Abstract
Esophageal adenocarcinoma (EAC) is the fastest growing cancer in the western world and the overall 5 year survival rate of EAC is below 20%. Most patients with EAC present with locally advanced or widespread metastatic disease, where current treatment is largely ineffective. Therefore, new therapeutic approaches are urgently needed. Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is a novel albumin-stabilized, cremophor-free and water soluble nanoparticle formulation of paclitaxel, and the potential role of nab-paclitaxel has not been tested yet in experimental EAC. Here we tested the antiproliferative and antitumor efficacy with survival advantage of nab-paclitaxel as monotherapy and in combinations in in-vitro, and in murine subcutaneous xenograft and peritoneal metastatic survival models of human EAC. Nab-paclitaxel significantly inhibited in-vitro cell proliferation with higher in-vivo antitumour efficacy and survival benefit compared to paclitaxel or carboplatin treatments both in mono- and combination therapies. Nab-paclitaxel treatment increased expression of mitotic-spindle associated phospho-stathmin, decreased expression of proliferative markers and enhanced apoptosis. This study demonstrates that nab-paclitaxel had stronger antiproliferative and antitumor activity in experimental EAC than the current standard chemotherapeutic agents which supports the rationale for its clinical use in EAC.
Introduction
Esophageal adenocarcinoma (EAC) has become the dominant type of esophageal cancer in the United States, and represents the fastest growing cancer in the western world [1], [2], [3], [4], [5], [6]. Adenocarcinoma of the distal esophagus, gastroesophageal (GE) junction, and proximal stomach is increasing in incidence and represents an emerging health epidemic in the United States and other Western countries [7]. Despite recent advances in surgical and radiation technique as well as in systemic medical treatment, prognosis remains poor [8], [9], [10]. 5-year relative survival rates for localized, regional and distal esophageal cancer are 40%, 21% and 4%, respectively [11]. Moreover, 50-60% of EAC cases are unresectable at diagnosis [12]. Paclitaxel (PT) has been used in combination with carboplatin (CP) as a conventional combination therapy for advanced EAC [13]. Although EAC seems to respond to conventional chemotherapy, clinical benefit is limited and most patients eventually die from metastatic disease [8], [9], [10], [14] while chemotherapy remains the mainstay of palliative treatment. Therefore, new therapeutic approaches are urgently needed. Currently, clinical trials are underway to explore combination treatment benefits of cytotoxic agents with targeted agents for the development of more effective therapeutic approaches [15], [16].
Paclitaxel is a classical microtubule inhibitor that promotes G2/M phase arrest and mitotic cell death by tubulin polymerization and stabilization of microtubules [17], [18], [19]. Paclitaxel phosphorylates stathmin, a phosphorylation-regulated tubulin-binding protein. Phosphorylation of stathmin reduces its microtubule destabilizing effects leading to mitotic arrest, a phenomenon that has been ascribed to taxane activity [20], [21], [22], [23]. Paclitaxel is a key cytotoxic agent in the first line and recurrent setting for most EAC. Paclitaxel has been frequently tested for advanced and recurrent EAC in combination with carboplatin [13]. However, paclitaxel requires emulsification with solvents to allow intravenous administration which has resulted in serious adverse effects in patients. Although active in EAC, some patients will not tolerate the drug secondary to hypersensitivity reactions (HSRs) [24], [25]. While the exact etiology of the HSRs is not specifically known, the Cremophor solvent required for the hydrophobic paclitaxel is thought to play a role [26]. Nanoparticle albumin-bound paclitaxel (nab-paclitaxel) is an albumin-stabilized, cremophor-free and water soluble nanoparticle formulation of paclitaxel consisting of 130 nm albumin-paclitaxel nanoparticles. The use of nanotechnology as a delivery system for paclitaxel was designed in part to neutralize paclitaxel’s hydrophobicity and thus eliminate the need for the Cremophor solvent. Nab-paclitaxel (NPT) treatment showed higher response rates and improved tolerability compared with solvent-based formulations in patients with advanced metastatic breast cancer, non-small cell-lung cancer and pancreatic adenocarcinoma [27], [28], [29].
Thus, Nab-paclitaxel is a novel microtubule-inhibitory cytotoxic agent and the potential role of nab-paclitaxel has not been tested yet in experimental EAC. Since the treatment cost of nab-paclitaxel is relatively higher than the commonly used paclitaxel, it is of substantial interest to know the comparative biological effects of nab-paclitaxel in experimental EAC. We therefore, in this study explored the antiproliferative and antitumor efficacy with survival advantage following carboplatin, paclitaxel and nab-paclitaxel as monotherapy and in combinations in in-vitro as well as in murine subcutaneous xenograft and peritoneal metastatic survival models of human EAC.
Materials and Methods
Cell Lines Culture and Reagents
Human esophageal adenocarcinoma cell lines OE19 and OE33 were obtained from Sigma Aldrich (St. Lois, MO). Both cell lines were cultured in RPMI-1640 medium (Gibco, Grand Island, New York, USA) supplemented with 10% fetal bovine serum (Hyclone), 2 mM GlutaMax (Gibco), 100 U/ml penicillin, 100 mg/ml streptomycin at 37 °C in a humidified atmosphere of 95% air – 5% CO2. Nab-paclitaxel was purchased from Abraxis Bioscience (Los Angeles, CA), paclitaxel, carboplatin and 5-flurouracil were obtained from local pharmacy.
Cell Viability Assay
Cell viability of esophageal adenocarcinoma OE19 and OE33 cell line was evaluated by the colorimetric WST-1 assay as previously described [30], [31]. The measurement is based on the ability of viable cells to cleave the sulfonated tetrazolium salt WST-1 (4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate) by mitochondrial dehydrogenases. OE19 and OE33 cells (5,000 cells/well) were plated in a 96-well plate in regular growth medium. After 16 hours the medium was replaced with 2% FBS containing medium and the cells were treated with nab-paclitaxel (NPT), paclitaxel (PT), carboplatin (CP) or 5-fluorouracil (5-FU) (1 nM to 5 μM). Cells were also treated with nab-paclitaxel or paclitaxel in combinations with carboplatin (all 1000 nM). After 72 hours, 10 μL WST-1 reagent was added in each well followed by additional incubation for 2 hours. The absorbance at 450 nm was measured using a microplate reader.
Western Blot Analysis
Western blot analyses were determined as described by us previously [32], [33]. OE 19 xenograft tissue samples were homogenized in a cold lysis buffer (20 mM HEPES, 150 mM NaCl, 1 mM EDTA, 0.5% Na+deoxycholate, 1% Nonidet P-40,and 1 mM DTT, pH 7.4) containing protease and phosphatase inhibitor cocktails (both from Sigma-Aldrich, St. Louis, MO) using a glass dounce tissue homogenizer. All other cell lysates were prepared by scraping cells from culture plates in cold lysis buffer containing protease and phosphatase inhibitor cocktails. For cell lysates OE19 and OE33 cells were cultured for 24 hours and then treated with 5 μM of nab-paclitaxel, paclitaxel, carboplatin and 5-fluorouracil alone or in combinations for 12 hours. Polyacrylamide gel electrophoresis was used to separate equal amounts of protein samples, which were then transferred to nitrocellulose membranes for analysis. The nitrocellulose membranes were blocked for 1 hour in PBS-T at room temperature and then incubated overnight at 4°C with the following primary antibodies: total stathmin, phospho stathmin (ser38), cleaved poly (ADP-ribose) polymerase-1, cleaved caspase-3 (all from Cell Signaling Technology, Beverly, MA) and β-actin (Sigma-Aldrich, St. Louis, MO). Blots were incubated with HRP-conjugated secondary antibodies (Pierce Biotechnologies, Santa Cruz, CA) for 1 hour at room temperature. Specific bands were detected using the enhanced chemiluminescence reagent (ECL, Perkin Elmer Life Sciences, Boston, MA.) Protein bands were quantified using ImageJ software (National Institutes of Health).
Subcutaneous Tumor Growth Model
All mouse experiments used in this study were carried out in accordance with the standards and guidelines of the Institutional Animal Care and Use Committee (IACUC) at the University of Notre Dame and confirmed to NIH guidelines. All animal researches used in this study were approved by the University of Notre Dame IACUC under protocol 15-08-263. At the end of experiments mice were euthanized by CO2 exposure followed by cervical dislocation according to University of Notre Dame IACUC-approved procedures. Female athymic nude mice (4 to 6 weeks old) were subcutaneously injected with OE19 esophageal adenocarcinoma cell lines (5X106). Measurements of subcutaneous tumor size were started when mice had measurable tumors. All mice had measurable tumor two weeks after OE19 cell injection. The mice were then randomly grouped (n = 5 per group) and treated intraperitoneally as described earlier with vehicle, nab-paclitaxel (10 mg/kg in 100 μl of PBS, 2 times a week) [34], paclitaxel (20 mg/kg, 2 times a week for 2 weeks) [35] or carboplatin (50 mg/kg, 2 times a week for 2 weeks) [36] alone and in combinations. The tumor size was measured twice a week for four weeks with slide calipers and tumor volume (TV) was calculated as (W2XL)/2, where W is width and L is length of the tumor [37]. Relative tumor volume (RTV) was calculated according to the following formula; RTV = TVn/TV0 where TVn is the tumor volume at the day of measurement and TV0 is the tumor volume on the first day of measurement [38]. Mice weight was measured twice a week during the period of the study. All mice were euthanized at the end of study and tumors were removed, weighted, dissected and processed for histological, immunohistochemical and western blot analysis.
Immunohistochemical Analysis
Antibody staining was performed on histological sections of 4% paraformaldehyde-fixed OE19 tumor xenografts. Prior to immunohistochemical staining, endogenous peroxidase activity was quenched with 3% (v/v) hydrogen peroxide for 30 min. Antigen retrieval was enhanced by microwaving in 10 mM sodium citrate, pH 6.0. Nonspecific binding was blocked with 3% normal horse serum in PBS for 30 minutes. Cleaved caspase-3 staining was performed with an anti–cleaved caspase-3 primary antibody (1:1,000 dilution; catalog 9661; Cell Signaling Technology) that specifically recognizes the large fragment (17 kDa) of the active protein but not full-length caspase-3. Similarly, Ki-67 immunostaining was performed with a polyclonal anti–Ki-67 (1:200 dilution; ab15580; Abcam) antibody. Staining was detected using an avidin-biotin horseradish peroxidase system (Vectastain Universal Elite ABC kit, Cat. PK-6200, Vector Laboratories, Burlingame, CA), with positive cells staining brown using diaminobenzidine chromogen and hydrogen peroxide substrate (two-component DAB pack HK542-XAK, BioGenex, San Ramon, CA). Slides were counterstained with modified Harris hematoxylin. Tissue sections were dehydrated through graded ethanol and mixed xylenes and mounted onto coverslips with mounting medium (Surgipath Micromount, Leica Biosystems, Richmond, IL).
Determination of Apoptotic and Proliferative Indices
For this study, activated cleaved caspase-3 labeling was used to identify apoptotic cells. The number of cleaved caspase-3–labeled cells in immunostained sections was counted relative to the total number of epithelial cells present in five different high power fields in a blinded manner. The apoptotic index for each treatments was expressed as the number of apoptotic cells per 100 cells. The Ki-67 proliferative index was determined similarly.
Peritoneal-Disseminated Animal Survival Analysis
Animal survival studies were performed using 6- to 8-week-old female non-obese diabetic/severe combined immunodeficient (NOD/SCID) mice bought from Charles River [30]. Mice were intraperitoneally injected with OE19 or OE33 (10X1010). Peritoneal tumor formation and animal survival were evaluated from the day of cancer cell injection until death. Two weeks after tumor cell injection mice were randomly grouped (n = 5 per group). For mice survival studies mice were treated intraperitoneally as described earlier with vehicle, nab-paclitaxel (10 mg/kg in 100 μl of PBS, 2 times a week) [34], paclitaxel (20 mg/kg, 2 times a week for 2 weeks) [35] or carboplatin (50 mg/kg, 2 times a week for 2 weeks) [36] alone and in combinations. Animals were examined daily for signs of distress or development of jaundice, and body weight was measured once a week. Animals were euthanized when they became moribund according to predefined criteria like rapid weight loss (>20%) or weight gain (>20% due to ascites), loss of ability to ambulate, labored respiration, or inability to drink or feed to avoid animal suffering [30] in line with the local animal care committee protocol.
Statistical Analysis
In vitro cell proliferation data are expressed as mean ± standard deviation. Statistical analysis was performed by ANOVA for multiple group comparison and Student’s t-test for the individual group comparison. The comparison of survival time between different groups was done by using the log-rank test, which is implemented in the "survdiff" function in the R package "survival" [39], [40]. The comparison of the relative tumor volume (RTV) between treatment groups was done by first normalizing the RTV values at day 14 by the mean TRV value of the corresponding group at day 0, and then applying the two-sample t test, implemented in the "t-test" R function. P < .05 was considered statistically significant.
Results
Effect of Drug Treatments on Human Esophageal Adenocarcinoma Cancer Cell Proliferation
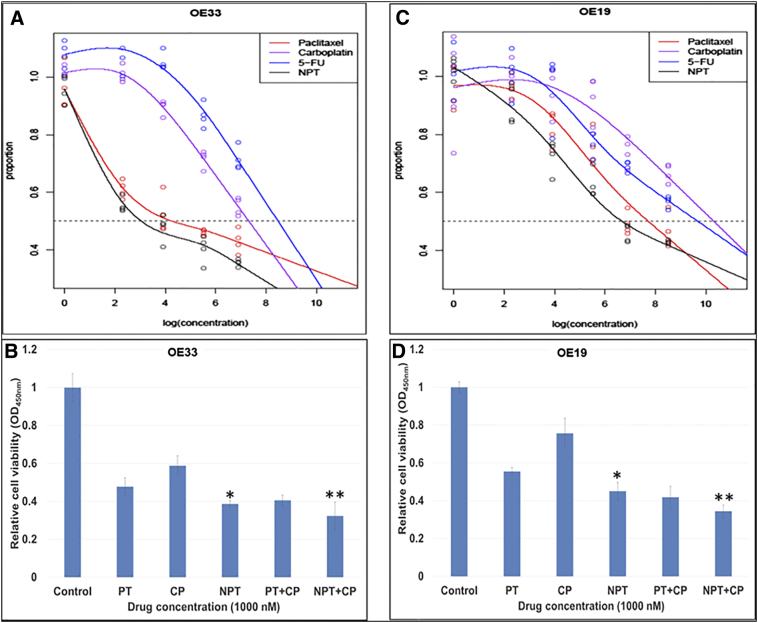
We first determined antiproliferative efficacy of each drug paclitaxel (PT), carboplatin (CP), 5-flurouracil (5-FU) and nab-paclitaxel (NPT) alone and in combination (PT+CP and NPT+CP). All these drugs inhibited esophageal adenocarcinoma cancer cell proliferation in a dose-dependent fashion (Figure 1, A and C). As shown in Figure 1A and C, NPT showed the highest antiproliferative potency with the lowest IC50 compared to paclitaxel, carboplatin and 5-FU in both OE33 and OE19 EAC cell lines. In addition, at 1000 nM concentration NPT in combination with CP showed significantly higher antiproliferative potency than that of PT in combination with CP (Figure 1B and D). The IC50 of nab-paclitaxel was 21 nM in OE33 and 805 nM in OE19, which was less than that of paclitaxel (72 nM in OE33 and 2.27 μM in OE19), carboplatin (1.51 μM in OE33 and 3.05 μM in OE19) or 5-flurouracil (4.82 μM in OE33 and 16.33 μM in OE19) (Figure 1A and C). Based on 10,000 bootstraps, the P value for IC50 of paclitaxel larger than of nab-paclitaxel in the OE19 cell line is less than 0.0001, and in the OE33 cell line is less than 0.0001.
Figure 1.
Higher antiprolifearive potency of nab-paclitaxel over paclitaxel in human esophageal adenocarcinoma cells. Human esophageal adenocarcinoma OE33 (A and B) and OE19 (C and D) cells were plated on 96-well plates and treated with (A and C) 1 nM to 5000 nM concentrations of nab-paclitaxel (NPT) (black points), paclitaxel (red points), carboplatin (violet points) and 5-flurouracil (blue points). Whereas in B and D, cells in 96 wells were treated with 1000 nM of paclitaxel (PT), carboplatin (CP) or nab-paclitaxel (NPT) alone or in combinations. After 72 hours, 10 μl WST-1 reagent was added in each well and incubated for 2 additional hours. The absorbance at 450 nm was measured using a microplate reader. The resulting number of viable cells was calculated by measuring absorbance of color produced in each well. Data are the mean ± SD of quadruplet determinations. * Represents NPT significantly different from Control, PT and CP; ** represents NPT+CP significantly different from Control, PT, CP, NPT and PT+CP.
In-Vitro Effect of Drug Treatments on the Expression of the Mitotic-Spindle Associated Phospho-Stathmin and Apoptosis Markers Cleaved PARP and Caspase 3
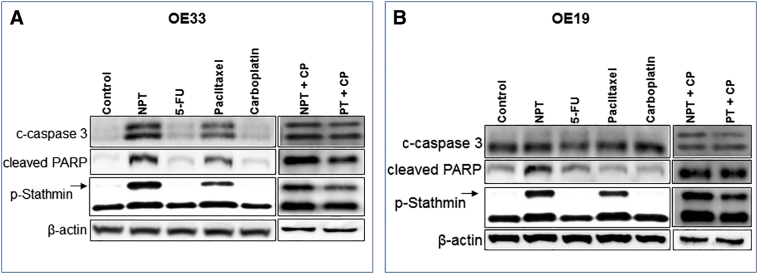
Both in OE33 (Figure 2A) and OE19 (Figure 2B) EAC cell lines microtubule destabilizing protein stathmin was strongly expressed, whereas phosphorylation of stathmin at Ser68, which is essential for function of the stathmin, was weakly expressed. In addition, phosphorylation of stathmin (p-Stathmin) was significantly enhanced after nab-paclitaxel (NPT) and paclitaxel treatments. Interestingly, we observed higher expression of p-Stathmin in nab-paclitaxel treatment compared to that of paclitaxel treatment in both cell lines. Expression of apoptosis related proteins PARP-1 and caspase-3 cleavages showed higher levels after nab-paclitaxel (NPT) treatment compared with paclitaxel, carboplatin or 5-flurouracil (5-FU) treatments (Figure 2A and B). In addition, expression of apoptotic-related proteins appeared higher in combination treatments of nab-paclitaxel with carboplatin (NPT+CP) compared to paclitaxel with carboplatin (PT+CP) in both EAC cell lines.
Figure 2.
In-vitro comparative effects of nab-paclitaxel on the expression of apoptosis related proteins and phospho-stathmin in esophageal adenocarcinoma cells. Subconfluent monolayer of human esophageal adenocarcinoma cells OE33 (A) and OE19 (B) were treated with 5 μM of nab-paclitaxel (NPT), 5-flurouracil (5-FU), paclitaxel (PT) and carboplatin (CP) alone or in combinations for 16 hours. Equal amounts of total cell extarcts were analyzed by western blots with antibodies to cleaved caspase 3 (c-caspase 3), cleaved poly (ADP-ribose) polymerase-1 (cleaved PARP), phospho ser38 stathmin (p-Stathmin) and β-actin. Data are representative of two independent experiments with similar results.
Effect of Drug Treatments on Human Esophageal Adenocarcinoma Xenograft Growth
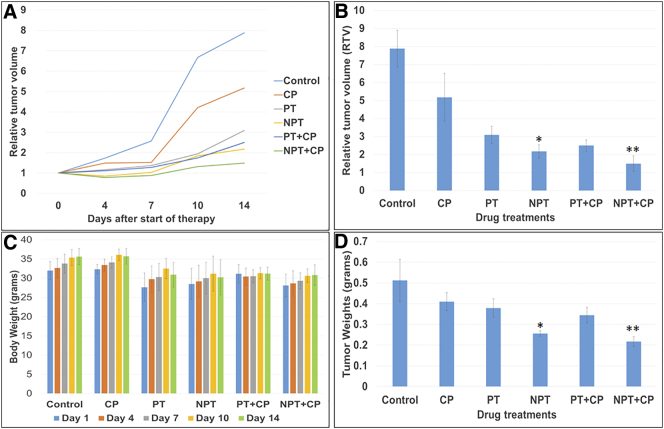
We then determined the in-vivo antitumor efficacy of nab-paclitaxel (NPT), paclitaxel (PT) and carboplatin (CP) alone and also nab-paclitaxel or paclitaxel in combination with carboplatin in a murine xenograft model using OE19 cells. Relative tumor volumes (RTV), tumor weights and response to treatment groups are shown in Figure 3. Nab-paclitaxel therapy at 10 mg/kg twice a week for two weeks was well tolerated without obvious signs of toxicity as judged by mouse weight and daily assessment. All treatment regimens tested in the OE19 subcutaneous tumor model showed statistically significant reductions in relative tumor volume (RTV) compared with control animals (Figure 3A and B). After two-week treatment nab-paclitaxel monotherapy was highly effective in reducing the RTV (P = 0.00006) compared with that of the control animals. The tumor growth inhibition rate after a 2-week treatment with nab-paclitaxel, paclitaxel and carboplatin was 72.38, 60.77 and 34.33 percent respectively (P < .05), supporting the higher efficacy of nab-paclitaxel in reducing xenograft tumor growth. Although both combination treatments (PT+CP and NPT+CP) were highly effective in reducing primary tumor growth, the tumor growth inhibition rate was significantly higher in the NPT plus CP (81.08%) vs. PT plus CP (68.24%) (P = 0.042), supporting the higher efficacy of NPT plus CP regimen. Mean tumor weight was significantly decreased by NPT treatment compared to control (0.25598 g vs. 0.51198 g, P = 0.0054) (Figure 3D). In addition, mean tumor weight was significantly lower by nab-paclitaxel compared to paclitaxel both in monotherapy (NPT vs. PT, 0.25598 g vs. 0.37894 g, P = 0.003) and in combination therapy (NPT+CP vs. PT+CP, 0.217 vs. 0.3444, P = 0.0025) (Figure 3D). There was no significant decrease in animal weight in both therapeutic groups (Figure 3C).
Figure 3.
Higher antitumor effect of nab-paclitaxel (NPT) on esophageal adenocarcinoma tumor xenografts inhibiting growth of established local tumor without significant change in mice body weight. OE19 cells were subcutaneously injected in athymic nude mice and treated with carboplatin (CP), paclitaxel (PT), nab-paclitaxel (NPT) as a monotherapy and in combinations. (A and B) Relative tumor volume (RTV) changes after treatments were compared. Relative tumor volume is calculated by dividing the tumor volume at any time by the tumor volume at the start of treatment. (A) RTV changes over a period of 2 weeks treatments of indicated drugs. (B) RTV changes after indicated treatments were compared at day 14. (C) Mouse body weight was measured twice a week and presented as bar chart for the 2-week therapy period. (D) Mean tumor weight was calculated from final day tumor weights in each group. Data are representative of mean values ± standard deviation. * indicates p < 0.05 in PT versus NPT, ** indicates p < 0.05 in PT+CP versus NPT+CP. All N=5 mice/group.
In-Vivo Effect of Drug Treatments on the Expression of the Mitotic-Spindle Associated Phospho-Stathmin, Proliferative Marker Ki-67 and Apoptosis Marker Cleaved Caspase 3
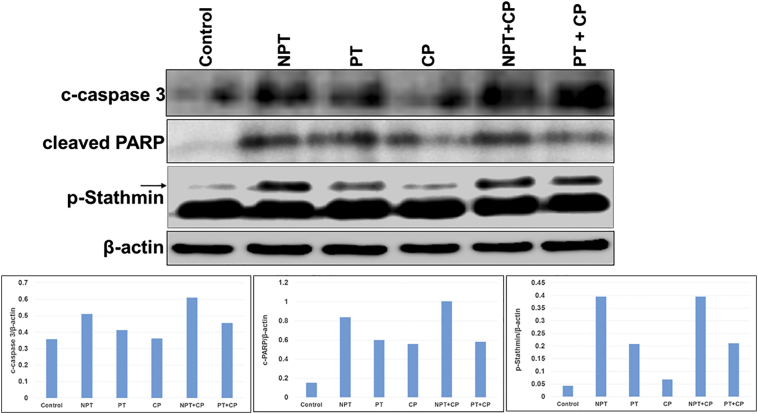
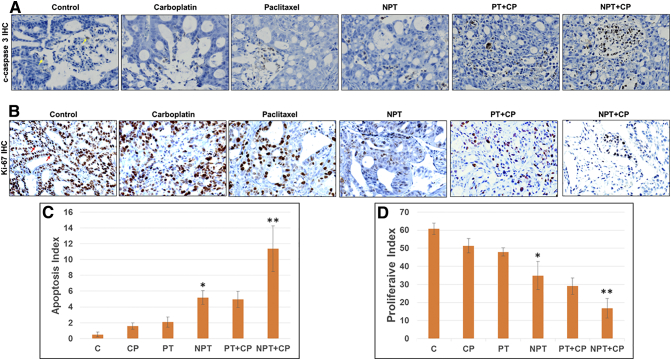
Similar to the in vitro experiments, mechanisms of antitumor activity were examined in OE19 subcutaneous tumor xenografts. Expression of phospho stathmin (P-Stathmin) with cleaved caspase-3 and PARP in OE19 xenograft tumor lysates were significantly increased after in vivo treatment with nab-paclitaxel (NPT) both in mono- and combination therapies as determined by western blot analysis (Figure 4). Immunohistochemical (IHC) analysis (Figure 5) of OE19 tumor xenografts with an antibody that recognizes carcinoma cells expressing the proliferative marker Ki-67 showed lower number of carcinoma cells expressing Ki-67 both in NPT mono- and combination therapies. In the NPT treated group, the proliferative index (PI) was significantly decreased by 42.58%, P = 0.005 compared to control, whereas in the paclitaxel (PT) treated group the PI reduction was 21.13%, P = 0.004 and in the carboplatin (CP) treated group the PI reduction was only 15.48%, P = 0.03. Interestingly, NPT plus CP effectively suppressed the PI to 75.32%, P = 0.002 whereas in the PT plus CP treated group the PI reduction was only 30.23%, P = 0.02. Similarly, apoptosis index was measured in OE19 xenograft tissues by an antibody that only recognizes cleaved caspase 3, not the full length caspase 3 [32]. NPT enhanced apoptosis index by 10.22 fold compared to control (P = 0.00003), whereas PT and CP enhanced apoptosis index by 4.11 (P = 0.0011) and 3 (P = .0016) fold, respectively. Apoptosis index was significantly (P = 0.004) higher in the NPT plus CP treated group (14.23 fold, P =0.0003) than in the PT plus CP treated group (6.45 fold, p = 0.0043). These results indicated that nab-paclitaxel had stronger in vivo antiproliferative and apoptotic effects compared to paclitaxel both as single agent and in combination with carboplatin.
Figure 4.
In-vivo comparative effects of nab-paclitaxel on the expression of apoptosis related proteins and phospho-stathmin in OE19 esophageal adenocarcinoma xenografts. Tumor lysates were prepared from OE19 xenograft tumor tissue samples obtained from tumor bearing mice after carboplatin (CP), paclitaxel (PT) or nab-paclitaxel (NPT) monotherapy or combination therapy. Tumor lysates were then analyzed by immunoblotting with antibodies to cleaved caspase 3 (c-caspase 3), cleaved poly (ADP-ribose) polymerase-1 (cleaved PARP), phospho ser38 stathmin (p-Stathmin) and β-actin. The intensity of bands was quantitated by densitometry and is represented in the bar graph after normalizing values with β-actin expression. Data are representative of pooled lysates obtained from tumors of 5 mice in each therapy group.
Figure 5.
Superior in-vivo antiproliferative and apoptotic effects of nab-paclitaxel on esophageal adenocarcinoma tumor xenografts. OE19 cells were subcutaneously injected in athymic nude mice and after 2 weeks treated with carboplatin (CP), paclitaxel (PT) or nab-paclitaxel (NPT) as a monotherapy and in combinations for 2 weeks. Tumors harvested from five mice of OE19 xenografts for each treatments were processed and stained with cleaved caspase 3 (c-caspase 3) and Ki-67 antibodies. (A) Intratumoral apoptosis was measured by immunohistochemistry (IHC) of tissue sections for cleaved caspase 3. (B) Intratumoral proliferation was measured by IHC for Ki-67 nuclear antigen. (C) Cleaved caspase 3 (apoptosis index) and (D) Ki-67 (proliferative index) +ve cells were counted in five different high power fields for each treatments. Data are expressed as the percent mean ± standard deviation. * indicates p < 0.05 in PT versus NPT, ** indicates p < 0.05 in PT+CP versus NPT+CP (N = 5). Representative photomicrographs of cleaved caspase 3 (A) or Ki-67 (B) stained tumor sections (X40) were shown.
Therapeutic Effect of Regimens on the Survival of Mice Harboring Esophageal Adenocarcinoma
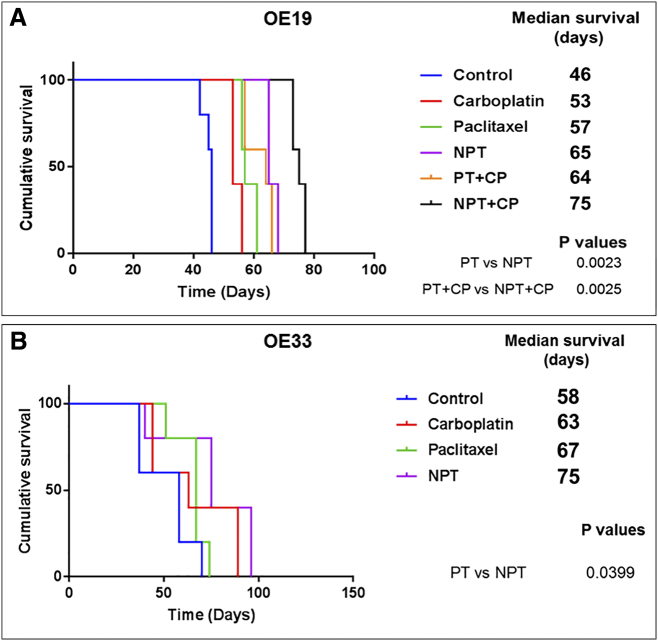
We evaluated the impact of drug treatments on the survival of mice harboring OE19 and OE33 peritoneal disseminated xenograft tumors as described earlier by us [30]. Kaplan-Meier curves of different treatment groups and the comparison are shown in Figure 6A (OE19) and Figure 6B (OE33). In the OE19 model, nab-paclitaxel (NPT) monotherapy prolonged the median animal survival from 46 days to 65 days, p = 0.0034 (Figure 6A) and in the OE33 model, NPT monotherapy prolonged the median animal survival from 58 days to 75 days, p = 0.0215 (Figure 6B). Nab-paclitaxel monotherapy showed significant survival advantage over paclitaxel (PT) monotherapy by extending median animal survival both in OE19 (NPT vs. PT, 65 vs. 57 days, p = 0.0023) and in OE33 (NPT vs. PT, 75 vs. 67 days, p = 0.0399) models. The combination of NPT with carboplatin (CP) treatment showed a median animal survival of 75 days, (p = 0.003 compared to control) in the OE19 model (Figure 6A). The combination of NPT plus CP was the most effective treatment and the increase in median mice survival was statistically significant compared to PT plus CP treatment (75 vs. 64 days, p = 0.0025) in the OE19 model. More interestingly, the median survival of animals in the NPT monotherapy group is equivalent to the PT plus CP combination treatment group (65 vs. 64 days, p = 0.138) in the OE19 model. (See Figure 6A).
Figure 7.
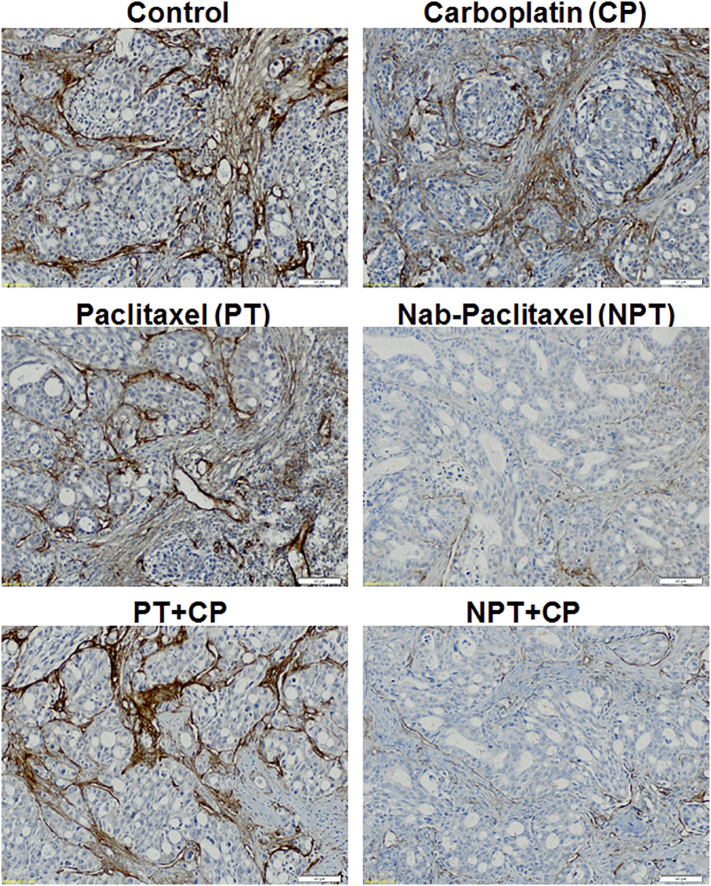
Superior effect of nab-paclitaxel alone and in combination on tumor stroma. Immunohistochemistry of OE19 xenograft tumors were performed in tumors harvested from untreated and treated with carboplatin (CP), paclitaxel (PT), nab-paclitaxel (NPT) alone and in combination using collagen IV antibody. Nab-paclitaxel monotherapy or combination with carboplatin depleted tumor stroma as evidenced by smaller numbers of collagen fibers in the xenograft tumors. Representative stained tumor sections (X20) were shown.
Figure 6.
Superior effect of nab-paclitaxel (NPT) on overall mice survival. 10X106 OE19 (A) and OE33 (B) cells were injected intraperitoneally in SCID mice, and carboplatin (CP), paclitaxel (PT) or nab-paclitaxel (NPT) monotherapy or combination therapy was started after 2 weeks and continued for another 2 weeks. The curve represents the animal survival time from the day of implantation. The comparison of survival time between different groups was done by using the log-rank test (GraphPad Prism 7.0) and P values were shown comparing PT versus NPT and PT+CP versus NPT+CP.
Effect of Drug Treatments on Esophageal Adenocarcinoma Xenografts Stroma
It has been known that collagen depletion can enhance delivery of cancer chemotherapeutics [41], [42] into the tumor. As stromal depletion has been reported after nab-paclitaxel, we therefore compared the intratumor collagen IV staining after drug treatments in OE19 xenografts by collagen IV immunohistochemistry (Figure 7). Nab-paclitaxel (NPT) monotherapy or combined with carboplatin (CP) robustly depleted tumor stroma compared to that of control, paclitaxel (PT) or CP treatments as evidenced by decreased numbers of collagen fibers in the xenograft tumors (Figure 7).
Discussion
The taxane paclitaxel (PT) was introduced more than two decades ago, and represented a revolution in cancer chemotherapy [43]. PT has become a core component of standard of care in several cancer types including EAC [13], [44], [45], [46]. The PT (Taxol®) and carboplatin (CP) combination, Carbo Taxol is a commonly used regimen for treating advanced EAC but can cause serious side effects and chemoresistance [13]. One of the disadvantages of PT is its solvent cremophor-ethanol, which has been shown to contribute to side effects such as hypersensitivity reactions in humans [47]. Therefore, nab-paclitaxel (NPT) was created as a solvent-free water soluble albumin-paclitaxel nanoparticle and has demonstrated improved tolerability and higher response rates compared to PT in patients with advanced metastatic breast, non-small-cell lung and pancreatic cancers [27], [28], [29]. This present study clearly demonstrates for the first time that nab-paclitaxel had stronger antiproliferative and antitumor activity in experimental EAC both in vitro and in vivo as measured by antiproliferative effects, apoptosis, localized antitumor responses and animal survival benefits than the current standard chemotherapeutic agents paclitaxel and carboplatin.
WST-1 in vitro colorimetric cell proliferation assay revealed that single agent treatment of nab-paclitaxel is more effective in inhibiting both OE19 and OE33 human EAC cancer cell proliferation than PT. We found that OE33 cells are more sensitive to the antiproliferative effect of the anticancer drug treatments we used including nab-paclitaxel than OE19 cells, supporting the more aggressive phenotype of OE19 as we mentioned previously [30]. In addition, nab paclitaxel combination with CP showed stronger inhibition of cell proliferation compared to that of PT combination with CP. High stathmin expression has been associated with human malignancies such as human breast cancer, non-small cell lung cancer, human hepatoma and gastric cancer [48], [49], [50], [51]. Stathmin contributes to poor prognosis, cancer progression and resistance to taxanes in patients [52], [53], [54] and phosphorylation of Stathmin inactivates the protein function [55]. In our experiments, we observed a strong expression of stathmin with weak expression of phospho stathmin in both OE19 and OE33 cells, indicating active mitosis in these cancer cells. During further evaluation of the mechanism of actions, we found both PT and nab-paclitaxel enhanced phosphorylation of stathmin and interestingly, expression of phospho stathmin correlated with the ability of nab-paclitaxel to inhibit in vitro cell proliferation with enhanced expression of cleaved PARP and cleaved caspase 3.
In this study, we then performed a xenograft study to compare the antitumor effects of nab-paclitaxel with paclitaxel and carboplatin on OE19 subcutaneous local tumors. Our results showed that nab-paclitaxel as a single agent significantly reduced primary tumor burden compared to that of control, PT and CP. This effect of reduction in primary tumor volume was further enhanced when nab-paclitaxel was combined with CP. RTV was significantly reduced in NPT plus CP combined treatment compared to PT plus CP treatment (RTV 2.5 vs. 1.6, p = 0.04). NPT compared to PT monotherapy significantly reduced the number of proliferating carcinoma cells as evidenced by reduced numbers of carcinoma cells expressing Ki-67, a nuclear protein expressed in proliferating cells; and also significantly enhanced apoptosis of carcinoma cells as evidenced by an increase in the number of cleaved caspase 3, an apoptotic marker, expressing carcinoma cells. In addition, NPT plus CP treatment was highly effective in reducing the Ki-67-positive carcinoma cells and in increasing the cleaved caspase 3 expressing apoptotic cells compared with PT plus CP treatment. Similar to the in vitro effect, higher expression of phosphor-stathmin was observed in xenograft tissues after in vivo nab-paclitaxel treatments, indicating that phosphor-stathmin can serve as a potential bio marker to predict nab-paclitaxel response in EAC. Previously it was reported that nab-paclitaxel effectively depleted tumor stroma in mouse models and patients tissues of pancreatic ductal adenocarcinoma [56]. In this study, we also observed reduced collagen IV staining only in nab-paclitaxel treated mice xenografts indicating that nab paclitaxel can be specifically effective in reducing tumor stroma in EAC.
Finally, we compared the survival benefits of nab-paclitaxel alone and in combination with CP. Subcutaneous and orthotropic xenograft models have been previously used for in-vivo experiments using EAC human cell lines for anticancer drug evaluation [30], [57], [58], [59], [60], [61]. However, subcutaneous implantation models rarely metastasize and are not patient-like. The establishment of an EAC orthotropic model is extremely difficult and is technically challenging to reproduce due to the anatomical location and small size of the mouse esophagus. In addition, it requires invasive procedures which can induce inflammation and thus may influence the efficacy of subsequent therapeutic interventions. Thus a simple, least invasive, patient-like EAC survival model with similar metastatic behavior has been used [30] and our results showed that nab-paclitaxel significantly enhanced mouse survival compared to that of PT, CP or control. When compared, combined treatment of NPT plus CP vs. PT plus CP, there was a significant increase in median survival of the animals. Thus our studies confirmed that nab-paclitaxel was remarkably effective in blocking tumor progression in experimental EAC. It appears to represent a potent new chemotherapeutic agent for clinical EAC treatment.
In conclusion, our results convincingly demonstrated that nab-paclitaxel both as mono- and combination therapies had stronger antitumor activity resulting in enhanced animal survival in experimental EAC than the current standard chemotherapeutic agents PT and CP, and appeared to be the superior taxane compared to PT. This strong antitumor activity supports the rationale for clinical application of nab-paclitaxel as a promising microtubule-inhibitory agent in EAC.
References
- 1.Chai J, Jamal MM. Esophageal malignancy: a growing concern. World J Gastroenterol. 2012;18:6521–6526. doi: 10.3748/wjg.v18.i45.6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rubenstein JH, Shaheen NJ. Epidemiology, Diagnosis, and Management of Esophageal Adenocarcinoma. Gastroenterology. 2015;149:302–317. doi: 10.1053/j.gastro.2015.04.053. [e301] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rustgi AK, El-Serag HB. vol. 371. 2014. Esophageal carcinoma; pp. 2499–2509. [Editor (ed)^(eds): City] [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Domper Arnal MJ, Ferrandez Arenas A, Lanas Arbeloa A. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933–7943. doi: 10.3748/wjg.v21.i26.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang HZ, Jin GF, Shen HB. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31:281–286. doi: 10.5732/cjc.011.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devesa SS, Blot WJ, Fraumeni JF., Jr. Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer. 1998;83:2049–2053. [PubMed] [Google Scholar]
- 8.Mawhinney MR, Glasgow RE. Current treatment options for the management of esophageal cancer. Cancer Manag Res. 2012;4:367–377. doi: 10.2147/CMAR.S27593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bollschweiler E, Holscher AH, Schmidt M, Warnecke-Eberz U. Neoadjuvant treatment for advanced esophageal cancer: response assessment before surgery and how to predict response to chemoradiation before starting treatment. Chin J Cancer Res. 2015;27:221–230. doi: 10.3978/j.issn.1000-9604.2015.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cools-Lartigue J, Spicer J, Ferri LE. Current status of management of malignant disease: current management of esophageal cancer. J Gastrointest Surg. 2015;19:964–972. doi: 10.1007/s11605-014-2701-3. [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society . 2016. Cancer Facts and Figures. [Atlanta, Ga. Available from: http://www.cancer.org/cancer/esophaguscancer/detailedguide/esophagus-cancer-survival-rates] [Google Scholar]
- 12.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 13.Chemotherapy for Cancer of the Esophagus. https://www.cancer.org/cancer/esophagus-cancer/treating/chemotherapy.html
- 14.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 15.Zhang L, Ma J, Han Y, Liu J, Zhou W, Hong L, Fan D. Targeted therapy in esophageal cancer. Expert Rev Gastroenterol Hepatol. 2016;10:595–604. doi: 10.1586/17474124.2016.1140036. [DOI] [PubMed] [Google Scholar]
- 16.Kordes S, Cats A, Meijer SL, van Laarhoven HW. Targeted therapy for advanced esophagogastric adenocarcinoma. Crit Rev Oncol Hematol. 2014;90:68–76. doi: 10.1016/j.critrevonc.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Risinger AL, Giles FJ, Mooberry SL. Microtubule dynamics as a target in oncology. Cancer Treat Rev. 2009;35:255–261. doi: 10.1016/j.ctrv.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowinsky EK, Cazenave LA, Donehower RC. Taxol: a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990;82:1247–1259. doi: 10.1093/jnci/82.15.1247. [DOI] [PubMed] [Google Scholar]
- 19.Stanton RA, Gernert KM, Nettles JH, Aneja R. Drugs that target dynamic microtubules: a new molecular perspective. Med Res Rev. 2011;31:443–481. doi: 10.1002/med.20242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou Q, Ching AK, Leung WK, Szeto CY, Ho SM, Chan PK, Yuan YF, Lai PB, Yeo W, Wong N. Novel therapeutic potential in targeting microtubules by nanoparticle albumin-bound paclitaxel in hepatocellular carcinoma. Int J Oncol. 2011;38:721–731. doi: 10.3892/ijo.2011.902. [DOI] [PubMed] [Google Scholar]
- 21.Belletti B, Baldassarre G. Stathmin: a protein with many tasks. New biomarker and potential target in cancer. Expert Opin Ther Targets. 2011;15:1249–1266. doi: 10.1517/14728222.2011.620951. [DOI] [PubMed] [Google Scholar]
- 22.Wosnitzer MS, Domingo-Domenech J, Castillo-Martin M, Ritch C, Mansukhani M, Petrylack DP, Benson MC, McKiernan JM, Cordon-Cardo C. Predictive value of microtubule associated proteins tau and stathmin in patients with nonmuscle invasive bladder cancer receiving adjuvant intravesical taxane therapy. J Urol. 2011;186:2094–2100. doi: 10.1016/j.juro.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 23.Mistry SJ, Bank A, Atweh GF. Synergistic antiangiogenic effects of stathmin inhibition and taxol exposure. Mol Cancer Res. 2007;5:773–782. doi: 10.1158/1541-7786.MCR-06-0290. [DOI] [PubMed] [Google Scholar]
- 24.Yared JA, Tkaczuk KH. Update on taxane development: new analogs and new formulations. Drug Des Devel Ther. 2012;6:371–384. doi: 10.2147/DDDT.S28997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kloover JS, den Bakker MA, Gelderblom H, van Meerbeeck JP. Fatal outcome of a hypersensitivity reaction to paclitaxel: a critical review of premedication regimens. Br J Cancer. 2004;90:304–305. doi: 10.1038/sj.bjc.6601303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajek R, Vorlicek J, Slavik M. Paclitaxel (Taxol): a review of its antitumor activity in clinical studies Minireview. Neoplasma. 1996;43:141–154. [PubMed] [Google Scholar]
- 27.Salgado M, Arevalo S, Hernando O, Martinez A, Yaya R, Hidalgo M. Management of unresectable, locally advanced pancreatic adenocarcinoma. Clin Transl Oncol. 2018;20(2):113–118. doi: 10.1007/s12094-017-1679-1. [DOI] [PubMed] [Google Scholar]
- 28.Montana M, Ducros C, Verhaeghe P, Terme T, Vanelle P, Rathelot P. Albumin-bound paclitaxel: the benefit of this new formulation in the treatment of various cancers. J Chemother. 2011;23:59–66. doi: 10.1179/joc.2011.23.2.59. [DOI] [PubMed] [Google Scholar]
- 29.Viudez A, Ramirez N, Hernandez-Garcia I, Carvalho FL, Vera R, Hidalgo M. Nab-paclitaxel: a flattering facelift. Crit Rev Oncol Hematol. 2014;92:166–180. doi: 10.1016/j.critrevonc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Hassan MS, Awasthi N, Li J, Schwarz MA, Schwarz RE, Holzen UV. A novel intraperitoneal metastatic xenograft mouse model for survival outcome assessment of esophageal adenocarcinoma. PLoS One. 2017;12:e0171824. doi: 10.1371/journal.pone.0171824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Awasthi N, Scire E, Monahan S, Grojean M, Zhang E, Schwarz MA, Schwarz RE. Augmentation of response to nab-paclitaxel by inhibition of insulin-like growth factor (IGF) signaling in preclinical pancreatic cancer models. Oncotarget. 2016;7:46988–47001. doi: 10.18632/oncotarget.9029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D'Agostino R, Jr., Danial N. Behavioral stress accelerates prostate cancer development in mice. J Clin Invest. 2013;123:874–886. doi: 10.1172/JCI63324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassan S, Karpova Y, Flores A, D'Agostino R, Jr., Kulik G. Surgical stress delays prostate involution in mice. PLoS One. 2013;8:e78175. doi: 10.1371/journal.pone.0078175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang C, Awasthi N, Schwarz MA, Hinz S, Schwarz RE. Superior antitumor activity of nanoparticle albumin-bound paclitaxel in experimental gastric cancer. PLoS One. 2013;8:e58037. doi: 10.1371/journal.pone.0058037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harada K, Ferdous T, Kobayashi H, Ueyama Y. Paclitaxel in combination with cetuximab exerts antitumor effect by suppressing NF-kappaB activity in human oral squamous cell carcinoma cell lines. Int J Oncol. 2014;45:2439–2445. doi: 10.3892/ijo.2014.2655. [DOI] [PubMed] [Google Scholar]
- 36.Karginova O, Siegel MB, Van Swearingen AE, Deal AM, Adamo B, Sambade MJ, Bazyar S, Nikolaishvili-Feinberg N, Bash R, O'Neal S. Efficacy of Carboplatin Alone and in Combination with ABT888 in Intracranial Murine Models of BRCA-Mutated and BRCA-Wild-Type Triple-Negative Breast Cancer. Mol Cancer Ther. 2015;14:920–930. doi: 10.1158/1535-7163.MCT-14-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Awasthi N, Zhang C, Ruan W, Schwarz MA, Schwarz RE. Evaluation of poly-mechanistic antiangiogenic combinations to enhance cytotoxic therapy response in pancreatic cancer. PLoS One. 2012;7:e38477. doi: 10.1371/journal.pone.0038477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zamai M, VandeVen M, Farao M, Gratton E, Ghiglieri A, Castelli MG, Fontana E, D'Argy R, Fiorino A, Pesenti E. Camptothecin poly[n-(2-hydroxypropyl) methacrylamide] copolymers in antitopoisomerase-I tumor therapy: intratumor release and antitumor efficacy. Mol Cancer Ther. 2003;2:29–40. [PubMed] [Google Scholar]
- 39.Therneau T. A Package for Survival Analysis in S. 2015. https://www.R-project.org/ version 2.38.
- 40.Therneau T, Grambsch P. 2000. Modeling Survival Data: Extending the Cox Model New York. [ISBN 0-387-98784-3] [Google Scholar]
- 41.Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science (New York, NY) 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99:1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 43.McGuire WP, Rowinsky EK, Rosenshein NB, Grumbine FC, Ettinger DS, Armstrong DK, Donehower RC. Taxol: a unique antineoplastic agent with significant activity in advanced ovarian epithelial neoplasms. Ann Intern Med. 1989;111:273–279. doi: 10.7326/0003-4819-111-4-273. [DOI] [PubMed] [Google Scholar]
- 44.Nowak AK, Wilcken NR, Stockler MR, Hamilton A, Ghersi D. Systematic review of taxane-containing versus non-taxane-containing regimens for adjuvant and neoadjuvant treatment of early breast cancer. Lancet Oncol. 2004;5:372–380. doi: 10.1016/S1470-2045(04)01494-9. [DOI] [PubMed] [Google Scholar]
- 45.Ramalingam S, Belani CP. Paclitaxel for non-small cell lung cancer. Expert Opin Pharmacother. 2004;5:1771–1780. doi: 10.1517/14656566.5.8.1771. [DOI] [PubMed] [Google Scholar]
- 46.Ajani JA, Ilson DH, Kelsen DP. Paclitaxel in the treatment of patients with upper gastrointestinal carcinomas. Semin Oncol. 1996;23:55–58. [PubMed] [Google Scholar]
- 47.Singla AK, Garg A, Aggarwal D. Paclitaxel and its formulations. Int J Pharm. 2002;235:179–192. doi: 10.1016/s0378-5173(01)00986-3. [DOI] [PubMed] [Google Scholar]
- 48.Wei SH, Lin F, Wang X, Gao P, Zhang HZ. Prognostic significance of stathmin expression in correlation with metastasis and clinicopathological characteristics in human ovarian carcinoma. Acta Histochem. 2008;110:59–65. doi: 10.1016/j.acthis.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Balasubramani M, Nakao C, Uechi GT, Cardamone J, Kamath K, Leslie KL, Balachandran R, Wilson L, Day BW, Jordan MA. Characterization and detection of cellular and proteomic alterations in stable stathmin-overexpressing, taxol-resistant BT549 breast cancer cells using offgel IEF/PAGE difference gel electrophoresis. Mutat Res. 2011;722:154–164. doi: 10.1016/j.mrgentox.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu SG, Yan PJ, Shao ZM. Differential proteomic analysis of a highly metastatic variant of human breast cancer cells using two-dimensional differential gel electrophoresis. J Cancer Res Clin Oncol. 2010;136:1545–1556. doi: 10.1007/s00432-010-0812-0. [DOI] [PubMed] [Google Scholar]
- 51.Gan L, Guo K, Li Y, Kang X, Sun L, Shu H, Liu Y. Up-regulated expression of stathmin may be associated with hepatocarcinogenesis. Oncol Rep. 2010;23:1037–1043. doi: 10.3892/or_00000730. [DOI] [PubMed] [Google Scholar]
- 52.Hsieh SY, Huang SF, Yu MC, Yeh TS, Chen TC, Lin YJ, Chang CJ, Sung CM, Lee YL, Hsu CY. Stathmin1 overexpression associated with polyploidy, tumor-cell invasion, early recurrence, and poor prognosis in human hepatoma. Mol Carcinog. 2010;49:476–487. doi: 10.1002/mc.20627. [DOI] [PubMed] [Google Scholar]
- 53.Jeon TY, Han ME, Lee YW, Lee YS, Kim GH, Song GA, Hur GY, Kim JY, Kim HJ, Yoon S. Overexpression of stathmin1 in the diffuse type of gastric cancer and its roles in proliferation and migration of gastric cancer cells. Br J Cancer. 2010;102:710–718. doi: 10.1038/sj.bjc.6605537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baquero MT, Hanna JA, Neumeister V, Cheng H, Molinaro AM, Harris LN, Rimm DL. Stathmin expression and its relationship to microtubule-associated protein tau and outcome in breast cancer. Cancer. 2012;118:4660–4669. doi: 10.1002/cncr.27453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Di Paolo G, Antonsson B, Kassel D, Riederer BM, Grenningloh G. Phosphorylation regulates the microtubule-destabilizing activity of stathmin and its interaction with tubulin. FEBS Lett. 1997;416:149–152. doi: 10.1016/s0014-5793(97)01188-5. [DOI] [PubMed] [Google Scholar]
- 56.Rajeshkumar NV, Yabuuchi S, Pai SG, Tong Z, Hou S, Bateman S, Pierce DW, Heise C, Von Hoff DD, Maitra A. Superior therapeutic efficacy of nab-paclitaxel over cremophor-based paclitaxel in locally advanced and metastatic models of human pancreatic cancer. Br J Cancer. 2016;115:442–453. doi: 10.1038/bjc.2016.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kresty LA, Weh KM, Zeyzus-Johns B, Perez LN, Howell AB. Cranberry proanthocyanidins inhibit esophageal adenocarcinoma in vitro and in vivo through pleiotropic cell death induction and PI3K/AKT/mTOR inactivation. Oncotarget. 2015;6:33438–33455. doi: 10.18632/oncotarget.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujihara S, Kato K, Morishita A, Iwama H, Nishioka T, Chiyo T, Nishiyama N, Miyoshi H, Kobayashi M, Kobara H. Antidiabetic drug metformin inhibits esophageal adenocarcinoma cell proliferation in vitro and in vivo. Int J Oncol. 2015;46:2172–2180. doi: 10.3892/ijo.2015.2903. [DOI] [PubMed] [Google Scholar]
- 59.Ford SJ, Obeidy P, Lovejoy DB, Bedford M, Nichols L, Chadwick C, Tucker O, Lui GY, Kalinowski DS, Jansson PJ. Deferasirox (ICL670A) effectively inhibits oesophageal cancer growth in vitro and in vivo. Br J Pharmacol. 2013;168:1316–1328. doi: 10.1111/bph.12045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Drenckhan A, Kurschat N, Dohrmann T, Raabe N, Koenig AM, Reichelt U, Kaifi JT, Izbicki JR, Gros SJ. Effective inhibition of metastases and primary tumor growth with CTCE-9908 in esophageal cancer. J Surg Res. 2013;182:250–256. doi: 10.1016/j.jss.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 61.Gros SJ, Kurschat N, Dohrmann T, Reichelt U, Dancau AM, Peldschus K, Adam G, Hoffman RM, Izbicki JR, Kaifi JT. Effective therapeutic targeting of the overexpressed HER-2 receptor in a highly metastatic orthotopic model of esophageal carcinoma. Mol Cancer Ther. 2010;9:2037–2045. doi: 10.1158/1535-7163.MCT-10-0209. [DOI] [PubMed] [Google Scholar]