group the median was also 89 (IQR 78–100; p=0·7232). 11 participants in the intervention group versus 15 participants in the control group were considered to be non-engagers; 51 participants in the intervention group versus 40 participants in the control group were considered to be sporadic attenders; and 287 participants in the intervention group versus 296 participants in the control group were considered to be regular users (p=0·3540).
Of the 488 participants eligible for ART at baseline, there was no difference between the intervention and control groups in the proportion who initiated ART by 3 months (table 2). Time to ART initiation was similar between the two groups (figure 2). Both groups initiated ART a median of 27 days after their first visit (IQR intervention 17–43; IQR control 18–43).
Figure 2. Kaplan–Meier estimate of ART initiation.
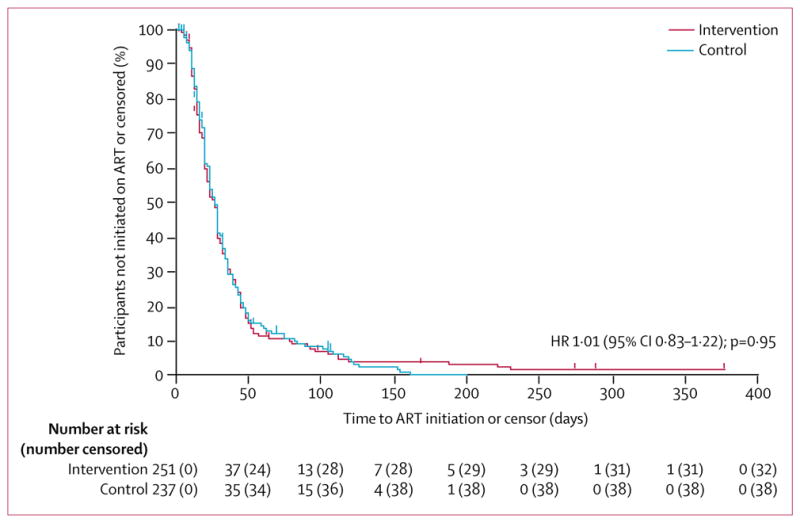
ART=antiretroviral therapy. HR=hazard ratio.
Between 10 and 14 months, 435 (96%) of 452 participants who returned to the clinic completed the follow-up questionnaire. This included 215 (61%) of 351 participants in the control group and 220 (63%) of 349 participants in the intervention group (p=0·6266). A comparison of key demographic (sex, age, and clinic) and clinical (baseline CD4 cell count, ART eligibility, and previous HIV diagnosis) characteristics between participants who completed the questionnaire with those who did not showed that baseline CD4 cell count was the only significant difference, with CD4 cell count higher among those who had completed the questionnaire (appendix).
Satisfaction with care was similar between the two groups (table 3). Although the median and IQR was the same in both groups for social support, participants in the intervention group had a significantly higher mean rank social support score than those in the control group, indicating greater perceived social support in the intervention group (table 3). Physical and mental composite quality-of-life scores were also greater in the intervention group than in the control group (table 3).
Table 3.
Outcomes based on 12-month follow-up questionnaire data
| Intervention (n=220) | Control (n=215) | Kruskal–Wallis χ2 or mean difference (95% CI) | p value | |
|---|---|---|---|---|
| Outcome (median, IQR) | ||||
|
| ||||
| Satisfaction with care | 7 (7–7) | 7 (7–7) | 0·912* | 0·34 |
| Level of social support | 5 (4–5) | 5 (4–5) | 4·910† | 0·0267 |
|
| ||||
| Health-related quality of life (mean, SD) | ||||
|
| ||||
| Physical composite score | 53·28 (4·68) | 51·01 (8·32)‡ | 2·27 (0·10–3·53) | 0·0005 |
| Mental composite score | 57·17 (5·53) | 55·32 (7·97)‡ | 1·86 (0·56–3·15) | 0·0050 |
There was one degree of freedom in the χ2 test.
Mean rank 213·80 (control), 222·11 (intervention).
Mean rank 206·97 (control), 228·78 (intervention).
n=214.
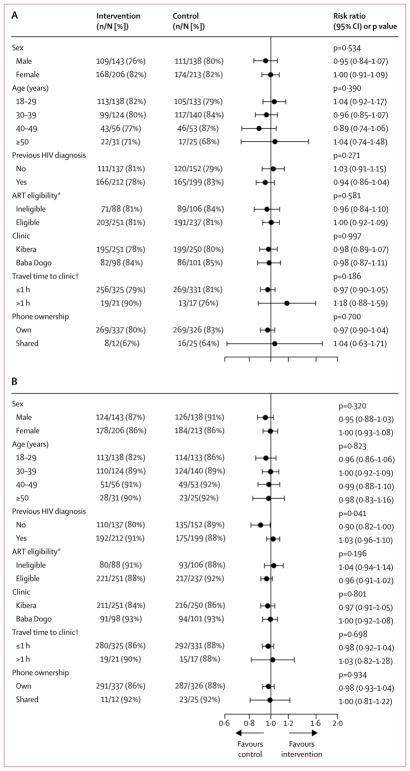
Subgroup analyses are depicted in figure 3. Apart from heterogeneity among those who were previously diagnosed versus newly diagnosed in stage 1 retention (p=0·0407), the effects of the intervention on retention in care at 12 months and in stage 1 HIV care were similar across subgroups, with p values for the tests for interaction greater than 0·1000.12
Figure 3. Forest plot of subgroup analyses for 12-month and stage 1 retention in care.
(A) 12-month retention in care. (B) Stage 1 retention in care. ART=antiretroviral therapy. *Data missing for 18 participants. †Data missing for six participants.
Of 349 participants in the intervention group, 220 completed the 12-month questionnaire. Participants who completed the questionnaire had a higher median CD4 cell count (302 [IQR 168–449]) than those who did not (240 [IQR 90–450]; p=0·0319; appendix). Participants in the intervention group viewed the service favourably: 204 (93%) strongly agreed that they liked the intervention, and 206 (94%) found the intervention helpful. Participants liked the frequency of text messages, with 203 (92%) preferring to receive the messages weekly; however, eight (4%) participants would have preferred to receive the messages less often, and nine (4%) participants would have liked to receive the messages more often. Most participants were not concerned that the messages would disclose their HIV status—eight (4%) participants indicated a concern. When asked what the largest barrier was to the intervention, 165 (75%) participants reported no barriers. Barriers noted included lack of network credit (22 [10%] of 220) and phone access (12 [5%] of 220). The greatest perceived benefits were convenient access to care and advice (88 [40%] of 219); regular contact with health-care providers (54 [25%] of 219); and feelings of care, support, or security (42 [19%] of 218).
At the end of the study, 213 (97%) of 220 respondents in the intervention group indicated that they would like the programme to continue and 200 (91%) would recommend the programme to someone with HIV. 153 (70%) of 220 participants in the intervention group thought that it would be helpful for health conditions other than HIV.
On Oct 16, 2013, a participant in the intervention group had a mild adverse event related to the intervention. A domestic dispute occurred when the participant’s partner became suspicious of the weekly text messages and follow-up calls. To our knowledge, no violence or injury occurred.
Discussion
The results of this study show that the WelTel service did not have an effect on retention during the first year of HIV care. It did not improve 12-month retention in care, and individuals who received the intervention were no more likely than those who did not to return to the clinic to receive their CD4 cell count results. Although the intervention was well liked by participants, there was no effect on additional secondary outcomes, such as 6-month retention in clinic or time to ART initiation. The clinics involved in the study followed up participants who did not attend clinic appointments and offered support services that might have helped to engage and retain participants in care. The intervention might have been more likely to have an effect in settings that did not have such services. Overall, 1 year retention in care in this trial population was 80%, substantially greater than retention in clinic (65%), showing the importance of considering those to be active in care elsewhere when quantifying retention in care.
Key strengths of this trial are its high participation rate and low loss to follow-up. Only 2% of eligible people declined to participate, and less than 5% of participants were lost to follow-up. Furthermore, there was no difference in loss to follow-up between the intervention and control groups, minimising the risk of bias. Another strength of this study is that by tracing participants who did not return to the clinic, we could determine a more valid assessment of retention in care than if we had used solely retention in clinic as a proxy measure for retention in care.
This study’s limitations include that only 435 (62%) of 700 participants completed the 12-month questionnaire; therefore, the validity of outcomes based on these data could be compromised. Despite that key demographic and clinical characteristics were similar between those who completed the questionnaire and those who did not, we cannot rule out the possibility of bias. Another limitation was the use of single-item questions for social support and satisfaction with care. Although this reduced participant burden, using social support and satisfaction with care scales with demonstrated validity and reliability might have provided stronger evidence in this regard. Finally, the study clinics already had mechanisms in place to support retention in care, such as calling patients who missed appointments. The intervention might have been more likely to show an effect on retention if tested in clinics without these support mechanisms.
At the end of the trial, HRQoL between the intervention and control groups differed, with mean physical and mental composite scores being approximately 2 points higher in the intervention group than in the control group. We had hypothesised that the intervention would improve HRQoL by enhancing patient–provider communication and retention,11 because individuals receiving consistent care could have greater HRQoL. Since retention in care was similar for the two groups, differences in physical composite scores might have been brought about by an effect of the intervention on ART adherence;3 although we did not measure adherence as an outcome in this trial, this particular intervention and other SMS interventions have been shown to improve ART adherence in other studies.8 The difference in mental composite score might be mediated by the increased social support reported by participants in the intervention group; findings from previous studies13 have shown an association between mental composite scores and social support. This possibility is strengthened by participants’ reports that one of the greatest benefits that they received from the intervention were feelings of care, support, and security.
In a previous study, we did a post-hoc analysis examining the effect of the intervention on adherence, and examined whether the service was associated with attrition from clinic at 6 and 12 months.14 Although the findings of this post-hoc analysis were not statistically significant, its direction of effect prompted us to investigate the intervention in this larger study, which was specifically designed to examine retention in care. We chose to test the same intervention in this study as in the previous one, including the “How are you?” text message. In the original trial, participants felt cared for and supported by the service.3 Our intention was to promote patient-centred care and engagement in care rather than remind participants to take medication, or in this case to attend appointments. However, in this current study, the intervention did not affect retention.
Studies of mHealth interventions in retention in HIV care have used text messages as appointment reminders, rather than as a general tool to support patients and engage them in care. Use of text messages and telephone calls as appointment reminders has shown effectiveness in trials in paediatric HIV follow-up care.15–18 Studies in other settings among general populations with HIV have not shown effectiveness,19–22 and have been limited by a before-and-after study design,20,21 analysing individuals with HIV as a subgroup,19,20 and selection bias.22
Most mHealth studies in HIV care have focused on ART adherence. Although findings have been mixed, findings from meta-analyses indicate that, overall, weekly text-message interventions improve adherence.8 Results from meta-analyses also indicate that less frequent messaging and messaging that requires a response from participants is more likely to be effective than both more frequent messaging and messages that don’t require a response.8,9,23 To our knowledge, this was the first study in a limited-resource setting to test whether interactive text messaging improves retention in a general population with HIV. Our findings do not necessarily conflict with those of our original study,3 the findings of which showed that the intervention improves treatment adherence and biological outcomes associated with better adherence. Although retention in care is necessary for adherence, it is plausible that two groups who are equally retained could have differing levels of treatment adherence.
WHO suggested the use of text messaging to improve retention in HIV care, both specifically from enrolment in care to ART eligibility and for lifelong retention.24 We have shown that this particular text-message intervention did not improve retention in what was previously defined as the first stage of HIV care. The cascade of HIV care has been changed to reflect a so-called treat all strategy, with stage 1 now referring to the period between testing and enrolment in care.25 Although CD4 cell count is no longer the basis of ART eligibility, there will remain a period between HIV diagnosis and linkage to care in which retention will remain crucial. Although we were unable to measure the effect of the intervention on life-long retention, there was no long-term effect on 6-month and 12-month retention in care. As millions more people become eligible for treatment under the treat-all strategy, different retention and adherence barriers could arise as new populations are treated—eg, asymptomatic individuals. It is not known whether the high rates of attrition previously seen among pre-ART populations who will now be eligible for treatment will reduce overall attrition, or if attrition will increase in this larger population. There are limitations in using a trial population to estimate retention in care; however, this study highlights the importance of considering silent transfers when quantifying retention, which substantially increased the proportion of participants retained in care. Despite this finding, retention was not high enough in our study, or in other estimates,26,27 to enable UNAIDS to meet global 90-90-90 targets.
Our results show that the WelTel service did not improve retention during the first year in HIV care. New ways to improve retention in care in general HIV populations are required, particularly at the start of the cascade of HIV care. In populations who are retained, evidence-based text-message interventions, including WelTel, could be used to improve adherence, which supports the third UNAIDS target to have 90% of people virally suppressed. Our findings also suggest that this intervention could make a modest contribution to the so-called fourth 90,28 to ensure that 90% of those who are virally suppressed have good HRQoL.
Supplementary Material
Acknowledgments
Funding: National Institutes of Health and Canadian Institutes of Health Research Canadian HIV Trials Network.
Research reported in this publication was supported by the National Institute of Mental Health (NIMH) of the NIH under award number R01MH097558, awarded to the University of British Columbia. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The CIHR Canadian HIV Trials Network (CTN 284) also provided funding for this trial. MLvdK was supported by a CIHR Doctoral Award–Doctoral Foreign Study Award (October, 2012), offered in partnership with the CIHR Strategy for Patient-Oriented Research and the CIHR HIV/ AIDS Research Initiative. None of the authors are employed by the NIH. We would like to thank the data manager, Richard Gichuki, for his dedicated work on the study, and all of the clinic staff, research team members, and participants who took part in the study. We are also grateful to Partners for Health and Development in Africa for supporting the study at the Baba Dogo Clinic, and to the members of the data and safety monitoring board for their oversight.
Footnotes
Contributors
RTL and MLvdK conceived the study. MLvdK designed the trial with input from RTL, LT, DIO, CM, and LBK. PIN and BA acquired the data. MLvdK did the statistical analyses and drafted the manuscript. MAB analysed the text message data and created figures 2 and 3. SM, PIN, LT, POA, MAB, BA, LBK, CM, AP, SK, DIO, EJM, AME, and RTL revised the manuscript for important intellectual content. All authors approved the submitted version of the manuscript.
For more on Amref Health Africa please see http://amref.org/
For more on Partners for Health and Development in Africa see http://www.phdaf.org/
Declaration of interests
This study uses a technology platform (WelTel/SMS) that was developed by a non-profit organisation and a private company. A co-investigator, RTL, has financial and professional interests in both organisations. RTL reports competing interests from his involvement in the WelTel International mHealth Society and WelTel Inc, grants from National Institutes of Health (NIH), Canadian Institutes of Health Research (CIHR), BC Lung Association, British Columbia Centre for Disease Control Foundation, and Grand Challenges Canada, and non-financial support from WHO and Task Force on Digital Health for TB Control, outside the submitted work. MLvdK reports grants from NIH, CIHR, and the CIHR Canadian HIV Trials Network during the conduct of the study. All other authors declare no competing interests.
References
- 1.UNAIDS. [accessed Dec 5, 2017];90-90-90 An ambitious treatment target to help end the AIDS epidemic. 2014 http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf.
- 2.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376:1838–45. doi: 10.1016/S0140-6736(10)61997-6. [DOI] [PubMed] [Google Scholar]
- 4.WHO. [accessed Dec 27, 2017];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013 http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. [PubMed]
- 5.WHO. [accessed Dec 27, 2017];Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. (2). 2016 http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf?ua=1. [PubMed]
- 6.Communications Authority of Kenya. [accessed Dec 5, 2017];Kenya’s mobile penetration hits 88 per cent. http://www.ca.go.ke/index.php/what-we-do/94-news/366-kenya-s-mobile-penetration-hits-88-per-cent.
- 7.Wesolowski A, Eagle N, Noor AM, Snow RW, Buckee CO. Heterogeneous mobile phone ownership and usage patterns in Kenya. PLoS One. 2012;7:e35319. doi: 10.1371/journal.pone.0035319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finitsis DJ, Pellowski JA, Johnson BT. Text message intervention designs to promote adherence to antiretroviral therapy (ART): a meta-analysis of randomized controlled trials. PLoS One. 2014;9:e88166. doi: 10.1371/journal.pone.0088166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mbuagbaw L, Kop ML, van der Lester RT, et al. Mobile phone text messages for improving adherence to antiretroviral therapy (ART): an individual patient data meta-analysis of randomised trials. BMJ Open. 2013;3:e003950. doi: 10.1136/bmjopen-2013-003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Kop ML, Ojakaa DI, Patel A, et al. The effect of weekly short message service communication on patient retention in care in the first year after HIV diagnosis: study protocol for a randomised controlled trial (WelTel Retain) BMJ Open. 2013;3:e003155. doi: 10.1136/bmjopen-2013-003155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox M, Larson B, Rosen S. Defining retention and attrition in pre-antiretroviral HIV care: Proposals based on experience in Africa. Boston: Center for Global Health and Development, Boston University; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? Updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340:c117. doi: 10.1136/bmj.c117. [DOI] [PubMed] [Google Scholar]
- 13.Degroote S, Vogelaers D, Vandijck DM. What determines health-related quality of life among people living with HIV: an updated review of the literature. Arch Public Health. 2014;72:40. doi: 10.1186/2049-3258-72-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van der Kop ML, Ekström AM, Awiti-Ujiji O, et al. Factors associated with attrition from HIV care during the first year after antiretroviral therapy initiation in Kenya. J AIDS Clin Res. 2014;5:10. [Google Scholar]
- 15.Bigna JJR, Noubiap JJN, Kouanfack C, Plottel CS, Koulla-Shiro S. Effect of mobile phone reminders on follow-up medical care of children exposed to or infected with HIV in Cameroon (MORE CARE): a multicentre, single-blind, factorial, randomised controlled trial. Lancet Infect Dis. 2014;14:600–08. doi: 10.1016/S1473-3099(14)70741-8. [DOI] [PubMed] [Google Scholar]
- 16.Odeny TA, Bukusi EA, Cohen CR, Yuhas K, Camlin CS, McClelland RS. Texting improves testing: a randomized trial of two-way SMS to increase postpartum prevention of mother-to-child transmission retention and infant HIV testing. AIDS. 2014;28:2307–12. doi: 10.1097/QAD.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kebaya L, Nduati R, Wamalwa D, et al. PO-0260a efficacy of mobile phone use on adherence to nevirapine prophylaxis and retention in care among the HIV-exposed infants in PMTCT: a randomised controlled trial. Arch Dis Child. 2014;99:A329. doi: 10.1186/s12887-021-02660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finocchario-Kessler S, Gautney BJ, Khamadi S, et al. If you text them, they will come: using the HIV infant tracking system to improve early infant diagnosis quality and retention in Kenya. AIDv. 28(suppl 3):S313–21. doi: 10.1097/QAD.0000000000000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perron NJ, Dao MD, Kossovsky MP, et al. Reduction of missed appointments at an urban primary care clinic: a randomised controlled study. BMC Fam Pract. 2010;11:79. doi: 10.1186/1471-2296-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kliner M, Knight A, Mamvura C, Wright J, Walley J. Using no-cost mobile phone reminders to improve attendance for HIV test results: a pilot study in rural Swaziland. Infect Dis Poverty. 2013;2:12. doi: 10.1186/2049-9957-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farmer T, Brook G, McSorley J, Murphy S, Mohamed A. Using short message service text reminders to reduce ‘did not attend’ rates in sexual health and HIV appointment clinics. Int J STD AIDS. 2013;25:289–93. doi: 10.1177/0956462413502325. [DOI] [PubMed] [Google Scholar]
- 22.Norton BL, Person AK, Castillo C, Pastrana C, Subramanian M, Stout JE. Barriers to using text message appointment reminders in an HIV clinic. Telemed J E Health Off. 2014;20:86–89. doi: 10.1089/tmj.2012.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wald DS, Butt S, Bestwick JP. One-way versus two-way text messaging on improving medication adherence: meta-analysis of randomized trials. Am J Med. 2015;128:1139. doi: 10.1016/j.amjmed.2015.05.035. [DOI] [PubMed] [Google Scholar]
- 24.WHO. Defining the challenges and identifying the solutions. Geneva: World Health Organization; 2012. Retention in HIV programmes. [Google Scholar]
- 25.Fox MP, Rosen S. A new cascade of HIV care for the era of ‘treat all’. PLoS Med. 2017;14:e1002268. doi: 10.1371/journal.pmed.1002268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen S, Fox MP. Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Med. 2011;8:e1001056. doi: 10.1371/journal.pmed.1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med. 2007;4:e298. doi: 10.1371/journal.pmed.0040298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lazarus JV, Safreed-Harmon K, Barton SE, et al. Beyond viral suppression of HIV—the new quality of life frontier. BMC Med. 2016;14:94. doi: 10.1186/s12916-016-0640-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.