Abstract
Many occupational and recreational settings require the use of protective and/or load-bearing apparatuses worn over the thoracic cavity, known as thoracic load carriage (LC). Compared to normal, unloaded exercise, thoracic LC exercise places an additional demand on the respiratory and limb locomotor systems by altering ventilatory mechanics as well as circulatory responses to exercise, thus accelerating the development of fatigue in the diaphragm and accessory respiratory muscles compared to unloaded exercise. This may be a consequence of the unique demands of thoracic LC, which places an additional mass load on the thoracic cavity and can restrict chest wall expansion. Therefore it is important to find effective strategies to ameliorate the detrimental effects of thoracic LC. Inspiratory muscle training is an intervention that aims to increase the strength and endurance of the diaphragm and accessory inspiratory muscle and may therefore be a useful strategy to optimize performance with thoracic LC.
Keywords: Exercise tolerance, Respiratory muscles, Occupational physiology, Backpack, Weighted exercise, Inspiratory muscle training
Introduction
Exercise while carrying an external load upon the thoracic cavity, such as backpacks or protective vests and pads (load carriage, or LC), imposes an extra stress on the cardiopulmonary and limb locomotor systems. This extra stress in turn negatively impacts exercise tolerance, exercise performance, and function of the pulmonary and respiratory muscles [10, 23, 72]. In particular, thoracic LC imposes an additional mass load to the thoracic cavity, restricts chest wall movement, and, consequent to restriction of chest wall movement, increases the elastic work of breathing. The combination of these factors makes it more difficult to adequately achieve pulmonary ventilation. Despite the negative effects of thoracic LC, the use of backpacks and similar cargo-carrying apparatuses is still commonplace as they remain one of the most optimal ways of carrying equipment and provisions. Additionally, the use of protective equipment in certain occupational settings, such as law enforcement, military, and firefighting, is also commonplace. While previous reviews have focused on the respiratory-related limitations consequent to load carriage [10], and the impact of thoracic load on Physical Employment Standards, including cardiopulmonary interactions [108], more recent evidence advances our understanding of the respiratory consequences of load carriage [1, 23, 27, 29, 30, 78–83, 116] and highlights the need to discover strategies to ameliorate impairments induced by load carriage. Therefore, this review will summarize the current understanding of the respiratory effects of load carriage and discuss inspiratory muscle training (IMT) as a strategy to counteract the negative effects of thoracic LC on pulmonary and respiratory muscle function may help to optimize comfort and exercise tolerance during thoracic LC exercise.
The Pulmonary System and its Limitations
The primary function of the pulmonary system during rest and exercise is to facilitate respiratory gas exchange, which in turn maintains arterial blood gas and acid–base homeostasis, and maintains cellular function throughout the body [20]. The muscles of the pulmonary system essentially act as a pump that facilitates the bulk flow of air in and out of the large airways in the conducting zone of the lungs by changing the volume of the thoracic cavity, and subsequently the intrapleural pressures [31, 98, 103]. Inspiration involves active contraction of the diaphragm and external intercostals (the sternocleidomastoid and scalene muscles may act as accessory muscles), resulting in an expansion of the thoracic cavity and a decrease in intrapleural pressure [98]. Air flows into the lung as intrapleural pressure falls below ambient atmospheric pressure. At rest, expiration is passive because the elastic recoil of the lung and chest wall compresses the alveolar gas, resulting in air flow out of the lung [98]. During exercise, expiration becomes an active process involving the rectus abdominis, internal and external obliques, and the transverse abdominis, which forcefully contract to increase intrapleural pressure and force air out of the lung [98].
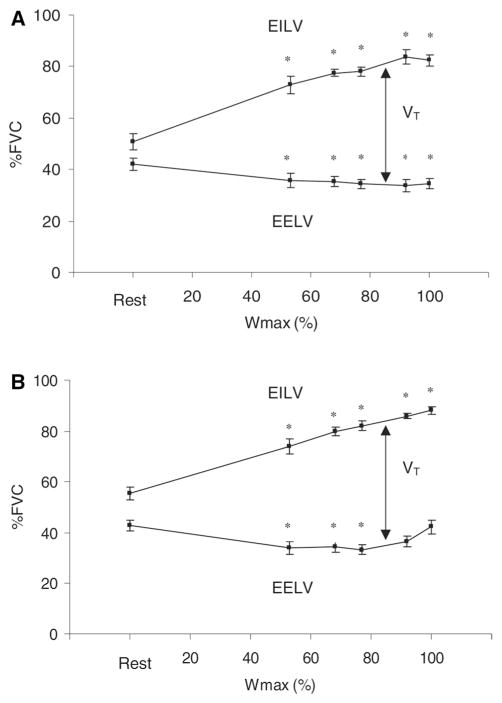
As ventilatory demand increases, typically both tidal volume (VT) and breathing frequency (fb) increase, with the increase in VT achieved primarily by decreasing end-expiratory lung volume (EELV) [45, 97, 122] at low-intensity exercise. By decreasing EELV, diaphragm length is optimized and the increase in end-inspiratory lung volume (EILV) is minimized, thereby reducing any hyperinflation (a shift in tidal breathing to a higher EELV at the expense of inspiratory capacity), and a consequent increase in the work of breathing, that might otherwise occur. In addition to optimizing diaphragm length, this adjustment in operational lung volume also minimizes the elastic work of breathing, which is greater at larger operational lung volumes. As exercise intensity increases, the capacity to decrease EELV is limited, thus EILV increases to allow VT to increase further. The work required by the respiratory muscle to breathe is detected by mechanoreceptors and is a second type of feedback that regulates the pulmonary system [19]. Figure 1 displays the changes in EILV, EELV, and VT during rest and progressive exercise to maximal workload. It is evident from these data that the reserve capacity to decrease EELV is quite small, compared to the reserve capacity to increase EILV. This reduction in ability to decrease EELV is likely due to the mechanical constraints on expiratory flow at low lung volumes. This limitation is effort independent and may be due in part to high intrapleural pressures compressing the small airways in the lung.
Fig. 1.
End-inspiratory lung volume (EILV), end-expiratory lung volume (EELV), and tidal volume (VT), expressed as a percentage of forced vital capacity (FVC) at rest and during progressive exercise to maximal workload at V̇O2max (Wmax) in men (a) and women (b) [38]. Reprinted with permission
The respiratory muscles are striated skeletal muscle, similar to the limb locomotor muscles [31]. As such, they are governed by the same length–tension and force–velocity relationships as other skeletal muscles [31]. Therefore, there is an ideal muscle length for the respiratory muscles, where their force-generating capacity is highest. Deviations from this length in either direction (longer or shorter) compromise this force-generating capacity, and thus are considered sub-optimal. As ventilatory demand increases, changes in operational lung volume may occur to optimize respiratory muscle length, and combat the occurrence of expiratory flow limitation.
Expiratory flow limitation (EFL) occurs when airflow out of the lungs is mechanically limited by the chest wall and conducting airways, thus compromising the ability to further increase ventilation at, or near, maximal exercise [15, 54, 117], which may limit the ability to increase pulmonary ventilation (V̇E) and consequently the ability to maintain blood gas homeostasis. EFL in highly trained athletes does not always represent a reduced ventilatory capacity however. Rather, in some cases, it is indicative of a ventilatory demand that approaches or exceeds the capacity of the respiratory system [55]. Although EFL may influence arterial oxygen saturation, not all athletes who exhibit EFL are susceptible to desaturation during exercise. Other factors reflecting abnormality in the pulmonary system’s ability to effectively facilitate gas exchange such as pulmonary capillary transit time, ventilation-perfusion mismatch, inadequate compensatory hyperventilation, and diffusion limitation may contribute to the development of exercise-induced arterial hypoxemia (EIAH) [22, 77, 84]. Thus, EIAH may compromise exercise tolerance due to its significant contribution to limb locomotor muscle fatigue development [90]. Taken together, the impact of EIAH in combination with the development of respiratory muscle fatigue, may activate a metaboreflex, which increases sympathetic vasoconstrictor outflow and compromises the perfusion of limb locomotor muscle. This in turn, may limit exercise tolerance and highlights the importance of a sufficient pulmonary system capable of adequately meeting the demands of exercise.
EFL may, in part, result from dynamic airway compression due to high intrathoracic pressures. Moreover, EFL promotes dynamic pulmonary hyperinflation [14], which may be a strategy to minimize the airflow limitation by shifting tidal breathing to a higher lung volume where maximal flow rates are higher. This dynamic hyperinflation in turn, causes further deviation from the optimal muscle length and increases the elastic work of breathing. Both of these effects may accelerate the development of respiratory muscle fatigue. EFL may be an important consideration in LC exercise because during LC, EELV is decreased compared with an unloaded state, thereby decreasing the reserve capacity to increase VT by decreasing EELV. Consequently, the low operational lung volume increases the likelihood of developing EFL [109], which may then result in dynamic pulmonary hyperinflation. LC, in particular chest wall restrictors such as ballistic vests, may prevent this compensatory hyperinflation; therefore, the ventilatory capacity of an individual may be compromised by LC.
Respiratory Muscle Fatigue
Historically, the healthy pulmonary system was considered “overbuilt” for exercise because it adequately meets the demands of exercise, even to exhaustion [19]. More recently, however, it has been documented that prolonged exercise (> 2 h), as well as severe exercise (> 80% V̇O2max), may induce respiratory muscle fatigue [53, 109]. Respiratory muscle fatigue refers to a transient decline in the force- or pressure-generating capacity of the respiratory muscles, particularly the diaphragm, which is reversible with rest. Generally, the gold-standard technique to assess respiratory muscle fatigue is through bilateral phrenic nerve stimulation in conjunction with esophageal balloon-tipped catheters to estimate pleural pressures [2, 24, 51, 53, 70, 113]. This technique involves advancing two flexible balloon-tipped catheters, which are connected to calibrated piezoelectric pressure transducers, through the nares and placing one in the esophagus to record esophageal pressure (Pes), and the second in the stomach to record gastric pressure (Pga). Trans-diaphragmatic pressure (Pdi) is calculated as the difference between Pga and Pes. Bilateral phrenic nerve stimulation at functional residual capacity (FRC, which controls for lung volume during stimulation) allows for determination of twitch Pdi (Pdi, tw), which is assessed pre- and post-exercise. Diaphragm fatigue is considered present with a post-exercise decline in Pdi, tw ≥ 15% [109]. Due to the invasive nature of esophageal balloon-tipped catheters, recent efforts have aimed to assess non-volitional declines in mouth pressure in response to cervical magnetic stimulation of the phrenic nerve [56, 119, 120], which were shown to be correlated with esophageal pressures [56]. Similarly, others have used a combination of sniff maneuvers and ultrasonography [79] to assess diaphragm fatigue. However, it is noted that the latter technique is a volitional maneuver and it is recommended that it is used in combination with cervical Pdi, tw to evaluate diaphragm strength [85]. It is noted that although these techniques are correlated to each other, the use of mouth pressures and sniff maneuvers are indirect estimates of diaphragm strength and thus the best measure of diaphragm fatigue remains Pdi, tw in response to phrenic nerve stimulation. Absent these non-volitional techniques, the change in voluntary mouth pressures has been used as a surrogate for respiratory muscle fatigue [27]. Recent evidence using voluntary mouth pressure measurements suggests that LC exercise induces significant global respiratory muscle fatigue [27], which may in part explain the negative impact of LC on exercise performance.
Respiratory Muscle Metabore ex
As with limb locomotor muscle fatigue, the genesis of respiratory muscle fatigue is highly complex and may originate both centrally and peripherally. As the respiratory muscles fatigue, it is likely that metabolites accumulate in the milieu of these muscles similar to the limb locomotor muscles [46], thereby resulting in the activation of unmyelinated type IV phrenic afferent nerves. The firing of these type IV afferents may act supra-spinally to trigger the respiratory muscle metaboreflex, thus compromising exercise performance [3, 21, 37, 42, 43, 89, 99]. Loading and unloading of the respiratory muscles has been shown to affect performance in fixed workload time to exhaustion exercise, and also to influence the perception of effort for both limb discomfort and dyspnea [44, 94]. It is clear that a significant contributing factor to the development of respiratory muscle fatigue is a high level of respiratory muscle work that is sustained throughout an exercise bout [41]. This is particularly important during heavy and severe exercise (50, and 70%, respectively, of the difference between ventilatory threshold and peak V̇O2 [86]) when V̇E is particularly high. The relationship between V̇E and work of breathing is exponential, therefore as V̇E rises exponentially above the gas exchange threshold, the concomitant increase in the work of breathing is larger in magnitude. Figure 2 displays the relationship between the work of breathing and minute ventilation (V̇E) in men and women.
Fig. 2.
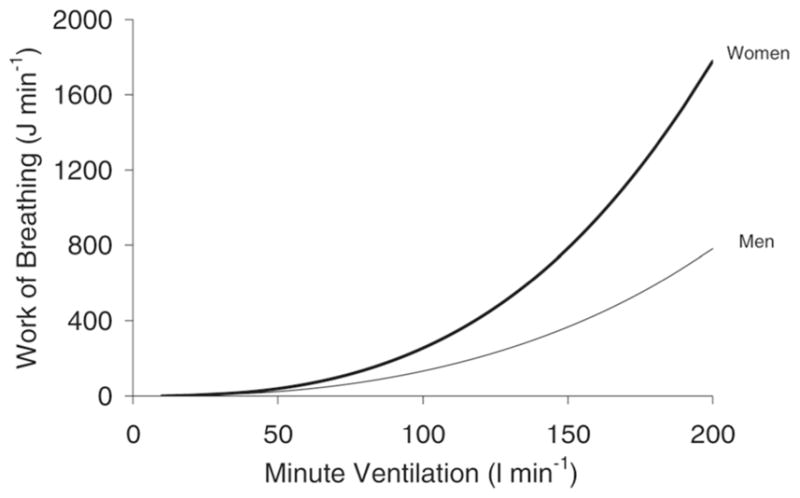
Relationship between work of breathing and minute ventilation (V̇E) in men and women [38]. Reprinted with permission
Mechanisms of Fatigue Development
Despite the classical partitioning of the origin of fatigue into central and peripheral pathways, recent investigations have proposed that afferent (interoceptive) feedback from the periphery, including effort perception, and exteroceptive input are integrated in the subconscious brain by the so-called “central governor” to produce feedforward signals, which are protective in nature [74, 75, 106]. This mechanism has been termed the Central Governor Model (CGM). The CGM differs from classical models of fatigue because it proposes that both central and peripheral factors serve as afferent input to the subconscious brain, which then integrates the afferent feedback with exteroceptive input and conscious input to optimally regulate exercise intensity. The subconscious brain then modulates motor output and may attempt to limit fatigue-related decrements in performance and protect the athlete from catastrophic physiological failure [100]. A key aspect of the CGM is that it is capable of accounting for both central and peripheral contributions to muscle fatigue.
Peripheral fatigue traditionally refers to changes distal to the neuromuscular junction such as metabolic changes in the muscle milieu [100], whereas central fatigue refers to alterations in the central nervous system such as decreased cortical excitability or decreased supraspinal drive [33, 57]. It is well documented that metabolic acidosis, which commonly occurs with exercise above 80% of V̇O2max [40], reduces twitch tension [32] and muscle contractility [63]. Metabolic acidosis causes accumulation of other metabolites such as ammonia, inorganic phosphate, and potassium which may also contribute to peripheral fatigue [4, 100]. Independent of these biochemical changes, feedback from the muscle spindles, Golgi tendon organs, and group III and IV muscle afferents may inhibit central motor drive [33]. Thus, it is clear that both central and peripheral factors likely contribute to the development of fatigue, as suggested by the CGM.
In the CGM, perceived exertion [7] may play a role in modulating central motor drive, therefore any disturbances in effort perception may alter the descending commands from the central nervous system [73]. Moreover, the motor cortex may concurrently transmit both motor outflow commands to both the limb locomotor muscles and the sensory cortex, which could lead to a sensation of muscle activation [12]. This sensation, in conjunction with afferent inputs, may continually adjust muscle contraction and motor performance via a feedforward/feedback process [12]. Therefore, the impact of increased respiratory muscle work on exercise performance may not be limited to just the respiratory muscle metaboreflex, as the increased perception of effort in both limb discomfort and dyspnea may be negatively interpreted by the brain, resulting in a down-regulation of motor output.
Respiratory Muscle Fatigue and Thoracic Load Carriage
Given that healthy humans typically carry a significant breathing reserve capacity, mild restriction to lung capacity, which could be induced by LC, may have little effect during light exercise given that V̇E is only mildly elevated beyond resting values. As exercise intensity increases, the ventilatory reserve capacity progressively declines [23] such that during high-intensity exercise, when ventilatory demand has increased 10 to 15-fold above resting, the impact of LC may be more pronounced because it further reduces the ventilatory reserve [23].
Recent evidence [27] suggests that LC exacerbates the respiratory muscle fatigue that has been clearly shown to occur during severe exercise [21, 53, 109]. The negative impact of LC on exercise tolerance could be reduced by finding interventions that specifically target the limitations of the respiratory system. Inspiratory muscle training (IMT) specifically attempts to increase respiratory muscle strength which may help reduce or delay the development of respiratory muscle fatigue [41, 66]. For example, Witt et al. [118] demonstrated that IMT reduced the increase in heart rate and mean arterial pressure that is commonly observed with heavy exercise and the consequent increase in the work of breathing.
Load Carriage
Thoracic load carriage (LC) refers to locomotion while bearing a mass upon the torso [27, 59] and may consist of a backpack supported by shoulder straps and/or a hip belt, as well as protective equipment such as ballistic vests, athletic pads, and self-contained breathing apparatuses. Thoracic LC is often essential in many recreational and occupational settings such as hiking, certain sports such as American football and lacrosse, military operations, and emergency services [6], and is one of the most economical avenues by which to carry necessary equipment and supplies [5]. When backpacks are used, the economy of carrying the load is particularly high when a hip belt aids in redistributing part of the load from the shoulders onto the pelvis [5]. Placement of mass is an important consideration with load carriage because of biomechanical, metabolic, and physiological considerations. Paradoxically, while placement of load on the thorax imposes the lowest relative metabolic cost per added kilogram of weight, this simultaneously surrounds the respiratory pump, subsequently impacting the respiratory function [35, 107, 108]. Load placement is beyond the scope of this review; however, readers are referred to Taylor et al. [108] for a more detailed discussion on load placement.
Load Carriage and Pulmonary Mechanics
Thoracic LC imposes two distinct stressors during exercise: the additional mass that must be transported and the restriction of chest wall movement from protective equipment and/or backpack sternum straps [23]. There is evidence of numerous physical effects of thoracic LC including the following: thoracic LC impairs pulmonary function [11, 61, 62, 72]; increases V̇E, HR and V̇O2 during exercise [64, 88]; accelerates the development of respiratory muscle fatigue [27]; and increases the energetic cost of walking [36, 49]. In particular, restriction of the chest wall during thoracic LC exercise may have unique deleterious effects on the respiratory system and consequent impairments in exercise capacity. While ‘load carriage’ is often used as a blanket term referring to any load carriage upon the thorax, the type of load (e.g., personal protective equipment vs. backpacks) as well as the weight and elasticity of the load has significant implications when considering the physiological and biomechanical consequences of load carriage.
Restriction of the chest wall decreases total lung volume, therefore if thoracic LC imposes a physical restriction to chest wall movement, total lung capacity would likely be decreased. However, this depends on the type of LC, with more restrictive devices such as protective vest and equipment likely having a larger effect compared to less restrictive equipment such as backpacks. For example, while previous studies using a weighted vest have suggested that reductions in TLC may be responsible for the decrease in forced vital capacity (FVC) [11, 62, 61, 72], a recent study on backpack thoracic LC demonstrated no difference in TLC despite both FVC and forced expiratory volume in one second (FEV1.0) being reduced with LC [80]. Alternatively, thoracic LC, in addition to impairing chest wall movement, may increase transpulmonary pressures, which in turn may increase the closing volume and consequently increase residual lung volume [52, 96]. However, it is controversial whether thoracic LC exercise induces these changes [23], therefore the definitive cause of the reductions in FVC is yet unknown. Therefore, further investigations are warranted to elucidate the mechanisms underpinning the reduction in FVC in subjects wearing backpacks. Specifically, full pulmonary function assessments, including closing volume, diffusing capacity, spirometry, and lung volumes with different modes of thoracic LC would aid in describing the effects of various types of LC on pulmonary function.
Investigations thus far have demonstrated that backpack thoracic LC reduces both FVC and FEV1.0 [11, 23, 61, 62, 72, 80], mimicking mild restrictive pulmonary disease [61]. These effects are likely a consequence of both the restrictive nature of the backpack straps as well as the mass load on the thorax, which may increase the force that the respiratory muscles need to generate in order to expand the thoracic cavity. It is important to note, however, that Dominelli et al [23] found no difference in FVC between a no backpack condition and a backpack with no additional weight condition. This finding suggests that the minimal weight of the backpack itself (3.1 kg) and restrictive nature of backpack straps were insufficient to alter FVC. With additional loads from 15 to 35 kg however, FVC progressively declined, as did FEV1.0. The authors postulated that the reduction in FVC while carrying the backpack may have been due to either increased residual lung volume or decreased total lung capacity (TLC), or both [23].
Despite the clear impairments to pulmonary function consequent to backpack LC, a recent investigation demonstrated no difference in the power of breathing at equivalent V̇E between no load and backpack LC load up to 35 kg [23]. The power of breathing, which is an estimation of the power output requirement of the respiratory system determined by multiplying the integral of several averaged pressure–volume loops (an estimate of work) by the breathing frequency, increased with V̇E, but no differences in power of breathing requirement were observed at a given level of ventilation as backpack weight increased. This study [23] was not controlled to compare power of breathing requirement at matched oxygen demands or ventilation rates, thus limiting the conclusions that can be drawn regarding load-dependent changes in power of breathing requirement. Figure 3 displays the power of breathing at different minute ventilations for unloaded and different thoracic LC conditions [23]. These findings are in contrast to previous studies that have demonstrated an increase in required power of breathing with loading and chest wall restriction [71, 76, 109]. It is important to note however, that carrying a backpack does not necessarily reflect the same stress as chest wall restriction only. The authors postulated that while the heaviest backpack condition (35 kg) in their study was enough to elicit mechanical changes in V̇E, it did not alter the power of breathing requirement because of other respiratory adaptive responses to the loading. Such adaptive changes may include changes in operational lung volume, which minimized the power of breathing requirement by decreasing EELV, thus allowing subjects to breathe at a lung volume with higher compliance.
Fig. 3.
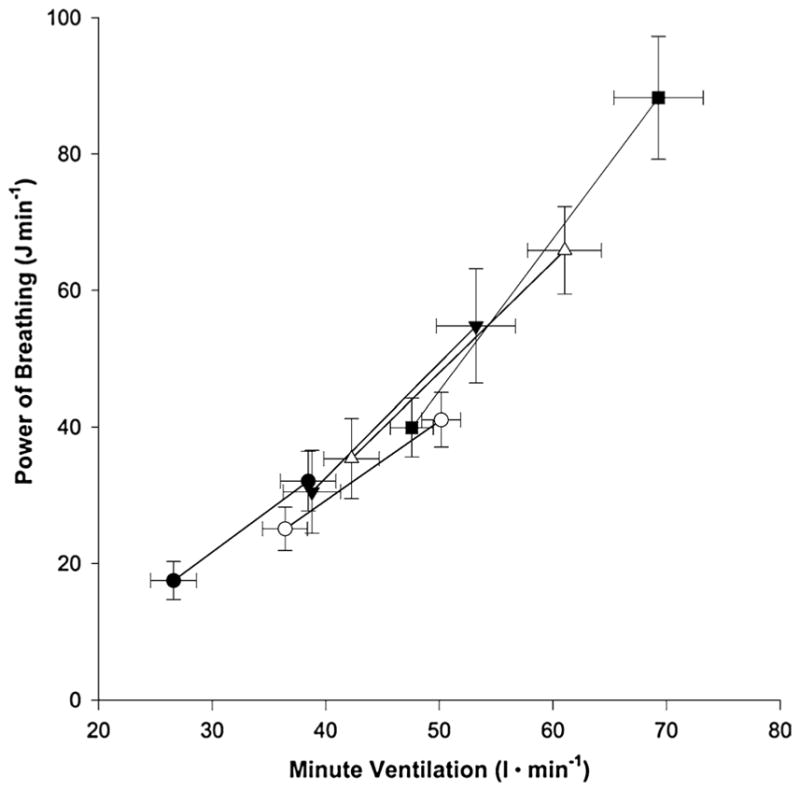
Power of breathing at different minute ventilations for five backpack conditions. Filled circles represent no backpack condition. Open circles represent unweighted backpack condition. Filled triangles represent 15-kg backpack condition. Open triangles represent 25-kg backpack condition. Filled squares represent 35-kg backpack condition [23]. Reprinted with permission
Although this adaptive decrease in EELV may offset the effects of thoracic LC on the power of breathing requirement, the shift to a lower operational lung volume also increases the likelihood of EFL. In fact, Dominelli et al. [23] observed EFL in two out of seven subjects (7 and 12% of tidal volume) with 35 kg LC during moderate exercise (4.0 km hr−1 at 15% grade, mean ± SE V̇E 69 ± 4.0). Had the subjects been required to exercise at a higher intensity, or higher level of V̇E, it is likely that either a greater degree of EFL would be observed, or EELV would increase to minimize the occurrence of EFL. However, EELV is constrained with thoracic LC, therefore the authors postulated that at higher V̇E, the subjects would likely experience a greater degree of EFL. Moreover, if EELV did indeed increase at higher V̇E levels, then the adaptive response to the thoracic LC would be reduced, thereby increasing the power of breathing requirement.
It is also important to note, that although power of breathing requirements may not differ with thoracic LC at the same level of V̇E, thoracic LC exercise has been shown to increase V̇E compared to unloaded exercise [23, 64, 80, 88]. Therefore, the elevated V̇E itself increases the degree of respiratory muscle work, and thus may accelerate the development of respiratory muscle fatigue. In fact, independent investigations into the effects of inelastic chest wall restriction with canvas straps and backpack LC on respiratory muscle fatigue have demonstrated that thoracic LC does in fact exacerbate respiratory muscle fatigue [27, 109].
Chest wall restriction with inelastic canvas straps increases the work of breathing and dyspnea during cycling exercise at 45% of maximum intensity, and reduces diaphragm contractility following 10 min of such exercise, indicating the presence of respiratory muscle fatigue [109]. Despite the moderate intensity and fixed duration of this exercise bout, the degree of diaphragm fatigue observed in this study was similar in magnitude to the findings in healthy subjects who exercise at intensities above 85% of their V̇O2max until exhaustion [53, 109]. The chest wall restriction in this study significantly increased the total work of breathing, which likely contributed to the development of diaphragm fatigue. The authors also noted that the physical restriction of the chest wall may have reduced diaphragm blood flow [109] and that cardiac output during exercise at 45% of maximal power output is reduced with chest wall restriction [71]. Aside from the increased work of breathing, the observed increase in dyspnea, as previously discussed, may also negatively impact exercise performance.
More recent investigations have described the cardiopulmonary and gross physiological responses to thoracic LC exercise [79–82], consistently demonstrating an additional strain on the cardiopulmonary system with thoracic LC compared to unloaded exercise. Thoracic LC with a heavy backpack (25 kg) produced significant changes in ventilatory mechanics including an increase in both V̇E and fb, and a decrease in VT and EILV [80]. Importantly, both the loaded (thoracic LC) and unloaded (control) exercise bouts were performed at equivalent oxygen demand, i.e., matched V̇O2, suggesting that the alterations in ventilatory mechanics were attributable to the thoracic LC and not differences in exercise intensity. Despite the matched workloads, V̇E was significantly greater with thoracic LC, largely due to an increase in deadspace ventilation. These findings were confirmed also to occur during a graded treadmill walking test with heavy LC (45 kg) in a similar population [82]. Further, PImax was reduced after 45 min of steady-state exercise with thoracic LC, but not after unloaded exercise at a matched V̇O2, supporting the hypothesis that thoracic LC induces respiratory muscle fatigue.
In a subsequent study [81], thoracic LC with a weighted backpack was shown to reduce V̇O2 and power output both at ventilatory threshold and at peak exercise during a graded treadmill walking exercise test. The reduction in energetic availability demonstrated a reduction in exercise capacity at two important exercise intensities, ventilatory threshold and peak exercise. Both of these intensities are relevant in many occupational settings that may require personnel such as search and rescue, firefighting, and military personnel to perform prolonged exercise at or near the anaerobic threshold and short exercise bouts at or near peak exercise. These findings are similar to those of Walker et al. [116] who demonstrated a reduction in V̇O2 with progressively heavier mass loads on a weighted vest. In contrast, others have shown no reduction in maximal aerobic power with LC in the form of firefighter personal protective equipment and a weighted vest [79, 107]. In both the latter studies, peak V̇E was not reduced with LC, but simply occurred at a lower work rate. Similarly, peak V̇E was not different between loaded (25 kg) and unloaded conditions in a study by Phillips et al. [80] despite a reduced V̇O2 at peak exercise. The same group, however, subsequently demonstrated with both 25 kg and 45 kg loads, that power output, V̇O2, and V̇E were both reduced at peak exercise in separate studies [81, 82]. Of note, in the latter study by Phillips et al. [82] PETCO2 was preserved between loaded and unloaded conditions, suggesting that subjects were able to maintain blood gas homeostasis. Therefore, other mechanisms may be responsible for the reduction in peak V̇O2. These equivocal findings make it difficult to determine the underlying causes that may result in reduced performance and work capacity with LC.
Load Carriage and Exercise Tolerance
Recently, Peoples et al. [79] demonstrated that load carriage in the form of a 22-kg vest reduced maximal work tolerance and reduced the maximal acceptable work duration compared to unloaded exercise. Despite observing impairments to exercise capacity with thoracic LC, however, Peoples et al. [79] observed no evidence of respiratory muscle fatigue as assessed using a sniff maneuver in tandem with ultrasonic imaging of the diaphragm. No direct measures of the pressure- or force-generating capacity of the diaphragm were taken, and the authors note that direct stimulation of the phrenic nerve, a non-volitional means of assessing diaphragm contractility, may have produced a difference outcome.
Backpack thoracic LC with a 25 kg load impairs running time trial (TT) performance compared with unloaded running and, unlike unloaded running, induces respiratory muscle fatigue following a 2.4-km running TT [27]. The design of this study required subjects to walk at a constant speed (6.5 km hr−1) at a 0% gradient for 60 min either with or without a 25-kg backpack (LC). They then rested for 15 min and completed a 2.4 km running TT on a treadmill, again, either with or without thoracic LC. Respiratory muscle fatigue in this case was assessed as the voluntary inspiratory and expiratory pressure-generating capacity of the respiratory muscles (PImax and PEmax, respectively). The authors of the study were the first to demonstrate a significant degree of respiratory muscle fatigue following thoracic LC exercise.
In contrast to the findings of Tomczak et al. [109], the authors in this study did not observe an increase in whole-body RPE, RPE specific to the legs, or RPE specific to breathing in the LC condition compared to the control at any point during the experimental trials [27]. Differences in whole-body RPE following the 60-min steady-state walk were 12 ± 3 with LC and 8 ± 1 without LC, which was not statistically significant. Similarly, RPE specific to the legs was 3 ± 2 with LC and 1 ± 1 without LC and RPE specific to breathing was 3 ± 2 with LC and 1 ± 1 without LC, neither of which was statistically significantly different. Therefore, despite the positive trend towards higher RPE scores with LC, the authors concluded that there were no differences in the perceptual responses to exercise with LC.
Methodological limitations may limit the internal and external validity of these studies given that in one study voluntary mouth pressures were used to quantify respiratory muscle fatigue [27], and in the other, custom-made canvas straps were used to mimic the chest wall restriction of protective equipment worn over the thoracic cavity [109]. The methods in the former study [27] are particularly problematic primarily because the time trial was performed on a treadmill, which required subjects to manually adjust the treadmill speed, and because respiratory muscle fatigue was measured using volitional measures of muscle force output, rather than non-volitional nerve stimulation techniques. Moreover, given that the authors measured only mouth pressures and did not utilize intrathoracic balloon-tipped catheters, no data regarding intrathoracic pressures, work of breathing, EILV, EELV, or total lung volumes were reported. Accordingly, it is prudent to further investigate the relationship between LC exercise and the development of respiratory muscle fatigue. Such an investigation should seek to duplicate recreational and occupational LC conditions and used non-volitional techniques such as trans-cranial magnetic stimulation to assess respiratory muscle fatigue.
Inspiratory Muscle Training
Inspiratory muscle training refers to a class of interventions aimed at enhancing the strength and/or endurance of the inspiratory muscles including the diaphragm and accessory muscles such as the external intercostals and scalene muscles [50]. These muscles are skeletal muscles, thus they may be trainable in a manner similar to limb locomotor muscles [91]. Several different loading protocols have been developed to place the specific training stress on the respiratory muscles required to induce adaptation. These loading protocols may be divided into three distinct categories. Flow-resistive loading typically involves a variable-diameter orifice that subjects must breathe through. The variable-diameter orifice in this type of device acts as a “leak” which increases the inspiratory pressure required to generate a given rate of airflow [101, 104]. Alternatively, pressure-threshold loading requires an individual to generate a predetermined amount of inspiratory pressure in order to open a one-way valve to enable airflow through the device [101, 104]. Both of these devices can be considered resistance loading devices. Separately, volume loading protocols such as normocapnic hyperpnea, have been employed, which require individuals to maintain a high ventilatory rate relative to their maximum voluntary ventilation [101, 104]. During this training, subjects engage in rebreathing to prevent hypocapnea from occurring. The common element of these three loading protocols is that they are all performed at rest, and not while exercising. For a full description of archetypal training protocols for each of these categories of IMT, the reader is referred to [101].
Briefly, flow-resistive loading protocols typically involve a hand-held training device that can be used either in a laboratory setting or at home. Training is conducted three times per week, with each session beginning with subjects performing three maximal inspiratory maneuvers initiated from residual lung volume and spanning the entire vital capacity. These maneuvers are performed against the inspiratory resistance (typically a small hole) and recorded on by computer software, which computes the area under the curve over the entire maneuver (abbreviated AuC or sustained maximal inspiratory pressure, abbreviated SMIP). A training template is generated from the best of the maneuvers, which is equal to 80% of the SMIP. Some protocols simply require subjects to reproduce the training template 30 times [114], whereas others utilize a progressively increasing work–rest ratio [16, 34, 68, 102]. In the latter, subjects complete blocks of six inspirations beginning with 1 min of rest between breaths. The rest period is then progressively decreased to 45-, 30-, 15-, 10-, and 5 s every six breaths, with training terminating either upon completion of all 36 breaths, or if subjects fail to achieve at least 90% of the target training template. For this type of training, a placebo control with resistance set to 30% SMIP rather than 80% SMIP has been demonstrated to have no ergogenic effect [16, 34].
Pressure-threshold protocols are varied; however, the most common protocol involves training twice daily for 30 breaths at a resistance equal to 50% of maximal inspiratory mouth pressure (PImax) [13, 90–93, 110, 111] with portable devices similar to flow-resistive training devices, which allows for training to be completed in either a laboratory setting, or at home. As with flow-resistive training, a validated placebo control has been developed, which requires control subjects to complete the same training except at a resistance set to 15% of PImax, rather than 50% PImax [13, 92].
Normocapnic hyperpnea is typically performed in 30-min sessions on two consecutive days, followed by one day of rest for a total of 20 sessions [8, 9, 47, 60, 67, 121]. Subjects are required to maintain a minute ventilation equal to 60% of their MVV15 at an inspiratory duty cycle of 0.5 and at a VT equal to 50–60% of vital capacity. During the training session, fb may be adjusted based upon feedback from subjects. Subsequent training sessions may have fb based upon subject performance during the previous training session.
The effects of flow-resistive and pressure-threshold IMT have been studied extensively [50] and it is well documented that chronic IMT can promote hypertrophy of the diaphragm and external intercostal muscles, increase the proportion of type II muscle fibers in the external intercostal muscles, and increase the oxidative capacity of the diaphragm [101]. Current evidence suggests that exercise performance in healthy individuals is improved with IMT [50]. Indeed, a number of studies have demonstrated positive effects of both flow-resistive and pressure-threshold IMT. Table 1 summarizes exercise studies involving IMT. Although the protocols between studies varied, collectively, these studies suggest that IMT may have an ergogenic effect on exercise capacity and performance. Despite this evidence, the debate regarding the efficacy of IMT on endurance performance is still ongoing [65, 78]. Furthermore, there is still contradictory evidence that does not demonstrate improvements to exercise performance with IMT [112, 114]. There is some evidence, however, that IMT reduces the development of respiratory muscle fatigue [93, 112, 114].
Table 1.
Summary of studies examining the impact of inspiratory muscle training on exercise performance
| Study | Population | Protocol | Outcome |
|---|---|---|---|
| Chatham et al. [16] | 22 healthy subjects (10 male, 12 female) | 8-week flow-resistive IMT; 3 times weekly using TIRE regimen (progressive work: rest ratio at 80% of sustained maximal inspiratory pressure, up to 36 breaths per session) | PImax increased by 42 cm H2O; 9.8% improvement in shuttle run performance |
| Volianitis et al. [115] | 14 female competitive rowers | 11-week pressure-threshold IMT; 30 breaths at 50% PImax twice daily | PImax increased by 44 ± 25 cm H2O; distance covered in 6-min all-out effort increased by 3.5 ± 1.2%, 5000 m time trial improved by 36 ± 9 s |
| Romer et al. [92] | 16 male trained cyclists | 6-week pressure-threshold IMT; 30 breaths at 50% PImax twice daily | PImax increased by 28 ± 7%; 40-km cycling time trial performance improved by 4.6 ± 1.9%, 20-km time trial improved by 3.8 ± 1.7% |
| Romer et al. [93] | 24 male repetitive sprint athletes | 6-week pressure-threshold IMT; 30 breaths at 50% PImax twice daily | PImax increased by 30.5 ± 2.2%; total recovery time during repetitive sprint test improved by 6.2 ± 1.1% |
| Gething et al. [34] | 15 apparently healthy subjects (10 male, 5 female) | 10-week flow-resistive IMT; 3 times weekly using TIRE regimen (progressive work: rest ratio at 80% of sustained maximal inspiratory pressure, up to 36 breaths per session) | PImax increased by 34%; cycling time to exhaustion at 75% of VO2peak improved by 36% |
| Enright et al. [26] | 20 moderately trained adults (9 male, 11 female) | 8-week flow-resistive IMT; 3 times weekly using TIRE regimen (progressive work: rest ratio at 80% of sustained maximal inspiratory pressure, up to 36 breaths per session) | PImax increased by 41%; Exercise capacity improved by 23% in progressive, incremental test on cycle ergometer (beginning with no resistance, then 8-W increases every minute until volitional exhaustion or reaching 80% of age-predicted maximum heart rate) |
| Kilding et al. [58] | 16 competitive club-level swimmers | 6-week pressure-threshold IMT; 30 breaths at 50% PImax twice daily | PImax increased by 9.1 ± 4.2%; 100-m swim time improved by 1.7 ± 1.4%, 200-m swim time improved by 1.5 ± 1.0% |
| Mickleborough et al. [69] | 24 recreational road runners | 6-week flow-resistive IMT; 3 times weekly using TIRE regimen (progressive work: rest ratio at 80% of sustained maximal inspiratory pressure, up to 36 breaths per session) | PImax increased by 43.9%; Endurance capacity during treadmill run set at 80% VO2max improved by 16.4% |
| Turner et al. [111] | 16 male highly trained cyclists | 6-week pressure-threshold IMT; 30 breaths at 50% PImax twice daily | PImax increased by 22.5 ± 8.7%; Oxygen cost of voluntary hyperpnea decreased by 1.5% at 75% of VO2max and 3.4% at 100% of VO2max |
IMT is thought to promote hypertrophy of the muscles activated during inspiration, including the diaphragm and external intercostal muscles [17, 25, 26]. Moreover, with IMT, the proportion of type I fibers and the cross-sectional area of type II fibers in the external intercostal muscles may increase [87]. Both of these morphological and physiological adaptations may improve inspiratory muscle endurance and strength. With increased respiratory muscle strength, the inspiratory muscles may be able to generate a given pressure with less respiratory motor drive [48]. Additionally, the increase in inspiratory muscle endurance may delay the onset of respiratory muscle fatigue, and by extension, the activation of the respiratory muscle metaboreflex and the increased effort perception of limb locomotor muscle discomfort and dyspnea. Therefore, IMT may facilitate improvements in exercise tolerance due to improvements in inspiratory muscle strength and endurance (Table 2).
Table 2.
Summary of studies examining the effects of load carriage exercise on pulmonary function and exercise tolerance
| Study | Population | Protocol | Outcome |
|---|---|---|---|
| Legg [61] | 30 healthy males | 6.2-kg bomb disposal searcher’s suit, 4.2-kg fragmentation vest, 2.9-kg body armor; pulmonary function data only (no exercise) | FVC reduced with bomb disposal searcher’s suit (−2.9%) and fragmentation vest (−2.4%); PEF reduced with bomb searcher’s suit (−4.9%) |
| Muza et al. [72] | 5 young males | 10- or 30-kg weighted backpacks; pulmonary function data only (no exercise) | FVC and FEV1.0 reduced with 10-kg pack (−2.5 and −3.0%, respectively) and with 30-kg pack (−6.0 and −6.7%, respectively), MVV decreased by 8.4% with 10-kg pack, but further decline with 30-kg pack |
| Majumdar et al. [64] | 6 and 16 male soldiers (phase 1 and 2, respectively) | Phase 1: 11-kg body armor; 10-min treadmill running at 2.2 m sec−1; phase 2: 9- and 11-kg body armor; pulmonary function testing only (no exercise) | HR, VE, and VO2 increased with 11-kg body armor (+ 15 beats min−1, + 9.4 L min−1, + 6.0 mL kg−1 min−1, respectively); FVC, FEV1.0, PEF all reduced with both 9- and 11-kg body armor; MVV reduced with 11-kg body armor; FEV1.0:FVC ratio increased with both 9- and 11-kg body armor |
| Bygrave et al. [11] | 12 healthy males | 15-kg weighted backpack—in “loose fit” and “tight fit” configurations; pulmonary function data only (no exercise) | Loose fit reduced FVC (−3.6%), FEV1.0 (−4.3%), and FEF25–75 (−8.4%); Tight fit reduced FVC (−8.1%), FEV1.0 (−9.1%), and FEF25–75 (−21%) |
| Legg and Cruz [62] | 13 university students (4 male, 9 female) | 6-kg weighted backpack—either with one or two shoulder straps worn across the shoulder and chest; pulmonary function data only (no exercise) | Single strap reduced FVC by 3.94%, no differences in FEV1.0 or PEF; Double strap reduced FVC by 1.94%, no differences in FEV1.0 or PEF |
| Ricciardi et al. [88] | 34 military personnel (17 male, 17 female) | 10-kg body armor; 30-min treadmill exercise at varying speeds and grades; stair stepping, pullup (male), and flexed arm hang (female) tests | Elevated VO2, blood lactate, heart rate, and perceived exertion with body armor compared to unloaded exercise; Reduction in ability to perform pull-ups, reduced hang time, and reduction in stair stepping performance with body armor |
| Tomczak et al. [109] | 7 healthy males | Inelastic chest wall restriction (via three or four inelastic canvas straps, 4 and 6 inches wide); 10-min cycling at 45% of maximum power output | Work of breathing and dyspnea increased with chest wall restriction; reduction in diaphragm contractility with chest wall restriction |
| Dominelli et al. [23] | 7 healthy males | 15-, 25-, and 35-kg weighted backpacks;2.5 min at 4.0 km h−1 at 10% grade followed by 2.5 min at 4.0 km h−1 at 15% grade | FVC reduced by 3, 5, and 8% with 15-, 25-, and 35-kg weighted backpacks, respectively; power of breathing increased with 35-kg weighted backpack compared to unloaded (88 ± 0.0 vs. 32 ± 4.3 J min−1, respectively); EELV decreased progressively with increasing pack weight |
| Faghy and Brown [27] | 19 physically active males | 25-kg weighted backpack; 60-min treadmill exercise at 0% grade, 6.5 km h−1 speed followed by 15-min rest, then 2.4-km treadmill running time trial | 11% reduction in PImax following constant-load exercise; further reduction of 5% following pre-loaded time trial; time trial performance 30 ± 5% slower with load carriage |
| Faghy and Brown [28] | 8 healthy males, experienced with regular recreational load carriage activities | 25-kg weighted backpack; 60-min treadmill exercise at 0% grade, 6.5 km h−1 speed followed by 15-min rest, then 2.4-km treadmill running time trial | 9 ± 7 cmH2O reduction in PImax following constant-load exercise; further reduction of 6 ± 16 cmH2O following pre-loaded time trial |
| Faghy and Brown [29] | 19 physically active males | 25-kg weighted backpack; 60-min treadmill exercise at 0% grade, 6.5 km h−1 speed followed by 15-min rest, then 2.4-km treadmill running time trial | 13 ± 7% reduction in PImax following constant-load exercise; 16 ± 8% reduction in PImax post-time trial |
| Peoples et al. [79] | 12 endurance-trained males | 22-kg weighted vest; incremental treadmill test and steady-state exercise trials from 30 to 80% of peak aerobic power | Maximal work tolerance reduced when loaded (13.44 ± 0.68 min vs. 17.21 ± 0.93 min unloaded control); maximal acceptable work duration reduced with load carriage |
| Phillips et al. [80] | 15 healthy, active males | 25-kg weighted backpack; 45-min treadmill walking at matched oxygen demand between loaded and unloaded exercise | Increased fb and VE (+ 21.7 and + 15.1%, respectively); VT and EILV (−6.3 and −6.4%, respectively); post-exercise reduction in PImax of 6.7% |
| Phillips et al. [81] | 50 healthy, active males | 25-kg weighted backpack; Incremental treadmill test | Significantly reduced VO2 at ventilatory threshold (−3.9%) and at peak exercise (−2.5%), and test duration (−29.5%) |
| Phillips et al. [82] | 19 healthy, active males | 45-kg weighted backpack; incremental treadmill test; then 15-, 30-, and 45-kg weighted backpacks; 10-min treadmill walking at 1.34 m s−1 and 4% grade | Significant reduction in VO2max and EILV, but EELV, fb, VT unchanged with 45-kg load carriage during incremental exercise; VO2 increased during submaximal exercise by 11% (unloaded to 15 kg), 14.5% (15–30 kg), and 18.0% (30–45 kg) |
Inspiratory Muscle Training and Load Carriage
Despite the potential benefits of IMT on LC exercise, only two studies to date have sought to identify whether respiratory muscle training improves physical performance during LC [29, 105]. In the first study [105], military personnel accustomed to wearing body armor completed 6 weeks of daily inspiratory muscle training (2 × 30 breath cycles set at 90% of maximal inspiratory mouth pressure). No differences were observed in endurance performance and V̇O2max between the IMT and control groups following the training period. However, several methodological limitations of this study may, in part, explain their findings. First, the exercise testing was performed without any LC; therefore the data give no indication whether respiratory muscle training affects performance with LC. Given that LC is documented to further elevate V̇E compared with unloaded exercise at the same workload, the additional respiratory muscle work required with LC is likely a significant factor that is unaccounted for in this study. Similarly, the pulmonary function testing in this study was carried out with no LC; so again, no data were available to demonstrate the effect of IMT on pulmonary function during LC. Finally, the training intervention differed from previous protocols (such as the established pressure-threshold protocol described above), therefore comparisons cannot be made.
An additional confounding factor may be that these personnel were accustomed to wearing body armor. The authors did not specify whether the subjects regularly engaged in exercise while wearing body armor. The subjects were described as German Special Forces Police Squad members so it is reasonable to surmise that they regularly participated in training operations, which likely involved exercise while wearing body armor. Their habituation to exercise while wearing body armor could be an important consideration because it has been previously documented that elite swimmers whose training volume and intensity is significantly greater than that of sub-elite swimmers, do not benefit from additional IMT combined with their swim training [68]. This may be due in part to the immersion of the thoracic cavity in water, which increases the hydrostatic pressure on the thorax and opposes the generation of force by the inspiratory muscles [18]. This additional stress may stimulate adaptations in the inspiratory muscles because of the additional force (pressure) generation that is required in water-based exercise compared to land-based exercise [39, 95]. Regularly exercising while wearing body armor may generate a similar stress on the inspiratory muscles due to the restriction of chest wall movement and additional mass loading, which could lessen the training effect of IMT.
It is important to note, however, that the specific stress placed upon the inspiratory muscles alone determines their adaptive response to the training stimuli. In the case of swimmers, recent data have demonstrated that IMT does improve respiratory muscle function in sub-elite swimmers whose training volume and intensity are significantly lower than those of elite swimmers [102]. This finding is in contrast to those of Mickleborough et al. [68], and highlights the importance of the specific stressors to which the inspiratory muscles are subjected. Therefore, although the subjects in the [105] study may have regularly participated in exercise with body armor, it is possible that the volume and/or intensity of that exercise was not significant enough to induce training adaptations in the inspiratory muscles. To date, no investigations have demonstrated an effect of LC exercise on inspiratory muscle adaptations. Despite this, the regular exercise routine of subject populations deserves consideration, as it could be a confounding factor.
The second study investigating the effects of IMT and LC exercise studied 19 healthy, physically active males who were habituated to recreational LC activities [29]. The authors found that 6 weeks of pressure-threshold IMT (2 × 30 breath cycles daily set at 50% of PImax) attenuated the cardiovascular and perceptual responses to steady-state exercise and improved running time trial performance while wearing a 25-kg backpack. They also showed no reduction in the fatigue-induced fall in maximal inspiratory pressure following LC exercise, although the magnitude of PImax during and following LC exercise was greater after the IMT intervention. Despite these novel findings, several key limitations ought to be considered. Two key limitations are the use of a treadmill for the running time trial and the decision to use a placebo that subjects were told mimicked altitude training, rather than a true sham IMT placebo. These critical flaws greatly reduce the merits of the study because maximal self-paced time trial exercise is difficult to mimic on a treadmill, which requires manual adjustments to increase or decrease the belt speed.
The other key flaw, the decision to tell placebo subjects that their training mimicked altitude training, introduced unnecessary experimental error. Employing an appropriate experimental control condition is an important consideration in study design. The choice of placebo in this study [29] is particularly concerning because alternative sham IMT placebo protocols that do not elicit any changes in inspiratory muscle function are well described in the literature [13, 91]. Therefore, the findings of this recent study [29], although novel, do not demonstrate significant internal and external validity when considered in the context of the limitations of the study. Thus, further investigation is warranted to confirm these findings and better describe the effects of IMT on LC exercise tolerance. Specifically, these investigations should aim to employ appropriate placebo controls as well as non-volitional means of assessing respiratory pressures.
Conclusions
Thoracic LC exercise has a distinct, deleterious effect on exercise performance and capacity. Specifically, the cardiovascular and pulmonary effects of thoracic LC are unique compared with other modes of exercise and impose additional stress on the cardiopulmonary system during exercise compared with intensity-matched unloaded exercise. Future studies should aim to describe specific impacts of thoracic LC on factors such as diaphragmatic fatigue, which may open new avenues by which targeted training may ameliorate some of the deleterious effects of thoracic LC on exercise performance and capacity.
Acknowledgments
R.-J.S. is supported by 5T32HL105346-08 from the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health.
Footnotes
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Armstrong NC, Gay LA. The effect of flexible body armour on pulmonary function. Ergonomics. 2016;59(5):692–696. doi: 10.1080/00140139.2015.1084052. https://doi.org/10.1080/00140139.2015.1084052. [DOI] [PubMed] [Google Scholar]
- 2.European Respiratory Society, and American Thoracic Society. ATS/ERS statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. https://doi.org/10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 3.Babcock MA, Johnson BD, Pegelow DF, Suman OE, Griffin D, Dempsey JA. Hypoxic effects on exercise-induced diaphragmatic fatigue in normal healthy humans. J Appl Physiol. 1995;78(1):82–92. doi: 10.1152/jappl.1995.78.1.82. [DOI] [PubMed] [Google Scholar]
- 4.Babcock MA, Pegelow DF, McClaran SR, Suman OE, Dempsey JA. Contribution of diaphragmatic power output to exercise-induced diaphragm fatigue. J Appl Physiol. 1995;78(5):1710–1719. doi: 10.1152/jappl.1995.78.5.1710. [DOI] [PubMed] [Google Scholar]
- 5.Bastien GJ, Willems PA, Schepens B, Heglund NC. Effect of load and speed on the energetic cost of human walking. Eur J Appl Physiol. 2005;94(1–2):76–83. doi: 10.1007/s00421-004-1286-z. https://doi.org/10.1007/s00421-004-1286-z. [DOI] [PubMed] [Google Scholar]
- 6.Birrell SA, Haslam RA. The effect of load distribution within military load carriage systems on the kinetics of human gait. Appl Ergon. 2010;41(4):585–590. doi: 10.1016/j.apergo.2009.12.004. https://doi.org/10.1016/j.apergo.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Borg GAV. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 8.Boutellier U, Büchel R, Kundert A, Spengler C. The respiratory system as an exercise limiting factor in normal trained subjects. Eur J Appl Physiol Occup Physiol. 1992;65(4):347–353. doi: 10.1007/BF00868139. https://doi.org/10.1007/BF00868139. [DOI] [PubMed] [Google Scholar]
- 9.Boutellier U, Piwko P. The respiratory system as an exercise limiting factor in normal sedentary subjects. Eur J Appl Physiol Occup Physiol. 1992;64(2):145–152. doi: 10.1007/BF00717952. https://doi.org/10.1007/BF00717952. [DOI] [PubMed] [Google Scholar]
- 10.Brown PI, McConnell AK. Respiratory-related limitations in physically demanding occupations. Aviat Space Environ Med. 2012;83(4):424–430. doi: 10.3357/asem.3163.2012. https://doi.org/10.3357/ASEM.3163.2012. [DOI] [PubMed] [Google Scholar]
- 11.Bygrave S, Legg SJ, Myers S, Llewellyn M. Effect of backpack fit on lung function. Ergonomics. 2004;47(3):324–329. doi: 10.1080/0014013031000157869. https://doi.org/10.1080/0014013031000157869. [DOI] [PubMed] [Google Scholar]
- 12.Cafarelli E. Peripheral contributions to the perception of effort. Med Sci Sports Exerc. 1982;14(5):382–389. [PubMed] [Google Scholar]
- 13.Caine MP, McConnell AK. Development and evaluation of a pressure threshold inspiratory muscle trainer for use in the context of sports performance. Sports Eng. 2000;3(3):149–159. https://doi.org/10.1046/j.1460-2687.2000.00047.x. [Google Scholar]
- 14.Calverley PMA, Koulouris NG. Flow limitation and dynamic hyperinflation: key concepts in modern respiratory physiology. Eur Respir J. 2005;25(1):186–199. doi: 10.1183/09031936.04.00113204. https://doi.org/10.1183/09031936.04.00113204. [DOI] [PubMed] [Google Scholar]
- 15.Chapman RF, Emery M, Stager JM. Extent of expiratory flow limitation influences the increase in maximal exercise ventilation in hypoxia. Respir Physiol. 1998;113(1):65–74. doi: 10.1016/s0034-5687(98)00043-7. https://doi.org/10.1016/S0034-5687(98)00043-7. [DOI] [PubMed] [Google Scholar]
- 16.Chatham K, Baldwin J, Griffiths H, Summers L, Enright S. Inspiratory muscle training improves shuttle run performance in healthy subjects. Physiotherapy. 1999;85(12):676–683. https://doi.org/10.1016/S0031-9406(05)61231-X. [Google Scholar]
- 17.Chiappa GR, Roseguini BT, Vieira PJC, Alves CN, Tavares A, Winkelmann ER, Ferlin EL, Stein R, Ribeiro JP. Inspiratory muscle training improves blood flow to resting and exercising limbs in patients with chronic heart failure. J Am Coll Cardiol. 2008;51(17):1663–1671. doi: 10.1016/j.jacc.2007.12.045. https://doi.org/10.1016/j.jacc.2007.12.045. [DOI] [PubMed] [Google Scholar]
- 18.Cordain L, Stager J. Pulmonary structure and function in swimmers. Sports Med. 1988;6(5):271–278. doi: 10.2165/00007256-198806050-00002. [DOI] [PubMed] [Google Scholar]
- 19.Dempsey JA. J.B. Wolffe memorial lecture. Is the lung built for exercise? Med Sci Sports Exerc. 1986;18(2):143–155. [PubMed] [Google Scholar]
- 20.Depsey JA, Adams L, Ainsworth DM, Fregosi RF, Gallagher CG, Guz A, Johnson BD, Powers SK. Airway, lung, and respiratory muscle function during exercise. In: Rowell LB, Shepard JT, editors. Handbook of physiology. Section 12. Exercise: regulation and integration of multiple systems. New York: Oxford University Press; 1996. pp. 448–514. [Google Scholar]
- 21.Dempsey JA, Romer L, Rodman J, Miller J, Smith C. Consequences of exercise-induced respiratory muscle work. Respir Physiol Neurobiol. 2006;151(2–3):242–250. doi: 10.1016/j.resp.2005.12.015. https://doi.org/10.1016/j.resp.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 22.Dempsey JA, Wagner PD. Exercise-induced arterial hypoxemia. J Appl Physiol. 1999;87(6):1997–2006. doi: 10.1152/jappl.1999.87.6.1997. [DOI] [PubMed] [Google Scholar]
- 23.Dominelli PB, Sheel AW, Foster GE. Effect of carrying a weighted backpack on lung mechanics during treadmill walking in healthy men. Eur J Appl Physiol. 2012;112(6):2001–2012. doi: 10.1007/s00421-011-2177-8. https://doi.org/10.1007/s00421-011-2177-8. [DOI] [PubMed] [Google Scholar]
- 24.Dominelli PB, Render JN, Molgat-Seon Y, Foster GE, Sheel AW. Precise mimicking of exercise hyperpnea to investigate the oxygen cost of breathing. Respir Physiol Neurobiol. 2014;201:15–23. doi: 10.1016/j.resp.2014.06.010. https://doi.org/10.1016/j.resp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 25.Downey AE, Chenoweth LM, Townsend DK, Ranum JD, Ferguson CS, Harms CA. Effects of inspiratory muscle training on exercise responses in normoxia and hypoxia. Respir Physiol Neurobiol. 2007;156(2):137–146. doi: 10.1016/j.resp.2006.08.006. https://doi.org/10.1016/j.resp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 26.Enright SJ, Unnithan VB, Heward C, Withnall L, Davies DH. Effect of high-intensity inspiratory muscle training on lung volumes, diaphragm thickness, and exercise capacity in subjects who are healthy. Phys Ther. 2006;86(3):345–354. [PubMed] [Google Scholar]
- 27.Faghy M, Brown P. Thoracic load carriage-induced respiratory muscle fatigue. Eur J Appl Physiol. 2014;114(5):1085–1093. doi: 10.1007/s00421-014-2839-4. https://doi.org/10.1007/s00421-014-2839-4. [DOI] [PubMed] [Google Scholar]
- 28.Faghy MA, Brown PI. Preloaded time trial to assess load carriage performance. J Strength Cond Res. 2014;28(12):3354–3362. doi: 10.1519/JSC.0000000000000555. https://doi.org/10.1519/jsc.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 29.Faghy MA, Brown PI. Training the inspiratory muscles improves running performance when carrying a 25 kg thoracic load in a backpack. Eur J Sport Sci. 2016;16(5):585–594. doi: 10.1080/17461391.2015.1071878. https://doi.org/10.1080/17461391.2015.1071878. [DOI] [PubMed] [Google Scholar]
- 30.Faghy MA, Blacker S, Brown PI. Effects of load mass carried in a backpack upon respiratory muscle fatigue. Eur J Sport Sci. 2016;16(8):1032–1038. doi: 10.1080/17461391.2016.1202326. https://doi.org/10.1080/17461391.2016.1202326. [DOI] [PubMed] [Google Scholar]
- 31.Farkas GA, Cerny FJ, Rochester DF. Contractility of the ventilatory pump muscles. Med Sci Sports Exerc. 1996;28(9):1106–1114. doi: 10.1097/00005768-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald RS, Hauer MC, Bierkamper GG, Raff H. Responses of in vitro rat diaphragm to changes in acid-base environment. J Appl Physiol. 1984;57(4):1202–1210. doi: 10.1152/jappl.1984.57.4.1202. [DOI] [PubMed] [Google Scholar]
- 33.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725–1789. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 34.Gething AD, Williams M, Davies B. Inspiratory resistive loading improves cycling capacity: a placebo controlled trial. Br J Sport Med. 2004;38(6):730–736. doi: 10.1136/bjsm.2003.007518. https://doi.org/10.1136/bjsm.2003.007518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goldman RF, Iampietro PF. Energy cost of load carriage. J Appl Physiol. 1962;17(4):675–676. doi: 10.1152/jappl.1962.17.4.675. [DOI] [PubMed] [Google Scholar]
- 36.Grenier JG, Peyrot N, Castells J, Oullion R, Messonnier L, Morin JB. Energy cost and mechanical work of walking during load carriage in soldiers. Med Sci Sports Exerc. 2012;44(6):1131–1140. doi: 10.1249/MSS.0b013e3182456057. https://doi.org/10.1249/MSS.0b013e3182456057. [DOI] [PubMed] [Google Scholar]
- 37.Guenette JA, Sheel AW. Physiological consequences of a high work of breathing during heavy exercise in humans. J Sci Med Sport. 2007;10(6):341–350. doi: 10.1016/j.jsams.2007.02.003. https://doi.org/10.1016/j.jsams.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 38.Guenette JA, Witt JD, McKenzie DC, Road JD, Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol. 2007;581(3):1309–1322. doi: 10.1113/jphysiol.2006.126466. https://doi.org/10.1113/jphysiol.2006.126466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hamilton P, Andrew GM. Influence of growth and athletic training on heart and lung functions. Eur J Appl Physiol Occup Physiol. 1976;36(1):27–38. doi: 10.1007/BF00421631. https://doi.org/10.1007/BF00421631. [DOI] [PubMed] [Google Scholar]
- 40.Hanson P, Claremont A, Dempsey J, Reddan W. Determinants and consequences of ventilatory responses to competitive endurance running. J Appl Physiol. 1982;52(3):615–623. doi: 10.1152/jappl.1982.52.3.615. [DOI] [PubMed] [Google Scholar]
- 41.Harms CA. Insights into the role of the respiratory muscle metaboreflex. J Physiol. 2007;584(3):711. doi: 10.1113/jphysiol.2007.145540. https://doi.org/10.1113/jphysiol.2007.145540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82(5):1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 43.Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P, Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998;85(2):609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- 44.Harms CA, Wetter TJ, St Croix CM, Pegelow DF, Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89(1):131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- 45.Henke KG, Sharratt M, Pegelow D, Dempsey JA. Regulation of end-expiratory lung volume during exercise. J Appl Physiol. 1988;64(1):135–146. doi: 10.1152/jappl.1988.64.1.135. [DOI] [PubMed] [Google Scholar]
- 46.Hill JM. Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res. 2000;856(1–2):240–244. doi: 10.1016/s0006-8993(99)02366-5. https://doi.org/10.1016/S0006-8993(99)02366-5. [DOI] [PubMed] [Google Scholar]
- 47.Holm P, Sattler A, Fregosi R. Endurance training of respiratory muscles improves cycling performance in fit young cyclists. BMC Physiol. 2004;4(1):1–14. doi: 10.1186/1472-6793-4-9. https://doi.org/10.1186/1472-6793-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang CH, Martin AD, Davenport PW. Effect of inspiratory muscle strength training on inspiratory motor drive and RREP early peak components. J Appl Physiol. 2003;94(2):462–468. doi: 10.1152/japplphysiol.00364.2002. https://doi.org/10.1152/japplphysiol.00364.2002. [DOI] [PubMed] [Google Scholar]
- 49.Huang T-wP, Kuo AD. Mechanics and energetics of load carriage during human walking. J Exp Biol. 2014;217(4):605–613. doi: 10.1242/jeb.091587. https://doi.org/10.1242/jeb.091587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Illi S, Held U, Frank I, Spengler C. Effect of respiratory muscle training on exercise performance in healthy individuals. Sports Med. 2012;42(8):707–724. doi: 10.1007/BF03262290. https://doi.org/10.1007/BF03262290. [DOI] [PubMed] [Google Scholar]
- 51.Janssens L, Brumagne S, McConnell AK, Raymaekers J, Goossens N, Gayan-Ramirez G, Hermans G, Troosters T. The assessment of inspiratory muscle fatigue in healthy individuals: a systematic review. Respir Med. 2013;107(3):331–346. doi: 10.1016/j.rmed.2012.11.019. https://doi.org/10.1016/j.rmed.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 52.Jenkins SC, Moxham J. The effects of mild obesity on lung function. Respir Med. 1991;85(4):309–311. doi: 10.1016/s0954-6111(06)80102-2. https://doi.org/10.1016/S0954-6111(06)80102-2. [DOI] [PubMed] [Google Scholar]
- 53.Johnson BD, Babcock MA, Suman OE, Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol. 1993;460(1):385–405. doi: 10.1113/jphysiol.1993.sp019477. https://doi.org/10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol. 1992;73(3):874–886. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- 55.Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest. 1999;116(2):488–503. doi: 10.1378/chest.116.2.488. https://doi.org/10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- 56.Kabitz HJ, Walker D, Walterspacher S, Windisch W. Controlled twitch mouth pressure reliably predicts twitch esophageal pressure. Respir Physiol Neurobiol. 2007;156(3):276–282. doi: 10.1016/j.resp.2006.10.007. https://doi.org/10.1016/j.resp.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 57.Kayser B. Exercise starts and ends in the brain. Eur J Appl Physiol. 2003;90(3–4):411–419. doi: 10.1007/s00421-003-0902-7. https://doi.org/10.1007/s00421-003-0902-7. [DOI] [PubMed] [Google Scholar]
- 58.Kilding A, Brown S, McConnell A. Inspiratory muscle training improves 100 and 200 m swimming performance. Eur J Appl Physiol. 2010;108(3):505–511. doi: 10.1007/s00421-009-1228-x. https://doi.org/10.1007/s00421-009-1228-x. [DOI] [PubMed] [Google Scholar]
- 59.Knapik J, Harman E, Reynolds K. Load carriage using packs: a review of physiological, biomechanical and medical aspects. Appl Ergon. 1996;27(3):207–216. doi: 10.1016/0003-6870(96)00013-0. https://doi.org/10.1016/0003-6870(96)00013-0. [DOI] [PubMed] [Google Scholar]
- 60.Leddy J, Limprasertkul A, Patel S, Modlich F, Buyea C, Pendergast D, Lundgren CG. Isocapnic hyperpnea training improves performance in competitive male runners. Eur J Appl Physiol. 2007;99(6):665–676. doi: 10.1007/s00421-006-0390-7. https://doi.org/10.1007/s00421-006-0390-7. [DOI] [PubMed] [Google Scholar]
- 61.Legg SJ. Influence of body armour on pulmonary function. Ergonomics. 1988;31(3):349–353. doi: 10.1080/00140138808966679. https://doi.org/10.1080/00140138808966679. [DOI] [PubMed] [Google Scholar]
- 62.Legg SJ, Cruz CO. Effect of single and double strap backpacks on lung function. Ergonomics. 2004;47(3):318–323. doi: 10.1080/0014013032000157878. https://doi.org/10.1080/0014013032000157878. [DOI] [PubMed] [Google Scholar]
- 63.Mainwood GW, Renaud JM. The effect of acid–base balance on fatigue of skeletal muscle. Can J Physiol Pharm. 1985;63(5):403–416. doi: 10.1139/y85-072. https://doi.org/10.1139/y85-072. [DOI] [PubMed] [Google Scholar]
- 64.Majumdar D, Srivastava KK, Purkayastha SS, Pichan G, Selvamurthy W. Physiological effects of wearing heavy body armour on male soldiers. Int J Ind Ergon. 1997;20(2):155–161. https://doi.org/10.1016/S0169-8141(96)00057-1. [Google Scholar]
- 65.McConnell AK. CrossTalk opposing view: respiratory muscle training does improve exercise tolerance. J Physiol. 2012;590(15):3397–3398. doi: 10.1113/jphysiol.2012.235572. https://doi.org/10.1113/jphysiol.2012.235572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McEntire SJ, Smith JR, Ferguson CS, Brown KR, Kurti SP, Harms CA. The effect of exercise training with an additional inspiratory load on inspiratory muscle fatigue and time-trial performance. Respir Physiol Neurobiol. 2016;230:54–59. doi: 10.1016/j.resp.2016.05.001. https://doi.org/10.1016/j.resp.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 67.McMahon ME, Boutellier U, Smith RM, Spengler CM. Hyperpnea training attenuates peripheral chemosensitivity and improves cycling endurance. J Exp Biol. 2002;205(24):3937–3943. doi: 10.1242/jeb.205.24.3937. [DOI] [PubMed] [Google Scholar]
- 68.Mickleborough T, Stager J, Chatham K, Lindley M, Ionescu A. Pulmonary adaptations to swim and inspiratory muscle training. Eur J Appl Physiol. 2008;103(6):635–646. doi: 10.1007/s00421-008-0759-x. https://doi.org/10.1007/s00421-008-0759-x. [DOI] [PubMed] [Google Scholar]
- 69.Mickleborough TD, Nichols T, Lindley MR, Chatham K, Ionescu AA. Inspiratory flow resistive loading improves respiratory muscle function and endurance capacity in recreational runners. Scan J Med Sci Sports. 2010;20(3):458–468. doi: 10.1111/j.1600-0838.2009.00951.x. https://doi.org/10.1111/j.1600-0838.2009.00951.x. [DOI] [PubMed] [Google Scholar]
- 70.Milic-Emili J, Mead J, Turner JM, Glauser EM. Improved technique for estimating pleural pressure from esophageal balloons. J Appl Physiol. 1964;19(2):207–211. doi: 10.1152/jappl.1964.19.2.207. [DOI] [PubMed] [Google Scholar]
- 71.Miller JD, Beck KC, Joyner MJ, Brice AG, Johnson BD. Cardiorespiratory effects of inelastic chest wall restriction. J Appl Physiol. 2002;92(6):2419–2428. doi: 10.1152/japplphysiol.00394.2001. https://doi.org/10.1152/japplphysiol.00394.2001. [DOI] [PubMed] [Google Scholar]
- 72.Muza SR, Latzka WA, Epstein Y, Pandolf KB. Load carriage induced alterations of pulmonary function. Int J Ind Ergon. 1989;3(3):221–227. https://doi.org/10.1016/0169-8141(89)90021-8. [Google Scholar]
- 73.Noakes TD. Fatigue is a brain-derived emotion that regulates the exercise behavior to ensure the protection of whole body homeostasis. Front Physiol. 2012;3(82):1–13. doi: 10.3389/fphys.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Noakes TD, St Clair Gibson A. Logical limitations to the “catastrophe” models of fatigue during exercise in humans. Br J Sport Med. 2004;38:648–649. doi: 10.1136/bjsm.2003.009761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Noakes TD, St Clair Gibson A, Lambert EV. From catastrophe to complexity: a novel model of integrative central neural regulation of effort and fatigue during exercise in humans: summary and conclusions. Br J Sport Med. 2005;39:120–124. doi: 10.1136/bjsm.2003.010330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O’Donnell DE, Hong HH, Webb KA. Respiratory sensation during chest wall restriction and dead space loading in exercising men. J Appl Physiol. 2000;88(5):1859–1869. doi: 10.1152/jappl.2000.88.5.1859. [DOI] [PubMed] [Google Scholar]
- 77.Olfert IM. Exercise and the lungs: nature or nurture? J Physiol. 2016;594(18):5037–5038. doi: 10.1113/JP272370. https://doi.org/10.1113/JP272370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel MS, Hart N, Polkey MI. CrossTalk proposal: training the respiratory muscles does not improve exercise tolerance. J Physiol. 2012;590(15):3393–3395. doi: 10.1113/jphysiol.2012.235408. https://doi.org/10.1113/jphysiol.2012.235408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Peoples GE, Lee DS, Notley SR, Taylor NAS. The effects of thoracic load carriage on maximal ambulatory work tolerance and acceptable work durations. Eur J Appl Physiol. 2016;116(3):635–646. doi: 10.1007/s00421-015-3323-5. https://doi.org/10.1007/s00421-015-3323-5. [DOI] [PubMed] [Google Scholar]
- 80.Phillips DB, Stickland MK, Petersen SR. Ventilatory responses to prolonged exercise with heavy load carriage. Eur J Appl Physiol. 2016;116(1):19–27. doi: 10.1007/s00421-015-3240-7. https://doi.org/10.1007/s00421-015-3240-7. [DOI] [PubMed] [Google Scholar]
- 81.Phillips DB, Stickland MK, Lesser IA, Petersen SR. The effects of heavy load carriage on physiological responses to graded exercise. Eur J Appl Physiol. 2016;116(2):275–280. doi: 10.1007/s00421-015-3280-z. https://doi.org/10.1007/s00421-015-3280-z. [DOI] [PubMed] [Google Scholar]
- 82.Phillips DB, Ehnes CM, Stickland MK, Petersen SR. The impact of thoracic load carriage up to 45 kg on the cardiopulmonary response to exercise. Eur J Appl Physiol. 2016;116(9):1725–1734. doi: 10.1007/s00421-016-3427-6. https://doi.org/10.1007/s00421-016-3427-6. [DOI] [PubMed] [Google Scholar]
- 83.Phillips DB, Stickland MK, Petersen SR. Physiological and performance consequences of heavy thoracic load carriage in females. Appl Physiol Nutr Metab. 2016;41(7):741–748. doi: 10.1139/apnm-2016-0002. https://doi.org/10.1139/apnm-2016-0002. [DOI] [PubMed] [Google Scholar]
- 84.Prefaut C, Durand F, Mucci P, Caillaud C. Exercise-induced arterial hypoxaemia in athletes. Sports Med. 2000;30(1):47–61. doi: 10.2165/00007256-200030010-00005. https://doi.org/10.2165/00007256-200030010-00005. [DOI] [PubMed] [Google Scholar]
- 85.Prigent H, Orlikowski D, Fermanian C, Lejaille M, Falaize L, Louis A, Faroux B, Lofaso F. Sniff and Muller manoeuvres to measure diaphragmatic muscle strength. Respir Med. 2008;102(12):1737–1743. doi: 10.1016/j.rmed.2008.07.004. https://doi.org/10.1016/j.rmed.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 86.Pringle JS, Doust JH, Carter H, Tolfrey K, Campbell IT, Sakkas GK, Jones AM. Oxygen uptake kinetics during moderate, heavy and severe intensity “submaximal” exercise in humans: the influence of muscle fibre type and capillarisation. Eur J Appl Physiol. 2003;89(3–4):289–300. doi: 10.1007/s00421-003-0799-1. https://doi.org/10.1007/s00421-003-0799-1. [DOI] [PubMed] [Google Scholar]
- 87.Ramírez-Sarmiento A, Orozco-Levi M, Güell R, Barreiro E, Hernandez N, Mota S, Sangenis M, Broquetas JM, Casan P, Gea J. Inspiratory muscle training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(11):1491–1497. doi: 10.1164/rccm.200202-075OC. https://doi.org/10.1164/rccm.200202-075OC. [DOI] [PubMed] [Google Scholar]
- 88.Ricciardi R, Deuster PA, Talbot LA. Metabolic demands of body armor on physical performance in simulated conditions. Mil Med. 2008;173(9):817–824. doi: 10.7205/milmed.173.9.817. [DOI] [PubMed] [Google Scholar]
- 89.Rodman JR, Henderson KS, Smith CA, Dempsey JA. Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol. 2003;95(3):1159–1169. doi: 10.1152/japplphysiol.00258.2003. https://doi.org/10.1152/japplphysiol.00258.2003. [DOI] [PubMed] [Google Scholar]
- 90.Romer LM, Haverkamp HC, Lovering AT, Pegelow DF, Dempsey JA. Effect of exercise-induced arterial hypoxemia on quadriceps muscle fatigue in healthy humans. Am J Physiol. 2006;290(2):R365–R375. doi: 10.1152/ajpregu.00332.2005. https://doi.org/10.1152/ajpregu.00332.2005. [DOI] [PubMed] [Google Scholar]
- 91.Romer LM, McConnell AK. Specificity and reversibility of inspiratory muscle training. Med Sci Sports Exerc. 2003;35(2):237–244. doi: 10.1249/01.MSS.0000048642.58419.1E. [DOI] [PubMed] [Google Scholar]
- 92.Romer LM, McConnell AK, Jones DA. Effects of inspiratory muscle training on time-trial performance in trained cyclists. J Sport Sci. 2002;20(7):547–590. doi: 10.1080/026404102760000053. https://doi.org/10.1080/026404102760000053. [DOI] [PubMed] [Google Scholar]
- 93.Romer LM, McConnell AK, Jones DA. Inspiratory muscle fatigue in trained cyclists: effects of inspiratory muscle training. Med Sci Sports Exerc. 2002;34(5):785–792. doi: 10.1097/00005768-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 94.Romer LM, Polkey MI. Exercise-induced respiratory muscle fatigue: implications for performance. J Appl Physiol. 2008;104(3):879–888. doi: 10.1152/japplphysiol.01157.2007. https://doi.org/10.1152/japplphysiol.01157.2007. [DOI] [PubMed] [Google Scholar]
- 95.Roussos C, Fixley M, Gross D, Macklem PT. Fatigue of inspiratory muscles and their synergic behavior. J Appl Physiol. 1979;46(5):897–904. doi: 10.1152/jappl.1979.46.5.897. [DOI] [PubMed] [Google Scholar]
- 96.Sharp JT, Barrocas M, Chokroverty S. The cardiorespiratory effects of obesity. Clin Chest Med. 1980;1(1):103–118. [PubMed] [Google Scholar]
- 97.Sharratt MT, Henke KG, Aaron EA, Pegelow DF, Dempsey JA. Exercise-induced changes in functional residual capacity. Respir Physiol. 1987;70(3):313–326. doi: 10.1016/0034-5687(87)90013-2. https://doi.org/10.1016/0034-5687(87)90013-2. [DOI] [PubMed] [Google Scholar]
- 98.Sheel AW. Respiratory muscle training in healthy individuals. Sports Med. 2002;32(9):567–581. doi: 10.2165/00007256-200232090-00003. https://doi.org/10.2165/00007256-200232090-00003. [DOI] [PubMed] [Google Scholar]
- 99.Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537(1):277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. https://doi.org/10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shei R-J, Mickleborough TD. Relative contributions of central and peripheral factors in human muscle fatigue during exercise: a brief review. J Exerc Physiol. 2013;16(6):1–17. [Google Scholar]
- 101.Shei R-J, Paris HLR, Wilhite DP, Chapman RF, Mickleborough TD. The role of inspiratory muscle training in the management of asthma and exercise-induced bronchoconstriction. Phys Sportsmed. 2016;44(4):327–334. doi: 10.1080/00913847.2016.1176546. https://doi.org/10.1080/00913847.2016.1176546. [DOI] [PubMed] [Google Scholar]
- 102.Shei R-J, Lindley M, Chatham K, Mickleborough TD. Effect of flow-resistive inspiratory loading on pulmonary and respiratory muscle function in sub-elite swimmers. J Sports Med Phys Fitness. 2016;56(4):392–398. [PubMed] [Google Scholar]
- 103.Shirley D, Hodges PW, Eriksson AEM, Gandevia SC. Spinal stiffness changes throughout the respiratory cycle. J Appl Physiol. 2003;95(4):1467–1475. doi: 10.1152/japplphysiol.00939.2002. https://doi.org/10.1152/japplphysiol.00939.2002. [DOI] [PubMed] [Google Scholar]
- 104.Silva IS, Fregonezi GA, Dias FA, Ribeiro CT, Guerra RO, Ferreira GM. Inspiratory muscle training for asthma. Cochrane Database Syst Rev. 2013;9:003792. doi: 10.1002/14651858.CD003792.pub2. https://doi.org/10.1002/14651858.cd003792.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sperlich PB, Fricke H, de Marées M, Linville JW, Mester J. Does respiratory muscle training increase physical performance? Mil Med. 2009;174(9):977–982. doi: 10.7205/milmed-d-04-6408. [DOI] [PubMed] [Google Scholar]
- 106.St Clair Gibson A, Noakes TD. Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br J Sport Med. 2004;38:797–806. doi: 10.1136/bjsm.2003.009852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Taylor NAS, Lewis MC, Notley SR, Peoples GE. A fractionation of the physiological burden of the personal protective equipment worn by firefighters. Eur J Appl Physiol. 2012;112(8):2913–2921. doi: 10.1007/s00421-011-2267-7. https://doi.org/10.1007/s00421-011-2267-7. [DOI] [PubMed] [Google Scholar]
- 108.Taylor NAS, Peoples GE, Petersen SR. Load carriage, human performance, and employment standards. Appl Physiol Nutr Metab. 2016;41(6):S131–S147. doi: 10.1139/apnm-2015-0486. https://doi.org/10.1139/apnm-2015-0486. [DOI] [PubMed] [Google Scholar]
- 109.Tomczak SE, Guenette JA, Reid WD, McKenzie DC, Sheel AW. Diaphragm fatigue after submaximal exercise with chest wall restriction. Med Sci Sports Exerc. 2011;43(3):416–424. doi: 10.1249/MSS.0b013e3181ef5e67. https://doi.org/10.1249/MSS.0b013e3181ef5e67. [DOI] [PubMed] [Google Scholar]
- 110.Turner LA, Mickleborough TD, McConnell AK, Stager JM, Tecklenburg-Lund S, Lindley MR. Effect of inspiratory muscle training on exercise tolerance in asthmatic individuals. Med Sci Sports Exerc. 2011;43(11):2031–2038. doi: 10.1249/MSS.0b013e31821f4090. https://doi.org/10.1249/MSS.0b013e31821f4090. [DOI] [PubMed] [Google Scholar]
- 111.Turner LA, Tecklenburg-Lund SL, Chapman RF, Stager JM, Wilhite DP, Mickleborough TD. Inspiratory muscle training lowers the oxygen cost of voluntary hyperpnea. J Appl Physiol. 2012;112(1):127–134. doi: 10.1152/japplphysiol.00954.2011. https://doi.org/10.1152/japplphysiol.00954.2011. [DOI] [PubMed] [Google Scholar]
- 112.Verges S, Lenherr O, Haner AC, Schulz C, Spengler CM. Increased fatigue resistance of respiratory muscles during exercise after respiratory muscle endurance training. Am J Physiol. 2007;292(3):R1246–R1253. doi: 10.1152/ajpregu.00409.2006. https://doi.org/10.1152/ajpregu.00409.2006. [DOI] [PubMed] [Google Scholar]
- 113.Verges S, Notter D, Spengler CM. Influence of diaphragm and rib cage muscle fatigue on breathing during endurance exercise. Respir Physiol Neurobiol. 2006;154(3):431–442. doi: 10.1016/j.resp.2005.12.007. https://doi.org/10.1016/j.resp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 114.Verges S, Renggli AS, Notter DA, Spengler CM. Effects of different respiratory muscle training regimes on fatigue-related variables during volitional hyperpnoea. Respir Physiol Neurobio. 2009;169(3):282–290. doi: 10.1016/j.resp.2009.09.005. https://doi.org/10.1016/j.resp.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 115.Volianitis S, McConnell AK, Koutedakis Y, McNaughton L, Backx K, Jones DA. Inspiratory muscle training improves rowing performance. Med Sci Sports Exerc. 2001;33(5):803–809. doi: 10.1097/00005768-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 116.Walker RE, Swain DP, Ringleb SI, Colberg SR. Effect of added mass on treadmill performance and pulmonary function. J Strength Cond Res. 2015;29(4):882–888. doi: 10.1519/JSC.0000000000000408. https://doi.org/10.1519/JSC.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 117.Weavil JC, Duke JW, Stickford JL, Stager JM, Chapman RF, Mickleborough TD. Endurance exercise performance in acute hypoxia is influenced by expiratory flow limitation. Eur J Appl Physiol. 2015;115(8):1653–1663. doi: 10.1007/s00421-015-3145-5. https://doi.org/10.1007/s00421-015-3145-5. [DOI] [PubMed] [Google Scholar]
- 118.Witt JD, Guenette JA, Rupert JL, McKenzie DC, Sheel AW. Inspiratory muscle training attenuates the human respiratory muscle metaboreflex. J Physiol. 2007;584(3):1019–1028. doi: 10.1113/jphysiol.2007.140855. https://doi.org/10.1113/jphysiol.2007.140855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wüthrich TU, Eberle E, Spengler CM. Locomotor and diaphragm muscle fatigue in endurance athletes performing time-trials of different durations. Eur J Appl Physiol. 2014;114(8):1619–1633. doi: 10.1007/s00421-014-2889-7. https://doi.org/10.1007/s00421-014-2889-7. [DOI] [PubMed] [Google Scholar]
- 120.Wüthrich TU, Marty J, Kerherve H, Millet GY, Verges S, Spengler CM. Aspects of respiratory muscle fatigue in a mountain ultramarathon race. Med Sci Sports Exerc. 2015;47(3):519–527. doi: 10.1249/MSS.0000000000000449. https://doi.org/10.1249/MSS.0000000000000449. [DOI] [PubMed] [Google Scholar]
- 121.Wylegala J, Pendergast D, Gosselin L, Warkander D, Lundgren CG. Respiratory muscle training improves swimming endurance in divers. Eur J Appl Physiol. 2007;99(4):393–404. doi: 10.1007/s00421-006-0359-6. https://doi.org/10.1007/s00421-006-0359-6. [DOI] [PubMed] [Google Scholar]
- 122.Younes M, Kivinen G. Respiratory mechanics and breathing pattern during and following maximal exercise. J Appl Physiol. 1984;57(6):1773–1782. doi: 10.1152/jappl.1984.57.6.1773. [DOI] [PubMed] [Google Scholar]