Abstract
In an attempt to elucidate the biological function of villin-like actin-binding proteins in plants we have cloned several genes encoding Arabidopsis proteins with high homology to animal villin. We found that Arabidopsis contains at least four villin-like genes (AtVLNs) encoding four different VLN isoforms. Two AtVLN isoforms are more closely related to mammalian villin in their primary structure and are also antigenically related, whereas the other two contain significant changes in the C-terminal headpiece domain. RNA and promoter/β-glucuronidase expression studies demonstrated that AtVLN genes are expressed in all organs, with elevated expression levels in certain types of cells. These results suggest that AtVLNs have less-specialized functions than mammalian villin, which is found only in the microvilli of brush border cells. Immunoblot experiments using a monoclonal antibody against pig villin showed that AtVLNs are widely distributed in a variety of plant tissues. Green fluorescent protein fused to full-length AtVLN and individual AtVLN headpiece domains can bind to both animal and plant actin filaments in vivo.
Only very few actin binding proteins have been characterized well in plants. With the exception of profilin and actin depolymerizing factor and some myosins, very little is known about the importance of actin cytoskeleton modulation by plant proteins (Staiger et al., 1997; Meagher et al., 1999). We have chosen Arabidopsis to study the role of the actin cytoskeleton in complex cellular processes such as cytokinesis and cell shape formation and maintenance.
Villin is an actin bundling, severing, nucleating, and capping protein (Bretscher and Weber, 1980; Craig and Powell, 1980; Mooseker et al., 1980; Glenney et al., 1981) that accumulates predominantly in the microvilli of absorptive epithelial cells of mammalian intestines (Bretscher and Weber, 1979; Matsudaira and Burgess, 1979; Robine et al., 1985). Together with the actin-bundling protein fimbrin, it is responsible for the formation of the rigid structure in the microvilli core (Fath and Burgess, 1995). Its importance in establishing microvilli has been described in cultured cells (Friederich et al., 1989, 1992; Franck et al., 1990) and in vitro (Coluccio and Bretscher, 1989); however, disruption of the villin gene does not impair microvilli morphogenesis (Pinson et al., 1998). Very recently, a novel gene with high homology to villin (65% identical amino acids) was isolated in a screen to identify further alleles for adseverin, a protein closely related to gelsolin (Marks et al., 1998). The same protein was also found in a screen for genes regulated by CHOP, a regulatory protein mediating stress responses (Wang et al., 1998). Villin homologs have not been found in the Saccharomyces cerevisiae genome.
Villin shares structural homology with gelsolin (Kwiatkowski et al., 1986), although the latter does not contain the headpiece, which is critical for the morphogenic function of villin (Friederich, et al., 1992). Both gelsolin and villin contain six evolutionarily conserved actin-binding modules, and three copies of this domain are also found in severin of Dictyostelium discoideum (Andre et al., 1988) and fragmin of Physarum polycephalum (Ampe and Vanderkerckhove, 1987; Arpin et al., 1988; Schleicher et al., 1988).
The specific and localized expression of mammalian villin suggests a very distinct function. This is in contrast to gelsolin, which is more ubiquitously expressed (Paunio et al., 1997). However, villin-like proteins have also been found in Drosophila, and their occurrence is not restricted to the intestines. The QUAIL gene is required for actin bundle assembly during oogenesis and is structurally very similar to villin (Mahajan-Miklos and Cooley, 1994). One of the FLIGHTLESS genes, which is involved in Drosophila gastrulation and muscle degeneration, encodes a protein more related to gelsolin and contains an additional N-terminal Leu-rich repeat domain (Campbell et al., 1993). Very recently, another member of the villin family was purified from bovine neutrophil plasma membranes and was called supervillin because of its exaggerated size. This molecule has been proposed to serve as a membrane anchor for actin and associated proteins (Pestonjamasp et al., 1997). A gelsolin-like gene has also been identified in the Caenorhabditis elegans genome sequencing project (Wilson et al., 1994).
Biochemical studies revealed that both villin and gelsolin contain a polyphosphoinositide binding domain (Janmey et al., 1992), and their functions are regulated by calcium (see Burtnick et al., 1997; Markus et al., 1997, and refs. therein). Calcium has been shown to modulate the protein's conformation and to bind to three distinct sites in villin (Hesterberg and Weber, 1983). Both the headpiece (the 8.5-kD C-terminal domain) and the core (the remainder of the protein) can bind F-actin, but they bind to different sites on the actin filament (Glenney et al., 1981; Pope et al., 1994).
Villin has been used as a marker for brush border cell development. An in vitro system has been established in which lymphocytes induce cell differentiation and, concomitantly, villin expression (Kerneis et al., 1997). Villin is also induced upon Glc starvation (Zweibaum et al., 1985) or acid modulation (Fitzgerald et al., 1997) in cultured cells.
We describe the primary structure of three members of an Arabidopsis gene family encoding villin-like proteins (AtVLN). All of these AtVLNs are expressed in all examined tissues; however, the expression of certain AtVLN genes appears to be higher in particular cell layers. We show that AtVLNs and individual AtVLN headpiece domains interact with both plant and mammalian actin filaments in vivo. Our results indicate that, compared with Drosophila or mammals, plant villin-like proteins have a more general role, probably in cell growth and differentiation.
MATERIALS AND METHODS
Plant Growth and Transformation
Plants (Arabidopsis, ecotype Landsberg erecta) were grown on Murashige and Skoog medium (JRH Biosciences, Lenexa, KS) containing 0.8% (w/v) Bacto-Agar (Difco Laboratories, Detroit) under constant white fluorescent light at 22°C. Kanamycin (50 μg/mL) for transgene selection and 3% (w/v) Suc were added as indicated. For seed collection, plants were grown on soil in constant light at 22°C.
Transgenic plants were generated by Agrobacterium tumefaciens-mediated root transformation as described previously (Valvekens et al., 1988). For the induction of the GVG system (Aoyama and Chua, 1997), transgenic plants carrying GFP-AtVLN constructs were grown on 30 μm dexamethasone (Sigma, St. Louis) under standard conditions.
Growth of Bright Yellow 2 (BY2) Tobacco Suspension Culture, Biolistic Bombardment, and Rhodamine-Phalloidin Staining
BY2 tobacco cells were grown as described previously (Kato et al., 1972; Newman et al., 1993). For biolistic bombardment, cells were transferred from liquid medium onto filter paper (type HA, 0.45-μm pore size, Millipore, Bedford, MA), preincubated on NT medium (Newman et al., 1993) for 2 d, and bombarded with a biolistic particle delivery system (Bio-Rad, Hercules, CA). DNA loading onto gold particles and delivery (1,100 psi) were performed according to the manufacturer's instructions. After bombardment, the samples were incubated overnight and analyzed by confocal microscopy. Rhodamine-phalloidin labeling was performed as described previously (Cooper 1987; Kost et al., 1998).
Southern and Northern Blotting
RNA was isolated from plant tissues as described previously (Kuhlemeier et al., 1988), and RNA gel blots were performed according to the method of Barnes et al. (1996). Ten micrograms of RNA was used per lane and loading was monitored using ethidium bromide and rehybridizing the blots with a 0.75-kb HindIII-EcoRI genomic fragment of Arabidopsis ACTIN 1 (Leu et al., 1995).
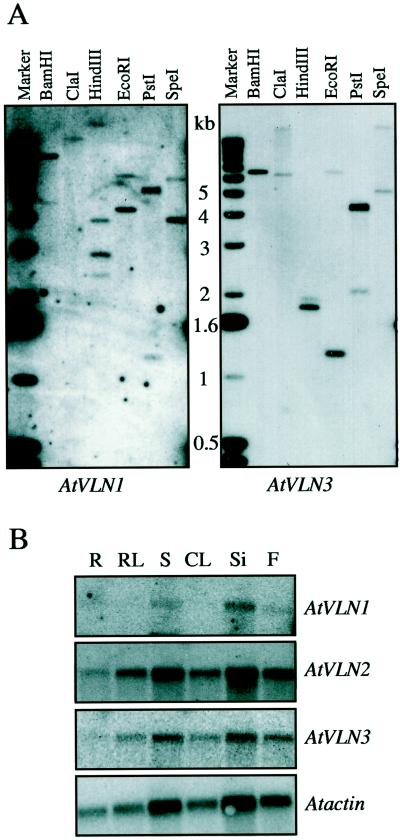
DNA for Southern-blot hybridization was isolated using CTAB, as described previously (Whitelam et al., 1993). Six micrograms of DNA was digested with the indicated enzymes (see Fig. 2) and resolved on an 0.7% (w/v) agarose gel. The DNA was denatured as described previously (Sambrook et al., 1989) and blotted onto a nylon membrane (Duralon UV, Stratagene, La Jolla, CA). The DNA was UV cross-linked to the membrane using a Stratalinker (Stratagene) according to the manufacturer's recommendations. Probe preparation, hybridization, and washing conditions of the membranes were performed as for RNA blots (Barnes et al., 1996).
Figure 2.
Abundance and expression of AtVLN genes. A, Genomic DNA gel-blot hybridizations. Six micrograms of total Arabidopsis DNA digested with the indicated restriction enzymes were loaded per lane. The left blot was hybridized with a cDNA fragment of AtVLN 1 and the right blot with a cDNA fragment of AtVLN 3. B, RNA gel-blot hybridizations. One microgram of poly(A+) RNA was loaded per lane and the blot was hybridized with cDNA fragments as indicated. The blot was rehybridized with a genomic fragment of Arabidopsis ACTIN 1 as a loading control (AtACTI). The size of transcripts was approximately 3 kb for AtVLN and 1.6 kb for AtACT1.
DNA Manipulations
DNA manipulations were performed according to standard methods. Genomic clones were isolated from a genomic library provided by the Arabidopsis Biological Resource Center (Ohio State University, Columbus) (CD-4). For sequencing, fragments were subcloned into pBluescript SK− (Stratagene) and sequenced using standard dideoxy methods (Sambrook et al., 1989). The initial fragment of genomic DNA was isolated using oligos 5′GCCCTTTGCCTTAGCCCTTGGTTCCCACC3′ and 5′GGTTGAGGAGATTTACAACTTTGCTC3′ derived from expressed sequence tag (EST) 136H9T7 from the Arabidopsis Biological Resource Center. cDNAs were isolated from an Arabidopsis cDNA library (Stratagene) or obtained from the Arabidopsis Biological Resource Center.
Recombinant DNAs used for this study were constructed as follows: For the expression of green fluorescent protein-fusions (GFPs), DNA fragments generated by PCR were inserted into the GFP-cloning vector pGFP(GA)5 II (P. Spielhofer and N.-H. Chua, unpublished data), which contains a cauliflower mosaic virus 35S promoter followed by GFP (CLONTECH, Palo Alto, CA) and a NOS polyadenylation site. The domains cloned downstream of GFP were nt 2,203-stop for AtVLN 1, 2,248-stop for AtVLN 2, and 2,185 for AtVLN 3, all relative to the ATG, respectively. These constructs were used for bombardment of BY2 cells directly. For in vitro translation the XhoI-SmaI fragments of GFP-headpiece-VLN constructs were subcloned into pSK− (Stratagene) digested with XhoI and SmaI. For plant transformation the XhoI-PacI fragment of the original GFP constructs was introduced into pTA211 (P. Spielhofer and N.-H. Chua, unpublished data), which contains the GVG system (Aoyama and Chua, 1997). For expression in mammalian cells the XhoI/SmaI fragments of the GFP-headpiece-VLN 1 and 2 constructs were blunt-ended with Klenow and introduced into the Ecl136II site of pCB6 (Friederich et al., 1995). For AtVLN3 the construct used for in vitro translation was digested with KpnI and BamHI and cloned into the KpnI/BamHI sites of pCB6. For β-glucuronidase (GUS) expression a 3.5-kb HindIII fragment of AtVLN 1 and a 2.7-kb BamHI fragment of AtVLN 2 were inserted into the HindIII site of pBI101 (CLONTECH) and the BamHI site of pBI101.2, respectively.
Reverse transcriptase (RT)-PCR amplifications on alternatively processed AtVLN1 mRNAs used oligos 3′-GGATGAGTTCAAGGAGGTTGC-5′ and 3′-CTAGCCTTCTACAATGAAGG-5′, spanning introns. Amplification products were subcloned to pSK− (Stratagene) and sequenced.
GUS Staining
For GUS staining, plants were grown under sterile conditions as described and were completely submerged in a staining solution (0.1 m Na-phosphate, pH 7, 0.5% [v/v] Triton X-100, 2 mm K-ferricyanide, 2 mm K-ferrocyanide, and 2 mm X-gluc). Samples were developed at 37°C for the indicated period of time.
Microscopy
Pictures of GUS-stained plants were taken on a stereomicroscope (model SMZ-U 1:10, Nikon, Tokyo) and an Axioscope using differential interference contrast imaging (Zeiss, Jena, Germany). Confocal images were taken on an Axiovert 100TV (Zeiss) using the Microsystem LSM 410 (Zeiss). For GFP images the excitation was at 488 nm, and emission was monitored at 515 to 565 nm.
Paraffin Sections
Tissue of GUS-stained plants was incubated for 3 h in a fixing solution (2% [v/v] paraformaldehyde and 10 mm Na-phosphate, pH 7.4), and dehydrated by 15-min incubations in 50%, 75%, 85%, 95%, 2× 100% (v/v) ethanol in water, 15 min of 50% (v/v) xylenes in ethanol, 2× 100% xylenes, 2 h of 50% (v/v) paraffin in xylenes, and overnight in 100% paraffin. Incubations in paraffin were carried out at 62°C. Samples were embedded in molds (Fisher Scientific, Pittsburgh) and sectioned (10 μm) on a histocut 820 (Jung, Heidelberg). Sections were dried and de-waxed by reversing the steps of the above dehydration procedure.
Gel Electrophoresis, Western Blotting, and in Vitro Translation
Protein concentrations were determined using a Bradford assay kit and by Coomassie staining of protein gels. Gel electrophoresis was performed as described previously (Sambrook et al., 1989). Gels were run on a mini gel apparatus (Bio-Rad) at 100 V. Proteins were transferred onto a nitrocellulose membrane (BA 85, Schleicher & Schuell, Keene, NH) using a semi-dry blotter (model EBU-4000, C.B.S. Scientific, Del Mar, CA) at 60 V at 4°C for 90 min. Blots were first treated with phosphate-buffered saline (PBS) containing 3% (w/v) milk powder (ALBA, high calcium, Heintz, Pittsburgh) before being incubated with the primary antibody in PBS containing 3% (w/v) milk powder for 4 to 16 h at 4°C. Anti-villin antibody (Dudouet et al., 1987) was used at a dilution of 1:10,000, and anti-GFP antibody was obtained from CLONTECH and used according to the manufacturer's recommendation. Blots were washed four times with PBS, incubated with secondary antibodies (Amersham, diluted 1:3,000 in 3% [w/v] milk powder in PBS) at room temperature for 1 h, washed four times with PBS, and assayed using enhanced chemiluminescence reagents as described previously (cat no. RPN 2108, Amersham). X-ray films (X-OMAT-AR) were from Kodak (Rochester, NJ). In vitro translation reactions were performed using a “TNT coupled wheat germ extract” (Promega, Madison, WI) according to the manufacturer's recommendations.
RESULTS
Isolation of Three Genes Encoding Proteins with High Homology to Villin
The Arabidopsis EST database listed a few cDNA clones encoding protein sequences with homology to animal gelsolin and villin. We used PCR to isolate a DNA fragment from a cDNA library that encodes amino acid sequences homologous to gelsolin and villin (see “Materials and Methods”). This fragment was then used as a probe to isolate full-length clones from a genomic library of Arabidopsis. The initial screen produced a large number of overlapping clones, which, after partial sequence determination, were shown to contain two related genes encoding proteins with high homology to animal villin (see Fig. 1, AtVLN 2 and 3). The sequence of AtVLN 2 has recently appeared in the database as part of the genome sequencing project (accession no. AC002339, chromosome II, BAC T11A7).
Figure 1.
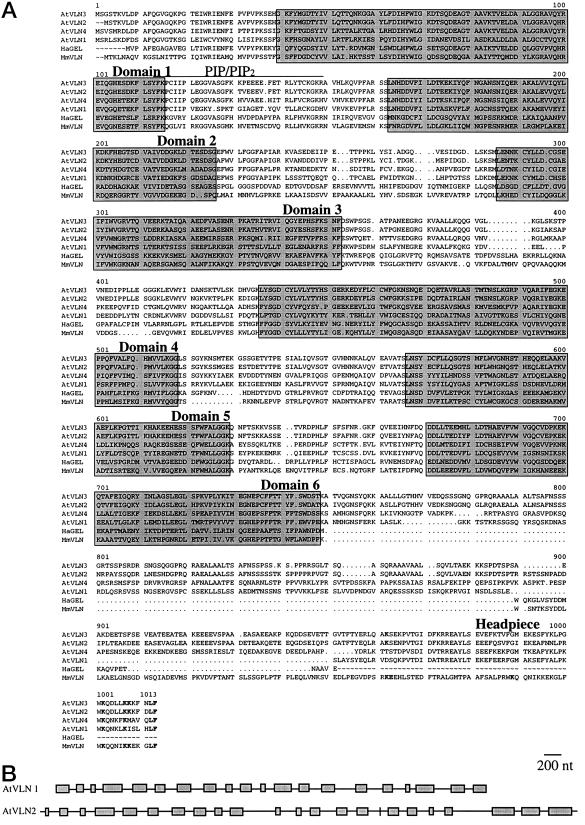
Deduced amino acid sequences and genomic structures of Arabidopsis VLN genes. A, Alignment of AtVLNs with animal villin and gelsolin. Four Arabidopsis amino acid sequences (AtVLN1–4) were compared with those of mouse villin (MmVLN) and lobster gelsolin (HaGEL). AtVLN 4 is a recent submission to GenBank (accession no. Y12782). The evolutionarily conserved domains 1 through 6 are boxed and indicated. Also indicated is the headpiece domain. Residues that are important for actin binding (Doering and Matsudaira, 1996) are printed in bold letters. The putative PIP/PIP2 binding domain is overlined. Note the major difference between animal villins and AtVLNs in the size of the linker domain between the core and the headpiece. This domain is rich in hydroxylated amino acids (18%–28%) and varies substantially between the isoforms. The sequences for cDNAs of AtVLNs 1 to 3 were deposited in GenBank and have the accession numbers. AF81201, AF81202, and AF81203, respectively. B, Genomic arrangement of AtVLN 1 and 2. Exons are shown as boxes and introns as lines.
Another EST clone (H5D9T7) that was made available during the course of this project was sequenced and used for the isolation of genomic fragments. The deduced protein sequence confirmed that this gene also encodes a villin-like protein (AtVLN 1), although the amino acid sequence homology was not as high as with AtVLN 2 and 3. Compared with the latter, the most C-terminal domain (the so-called “headpiece”) of AtVLN 1 showed significant amino acid exchanges (see below).
Table I compares Arabidopsis VLNs and villin and gelsolin from animals, both at the nucleotide and the amino acid levels. Clearly, among the Arabidopsis genes, AtVLN 2 and 3 seem to have arisen from a more recent gene duplication and share 84% of the nucleotides and 79% of the deduced amino acids. Although AtVLN 1 and 4 (a recent submission to GenBank, accession no. Y12782) are more related in the C-terminal domain (see Fig. 1A, headpiece domain), their overall amino acid homology is only 56%. Both AtVLN 1 and 4 lack Lys residues implicated in the morphological function of villin, whereas AtVLN 2 and 3 contain most of these residues (see Fig. 1A; Friederich et al., 1992; Doering and Matsudaira, 1996). We note that among the Arabidopsis VLNs, AtVLN 4 has the highest percentage of similar amino acids compared with gelsolin.
Table I.
Comparison of DNA and peptide sequences of Arabidopsis villins with animal villin and gelsolin
| AtVLN1 | AtVLN2 | AtVLN3 | AtVLN4 | MmVIL | HaGEL | |
|---|---|---|---|---|---|---|
| AtVLN1 | – | 53/43 | 54/43 | 56/47 | 40/31 | 42/30 |
| AtVLN2 | 57 | – | 86/82 | 58/48 | 44/35 | 46/35 |
| AtVLN3 | 57 | 84 | – | 58/48 | 45/35 | 45/34 |
| AtVLN4 | 61 | 60 | 61 | – | 43/33 | 47/35 |
| Mm VIL | 42 | 44 | 45 | 44 | – | 50/38 |
| HaGEL | 44 | 44 | 43 | 43 | 48 | – |
Comparison of DNA (lower half) and peptide (upper half) of sequences of villin from Arabidopsis (AtVLN) with villin from mouse (MmVIL) and gelsolin from lobster (HaGEL). Percent similarities/identities were calculated using the “Bestfit” program for peptide sequence comparisons and of Arabidopsis DNA sequences, and the “Gap” program for interspecies comparison of DNA sequences (both programs from Genetics Computer Group, Madison, WI).
Figure 1A shows a comparison of the amino acid sequences deduced from genomic and cDNA clones. The overall structure of animal villin is generally very well conserved in Arabidopsis VLNs. Major differences are found in the portion of the protein that links the six actin-binding modules, which are also found in gelsolin, and the villin-specific headpiece domain. Unlike in mammalian villins, the linker domain of AtVLNs is rather large and contains a high proportion of hydroxylated amino acids. Both in terms of amino acid similarity and structure, Arabidopsis VLNs are more related to mammalian villin than to Drosophila villin-like proteins (data not shown).
Arabidopsis Probably Contains Four AtVLN Genes
We performed genomic Southern-blot analysis to estimate the number of AtVLN genes in the Arabidopsis genome. Figure 2A shows that there are at least four AtVLN genes comprised of two subgroups (one gene being more similar to AtVLN 1 and the other to AtVLN 2), probably of two members each. Analysis of EST clones deposited in the database showed that all available clones that encode proteins of sequence homology to villin or gelsolin fall within the four classes described here. We therefore assume that Arabidopsis contains four genes encoding villin-like proteins. Until recently, only one gene was identified for villin from mammalian cells; however, the discovery of advillin/DOC6 (Marks et al., 1998; Wang et al., 1998) indicates that several villin-like genes might exist in animals as well. Therefore, both plants and animals appear to have several villin isoforms that may be responsible for related but specific functions.
Isolation of cDNA Clones: AtVLN1 Appears to Be Alternatively Processed
To examine the function of VLN in plants, we used the sequence information from the genomic clones to obtain full-length cDNA clones of one member from each subgroup. First, the sequence of AtVLN 1 was reconstituted from RT-PCR-amplified partial cDNA sequences encoding the amino-terminal region of AtVLN1 using information from genomic sequences and the partial cDNA clone EST H5D9T7 (starts at nt 144 of AtVLN1, see GenBank accession no. AF81201). However, in vitro translation assays using this reconstituted cDNA as a template did not produce a protein of the expected size (110 kD). Detailed sequence analysis showed no sequence difference between H5D9T7 and genomic clones. However, H5D9T7 encodes a protein of a substantially lower molecular mass due to a premature stop codon. This alternate form of AtVLN1 would lack domains 4 to 6 and the headpiece and have a calculated molecular mass of approximately 50.7 kD. Therefore, we used RT-PCR amplification of the region in question to determine whether the cDNA reflects the sequence of the AtVLN 1 mRNA (see “Materials and Methods” for details). Interestingly, among three independent RT-PCR products, one showed the same sequence as H5D9T7, whereas the other two contained an additional 13 bp inserted at nt 1,178 (relative to the ATG). The protein sequence deduced from a full-length cDNA that contained this insertion had the expected molecular mass of approximately 110 kD. The reason for the variability of cDNA production at this position of the mRNA is unclear (see “Discussion”).
A full-length cDNA of AtVLN 2 was reconstituted using RT-PCR-amplified partial cDNA sequences encoding the amino-terminal region of AtVLN2 using information from genomic sequences and the partial cDNA clone EST H10C6T7 (starts at nucleotide 276 of AtVLN2, see GenBank accession no. AF81202) that appeared in the database during the course of this project. Figure 1A also shows the amino acid sequence encoded by a third, full-length cDNA (EST H2G4T7) that was recently made available by the Arabidopsis Resource Center, and which corresponds to the gene designated AtVLN 3. The proteins encoded by all three cDNAs are similar in size to human villin, suggesting that the cDNAs are likely to be full-length clones. EST H2G4T7 also contains an in frame stop codon upstream of the presumptive first ATG.
Genomic Organization of AtVLN Genes
We determined the complete nucleotide sequence of AtVLN 1 and 2. AtVLN 2 has also been independently sequenced by the genome sequencing project (accession no. AC002339). Figure 1B shows the structure of AtVLN genes in a schematic form. Two aspects of the genomic structure and the spliced exons are interesting. First, we found cDNA clones that are derived from the same genes but differ in the presence or absence of an intron in the 3′ untranslated region. Because the intron is situated in a non-coding region of the mRNA, the alternative splicing event can only be relevant in terms of RNA secondary structure, stability, or recognition by proteins. Second, a comparison of genomic and cDNA sequence for AtVLN 2 reveals that exon 16 is extremely small (also see “Discussion”). The intron preceding exon 16 is not present in AtVLN 1 and the intron preceding exon 13 is absent as well. Generally, though, the positions of the introns are very well conserved.
Compared with the genomic organization of human villin (Pringault et al., 1991), the position of introns are better conserved in the 5′ half of the genes. Introns are conserved in AtVLN1, AtVLN2, and the human villin gene in the region encoding the beginning of the first domain, just prior to the second domain, between the second and third domain, within the third domain, and two introns between the third and fourth domain. The position of one intron is conserved between AtVLN2 and the human villin gene (end of domain 4), but is absent from AtVLN1. No conservation between these three genes exists in the 3′ region encoding the C-terminal three domains and the headpiece. The genes have six introns in common out of 18 (human villin gene), 17 (AtVLN1), and 21 (AtVLN2).
Expression Pattern of AtVLN Genes
In mammals and Drosophila, villin-like proteins are expressed in highly specialized tissues. We wanted to investigate whether plant VLNs also play a role in establishing the differentiation of one particular tissue. As a first step, we analyzed AtVLN mRNA levels in different organs by northern-blot hybridization. Figure 2B shows that AtVLN 2 and 3 are expressed at higher levels than AtVLN 1, but all three genes were expressed in all organs tested. Because of the high sequence homology between AtVLN 2 and 3, we used an RNase protection assay and verified that all mRNA species tested were indeed present at comparable amounts in all tissues (data not shown).
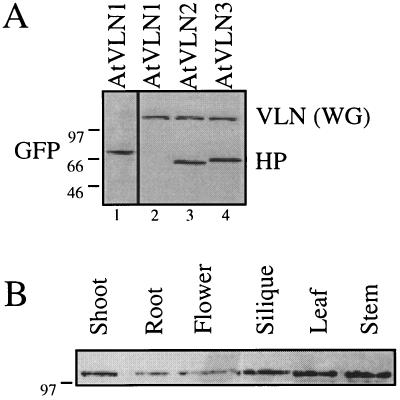
To determine whether the proteins showed a similar tissue distribution as the mRNAs, we performed immunoblotting experiments using a monoclonal antibody against the pig villin headpiece (Dudouet et al., 1987). Figure 3A shows that the C-terminal domains of both AtVLN 2 and AtVLN 3 are antigenically related to pig villin, but the antibody failed to bind to the equivalent portion of AtVLN 1. Western blotting of tissue extracts showed that the protein was expressed at comparable levels in all tissues examined (Fig. 3B), confirming results obtained from mRNA analyses. Tissue extracts from several crop plants, including banana, carrot, bean, and pear, contained antigenically related proteins (data not shown), indicating that VLNs are not only present in all of the Arabidopsis tissues examined, but are also conserved throughout the plant kingdom.
Figure 3.
Detection of AtVLN by a monoclonal antibody. A, A monoclonal antibody against pig villin headpiece (Dudouet et al., 1987) recognizes AtVLN 2 and 3, but not AtVLN 1. GFP-headpiece fusion proteins were synthesized by wheat germ in vitro translation reactions and treated on a western blot with an anti-villin antibody (HP; lanes 2–4). The presence of GFP-AtVLN 1 was verified using an antibody raised against GFP (GFP, lane 1, CLONTECH). The anti-villin antibody also recognizes VLN(WG) (the wheat germ VLN). B, AtVLNs can be detected in all organs. Western blots of crude protein extracts (approximately 30 μg per lane) were treated with the monoclonal anti-villin antibody. Molecular masses (in kD) are indicated on the left of the panels.
Cell-Layer Specific Expression of AtVLN1 and AtVLN2
To determine the expression of the different AtVLN genes at the cellular level, we fused the genomic fragments preceding the coding regions of AtVLN 1 and AtVLN 2 to a GUS reporter gene (Jefferson, 1987). In the case of AtVLN 1, an approximately 3.5-kb genomic fragment was fused to GUS as a translational fusion at amino acid 237. Notably, this fusion resulted in the expression of the actin-binding site that is present between the first and the second conserved actin-binding domain. For AtVLN 2, a 2.7-kb BamHI fragment terminating very close to the beginning of the coding region (including the first nine amino acids) was used to produce a translational fusion between the AtVLN 2 N terminus and GUS (see “Materials and Methods”). Both constructs were introduced into wild-type Arabidopsis plants by A. tumefaciens-mediated transformation. Many lines were obtained for both constructs, and initial staining experiments revealed that all lines transformed with the same construct had very similar GUS staining patterns. Segregation of the transgenes and the intensity of the staining indicated that some lines contained multiple transgenes (data not shown). We selected three representative lines of each construct for more detailed analyses and the results are shown in Figure 4. To address the issue of preferred substrate availability to certain tissues, we used both vacuum infiltration and pretreatment with acetone (Hemerly et al., 1993) and obtained identical results as when no pretreatment was performed (data not shown). We therefore concluded that the observed staining pattern reflects the expression of the GUS reporter gene.
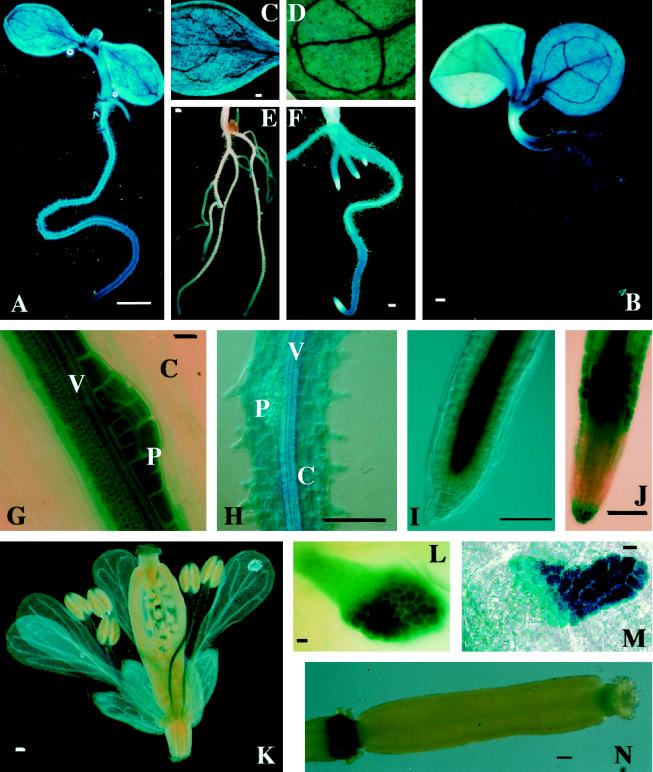
Figure 4.
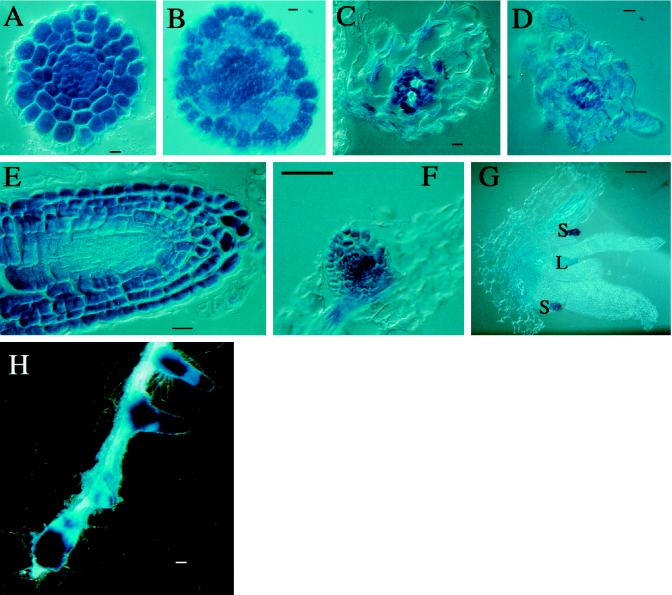
Expression pattern of representative AtVLN::GUS transgenic plants. A, C, E, G, I, K, L, and N are from AtVLN 1::GUS plants; B, D, F, H, J, and M are from AtLVN 2::GUS plants. A and B, Entire seedling. C and D, Cotyledon. E and F, Roots. G and H, Root, high magnification; C, cortex; P, pericycle; V, vasculature. I and J, Root tip, high magnification. K, Flower. L and M, Stipule. N, Silique. GUS staining was performed for 1 h in I, K, and N; for 2 h in E, I, F, J, and H; and for 24 h in B, D, and M. Scale bars represent 1 mm in A and E; 100 μm in B through D, F, H through K, and N; and 10 μm in G, L, and M.
For AtVLN 1, GUS staining was mainly found in the vascular tissue and the pericycle of roots, as well as the vasculature of leaves (Fig. 4A). Staining was strongest in the root tip and in locations where lateral roots emerge (Fig. 4E). In the latter regions GUS expression was detectable as soon as cell division was observed in the pericycle, before a minute bulge was formed by the lateral root bud (Fig. 4G). The root cap was not stained, but cells immediately above the root meristem strongly expressed GUS. While expression was stronger in the central cylinder of the root tip (Fig. 4I), prolonged incubation produced a blue color throughout the root tip (data not shown). Among the aerial organs with markedly stronger staining were the guard cells, the vasculature (Fig. 4C), and the trichomes (data not shown). Very intense staining was found in stipules (Fig. 4M). Unlike in AtVLN 2::GUS (see below), both the base and the “berry” of the stipule were stained. In flowers, strong GUS activity was observed in the vascular tissue of the filaments of the anthers, whereas weaker staining was detected in the vasculature of the sepals. No staining was found in petals (Fig. 4K) and pollen (data not shown).
Figure 4B shows that AtVLN 2::GUS was also strongly expressed in the root vasculature. However, unlike AtVLN 1::GUS, which was expressed in the pericycle, AtVLN 2::GUS was expressed in the epidermal layer of the root (Fig. 4H). Furthermore, in contrast to AtVLN 1::GUS, AtVLN 2::GUS was expressed in the root cap and in cells farther away from the meristem, leaving a region in the root tip that did not show any GUS activity (Fig. 4J). AtVLN 1 and AtVLN 2 appear to complement each other in their expression patterns throughout the root. The expression of both genes was stronger in the actively growing cells than in more mature tissue. The expression of AtVLN 2::GUS in the aerial parts of the plant was also weaker than in the roots and more pronounced in the vascular tissue (Fig. 4, B and D). The only location where very strong staining was observed was (as for AtVLN 1::GUS) in the stipules. This gene was specifically expressed in the “berry” of the stipule (Fig. 4M). Unlike AtVLN 1::GUS, no specific GUS staining was observed in flowers of transgenic lines carrying the AtVLN 2::GUS construct, and no staining was found in pollen (data not shown).
To assess the exact staining patterns within single organs, we made paraffin sections of stained tissues. Figure 5 shows that both AtVLN 1 and 2 promoters direct strong expression in the central cylinder of the root. In rapidly expanding cells of young roots all cells were more or less stained (Fig. 5, A and B). A predominant expression was observed in epidermal cells of AtVLN 2::GUS plants (Fig. 5B). More mature roots showed strong GUS activity in the vascular cylinder, but cortical cells expressed little GUS (Fig. 5, C and D). As expected, no GUS activity was found in the mature xylem. AtVLN 1::GUS was expressed in most cells of the root tip. However, less staining was observed in the center of the root tip, which contains the most actively dividing cells of the meristem, indicating that undifferentiated cells express little or no AtVLN (Fig. 5E, see also below, Fig. 5H). The cross-section through the apical meristem shown in Figure 5G demonstrates that strong AtVLN 1::GUS expression was again found in stipules. In addition, young leaf primordia were also stained, while the actively dividing and undifferentiated cells of the meristem showed little or no staining. The expression patterns of the two AtVLN genes indicate that most differentiating cells express the putative actin-bundling protein. Higher expression levels are found in younger tissues.
Figure 5.
Cell-specific expression in AtVLN 1::GUS (A, C, E, and G) and AtVLN 2::GUS (B, D, and F) transgenic plants. A and B, Cross-sections of root tips. C and D, Cross-sections through adult root. E, Longitudinal section of root tip. F, Longitudinal section of lateral root bud. G, Section through apical meristem; S, stipules; L, leaf primordium. GUS staining was performed for 16 h. H, Staining of a root of an AtVLN 1::GUS plant. Two days after treatment with 2,4-D, GUS staining was performed for 2 h. Scale bars represent 10 μm in A through E and 100 μm in F through H.
Because mammalian villin expression is strongly regulated developmentally and in response to sugar and pH variations, we tested the response of plants carrying promoter-GUS transgenes to various compounds. First, the addition of Suc to the culture medium did not change the intensity nor the tissue distribution of GUS expression (data not shown). Next, we investigated the effects of hormones such as gibberellic acid, abscisic acid, auxin (2,4-dichlorophenoxyacetic acid [2,4-D]), methyljasmonate, brassinolide, and cytokinin (benzyladenine). With the exception of auxin, none of the treatments had an effect on either AtVLN::GUS expression. Auxin is known to strongly affect root differentiation and to promote lateral root initiation (e.g. Hemerly et al., 1993). Therefore, it was not surprising that the expression pattern of AtVLN 1::GUS in particular was altered. After 2 d of incubation on 50 μm 2,4-D, GUS expression was redistributed due to a de-differentiation of cells of the central cylinder (Fig. 5H). To test whether the expression of AtVLNs was changed by hormone application, RNAs from treated and untreated plants were isolated and analyzed by gel-blot hybridization. None of the treatments above changed mRNA levels significantly (data not shown).
AtVLNs Interact with Plant and Animal Actin Filaments
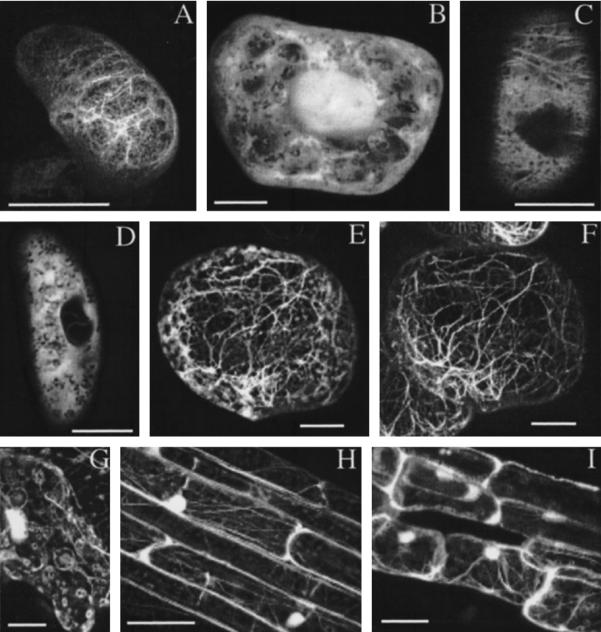
Animal villin has several actin binding sites, one F-actin binding site was found in the N terminus and one in the headpiece. The latter has been thoroughly studied and amino acid residues critical for its function have been identified (Friederich et al., 1992; Doering and Matsudaira, 1996). To examine whether AtVLNs can indeed interact with actin filaments, and because available antibodies did not recognize AtVLN in immunofluorescence assays, we constructed fusion genes encoding proteins of AtVLN headpiece domains fused to the GFP as well as a fusion gene of AtVLN 3 and GFP. These constructs were introduced into Arabidopsis by A. tumefaciens-mediated transformation and into tobacco BY2 cell lines by biolistic bombardment. All three headpiece-GFP fusion proteins, as well as the entire AtVLN 3 protein fused to GFP, showed similarly localized GFP signals and appeared to have no obvious morphological effect on plant cells. Positive cells showed fluorescent filaments that resembled actin filaments. Figure 6A shows a BY2 cell expressing a full-length AtVLN 3-GFP. Control experiments in cells expressing GFP alone showed diffuse staining throughout cells, with strong staining in the nucleus (Fig. 6B). A comparison of optical sections through the cortex of cells expressing GFP-AtVLN or GFP alone demonstrated that stained actin filaments were clearly distinguishable from cytoplasmic strands (Fig. 6, C and D, respectively). Similar results were obtained when a GFP-headpiece AtVLN 3 construct was used (Fig. 6E). Furthermore, as seen in Figure 6F, the GFP fluorescence co-localized precisely with the rhodamine-phalloidin staining of actin filaments. However, the treatment with the staining solution slightly changed the appearance of the fluorescence (compare Fig. 6, A and E), probably due to the permeabilization of the cells. As seen in BY2 cells, transgenic Arabidopsis showed staining of actin filaments in all tissues examined. Actin filaments were easily visible in leaf, hypocotyl, and root tissues (Fig. 6, G–I, respectively). These results indicate that plant VLN headpiece domains can decorate F-actin in vivo. Because AtVLN 1 lacks certain crucial amino acids compared with the mammalian villin headpiece (see also Fig. 1), it is surprising that all three fusion proteins interacted with actin similarly. Full-length AtVLN 3-GFP was equally capable of labeling actin filaments.
Figure 6.
AtVLNs interact with F-actin in vivo. A, GFP-derived fluorescence from a BY2 cell expressing GFP-AtVLN 3. B, Fluorescence of a BY2 cell expressing GFP alone. C, Optical section through a cell expressing GFP-AtVLN 2-headpiece. D, Optical section through a cell expressing GFP alone. E, GFP-derived fluorescence from a BY2 cell expressing GFP-AtVLN 3-headpiece. F, Same cell as E, but counterstained with rhodamine-phalloidin. G through I, GFP-derived fluorescence from transgenic plant carrying GFP-AtVLN 1-headpiece. G, Epidermis; H, hypocotyl; I, root. Circular structures in epidermal cells were regularly observed, but are of unknown origin. Scale bar represents 10 μm in A through F and 50 μm in G through I.
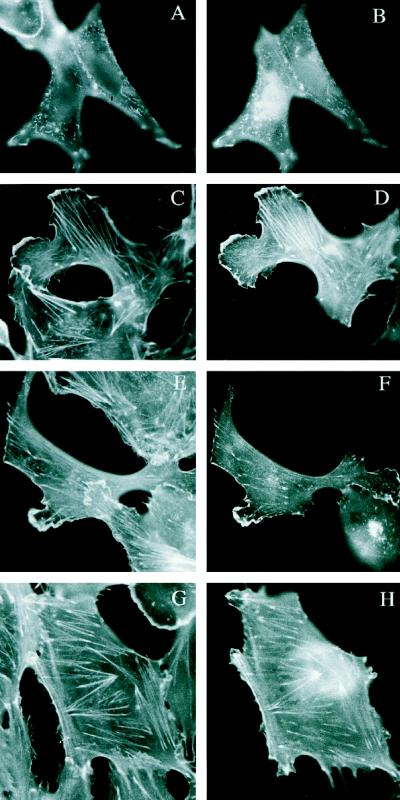
To determine whether the properties of plant VLNs are similar to those of animal villin, the plasmids carrying the transgene encoding headpiece-GFP fusion proteins were also introduced into animal cells. Transfection of Vero cells (monkey kidney fibroblasts) with plasmids expressing these proteins under the control of the mammalian cytomegalovirus promoter (see “Materials and Methods”) showed similar results as in plants. Headpiece domains of AtVLN 1 and 3, which are representative for the two groups of AtVLN, co-localized with F-actin structures visualized by staining with rhodamine-phalloidin (Fig. 7). Compared with human villin headpiece, which preferentially co-localizes with F-actin of microvilli (Friederich et al., 1989), strong GFP signals were found in stress fibers, microvilli, and plasma membrane extensions (Fig. 7, B, D, and F, respectively). Even the headpiece of AtVLN 1, which lacks many of the highly conserved residues in this domain, was able to interact with mammalian actin. Counterstaining with rhodamine-phalloidin confirmed the association with F-actin (Fig. 7, A, C, E, and G).
Figure 7.
Expression of GFP-fusion proteins in mammalian Vero cells. A, Rhodamine-phalloidin staining of B. B, GFP-headpiece-AtVLN3 fusion protein, dorsal face of cell, showing microvilli. C, Rhodamine-phalloidin staining of D. D, GFP-headpiece-AtVLN1 fusion protein, base of cell, showing membrane extensions. E, Rhodamine-phalloidin staining of F. F, GFP-headpiece-AtVLN3 fusion protein, base of cell, showing membrane extensions. G, Rhodamine-phalloidin staining of H. H, GFP-headpiece-AtVLN3 fusion protein, base of cell, showing stress fibers.
DISCUSSION
Considering the specific function of mammalian villin in absorptive tissues, as well as that of the villin-like protein QUAIL in Drosophila, the mere existence of a highly conserved version of this class of proteins in plants comes as a surprise. In this context, evolutionary considerations might help to understand the function of villin, but maybe even aspects of the role of the actin cytoskeleton in general. The introduction of actin into primitive cells supposedly enabled them to enlarge their volume and organize their interior into compartments. Actin-binding proteins, in turn, appear to be instrumental for the evolution of specialized morphological traits and multicellular organisms per se. The fact that villin-like genes have been isolated only from multicellular organisms and have no homolog in S. cerevisiae indicates that this protein appeared at the onset of the existence of multicellular structures.
Intron positions in the 5′ half of villin genes are rather well conserved between plants and mammals. Six introns are found at identical positions, which indicates that the first half of the protein is substantially more conserved throughout evolution than the second half, which includes domains 4 through 6 and the headpiece. This is also reflected at the amino acid level. It is possible that functional domains in the N terminus (severing, F-actin binding, calcium regulation, and phosphoinositide binding) allow little variation in this part of the protein.
AtVLN 1 cDNAs (EST H5D9T7 and our RT-PCR clones) showed variability either in their splicing pattern or in the fidelity of reverse transcription. It has been previously reported that mammalian villin contains secondary structures within its mRNA (Arpin et al., 1988), which might be a cause for unfaithful polymerization by reverse transcriptase. This could indicate that secondary structures are important for the regulation of the expression and/or localization of villin-like mRNAs. Mammalian actin mRNA is localized by a mechanism that involves a “zip code” recognition sequence (Singer, 1996) and a protein complex that includes gelsolin (Ross et al., 1997). Actin mRNA localization itself is dependent on actin filaments (Singer, 1996).
Alternatively, it is possible that the mRNA that is missing the 13 nucleotides does indeed produce a functional protein. The AtVLN1 mRNA that lacks 13 nucleotides encodes a protein that comprises only the first three domains and would therefore resemble the shorter capping proteins found in several organisms. It would therefore be interesting to know whether the production of alternative forms of AtVLN1 is regulated in plants.
Villin in mammals is involved in the correct formation of microvilli in intestines. Together with fimbrin and a myosin, it promotes the formation of densely packed actin filaments that define this microstructure. Similarly, in Drosophila oocytes QUAIL, together with SINGED (a fascin homolog) and CHICKADEE (a profilin), forms thick actin cables that are required for the retention of nuclei within nurse cells (Robinson and Cooley, 1997). In both cases actin bundling proteins serve to form a structural element in a specific tissue. Likewise, AtVLN might execute a cell morphological task to form elongated cells and/or specialized intracellular surfaces. The lack of AtVLN expression in undifferentiated tissue and high expression levels in rapidly elongating tissues are in good agreement with such a model.
As yet, no gelsolin-like protein has been isolated from Arabidopsis. However, some of the AtVLNs share a higher amino acid homology to animal gelsolin within the core sequence compared with animal villin. Moreover, the expression of AtVLNs throughout the plant is a feature that is more similar to gelsolin, which is expressed in most animal tissues (Paunio et al., 1997). It is therefore possible that AtVLNs have gelsolin-like functions. All three AtVLN headpiece domains tested, however, were able to associate with F-actin in vivo, making it likely that AtVLNs have an actin bundling function. As this capability is lacking in gelsolin, we assume that AtVLNs are true functional homologs of mammalian villin. We note, however, that the bundling activity of AtVLNs has to be demonstrated, especially since no such activity was observed in vitro for villin of Dictyostelium discoideum (Hofmann et al., 1993).
The expression of AtVLN-GFP fusions also showed that the plant proteins associate with a variety of actin filaments within plant and animal cells. This suggests that plant villin-like proteins bind less specifically to a particular set of actin filaments than animal villin, which is found in the brush borders of the intestines. It will be interesting to determine the binding specificity of advillin/DOC6 (Marks et al., 1998; Wang et al., 1998) to determine whether this protein has similar properties as the plant proteins. Furthermore, unlike animal villin, fusions of plant villin-like proteins to GFP are useful markers for actin filaments in animal cells in vivo. We recently described a similar system (Kost et al., 1998, 1999) using a GFP-talin construct for the detection of actin filaments. Labeling of filaments was similar in both systems, with the difference that in live cells GFP-AtVLN constructs also produced some diffuse staining.
RNA and protein analyses showed that AtVLN genes are expressed in most tissues of Arabidopsis. In addition, immunologically related proteins can be detected in extracts of a number of crop plants. This indicates that villin-like proteins may serve a more general function in plants than in the organisms for which it has been previously described. The fact that the expression seems to be lower in meristematic tissues (see Figs. 4 and 5) suggests that it is not important for cell division but rather for cell differentiation and, more specifically, in tissues that are composed of elongated cells.
ACKNOWLEDGMENTS
We would like to thank Li-Fang Huang, Qi-Wen Niu, and Bernadette Menichi for technical assistance, the Arabidopsis Biological Resource Center for EST clones, and Greg Jedd, Pius Spielhofer, Emmanuel Lemichez, and Yang Sun Chan for helpful discussions and suggestions.
Footnotes
This paper was supported in part by fellowships from the Swiss National Science Foundation to U.K. and B.K. and by a grant from the Department of Energy (no. DOE94ER20143) to N.-H.C.
LITERATURE CITED
- Ampe C, Vanderkerckhove J. The F-actin capping proteins of Physarum polycephalum: cap42(a) is very similar, if not identical, to fragmin and is structurally and functionally very homologous to gelsolin; cap42(b) is Physarum actin. EMBO J. 1987;6:4149–4157. doi: 10.1002/j.1460-2075.1987.tb02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre E, Lottspeich F, Schleicher M, Noegel A. Severin, gelsolin, and villin share a homologous sequence in regions presumed to contain F-actin severing domains. J Biol Chem. 1988;263:722–727. [PubMed] [Google Scholar]
- Aoyama T, Chua N-H. A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 1997;11:605–612. doi: 10.1046/j.1365-313x.1997.11030605.x. [DOI] [PubMed] [Google Scholar]
- Arpin M, Pringault E, Finidori J, Garcia A, Jeltsch J-M, Vandekerckhove J, Louvard D. Sequence of human villin: a large duplicated domain homologous with other actin-severing proteins and a unique small carboxy-terminal domain related to villin specificity. J Cell Biol. 1988;107:1759–1766. doi: 10.1083/jcb.107.5.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes SA, Nishizawa NK, Quaggio RB, Whitelam GC, Chua N-H. Far-red light blocks greening of Arabidopsis seedlings via a phytochrome A-mediated change in plastid development. Plant Cell. 1996;8:601–615. doi: 10.1105/tpc.8.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher A, Weber K. Villin is a major protein of the microvillus cytoskeleton which binds both G and F actin in a calcium-dependent manner. Cell. 1980;20:839–847. doi: 10.1016/0092-8674(80)90330-x. [DOI] [PubMed] [Google Scholar]
- Bretscher A, Weber K. Villin: the major microfilament-associated protein of the intestinal microvillus. Proc Natl Acad Sci USA. 1979;76:2321–2325. doi: 10.1073/pnas.76.5.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtnick LD, Koepf EK, Grimes J, Jones EY, Stuart DI, McLaughlin PJ, Robinson RC. The crystal structure of plasma gelsolin: implications for actin sequestering, capping, and nucleation. Cell. 1997;90:661–670. doi: 10.1016/s0092-8674(00)80527-9. [DOI] [PubMed] [Google Scholar]
- Campbell HD, Schimansky T, Claudianos C, Ozsarac N, Kasprzak AB, Cotsell JN, Young IG, De Couet HG, Miklos GLG. The Drosophila melanogaster flightless-I gene involved in gastrulation and muscle degeneration encodes gelsolin-like and leucine-rich repeat domains and is conserved in Caenorhabditis elegans and humans. Proc Natl Acad Sci USA. 1993;90:11386–11390. doi: 10.1073/pnas.90.23.11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coluccio LM, Bretscher A. Reassociation of microvillar core proteins: making a microvillar core in vitro. J Cell Biol. 1989;108:495–502. doi: 10.1083/jcb.108.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig SW, Powell LD. Regulation of actin polymerization by villin, a 95,000 Dalton cytoskeletal component of intestinal brush borders. Cell. 1980;22:739–746. doi: 10.1016/0092-8674(80)90550-4. [DOI] [PubMed] [Google Scholar]
- Doering DS, Matsudaira P. Cysteine scanning mutagenesis at 40 of 76 positions in villin headpiece maps the F-actin binding site and structural feature of the domain. Biochemistry. 1996;35:12677–12685. doi: 10.1021/bi9615699. [DOI] [PubMed] [Google Scholar]
- Dudouet B, Robine S, Huet C, Sahuquillo-Merino C, Blair L, Coudrier E, Louvard D. Changes in villin synthesis and subcellular distribution during intestinal differentiation of HT29–18 clones. J Cell Biol. 1987;105:359–369. doi: 10.1083/jcb.105.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fath KR, Burgess DR. Not actin alone. Curr Biol. 1995;5:591–593. doi: 10.1016/s0960-9822(95)00117-5. [DOI] [PubMed] [Google Scholar]
- Fitzgerald RC, Omary MB, Triadafilopoulos G. Acid modulation of HT29 cell growth and differentiation. J Cell Sci. 1997;110:663–671. doi: 10.1242/jcs.110.5.663. [DOI] [PubMed] [Google Scholar]
- Franck Z, Footer M, Bretscher A. Microinjection of villin into cultured cells induces rapid and long-lasting changes in cell morphology but does not inhibit cytokinesis, cell motility, and membrane ruffling. J Cell Biol. 1990;111:2475–2485. doi: 10.1083/jcb.111.6.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich E, Gouin E, Hellio R, Kocks C, Cossart P, Louvard D. Targeting of Listeria monocytogenes ActA protein to the plasma membrane as a tool to dissect both actin-based cell morphogenesis and ActA function. EMBO J. 1995;15:2731–2744. doi: 10.1002/j.1460-2075.1995.tb07274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederich E, Huet C, Arpin M, Louvard D. Villin induces microvilli growth and actin redistribution in transfected fibroblasts. Cell. 1989;59:461–475. doi: 10.1016/0092-8674(89)90030-5. [DOI] [PubMed] [Google Scholar]
- Friederich E, Vancompernolle K, Huet C, Goethals M, Finidori J, Vanderkerckhove J, Louvard D. An actin-binding site containing a conserved motif of charged amino acid residues is essential for the morphogenic effect of villin. Cell. 1992;70:81–92. doi: 10.1016/0092-8674(92)90535-k. [DOI] [PubMed] [Google Scholar]
- Glenney JR, Kaulfus P, Weber K. F actin assembly modulated by villin: Ca++-dependent nucleation and capping of the barbed end. Cell. 1981;24:471–480. doi: 10.1016/0092-8674(81)90338-x. [DOI] [PubMed] [Google Scholar]
- Hemerly AS, Ferreira P, deAlmeida Engler J, Van Montagu M, Engler G, Inze D. cdc2a expression in Arabidopsis is linked with competence for cell division. Plant Cell. 1993;5:1711–1723. doi: 10.1105/tpc.5.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesterberg LK, Weber K. Ligand-induced conformational changes in villin, a calcium-controlled actin-modulating protein. J Biol Chem. 1983;258:359–364. [PubMed] [Google Scholar]
- Hofmann A, Noegel AA, Bomblies L, Lottspeich F, Schleicher M. The 100 kDa F-actin capping protein in Dictyostelium amoeba is a villin prototype (“protovillin”) FEBS Lett. 1993;328:71–76. doi: 10.1016/0014-5793(93)80968-z. [DOI] [PubMed] [Google Scholar]
- Janmey PA, Lamb J, Allen PG, Matsudaira PT. Phosphoinositide-binding peptides derived from the sequences of gelsolin and villin. J Biol Chem. 1992;267:11818–11823. [PubMed] [Google Scholar]
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5:387–405. [Google Scholar]
- Kato K, Matsumoto T, Kowai A, Mizusaki S, Nishida K, Noguchi M, Tamaki E. Liquid suspension culture of tobacco cells. Ferment Technol Today. 1972. pp. 689–695. [Google Scholar]
- Kerneis S, Bogdanova A, Kraehenbuhl J-P, Pringault E. Conversion by Peyer's patch lymphocytes of human enterocytes into M cells that transport bacteria. Science. 1997;277:949–952. doi: 10.1126/science.277.5328.949. [DOI] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, Chua N-H. Rac homologues and compartmentalized phosphatidylinositol 4,5-bisphosphate act in a common pathway to regulate polar pollen tube growth. J Cell Biol. 1999;19:317–330. doi: 10.1083/jcb.145.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kost B, Spielhofer P, Chua N-H. A GFP-mouse talin fusion protein labels plant actin filaments in vivo and visualizes the actin cytoskeleton in growing pollen tubes. Plant J. 1998;16:393–401. doi: 10.1046/j.1365-313x.1998.00304.x. [DOI] [PubMed] [Google Scholar]
- Kuhlemeier C, Fluhr R, Chua N-H. Upstream sequences determine the difference in transcript abundance of pea rbcS genes. Mol Gen Genet. 1988;212:405–411. [Google Scholar]
- Kwiatkowski DJ, Stossel TP, Orkin SH, Mole JE, Colten HR, Yin HL. Plasma and cytoplasmic gelsolins are encoded by a single gene and contain a duplicated actin-binding domain. Nature. 1986;323:455–458. doi: 10.1038/323455a0. [DOI] [PubMed] [Google Scholar]
- Leu W-M, Cao J-L, Wilson TJ, Snustad P, Chua N-H. Phytochrome A and phytochrome B mediate the hypocotyl-specific downregulation of TUB1 by light in Arabidopsis. Plant Cell. 1995;7:2187–2196. doi: 10.1105/tpc.7.12.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan-Miklos S, Cooley L. The villin-like protein encoded by the Drosophila quail gene is required for actin bundle assembly during oogenesis. Cell. 1994;78:291–301. doi: 10.1016/0092-8674(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Marks PW, Arai M, Bandura JL, Kwiatkowski DJ. Advillin (p92): a new member of the gelsolin/villin family of actin regulatory proteins. J Cell Sci. 1998;111:2129–2136. doi: 10.1242/jcs.111.15.2129. [DOI] [PubMed] [Google Scholar]
- Markus MA, Matsudaira P, Wagner G. Refined structure of villin 14T and a detailed comparison with other actin-severing domains. Protein Sci. 1997;6:1197–1209. doi: 10.1002/pro.5560060608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira PT, Burgess DR. Identification and organization of the components in the isolated microvillus cytoskeleton. J Cell Biol. 1979;83:667–673. doi: 10.1083/jcb.83.3.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meagher RB, McKinney EC, Vitale AV. The evolution of new structures: clues from plant cytoskeletal genes. Trends Genet. 1999;15:278–284. doi: 10.1016/s0168-9525(99)01759-x. [DOI] [PubMed] [Google Scholar]
- Mooseker MS, Graves TA, Wharton KA, Falco N, Howe CL. Regulation of microvillus structure: calcium-dependent solation and cross-linking of actin filaments in the microvilli of intestinal epithelial cells. J Cell Biol. 1980;87:809–822. doi: 10.1083/jcb.87.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman TC, Ohme-Takagi M, Taylor CB, Green PJ. DST sequences, highly conserved among plant SAUR genes, target reporter transcripts for rapid decay in tobacco. Plant Cell. 1993;5:701–714. doi: 10.1105/tpc.5.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunio T, Kangas H, Kiuru S, Peltonen L, Syvanen AC. Tissue distribution and levels of gelsolin mRNA in normal individuals and patients with gelsolin-related amyloidosis. FEBS Lett. 1997;406:49–55. doi: 10.1016/s0014-5793(97)00237-8. [DOI] [PubMed] [Google Scholar]
- Pestonjamasp KN, Pope RK, Wulfkuhle JD, Luna EJ. Supervillin(p205): a novel membrane-associated, F-actin-binding protein in the villin/gelsolin superfamily. J Cell Biol. 1997;139:1255–1269. doi: 10.1083/jcb.139.5.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Dunbar L, Samuelson L, Gumucio DL. Targeted disruption of the mouse villin gene does not impair the morphogenesis of microvilli. Dev Dyn. 1998;211:109–121. doi: 10.1002/(SICI)1097-0177(199801)211:1<109::AID-AJA10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Pope B, Way M, Matsurdaira PT, Weeds A. Characterisation of the F-actin binding domains of villin: classification of F-actin binding proteins into two groups according to their binding sites on actin. FEBS Lett. 1994;338:58–62. doi: 10.1016/0014-5793(94)80116-9. [DOI] [PubMed] [Google Scholar]
- Pringault E, Robine S, Louvard D. Structure of the human villin gene. Proc Natl Acad Sci USA. 1991;88:10811–10815. doi: 10.1073/pnas.88.23.10811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robine S, Huet C, Moll R, Sahuquillo-Merino C, Coudrier E, Zweibaum A, Louvard D. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? Proc Natl Acad Sci USA. 1985;82:8488–8492. doi: 10.1073/pnas.82.24.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DN, Cooley L. Genetic analysis of the actin cytoskeleton in the Drosophila ovary. Annu Rev Cell Dev Biol. 1997;13:147–170. doi: 10.1146/annurev.cellbio.13.1.147. [DOI] [PubMed] [Google Scholar]
- Ross AF, Oleynikov Y, Kislauskis ED, Taneja KL, Singer RH. Characterization of a β-actin mRNA zipcode-binding protein. Mol Cell Biol. 1997;17:2158–2165. doi: 10.1128/mcb.17.4.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Schleicher M, Andre E, Hartmann H, Noegel AA. Actin-binding proteins are conserved from slime molds to man. Dev Genet. 1988;9:521–530. doi: 10.1002/dvg.1020090428. [DOI] [PubMed] [Google Scholar]
- Singer RH. RNA: traffic transport. Trends Cell Biol. 1996;6:486–489. doi: 10.1016/0962-8924(96)84947-6. [DOI] [PubMed] [Google Scholar]
- Staiger CJ, Gibbon BC, Kovar DR, Zonia LE. Profilin and actin-depolymerizing factor: modulators of actin organization in plants. Trends Plant Sci. 1997;2:275–281. [Google Scholar]
- Valvekens D, Van Montagu M, Lijsebettens M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants using kanamycin selection. Proc Natl Acad Sci USA. 1988;85:5536–5540. doi: 10.1073/pnas.85.15.5536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X-Z, Kuroda M, Sok J, Batchvarova N, Kimmel R, Chung P, Zinszner H, Ron D. Identification of novel stress-induced genes downstream of chop. EMBO J. 1998;17:3619–3630. doi: 10.1093/emboj/17.13.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R, Ainscough R, Anderson K, Baynes C, Berks M, Bonfield J, Burton J, Connell M, Copsey T, Cooper J, Coulson A, Craxton M, Dear S, Du Z, Durbin R, Favello A, Fraser A, Fulton L, Gardner A, Green P, Hawkins T, Hillier L, Jier M, Johnston L, Jones M, Kershaw J, Kirsten J, Laisster N, Latreille P, Lightning J, Lloyd C, Mortimore B, O'Callaghan M, Parsons J, Percy C, Rifken L, Roopra A, Saunders D, Shownkeen R, Sims M, Smaldon N, Smith A, Smith M, Sonnhammer E, Staden R, Sulston J, Thierry-Mieg J, Thomas K, Vaudin M, Vaughan K, Waterston R, Watson A, Weinstock L, Wilkinson-Sproat J, Wohldman P. 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature. 1994;368:32–38. doi: 10.1038/368032a0. [DOI] [PubMed] [Google Scholar]
- Zweibaum A, Pinto M, Chevalier G, Dussaux E, Triadou N, Lacroix B, Haffen K, Brun J-L, Rousset M. Enterocytic differentiation of a subpopulation of the human colon tumor cell line HT-29 selected for growth in sugar-free medium and its inhibition by glucose. J Cell Physiol. 1985;122:21–29. doi: 10.1002/jcp.1041220105. [DOI] [PubMed] [Google Scholar]