Abstract
Breast cancer is the most common malignancy in women worldwide, with a developmental process spanning decades. The malignant cells recruit a variety of cells including fibroblasts, endothelial cells, immune cells, and adipocytes, creating the tumor microenvironment. The tumor microenvironment has emerged as active participants in breast cancer progression and response to treatment through autocrine and paracrine interaction with the malignant cells. Adipose tissue is abundant in the breast cancer microenvironment; interactions with cancer cells create cancer-associated adipocytes which produce a variety of adipokines that influence breast cancer initiation, metastasis, angiogenesis, and cachexia. Interleukin (IL)-6 has emerged as key compound significantly produced by breast cancer cells and adipocytes, with the potential of inducing proliferation, epithelial-mesenchymal phenotype, stem cell phenotype, angiogenesis, cachexia, and therapeutic resistance in breast cancer cells. Our aim is to present a brief knowledge of IL-6’s role in breast cancer. This review summarizes our current understanding of the breast microenvironment, with emphasis on adipocytes as key players in breast cancer tumorigenesis. The effects of key adipocytes such as leptin, adipokines, TGF-b, and IL-6 are discussed. Finally, we discuss the role of IL-6 in various aspects of cancer progression.
Introduction
Breast cancer is the most common malignancy in women worldwide, with nearly 1.7 million new cases diagnosed in 2012 (second most common cancer overall), and the leading cause of cancer-related death in women worldwide. This represents about 12% of all new cancer cases and 25% of all cancers in women [1]. The developmental process spanning decades has a multifactorial etiology and a heterogeneous genetic background. Advances in molecular testing have allowed various markers to be analyzed including the human epidermal receptor 2 (HER2) expression status, and estrogen receptor (ER) and progesterone receptor (PR) status [2], [3]. Localized and early diagnosis of the the disease has better clinical outcome, whereas advanced/metastatic disease usually has an abysmal prognosis despite advances in treatment methods [3]. This has heightened the need to identify new and effective targets for treatment. The stromal cells of the breast cancer microenvironment have emerged as active participants in the development of breast cancer and a potential target for future treatment. The breast cancer microenvironment comprises stromal cells including fibroblasts, endothelial cells, immune cells, and adipocytes with altered phenotype and function from the normal state. The cell-to-cell and cells-to-tumor cell interaction between the cells creates a complex tumor microenvironment (TME) [4], [5]. The stromal cells in the breast cancer microenvironment are not just passive participants but contribute actively to influence disease progression and response to treatment [5]. Paracrine interactions between the stromal cell and malignant cells are the main mechanism by which stromal cells influence tumor cell behavior [5]. Hence, the TME is presently an active area of research, particularly in understanding how the various components influence cancer progression and the possibility of developing novel therapies targeting the microenvironment [5], [6]. The influence of cancer-associated fibroblast (CAF) on breast cancer cells is the most studied microenvironment interaction. These studies reveal significant alteration in genetic and epigenetic signatures in the CAF, which can potentially predict clinical outcomes [7], [8]. These findings have increased interest in the other components in the breast cancer microenvironment and their potential role as prognostic and therapeutic targets. Surprisingly, white adipose tissue [comprising of mature adipocytes and progenitors (preadipocytes and adipose-derived stem cells)], which accounts for 80% of the adult breast volume and forms the site of early local invasion of breast cancer cells, has received relatively little attention [9], [10]. The emergence of the endocrine function of adipocytes, i.e., their ability to produce and secrete a diverse group of molecules called adipokines (i.e., hormones, growth factors, cytokines), has brought the potential influence of adipocytes and breast cancer behavior to the forefront [10]. The interaction between adipocytes and breast cancer cells is reciprocal; hence, both adipocytes and breast cancer cells are altered during their interactions. Adipocytes during this interaction assume an inflammatory phenotype and are termed “cancer-associated adipocytes” (CAAs) [10], [11]. Among the myriad of cytokines secreted by adipocytes, the inflammatory cytokine interleukin-6 (IL-6) is significantly produced [12]. IL-6 is associated with the development of stem cell phenotype [13], angiogenesis [14], cachexia [15], and resistance to therapy [16] in breast cancer and other solid tumors. In this review, we focus on the adipocyte–breast cancer cell interaction, with emphasis on the current knowledge on the influence of adipocyte-derived IL-6 on breast cancer progression, and subsequently discuss the potential roles for adipocyte-derived IL-6 based on emerging evidence from various stromal cells. We also discuss the reciprocal effects of breast cancer cells on adipocyte phenotype and function. This has implication for the development of novel therapy targeting adipocytes in the breast cancer microenvironment.
Adipocytes as Components of the Breast Cancer Microenvironment
The myriad of cytokines produced by cancer cells recruits various host cells such as macrophages, fibroblast, and dendritic cells to the microenvironment [17]. The various components of the microenvironment function to promote tumorigenesis, protect the tumor from the host immunity and therapeutic response [12]. The ensuing paracrine interaction between host cells and tumor cells in the microenvironment results in genetic alterations in stromal cells that enable them to produce growth factors, cytokines, and ECM-remodeling proteins that enhance tumor cell proliferation and invasion into surrounding tissues [5], [18]. The aggregation of host and tumor cells creates a hypoxic microenvironment; the tumor cells adapt to the hypoxic condition by upregulation of hypoxia-inducible factor-a (HIF-1a) gene [19]. HIF-1a upregulation results in an alteration in the expression of genes involved in migration, metabolism, angiogenesis, dedifferentiation, and apoptosis, enabling tumor cells to adapt and survive in low oxygen concentration [20], [21]. There is presently a great interest in understanding how the individual microenvironment populations of cells are recruited and modulated and how they influence breast cancer behavior. In breast cancer, the tumor originates in the mammary glands, which are composed of highly branched ducts and lobuloalveolar differentiated units embedded in a complex stroma termed mammary fat pad [22]. Adipose tissue in the mammary gland is composed of adipocytes, fibroblast, and immune cells. In the normal breast, the basement membrane separates mature adipocytes from the epithelial cells lining the mammary gland, serving as a barrier for heterotypic interaction/cross talk between the two cell types [9], [10]. However, during invasive breast cancer, the basement membrane separation is disrupted, and the two cell populations appear mixed together in the ECM, allowing for paracrine interaction to occur [10]. Histological sections from human breast carcinomas show that early invasion of tumor cells occur in the localized adipocytes [11], [23]. This area also has a high ratio of adipocytes to fibroblasts; however, the adipocytes are reduced in size compared with those observed at a distance [11], [23]. This close association indicates a potential for adipocytes to influence the invasive behavior of breast tumor cells through the secretion of various adipokines [11], [23]. This makes adipocytes an interesting member of breast tumor microenvironment, and it is important to understand clearly the role these adipocytes play in the breast cancer development. Recently, studies have emerged indicating how adipocytes may influence breast cancer proliferation, migration, invasion, and metabolism (discussed below).
The Evolution of Adipocytes in the Breast Cancer Microenvironment
The two main types of adipose tissue present in humans are white adipose tissue (WAT) and brown adipose tissue (BAT) [24]. The predominate population of adipose tissues in an adult human is the WAT composed of adipocytes, along with other cells in the stroma vascular fraction (adipose-derived stem cells, preadipocytes, macrophages, fibroblasts, and vascular endothelial cells) [24]. White adipocytes have a spherical shape with a single large lipid droplet surrounded by a reduced rim of cytoplasm, which essentially occupies the fat cell volume and with very few mitochondria [22], [24]. The WAT is mainly responsible for storing energy in the form of triglycerides and also functions as an essential endocrine organ that secretes a myriad of cytokines, hormones, and growth factors [22], [24]. The other adipose population, BAT, is predominantly found in newborn children but is replaced by WAT during development [24]. Recent studies with positron emission tomography imaging have identified BAT in adult individuals [25]. Brown adipocytes have a multilocular lipid inclusions and numerous well-developed mitochondria. BATs are highly innervated and associated with thermogenesis due to the presence of a unique mitochondrial protein, the uncoupling proteins (UCPs) [26]. UCPs regulate proton leakage across the inner mitochondrial membrane, thus decreasing the coupling of respiration to ADP phosphorylation and shifting the proton imbalance towards heat production [27]. Another class of adipose cells recently identified develops in WAT in response to various stimuli and is referred to as brite (or beige or systemic) adipose cells and is located in the much larger area of WAT tissue [28]. Beige cells in mouse are characterized by their multilocular lipid droplet and high mitochondrial content and express genes similar to brown fat (for example, Ucp1, Cidea, and Pgc1α) at lower levels [28]. They are thought to have the same origin as WAT cells but function more like BAT in expressing UCP and having thermogenic capacity similar to brown adipocytes although genetically different [28].
Histological sections of breast cancers invading surrounding adipose tissues show the large spherical and single lipid droplet WAT replaced by reduced lipid droplets in adipocytes [11]. This observation is characteristic of the in vitro cross talk between breast cancer cells and adipocytes, where adipocytes are observed to undergo a kind of “adipocyte dedifferentiation” where they acquire a fibroblast-like phenotype with reduced lipid droplets [29]. Recent in vitro and in vivo studies indicate that this activated state of adipocytes is characterized by a decreased expression of adipocyte markers and an overexpression of proinflammatory cytokines [11], [30]. The new population of adipocytes presenting this unique phenotype has been referred to as “cancer-associated adipocytes” (CAAs) [11]. Controversies exist pertaining to the role and nature of these CAAs. In an in vitro and in vivo study by Petruzzelli et al. on the effects of tumor cells on adipocytes, the authors associated this alteration in WAT phenotype to “WAT browning,” the process by which WAT obtains characteristics of BAT [31]. IL-6 secreted by tumor cells was identified as the main cytokine driving the process and linked the adipocyte delipidation to early events in cancer-associated cachexia. The change has also been associated with lipolysis of the lipid stores to support tumor cell metabolism. A recent in vitro study clearly demonstrated that co-culture between mouse 3T3-F442A–derived adipocytes and breast cancer cells (ZR-75-1 and SUM159PT) resulted in lipolysis and transfer of free fatty acids (FFAs) to tumor cells. The transferred FFAs are used in fatty acid β-oxidation by tumor cells to drive their migration and invasion [32]. These studies clearly indicate that the heterotypic cross talk between adipocytes and tumor cells, at least in invasive primary tumors, results in modification of adipocytes. These changes significantly involve changes to lipid stores, gene expression, and secretory profile of adipocytes [33], suggesting a release of fatty acids from adipocytes and a reduction in the triacylglycerol (TAG) stores [33]. Delipidation of adipocytes has been associated with increased expression of TAG lipases adipose triglyceride lipase and hormone-sensitive lipase and reduced expression of adipocytes differentiation markers such as LIPE and FABP4 [32], [33], [34]. Hence, cancer cells may induce delipidation in adipocytes via the secretion cytokines that alter adipocytes function. Although the specific cytokine and the mechanism involved in the process have not been completely deciphered, IL-6 has been associated with this delipidation process. Petruzzelli et al. identified IL-6 as the key cytokine driving lipid lipolysis in WAT browning [31]. Wang et al. also observed IL-6 as the only lipolytic factor increased in cancer cells after co-culture, although treatment with anti–IL-6 monoclonal antibodies did not inhibit fatty acids lipolysis [32]. Changes in adipocyte secretory profile are also observed after co-culture with breast cancer cell or in isolated adipocytes from breast tumors. These changes include increased expression of genes regulating adipokines and adipocytokines (TNF, IL-6, and IL-1B), proteases, and inhibitors (MMP11 and SERPine1). These changes are accompanied by altered protein secretion [30]. Dirat et al. using a co-culture system demonstrated that cocultured adipocytes underwent delipidation with overexpression of IL-6 and IL-1β. Immunohistochemical staining and quantitative polymerase chain reaction of human breast tumors confirmed the presence of modified adipocytes with increased IL-6 secretion [11]. In another study, human preadipocytes collected from fat pad immediately adjacent to malignant breast tumors had a lower lipid formation and reduced differentiation capacity [30]. Reviewing pathology specimens of human breast cancer patients revealed morphologic characteristics of dedifferentiation in CAAs and upregulation of IL-6 [30]. Additionally, indirectly co-culture of 3T3-L1 adipocytes with breast cancer cells resulted in an upregulation of inflammation-related genes including IL-6 [30]. These studies clearly highlight a role of adipocytes in breast cancer progression, with the CAAs transitioning into proinflammatory states that enhance breast cancer progression.
Adipose Signaling
Adipocytes present in the breast cancer microenvironment secrete adipokines that affect various aspects of cancer development. Various studies using human- and mouse-derived adipocytes have demonstrated that adipocytes enhance the proliferation, migration, and invasive characteristics of breast cancer cells in co-culture [11], [35], [36]. Understanding the role of adipocytes and the effect of the various adipokines on breast cancer cells has been obtained through studies using adipocytes conditioned media to culture breast cancer cells with different molecular characteristics or using a co-culture system with adipocytes and breast cancer cells [32], [35], [37], [38], [39]. Three of the best-characterized adipokine molecules in the adipocyte–breast cancer interactions are leptin, adiponectin, and IL-6. These factors regulate the inflammatory tumor microenvironment and the tumor behavior (such as stimulating cancer cell proliferation and invasion) through the activation of various intracellular signaling pathways and transcription factors that affect various aspect of the cell function [40], [41].
Leptin
Leptin is a multifunctional neuroendocrine peptide hormone with a molecular weight of 16 kDa secreted by adipocytes and generally functions in appetite control, energy reserve metabolism, immune response, and reproductive processes [42], [43]. Breast tumors have increased expression of leptin and its receptors; this increase is associated with distant metastasis [44]. Leptin activates the JAK/STAT, PI3K, and mitogen-activated protein kinase (MAPK) pathways and regulates the cellular activity through various downstream targets [43], [45], [46]. Leptin expression results in increased cell survival and proliferation by inducing c-MYC expression [47]. Leptin is also an activator of stem cells signaling pathways such as Notch and Wnt, and both leptin and its receptors are upregulated in breast cancer stem cells [45], [48], [49]. The role of leptin in breast cancer cells is emphasized in an in vitro study where silencing the leptin receptor via shRNA lentivirus inhibited the expression of stem cell self-renewal transcription factors Nanog, sox2, and oct4 in MDA-MB-231 breast cancer cells [49], [50]. Leptin receptor silencing also exhibited a mesenchymal to epithelial transition morphologically with increased E-cadherin and decreased vimentin expression, suggesting a role in mesenchymal-epithelial transition (MET) [49]. Leptin also regulates angiogenesis by increasing endothelial cell proliferation and by VEGF signaling [45], [51]. Hence, leptin has a proliferative, self-renewal, and survival function in adipocyte–breast cancer interaction.
Adiponectin
Adiponectin is a unique adipokine known for its antiproliferative effects on human breast cancer cells. It has a molecular weight of 28 to 30 kDa, with several identified isoforms [52]. Adiponectin activates AMP-activated protein kinase, and the inactivation of p42/p44 MAPK results in the initiation of apoptosis and inhibition of the cell survival [53], [54], [55]. In vitro studies in breast cancer cells indicate that adiponectin increased apoptosis by increasing poly(ADP-ribose) polymerase cleavage [56]. Adiponectin also regulates the expression of tumor suppressor genes, oncogenes, pro- and antiapoptotic genes, and cell cycle regulatory genes including p53, Bax, Bcl-2, c-myc, cyclin D1, MAPK3, and ataxia telangiectasia mutated [40], [57]. Cell proliferation induced by leptin and oxidized low-density lipoprotein can be inhibited by adiponectin [56]. The glycogen synthase kinase-3b (GSK-3b)/β-catenin signaling pathways are involved in adiponectin-mediated growth inhibition of breast cancer cell lines, where adiponectin inhibits GSK-3b phosphorylation and suppresses the intracellular accumulation of β-catenin [56]. Several studies have also shown a significant association between low serum adiponectin levels and increased risk of breast cancer [58]. These studies clearly elucidate the antiproliferative and proapoptotic functions of adiponectin and its potential to inhibit breast cancer progression.
IL-6 and the IL-6/JAK/STAT3 Signaling Pathway in the Tumor Microenvironment
Approximately one-third of plasma IL-6 is produced by adipose tissues. In healthy adipose tissue, the major source of IL-6 are the nonadipocyte members; however, under pathological conditions like obesity and cancer, the levels of IL-6 secreted from adipocytes increase significantly [59], [60]. In breast cancer, the increase in adipocyte IL-6 secretion is regulated by paracrine interaction with tumor cells. The increased secretion of IL-6 in CAAs and the pleiotropic roles associated with IL-6 make the potential effects of adipocyte-derived IL-6 on breast cancer cells an interesting area of research [30]. IL-6 is an inflammation-associated cytokine, with pleiotropic effects produced by a diverse cell population including T cells, B cells, macrophages, monocytes, fibroblasts, endothelial cells, and several tumor cells. IL-6 is involved in diverse cellular and physiological responses including immune response, inflammation, hematopoiesis, and oncogenesis [61].
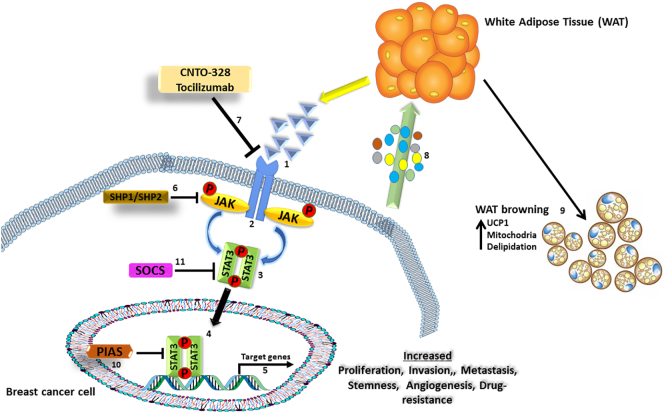
IL-6 gene expression is NF-κB dependent and produces a 26-kDa protein [62]. IL-6 signaling occurs via two types of cellular receptors: the membrane-bound ligand-binding chain, IL-6R (IL-6Ra, CD126), which ensures ligand specificity, and a signal-transducing chain [63], [64]. A receptor subunit is shared with other cytokines in the IL-6 family, gp130 homodimer (IL-6Rb, CD130), which is the signal-transducing component [63], [64]. IL-6R and gp130 dimerization activates the Janus kinase 1/2 (JAK1/2) and the subsequent phosphorylation of the signal transducer and activator of transcription 3 (STAT3) by JAK1/2 in the cytoplasm [65], as shown in Figure 1. Phosphorylated STAT3 dimerizes with other STAT family members, and the activated STAT3 complex translocates into the nucleus, activating the transcription of genes under STAT3 control including cyclin D1, Bcl-xL, c-myc, Mcl1, and VEGF [62], [63], [64]. Genes controlled by STAT3 are associated cancer traits such as angiogenesis, cell survival, cell proliferation, and cell transformation [62], [63], [64]. The JAK/STAT3 signaling pathway is regarded as the “classical” signaling pathway for IL-6. IL-6 signaling is terminated by the upregulation of suppressor of cytokine signaling 3 gene via homologous or heterologous feedback regulation [62], [63]. The extracellular signal-regulated kinase 1 and 2 signaling pathways are also activated by JAK1 through phosphorylation of the protein tyrosine phosphatase nonreceptor type 2 [62], [63].
Figure 1.
Signaling pathway of IL-6. IL-6 secreted by cancer-associated adipocytes binds to the IL-6 receptor [1] on breast cancer cell and induces a cascade of JAK phosphorylation [2] that results in phosphorylation and activation of Stat3 [3]. Activated stat3 dimerizes with other phosphorylated stat3 [4], translocates into the nucleus, binds to target DNA sequences, and activates the transcription of genes under its control [5], which are usually involved with invasion, proliferation, metastasis, stemness, angiogenesis, immune suppression, and drug resistance. SHP1/SHP2, PIAS, and SOCs are negative feedback regulated of the JAK/STAT pathway [[6], [10], [11]]. The tumor cells also secrete IL-6 and other inflammatory molecules [8] that stimulate WAT browning [9] with increase in UCP1 expression, mitochondria, and thermogenesis. CNTO-328 and tocilizumab are monoclonal antibodies that target and inhibit IL-6 activation of the JAK/Stat3 signaling [7].
In cells that express gp130 but not IL6-Ra, an alternative to classical IL-6 signaling occurs: the soluble type of the IL-6 receptor (sIL-6R) produced by proteolytic cleavage of membrane-bound IL-6R or alternative splicing of IL-6R mRNA [66], [67]. SIL-6R binds to IL-6 and recruits gp130, allowing IL-6 signaling to occur [66], [67]. This alternate pathway is referred to as IL-6 trans-signaling, and it is proposed to act as a danger signal to enhance IL-6 responsiveness and drive inflammatory events [67], [68]. The IL-6sR expression has been demonstrated in human breast cancer cell lines, suggesting the potential of IL-6 trans-signaling to mediate the effects of IL-6 in breast cancer cells [67], [69]. IL-6 signaling can also occur through other kinase-dependent pathways including the MAPK pathway and the PI3K/Akt pathway [62], [63], [70]. IL-6 expression is upregulated in response to other adipokines, IL-1β, TNF-α, and TGF-β1, and a wide range of transcription factors such as NF-kB, Jun D proto-oncogene (Jun D), cAMP responsive element binding protein 1 (CREB1), and Jun oncogene (c-Jun), depending on cell type and ligand specificity [10], [71].
The JAK/STAT signaling pathway regulates the expression of over 40 different cytokines or growth factors [70], [72], [73]. The pathway also plays an important role in promoting tumorigenesis of diverse human cancers. The effect of IL-6 on cancer cells is accomplished by both autocrine and paracrine mechanisms [70], [72]. Certain cancers (breast cancer, lung cancer) have increased IL-6 gene expression, produce large amounts of IL-6, and also express the IL-6R and gp130 receptor subunits; this allows for autocrine stimulation [61], [74], [75]. In other cancers (myeloma and neuroblastoma), IL-6 stimulation occurs via paracrine stimulation since the tumor cells do not produce IL-6 but express functional IL-6/gp130 receptor complex and thus respond to IL-6 produced in the TME [76]. Paracrine signaling can still occur in cancer cells that produce IL-6 and express its receptors since several components of the TME also secrete IL-6 [76]. The possibility of paracrine signaling means the IL-6 secretion from stromal cells in the TME is essential for these cells. Hence, IL-6 secreted by adipocytes may be essential in these types of cancers.
Role of adipocyte-derived IL-6 in breast cancer progression
It is now well established that breast cancer cell lines produce IL-6 ER-positive cells and secrete lower levels than ER-negative cells] [77]. Other cells in the TME such as fibroblasts and macrophages secrete IL-6, which stimulates the growth and invasiveness and induces epithelial-mesenchymal transition (EMT) in breast cancer cells [68], [78]. IL-6 also regulates the formation and maintenance of breast cancer stem cells through STAT3 activation [78]. The pleiotropic roles of IL-6 in cancer progression make it a potential target for therapy; however, these functions are not linked directly to adipocytes. With attention directed at the role adipocytes play in the breast cancer microenvironment, the potential role of adipocyte-derived IL-6 in breast cancer progression is beginning to emerge. Several members of the adipokines secreted by adipocytes have been demonstrated independently to regulate various aspects of breast cancer progression. It can be hypothesized that adipokines secreted by adipocytes may promote breast cancer proliferation, migration, and invasion by similar established mechanism or other yet to be identified mechanisms. The remaining section of the review focuses on the established role of IL-6 in the adipocyte–breast cancer cell interaction and the potential role adipocyte-derived IL-6 may play in cancer progression based on established functions of IL-6.
Role of Adipocyte-Derived IL-6 in EMT
The ability for epithelial cells to transit into a mesenchymal status is key in development, wound healing, stem cell behavior, and tumorigenesis; this process is known as EMT [79]. It is a reversible biologic process where polarized epithelial cells undergo molecular, genetic, and biochemical changes and acquire a mesenchymal phenotype with enhanced tumorigenic capacity [79]. EMT is characterized by the loss of cell junctions, loss of apical-basal polarity, reorganization of cytoskeleton, and reprogrammed gene expression [79]. The completion of EMT is marked by the degradation of the basement membrane and the formation of a mesenchymal cell with enhanced migratory and invasion ability [79], [80]. A reverse process known as MET that involves the conversion of mesenchymal cells to their epithelial derivatives can also occur when circulatory mesenchymal tumor cells settle in favorable environments [79], [81]. The process of EMT and MET illustrates the inherent plasticity of the epithelial phenotype. Understanding of the EMT process is important since about 90% of cancer-associated death is due to metastasis and EMT is an important step in cancer cell metastasis [80], [82].
A number of distinct molecular signals are required to induce EMT; the successful completion of the process requires the activation of transcription factors, expression of specific cell-surface proteins, reorganization and expression of cytoskeletal proteins, production of ECM-degrading enzymes, and changes in the expression of specific microRNAs [79], [81], [83]. EMT is identified experimentally by the loss of E-cadherin expression (associated with epithelial phenotype) and the increased expression of vimentin (associated with mesenchymal phenotype) [79], [81]. Several genes are also involved in the EMT process including Twist, Snail (SNAI1), Slug (SNAI2), SIP1, or zinc finger E-box-binding (ZEB) and basic helix-loop-helix (bHLH) transcription factors [81]. The EMT process is initiated by various extracellular signals such as TGF-β and IL-6; these molecules activate various downstream signals such as the SMADs, PI3K/AKT, and JAK/STAT3 that regulate EMT [83], [84]. In breast cancer, the evidence of EMT is well documented; impaired e-cadherin expression in human breast tumors correlates with enhanced invasiveness, metastatic potential, and decreased breast cancer patient survival [85].
Emerging evidence indicates that adipocytes are capable of inducing similar effects when cultured with breast cancer cells. A recent in vitro study demonstrated that a direct co-culture between murine 3T3-L1–derived adipocytes and breast cancer cells of various molecular subtypes [ER+, ER−, and triple-negative breast cancer (TNBC)] induced an EMT phenotype and enhanced their proliferation, migration, and invasion capabilities [35]. Although the study did not demonstrate adipocyte-derived IL-6 as the cytokine inducing EMT in the cells, results from similar studies with adipocyte-conditioned media both in vitro and in vivo indicate the potential for IL-6 to induce EMT and enhance the migratory and invasion capabilities of breast cancer cells [11], [86], [87]. Most interestingly, adipose stromal cells co-cultured with ER-negative MDA-MB-231 breast cancer cells enhanced their migratory and invasion capabilities. Co-injecting adipose stromal cell with MDA-MB-231 cells exhibited a more aggressive phenotype compared to MDA-MB-231 cells alone in mouse models [38], [88]. Depletion of IL-6 from adipose stromal cells (ASC)–conditioned medium abrogated the stimulatory effect of ASCs on the migration and invasion of breast tumor cells [38]. (See Table 1). Collectively, these studies demonstrate that adipocytes can induce EMT and enhance the aggressive behavior of breast cancer cells, potentially via IL-6 secretion. Using human-derived adipocytes in co-culture with breast cancer cells, we demonstrated similar adipocytes-enhanced proliferation, migration, and invasion in MCF-7 and MDA-MB-468 breast cancer cells. Human adipocytes also reduced E-cadherin expression and increased vimentin and ZEB1, which are characteristics of cells in EMT. We also showed that neutralizing adipocytes-derived IL-6 with a blocking antibody reduced the proliferation, migration, and invasion capabilities of MCF-7 and MDA-MB-468 breast cancer cells. Furthermore, blocking IL-6 decreased the expression of EMT-regulated transcription factors such as TWIST and SNAIL. These findings indicate that adipocyte-secreted IL-6 can induce EMT in breast cancer cells. Collectively, these findings indicate the increased secretion of IL-6 by adipocytes and of interleukin-6 highlights the potential role of IL-6 in the adipocyte–breast cancer interaction specifically in inducing EMT, which enhances the migratory and invasion capabilities of breast cancer cells.
Table 1.
Effects of Adipocytes–Cancer Cell Interactions
| Mechanism | Important Findings | References |
|---|---|---|
| EMT | (i) 3T3-L1 adipocytes induced an EMT phenotype and enhanced proliferation, migration, and invasion n TNBC cells. | Lee et al., 2015 |
| (ii) Depletion of IL-6 in adipose stromal cells inhibited migration and invasion in ER-negative breast cancer cells. | Walter et al., 2009 | |
| Cancer stem cells | (i) Matured adipocytes enhanced the mammosphere-forming cells and cells expressing stem-like markers in MCF-7, MDA-MB-231, and T47D. | Picon-Ruiz et al., 2016 |
| Cancer-associated cachexia | (i) IL-6 from tumor cells induced a rapid weight loss and cachexia in xenograft mouse models; blocking IL-6 secretion with shRNA rescued the cachectic phenotype. | Petruzzelli et al., 2016 |
| (ii) Co-culture of breast cancer cells and adipocytes resulted in delipidation of adipocytes with overexpression of IL-6. | Dirat et al., 2011 | |
| Therapeutic resistance | (i) Co-culture of preadipocytes and mature human adipocytes with HER2-expressing breast cancer cells inhibited trastuzumab-mediated ADCC via the secretion of soluble factors. | Duong et al., 2015 |
| (ii) A 2D co-culture of mature adipocyte induced radioresistance in breast tumor cells; radioresistance in breast cancer cells was associated with adipocyte-induced increase in IL-6 expression in tumor cells. | Bochet et al., 2011 |
Role of Adipocyte-Derived IL-6 in Breast Cancer Stem Cell
Cancer stem cells (CSCs; also called tumor initiating cells) are essential players in tumorigenesis; they are considered as “tumor-initiating cells” because of their ability to self-renewal and initiate a new whole tumor, like normal stem cells [89]. CSCs are highly tumorigenic cells that exist within tumors; they produce daughter cells that form the bulk of the tumor cells while maintaining their unique ability of self-replication [89]. Characteristics of CSCs identified include their ability to self-renew under nondifferentiation conditions and differentiate into non–stem cancer cells, they have a high tumorigenicity, and they form spheres (e.g., mammospheres for breast CSCs) and are resistant to chemotherapeutic drugs [89]. CSCs usually constitute only a small proportion (0.05%-1%) of the tumor mass and are present in a variety of cancers including breast cancer [termed breast cancer stem cells (bCSCs)] [90]. The acquisitions of stem cell traits that influence the tumor initiation capability by breast cancer cells has been linked to the induction of EMT programs [91], [92]. Recent evidence supports a link between induction of EMT and the acquisition of molecular and functional properties of stem cells. In immortalized and transformed human mammary epithelial cells, induction of EMT enhances their self-renewal and tumor-initiating capabilities characterized by the expression of various markers associated with bCSCs [93], [94] (a more detailed relationship between EMT and bCSCs is discussed in May et al. [95]). Breast cancer stem cells influence cancer growth and the formation of metastasis and have been hypothesized to be key drivers of cancer [90]. BRCA1 gene mutations are associated with an increased risk of breast cancer, and mutation in BRCA1 may contribute to the accumulation of genetically unstable breast cancer stem cells [96]. Various biomarkers have been identified in BCSCs, such as high expression of CD44, aldehyde dehydrogenase 1, CD133 (prominin-1), CD49f, and ITGA6 and low or undetectable levels of CD24 [96], [97], [98]. These markers are not expressed universally across bCSCs isolated from different breast cancer subtypes, but are expressed differentially according to subtype and are influenced by signals from the TME [96], [97], [98]. There is great interest in identifying factors that activate the development of bCSCs and the role of bCSCs in metastasis. As no conventional therapy presently targets CSCs, CSCs may be potentially responsible for resistance to therapy and tumor recurrence.
The myriad of regulatory factors in the TME can modulate the activities of transcription factors and genes involved in the development of CSCs. Emerging studies indicate that IL-6 has potential to regulate CSCs self-renewal and may be responsible for maintaining a stable balance between CSCs and non–stem cancer cell [13], [99]. Several signaling pathways such as Notch, Hedgehog, Wnt, and Oct-4/SOX2/Nanog axis have been identified as regulators of CSCs survival and proliferation [48], [84], [100]. IL-6 secretion is linked to all four pathways, and these pathways are frequently dysregulated in human cancers [84], [100]. In a recent study, Kim et al. using MDA-MB-231 and MDA-MB-453 breast cancer cells expressing stemness genes and control cells demonstrated that nonstem cells but not CSC-like cells produced IL-6, which activated the JAK1-STAT3 signal transduction pathway, resulting in the conversion of nonstem cells into stem-like cells through upregulation of Oct-4 [13], (See Table 1). This study indicates that IL-6 secreted from nonstem cells plays an important role in the conversion of non-CSCs to CSCs through activation of the JAK1-STAT3-Oct-4 signal transduction pathway. Picon-Ruiz et al. present the strongest evidence in support of a role of adipocyte-derived IL-6 in driving breast cancer stem cells. They demonstrated that co-culturing matured adipocytes with breast cancer cells (MCF-7, MDA-MB-231, and T47D) with different molecular traits resulted in an increased secretion of proinflammatory cytokines by adipocytes [36]. Prolonged co-culture of cancer cells with adipocytes increased the proportion of mammosphere-forming cells and of cells expressing stem-like markers in vitro [36]. Hence, they proposed an increased secretion of cytokines such as IL-6 in adipocyte co-cultured cancer cells and activated Src, thus promoting Sox2, c-Myc, and Nanog upregulation resulting in the emergence of stem cell traits [36]. This demonstrates the potential for adipose-derived IL-6 to activate various signal pathways linked to the development of breast cancer stem cells. However, further studies are required clearly elucidate the role of adipocytes in promoting bCSC characteristics.
Role of Adipocyte-Derived IL-6 in Cancer-Associated Cachexia
Cachexia is a life-threatening condition associated with several pathologies, including cancer, acquired immunodeficiency syndrome, surgery, malabsorption, and severe sepsis [101]. Understanding of the cellular and physiological mechanism of cachexia is particularly key in cancer since it occurs in approximately 80% of all cancers and accounts for 20% of cancer-associated deaths [102]. Depletion of adipose tissue stores, loss of skeletal mass in several organs, and weight loss are key features of cachexia [101], [102]. Symptoms of cachexia include loss of total body mass, anorexia, general inflammation, and pronounced muscle wasting [101], [102]. Muscle wasting in cachexia involves chest, diaphragm, and cardiac muscle, accounting for the high levels of respiratory or cardiac failure deaths associated with cachexia [101], [102]. Cancer-associated cachexia patients are usually less tolerant to radio- and chemotherapy, limiting therapeutic options for patients. Hence, a detailed understanding of the molecular and regulatory mechanism of cancer-associated cachexia is needed to develop potential therapies [101], [102], [103]. Cachexia is a considered a generalized inflammatory state, with several inflammatory cytokines implicated including IL-6, TNF-α, IL-1β, and interferon-γ [104]. Among the different cytokines associated with cachexia, IL-6 is identified as an essential regulator in the maintenance of body mass during disease and a key regulator of cancer cachexia [104]. The potential role of IL-6 in cancer cachexia stems from its potential role in WAT browning, as shown in Figure 1. WAT browning is the development of BAT markers in WAT in response to specific regulatory stimuli [105]. It has been demonstrated that with an appropriate stimulus such as chronic cold exposure, hormonal stimuli, and pharmacological treatment, brown fat–like genes can be expressed in WAT, inducing a BAT phenotype [105], [106]. This phenomenon is reported in cancer cachexia, the progressive transdifferentiation of WAT into BAT, which expresses UCP-1, promotes thermogenesis, and results in the loss of adipose tissue [31]. White adipose tissue browning strongly contributes to the increased energy expenditure and loss of adipose tissue common in cachectic patients [107]. Proinflammatory factors such as IL-1, IL-6, and TNFα derived from the host immune system or the tumor have been shown to contribute WAT browning [104].
IL-6–induced development of cachexia is also attributed to the altered metabolism that occurs in adipocytes co-cultured with cancer cells. CAAs exhibit inhibiting lipid biosynthesis and an increase the rate of lipid catabolism [11], [30], [32]. IL-6 is also associated with atrophy and increased catabolism of muscle protein [108]. Cachectic cancer patients and weight-losing patients have elevated systemic IL-6 levels compared to control groups [15]. A study by Iwase et al. reported IL-6 as the only cytokine elevated in 28 cachectic patients and the levels of IL-6 further increased as the patients approached death [15]. While this study does not establish adipocytes as the sole source of increased IL-6, the increased secretion of IL-6 by adipocytes makes them a prime candidate. A more confirmatory role of IL-6 in cancer-associated cachexia comes from a recent study by Petruzzelli et al. In their elegant study, they showed that IL-6 from C26 tumor cells induced a rapid weight loss and cachexia in xenograft mouse models; blocking IL-6 secretion with shRNA rescued the cachectic phenotype [31] (See Table 1). More importantly silencing IL-6 blocked increased UCP1 expression in adipose tissue and reduced the increased mitochondrial respiration induced by IL-6–secreting tumor cells [31]. In another study, removal of tumors from cachectic rodents resulted in body mass returning to normal and circulating IL-6 levels significantly decreasing [109]. These studies illustrate the potential role of adipocyte-derived IL-6 in cachexia development via activation of IL-6 signaling. Agents like Suramin, a polysulfonated naphthyl urea, inhibit the binding of IL-6 to cell surface receptor subunit, and inhibit the development of cachexia cancer models, indicating the potential for agents targeting IL-6 to inhibit cachexia [101]. These studies indicate that IL-6 may play an active role in the cachectic process; however, further research is needed to understand if IL-6 acts alone or functions in synergy with other inflammatory factors to induce cachexia development.
Potential Role of Adipocyte-Derived IL-6 in Therapeutic Resistance
The emergence of evidence that the TME influences cancer initiation, progression, and metastasis has raised interest in the potential role of the TME and its stromal cell populations in targeted drug resistance. Therapeutic resistance has been linked to the increased production of inflammatory cytokines including IL-6, IL-8, and TNFα [110]. In various cancers, increased IL-6 levels contribute to poor therapeutic gain, tumor relapse, and aggressive tumor growth [110], [111]. In ovarian cancer, patients with reduced levels of IL-6 respond better to therapy than those with higher levels [112]. In prostate cancer, inhibition of IL-6 secretion increased the sensitivity of cancer cells to anticancer drugs [113]. These studies indicate that increased IL-6 production by tumor cells may offer a protective mechanism against drug-induced cell death. In breast cancer patients, elevated serum levels of IL-6 have been associated with poorer prognosis and therapeutic resistance [114]. The role of IL-6 in therapeutic resistance in breast cancer is supported by the observation that multidrug-resistant cancer cells produced high levels of IL-6, whereas drug-sensitive counterparts do not express IL-6 [115]. Treatment with exogenous IL-6 rendered drug-sensitive cells resistant to several chemotherapy agents (doxorubicin, vincristine, and taxol), indicating that the expression of IL-6 in cancer cells may be key to cancer cells becoming resistant to multiple therapeutic agents [116]. The exact mechanism by which IL-6 induced resistance has not been fully elucidated; however, it seems that IL-6–mediated STAT3 activation induces increased expression of the multidrug resistance genes MDR1 and C/EBPβ and C/EBPδ (CCAAT enhancer-binding protein family of transcription factors) [111]. These findings clearly indicate a role of IL-6 in therapeutic resistance but not directly linked to adipocyte-derived IL-6. In a recent study, co-culture of preadipocytes and mature human adipocytes with HER2-expressing breast cancer cells inhibited trastuzumab-mediated antibody-dependent cellular cytotoxicity (ADCC) via the secretion of soluble factors in vitro and was also validated in vivo in a mouse xenograft model [117]. The study, however, did not identify IL-6 as the main factor responsible for the effect but underlines the potential role of adipocytes in breast cancer cell resistance to trastuzumab [117]. In a similar study, Bochet et al. demonstrated using a 2D co-culture system mature adipocyte-induced radioresistance in breast tumor cells [118]. Interestingly, radioresistance in breast cancer cells was associated with adipocyte-induced increase in IL-6 expression in tumor cells, and postincubation of irradiated tumor cells with recombinant IL-6 was able to reproduce the radioprotective effect [118]. (See Table 1). These observations, although not conclusive, heighten the potential role of adipocyte-derived secretory factors (particularly IL-6) in adipocytes-induced therapeutic resistance in breast cancer cells. Hence, targeting IL-6 may offer an alternative approach to minimizing therapeutic resistance, and the levels of IL-6 could serve as a key marker for the development of resistance.
Potential Role of Adipocyte-Derived IL-6 in Angiogenesis
In addition to the catalog of tumor-promoting functions played by IL-6, it has also been reported to play an active role in angiogenesis and vascular remodeling in the TME [119]. The downstream signaling pathways associated with IL-6, particularly the targets of STAT3, have been linked to angiogenesis [120]. Tumor neoangiogenesis is essential for the growth of tumors beyond 1 mm; the formation of these vessels is regulated by both pro- and antiangiogenic factors [121]. The presence of high levels of proangiogenic molecules over antiangiogenic molecules, coupled with the hypoxic and inflammatory conditions in the TME, causes a switch in favor of proangiogenic state [121]. The resulting effect is the development of new vasculature to support the tumor growth. A key player in the angiogenesis process is vascular endothelial growth factor (VEGF). a 45-kDa glycoprotein widely accepted to be the essential driver of angiogenesis [121]. VEGF also increases tumor vessel dilatation, permeability, and leaking [121]. The effects of VEGF are potentiated by the hypoxic condition present in the tumor, where the low oxygen level results in increased expression of HIF-1α, which upregulates the expression of VEGF mRNA, promoting angiogenesis. Induction of HIF-1α and VEGF upregulation have been shown to occur under inflammatory conditions, which are common in tumors [120], [122]. Hence, it is logical to think that mediators of inflammation in the tumor microenvironment may be key regulators of HIF-1α and VEGF. The pleiotropic role played by IL-6 in tumorigenesis makes it an ideal candidate in this relationship, and various studies have revealed the ability of IL-6 to regulate HIF-1α and VEGF expression through the JAK/STAT3 signaling [123]. Studies in malignant cell lines showed that IL-6 can stimulate VEGF signaling, and RNA-seq experiments reported a positive association between IL-6 and VEGF mRNA levels [119].
Niu et al. showed by chromatin immunoprecipitation that the downstream target of IL-6, STAT3, binds directly to the VEGF promoter, upregulates VEGF, and influences tumor angiogenesis. The study also demonstrated that treatment of cells with IL-6 significantly induced VEGF mRNA [122]. The effects of IL-6 on angiogenesis are also associated with several other processes including promoting endothelial progenitor cell migration, regulation of bFGF, stimulation of vascular smooth muscle cell (VSMC) migration, and induction of platelet-derived growth factor–mediated VSMC proliferation [14]. The notch signaling pathway and its ligands jagged-1 and Delta-like ligand 4 also regulate various aspects of tumor angiogenesis [14], Sansone et al. showed in breast cancer cells that IL-6 is able to stimulate notch-dependent upregulation of jagged-1 resulting in their aggressive phenotype; hence, IL-6 may regulate angiogenesis via the Notch-dependent signaling [124]. In ovarian cancer xenografts models treated with anti–IL-6 antibody, tumor vasculature was reduced significantly with the inhibition of the Notch ligand Jagged-1 [112]. In clinical studies in ovarian cancer, therapeutic neutralizing anti–IL-6 antibody reduced systemic VEGF levels [112]. Collectively, studies in humans and animal models indicate the potential for IL-6 to drive abnormal angiogenesis and that the anti–IL-6 antibody holds potential as an antiangiogenic agent. Correlating these independent functions of IL-6 to the increased secretion of IL-6 from adipocytes in breast cancer, it is logical to assume a similar function for adipocyte-derived IL-6. The acquisition of an inflammatory state with increased IL-6 secretion in adipocytes, combined with the hypoxic microenvironment, creates an ideal environment for adipocyte IL-6 to drive angiogenesis via upregulation of STAT3 and VEGF expression.
Potential for Targeting the IL-6 Pathways for Targeted Therapy in Breast Cancer
The plethora of regulatory and modulatory functions played by IL-6 in cancer makes it a potential target for targeted therapy. This potential has resulted in the development of various anti–IL-6 agents, including the anti–IL-6R mAb tocilizumab, as a robust inhibitor of IL-6/STAT3 activity [125]. Overexpression of both IL-6 and its receptors (IL-6R and sIL-6R) occurs in several cancers including multiple myeloma, lung cancer, colorectal cancer, cervical cancer, breast cancer, and ovarian carcinoma [126]. Elevated levels of IL-6 have been found in the serum of cancer patients and the culture supernatant of multidrug-resistant cell lines [50]. Hence, blocking IL-6 may serve as a therapeutic option for cancer with increased expression of IL-6. Various cancer clinical trials (in ovarian cancer, breast cancer, and multiple myeloma) indicate a potential for targeted anti–IL-6 antibody therapy [127]. These studies used various targeted agents against IL-6, including IL-6–conjugated toxins and murine or humanized monoclonal antibodies (mAbs) against IL-6 and IL-6R. IL-6–conjugated toxin therapy involved conjugating the IL-6 gene to a pseudomonas exotoxin or diphtheria toxin genes [125]. The approach may not be viable since normal cells expressing IL-6R are also targeted. An alternative approach includes using mAbs against IL-6, either a murine humanized anti–IL-6 monoclonal antibody or a human anti–IL-6 monoclonal antibody [128]. CNTO 328 (Siltuximab), a human-mouse chimeric antibody, has shown considerable benefit clinically. CNTO 328 is constructed from the variable antigen-binding region of a murine anti–IL-6 mAbs (with antitumor and anti-inflammatory activities) containing the antigen binding region of the human immunoglobulin G kappa (IgG κappa) immunoglobulin [128], [129], [130]. CNTO 328 has been shown to be without any significant immunogenicity and with a long half-life (approximately 2 weeks) [129]. CNTO 328 effectively inhibited binding of IL-6 to the IL-6R; suppressing IL-6 induced JAK/Stat3 signaling, effectively blocking IL-6/IL-6R/gp130 signal transduction [125], [130], [131], as shown in Figure 1. Treatment with CNTO 328 decreases activation of Stat3 downstream proteins, including MCL-1 and Bcl-XL. CNTO 328 thus successfully neutralizes the function of IL-6, offering an antitumor and anti-inflammatory effect, and reduces cancer-related anorexia and cachexia [125], [130], [131]. These beneficial effects of CNTO 328 antibody have been shown in different cancers including multiple myeloma, ovarian cancer, and prostate cancer [131]. In a CNTO 328 ovarian cancer trial, patients treated for 6 months have a significant decline in plasma levels of IL-6–regulated CCL2, CXCL12, and VEGF [132]. CNTO 328 was well tolerated and stabilized disease in >50% of progressive metastatic renal cell cancer patients [133], [134]. No adverse events related to CNTO 328 treatment were observed in prostate cancer patients, with patients receiving CNTO 328 showing higher levels of apoptosis markers, with a decrease in pStat3 and p44/p42 mitogen-activated protein kinases [135], [136]. Tocilizumab (namely, MRA) has emerged as a robust inhibitor of IL-6/STAT3 activity since it inhibits both the classical and trans–IL-6 signaling [137]. Tocilizumab is a humanized anti–human IL-6R antibody engineered by complexing the complementarily determining regions of a mouse anti-human IL-6R antibody into human IgG1k to create a human antibody with a human IL-6R binding site [127], [137]. Therapies strictly targeting IL-6R using tocilizumab have been shown to be effective in treating oral squamous cell carcinoma through inhibiting angiogenesis [127], [138]. The success of the various anti–IL-6 therapies emphasizes the central role of IL-6 in cancers; however, more research is still required to better understand the mechanism of action of these therapies and the likelihood of patients developing resistance to the therapies.
Conclusion
The tumor microenvironment is composed of various components and cells that have been demonstrated to influence breast cancer tumorigenesis via paracrine interaction with the tumor cells. Adipocytes are abundant in the breast cancer microenvironment and secrete an array of growth factors, hormones, and cytokines that have been implicated in cancer initiation, migration, metastasis, and therapeutic resistance. The pleiotropic cytokine IL-6 has been demonstrated to regulate several of the processes in tumorigenesis. As research continues to identify unique biomarkers and novel targets for targeted therapy, the tumor microenvironment has begun to gain much attention. An understanding of the functions played by the various components in the tumor microenvironment may provide alternative targets for future therapy. The abundance of adipocytes in the tumor microenvironment makes them key targets for such studies. Much research is required to understand the heterotypic crosstalk between tumor cells and adipocytes, particularly in understanding the key adipokines that stimulate tumor growth and those that inhibit tumor progression. Such studies would serve as a means for screening potential candidates in tumor growth or inhibitions and provide alternative targets for breast cancer therapy.
Funding
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2017R1D1A1B03033362 and NRF 2014R1A1A1002443); by the Brain Research Program through the NRF funded by the Ministry of Science, ICT & Future Planning (2016M3C7A1913844) and by the Faculty research grant of Yonsei University, Wonju College of Medicine.
Conflicts of Interest
The authors indicate no potential conflicts of interest.
Contributor Information
Ja-Seung Koo, Email: kjs1976@yuhs.ac.
Junjeong Choi, Email: junjeong@yonsei.ac.kr.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas N Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 4.Place AE, Jin Huh S, Polyak K. The microenvironment in breast cancer progression: biology and implications for treatment. Breast Cancer Res. 2011;13(6):227–238. doi: 10.1186/bcr2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao Y, Keller ET, Garfield DH, Shen K, Wang J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013;32(1–2):303–315. doi: 10.1007/s10555-012-9415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Artacho-Cordon A, Artacho-Cordon F, Rios-Arrabal S, Calvente I, Nunez MI. Tumor microenvironment and breast cancer progression: a complex scenario. Cancer Biol Ther. 2012;13(1):14–24. doi: 10.4161/cbt.13.1.18869. [DOI] [PubMed] [Google Scholar]
- 7.Aboussekhra A. Role of cancer-associated fibroblasts in breast cancer development and prognosis. Int J Dev Biol. 2011;55(7–9):841–849. doi: 10.1387/ijdb.113362aa. [DOI] [PubMed] [Google Scholar]
- 8.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 9.Wang YY, Lehuede C, Laurent V, Dirat B, Dauvillier S, Bochet L. Adipose tissue and breast epithelial cells: a dangerous dynamic duo in breast cancer. Cancer Lett. 2012;324(2):142–151. doi: 10.1016/j.canlet.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 10.Tan J, Buache E, Chenard MP, Dali-Youcef N, Rio MC. Adipocyte is a non-trivial, dynamic partner of breast cancer cells. Int J Dev Biol. 2011;55(7–9):851–859. doi: 10.1387/ijdb.113365jt. [DOI] [PubMed] [Google Scholar]
- 11.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 2011;71(7):2455–2465. doi: 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 12.Esquivel-Velazquez M, Ostoa-Saloma P, Palacios-Arreola MI, Nava-Castro KE, Castro JI, Morales-Montor J. The role of cytokines in breast cancer development and progression. J Interferon Cytokine Res. 2015;35(1):1–16. doi: 10.1089/jir.2014.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim SY, Kang JW, Song X, Kim BK, Yoo YD, Kwon YT. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell Signal. 2013;25(4):961–969. doi: 10.1016/j.cellsig.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middleton K, Jones J, Lwin Z, Coward JI. Interleukin-6: an angiogenic target in solid tumours. Crit Rev Oncol Hematol. 2014;89(1):129–139. doi: 10.1016/j.critrevonc.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Iwase S, Murakami T, Saito Y, Nakagawa K. Steep elevation of blood interleukin-6 (IL-6) associated only with late stages of cachexia in cancer patients. Eur Cytokine Netw. 2004;15(4):312–316. [PubMed] [Google Scholar]
- 16.Eichten A, Su J, Adler AP, Zhang L, Ioffe E, Parveen AA. Resistance to anti-VEGF therapy mediated by autocrine IL6/STAT3 signaling and overcome by IL6 blockade. Cancer Res. 2016;76(8):2327–2339. doi: 10.1158/0008-5472.CAN-15-1443. [DOI] [PubMed] [Google Scholar]
- 17.van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82(11):539–548. doi: 10.1078/0171-9335-00346. [DOI] [PubMed] [Google Scholar]
- 18.Soysal SD, Tzankov A, Muenst SE. Role of the tumor microenvironment in breast cancer. Pathobiology. 2015;82(3–4):142–152. doi: 10.1159/000430499. [DOI] [PubMed] [Google Scholar]
- 19.Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9(Suppl. 5):10–17. doi: 10.1634/theoncologist.9-90005-10. [DOI] [PubMed] [Google Scholar]
- 20.Heinecke JL, Ridnour LA, Cheng RY, Switzer CH, Lizardo MM, Khanna C. Tumor microenvironment-based feed-forward regulation of NOS2 in breast cancer progression. Proc Natl Acad Sci U S A. 2014;111(17):6323–6328. doi: 10.1073/pnas.1401799111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez-Outschoorn U, Sotgia F, Lisanti MP. Tumor microenvironment and metabolic synergy in breast cancers: critical importance of mitochondrial fuels and function. Semin Oncol. 2014;41(2):195–216. doi: 10.1053/j.seminoncol.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 22.Hovey RC, Aimo L. Diverse and active roles for adipocytes during mammary gland growth and function. J Mammary Gland Biol Neoplasia. 2010;15(3):279–290. doi: 10.1007/s10911-010-9187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andarawewa KL, Motrescu ER, Chenard MP, Gansmuller A, Stoll I, Tomasetto C. Stromelysin-3 is a potent negative regulator of adipogenesis participating to cancer cell-adipocyte interaction/crosstalk at the tumor invasive front. Cancer Res. 2005;65(23):10862–10871. doi: 10.1158/0008-5472.CAN-05-1231. [DOI] [PubMed] [Google Scholar]
- 24.Fletcher SJ, Sacca PA, Pistone-Creydt M, Colo FA, Serra MF, Santino FE. Human breast adipose tissue: characterization of factors that change during tumor progression in human breast cancer. J Exp Clin Cancer Res. 2017;36(1):26–38. doi: 10.1186/s13046-017-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol. 2014;76:225–249. doi: 10.1146/annurev-physiol-021113-170252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rousset S, Alves-Guerra MC, Mozo J, Miroux B, Cassard-Doulcier AM, Bouillaud F. The biology of mitochondrial uncoupling proteins. Diabetes. 2004;53(Suppl. 1):S130–S135. doi: 10.2337/diabetes.53.2007.s130. [DOI] [PubMed] [Google Scholar]
- 28.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–1263. doi: 10.1038/nm.3361. [DOI] [PubMed] [Google Scholar]
- 29.Bochet L, Lehuede C, Dauvillier S, Wang YY, Dirat B, Laurent V. Adipocyte-derived fibroblasts promote tumor progression and contribute to the desmoplastic reaction in breast cancer. Cancer Res. 2013;73(18):5657–5668. doi: 10.1158/0008-5472.CAN-13-0530. [DOI] [PubMed] [Google Scholar]
- 30.Lee J, Hong BS, Ryu HS, Lee HB, Lee M, Park IA. Transition into inflammatory cancer-associated adipocytes in breast cancer microenvironment requires microRNA regulatory mechanism. PLoS One. 2017;12(3):e0174126. doi: 10.1371/journal.pone.0174126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petruzzelli M, Schweiger M, Schreiber R, Campos-Olivas R, Tsoli M, Allen J. A switch from white to brown fat increases energy expenditure in cancer-associated cachexia. Cell Metab. 2014;20(3):433–447. doi: 10.1016/j.cmet.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Wang YY, Attane C, Milhas D, Dirat B, Dauvillier S, Guerard A. Mammary adipocytes stimulate breast cancer invasion through metabolic remodeling of tumor cells. JCI Insight. 2017;2(4):e87489. doi: 10.1172/jci.insight.87489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoy AJ, Balaban S, Saunders DN. Adipocyte-tumor cell metabolic crosstalk in breast cancer. Trends Mol Med. 2017;23(5):381–392. doi: 10.1016/j.molmed.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Wen YA, Xing X, Harris JW, Zaytseva YY, Mitov MI, Napier DL. Adipocytes activate mitochondrial fatty acid oxidation and autophagy to promote tumor growth in colon cancer. Cell Death Dis. 2017;8(2):e2593. doi: 10.1038/cddis.2017.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee Y, Jung WH, Koo JS. Adipocytes can induce epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res Treat. 2015;153(2):323–335. doi: 10.1007/s10549-015-3550-9. [DOI] [PubMed] [Google Scholar]
- 36.Picon-Ruiz M, Pan C, Drews-Elger K, Jang K, Besser AH, Zhao D. Interactions between adipocytes and breast cancer cells stimulate cytokine production and drive Src/Sox2/miR-302b–mediated malignant progression. Cancer Res. 2016;76(2):491–504. doi: 10.1158/0008-5472.CAN-15-0927. [DOI] [PubMed] [Google Scholar]
- 37.Muller C. Tumour-surrounding adipocytes are active players in breast cancer progression. Ann Endocrinol (Paris) 2013;74(2):108–110. doi: 10.1016/j.ando.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Walter M, Liang S, Ghosh S, Hornsby PJ, Li R. Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells. Oncogene. 2009;28(30):2745–2755. doi: 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Gao C, Meng K, Qiao H, Wang Y. Human adipocytes stimulate invasion of breast cancer MCF-7 cells by secreting IGFBP-2. PLoS One. 2015;10(3):e0119348. doi: 10.1371/journal.pone.0119348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jarde T, Perrier S, Vasson MP, Caldefie-Chezet F. Molecular mechanisms of leptin and adiponectin in breast cancer. Eur J Cancer. 2011;47(1):33–43. doi: 10.1016/j.ejca.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Nalabolu MR, Palasamudram K, Jamil K. Adiponectin and leptin molecular actions and clinical significance in breast cancer. Int J Hematol Oncol Stem Cell Res. 2014;8(1):31–40. [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang F, Chen Y, Heiman M, Dimarchi R. Leptin: structure, function and biology. Vitam Horm. 2005;71:345–372. doi: 10.1016/S0083-6729(05)71012-8. [DOI] [PubMed] [Google Scholar]
- 43.Jarde T, Caldefie-Chezet F, Damez M, Mishellany F, Penault-Llorca F, Guillot J. Leptin and leptin receptor involvement in cancer development: a study on human primary breast carcinoma. Oncol Rep. 2008;19(4):905–911. [PubMed] [Google Scholar]
- 44.Strong AL, Ohlstein JF, Biagas BA, Rhodes LV, Pei DT, Tucker HA. Leptin produced by obese adipose stromal/stem cells enhances proliferation and metastasis of estrogen receptor positive breast cancers. Breast Cancer Res. 2015;17:112–128. doi: 10.1186/s13058-015-0622-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ando S, Barone I, Giordano C, Bonofiglio D, Catalano S. The multifaceted mechanism of leptin signaling within tumor microenvironment in driving breast cancer growth and progression. Front Oncol. 2014;4:340–346. doi: 10.3389/fonc.2014.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caldefie-Chezet F, Damez M, de Latour M, Konska G, Mishellani F, Fusillier C. Leptin: a proliferative factor for breast cancer? Study on human ductal carcinoma. Biochem Biophys Res Commun. 2005;334(3):737–741. doi: 10.1016/j.bbrc.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien SN, Welter BH, Price TM. Presence of leptin in breast cell lines and breast tumors. Biochem Biophys Res Commun. 1999;259(3):695–698. doi: 10.1006/bbrc.1999.0843. [DOI] [PubMed] [Google Scholar]
- 48.Wolfson B, Eades G, Zhou Q. Adipocyte activation of cancer stem cell signaling in breast cancer. World J Biol Chem. 2015;6(2):39–47. doi: 10.4331/wjbc.v6.i2.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Q, Banaszak L, Fracci S, Basali D, Dunlap SM, Hursting SD. Leptin receptor maintains cancer stem-like properties in triple negative breast cancer cells. Endocr Relat Cancer. 2013;20(6):797–808. doi: 10.1530/ERC-13-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38(7):904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 51.Vona-Davis L, Rose DP. Angiogenesis, adipokines and breast cancer. Cytokine Growth Factor Rev. 2009;20(3):193–201. doi: 10.1016/j.cytogfr.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 52.Obeid S, Hebbard L. Role of adiponectin and its receptors in cancer. Cancer Biol Med. 2012;9(4):213–220. doi: 10.7497/j.issn.2095-3941.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X, Wang Y. Adiponectin and breast cancer. Med Oncol. 2011;28(4):1288–1295. doi: 10.1007/s12032-010-9617-x. [DOI] [PubMed] [Google Scholar]
- 54.Dos Santos E, Benaitreau D, Dieudonne MN, Leneveu MC, Serazin V, Giudicelli Y. Adiponectin mediates an antiproliferative response in human MDA-MB 231 breast cancer cells. Oncol Rep. 2008;20(4):971–977. [PubMed] [Google Scholar]
- 55.Kang JH, Lee YY, Yu BY, Yang BS, Cho KH, Yoon DK. Adiponectin induces growth arrest and apoptosis of MDA-MB-231 breast cancer cell. Arch Pharm Res. 2005;28(11):1263–1269. doi: 10.1007/BF02978210. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Lam KS, Xu JY, Lu G, Xu LY, Cooper GJ. Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J Biol Chem. 2005;280(18):18341–18347. doi: 10.1074/jbc.M501149200. [DOI] [PubMed] [Google Scholar]
- 57.Jarde T, Caldefie-Chezet F, Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr Relat Cancer. 2009;16(4):1197–1210. doi: 10.1677/ERC-09-0043. [DOI] [PubMed] [Google Scholar]
- 58.Miyoshi Y, Funahashi T, Kihara S, Taguchi T, Tamaki Y, Matsuzawa Y. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9(15):5699–5704. [PubMed] [Google Scholar]
- 59.Galic S, Oakhill JS, Steinberg GR. Adipose tissue as an endocrine organ. Mol Cell Endocrinol. 2010;316(2):129–139. doi: 10.1016/j.mce.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 60.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 61.Dethlefsen C, Hojfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat. 2013;138(3):657–664. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 62.Kishimoto T. Interleukin-6: discovery of a pleiotropic cytokine. Arthritis Res Ther. 2006;8(Suppl. 2):S2. doi: 10.1186/ar1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374(Pt 1):1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334(Pt 2):297–314. doi: 10.1042/bj3340297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grivennikov S, Karin M. Autocrine IL-6 signaling: a key event in tumorigenesis? Cancer Cell. 2008;13(1):7–9. doi: 10.1016/j.ccr.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 66.Wang SW, Sun YM. The IL-6/JAK/STAT3 pathway: potential therapeutic strategies in treating colorectal cancer (Review) Int J Oncol. 2014;44(4):1032–1040. doi: 10.3892/ijo.2014.2259. [DOI] [PubMed] [Google Scholar]
- 67.Kuang Y, Zhang Z, Zhang X. Interleukin-6 and its soluble receptors in human breast cancer. Zhonghua Zhong Liu Za Zhi. 1998;20(4):305–307. [PubMed] [Google Scholar]
- 68.Sanguinetti A, Santini D, Bonafe M, Taffurelli M, Avenia N. Interleukin-6 and pro inflammatory status in the breast tumor microenvironment. World J Surg Oncol. 2015;13:129–135. doi: 10.1186/s12957-015-0529-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurebayashi J. Regulation of interleukin-6 secretion from breast cancer cells and its clinical implications. Breast Cancer. 2000;7(2):124–129. doi: 10.1007/BF02967443. [DOI] [PubMed] [Google Scholar]
- 70.Chang Q, Daly L, Bromberg J. The IL-6 feed-forward loop: a driver of tumorigenesis. Semin Immunol. 2014;26(1):48–53. doi: 10.1016/j.smim.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 71.Path G, Bornstein SR, Gurniak M, Chrousos GP, Scherbaum WA, Hauner H. Human breast adipocytes express interleukin-6 (IL-6) and its receptor system: increased IL-6 production by beta-adrenergic activation and effects of IL-6 on adipocyte function. J Clin Endocrinol Metab. 2001;86(5):2281–2288. doi: 10.1210/jcem.86.5.7494. [DOI] [PubMed] [Google Scholar]
- 72.Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia. 2013;15(7):848–862. doi: 10.1593/neo.13706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bournazou E, Bromberg J. Targeting the tumor microenvironment: JAK-STAT3 signaling. JAKSTAT. 2013;2(2):e23828. doi: 10.4161/jkst.23828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knupfer H, Preiss R. Significance of interleukin-6 (IL-6) in breast cancer (review) Breast Cancer Res Treat. 2007;102(2):129–135. doi: 10.1007/s10549-006-9328-3. [DOI] [PubMed] [Google Scholar]
- 75.Lin S, Gan Z, Han K, Yao Y, Min D. Interleukin-6 as a prognostic marker for breast cancer: a meta-analysis. Tumori. 2015;101(5):535–541. doi: 10.5301/tj.5000357. [DOI] [PubMed] [Google Scholar]
- 76.Ara T, Declerck YA. Interleukin-6 in bone metastasis and cancer progression. Eur J Cancer. 2010;46(7):1223–1231. doi: 10.1016/j.ejca.2010.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sasser AK, Sullivan NJ, Studebaker AW, Hendey LF, Axel AE, Hall BM. Interleukin-6 is a potent growth factor for ER-alpha-positive human breast cancer. FASEB J. 2007;21(13):3763–3770. doi: 10.1096/fj.07-8832com. [DOI] [PubMed] [Google Scholar]
- 78.Xie G, Yao Q, Liu Y, Du S, Liu A, Guo Z. IL-6–induced epithelial-mesenchymal transition promotes the generation of breast cancer stem-like cells analogous to mammosphere cultures. Int J Oncol. 2012;40(4):1171–1179. doi: 10.3892/ijo.2011.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–1428. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Heerboth S, Housman G, Leary M, Longacre M, Byler S, Lapinska K. EMT and tumor metastasis. Clin Transl Med. 2015;4:6–19. doi: 10.1186/s40169-015-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15(3):178–196. doi: 10.1038/nrm3758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beerling E, Seinstra D, de Wit E, Kester L, van der Velden D, Maynard C. Plasticity between epithelial and mesenchymal states unlinks EMT from metastasis-enhancing stem cell capacity. Cell Rep. 2016;14(10):2281–2288. doi: 10.1016/j.celrep.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moustakas A, Heldin CH. Signaling networks guiding epithelial-mesenchymal transitions during embryogenesis and cancer progression. Cancer Sci. 2007;98(10):1512–1520. doi: 10.1111/j.1349-7006.2007.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takebe N, Warren RQ, Ivy SP. Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. Breast Cancer Res. 2011;13(3):211–222. doi: 10.1186/bcr2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T. Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res. 1993;53(7):1696–1701. [PubMed] [Google Scholar]
- 86.Carter JC, Church FC. Mature breast adipocytes promote breast cancer cell motility. Exp Mol Pathol. 2012;92(3):312–317. doi: 10.1016/j.yexmp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 87.Chamras H, Bagga D, Elstner E, Setoodeh K, Koeffler HP, Heber D. Preadipocytes stimulate breast cancer cell growth. Nutr Cancer. 1998;32(2):59–63. doi: 10.1080/01635589809514719. [DOI] [PubMed] [Google Scholar]
- 88.Nieman KM, Romero IL, Van Houten B, Lengyel E. Adipose tissue and adipocytes support tumorigenesis and metastasis. Biochim Biophys Acta. 2013;1831(10):1533–1541. doi: 10.1016/j.bbalip.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O'Brien CA, Kreso A, Jamieson CH. Cancer stem cells and self-renewal. Clin Cancer Res. 2010;16(12):3113–3120. doi: 10.1158/1078-0432.CCR-09-2824. [DOI] [PubMed] [Google Scholar]
- 90.Owens TW, Naylor MJ. Breast cancer stem cells. Front Physiol. 2013;4:225–235. doi: 10.3389/fphys.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pradella D, Naro C, Sette C, Ghigna C. EMT and stemness: flexible processes tuned by alternative splicing in development and cancer progression. Mol Cancer. 2017;16(1):8. doi: 10.1186/s12943-016-0579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang SS, Jiang J, Liang XH, Tang YL. Links between cancer stem cells and epithelial-mesenchymal transition. Onco Targets Ther. 2015;8:2973–2980. doi: 10.2147/OTT.S91863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One. 2008;3(8):e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.May CD, Sphyris N, Evans KW, Werden SJ, Guo W, Mani SA. Epithelial-mesenchymal transition and cancer stem cells: a dangerously dynamic duo in breast cancer progression. Breast Cancer Res. 2011;13(1):202–212. doi: 10.1186/bcr2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wright MH, Calcagno AM, Salcido CD, Carlson MD, Ambudkar SV, Varticovski L. Brca1 breast tumors contain distinct CD44+/CD24− and CD133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008;10(1):R10. doi: 10.1186/bcr1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69(4):1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petersen OW, Polyak K. Stem cells in the human breast. Cold Spring Harb Perspect Biol. 2010;2(5):a003160. doi: 10.1101/cshperspect.a003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bharti R, Dey G, Mandal M. Cancer development, chemoresistance, epithelial to mesenchymal transition and stem cells: a snapshot of IL-6 mediated involvement. Cancer Lett. 2016;375(1):51–61. doi: 10.1016/j.canlet.2016.02.048. [DOI] [PubMed] [Google Scholar]
- 100.Takebe N, Harris PJ, Warren RQ, Ivy SP. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat Rev Clin Oncol. 2011;8(2):97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 101.Tisdale MJ. Biology of cachexia. J Natl Cancer Inst. 1997;89(23):1763–1773. doi: 10.1093/jnci/89.23.1763. [DOI] [PubMed] [Google Scholar]
- 102.Vaughan VC, Martin P, Lewandowski PA. Cancer cachexia: impact, mechanisms and emerging treatments. J Cachexia Sarcopenia Muscle. 2013;4(2):95–109. doi: 10.1007/s13539-012-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Porporato PE. Understanding cachexia as a cancer metabolism syndrome. Oncogene. 2016;5:e200. doi: 10.1038/oncsis.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fearon KC, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–166. doi: 10.1016/j.cmet.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 105.Nedergaard J, Cannon B. The browning of white adipose tissue: some burning issues. Cell Metab. 2014;20(3):396–407. doi: 10.1016/j.cmet.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 106.Lo KA, Sun L. Turning WAT into BAT: a review on regulators controlling the browning of white adipocytes. Biosci Rep. 2013;33(5):33–42. doi: 10.1042/BSR20130046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Abdullahi A, Jeschke MG. White adipose tissue browning: a double-edged sword. Trends Endocrinol Metab. 2016;27(8):542–552. doi: 10.1016/j.tem.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carson JA, Baltgalvis KA. Interleukin 6 as a key regulator of muscle mass during cachexia. Exerc Sport Sci Rev. 2010;38(4):168–176. doi: 10.1097/JES.0b013e3181f44f11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Strassmann G, Fong M, Kenney JS, Jacob CO. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J Clin Invest. 1992;89(5):1681–1684. doi: 10.1172/JCI115767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jones VS, Huang RY, Chen LP, Chen ZS, Fu L, Huang RP. Cytokines in cancer drug resistance: cues to new therapeutic strategies. Biochim Biophys Acta. 2016;1865(2):255–265. doi: 10.1016/j.bbcan.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 111.Kumari N, Dwarakanath BS, Das A, Bhatt AN. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37(9):11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 112.Coward J, Kulbe H, Chakravarty P, Leader D, Vassileva V, Leinster DA. Interleukin-6 as a therapeutic target in human ovarian cancer. Clin Cancer Res. 2011;17(18):6083–6096. doi: 10.1158/1078-0432.CCR-11-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu CT, Chen MF, Chen WC, Hsieh CC. The role of IL-6 in the radiation response of prostate cancer. Radiat Oncol. 2013;8:159–170. doi: 10.1186/1748-717X-8-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bachelot T, Ray-Coquard I, Menetrier-Caux C, Rastkha M, Duc A, Blay JY. Prognostic value of serum levels of interleukin 6 and of serum and plasma levels of vascular endothelial growth factor in hormone-refractory metastatic breast cancer patients. Br J Cancer. 2003;88(11):1721–1726. doi: 10.1038/sj.bjc.6600956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Conze D, Weiss L, Regen PS, Bhushan A, Weaver D, Johnson P. Autocrine production of interleukin 6 causes multidrug resistance in breast cancer cells. Cancer Res. 2001;61(24):8851–8858. [PubMed] [Google Scholar]
- 116.Jiang XP, Yang DC, Elliott RL, Head JF. Down-regulation of expression of interleukin-6 and its receptor results in growth inhibition of MCF-7 breast cancer cells. Anticancer Res. 2011;31(9):2899–2906. [PubMed] [Google Scholar]
- 117.Duong MN, Cleret A, Matera EL, Chettab K, Mathe D, Valsesia-Wittmann S. Adipose cells promote resistance of breast cancer cells to trastuzumab-mediated antibody-dependent cellular cytotoxicity. Breast Cancer Res. 2015;17:57–71. doi: 10.1186/s13058-015-0569-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bochet L, Meulle A, Imbert S, Salles B, Valet P, Muller C. Cancer-associated adipocytes promotes breast tumor radioresistance. Biochem Biophys Res Commun. 2011;411(1):102–106. doi: 10.1016/j.bbrc.2011.06.101. [DOI] [PubMed] [Google Scholar]
- 119.Gopinathan G, Milagre C, Pearce OM, Reynolds LE, Hodivala-Dilke K, Leinster DA. Interleukin-6 stimulates defective angiogenesis. Cancer Res. 2015;75(15):3098–3107. doi: 10.1158/0008-5472.CAN-15-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24(36):5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 121.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 122.Niu G, Wright KL, Huang M, Song L, Haura E, Turkson J. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21(13):2000–2008. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 123.Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22(10):1517–1527. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- 124.Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest. 2007;117(12):3988–4002. doi: 10.1172/JCI32533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Trikha M, Corringham R, Klein B, Rossi JF. Targeted anti–interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clin Cancer Res. 2003;9(13):4653–4665. [PMC free article] [PubMed] [Google Scholar]
- 126.Lukaszewicz M, Mroczko B, Szmitkowski M. Clinical significance of interleukin-6 (IL-6) as a prognostic factor of cancer disease. Pol Arch Med Wewn. 2007;117(5–6):247–251. [PubMed] [Google Scholar]
- 127.Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121(9):3375–3383. doi: 10.1172/JCI57158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rossi JF, Lu ZY, Jourdan M, Klein B. Interleukin-6 as a therapeutic target. Clin Cancer Res. 2015;21(6):1248–1257. doi: 10.1158/1078-0432.CCR-14-2291. [DOI] [PubMed] [Google Scholar]
- 129.Li J, Hu XF, Xing PX. CNTO-328 (Centocor) Curr Opin Investig Drugs. 2005;6(6):639–645. [PubMed] [Google Scholar]
- 130.Zaki MH, Nemeth JA, Trikha M. CNTO 328, a monoclonal antibody to IL-6, inhibits human tumor-induced cachexia in nude mice. Int J Cancer. 2004;111(4):592–595. doi: 10.1002/ijc.20270. [DOI] [PubMed] [Google Scholar]
- 131.Roxburgh CS, McMillan DC. Therapeutics targeting innate immune/inflammatory responses through the interleukin-6/JAK/STAT signal transduction pathway in patients with cancer. Transl Res. 2016;167(1):61–66. doi: 10.1016/j.trsl.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 132.Yanaihara N, Hirata Y, Yamaguchi N, Noguchi Y, Saito M, Nagata C. Antitumor effects of interleukin-6 (IL-6)/interleukin-6 receptor (IL-6R) signaling pathway inhibition in clear cell carcinoma of the ovary. Mol Carcinog. 2016;55(5):832–841. doi: 10.1002/mc.22325. [DOI] [PubMed] [Google Scholar]
- 133.Rossi JF, Negrier S, James ND, Kocak I, Hawkins R, Davis H. A phase I/II study of siltuximab (CNTO 328), an anti–interleukin-6 monoclonal antibody, in metastatic renal cell cancer. Br J Cancer. 2010;103(8):1154–1162. doi: 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Puchalski T, Prabhakar U, Jiao Q, Berns B, Davis HM. Pharmacokinetic and pharmacodynamic modeling of an anti–interleukin-6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2010;16(5):1652–1661. doi: 10.1158/1078-0432.CCR-09-2581. [DOI] [PubMed] [Google Scholar]
- 135.Fizazi K, De Bono JS, Flechon A, Heidenreich A, Voog E, Davis NB. Randomised phase II study of siltuximab (CNTO 328), an anti–IL-6 monoclonal antibody, in combination with mitoxantrone/prednisone versus mitoxantrone/prednisone alone in metastatic castration-resistant prostate cancer. Eur J Cancer. 2012;48(1):85–93. doi: 10.1016/j.ejca.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 136.Karkera J, Steiner H, Li W, Skradski V, Moser PL, Riethdorf S. The anti-interleukin-6 antibody siltuximab down-regulates genes implicated in tumorigenesis in prostate cancer patients from a phase I study. Prostate. 2011;71(13):1455–1465. doi: 10.1002/pros.21362. [DOI] [PubMed] [Google Scholar]
- 137.Alten R, Maleitzke T. Tocilizumab: a novel humanized anti–interleukin 6 (IL-6) receptor antibody for the treatment of patients with non-RA systemic, inflammatory rheumatic diseases. Ann Med. 2013;45(4):357–363. doi: 10.3109/07853890.2013.771986. [DOI] [PubMed] [Google Scholar]
- 138.Kang S, Tanaka T, Kishimoto T. Therapeutic uses of anti-interleukin-6 receptor antibody. Int Immunol. 2015;27(1):21–29. doi: 10.1093/intimm/dxu081. [DOI] [PubMed] [Google Scholar]