Abstract
OBJECT: Our purpose was to provide data regarding relationships between different imaging and histopathological parameters in HNSCC. METHODS: MEDLINE library was screened for associations between different imaging parameters and histopathological features in HNSCC up to December 2017. Only papers containing correlation coefficients between different imaging parameters and histopathological findings were acquired for the analysis. RESULTS: Associations between 18F-FDG positron emission tomography (PET) and KI 67 were reported in 8 studies (236 patients). The pooled correlation coefficient was 0.20 (95% CI = [−0.04; 0.44]). Furthermore, in 4 studies (64 patients), associations between 18F-fluorothymidine PET and KI 67 were analyzed. The pooled correlation coefficient between SUVmax and KI 67 was 0.28 (95% CI = [−0.06; 0.94]). In 2 studies (23 patients), relationships between KI 67 and dynamic contrast-enhanced magnetic resonance imaging were reported. The pooled correlation coefficient between Ktrans and KI 67 was −0.68 (95% CI = [−0.91; −0.44]). Two studies (31 patients) investigated correlation between apparent diffusion coefficient (ADC) and KI 67. The pooled correlation coefficient was −0.61 (95% CI = [−0.84; −0.38]). In 2 studies (117 patients), relationships between 18F-FDG PET and p53 were analyzed. The pooled correlation coefficient was 0.0 (95% CI = [−0.87; 0.88]). There were 3 studies (48 patients) that investigated associations between ADC and tumor cell count in HNSCC. The pooled correlation coefficient was −0.53 (95% CI = [−0.74; −0.32]). Associations between 18F-FDG PET and HIF-1α were investigated in 3 studies (72 patients). The pooled correlation coefficient was 0.44 (95% CI = [−0.20; 1.08]). CONCLUSIONS: ADC may predict cell count and proliferation activity, and SUVmax may predict expression of HIF-1α in HNSCC. SUVmax cannot be used as surrogate marker for expression of KI 67 and p53.
Introduction
Head and neck squamous cell carcinoma (HNSCC) is one of the most frequent malignancies [1]. HNSCC shows often a worse prognosis, with a 5-year survival rate of 50% [2]. Multiple factors influence tumor biology in HNSCC. According to the literature, different molecular markers play a key role here [3]. Previous reports investigated numerous biomarkers and suggested that some histopathological parameters can predict tumor behavior in HNSCC [3], [4]. It has been shown that they provide information about tumor aggressiveness, prognosis, and therapy response [3], [4], [5]. For instance, proliferation index KI 67 predicts tumor aggressiveness in HNSCC [3]. Another biomarker, hypoxia-inducible factor (HIF)-1α, has been reported as predictor of worse prognosis of HNSCC [5].
Previously, some reports described significant associations between imaging parameters and histopathological features in HNSCC [6], [7], [8]. It has been shown that parameters of positron emission tomography (PET) like standardized uptake values (SUVs) correlated with KI 67 [7]. Furthermore, some reports indicated that apparent diffusion coefficient (ADC) as quantitative parameter of diffusion-weighted imaging (DWI) is associated with several biomarkers in HNSCC [8]. Finally, also some parameters of dynamic contrast-enhanced magnetic resonance imaging (DCE MRI), especially volume transfer constant Ktrans, have been reported to be associated with different histopathological features in HNSCC [9]. However, the reported data were inconsistent. While some authors found an association between imaging parameters and histological findings in HNSCC, others did not [6], [7], [8], [9], [10], [11], [12]. Furthermore, most studies investigated small number of patients only.
Therefore, the purpose of this meta-analysis was to provide data regarding relationships between different imaging and histopathological parameters in HNSCC.
Material and Methods
Data Acquisition
MEDLINE library was screened for associations between different imaging parameters and histopathological features in HNSCC up to December 2017. Firstly, for association between PET and histopathology, the following search words were used: “PET or positron emission tomography AND neck AND squamous cell carcinoma”. Overall, 1044 records were identified. Review articles (n=190), case reports (n=75), and non-English publications (n=30) were excluded. Thereafter, abstracts of the remaining 749 articles were checked, and only papers containing correlation coefficients between PET and histopathological parameters were acquired for further analysis. There were 12 publications.
Secondly, for associations between DWI MRI and histopathology, the following search words were used: “DWI or diffusion weighted imaging or ADC or apparent diffusion coefficient or positron emission tomography AND neck AND squamous cell carcinoma”. Here, 107 records were found. After exclusion of reviews (n=9), case reports (n=2), and non-English publications (n=1), abstracts of 95 publications were analyzed. Papers (n=91) which did not contain correlation coefficients between ADC and histopathology were excluded. Therefore, four articles were included into this meta-analysis.
Thirdly, data about associations between DCE MRI parameters and histopathological findings were acquired. For this search, the following words were used: “DCE or dynamic contrast enhancement AND neck AND squamous cell carcinoma”. Overall, 64 records were identified. Reviews (n=5) and non-English publications (n=1) were excluded. Thereafter, we checked abstracts of the remaining 59 publications. In 57 articles, no correlation coefficients between DCE MRI and histopathological parameters were reported, and these publications were also excluded. Therefore, only two articles were included into the meta-analysis.
One article contained correlation coefficients both between ADC versus histopathology and PET versus histopathology (n=9). Therefore, the present meta-analysis involved 17 publications [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23]. In these articles, correlations between different imaging and histopathological features were analyzed (Table 1).
Table 1.
Involved Studies and Analyzed Parameters
| Author, Year | Included Patients | Study Design | Imaging Modality | Analyzed Imaging Parameters |
|---|---|---|---|---|
| KI 67 | ||||
| Surov et al., 2016 [8] | 11 | Prospective | 18F-FDG PET | SUVmax |
| Rasmussen et al., 2015 [15] | 102 | Retrospective | 18F-FDG PET | SUVmax |
| Grönroos et al., 2014 [10] | 15 | Retrospective | 18F-FDG PET | SUVmax |
| Hoshikawa et al., 2011 [20] | 31 | Prospective |
18F-FDG PET 18F-FLT PET |
SUVmax |
| Deron et al., 2011 [17] | 25 | Retrospective | 18F-FDG PET | SUVmax |
| Linecker et al., 2008 [11] | 18 | Retrospective |
18F-FDG PET 18F-FLT PET |
SUVmax |
| Kitagawa et al., 2003 [21] | 20 | Retrospective | 18F-FDG PET | SUVmax |
| Jacob et al., 2001 [7] | 14 | Retrospective | 18F-FDG PET | SUVmax |
| Hoeben et al., 2014 [19] | 5 | Prospective | 18F-FLT PET | SUVmax |
| Troost et al., 2007, [22] | 10 | Retrospective | 18F-FLT PET | SUVmax |
| Surov et al., 2017 [9] | 11 | Prospective | DCE MRI | Ktrans |
| Jansen et al., 2012 [14] | 12 | Retrospective | DCE MRI | Ktrans |
| Surov et al., 2016 [8] | 11 | Prospective | MRI DWI | ADC |
| Swartz et al., 2018 [23] | 20 | Retrospective | MRI DWI | ADC |
| P53 | ||||
| Rasmussen et al., 2015 [15] | 102 | Retrospective | 18F-FDG PET | SUVmax |
| Grönroos et al., 2014 [10] | 15 | Retrospective | 18F-FDG PET | SUVmax |
| Cell count | ||||
| Surov et al., 2016 [8] | 11 | Prospective | MRI DWI | ADC |
| Driessen et al., 2014 [12] | 16 | Prospective | MRI DWI | ADC |
| White et al., 2006 [13] | 21 | Retrospective | MRI DWI | ADC |
| HIF-1α | ||||
| Grönroos et al., 2014 [10] | 15 | Retrospective | 18F-FDG PET | SUVmax |
| Han et al., 2012 [18] | 33 | Retrospective | 18F-FDG PET | SUVmax |
| Zhao et al., 2014 [16] | 24 | Prospective | 18F-FDG PET | SUVmax |
18F-FDG PET, fluorine-18 fluorodeoxyglucose positron emission tomography; 18F-FLT PET, fluorine-18 fluorothymidine positron emission tomography; SUVmax, maximal standardized uptake value; DCE MRI, dynamic contrast-enhanced magnetic resonance imaging; DWI, diffusionweighted imaging; ADC, apparent diffusion coefficient.
The following data were extracted from the literature: authors, year of publication, number of patients, imaging parameters, histopathological parameters, and correlation coefficients.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement was used for the research [24].
Meta-Analysis
The methodological quality of the acquired 15 studies was independently checked by two observers (A.S. and H.J.M.) using the Quality Assessment of Diagnostic Studies (QUADAS) instrument according to previous descriptions [25]. Table 2 shows the results of QUADAS proving.
Table 2.
Methodological Quality of the Involved Studies According to the QUADAS Criteria
| Study | Patient Spectrum | Selection Criteria | Reference Standard | Disease Progression Bias | Partial Verification Bias | Differential Verification Bias | Incorporation Bias | Text Details | Reference Standard Details | Text Review Details | Diagnostic Review Bias | Clinical Review Bias | Uninterpretable Results | Withdrawals Explained |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deron et al., 2011 [17] | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Driessen et al., 2014 [12] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yse | No | No | Yes | Yes | Yes |
| Grönroos et al., 2014 [10] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Han et al., 2012 [18] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Hoeben et al., 2014 [19] | Yes | Yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | yes |
| Hoshikawa et al., 2011 [20] | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Jacob et al., 2001 [7] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Jansen et al., 2012 [14] | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | Yes |
| Kitawaga et al., 2003 [21] | Yes | yes | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Linecker et al., 2008 [11] | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Rasmussen et al., 2015 [15] | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Swartz et al, 2018 [23] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Surov et al, 2016 [8] | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Unclear |
| Surov et al, 2017 [9] | Yes | Unclear | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Unclear |
| Troost et al., 2007 [22] | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| White et al., 2006 [13] | Yes | Unclear | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Unclear | Unclear | Yes | Yes | yes |
| Zhao et al., 2014 [16] | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | yes |
Associations between imaging parameters and histopathological findings were analyzed by Spearman's correlation coefficient. The reported Pearson's correlation coefficients in some studies were converted into Spearman's correlation coefficients according to the previous description [26].
Furthermore, the meta-analysis was undertaken by using RevMan 5.3 (Computer program, version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). In addition, heterogeneity was calculated by means of the inconsistency index I2 [27], [28]. Also DerSimonian and Laird random-effects models with inverse-variance weights were used without any further correction [29].
Results
KI 67
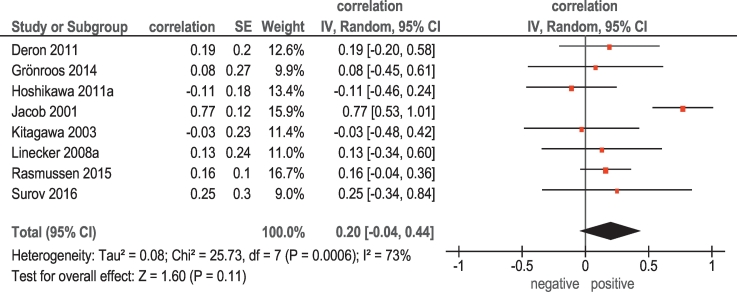
Associations between 18F-FDG PET and KI 67were reported in 8 studies (236 patients) (Table 1). Here, the calculated correlation coefficients between SUVmax and KI 67 ranged from −0.11 to 0.77 (Figure 1). The pooled correlation coefficient was 0.20 (95% CI =[−0.04; 0.44]).
Figure 1.
Forest plots of correlation coefficients between SUVmax retrieved from 18F-FDG PET and KI 67.
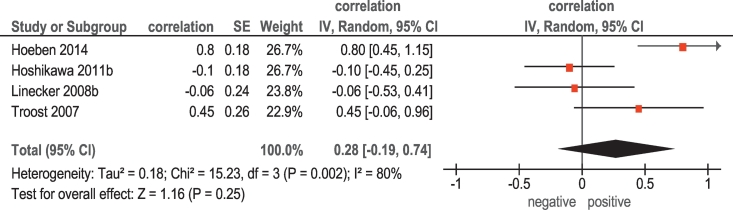
Furthermore, in 4 studies (64 patients), associations between 18F-fluorothymidine (FLT) PET and KI 67 were analyzed (Table 1). The pooled correlation coefficient between SUVmax and KI 67 was 0.28 (95% CI = [−0.06; 0.94]) (Figure 2).
Figure 2.
Forest plots of correlation coefficients between SUVmax retrieved from 18F-FLT PET and KI 67.
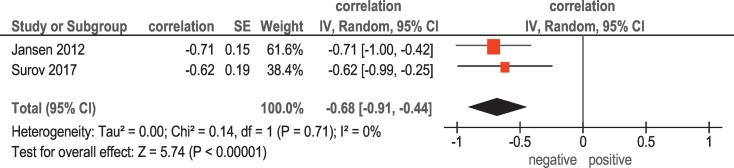
In 2 studies (23 patients), relationships between KI 67 and DCE MRI (Ktrans) were reported (Table 1). The pooled correlation coefficient between Ktrans and KI 67 was −0.68, (95% CI = [−0.91; −0.44]) (Figure 3).
Figure 3.
Forest plots of correlation coefficients between Ktrans and KI 67.
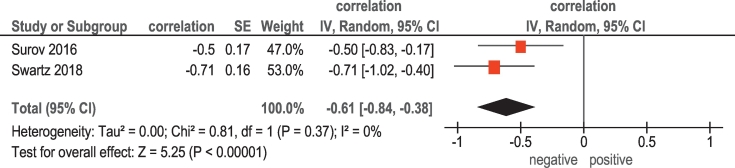
Finally, in 2 studies (31 patients), associations between ADC and KI 67 were analyzed (Table 1). The pooled correlation coefficient between the investigated parameters was −0.61, (95% CI = [−0.84; −0.38]) (Figure 4).
Figure 4.
Forest plots of correlation coefficients between ADC and KI 67.
P53
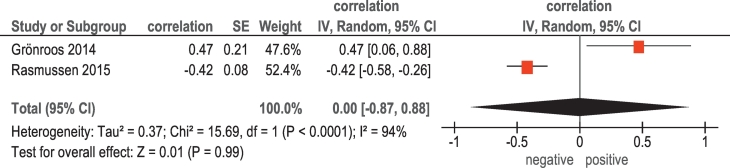
In 2 studies (117 patients), relationships between 18F-FDG PET and p53 were analyzed (Table 1). The pooled correlation coefficient between these parameters was 0.0 (95% CI = [−0.87; 0.88]) (Figure 5).
Figure 5.
Forest plots of correlation coefficients between SUVmax retrieved from 18F-FDG PET and expression of p53.
Cell Count
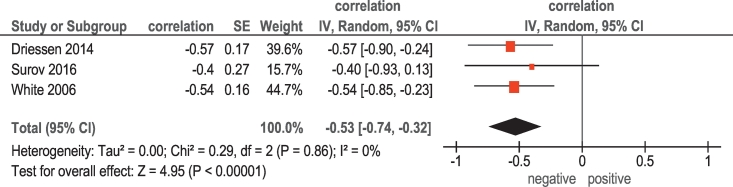
There were 3 studies (48 patients) that investigated associations between ADC and tumor cell count in HNSCC (Table 1). The reported correlation coefficients ranged from −0.57 to 0.40 (Figure 6). The pooled correlation coefficient was −0.53 (95% CI = [−0.74; −0.32]).
Figure 6.
Forest plots of correlation coefficients between ADC and cell count in HNSCC.
HIF-1α
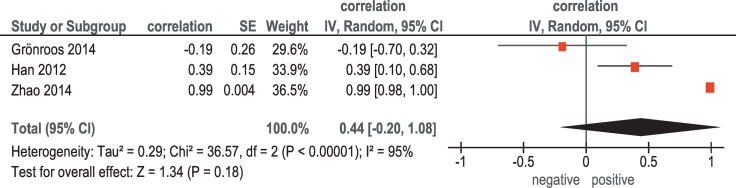
Associations between 18F-FDG PET and HIF-1α were investigated in 3 studies (72 patients) (Table 1). The reported correlation coefficients ranged from −0.19 to 0.99 (Figure 7). The pooled correlation coefficient was 0.44 (95% CI = [−0.20; 1.08]).
Figure 7.
Forest plots of correlation coefficients between SUVmax retrieved from 18F-FDG PET and expression of HIF-1α in HNSCC.
Discussion
To the best of our knowledge, this is the first meta-analysis about relationships between different imaging parameters and histopathology in HNSCC.
Previously, only few reports investigated this question. Our meta-analysis showed that different imaging parameters reflect different histopathological features. Furthermore, some findings are very surprisingly. As seen, neither 18F-FDG nor 18F-FLT PET reflects proliferation activity in HNSCC estimated by KI 67 expression. KI 67 is a nonhistone nuclear protein synthesized throughout the whole cell cycle except the G0 phase and has been shown to be responsible for cell proliferation [3], [4]. It is an established biomarker in HNSCC. Our finding is difficult to explain. Theoretically, PET parameters, reflecting metabolic activity, should be associated with the proliferation index. However, almost all reports involved in the present work did not identify statistically significant correlations between SUVmax and KI 67. Previously, this phenomenon was observed also in other malignancies like thyroid cancer, esophageal carcinoma, gastric cancers, and malignant melanoma [30]. Overall, our meta-analysis suggests that SUVmax cannot be used as a surrogate marker for proliferation activity in HNSCC.
Furthermore, we found that Ktrans correlated inversely strong with KI 67. According to the literature, Ktrans represents vessel permeability and reflects the diffusion of contrast medium from the plasma through the vessel wall into the interstitial space [9]. It has been shown that this parameter can distinguish malignant and benign lesions. For instance, benign breast lesions showed statistically significant lower Ktrans values than malignant tumors [31]. Furthermore, Ktrans correlated also with tumor grading and can discriminate low-grade and high-grade tumors [31]. Based on these data, presumably, Ktrans should correlate positively with proliferation activity of several tumors, in particular, in HNSCC. However, our results did not confirm this assumption. Furthermore, both involved studies showed inverse statistically significant correlations between Ktrans and KI 67 in HNSCC [9], [14]. The exact cause of this phenomenon is unclear. Hypothetically, high proliferative lesions may have a small number of vessels in relation to tumor cells and/or proliferation index. This may result in the calculated inverse correlation between Ktrans and KI 67. Independent of possible pathomechanisms of interaction between Ktrans and KI 67, our meta-analysis showed that Ktrans may be used as a surrogate marker for proliferation potential in HNSCC. However, our statement is based on 2 studies with 23 patients only. Therefore, further studies are needed to proof our results.
Furthermore, our analysis identified that ADC values may be used as a surrogate marker for tumor cellularity and proliferation activity. This finding seems to be logical. In fact, ADC reflects diffusion of water molecules in tissues and depends on tissues barriers like cell membranes [32]. Previously, numerous studies showed that ADC values inversely correlated with cell count and expression of KI 67 in several tumors [33], [34]. However, these results are based on small number of patients and should be proven in further studies.
Our meta-analysis did not find significant associations between tumor suppressor protein p53 and SUVmax. There were only two reports regarding relationships between SUVmax and p53 [10], [15]. This protein plays an important role in the development of cancer [35]. It regulates the activity of several pathways, which lead variously to cell cycle arrest, DNA repair, senescence, or apoptosis following exposure of cells to endogenous or exogenous cellular stresses [35]. However, according to the literature, current data regarding the role of p53 in HNSCC are inconclusive [35].
Finally, the present meta-analysis identified moderate pooled correlation between SUVmax and HIF-1α. According to the literature, HIF-1α characterizes cellular responses to hypoxic stress [5]. Furthermore, overexpression of HIF-1α is reported to be associated with increased mortality and worse prognosis of HNSCC [5]. Our finding showed that SUVmax derived from F-FDG PET may predict expression of HIF-1α.
The present meta-analysis identified also several problems. Firstly, as mentioned above, only few reports with small number of patients investigated associations between different imaging parameters and histopathological features in HNSCC. Secondly, most of the acquired studies were retrospective. Thirdly, according the QUADAS criteria, all involved studies showed partial verification bias, differential verification bias, and incorporation bias. Furthermore, most of the studies had clinical review bias and diagnostic review bias. In addition, the acquired data were obtained using different PET and MRI scanners with different technical parameters like tesla strength, b values, and acquisition time. Also, the involved studies used different ways of SUV, ADC and Ktrans measurements. This relativizes the identified results. Clearly, further prospective studies with more patients are needed to investigate associations between imaging and histopathology in HNSCC.
Recently, it has been shown that other histopathological markers like cyclin D1, human papilloma virus, vascular endothelial growth factor, and epidermal growth factor receptor play also a great role in prognosis of HNSCC [3], [4]. However, there were either no data or each with one report about relationships between imaging parameters and these histopathological factors. This is also the purpose for further investigations.
In conclusion, our meta-analysis showed that ADC may predict cell count and expression of KI 67, and SUVmax may predict expression of HIF-1α in HNSCC. Furthermore, SUVmax cannot be used as surrogate marker for expression of KI 67 and p53.
References
- 1.Braakhuis BJ, Leemans CR, Visser O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol. 2014;50(7):670–675. doi: 10.1016/j.oraloncology.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 2.Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM, Cullen K, Ervin TJ. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357(17):1705–1715. doi: 10.1056/NEJMoa070956. [DOI] [PubMed] [Google Scholar]
- 3.Hoogsteen IJ, Marres HA, Bussink J, van der Kogel AJ, Kaanders JH. Tumor microenvironment in head and neck squamous cell carcinomas: predictive value and clinical relevance of hypoxic markers. A review. Head Neck. 2007;29(6):591–604. doi: 10.1002/hed.20543. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira LR, Ribeiro-Silva A. Prognostic significance of immunohistochemical biomarkers in oral squamous cell carcinoma. Int J Oral Maxillofac Surg. 2011;40(3):298–307. doi: 10.1016/j.ijom.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Gong L, Zhang W, Zhou J, Lu J, Xiong H, Shi X, Chen J. Prognostic value of HIFs expression in head and neck cancer: a systematic review. PLoS One. 2013;8(9) doi: 10.1371/journal.pone.0075094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokobori Y, Toyoda M, Sakakura K, Kaira K, Tsushima Y, Chikamatsu K. (18)F-FDG uptake on PET correlates with biological potential in early oral squamous cell carcinoma. Acta Otolaryngol. 2015;135(5):494–499. doi: 10.3109/00016489.2014.969385. [DOI] [PubMed] [Google Scholar]
- 7.Jacob R, Welkoborsky HJ, Mann WJ, Jauch M, Amedee R. [Fluorine-18] fluorodeoxyglucose positron emission tomography, DNA ploidy and growth fraction in squamous-cell carcinomas of the head and neck. ORL J Otorhinolaryngol Relat Spec. 2001;63(5):307–313. doi: 10.1159/000055764. [DOI] [PubMed] [Google Scholar]
- 8.Surov A, Stumpp P, Meyer HJ, Gawlitza M, Hoehn AK, Boehm A, Sabri O, Kahn T, Purz S. Simultaneous 18F-FDG-PET/MRI: associations between diffusion, glucose metabolism and histopathological parameters in patients with head and neck squamous cell carcinoma. Oral Oncol. 2016;58(3):14–20. doi: 10.1016/j.oraloncology.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Surov A, Meyer HJ, Gawlitza M, Höhn AK, Boehm A, Kahn T, Stumpp P. Correlations between DCE MRI and histopathological parameters in head and neck squamous cell carcinoma. Transl Oncol. 2017;10(1):17–21. doi: 10.1016/j.tranon.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grönroos TJ, Lehtiö K, Söderström KO, Kronqvist P, Laine J, Eskola O, Viljanen T, Grénman R, Solin O, Minn H. Hypoxia, blood flow and metabolism in squamous-cell carcinoma of the head and neck: correlations between multiple immunohistochemical parameters and PET. BMC Cancer. 2014;14:876. doi: 10.1186/1471-2407-14-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Linecker A, Kermer C, Sulzbacher I, Angelberger P, Kletter K, Dudczak R, Ewers R, Becherer A. Uptake of (18)F-FLT and (18)F-FDG in primary head and neck cancer correlates with survival. Nuklearmedizin. 2008;47(2):80–85. doi: 10.3413/nukmed-0092. [DOI] [PubMed] [Google Scholar]
- 12.Driessen JP, Caldas-Magalhaes J, Janssen LM, Pameijer FA, Kooij N, Terhaard CH, Grolman W, Philippens ME. Diffusion-weighted MR imaging in laryngeal and hypopharyngeal carcinoma: association between apparent diffusion coefficient and histologic findings. Radiology. 2014;272(2):456–463. doi: 10.1148/radiol.14131173. [DOI] [PubMed] [Google Scholar]
- 13.White ML, Zhang Y, Robinson RA. Evaluating tumors and tumor like lesions of the nasal cavity, the paranasal sinuses, and the adjacent skull base with diffusion-weighted MRI. J Comput Assist Tomogr. 2006;30(3):490–495. doi: 10.1097/00004728-200605000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Jansen JF, Carlson DL, Lu Y, Stambuk HE, Moreira AL, Singh B, Patel SG, Kraus DH, Wong RJ, Shaha AR. Correlation of a priori DCE-MRI and (1)H-MRS data with molecular markers in neck nodal metastases: Initial analysis. Oral Oncol. 2012;48(8):717–722. doi: 10.1016/j.oraloncology.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rasmussen GB, Vogelius IR, Rasmussen JH, Schumaker L, Ioffe O, Cullen K, Fischer BM, Therkildsen MH, Specht L, Bentzen SM. Immunohistochemical biomarkers and FDG uptake on PET/CT in head and neck squamous cell carcinoma. Acta Oncol. 2015;54(9):1408–1415. doi: 10.3109/0284186X.2015.1062539. [DOI] [PubMed] [Google Scholar]
- 16.Zhao K, Yang SY, Zhou SH, Dong MJ, Bao YY, Yao HT. Fluorodeoxyglucose uptake in laryngeal carcinoma is associated with the expression of glucose transporter-1 and hypoxia-inducible-factor-1α and the phosphoinositide 3-kinase/protein kinase B pathway. Oncol Lett. 2014;7(4):984–990. doi: 10.3892/ol.2014.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deron P, Vangestel C, Goethals I, De Potter A, Peeters M, Vermeersch H, Van de Wiele C. FDG uptake in primary squamous cell carcinoma of the head and neck. The relationship between over expression of glucose transporters and hexokinases, tumour proliferation and apoptosis. Nuklearmedizin. 2011;50(1):15–21. doi: 10.3413/nukmed-0324-10-06. [DOI] [PubMed] [Google Scholar]
- 18.Han MW, Lee HJ, Cho KJ, Kim JS, Roh JL, Choi SH, Nam SY, Kim SY. Role of FDG-PET as a biological marker for predicting the hypoxic status of tongue cancer. Head Neck. 2012;34(10):1395–1402. doi: 10.1002/hed.21945. [DOI] [PubMed] [Google Scholar]
- 19.Hoeben BA, Troost EG, Bussink J, van Herpen CM, Oyen WJ, Kaanders JH. 18F-FLT PET changes during radiotherapy combined with cetuximab in head and neck squamous cell carcinoma patients. Nuklearmedizin. 2014;53(2):60–66. doi: 10.3413/Nukmed-0625-13-09. [DOI] [PubMed] [Google Scholar]
- 20.Hoshikawa H, Nishiyama Y, Kishino T, Yamamoto Y, Haba R, Mori N. Comparison of FLT-PET and FDG-PET for visualization of head and neck squamous cell cancers. Mol Imaging Biol. 2011;13(1):172–177. doi: 10.1007/s11307-010-0331-z. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa Y, Sano K, Nishizawa S, Nakamura M, Ogasawara T, Sadato N, Yonekura Y. FDG-PET for prediction of tumour aggressiveness and response to intra-arterial chemotherapy and radiotherapy in head and neck cancer. Eur J Nucl Med Mol Imaging. 2003;30(1):63–71. doi: 10.1007/s00259-002-0978-z. [DOI] [PubMed] [Google Scholar]
- 22.Troost EG, Vogel WV, Merkx MA, Slootweg PJ, Marres HA, Peeters WJ, Bussink J, van der Kogel AJ, Oyen WJ, Kaanders JH. 18F-FLT PET does not discriminate between reactive and metastatic lymph nodes in primary head and neck cancer patients. J Nucl Med. 2007;48(5):726–735. doi: 10.2967/jnumed.106.037473. [DOI] [PubMed] [Google Scholar]
- 23.Swartz JE, Driessen JP, van Kempen PMW, de Bree R, Janssen LM, Pameijer FA, Terhaard CHJ, Philippens MEP, Willems S. Influence of tumor and microenvironment characteristics on diffusion-weighted imaging in oropharyngeal carcinoma: a pilot study. Oral Oncol. 2018;77(7):9–15. doi: 10.1016/j.oraloncology.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol. 2003;3:25. doi: 10.1186/1471-2288-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chalkidou A, Landau DB, Odell EW, Cornelius VR, O'Doherty MJ, Marsden PK. Correlation between Ki-67 immunohistochemistry and 18F-fluorothymidine uptake in patients with cancer: a systematic review and meta-analysis. Eur J Cancer. 2012;48(18):3499–3513. doi: 10.1016/j.ejca.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Leeflang MM, Deeks JJ, Gatsonis C, Bossuyt PM. Systematic reviews of diagnostic test accuracy. Ann Intern Med. 2008;149(12):889–897. doi: 10.7326/0003-4819-149-12-200812160-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zamora J, Abraira V, Muriel A, Khan K, Coomarasamy A. Meta-DiSc: A software for meta-analysis of test accuracy data. BMC Med Res Methodol. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 30.Deng SM, Zhang W, Zhang B, Chen YY, Li JH, Wu YW. Correlation between the uptake of 18F-fluorodeoxyglucose (18F-FDG) and the expression of proliferation-associated antigen Ki-67 in cancer patients: a meta-analysis. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li L, Wang K, Sun X, Wang K, Sun Y, Zhang G, Shen B. Parameters of dynamic contrast-enhanced MRI as imaging markers for angiogenesis and proliferation in human breast cancer. Med Sci Monit. 2015;21:376–382. doi: 10.12659/MSM.892534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fornasa F. Diffusion-weighted magnetic resonance imaging: what makes water run fast or slow? J Clin Imaging Sci. 2011;1:27. doi: 10.4103/2156-7514.81294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Surov A, Meyer HJ, Wienke A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: a meta-analysis. Oncotarget. 2017;8(35):59492–59499. doi: 10.18632/oncotarget.17752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Surov A, Meyer HJ, Wienke A. Associations between apparent diffusion coefficient (ADC) and KI 67 in different tumors: a meta-analysis. Part 1: ADCmean. Oncotarget. 2017;8(43):75434–75444. doi: 10.18632/oncotarget.20406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tandon S, Tudur-Smith C, Riley RD, Boyd MT, Jones TM. A systematic review of p53 as a prognostic factor of survival in squamous cell carcinoma of the four main anatomical subsites of the head and neck. Cancer Epidemiol Biomark Prev. 2010;19(2):574–587. doi: 10.1158/1055-9965.EPI-09-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]