Abstract
OBJECTIVE: To investigate promoting factors for background parenchymal enhancement (BPE) in MR mammography (MRM). METHODS: 146 patients were retrospectively evaluated, including 91 high-risk patients (50 BRCA patients, 41 patients with elevated lifetime risk). 56 screening patients were matched to the high-risk cases on the basis of age. The correlation of BPE with factors such as fibroglandular tissue (FGT), age, menopausal status, breast cancer, high-risk precondition as well as motion were investigated using linear regression. RESULTS: BPE positively correlated with FGT (P < .001) and negatively correlated with menopausal status (P < .001). Cancer did not show an effect on BPE (P > .05). A high-risk precondition showed a significant impact on the formation of BPE (P < .05). However, when corrected for motion, the correlation between BPE and a high-risk precondition became weak and insignificant, and a highly significant association between BPE and motion was revealed (P < .01). CONCLUSION: BPE positively correlated with FGT and negatively correlated with age. Cancer did not have an effect on BPE. A high-risk precondition appears to have a negative effect on BPE. However, when corrected for motion, high-risk preconditions became insignificant. Technical as well as physiological influences seem to play an important role in the formation of BPE.
Introduction
Breast parenchymal enhancement (BPE) is defined as normal breast tissue enhancement on the magnetic resonance mammography (MRM) and was recently added to the updated American College of Radiology Breast Imaging Reporting and Data System (BI-RADS) atlas [1].
Destinct BPE is considered to lead to false positive and sometimes even false negative results. Even though every breast radiologist faces BPE in clinical routine, little is known about its origin.
Understanding BPE can thus be considered key to understanding underlying breast physiology and thus improved diagnosis in MRM.
Some authors have analyzed possible factors influencing BPE, including the dose and rate of contrast medium [2] the vascular supply of the breast [3], and a variety of hormone effects and antihormonal treatment [4], [5], [6], [7], [8], [9], [10]. BPE is thought to coincide with the amount of blood flow in fibroglandular tissue and may reflect breast activity [11]. Other studies [12] have suggested BPE to be a major risk factor for the development of breast cancer, possibly as important as and independent of mammographic density, which could serve as an imaging biomarker of malignant transformation. Some studies have proposed [13], [14] proposed data in which BPE was associated with a higher chance of developing breast cancer in women at high risk for cancer. Other recent studies [15], [16], [17], [18] have contradictorily suggested BPE to be an imaging characteristic without increased cancer coincidence in asymptomatic or high-risk patients.
This uncertainty about the origin of BPE raised the question of this study: What causes BPE, and is there truly an association between BPE and breast cancer risk?
The purpose of this study was to investigate promoting factors for BPE in a high-risk patient collective.
Materials and Methods
Patient Collective
The institutional review board granted a waiver of authorization and patient consent for our retrospective study, which was complaint with the Health Insurance Portability and Accountability Act by the University Medical Centre Mannheim. For this retrospective study, all high-risk patients examined in our university hospital between December 2008 and June 2015 were considered. High-risk criteria included the presence of a known genetic mutation (e.g., BRCA 1 or BRCA 2) and determination of a lifetime risk of breast of greater than 20% by using a risk assessment tool that is primarily based on family history.
In total, 146 women with at least 1 MRM were included in this study. Of the 91 included high-risk patients, 11 were diagnosed with breast cancer, 30 patients had received prior unilateral mastectomy upon a previous breast cancer diagnosis, and 50 patients served as a high-risk control group without suspicious findings (Table 1). Additionally, 56 non–high-risk screening patients, for whom MR served as problem-solver, were selected and matched to the high-risk cases on the basis of age (mean age 48.7 ± 25 years). For the cancer cohort, the examination date of cancer detection was considered. For the control cohort, the postoperative cohort, and the screening population, only data from the latest examination were considered if multiple MRM images were obtained during the study period.
Table 2.
Results Various Models of Impact Factors on BPE
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | |
|---|---|---|---|---|---|---|---|
| FGT (interreader average) | 0.55 (.09) ⁎⁎⁎ | 0.47 (0.09) ⁎⁎⁎ | 0.47 (.09) ⁎⁎⁎ | 0.48 (.09) ⁎⁎⁎ | 0.44 (0.9) ⁎⁎⁎ | 0.39 (.09) ⁎⁎⁎ | 0.4 (.1) ⁎⁎⁎ |
| Age | −0.01 (.01) | ||||||
| Status after menopause | −0.46 (.09)⁎⁎⁎ | −0.48 (.17)⁎⁎ | −0.54 (.18)⁎⁎ | −0.55 (.16)⁎⁎ | −0.56 (.17) ⁎⁎⁎ | ||
| Cancer | −0.46 (.29) | −0.3 (.29) | −0.22 (.29) | −0.19 (.3) | |||
| High-risk status | −0.4 (.16)⁎ | −0.27 (.16) | −0.32 (.19) | ||||
| Motion | −0.25 (.09)⁎⁎ | −0.24 (.09)⁎⁎ | |||||
| Status after operation | 0.10 (.22) | ||||||
| Intercept | 0.91 (.26)⁎⁎⁎ | 1.75 (.52)⁎⁎⁎ | 1.29 (.29)⁎⁎⁎ | 1,3 (.28)⁎⁎⁎ | 1.66 (.31)⁎⁎⁎ | 1.22 (.34)⁎⁎⁎ | 1.22 (.34)⁎⁎⁎ |
Coefficients with standard errors in brackets.
Significance codes:
<.05.
<.01.
<.001. For example: model 1, impact of FGT on BPE; model 2, Impact of FGT, corrected for age.
Table 1.
High-Risk Population Characteristics
| Patient Collective | N (= 147) | |
|---|---|---|
| BRCA 1 | 27 (18.37%) | 91 high-risk patients |
| BRCA 2 | 23 (15.65%) | |
| Lifetime risk | 41 (27.9%) | |
| Non–high-risk | 56 (38.1%) | |
| High-risk patients | N (=91) | |
| Cancer diagnosed | 11 (12.09%) | |
| Postoperative status | 30 (32.97%) | |
| No cancer or previous surgery | 50 (54.94%) | |
Technique
MRM was performed with a 1.5-T system (Magnetom Avanto, Siemens Healthcare, Erlangen, Germany) by using a standard bilateral breast coil. The standardized protocol consisted of a T2-weighted turbo spin-echo sequence (2-mm axial slices, FOV 320 mm, matrix 512 × 512, TR/TE 7410/104 milliseconds), a short tau inversion recovery sequence (2-mm axial slices, FOV 320 mm, matrix 512 × 512, TR/TE 2350/50 milliseconds), and a T1-weighted 3D gradient-echo sequence (2-mm axial slices, FOV 320 mm, matrix 512 × 512, TR/TE 4.1/1.4 milliseconds) once before and six times after bolus injection of 0.1 mmol of GD-DTPA or Gd-DOTA per kilogram of body weight. The temporal resolution was 59 seconds for each dynamic acquisition. Sequence acquisition parameters have been previously reported [19].
Imaging Acquisition and Interpretation
All MRM images were independently reviewed by two fellowship-trained radiologists with 8 and 4 years of experience who specialized in breast imaging in accordance with BI-RADS [20]. The amount of fibroglandular tissue (FGT) on MR was evaluated as 1 = almost entirely fat (<25% glandular), 2 = scattered fibroglandular density (25%-50% glandular), 3 = heterogeneously dense (51%-75% glandular), 4 = extremely dense (>75% glandular). BPE was considered in the entire breast parenchyma and evaluated in the early post contrast set of the dynamic images according to the BI-RADS criteria. The evaluation of BPE was performed on the original set of images. Subtractions were not considered as we found them susceptible to artifacts, such as motion, potentially camouflaging BPE. BPE was defined as enhancement of the normal breast parenchyma: 1 = minimal, 2 = mild, 3 = moderate, or 4 = marked (Figure 1, Figure 2). Additionally, patient motion as an additional possible influence on BPE was assessed in the dynamic set of images by both readers as 1 = minimal, 2 = mild, 3 = moderate, or 4 = marked.
Figure 1.
Examples of minimal BPE in the dynamic set of images (first row left to right: precontrast, 1 minute, 2 minutes post injection of 0,1 mmol/kg; second row left to right: third, fourth, and fifth minute post injection of 0,1 mmol/kg gadolinium).
Figure 2.
Examples of marked BPE in the dynamic set of images (first row left to right: precontrast, 1 minute, 2 minutes post injection of 0,1 mmol/kg; second row left to right: third, fourth, and fifth minute post injection of 0,1 mmol/kg gadolinium).
Statistical Analysis
Linear regression was used to investigate the correlation between BPE and multiple factors. These are FGT, age, breast cancer factor, high-risk precondition, as well as motion in a cohort. The stepwise inclusion of these factors induces seven models as shown in table 2. Since there was scant information on the menopausal status of the women in this study, age of 50 years was defined as the boundary to assess the menopausal status (≥50 years postmenopausal, <50 years premenopausal), as suggested in other studies [21]. Kappa statistics were used to evaluate interreader interpretation [10]. The strength of the kappa agreement was defined as <0.00 = poor, 0.00-0.20 = slight, 0.21-0.40 = fair, 0.41-0.60 = moderate, 0.61-0.81 = substantial, or 0.81-1.00 = almost perfect. P < .05 was considered to indicate a significant difference for all comparisons. All computations were performed by using “R” statistical software (Project for statistical Computing 3.2.2).
Results
Interreader Agreement
Interobserver agreement can be considered substantial with ϰ = 0.653 for BPE and 0.794 for FGT.
BPE Promoting Factors
Linear regression analysis revealed that FGT is positively correlated with BPE both when investigating the bivariate relationship to BPE as well as when controlling for all other factors considered in this study (P < .001 in all seven models). The effect of FGT is moderately high with an increase of BPE of a little less than half a point for every unit increase of FGT. Patients with the highest FGT value show on average 1.2 points more in BPE compared to patients with the lowest value in FGT, holding all else constant. Age has no effect whatsoever on BPE (see model 2), while status after menopause has a consistent and significant effect. Holding all other factors constant, women after menopause exhibit on average 0.56 (P < .001) point less on the BPE scale than women before menopause. Following model 2, age has been dropped in subsequent models because status after menopause and age are highly correlated.
In our study, cancer was not associated with BPE (P > 0,05). A high-risk status at first seemed to show a negative effect on BPE. Model 5 shows that patients with BRCA1, BRCA2, or elevated lifetime risk present with BPE values that are on average 0.4 point (P < .05) lower than those of nonrisk patients. This effect dissolves, however, when adjusting for the degree of patient motion during the MR examination.
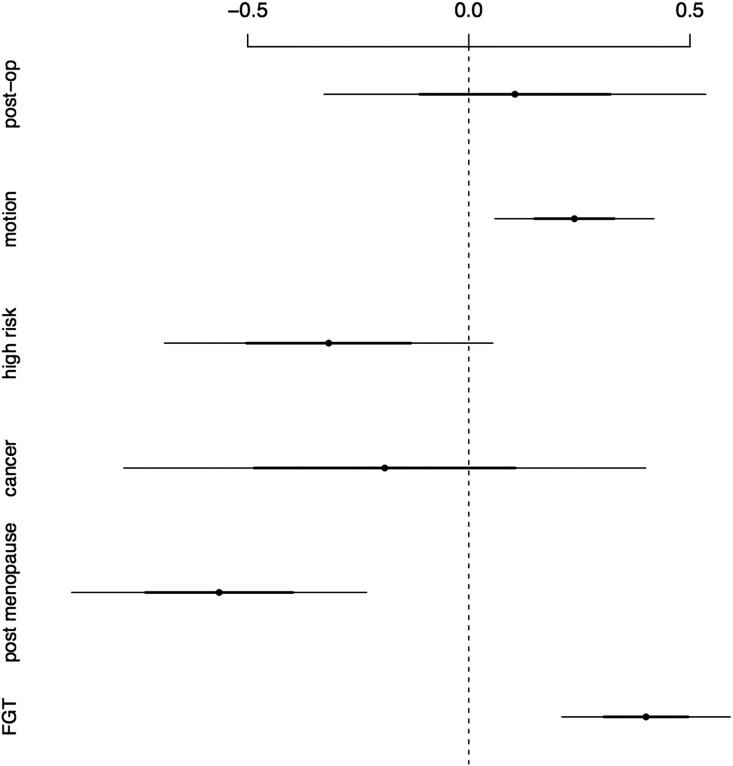
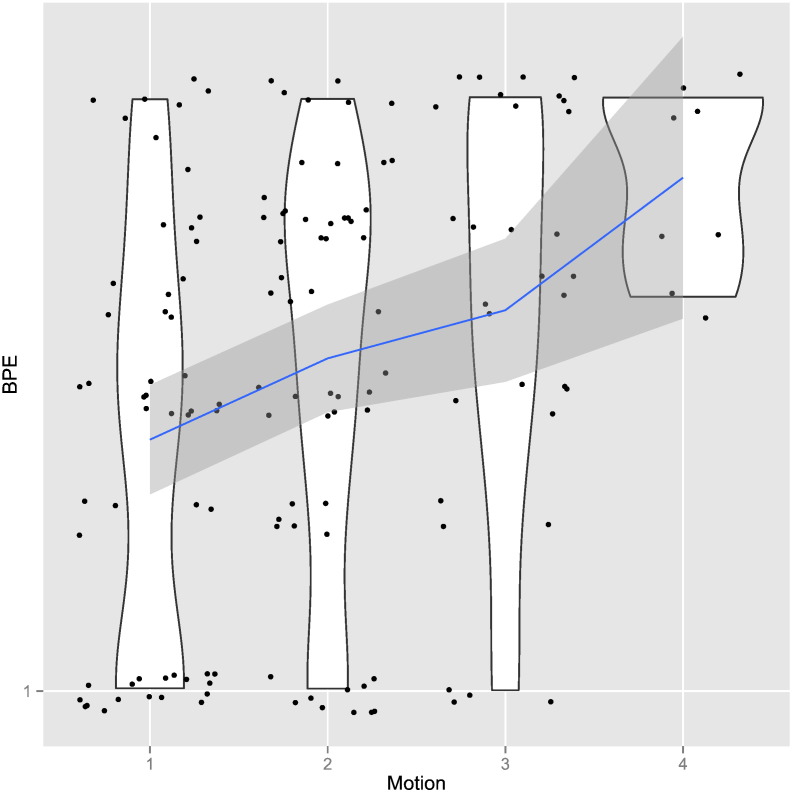
Motion in fact explains a considerable part of the variation in BPE. The difference between patients with the least movement and patients with the most movement is on average 0.7 increment in BPE. Motion has therefore the second strongest impact on BPE after FGT (recall that both are measured on a four-point scale). Whether patients had a status after surgery or not made neither a substantial nor statistically distinguishable difference in regards to BPE. Figure 3 visualizes the effect of various factors on BPE. Figure 4 graphically shows in detail our results for the effect of motion on the formation of BPE.
Figure 3.
Regression estimates with confidence intervals. The graph shows the coefficients along with their standard errors from the complete fitted model in Table 2. Dots represent coefficients and the size of the effect of a specific factor on BPE. Dots on the right-hand side indicate positive effects; dots on the left-hand side indicate negative effects on BPE. Where bars cross through the zero line, effects are not statistically distinguishable.
Figure 4.
Violin plot of motion on BPE: The blue line shows the fitted association between BPE and motion. The gray area indicates the 95% confidence interval. The width of the white area indicates distributional properties within each category. The broader these areas are, the more observations are associated with the respective value of BPE. It is evident from this plot that the relationship between motion and BPE is not deterministic. A number of patients exhibit high levels of BPE despite a low degree of motion. However, there are almost no patients with high levels of motion who do not show high levels of BPE, indicating that motion is a sufficient but not a necessary condition for high BPE levels.
Discussion
Background parenchymal enhancement has been a matter of scientific discussion for some time. As it can often be detected as contrast uptake of unspecific breast parenchyma, radiologists have ever since been trying to understand the diagnostic meaning of BPE as it distracts from truly enhancing lesions, causing false-positive and—even worse—false-negative diagnoses [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22]. Recent research has demonstrated only little effect of BPE on the diagnostic performance of breast MRI [15], [16]. This leaves the question whether hormonal or otherwise activated breast tissue as reflected by increeased BPE is associated with a higher risk of breast cancer, with BPE acting as an imaging biomarker in this respect.
Breast MRI screening today is mainly suggested only for women with high-risk backgrounds in current international guidelines, such as those published by the European Society of Breast Imaging, the European Society of Breast Cancer Specialists, and the American College of Radiology [20], [23], [24]. At the same time, there are only few studies examining BPE promoting factors in high-risk populations [6], [23], [25], [26].
To our knowledge, this is the first study to structurally examine factors on their effect on BPE in their codependence in a high-risk population. The correlation between BPE and FGT, age, and postmenopausal status has been investigated in other studies [12], [13], [14], [15], [16], [17], [18], [19], [20], [27], [28]. In line with some of these previous studies [5], [20], we have confirmatively found that BPE positively correlates with FGT in our high-risk patient collective. BPE is commonly considered to reflect blood flow in dense tissue, while FGT may represent the connective and epithelial tissues on MRM before the administration of the contrast agent [27].
Both BPE and FGT together may demonstrate normal breast epithelial cell proliferation. Therefore, they may have a similar tendency to change in breast MRI. Only few women in our study with dense breasts showed little or no BPE, whereas women with scattered fibroglandular tissue may show marked BPE. Upon review of the literature, some authors have suggested that there is no correlation between BPE and mammographic density when MRM was performed in the first part of the menstrual cycle [26], [27]. This finding could be a hint towards other influences on the degree of BPE essentially independent of the amount of FGT.
In our study, there was no correlation between BPE and age. However, when we assessed 50 years of age as the postmenopausal threshold for further assessment as suggested in other studies [21], BPE correlated negatively with menopausal status. Our findings are therefore in line with King et al., strongly suggesting that the effect of menopausal status on BPE is substantially greater than the effect of age alone [6]. BPE may therefore be assumed as a sensitive parameter to the hormonal changes coinciding with menopause. Status after operation or radiotherapy has no effect on BPE in our study, which is somewhat contradictory to the findings of Dontchos et al., suggesting decreased BPE levels in patients having undergone prior treatment with radiation after surgery [13]. One reason may be that changes in breast tissue are transitional after surgery. The correlation between BPE levels and patients’ risk for developing breast cancer is still a much-discussed topic. Several studies have investigated a possible connection between BPE and cancer. However, results were variable and inconsistent [6], [7], [8], [9], [10], [11], [12], [13], [16], [18], [25].
King et al. as well as Dontchos et al. proposed increased levels of BPE as an important risk factor for breast cancer. Hambly et al., DeMartini et al., and Albert et al. oppositely suggested that the incidence of breast cancer would not increase with BPE levels. In our data, there was no significant association between BPE and an elevated breast cancer risk, although it has to be critically added that our sample size of 11 cancer cases is rather small and may not allow for reliable statistical results (see study limitations). In line with Humbly and DeMartini et al., a high-risk precondition even has a negative effect on BPE, with general BPE levels in the high-risk population significantly below the screening population.
Yet, when corrected for motion, the effect of a high-risk precondition on the formation of BPE becomes insignificant and weak. At the same time, a highly significant association between BPE and motion was revealed. Motion as a clinically measurable parameter to some degree represents the patients’ physiological vital status (e.g., elevated blood pressure due to patient anxiety) and may be seen a proxy for “patient-external” factors, i.e., technique. Vice versa, we may hypothesize that elevated motion levels are somewhat connected to a non–high-risk patient collective. To our knowledge, this is the first study revealing a possible connection of BPE and external factors like patient motion.
Study Limitations
The estimate for cancer has to be considered with some caution owing to the fact that only 11 patients in our sample were diagnosed with carcinoma. At the same time, a larger sample size of cancer patients is needed to conduct further subgroup analyses in a high-risk population, such as BRCA gene mutations and family history. A second limitation of our study was the qualitative evaluation of BPE, FGT, and motion, making it potentially prone to interobserver variability. Even though the interobserver agreement was substantial in our study, it is still necessary to confirm our data with quantitative measurements, such as fully automated computerized methods. An additional limitation of this study was its retrospective nature. We depended on patient recollection and the electronic medical record for information. Therefore, this study did not control for patient-related issues such as menstrual cycle, menopausal status, and weight. However, as the state of the menstrual cycle is not systematically correlated/associated with any of the coefficients (except menopause) within our model, the effects of the regression analysis are not affected by menstrual effects. Our data were acquired with our specific technique in our university hospital. Further studies are needed to confirm our data with other techniques, e.g., using 2D sequences, known to be less susceptible to background enhancement. In our study, all women underwent MRM in the same unit with the same sequences, which will decrease the influence of oretical differences in T1 relaxation times on BPE.
In conclusion, BPE is associated with FGT and patient motion as well as status after menopause. We found no correlation of BPE and cancer, high-risk status, and status after surgery. Our results present “patient-external factors” (i.e., motion) as a novel set of factors to have a strongly positive impact on BPE. Physiological changes as well as technical examination parameters seem to play an important role in the formation of BPE. Further studies are needed.
Conflicts of Interest
None declared.
Grant Support
None declared.
Authors' Contributions
All authors contributed fundamentally to this study and participated sufficiently to take public responsibility for its content. Chao You did the literature review, interpreted the results, and drafted the manuscript. Chao You and Clemens G. Kaiser designed the case-control study used for this analysis. Chao You, and Clemens G. Kaiser collected the data and were responsible for quality control of data and interpretation. Anna K. Kaiser performed the statistical analysis and interpreted the results. Yajia Gu, Weijun Peng, and Stefan O. Schönberg interpreted some of the results and edited the manuscript. Clemens G. Kaiser and Pascal Baltzer edited and finalized the manuscript. Publication is approved by all authors. There are no conflicts of interests associated with this work.
Acknowledgements
We thank all the patients of this study for their participation.
References
- 1.Burnside ES, Sickles EA, Bassett LW, Rubin DL, Lee CH, Ikeda DM, Mendelson EB, Wilcox PA, Butler PF, D’Orsi CJ. The ACR BI-RADS® Experience: Learning From History. J Am Coll Radiol. 2009;6:851–860. doi: 10.1016/j.jacr.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356–378. doi: 10.1148/radiol.2442051620. [DOI] [PubMed] [Google Scholar]
- 3.Giess CS, Yeh ED, Raza S, Birdwell RL. Background parenchymal enhancement at breast MR imaging: normal patterns, diagnostic challenges, and potential for false-positive and false-negative interpretation. Radiographics. 2014;34:234–247. doi: 10.1148/rg.341135034. [DOI] [PubMed] [Google Scholar]
- 4.Kuhl CK, Bieling HB, Gieseke J, Kreft BP, Sommer T, Lutterbey G, Schild HH. Healthy premenopausal breast parenchyma in dynamic contrast-enhanced MR imaging of the breast: normal contrast medium enhancement and cyclical-phase dependency. Radiology. 1997;203:137–144. doi: 10.1148/radiology.203.1.9122382. [DOI] [PubMed] [Google Scholar]
- 5.Taylor D, Lazberger J, Ives A, Wylie E, Saunders C. Reducing delay in the diagnosis of pregnancy-associated breast cancer: how imaging can help us. J Med Imaging Radiat Oncol. 2011;55:33–42. doi: 10.1111/j.1754-9485.2010.02227.x. [DOI] [PubMed] [Google Scholar]
- 6.King V, Gu Y, Kaplan JB, Brooks JD, Pike MC, Morris EA. Impact of menopausal status on background parenchymal enhancement and fibroglandular tissue on breast MRI. Eur Radiol. 2012;22:2641–2647. doi: 10.1007/s00330-012-2553-8. [DOI] [PubMed] [Google Scholar]
- 7.Pfleiderer SOR, Sachse S, Sauner D, Marx C, Malich A, Wurdinger S, Kaiser WA. Changes in magnetic resonance mammography due to hormone replacement therapy. Breast Cancer Res. 2004;6:R232–238. doi: 10.1186/bcr779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delille J-P, Slanetz PJ, Yeh ED, Kopans DB, Halpern EF, Garrido L. Hormone replacement therapy in postmenopausal women: breast tissue perfusion determined with MR imaging--initial observations. Radiology. 2005;235:36–41. doi: 10.1148/radiol.2351040012. [DOI] [PubMed] [Google Scholar]
- 9.King V, Goldfarb SB, Brooks JD, Sung JS, Nulsen BF, Jozefara JE, Pike MC, Dickler MN, Morris EA. Effect of aromatase inhibitors on background parenchymal enhancement and amount of fibroglandular tissue at breast MR imaging. Radiology. 2012;264:670–678. doi: 10.1148/radiol.12112669. [DOI] [PubMed] [Google Scholar]
- 10.King V, Kaplan J, Pike MC, Liberman L, David Dershaw D, Lee CH, Brooks JD, Morris EA. Impact of tamoxifen on amount of fibroglandular tissue, background parenchymal enhancement, and cysts on breast magnetic resonance imaging. Breast J. 2012;18:527–534. doi: 10.1111/tbj.12002. [DOI] [PubMed] [Google Scholar]
- 11.Pike MC, Pearce CL. Mammographic density, MRI background parenchymal enhancement and breast cancer risk. Ann Oncol. 2013;24(Suppl. 8):viii37–viii41. doi: 10.1093/annonc/mdt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King V, Brooks JD, Bernstein JL, Reiner AS, Pike MC, Morris EA. Background parenchymal enhancement at breast MR imaging and breast cancer risk. Radiology. 2011;260:50–60. doi: 10.1148/radiol.11102156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dontchos BN, Rahbar H, Partridge SC, Korde LA, Lam DL, Scheel JR, Peacock S, Lehman CD. Are Qualitative Assessments of Background Parenchymal Enhancement, Amount of Fibroglandular Tissue on MR Images, and Mammographic Density Associated with Breast Cancer Risk? Radiology. 2015;276:371–380. doi: 10.1148/radiol.2015142304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G, Moschetta M. Breast MRI background parenchymal enhancement (BPE) correlates with the risk of breast cancer. Magn Reson Imaging. 2016;34:173–176. doi: 10.1016/j.mri.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Baltzer PA, Dietzel M, Vag T, Burmeister H, Gajda M, Camara O, Pfleiderer SO, Kaiser WA. Clinical MR mammography: impact of hormonal status on background enhancement and diagnostic accuracy. Rofo. 2011;183:441–447. doi: 10.1055/s-0029-1246072. [DOI] [PubMed] [Google Scholar]
- 16.DeMartini WB, Liu F, Peacock S, Eby PR, Gutierrez RL, Lehman CD. Background parenchymal enhancement on breast MRI: impact on diagnostic performance. AJR Am J Roentgenol. 2012;198:W373–380. doi: 10.2214/AJR.10.6272. [DOI] [PubMed] [Google Scholar]
- 17.Bennani-Baiti B, Dietzel M, Baltzer PA. Correction: MRI Background Parenchymal Enhancement Is Not Associated with Breast Cancer. PLoS One. 2016;11:e0162936. doi: 10.1371/journal.pone.0158573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hambly NM, Liberman L, Dershaw DD, Brennan S, Morris EA. Background parenchymal enhancement on baseline screening breast MRI: impact on biopsy rate and short-interval follow-up. AJR Am J Roentgenol. 2011;196:218–224. doi: 10.2214/AJR.10.4550. [DOI] [PubMed] [Google Scholar]
- 19.Krammer J, Wasser K, Schnitzer A, Henzler T, Schoenberg SO, Kaiser CG. Axillary lymph node characterization in breast cancer patients using magnetic resonance mammography: a prospective comparative study with FDG PET-CT and healthy women. Eur J Radiol. 2013;82:2194–2198. doi: 10.1016/j.ejrad.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 20.A.C. of Radiology . Springer-Verlag; 2016. ACR BI-RADS®-Atlas der Mammadiagnostik: Richtlinien zu Befundung, Handlungsempfehlungen und Monitoring. [Google Scholar]
- 21.Müller-Schimpfle M, Ohmenhaüser K, Stoll P, Dietz K, Claussen CD. Menstrual cycle and age: influence on parenchymal contrast medium enhancement in MR imaging of the breast. Radiology. 1997;203:145–149. doi: 10.1148/radiology.203.1.9122383. [DOI] [PubMed] [Google Scholar]
- 22.Müller-Schimpfle M, Ohmenhäuser K, Claussen CD. Effect of age and menstrual cycle on mammography and MR mammography. Radiology. 1997;37:718–725. doi: 10.1007/s001170050273. [DOI] [PubMed] [Google Scholar]
- 23.Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008;18:1307–1318. doi: 10.1007/s00330-008-0863-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sardanelli F, Boetes C, Borisch B, Decker T, Federico M, Gilbert FJ, Helbich T, Heywang-Köbrunner SH, Kaiser WA, Kerin MJ. Magnetic resonance imaging of the breast: recommendations from the EUSOMA working group. Eur J Cancer. 2010;1990(46):1296–1316. doi: 10.1016/j.ejca.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Albert M, Schnabel F, Chun J, Schwartz S, Lee J, Klautau Leite AP, Moy L. The relationship of breast density in mammography and magnetic resonance imaging in high-risk women and women with breast cancer. Clin Imaging. 2015;39:987–992. doi: 10.1016/j.clinimag.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu S, Weinstein SP, DeLeo MJ, Conant EF, Chen J, Domchek SM, Kontos D. Quantitative assessment of background parenchymal enhancement in breast MRI predicts response to risk-reducing salpingo-oophorectomy: preliminary evaluation in a cohort of BRCA1/2 mutation carriers. Breast Cancer Res. 2015;17:67. doi: 10.1186/s13058-015-0577-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cubuk R, Tasali N, Narin B, Keskiner F, Celik L, Guney S. Correlation between breast density in mammography and background enhancement in MR mammography. Radiol Med. 2010;115:434–441. doi: 10.1007/s11547-010-0513-4. [DOI] [PubMed] [Google Scholar]
- 28.Bennani-Baiti B, Bennani-Baiti N, Baltzer PA. Diagnostic Performance of Breast Magnetic Resonance Imaging in Non-Calcified Equivocal Breast Findings: Results from a Systematic Review and Meta-Analysis. PLoS One. 2016;11:e0160346. doi: 10.1371/journal.pone.0160346. [DOI] [PMC free article] [PubMed] [Google Scholar]