Abstract
William Coley, between 1895 and 1936, treated hundreds of cancer patients using infusions of fever inducing bacerial extracts. Similar experiments were done by Klyuyeva and co-workers in the 1940ies in Russia using trypanosoma extracts. Many remissions and cures were reported. We have conjectured that pathogen associated molecular pattern substances (PAMP) are the molecular explanation for the beneficial treatments in both groups. We could show that a combination of PAMP can eradicate solid tumours in cancer mice if applied several times. Accordingly, we suggested to combine PAMP containing approved drugs to treat cancer patients using a protocol similar to the old fever induction regimen. In this retrospective phase-1 study we report on the fever induction capacity and safety of applications of bacterial extracts, combinations of bacterial extracts with approved drugs, and combinations of approved drugs in 131 mainly cancer patients. Adverse reactions were those which can be expected during a feverish infection and mild. Over 523 fever inductions, no severe adverse reaction was observed.
Introduction
PAMP Presumably are the Molecular Explanation for a Range of Diverse Observations
Several observations combined led us to suggest [1], [2] that pathogen associated molecular pattern (PAMP, pathogenic danger signals) are at the core of an immune reaction against cancer cells induced by bacterial extracts, by combinations of PAMP and by combinations of PAMP containing approved drugs:
-
•
Coley’s fever therapy using extracts from Streptococcus in hundreds of patients more than 100 years ago [3], [4], [5] and Klyuyevas fever therapy using extracts from Trypanosoma in the 1940ies in Russia [6] undoubtedly led to numerous amazing cures. Both organisms, a bacterium and a nucleated single cell pathogen, have nothing much in common, except both provide several PAMP upon infection.
-
•
A large fraction (25%-80%) of spontaneous cancer regressions can be correlated with a hefty feverish infection [7], [8]. Most if not all infectious agents deliver PAMP.
-
•
A personal history of feverish infections reduces the likelihood to develop cancer later, this protective effect diminishes with length of infection-free periods [9], [10].
-
•
Strong protection from lung cancer in dairy but not orchard farms; dairy barn dust can be contaminated with bacterial toxins [11]. Both exotoxins and endotoxins may act as PAMP.
-
•
Mistletoe lectin is a PAMP of bacterial origin [12]. Complete remissions after multimodal high-dosage mistletoe therapy have been observed in otherwise therapy naive patients [13], [14].
-
•
Fever / external heat generates a higher rate of tumor/normal cell debris [15], [16], i.e. likely delivers more tumor antigens. Some PAMP, e.g. LPS, are potent fever inducers; fever stimulates dendritic cells [17].
-
•
In many if not all cancer patients, tumor-specific T-cells can be found in or around tumor tissue [18]. These T-cells usually are anergic or not activated, presumably because co-stimulatory signals are missing [19]. PAMP are required for DC maturation and expression of co-simulatory signals, mature DC are needed to induce proper T-cell activation and clonal expansion [20].
-
•
Compared to single PAMP, PAMP combined act synergistically [12], [21], [22], [23].
-
•
Repeated treatment using to a combination of PAMP can lower myeloid derived suppressor cell (MDSC) numbers in cancer mice [12], potentially suppressing tumour escape.
-
•
A combination of PAMP applied metronomically (10x over 3 weeks) lead to complete remissions in cancer mice, while metronomically applied single PAMP could slow tumour growth [12].
These observations led us to suggest to re-evaluate Coley's therapy. Since bacterial extracts can hardly be approved any more, and since pre-clinical experiments using bacterial extracts would require considerable time and expense, we suggested to combine approved PAMP containing drugs [24] and apply them using a regimen similar to Coley's. Ideally this means infusing fever inducing PAMP drugs 2-3 times per week over several weeks. Due to practical restrictions and patients requests, a less frequent application regimen has been applied in the majority of cases presented here.
Material and Methods
Drugs Used for Fever Induction
We used 7 PAMP containing drugs (Table 1) in 19 different combinations (Table 2) to induce therapeutic fever in patients. Most of the patients were cancer patients. Others were treated for borreliosis (n=9), inflammation (n=9) and infection liability (n=4).
Table 1.
Selection of PAMP Drugs
| Name | Manufacturer | Content | Abbreviation | OTC Price Per Application (€) |
|---|---|---|---|---|
| Colibiogen | Laves, Switzerland | Metabolic products of Escherichia coli laves | Co | 8 |
| Iscador (apple tree 10mg) | Weleda, Switzerland | Mistletoe extract | Is | 9 |
| Picibanil | Chugai, Japan | Lyophilised Streptococcus pyogenes | Pi | 34 |
| Polyvaccinum forte | IBS Biomed, Poland | Inactivated extract from Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus salivarius, Streptococcus pneumoniae, Streptococcus pyogenes, Escherichia coli, Klebsiella pneumoniae, Haemophilus influenzae, Corynebacterium pseudodiphtheriticum, Moraxella catarrhalis | Po | not any more available |
| Pseudomonas/ Streptococcus | Organomed, Dr.Neumeyer, Germany | Sterile solution of Pseudomonas aeruginosa, Streptococcus pyogenes | Ps | not any more available |
| Serratia/ Strepococcus | Organomed, Dr.Neumeyer, Germany | Sterile solution of Serratia marcescens, Streptococcus pyogenes | Se | not any more available |
| Strovac | Strathmann, Germany | Inactivated Escherichia coli, Morganella morganii, Proteus mirabilis, Klebsiella pneumoniae, Enterococcus faecalis | St | 36 |
Table 2.
Combinations of PAMP Drugs Tested for Safety and Fever Induction Capacity in Group B
| Combination | Number of Applications (n=100) | Average Peak Body Temperature Induced |
|---|---|---|
| Co,Is,Pi | 2 | 39.1 |
| Co,Is,Pi,Po | 3 | 38.8 |
| Co,Is,Po | 1 | 40.0 |
| Co,Is,Po,Se | 2 | 40.2 |
| Co,Is,Po,St | 1 | 40.5 |
| Co,Is,Se | 3 | 39.5 |
| Co,Is,St | 12 | 40.0 |
| Co,Pi,Po | 6 | 39.9 |
| Co,Po,Ps | 4 | 40.7 |
| Co,Po,Ps,Se,St | 1 | 40.5 |
| Co,Po,Se | 9 | 39.5 |
| Co,Po,Se,St | 9 | 39.3 |
| Co,Po,St | 24 | 39.7 |
| Co,Se | 2 | 38.2 |
| Co,Se,St | 16 | 39.3 |
| Po,Se | 1 | 38.4 |
| Po,Se,St | 1 | 40.0 |
| Se,Ps | 1 | 39.5 |
| Se,St | 2 | 41.0 |
Two drugs were bacterial extracts manufactured in close accordance with Coley's descriptions ("Se", sterile solution of Serratia marcescens, Streptococcus pyogenes; "Ps", sterile solution of Pseudomonas aeruginosa, Streptococcus pyogenes). Three drugs are approved for cancer therapy ("Co", Colibiogen; "Is", Iscador; "Pi", Picibanil). One drug is approved for i.v. application (Co), one other drug has been tested for i.v. application extensively (Is) [25]. So part of the drugs were used off-label with respect to disease approval and/or way of transmission (i.v.). All patients were informed accordingly and signed informed consent. For all drugs used, fever is described as frequent side reaction in the respective instruction leaflets. All drugs contain, as jugded by ingredients, PAMP. All drugs are approved in at least one EU state and can therefore be applied EU wide.
Dosage of Drugs
The starting dose of bacterial extracts (Se, Ps) was determined according to the recommendations of the AG Fevertherapy of the German Society for hyperthermia (DGHT) and the manufacturers instruction leaflet. Typically it was 1/4 to 1/10 of final dose and guided by patient vitality, the latter estimated by computer regulation thermography (Alpha Thermodiagnostics, Canada) and heart rate variability (HRV Coprevent, Grimm, Germany). In case of good tolerance and repeated application, subsequent doses were increased by about 25%-50% until robust fever of >39oC could be achieved. Once this patient specific dose was determined, it was held constant for subsequent applications. In case of larger peak body temperature deviations from the target temperature of 39oC-40oC, doses were adjusted for subsequent applications accordingly. The dosage of the other drugs (Co, Is, Pi, Po, St; see Table 1) was determined along similar upward titration rules in the first patients. Later we had enough experience to start early with an appropriate dose. Typical starting and repetition doses are given in Table 3. In many cases fever therapy was preceded by 30 min whole-body hyperthermia (IRA 1000, Fa. Von Ardenne, Dresden, Germany), which in our experience severely reduces burdening side effects such as chills and vomiting. Infusions typically started between 8 and 9 in the morning and lasted about 30-60 minutes. Number of applications per patient was integrative result from physicians recommendations and patients demand.
Table 3.
Typical Dose Per Infusion
| Drug | Typical Starting Dose | Typical Repetition Dose | Route of Application |
|---|---|---|---|
| Colibiogen | 1ml | 2ml | i.v. |
| Iscador | 5mg | 20mg | i.v. |
| Picibanil | 1ml | 3ml | i.v. |
| Polyvaccinum | 100mg | 300mg | i.v. |
| Pseudomonas | 0.5ml | 3-10ml | i.v. |
| Serratia | 0.5ml | 3-10ml | i.v. |
| Strovac | 0.5ml | 0.5-1ml | i.v. |
Results
Group A - Safety and Fever Induction Using Bacterial Extracts
We treated 106 patients with 350 fever inductions using bacterial extracts alone (Se or Ps). In the course of repetitive applications we typically changed from Se to Ps and vice versa each 3-4 applications.
Patients were divided into two groups. Group A1 (starting 2006, n=44, 135 applications) had no preceding hyperthermia, while patients in group A2 were given 30min whole body hyperthermia before application of extract (starting 2011, n=62, 215 applications). A majority of patients had been pre-treated by chemotherapy and/or radiation and were in a palliative situation. Results on peak fever height and adverse reactions can be found in Table 4. Both drugs Se and Ps routinely induced robust temperature rises of >=2oC.
Table 4.
Peak Fever Height and Adverse Reactions Upon Fever Induction
| Temperature and Side Effects | Group A1 Bacterial Extracts, No Preceding Hyperthermia, 135 Applications |
Group A2 Bacterial Extracts, Preceding Whole Body Hyperthermia, 215 Applications |
Group B Combinations of Approved Drugs, Preceding Whole-Body Hyperthermia, 100 Applications |
|---|---|---|---|
| Peak body temperature (oC±SD) | Ps 39,1 ± 0,71 Se 39,2 ± 0,81 |
Ps 39,2 ± 0,67 Se 39,4 ± 0,78 |
39,6 ± 0.86 |
| Nausea/vomitting (%) | Ps 15, Se 24,9 |
Ps 6,1 Se 8,2 |
26 |
| Headache (%) | Ps 12, Se 19,3 |
Ps 5,5 Se 6,1 |
25 |
| Back pain (%) | Ps 5,4 Se 7,4 |
Ps 2,4 Se 2,3 |
12 |
| Circulatory reactions (%) | Ps 7,7 Se 10,9 |
Ps 3,1 Se 3,2 |
0,5 |
| Weakness next day (%) | Ps 17,7 Se 21,2 |
Ps 13,1 Se 16,1 |
0,5 |
Group A1: application of bacterial extracts (Se: Serratia marcescens+Streptococcus pyogenes, Ps: Pseudomonas aeruginosa+Streptococcus pyogenes) without preceding hyperthermia. Group A2: application of bacterial extracts preceded by 30min whole body hyperthermia. Group B: application of combinations of approved drugs (Colibiogen, Iscador, Picibanil, Polyvaccinum forte, Strovac) usually preceded by whole body hyperthermia (intermittend single drug applications of bacterial extracts excluded).
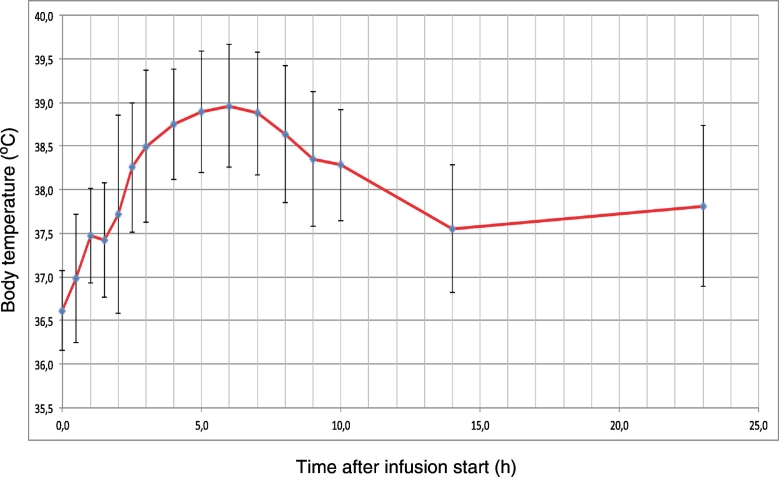
While the capability to rise body temperature is similar for both bacterial preparations Se and Ps, without hyperthermia Se has a higher rate of adverse reactions compared to Ps. Both number of side reactions and the difference between Se and Ps is drastically reduced when whole body hyperthermia precedes fever induction. No severe adverse reactions, for instance seizures or circulatory problemes requiring intervention, have been observed over 350 fever inductions using bacterial extracts. Kinetics of fever rise and fall are similar for both preparations Se and Ps (Figure 1). In about one third of cases a second lower fever peak can occur during the following night. Coley has recommended to avoid injections of bacterial extracts when fever is not completely settled, so at least one day should be given for recovery between applications.
Figure 1.
Average fever kinetics of 215 fever inductions using bacterial extracts (Se, Ps).
Group B - Safety and Fever Induction Using a Combination of Approved PAMP Drugs
Here we present safety data for 25 patients treated by fever induction using a combination of approved PAMP drugs. Number of treatments per patient using combinations varied between 2 and 26 times, number of drugs combined between 2 an 5 (Table 5). Some patients received intermittend single drug applications of bacterial extracts (n=73) rather than combinations (n=100). These contributed to the total number of PAMP drug applications over both groups A and B (n=523) without severe adverse reactions.
Table 5.
Fever Induction Using a Combination of Approved PAMP Drugs (see Table 1 for Drug Abbreviations) in Group B
| Patient | Diagnosis | Treatment (from Month to Month) | Drug Combinations Applied | Number of Applications | Average Number of Days Between Applications |
|---|---|---|---|---|---|
| 1 | mamma-CA 12.2003; lung metastasis 2007; mamma-CA; lung, kidney and eye metastases 9.2015 | 11.2015 | Co Se St | 2 | 5 |
| 2 | mamma-CA 11.2013; kidney and eye metastases 2015 | 10.2015-11.2015 | Co Se, Co Se St | 2 | 21 |
| 3 | rectum adeno-CA; liver metastasis 01.2012; enlarged central lymph nodes | 06.2014-10.2015 | Se, Se St | 9 | 58 |
| 4 | prostate-CA 11.2014 | 02.2015-07.2015 | Se, Co Se St | 4 | 50 |
| 5 | mamma-CA 09 2014; skin metastases 10.2015 | 02.2016-05.2016 | Co Se St,Co Po Se St | 5 | 32 |
| 6 | leyomyosarcoma 01.2015; lung liver metastases 09.2015; 15 metastases one abdominal 18cm 01.2016 | 02.2016 | Co Po St | 2 | 4 |
| 7 | mamma-CA 012.2012 | 02.2016-09.2016 | Se, Co Se St | 12 | 70 |
| 8 | mamma-CA 03.2003 | 04.2005-03.2016 | Se, Ps, Se Ps, Co Po Ps Se St | 25 | 185 |
| 9 | mamma-CA 08.2012 | 03.2016-02.2017 | Co Po Se St, Co Po Se, Co Is Po Se | 13 | 28 |
| 10 | hepatocell-CA 01.2015 | 12.2015-03.2016 | Co Se St, Co Po Se St | 2 | 91 |
| 11 | papill.thyroid-CA with locoregional and distant metastases 2008 | 12.2016 | Co Pi Po | 6 | 2 |
| 12 | mamma-CA 09.2013 | 07.2016-05.2017 | Co Po St, Ps, Co Po Ps | 14 | 23 |
| 13 | bronchial-CA; metastases in lung and brain 03.2015 | 10.2015-05.2016 | Co Se, Co Se St, Po Se, Co Po St, Co Po Se St | 6 | 40 |
| 14 | adenocarcinoma 10.2016 | 11.2016 | Co Is Pi Po, Co Is Pi | 5 | 2 |
| 15 | bladder-CA 06.2015 | 04.2016-05.2016 | Co Po St, Co Po Se St | 2 | 41 |
| 16 | ovarial-CA 2012 | 05.2016-06.2016 | Co Po St, Co Po Se St | 3 | 17 |
| 17 | melanoma 01.2007; liver and lymph node and brain metastases 04.2016 | 05.2016-06.2016 | Co Po St, Po Se St | 7 | 1 |
| 18 | colon-CA 03.2014 | 08.2014-01.2017 | Se, Co Se St, Co Is Po St | 13 | 74 |
| 19 | mamma-CA 2012 | 07.2016-10.2016 | Co Po Se St, Co Po St | 2 | 100 |
| 20 | prostate-CA metastases 07.2012 | 01.2017-02.2017 | Co Po St, Co Is Po | 3 | 67 |
| 21 | high-grade lymphoma suspicion 12.2012 | 07.2013-11.2016 | Ps, Se, Co Se St, Se St | 26 | 48 |
| 22 | mamma-CA 05.2015 | 10.2015-01.2016 | Se | 2 | 89 |
| 23 | invasive mamma-CA 09.2016 | 02.2017-03.2017 | Co Is Se | 3 | 5 |
| 24 | borreliosis, rheumatic pain since 09.2013 | 04.2017-06.2017 | Co Is St | 3 | 32 |
| 25 | rhabdomyosarcoma 07.2014; liver metastasis 10.2015; relapse 05.2016 | 03.2017-05.2017 | Co Is St | 9 | 7 |
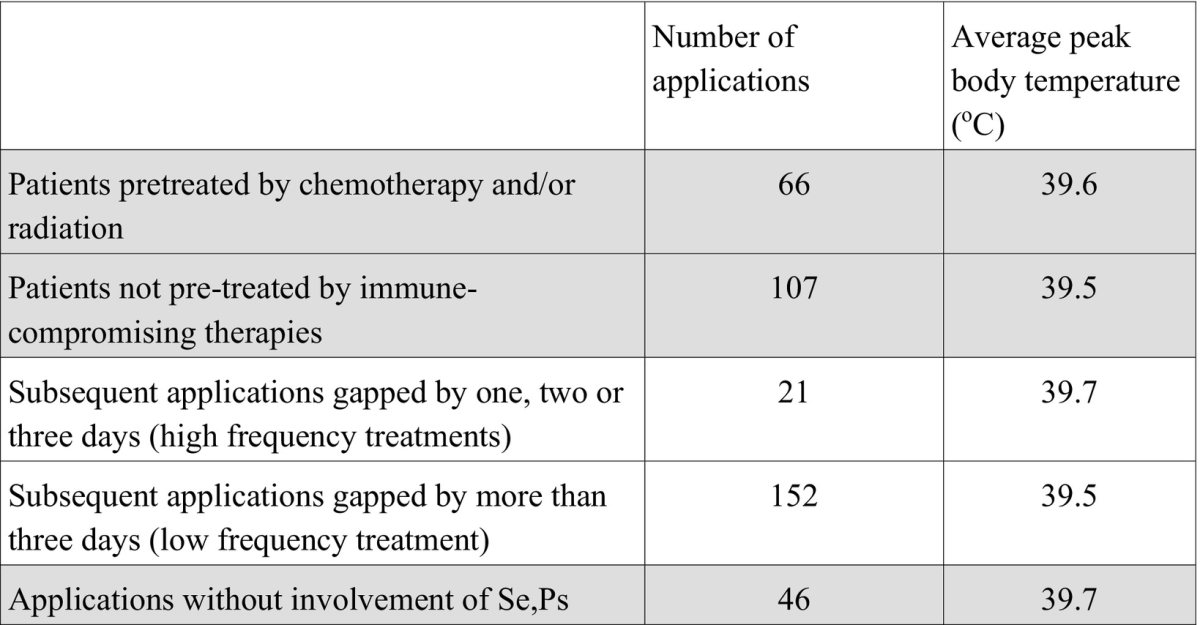
Usually we recommend cancer patients to continue fever therapy for a couple of weeks in a high-frequency "metronomic" setting, however, in many cases patients requested larger intervalls and/or stopped treatment for a variety of personal reasons. 8/25 patients were pre-treated by chemotherapy, 7 by radiation, 17 by surgery. Average peak body temperature in pre-treated patients was 39.6oC, in patients without chemotherapy and radiation 39.5oC, hence there was no significant difference in the capability to raise body temperature depending on prior immune compromising treatment.
It might be possible that treatments in close succession could lead to an exhaustion of the capacity to raise body temperature upon subsequent treatments, however, we did not find any indication for exhaustion. If we compare those treatments gapped by one, two or three days (n=21) with combination treatments gapped by more than three days (n=152), the average peak body temperature we could achieve in the high frequency setting was 39.7oC, compared to the average of 39.5oC. Thus no significant difference was found for treatments in close succession compared to treatments with longer intervalls.
Mild adverse reactions occured upon about a quarter of applications (Table 4).
Looking at all applications without involvement of bacterial extracts manufactured according to Coley, i.e. those combinations without Se or Ps (n=46), the average peak body temperature achieved was 39.7oC. Therefore, combinations of approved PAMP drugs have the same fever induction potency as bacterial extracts.
Compared to the application of single bacterial extracts, adverse reactions were more frequent using combinations of approved drugs, despite preparatory hyperthermia (Table 4). Possibly, PAMP substances combined by drug combinations might be more diverse and / or of higher concentration compared to heat sterilized bacterial extracts, leading to a higher frequency of mild adverse reactions and a slightly higher average peak body temperature, and potentially a stronger immune response.
A summary of fever induction results stratified by sub-goups can be found in Table 6.
Table 6.
Potency of Fever Induction Stratified by Sub-Groups Within Ggroup B (Intermittend Single Drug Applications of Bacterial Extracts Included)
Outcome
In this work we present observations with a focus on the safety of fever therapy in cancer patients using combinations of approved drugs. To monitor outcome is, other than during a clinical study, difficult in private clinics, since in general patients are lost on follow-up. On top of the numerous impressive healings achieved by Coley, contemporaries, Klyuyeva and others long ago, we have reported 17 successful recent treatments using fever therapy [2] and add two cases here.
Patient A, age 71 years at diagnosis, was diagnosed with prostate carcinoma and multiple bone metastases in November 2009. He underwent prostectomy and hormone therapy. In May 2011 PSA was rising and bone metastases were confirmed by scintigraphy. In June 2012 PSA reached 387mmol/l. Patient refused chemotherapy. Between mid 2012 and September 2014 he received 15 fever inductions. PSA fell down to 1 mmol/l in October 2014. He refused confirmatory NMR.
Patient B, age 56 years at time of diagnosis in June 2006, was diagnosed with a primary mamma carcinoma of 8x6x4.5cm, confirmed by biopsy. One lymph node of walnut size was determined in the left axilla. Tumor marker CA-15-3 was measured at 46.2 U/ml. The patient refused surgery and standard treatment. NMR in February 2009 showed an increase in size of the primary tumour. She received 16 active fever therapies and infusions of anti-oxidants until June 2012. NMR in February 2014 showed a significant decrease in tumour size and palpation showed a softening of neoplastic tissue. The axillary lymph node was not palpable any more. Tumor marker CA-15-3 was down to 12.1 U/ml.
Discussion
An Immunological Explanation
Heat, both in the form of passive hyperthermia and active fever can induce or support several immune reactions. Heat enhances the Fas ligand CD95 gene expression in T lymphocytes [26]. Fas ligand is a type II transmembrane protein able to trigger apoptosis. Activated Fas receptor may induce functional maturation of dendritic cells (DC) and preferential T cell polarization towards Th1 [27]. Heat can induce several heat shock proteins (HSP), which can mediate immune reactions such as IL-6, IL-8, IL-12, nitric oxide (NO) and TNFα production via monocytes and macrophages, DC maturation, B-cell proliferation and IL-10 production, upregulation of toll-like receptors (TLR). Some HSP work hand in hand with PAMP (see [28] for overview). For instance, HSP70 potentiates lipopolysaccaride-(LPS)-stimulated THFα production. Immunogenic HSP-peptide complexes are displayed to a larger extent on cancer cells after heat treatment [29], [30]. DC treated with fever-like heat (41oC 6h) were significantly more effective compared to non-heat treated DC in stimulating T-cells both in the presence and absence of antigen [17]. Interleukin-2 (IL2) treated cancer patients had an almost doubled survival rate compared to IL2 plus paracetamol treated cancer patients [31]. Zoledronic acid-induced fever was the most important prognostic factor in a cohort of lung cancer patients with bone metastases [32]. Cancer cells are more vulnerable to heat than normal cells and die from necrosis to a larger extent [15], [16]. A tumour with a higher death rate is presumed to be more immunogenic [33].
Most of the positive effects of passive heat most likely will fade without PAMP synergy. For instance, hyperthermia induced increased transcription of several tumour associated antigens is only transient [34]. Hyperthermia alone has, to the best of our knowledge, not been shown to increase cancer survival rates significantly. However, passive and active fever therapy joined together can, in our opinion, be beneficial.
In many, if not most, cases of cancer, a more or less pronounced immune reaction appears to be present, demonstrated for instance by tumour infiltrating lymphocytes (TIL) within tumour and stroma, or by tumour specific antibodies. Since presence and amount of TIL is one of the strongest predictors for longer survival [35], [36], [37], [38], at least a fraction of TIL must represent an active anti-tumour response. Yet, in the majority of cases, obviously, this immune response is too weak to induce complete erradication of tumour cells. Fever, by providing necrotic cancer cell death and by inference an increase in tumour antigens, could extend a pre-existing T-cell response. PAMP, not provided by cancer cells, might be a missing link towards complete erradication, since PAMP are the most potent activators of DC. DC activation is stricly required for proper activation of B-and T-cells. Combinations of PAMP show more pronounced effects compared to single PAMP [12], [21], [22], [23], therefore different PAMP should be combined to maximise immune stimulation. It is fortunate that PAMP, at the same time, might help against tumour escape. PAMP activated toll-like receptor (TLR) signalling can directly affect regulatory T-cells (Treg) and activate the PI3K/Akt pathway, consistent with Treg resistance [39]. The recruitment of myeloid-derived suppressor cells (MDSC) by the tumour bed is a typical escape mechanism; PAMP can downregulate MDSC numbers [12].
General Experiences and Recommendations for Intravenous Application
Fever induction in cancer patients using GMP approved drugs which, judged by content, contain PAMP substances, is a safe and steerable treatment. Over several applications, sometimes small dose adjustments are needed to maintain a target peak body temperature of 39oC-40oC. Along 523 intravenous applications in 131 patients, not a single severe adverse reaction such as seizures, heavy circulatory problems or tumour lysis syndrome has been observed. Mild adverse reactions were similar to those which can be observed during a proliferative infection and included nausea, vomitting, chills, headache, back pain, weakness, brief periods of increases or decreases in blood pressure. Thus patients should be advised to stand up and move slowly. Reactions in some patients relaxed over the course of several applications. On the other side, patients may report a period of pronounced physical and mental strength one or two days after fever therapy.
Despite excellent safety, we recommend to monitor blood pressure and circulation at least until fever declines below 38oC. We recommend first treatments under stationary setting, while subsequent treatments can be done under ambulatory setting, making fever therapy using PAMP drugs more convenient and less expensive. Some experienced patients, at own risk, even requested to leave the clinic shortly after infusion and undergo fever at home under supervision of a relative.
Since a preceding whole body hyperthermia reduces both patient stress and adverse reactions, and since hyperthermia can help patients which have difficulties to ignite internal heat and realize fever upon PAMP stimulation (low responders), we recommend hyperthermia preceding infusion in general. Hyperthermia should not be applied after infusion during active fever.
A high frequency therapy regimen with two to three applications per week might be optimal in otherwise therapy naive patients. This conclusion can be drawn from Coley's recommendations, from retrospective analyses of his cases [3], [4], from the observation that many spontaneous cancer regressions occured after a hefty feverish infection [7] providing PAMP over several days continuously, and since the innate immune response triggered by PAMP has no memory and needs permanent stimulation [20]. Even if a high-frequency regimen likely is the most effective fever therapy regimen, less frequent applications, for instance once per week or less, may as well exert beneficial outcomes [2]. The majority of the patients presented here requested less frequent applications for personal reasons, for instance, to be able to continue their jobs.
In patients, who had immune compromising treatments such as chemotherapy or raditation less than two years before fever therapy, we cannot exclude the possibility that fever kinetics and outcome may be less predictable and more erratic. For instance, we have seen pre-treated cases where body temperature transiently fell after hyperthermia or the onset of fever was delayed. Thus, although we have seen positive courses after low-frequency fever therapy also in pretreated patients, at present, we cannot recommend fever therapy in general to those patients. Yet, often, pretreated patients strongly demand fever therapy. In these cases, careful patient information, narrow disease monitoring and treatment under a less frequent therapy regimen may be considered.
Fever therapy using a combination of approved drugs is inexpensive. A recommended combination is Colibiogen+Iscador+Strovac, which costs about 50 Euro per application in Germany, with Strovac warranting a share of 36 Euro. A systematic scan of the Red List database of drugs revealed Begripal (Seqirus, Germany) or Mutaflor (Ardeypharm, Germany) as cheaper potential substitutes for Strovac.
Efficacy and Safety of Intra-Tumoural Application
The optimal route for application still has to be determined. While the intravenous route allows slow conveyance of drug and is well suited to avoid potential sudden reactions, intratumoural or peritumoural bolus injections might be more effective, according to Helen Coley-Nauts extensive 1953-review about applications of bacterial toxins during William Coley's time. In this publication, 30 inoperable cancer cases were reviewed, which were treated by Coley and contemporaries in the early 20th century. About half of them were treated by intratumoural injection of bacterial extracts, another half using intramuscular injections. All treatments led to complete regressions of sometimes huge tumor masses. Yet, she took a broader view to write as a corollary: "A study of over 1200 cases of various types of neoplasms treated with the toxins indicates that the site of injection apparently is of importance in determining the success or failure of the treatment. Undoubtedly, the toxins do exert a favorable effect on tumors through intramuscular or intravenous injections remote from the growth, but these alone take longer to accomplish the complete destruction of the neoplasm [compared to intratumoural and pre-tumoural injections]" [4]. For instance, case 19 in Coley-Nauts review was a 16-year old boy with a giant cell tumour near the tenth dorsal vertebra. Initial intramuscular injections of bacterial extract did not stop tumour growth, while subsequent intratumoural applications led into complete and durable remission. The application of PAMP at the site, where the highest concentration of tumour antigens can be expected, is immunologically plausible. In Helen Coley-Nauts review no general safety problems of intramuscular injections were reported, except when the injection accidently hit a vene rather than dense tissue. In these cases sudden circulatory reactions could occur. As we have reported before [24], over several thousands of applications, Coley mentioned six treatment related fatalities in his own department and another three from colleagues; all these patients had inoperable tumours. He concluded that these nine cases were "probably or possibly" caused by the treatment with bacterial extracts. Two i.v.-injected patients died from embolism. Three patients got a too high initial dosage, in one case a second injection was applied during high fever. Since he always cautioned not to start with high-dosage i.t. injections but rather increase dose gradually, and again cautioned not to apply bacterial extract when fever is still high, these six fatalities could probably be called medical malpractice. Three patients died from kidney failure, most likely caused by tumour lysis syndrome, which probably could be avoided in a modern clinical setting.
Peri-tumoural and intra-tumoural applications warrant further investigation both with respect to safety and outcome.
Monitoring
Following PAMP fever therapy, a transient increase in size of primary lesions, with skin becoming red and tense in case of lesions close to the surface, may be observed. An activated immune reaction can lead to a massive influx of immune cells into the tumour (tumour infiltrating lymphocytes, TIL), comprising up to 40% of tumour volume. More TIL correspond with better prognosis [18], [35], [36], [37], [40], [41], [42], [43], [44]. Also, metastases not recognised by palpation or imaging before fever therapy may show up briefly after fever therapy, presumably as a result of an inflammatory immune response and not de novo dissemination and growth, which is hardly possible within a few days. Classically, to judge cancer therapy outcomes, RECIST-criteria are applied. Meanwhile it became apparent that RECIST criteria developed for chemotherapy and radiation are not directly applicable to cancer immune therapy. Melanoma treatment with the antibody drug Ipilimumab can lead to four types of response, which all correlate with increased survival: shrinkage of the primary tumour without development of new lesions, growth stop ("stable disease"), shrinkage of a tumour after a transient increase in size, shrinkage of tumour with development of new lesions [45]. It turned out that "new lesions" might be lesions which were too small to become apparent by X-ray before therapy but show up due to the immune response. Accordingly, a revised list of criteria named irRC (immune related Response Criteria) has been developed. According to irRC a CT or X-ray check every 4 weeks should substantiate whether growth of a lesion is final, and the decision to stop treatment should be taken not before 12 weeks after treatment begin [46]. At any rate, treatment payoff should be closely monitored by tumour markers, imaging, innate cytokine markers (e.g. TNF-α, IL-1, IL-1β,IL-6, IL-12, IF-γ), immuno suppression markes (e.g. IL-10, TGF-β) the neutrophil-lymphocyte ratio [47], [48], and subjective well-being of the patient.
Conclusion
Active fevertherapy using a combination of PAMP containing drugs, ideally combined with preceding hyperthermia, is a cancer treatment that is safe, cheap, has induced spectacular remissions in the past which "would be difficult to achieve now" [49] with present standard therapy, and induced remissions in several patients in the recent past both in our and in other private clinics in Germany and other countries [2]. In contrast to the bacterial extracts used by us and other private clinics in Germany, which were manufactured according to Coley's receipes and which experienced increasing scrutiny by the authorities, leading to their withdrawal from the market, similar impediments are not to be expected for GMP approved drugs such as those introduced for fever therapy here. The time is ripe to engage in more case studies and in a formal clinical study on active fever therapy.
With respect to case studies, several obstacles need to be hurdled. First, other than in private clinics, the implementation of fever therapy in governmental hospitals is hampered by a lack of health insurance cost categories and thus undefined financing. Second, one has to admit that cancer treatment guidelines ("Leitlinien") often impede fever therapy. Guidelines, for most forms of cancer, recommend immune compromising treatments such as chemotherapy and radiation shortly after diagnosis, however, for fever therapy a window of 4-5 weeks high-frequency treatment (3x per week) or longer is needed, requiring an uncompromised immune system during this time. Since cancer usually is a disease that develops over years and even decades [50], the insertion of such a time window in front of more drastic measures seems justifiable, yet difficult to implement against common habitude. Patient individual dose finding and off-label usage of approved drugs also stand against a distinctly formalized and time optimized schedule in large clinics. Although guidelines formally are recommendations rather than strict rules, physicians in governmental institutions usually adhere to guidelines obediently, because deviations may require verbal or written justification. The deviation from guidelines is widely accepted only for those types of cancer where the chances of lasting cure are small, including liver metastases, brain tumours, pancreatic cancer and merkel cell carcinoma. In these cases, and for the growing number of patients who deny chemotherapy and radiation, we recommend fever therapy as an option to be offered to and discussed with patients. Fever therapy is also an option in the palliative situation for many patients.
Considering expense, fever therapy is much cheaper (Table 1) compared to standard treatments and ordes of magnitudes cheaper than recent antibody therapies, including checkpoint inhibitors, which might threaten health budgets in the near future even in developed countries [51] and are unaffordable in developing countries.
More than hundred years after Busch [52], Fehleisen [53], Coley [54], [55] and others [56] and more than 50 years after Klyuyeva et al. [6] have published surprisingly beneficial regimes upon fever therapy, we hope that more physicians learn to valuate fever therapy as a safe and inexpensive yet potentially powerful option to treat cancer.
Declarations
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Ethics Approval and Consent to Participate
Not need for ethics approval and consent.
Consent for Publication
All authors have read, and confirm that they meet, ICMJE criteria for authorship. All authors agree with the manuscript’s results and conclusions.
Availability of Data and Material
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Funding
Not applicable.
Author's Contributions
Suggested combining approved PAMP drugs and wrote the first draft of the manuscript: UH. Contributed to the writing of the manuscript: UR. Fever inductions in patients: UR 404, RO 133.
Acknowledgments
Acknowledgements
Contributions.
UH developed the concept, MO and UR tested augmented mistletoe therapy.
Conflict of Interest
None.
Not applicable.
Contributor Information
Uwe Rudolf Max Reuter, Email: uwe.reuter@klinik-imleben.de.
Ralf Oettmeier, Email: Ralf.Oettmeier@gmx.de.
Uwe Hobohm, Email: uwe.hobohm@mni.thm.de.
References
- 1.Hobohm U, Stanford JL, Grange JM. Pathogen-associated molecular pattern in cancer immunotherapy. Crit Rev Immunol. 2008;28(2):95–107. doi: 10.1615/critrevimmunol.v28.i2.10. [DOI] [PubMed] [Google Scholar]
- 2.Hobohm HU. BoD; Norderstedt: 2016. Healing Heat. [140 pp.] [Google Scholar]
- 3.Wiemann B, Starnes CO. Coley's toxins, tumor necrosis factor and cancer research: a historical perspective. Pharmacol Ther. 1994;64(3):529–564. doi: 10.1016/0163-7258(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 4.Coley-Nauts HC, Fowler GAA, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley's toxins) on malignant tumors in man. Acta Med Scand. 1953;145:5–102. [PubMed] [Google Scholar]
- 5.Mantovani A, Pierotti MA. Cancer and inflammation: A complex relationship. Cancer Lett. 2008;267(2):180–181. doi: 10.1016/j.canlet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 6.Klyuyeva NG, Roskin GI. Pergamon Press; Oxford: 1963. Biotherapy of malignant tumours. [314 pp.] [Google Scholar]
- 7.Hobohm U. Fever and cancer in perspective. Cancer Immunol Immunother. 2001;50(8):391–396. doi: 10.1007/s002620100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobohm U. Fever therapy revisited. Br J Cancer. 2005;92(3):421–425. doi: 10.1038/sj.bjc.6602386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koelmel KF, Pfahlberg A, Mastrangelo G, Niin M, Botev I, Seebacher C, Schneider D, Lambert D, Shafir R, Kokoschka E. Infections and melanoma risk: Results of a multicenter EORTC case study. Melanoma Res. 1999;9:511–519. [PubMed] [Google Scholar]
- 10.Hobohm U. Toward general prophylactic cancer vaccination. Bioessays. 2009;31(10):1071–1079. doi: 10.1002/bies.200900025. [DOI] [PubMed] [Google Scholar]
- 11.Mastrangelo G, Grange JM, Fadda E, Fedeli U, Buja A, Lange JH. Lung cancer risk: effect of dairy farming and the consequence of removing that occupational exposure. Am J Epidemiol. 2005;161(11):1037–1046. doi: 10.1093/aje/kwi138. [DOI] [PubMed] [Google Scholar]
- 12.Maletzki C, Linnebacher M, Savai R, Hobohm U. Mistletoe lectin has a shiga toxin-like structure and should be combined with other Toll-like receptor ligands in cancer therapy. Cancer Immunol Immunother. 2013;62(8):1283–1292. doi: 10.1007/s00262-013-1455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orange M, Fonseca M, Lace A, Laue HB, Geider S. Durable tumour responses following primary high dose induction with mistletoe extracts: Two case reports. Eur J Integr Med. 2010;2:63–69. [Google Scholar]
- 14.Orange M, Lace A, Fonseca MP, Laue BH, Geider S, Kienle GS. Durable Regression of primary Cutaneous B-Cell Lymphoma Following Fever-inducing Mistletoe treatment: two Case Reports. Glob Adv Health Med. 2012;1(1):18–25. doi: 10.7453/gahmj.2012.1.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giovanella BC, Stehlin JS, Jr., Morgan AC. Selective lethal effect of supranormal temperatures on human neoplastic cells. Cancer Res. 1976;36(11 Pt 1):3944–3950. [PubMed] [Google Scholar]
- 16.Trieb K, Sztankay A, Amberger A, Lechner H, Grubeck-Loebenstein B. Hyperthermia inhibits proliferation and stimulates the expression of differentiation markers in cultured thyroid carcinoma cells. Cancer Lett. 1994;87(1):65–71. doi: 10.1016/0304-3835(94)90410-3. [DOI] [PubMed] [Google Scholar]
- 17.Basu S, Srivastava PK. Fever-like temperature induces maturation of dendritic cells through induction of hsp90. Int Immunol. 2003;15(9):1053–1061. doi: 10.1093/intimm/dxg104. [DOI] [PubMed] [Google Scholar]
- 18.Romero P, Dunbar PR, Valmori D, Pittet M, Ogg GS, Rimoldi D, Chen JL, Liénard D, Cerottini JC, Cerundolo V. Ex vivo staining of metastatic lymph nodes by class-I major histocompatibility complex tetramers reveals high numbers of antigen-experienced tumor-specific cytolytic T-lymphocytes. J Exp Med. 1998;188(9):1641–1650. doi: 10.1084/jem.188.9.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 20.Murphy K, Weaver C. 9 ed. Garland Science; New York: 2017. Janeways Immunobiology. [Google Scholar]
- 21.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kornbluth RS, Stone GW. Immunostimulatory combinations: designing the next generation of vaccine adjuvants. J Leukoc Biol. 2006;80(5):1084–1102. doi: 10.1189/jlb.0306147. [DOI] [PubMed] [Google Scholar]
- 23.Dunne MR, Madrigal-Estebas L, Tobin LM, Doherty DG. (E)-4-hydroxy-3-methyl-but-2 enyl pyrophosphate-stimulated Vgamma9Vdelta2 T cells possess T helper type 1-promoting adjuvant activity for human monocyte-derived dendritic cells. Cancer Immunol Immunother. 2010;59(7):1109–1120. doi: 10.1007/s00262-010-0839-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orange M, Reuter U, Hobohm U. Coley's Lessons Remembered: Augmenting Mistletoe Therapy. Integr Cancer Ther. 2016;15(4):502–511. doi: 10.1177/1534735416649916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlappi M, Ewald C, Kuehn JJ, Weinert T, Huber R. Fever Therapy With Intravenously Applied Mistletoe Extracts for Cancer Patients: A Retrospective Study. Integr Cancer Ther. 2017;16(4):479–484. doi: 10.1177/1534735416658121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cippitelli M, Fionda C, Di Bona D, Piccoli M, Frati L, Santoni A. Hyperthermia enhances CD95-ligand gene expression in T lymphocytes. J Immunol. 2005;174(1):223–232. doi: 10.4049/jimmunol.174.1.223. [DOI] [PubMed] [Google Scholar]
- 27.Rescigno M, Piguet V, Valzasina B, Lens S, Zubler R, French L, Kindler V, Tschopp J, Ricciardi-Castagnoli P. Fas engagement induces the maturation of dendritic cells (DCs), the release of interleukin (IL)-1beta, and the production of interferon gamma in the absence of IL-12 during DC-T cell cognate interaction: a new role for Fas ligand in inflammatory responses. J Exp Med. 2000;192(11):1661–1668. doi: 10.1084/jem.192.11.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Osterloh A, Breloer M. Heat shock proteins: linking danger and pathogen recognition. Med Microbiol Immunol. 2008;197(1):1–8. doi: 10.1007/s00430-007-0055-0. [DOI] [PubMed] [Google Scholar]
- 29.Udono H, Srivastava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178(4):1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, Issels R D. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61(2):272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- 31.Kostner AH, Ellegaard MB, Christensen IJ, Bastholt L, Schmidt H. Fever and the use of paracetamol during IL-2-based immunotherapy in metastatic melanoma. Cancer Immunol Immunother. 2015;64(3):349–355. doi: 10.1007/s00262-014-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sunaga T, Shimamoto K, Nakamura S, Takahashi N, Higashino M, Hozumi T, Matsui M, Nagatani A, Kokubu F, Kogo M. The Association between Fever and Prognosis in Lung Cancer Patients with Bone Metastases Receiving Zoledronic Acid. Chemotherapy. 2017;62(6):327–333. doi: 10.1159/000476055. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava N, Srivastava PK. Modeling the repertoire of true tumor-specific MHC I epitopes in a human tumor. PLoS One. 2009;4(7):e6094. doi: 10.1371/journal.pone.0006094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi H, Cao T, Connolly JE, Monnet L, Bennett L, Chapel S, Bagnis C, Mannoni P, Davoust J, Palucka AK. Hyperthermia enhances CTL cross-priming. J Immunol. 2006;176(4):2134–2141. doi: 10.4049/jimmunol.176.4.2134. [DOI] [PubMed] [Google Scholar]
- 35.MacCarty WC, Mahle AE. Relation of differentiation and lymphocytic infiltration to postoperative longevity in gastric carcinoma. J Lab Clin Med. 1921;6:473–480. [Google Scholar]
- 36.Black MS, Opler S, Speer S. Structural representations of tumor-host relationships in gastric carcinoma. Surg Gynecol Obstet. 1956;102:599–603. [PubMed] [Google Scholar]
- 37.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 38.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- 39.Mercadante ER, Lorenz UM. Breaking Free of Control: How Conventional T Cells Overcome Regulatory T Cell Suppression. Front Immunol. 2016;7:193. doi: 10.3389/fimmu.2016.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aaltomaa S, Lipponen P, Eskelinen M, Kosma VM, Marin S, Alhava E, Syrjanen K. Lymphocyte infiltrates as a prognostic variable in female breast cancer. Eur J Cancer. 1992;28A(4-5):859–864. doi: 10.1016/0959-8049(92)90134-n. [DOI] [PubMed] [Google Scholar]
- 41.McCoy JL, Rucker R, Petros JA. Cell-mediated immunity to tumor-associated antigens is a better predictor of survival in early stage breast cancer than stage, grade or lymph node status. Breast Cancer Res Treat. 2000;60(3):227–234. doi: 10.1023/a:1006405504158. [DOI] [PubMed] [Google Scholar]
- 42.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A, Gray H, Schlienger K, Liebman MN. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 43.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29(6):610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 44.Fridman WH, Galon J, Pages F, Tartour E, Sautes-Fridman C, Kroemer G. Prognostic and predictive impact of intra- and peritumoral immune infiltrates. Cancer Res. 2011;71(17):5601–5605. doi: 10.1158/0008-5472.CAN-11-1316. [DOI] [PubMed] [Google Scholar]
- 45.Wolchok JD, Hoos A, O'Day S, Weber JS, Hamid O, Lebbe C, Maio M, Binder M, Bohnsack O, Nichol G. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15(23):7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 46.Hoos A, Wolchok JD, Humphrey RW, Hodi FS. CCR 20th Anniversary Commentary: Immune-Related Response Criteria-Capturing Clinical Activity in Immuno-Oncology. Clin Cancer Res. 2015;21(22):4989–4991. doi: 10.1158/1078-0432.CCR-14-3128. [DOI] [PubMed] [Google Scholar]
- 47.Templeton AJ, Knox JJ, Lin X, Simantov R, Xie W, Lawrence N, Broom R, Fay AP, Rini B, Donskov F. Change in Neutrophil-to-lymphocyte Ratio in Response to Targeted Therapy for Metastatic Renal Cell Carcinoma as a Prognosticator and Biomarker of Efficacy. Eur Urol. 2016;70(2):358–364. doi: 10.1016/j.eururo.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 48.Templeton AJ, McNamara MG, Seruga B, Vera-Badillo FE, Aneja P, Ocana A, Leibowitz-Amit R, Sonpavde G, Knox JJ, Tran B. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124. doi: 10.1093/jnci/dju124. [DOI] [PubMed] [Google Scholar]
- 49.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 50.Weinberg RA. Second edition. Garland Science; NY: 2014. The Biology of Cancer. [Google Scholar]
- 51.Lauterbach K, editor. Die Krebs-Industrie: Wie eine Krankheit Deutschland erobert. Rowohlt; Berlin: 2015. [Google Scholar]
- 52.Busch W. Aus der Sitzung der medicinischen Section vom 13.November 1867. Berl Klin Wochenschr. 1868;5:137. [Google Scholar]
- 53.Fehleisen F. Über die Züchtung der Erysipelkokken auf künstlichem Nährboden und ihre Übertragbarkeit auf den Menschen. Dtsch Med Wochenschr. 1882;85:553–554. [Google Scholar]
- 54.Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the streptococcus of erysipelas and the bacillus prodigiosus). Practitioner. 1909;83:589–613. [PMC free article] [PubMed] [Google Scholar]
- 55.Coley WB, Coley BL. Primary malignant tumors of the long bones: end results in one hundred and seventy operable cases. Ann Surg. 1926 and 1927;13 and 14:779–836. [and 63-141] [Google Scholar]
- 56.Christian S, Palmer L. An apparent recovery from multiple sarcoma with involvement of both bone and soft parts treated by toxin of erysipelas and bacillus prodigiosus. Am J Surg. 1928;43:188–197. [Google Scholar]