Abstract
Background
Echocardiography plays an important role in the diagnostic work up of heart failure with preserved ejection fraction (HFpEF). We sought to determine the left ventricular (LV) diastolic profile by echocardiography in patients diagnosed with pulmonary hypertension (PH) due to PH‐HFpEF.
Hypothesis
The study of LV diastolic function by echocardiography has limitations in patients with HFpEF and PH, and certain LV diastolic determinations convey a worse prognosis.
Methods
We included patients with postcapillary PH and diagnosis of PH‐HFpEF. Investigators reviewed Doppler echocardiograms completed within 3 months of the diagnostic right heart catheterization.
Results
We included 149 patients with a mean ± standard deviation age of 63 ± 14 years; 58% were women. LV diastolic function profile was determined as normal (41%), grade I (34%), and grade II and grade III (25%). Pulmonary artery pressure and pulmonary vascular resistance were higher and cardiac output lower in patients with LV diastolic dysfunction profile; however, pulmonary artery wedge pressure was not significantly different among grades of LV diastolic function. Although there was an association between the presence of LV diastolic dysfunction profile and long‐term survival (P = 0.03), it disappeared when adjusting for age and gender. Right ventricular (RV) dysfunction, paradoxical septal motion, and higher RV systolic pressure remained the only variables significantly associated with poor survival.
Conclusions
The profile of LV diastolic dysfunction by conventional echocardiography is highly variable in patients with PH‐HFpEF and has no significant impact on long‐term survival. A more severe RV function and higher right ventricle systolic pressure were associated with worse survival.
Keywords: Pulmonary hypertension, Heart failure with preserved ejection fraction, Imaging, echocardiography, Survival, Outcomes
1. INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) is defined as symptoms and signs of heart failure in the setting of abnormal left ventricular (LV) diastolic but normal systolic function.1 Patients with HFpEF commonly (68%–78%) have pulmonary hypertension (PH)2 and, in fact, HFpEF constitutes the most common cause of PH in the developed world.3 More importantly, PH in patients with HFpEF is associated with worse short‐ and long‐term mortality.4 The 5th World Symposium places PH due to HFpEF as part of group 2 in their classification (ie, PH due to left heart disease).5
Echocardiography has a pivotal role in the diagnostic of HFpEF6; however, it lacks sufficient sensitivity in earlier stages of disease and can be influenced by changes in heart rate and filling pressures.7 Current guidelines recommend right heart catheterization (RHC) for diagnosis of PH,8, 9 but the more specific identification of PH due to HFpEF requires a comprehensive clinical, echocardiographic, and hemodynamic evaluation, including performing fluid and/or exercise challenges in certain situations.
It remains unclear what is the LV diastolic function profile in patients with PH associated with HFpEF. Furthermore, it is unknown whether parameters that assess LV diastolic function in this group of patients have an impact on survival, particularly when added to traditional echocardiographic determinations. The purpose of our study was to describe the LV diastolic function profile of patients diagnosed with PH due to HFpEF by RHC, and identify if echocardiographic parameters that assess the LV diastolic function predict survival. We hypothesize that in the traditional echocardiographic evaluation of the LV diastolic function has limitations in patients with HFpEF and PH. We further hypothesize that certain LV diastolic determinations that represent a more advanced degree of LV diastolic dysfunction convey a worse prognosis.
2. METHODS
2.1. Study design, hemodynamics, inclusion and exclusion criteria
This study was approved by the Cleveland Clinic Institutional Review Board (IRB #10‐1127). Subjects with PH attributable to HFpEF were identified using the Cleveland Clinic Pulmonary Hypertension Registry. This retrospective cohort study included individuals who had undergone a diagnostic RHC for suspected PH from April 2004 to September 2011. We began our study in 2004 because at this time all echocardiograms were digitally recorded (syngo Dynamics; Siemens Medical Solutions USA, Inc., Malvern, PA), allowing for offline review.
We selected patients with mean pulmonary artery pressure (PAP) ≥25 mm Hg and pulmonary artery wedge pressure (PAWP) >15 mm Hg during RHC, including isolated postcapillary (diastolic pulmonary gradient [DPG] <7 mm Hg and/or pulmonary vascular resistance (PVR) ≤3 Wood units) and combined pre‐ and postcapillary PH (DPG ≥7 mm Hg and/or PVR >3 Wood units).9 Patients needed to have an echocardiogram performed within 3 months of the hemodynamic evaluation and the diagnosis of HFpEF as the main reason for their PH as assessed by 2 PH experts; the primary PH physician plus a second unbiased reviewer who had access to clinical, laboratory, radiographic, echocardiographic, and hemodynamic data.9 If disagreement occurred, the case was presented to the PH team for consensus.10
We excluded individuals with impaired LV systolic function defined by an ejection fraction of <50%, individuals with pulmonary arterial hypertension (5th World Symposium group I),8 moderate‐to‐severe left‐sided valvular disease (including mitral stenosis, mitral regurgitation, aortic stenosis, and aortic insufficiency), previous history of heart valve replacement, or cardiac and/or lung transplantation. We also excluded patients with advanced parenchymal lung disease or chronic thromboembolic pulmonary hypertension.
Right heart catheterization was performed using local anesthesia and no sedation. Oxygen was provided to those patients already on oxygen supplementation or when the pulse oxygen saturation was <90%. Cardiac output (CO) was determined by thermodilution and indirect Fick methodology. Body surface area was calculated according to the formula of DuBois and DuBois.11 Mixed venous oxygenation was measured in the blood obtained from the pulmonary artery during RHC. The transpulmonary pressure gradient (TPG) and DPG were calculated as mean PAP–PAWP and diastolic PAP–PAWP, respectively. Pulmonary vascular resistance was obtained by dividing the TPG over CO.
2.2. Echocardiography measurement and calculations
Echocardiograms were reviewed independently by 3 investigators (L.L., A.R.T., and B.T.), unaware of the patients’ clinical or hemodynamic status. We measured transmitral peak early diastolic velocity (peak E velocity), E velocity deceleration time, peak late diastolic velocity (peak A velocity), E/A ratio, left atrial (LA) area, interventricular septum and posterior wall dimensions, left ventricular end‐diastolic diameter (LVEDD) and left ventricular end‐systolic diameter (LVESD), LV ejection fraction, right atrium (RA) area, right ventricular (RV) function based on visual estimation and tricuspid annular plane systolic excursion,12, 13 tricuspid regurgitation jet velocity, right ventricular systolic pressure (RVSP), and inferior vena cava diameter.14 Left and right atrial areas were measured using planimetry at end‐ventricular systole in the apical 4‐chamber view. We also determined the presence of midsystolic or late‐systolic right ventricular outflow tract notch.15
A variety of echocardiographic measurements (2‐dimensional and Doppler) were used to grade the LV diastolic function following recommendations from the American Society of Echocardiography (ASE)/European Society of Echocardiography (grade I: impaired LV relaxation; grade II: pseudonormal LV filling; and grade III: restrictive LV filling).16 When appropriate, the Valsalva maneuver was performed to distinguish a normal from a pseudonormal diastolic filling pattern. For echocardiographic determinations, in patients with atrial fibrillation, we averaged 3 beats with cardiac cycle lengths between 10% and 20% of the average.16
2.3. Statistics
Two‐group comparisons were performed by Student t test or Mann–Whitney‐Wilcoxon test when appropriate. For more than 2 groups’ comparison, we used 1‐way analysis of variance. Categorical data were tested using Fisher exact or χ2 test. We used linear regression analysis to compare continuous variables. Survival was assessed by Kaplan‐Meier methodology. The end of follow‐up was March 2016 or the time of the participant's death. Death of the study participants was ascertained by reviewing our records and querying the US Social Security Death Index. Cox proportional hazards analysis, adjusted by age and gender, was used to investigate the impact of echocardiographic variables on survival. All P values reported are 2‐tailed. A P value of <0.05 was considered significant. The statistical analyses were performed using the statistical package SPSS version 17 (IBM, Armonk, NY).
3. RESULTS
Of 157 patients with PH due to HFpEF confirmed by RHC, LV diastolic function could not be confidently graded in 8 patients; therefore, our study population comprised 149 subjects. The mean ± standard deviation age was 67 ± 14 years, and 57% of the patients were women. Baseline demographics, and hemodynamic and echocardiographic parameters are shown in Tables 1 and 2.
Table 1.
Baseline demographics and hemodynamic parameters
| Variables | Overall, Mean ± SD or No. (%), N = 149 | No LV DD, Mean ± SD or No. (%), N = 61 | Grade I DD, Mean ± SD or No. (%), N = 51 | Grade II to III LV DD, Mean ± SD or No. (%), N = 37) | P Value, ANOVA or χ2 |
|---|---|---|---|---|---|
| Age, y | 63.2 ± 14 | 61.1 ± 15 | 62.0 ± 12 | 68.1 ± 12 | 0.04 |
| Female gender | 86 (58) | 30 (49) | 30 (59) | 26 (70) | 0.12 |
| Race | 0.41 | ||||
| Caucasian | 117 (79) | 46 (75) | 41 (80) | 30 (81) | |
| African American | 30 (20) | 13 (21) | 10 (20) | 7 (19) | |
| Other | 2 (1) | 2 (3) | 0 (0) | 0 (0) | |
| BMI, kg/m2 | 35 ± 10 | 38 ± 11 | 31 ± 7 | 36 ± 12 | 0.005 |
| Atrial fibrillation | 24 (16) | 17 (28) | 1 (2) | 6 (16) | 0.001 |
| NYHA functional class | 2.9 ± 0.8 | 2.8 ± 0.8 | 2.9 ± 0.8 | 2.9 ± 0.7 | 0.83 |
| 6MWD % of predicted | 52.1 ± 22 | 51.7 ± 19 | 53 ± 25 | 51.2 ± 24 | 0.95 |
| RHC | |||||
| SBP, mm Hg | 138 ± 24 | 135 ± 25 | 132 ± 20 | 150.8 ± 21 | <0.001 |
| DBP, mm Hg | 79 ± 14 | 79 ± 16 | 79 ± 12 | 79 ± 13 | 1 |
| HR, bpm | 78 ± 16 | 80 ± 16 | 83 ± 15 | 68 ± 13 | <0.001 |
| RA, mm Hg | 15 ± 7 | 16 ± 8 | 12 ± 5 | 16 ± 8 | 0.01 |
| Systolic PAP, mm Hg | 73 ± 22 | 67 ± 22 | 77 ± 22 | 77 ± 20 | 0.02 |
| Diastolic PAP, mm Hg | 31 ± 10 | 28 ± 10 | 33 ± 11 | 31 ± 9 | 0.05 |
| Mean PAP, mm Hg | 45 ± 13 | 42 ± 13 | 48 ± 13 | 46 ± 12 | 0.04 |
| PAWP, mm Hg | 22 ± 6 | 22 ± 6 | 21 ± 6 | 22 ± 6 | 0.45 |
| CO, L/min | 5.7 ± 2 | 6.5 ± 3 | 5.0 ± 2 | 5.2 ± 1 | 0.001 |
| CI, L/min/m2 | 2.7 ± 1 | 3.1 ± 1 | 2.4 ± 1 | 2.6 ± 1 | 0.001 |
| PVR, Wood units | 5.0 ± 4 | 3.7 ± 3 | 6.7 ± 5 | 4.8 ± 2 | <0.001 |
| TPG, mm Hg | 24 ± 11 | 20 ± 12 | 27 ± 12 | 24 ± 10 | 0.005 |
| DPG, mm Hg | 9 ± 10 | 6 ± 9 | 12 ± 11 | 9 ± 8 | 0.008 |
| Mixed venous oxygenation, % | 65 ± 8 | 65 ± 8 | 65 ± 9 | 64 ± 8 | 0.84 |
| Type of PH | |||||
| Isolated postcapillary | 47 (32) | 30 (49) | 10 (20) | 7 (19) | 0.001 |
| Combined pre‐ and postcapillary | 102 (68) | 31 (51) | 41 (80) | 30 (81) | |
Abbreviations: 6MWD: 6‐minute walk distance; ANOVA, analysis of variance; BMI, body mass index; CI, cardiac index; CO, cardiac output; DBP, diastolic arterial blood pressure; DD, diastolic dysfunction; DPG, diastolic pulmonary gradient; HR, heart rate; LV, left ventricular; NYHA, New York Heart Association; PAP, pulmonary artery pressure; PAWP, pulmonary artery wedge pressure; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RA, right atrium; RHC, right heart catheterization; SBP, systolic arterial blood pressure; SD, standard deviation; TPG, transpulmonary gradient.
Table 2.
Echocardiographic parameters
| Variables | Overall, Mean ± SD or No. (%), N = 149 | No LV DD,Mean ± SD or No. (%), N = 61 | Grade I DD,Mean ± SD or No. (%), N = 51 | Grade II to III LV DD, Mean ± SD or No. (%), N = 37 | P Value, ANOVA or χ2 |
|---|---|---|---|---|---|
| HR, bpm | 76 ± 15 | 77 ± 14 | 81 ± 14 | 66 ± 12 | <0.001 |
| PR interval, msec | 166 ± 38 | 166 ± 36 | 160 ± 36 | 174 ± 44 | 0.31 |
| LA diameter, cm | 4.5 ± 3 | 4.3 ± 1 | 4.3 ± 4 | 4.8 ± 2 | 0.62 |
| LA area, cm2 | 20.4 ± 6 | 21.3 ± 7 | 17.2 ± 5 | 23.3 ± 5 | <0.001 |
| RA area, cm2 | 21.7 ± 8 | 21.7 ± 8 | 21.3 ± 7 | 22.2 ± 7 | 0.85 |
| LVEDD, cm | 4.3 ± 0.7 | 4.5 ± 0.7 | 3.9 ± 0.7 | 4.6 ± 0.6 | <0.001 |
| LVESD, cm | 2.8 ± 0.7 | 3 ± 0.8 | 2.6 ± 0.7 | 3.0 ± 0.6 | 0.006 |
| IVS, cm | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.2 ± 0.2 | 1.3 ± 0.3 | 0.16 |
| PW, cm | 1.2 ± 0.2 | 1.1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 | 0.49 |
| LVEF, % | 57 ± 4 | 57 ± 4 | 58 ± 4 | 57 ± 5 | 0.2 |
| RV function | 0.04 | ||||
| Normal | 65 (44) | 31 (51) | 15 (29) | 19 (51) | |
| Mild | 25 (17) | 12 (20) | 6 (12) | 7 (19) | |
| Moderate | 47 (31) | 14 (23) | 23 (45) | 10 (27) | |
| Severe | 12 (8) | 4 (7) | 7 (14) | 1 (3) | |
| TR jet, m/s | 3.9 ± 0.9 | 3.6 ± 0.7 | 4.1 ± 1 | 4 ± 0.8 | 0.02 |
| RVSP, mmHg | 68.5 ± 28 | 60.4 ± 23 | 76.3 ± 31 | 70 ± 28 | 0.02 |
| IVC diameter, cm | 2.1 ± 0.5 | 2.3 ± 0.5 | 1.9 ± 0.5 | 2.2 ± 0.5 | 0.01 |
| RVOT notch 1 | 11 (10) | 4 (9) | 4 (10.5) | 3 (12) | 0.81 |
| Paradoxical septal motion | 55 (37) | 20 (33.3) | 26 (51) | 9 (24.3) | 0.028 |
Abbreviations: ANOVA, analysis of variance; DD, diastolic dysfunction; HR, heart rate at the time of echocardiography; IVC, inferior vena cava; IVS, interventricular septum; LA, left atrium; LV, left ventricular; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end‐diastolic dimension; LVESD, left ventricular end‐systolic dimension; PW, posterior wall; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; RVSP, right ventricular systolic pressure; SD, standard deviation; TR, tricuspid regurgitation.
Evaluated in 109 patients. All but 1 of the RVOT flow notches were in late systole.
3.1. Degree of diastolic dysfunction
Eighty‐five percent and 98% of the echocardiograms were performed within 1 to 2 months of the diagnostic RHC, respectively. We noted that 41% (n = 61) of the patients had apparently normal LV diastolic function on echocardiography; meanwhile, 34% (n = 51) had grade I and 25% (n = 37) had either grade II or III LV diastolic dysfunction. At the time of echocardiography, 24 patients had chronic atrial fibrillation. These patients more commonly had normal LV diastolic function than patients with normal sinus rhythm (71% vs 35%, P = 0.001).
Seventy‐eight patients (52%) had tissue Doppler examination, and of these 39% had apparently normal LV diastolic function on echocardiography, which is similar to the 41% reported in the entire cohort. Interestingly, patients with combined PH9 had higher prevalence of LV diastolic dysfunction (across all grades) than subjects with isolated PH (70% vs 36%, P < 0.001) (Table 3). In the entire cohort, LA enlargement and left ventricular hypertrophy (LVH) were noted in 41% and 85% of patients, respectively. LA enlargement was significantly more common in patients with atrial fibrillation compared with those in normal sinus rhythm (P = 0.02) (Table 3).
Table 3.
Distribution of LV diastolic dysfunction, LA enlargement, and LVH
| All Patients,No. (%), N = 149 | Patients With NSR, No. (%), N = 125 | Patients With Atrial Fibrillation No. (%), N = 24 | Patients With Tissue Doppler,No. (%), N = 78 | Isolated PH No. (%), N = 47 | Combined PH, No. (%), N = 102 | |
|---|---|---|---|---|---|---|
| Normal diastolic function | 61 (41) | 44 (35) | 17 (71) | 30 (39) | 30 (64) | 31 (30) |
| Grade I | 51 (34) | 50 (40) | 1 (4) | 23(29) | 10 (21) | 41 (40) |
| Grade II | 32 (22) | 26 (21) | 6 (25) | 23 (29) | 6 (13) | 26 (26) |
| Grade III | 5 (3) | 5 (4) | 0 (0) | 2 (3) | 1 (2) | 4 (4) |
| LA enlargement 1 | 60 (41) | 45 (37) | 15 (63) | 32 (42) | 21 (47) | 39 (39) |
| Presence of LVH 2 | 123 (85) | 101 (84) | 22 (92) | 67 (87) | 41 (89) | 82 (84) |
Abbreviations: LA, left atrium; LVH, left ventricular hypertrophy; NSR, normal sinus rhythm.
3 measurements could not be obtained.
5 measurements were not obtained.
When compared to grade I, patients with grade II to III diastolic dysfunction were older (mean difference: 6.2 years; 95% confidence interval [CI]: 1.2‐11.3, P = 0.02), had higher systolic blood pressure (mean difference: 19.2 mm Hg, 95% CI: 10.3‐28.1, P < 0.001) and BMI (mean difference: 4.8 kg/m2, 95% CI: 0.8‐8.8, P = 0.02) as well as lower heart rate (mean difference: 15.2 bpm, 95% CI: 8.9‐21.5, P < 0.001) (Table 1). The proportion of patients with isolated postcapillary and combined pre‐ and postcapillary PH was similar (49% vs 51%) in subjects with apparently normal LV diastolic function on echocardiogram; however, in patients with abnormal LV diastolic function, around 80% had the combined form of PH (Table 1).
3.2. Hemodynamic measurements
Mean PAP was higher in patients with LV diastolic dysfunction (mean difference: 5.3 mm Hg, 95% CI: 1.2‐9.4, P = 0.01); however, no specific trend was noted between mean PA pressure and severity of LV diastolic dysfunction. Cardiac index was lower in patients with LV diastolic dysfunction than individuals with normal function by echocardiography (mean difference: 0.6 (95% CI: 0.3‐0.9, P < 0.001). Transpulmonary and diastolic gradients as well as PVR were significantly higher in patients with LV diastolic dysfunction (mean difference: 5.2 mm Hg, 95% CI: 1.2‐9.2, P = 0.01; 4.3 mm Hg, 95% CI: 1.2‐7.4, P = 0.007; and 2.1 Wood units, 95% CI: 0.9‐3.3, P = 0.001, respectively). Remarkably, there was no significant difference in PAWP among different grades of LV diastolic function (Table 1).
3.3. Echocardiographic determinations
Groups with LV diastolic dysfunction grades II and III had greater LA area when compared to grade I (mean difference: 6.0 cm2, 95% CI: 3.8‐8.2, P < 0.001) (Table 2). Worse RV dysfunction (moderate to severe dysfunction 59% vs 30%, P = 0.04) and paradoxical septal motion (51% vs 24%, P = 0.01) were more common in grade I LV diastolic dysfunction than in grades II and III (Table 2). The LVEDD and LVESD were smaller in grade I than in grades II and III LV diastolic dysfunction (mean difference: 0.6 cm, 95% CI: 0.4‐0.9, P < 0.001; and mean difference: 0.4 cm, 95% CI: 0.1‐0.7, P = 0.006), respectively).
3.4. Survival analysis
Patients were followed for a median (interquartile range) of 4.6 (1.5–7.3) years. Survival rate was 45.2% at 5 years and 31.8% at 10 years. Kaplan‐Meier analysis showed a survival difference between patients with normal and abnormal LV diastolic function (P = 0.03) (Figure A). However, when adjusted by age and gender, the echocardiographic parameters that predicted long‐term survival included measurements of RV function and pulmonary pressure but not of LV diastolic function (P = 0.12) (Table 4; Figure, B). We did not observe a survival difference among patients with normal LV diastolic function, LV diastolic dysfunction grade I and II to III; either by log‐rank test (P = 0.12) (Figure C) or Cox regression adjusted by age and gender (P = 0.38) (Figure D).
Table 4.
Long‐term survival analysis adjusted for age and gender
| Variables | HR | 95% CI | P |
|---|---|---|---|
| Predicted 6‐minute walk distance (per 10 m) | 0.97 | 0.95–0.99 | 0.01 |
| Mixed venous O2 sat (per 1 % change) | 0.94 | 0.92–0.97 | <0.001 |
| PVR (per Wood unit) | 1.07 | 1.01–1.13 | 0.02 |
| Combined pre‐ and postcapillary PH (vs isolated PH) | 1.77 | 1.08–2.90 | 0.03 |
| RA area (per 1 cm2) | 1.04 | 1.01–1.06 | 0.003 |
| RV function (per 1° worsening) | 1.37 | 1.14–1.64 | 0.001 |
| Paradoxical septal motion (yes) | 1.79 | 1.21–2.67 | 0.004 |
| RVSP (per mm Hg) | 1.01 | 1.00–1.02 | 0.03 |
Abbreviations: CI, confidence interval; HR, hazard ratio; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RA, right atrium; RV, right ventricle; RVSP, right ventricle systolic pressure.
Figure 1.
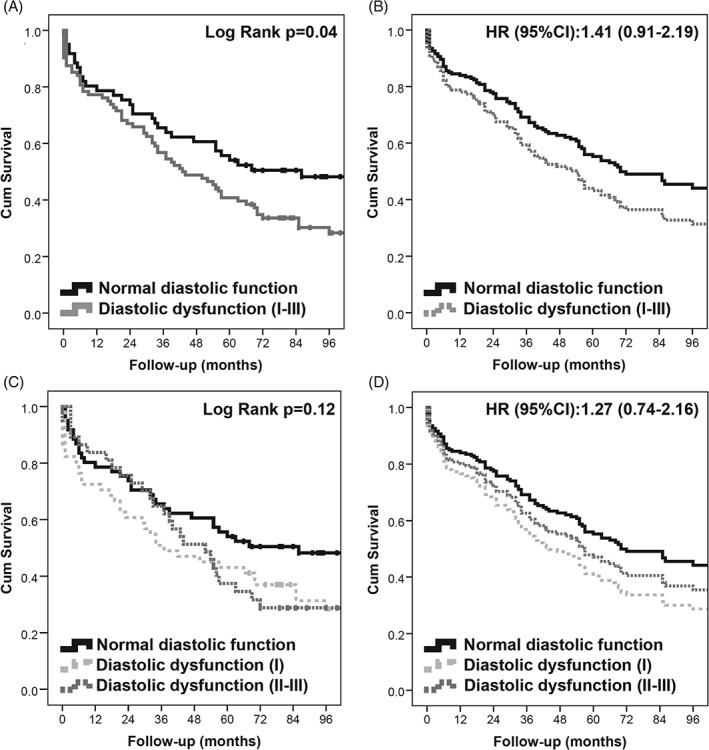
Analyses testing the effect of LV diastolic dysfunction on survival. (A) The Kaplan‐Meier survival curves of patients with normal or abnormal LV diastolic function on echocardiography. (B) The survival curves in patients with normal or abnormal LV diastolic function using a Cox regression model adjusted by age and gender. (C) The Kaplan‐Meier survival curves of patients with normal and abnormal (stratified by severity grade I or grade II–III) LV diastolic function. (D) The survival curves of the same groups in (C), but adjusted by age and gender using a Cox regression model. Abbreviations: CI, confidence interval; Cum, cumulative; HR, hazard ratio; LV, left ventricular.
4. DISCUSSION
In the present study we found that 41% of patients with PH due to HFpEF confirmed by RHC had normal LV diastolic function on echocardiography. Patients with more advanced degrees of LV diastolic dysfunction were older, with higher systolic blood pressure, higher mean PAP, but lower cardiac index. Interestingly, a higher degree of precapillary PH, lower cardiac index, and worse RV function were observed in individuals with grade I LV diastolic dysfunction when compared with subjects who had apparently normal LV diastolic function or grade II or III dysfunction. Although patients with LV diastolic dysfunction on echocardiogram appeared to have worse survival compared to patients with normal diastolic function, this association disappeared when adjusting for age and gender. Notably, echocardiographic parameters that assess RV function and afterload were associated with poor long‐term survival.
We described the LV diastolic function profile in patients with PH due to HFpEF in whom the diagnosis was supported by clinical, laboratory, radiological, and hemodynamic evaluation. We noted that approximately 40% of patients considered to have PH due to HFpEF had normal LV diastolic function by Doppler mitral inflow analysis with and without the addition of tissue Doppler imaging of mitral annular motion. In fact, even when using tissue Doppler, 38.5% of the patients had normal LV diastolic function by echocardiography.17, 18 This research highlights the limitations of echocardiography in the LV diastolic evaluation of patients with PH due to HFpEF given the particular interplay of LV relaxation, compliance, dynamic synchrony,7 loading conditions, LA function, heart rate, and difficulties in obtaining adequate mitral inflow signals during Valsalva maneuver.19 It is possible the different LV diastolic profiles represent a dynamic continuum with the potential to change depending on filling pressures, medical treatments, and disease progression.20
The condition HFpEF is characterized by elevated filling pressures of the LV and pulmonary venous congestions, which with time may lead to remodeling of pulmonary arteries and development of combined pre‐ and postcapillary PH.9 Following this line of thinking, one would expect patients with higher degrees of LV diastolic dysfunction to have more advanced PH and possibly a higher prevalence of combined pre‐ and postcapillary PH. In contrast, we noticed more advanced PH in patients with grade I LV diastolic dysfunction, characterized by a lower cardiac index and higher TPG, DPG, PVR as well as higher prevalence of moderate to severe RV dysfunction. Potential explanations for our findings include (1) combined pre‐ and postcapillary PH may developed in patients with grade I LV diastolic dysfunction when there are other insults predisposing to the development of PH (multiple‐hit hypothesis21), and (2) the development of PH in patients with HFpEF with potential reduction in cardiac output may have concealed the severity of LV diastolic dysfunction assessed by echocardiography.
As expected, patients with higher degrees of LV diastolic dysfunction were older with higher blood pressure. Remarkably, those with grade I LV diastolic dysfunction had worse pulmonary hemodynamics and RV function, suggesting that this group may represent a different phenotype characterized by a more pronounced component of a precapillary PH with higher diastolic pulmonary gradient and PVR.9 We have previously described that a large proportion of patients with pulmonary arterial hypertension had grade I diastolic dysfunction, likely related to RV dilation with reduction of LV size and impairment of LV relaxation.22
Previous studies have shown that LA size can be used as a marker of LV diastolic dysfunction.23, 24 We sought to determine whether surrogate markers such as LA enlargement and LVH were useful in the detection of LV diastolic dysfunction.25 We found that 42% of our patients had LA enlargement and 85% had LVH. When combining transmitral flow determinations with LA enlargement, LV diastolic dysfunction was observed in 74.5% of the patients in our cohort. Furthermore, when LVH was combined with the previous findings, we increased the detection of LV diastolic dysfunction to 96% of the patients. These findings are supported by recent guidelines.26, 27 The lack of LA enlargement in a large percentage of patient in our cohort could be explained by higher RA pressure in patient with PH, which may affect the relative size of the atria.28
In our study, the overall survival at 5 and 10 years was 45% and 31%, respectively. Survival at 5 years is similar to other studies of patients with HFpEF29, 30 but higher than the Framingham Heart Study.31 We found that when adjusted by age and gender, echocardiographic parameters assessing LV diastolic dysfunction did not predict long‐term survival; however, RV function and RVSP did. A few echocardiographic parameters have been associated with worse survival in HFpEF such as RV dysfunction.32 Burke et al33 showed that reduced LV compliance and RV remodeling were the strongest predictors of adverse outcomes in HFpEF. Overall, scarce information exists about the utility of LV diastolic function parameters to predict outcomes in patients with PH related to HFpEF. In our cohort, patients with grade I LV diastolic dysfunction had a more pronounced component of precapillary PH, which could explain the nonsignificant trend to worse survival.
There are several limitations to our study including (1) retrospective analysis of data from a PH center at a tertiary care institution; (2) the possibility of overestimation of PAWP during RHC, even after taking all the precautions for its measurement34; (3) tissue Doppler examination was obtained in about half of the patients, because this assessment started to be routinely performed a few years after the initiation of our study; (4) echocardiography was not done simultaneously with RHC; however, 85% of the patients had these tests done within 1 month; (5) use and dose of certain medications (eg, diuretics, β‐blockers) at the time of RHC and echocardiogram are not available; and (6) LV diastolic dysfunction was graded based on 200916 and not the updated 2016 ASE/European Association of Cardiovascular Imaging guidelines.26 In spite these limitations, we present a study that included a large number of carefully phenotyped patients with PH due to HFpEF who were followed up to 10 years from their initial diagnostic RHC. Further investigations are needed to confirm our findings, but a substantial proportion of patients with PH due to HFpEF diagnosed by RHC appear to have a normal LV diastolic function profile by echocardiography.
5. CONCLUSION
In patients with PH due to HFpEF diagnosed by RHC, the LV diastolic function profile by conventional echocardiography is highly variable. Parameters that assess LV diastolic function by echocardiography were not associated with long‐term survival. A more severe RV function and higher RVSP indicated worse survival.
5.1. Conflict of interests
The authors declare no potential conflict of interests.
Raeisi‐Giglou P, Lam L, Tamarappoo B, Newman J, Dweik RA and Tonelli AR. Evaluation of left ventricular diastolic function profile in patients with pulmonary hypertension due to heart failure with preserved ejection fraction. Clin Cardiol. 2017;40:356–363. 10.1002/clc.22664
Funding information National Institute of Health, Clinical Translational Science Award KL2, Grant/Award numbers: TR000440 and R01HL130307.
REFERENCES
- 1. Paulus WJ, Tschope C, Sanderson JE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28:2539–2550. [DOI] [PubMed] [Google Scholar]
- 2. Ghio S. Pulmonary hypertension in advanced heart failure. Herz. 2005;30:311–317. [DOI] [PubMed] [Google Scholar]
- 3. Hoeper MM, Barbera JA, Channick RN, et al. Diagnosis, assessment, and treatment of non‐pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 suppl):S85–S96. [DOI] [PubMed] [Google Scholar]
- 4. Kjaergaard J, Akkan D, Iversen KK, et al. Prognostic importance of pulmonary hypertension in patients with heart failure. Am J Cardiol. 2007;99:1146–1150. [DOI] [PubMed] [Google Scholar]
- 5. Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D34–D41. [DOI] [PubMed] [Google Scholar]
- 6. Vachiery JL, Adir Y, Barbera JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 suppl):D100–D108. [DOI] [PubMed] [Google Scholar]
- 7. Penicka M, Bartunek J, Trakalova H, et al. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure‐volume loop analysis. J Am Coll Cardiol. 2010;55:1701–1710. [DOI] [PubMed] [Google Scholar]
- 8. Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 suppl):D42–D50. [DOI] [PubMed] [Google Scholar]
- 9. Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. [DOI] [PubMed] [Google Scholar]
- 10. Tonelli AR, Arelli V, Minai OA, et al. Causes and circumstances of death in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5:303–311; discussion 312–313. [PubMed] [Google Scholar]
- 12. Ahmed M, Dweik RA, Tonelli AR. What is the best approach to a high systolic pulmonary artery pressure on echocardiography? Cleve Clin J Med. 2016;83:256–260. [DOI] [PubMed] [Google Scholar]
- 13. Rudski LG, Lai WW, Afilalo J, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713; quiz 786–788. [DOI] [PubMed] [Google Scholar]
- 14. Lang RM, Badano LP, Mor‐Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 15. Kushwaha SP, Zhao QH, Liu QQ, et al. Shape of the pulmonary artery doppler‐flow profile predicts the hemodynamics of pulmonary hypertension caused by left‐sided heart disease. Clin Cardiol. 2016;39:150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. J Am Soc Echocardiogr. 2009;22:107–133. [DOI] [PubMed] [Google Scholar]
- 17. Cahill JM, Horan M, Quigley P, Maurer B, McDonald K. Doppler‐echocardiographic indices of diastolic function in heart failure admissions with preserved left ventricular systolic function. Eur J Heart Fail. 2002;4:473–478. [DOI] [PubMed] [Google Scholar]
- 18. Huis In 't Veld AE, de Man FS, van Rossum AC, Handoko ML. How to diagnose heart failure with preserved ejection fraction: the value of invasive stress testing. Neth Heart J. 2016;24:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khouri SJ, Maly GT, Suh DD, Walsh TE. A practical approach to the echocardiographic evaluation of diastolic function. J Am Soc Echocardiogr. 2004;17:290–297. [DOI] [PubMed] [Google Scholar]
- 20. Appleton CP, Hatle LK, Popp RL. Relation of transmitral flow velocity patterns to left ventricular diastolic function: new insights from a combined hemodynamic and Doppler echocardiographic study. J Am Coll Cardiol. 1988;12:426–440. [DOI] [PubMed] [Google Scholar]
- 21. Yuan JX, Rubin LJ. Pathogenesis of pulmonary arterial hypertension: the need for multiple hits. Circulation. 2005;111:534–538. [DOI] [PubMed] [Google Scholar]
- 22. Tonelli AR, Plana JC, Heresi GA, Dweik RA. Prevalence and prognostic value of left ventricular diastolic dysfunction in idiopathic and heritable pulmonary arterial hypertension. Chest. 2012;141:1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Segers VF, Brutsaert DL, De Keulenaer GW. Pulmonary hypertension and right heart failure in heart failure with preserved left ventricular ejection fraction: pathophysiology and natural history. Curr Opin Cardiol. 2012;27:273–280. [DOI] [PubMed] [Google Scholar]
- 24. Pritchett AM, Mahoney DW, Jacobsen SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population‐based study. J Am Coll Cardiol. 2005;45:87–92. [DOI] [PubMed] [Google Scholar]
- 25. Thenappan T, Shah SJ, Gomberg‐Maitland M, et al. Clinical characteristics of pulmonary hypertension in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2011;4:257–265. [DOI] [PubMed] [Google Scholar]
- 26. Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 27. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 28. Tonelli AR, Dweik RA. Response. Chest. 2013;143:273–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tribouilloy C, Rusinaru D, Mahjoub H, et al. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population‐based study. Eur Heart J. 2008;29:339–347. [DOI] [PubMed] [Google Scholar]
- 30. Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee DS, Gona P, Vasan RS, et al. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Agarwal R, Shah SJ, Foreman AJ, et al. Risk assessment in pulmonary hypertension associated with heart failure and preserved ejection fraction. J Heart Lung Transplant. 2012;31:467–477. [DOI] [PubMed] [Google Scholar]
- 33. Burke MA, Katz DH, Beussink L, et al. Prognostic importance of pathophysiologic markers in patients with heart failure and preserved ejection fraction. Circ Heart Fail. 2014;7:288–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tonelli AR, Mubarak KK, Li N, Carrie R, Alnuaimat H. Effect of balloon inflation volume on pulmonary artery occlusion pressure in patients with and without pulmonary hypertension. Chest. 2011;139:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]


