Abstract
OBJECT: Nowadays, there is increasing evidence that functional magnetic resonance imaging (MRI) modalities, namely, diffusion-weighted imaging (DWI) and dynamic-contrast enhanced MRI (DCE MRI), can characterize tumor architecture like cellularity and vascularity. Previously, two formulas based on a logistic tumor growth model were proposed to predict tumor cellularity with DWI and DCE. The purpose of this study was to proof these formulas. METHODS: 16 patients with head and neck squamous cell carcinomas were included into the study. There were 2 women and 14 men with a mean age of 57.0 ± 7.5 years. In every case, tumor cellularity was calculated using the proposed formulas by Atuegwu et al. In every case, also tumor cell count was estimated on histopathological specimens as an average cell count per 2 to 5 high-power fields. RESULTS: There was no significant correlation between the calculated cellularity and histopathologically estimated cell count by using the formula based on apparent diffusion coefficient (ADC) values. A moderate positive correlation (r=0.515, P=.041) could be identified by using the formula including ADC and Ve values. CONCLUSIONS: The formula including ADC and Ve values is more sensitive to predict tumor cellularity than the formula including ADC values only.
Introduction
Nowadays, there is a changing behavior regarding clinical oncologic imaging techniques and their possible role in daily routine. Previously, radiologic imaging like computed tomography (CT) and magnetic resonance tomography (MRI) was only used for tumor detection and tumor staging. However, emergent functional imaging modalities like diffusion-weighted imaging (DWI) and dynamic contrast enhanced MRI (DCE-MRI) can not only detect malignant lesions but also characterize tumor microstructure [1], [2], [3], [4], [5].
DWI measures the random water movement in tissues, the so-called Brownian motion, which can be quantified by apparent diffusion coefficient (ADC) [2]. The underlying principle is that the free movement is hindered by cells and, therefore, ADC may predict cell density [2], [4], [6].
Another imaging modality is DCE MRI, which can measure the perfusion in tissue using contrast media agents [8]. Several parameters can be obtained with this technique, namely, Ktrans, Kep, and Ve [8]. Ktrans is the volume transfer constant, Ve is the extravascular extracellular volume fraction, and Kep is the flux rate constant [8]. It is widely acknowledged that DCE parameters, especially Ktrans, are associated with microvessel density in tissues, [8], [9]. Interestingly, Ve as a parameter reflecting the extracellular volume fraction might also be linked to cell count [9], [10]. In fact, previously, it has been shown that Ve correlated with ADC in head and neck cancer [11]. Furthermore, some studies indicated that Ve correlated with cellularity [9], [10].
Prediction of tumor behavior by imaging modalities is of increasing interest. Atuegwu et al. proposed formulas by which cellularity might be calculated by using of ADC values (formula 1) and ADC and Ve values (formula 2) [12]. However, the authors only used breast cancer patients to evaluate their results [12]. Recently, the results of cellularity calculation based on ADC values (formula 1) were analyzed in different tumors [13]. It has been shown that this formula did not apply for all lesions [13].
Therefore, the aim of this study was to compare results of both formulas for cellularity calculation with the histopathologically estimated cell count.
Material and Methods
Patients
Sixteen patients with head and neck sqamous cell carcinoma (HNSCC) were included into the study. There were 2 women and 14 men with a median age of 57 years, mean age of 57.0 ± 7.5 years, and age range 49-79 years. In 11 cases, primary HNSCC and, in 5 patients, local tumor recurrences were diagnosed by histopathology.
DWI
DWI was obtained with an axial DWI-EPI sequence (TR/TE 8620/73 milliseconds, slice thickness 4 mm, voxel size 3.2 × 2.6 × 4.0 mm, b-values of 0 and 800 s/mm2). ADC maps were automatically generated by the implemented software. Regions of interest were manually drawn on the ADC maps along the contours of the tumor on each slice. In all lesions, minimal ADC values (ADCmin), mean ADC values (ADCmean), and maximal ADC values (ADCmax) were estimated.
DCE
DCE imaging was performed using T1w DCE sequences according to a protocol reported previously [9]. The following pharmacokinetic parameters were calculated:
-
-
Ktrans: volume transfer constant which estimates the diffusion of contrast medium from the plasma through the vessel wall into the interstitial space, representing vessel permeability;
-
-
Ve: volume of the extravascular extracellular leakage space;
-
-
Kep: parameter for diffusion of contrast medium from the extracellular leakage space back to the plasma. It is in close relation with Ktrans and Ve and is calculated by the formula:
Calculation of Cellularity
As previously described by Atuegwu et al. (2013) [12], the number of tumor cells can be calculated from ADC values taking into account tumor volume fractions estimated from extended Tofts model (ETM) analysis of DCE-MRI data. For the cell number calculation, the following relationship has been used:
Where ADCw is the ADC of free water (ADCw = 3 × 10−3 mm2/s) and ADCmin is the minimum and ADCmean is the mean ADC value within the region of interest, respectively. θ is the carrying capacity, i.e., maximum number of cells within a given volume [12]. To calculate θ, we converted the given volumes to a standard volume of 1 mm3and used the tumor cell volume of 4189 μm3 [12]. Tumor volume fractions vTC can be calculated from the extravascular extracellular (ve) and plasma volume (vp) fractions using the equation:
ve and vp can be estimated from ETM. In our study, we used the Tofts model (TM), which assumes negligible plasma volume (vp= 0).
We then computed the number of tumor cells per cubic millimeter in two ways: 1) using ADC values only, i.e., assuming vTC = 1, and 2) taking into account volume fractions vTC = 1 − ve.
Estimation of Cellularity
For this study, we reanalyzed our previous data regarding associations between ADC parameters and histopathological findings [9]. Here, KI 67 antigen stained specimens (MIB-1 monoclonal antibody, Dako Cytomation, Denmark) were used as reported previously [9]. In every case, cellularity was estimated as an average cell count per 2 to 5 high-power fields (×400; 0.16 mm2 per field). All images were analyzed by using a research microscope, Jenalumar, with camera Diagnostic instruments 4.2 as reported previously [9].
Statistical Analysis
Because the fact that the formula calculated cells in a volume and previously reported data were based on cell count on high-power fields, a correlation analysis between the calculated and estimated cellularity was performed. Spearman’s correlation coefficient was used, and P values <.05 were taken to indicate statistical significance in all instances.
Results
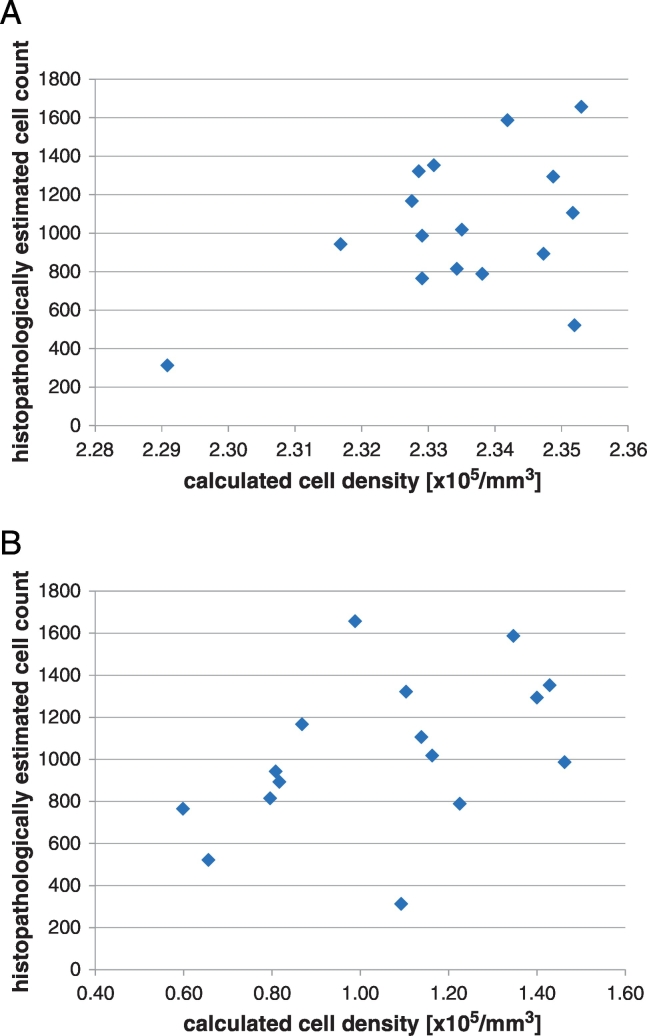
Table 1 displays the correlation coefficients between calculated and estimated cell count. There was no significant correlation between the calculated cellularity and histopathologically estimated cell count by using the formula based on ADC values (formula 1) (Figure 1A). A moderate positive correlation of r=0.515, P=.041 could be identified by using of the formula including both ADC and Ve values (formula 2) (Figure 1B).
Table 1.
Correlation between the calculated cellularity and histopathologically estimated cell count.
| Correlation with Histopathologically Estimated Cell Count | |
|---|---|
| ADC only (formula 1) | r=0.243, P=.365 |
| ADC and Ve (formula 2) | r=0.515, P=.041 |
Figure 1.
(A) Relationships between the histopathologically estimated cellularity and calculated cell counts based on the ADC formula (formula 1).
(B) Relationships between the histopathologically estimated cellularity and calculated cell counts based on the ADC/Ve formula (formula 2).
Discussion
The present study identified a statistically significant correlation between the calculated cellularity using the formula based on ADC and Ve values and the estimated cellularity using histopathology specimens in HNSCC.
Recently, there has been increasing evidence that MRI, using functional imaging modalities, namely, DWI and DCE, can predict tumor behavior and microstructure [1], [2], [3], [4], [5]. Especially ADC values acquired by DWI correlate with cellularity [2], [4], [7]. In a recent meta-analysis, a moderate correlation coefficient of r=−0.56 between ADC values and cell count could be identified [4], [7]. However, this association seems to be different in different tumor entities [4], [7]. For example, in gliomas, the correlation coefficient was higher (r=−0.66), whereas in lymphomas, it was −0.25 [4]. This seems to be related to the fact that ADC values are mainly influenced by cellularity, but also, other cellular structures such as [15] extracellular matrix can also cause diffusion restriction in tissues [6], [13], [14].
The underlying hypothesis is that due to increasing cell density, the free diffusion of protons is hindered and therefore the ADC is lowered [2], [6]. Another aspect seems to be that the intracellular protons have a slower diffusion than the extracellular protons due to higher viscous intracellular milieu [6]. As a recent example, different correlation coefficients between ADC values and various histopathology parameters in a murine prostate model could be identified [16]. The values ranged from r=−0.23 with nuclear spaces up to r=0.74 with extracellular spaces [16]. Furthermore, a strong inverse correlation between nuclear count and ADC values was identified (r=−0.82) [16].
Regarding DCE, there is weaker evidence regarding correlation analysis between DCE parameters and their underlying tissue structures. In a study using 7-T MRI in a glioma mouse model, a strong inverse correlation between Ve and cellularity could be identified (r=−0.75) [10]. Interestingly, this correlation was even stronger than that for ADC values (r=−0.54) [10].
However, in another study that investigated head and neck cancer, only a trend could be identified between Ve and cellularity (r=−0.48, P=.058), [9].
Contrarily, a study on breast cancer murine models even identified that ADC might be better correlated with extracellular spaces than Ve [17].
DCE MRI primarily measures the vascularity of tissues and is thusly strongly associated with vessel densities in tissues [9], [10]. Ve is a parameter which measures the interstitial space and thus might be associated with cellularity [10]. Due to increasing cell density, the interstitial space is narrow, and therefore, Ve might be also lower accordingly.
Previously, it has been shown that especially Ve and ADC are linked to each other and might be influenced by the same histopathology parameters. However, conflicting results were published here. In a recent study investigating head and neck cancer, a moderate correlation coefficient was identified between Ve and ADC using histogram-based analysis [11]. In glioblastoma and in breast cancer, however, no correlation was identified between these parameters, and therefore, they might reflect different tumor aspects [18], [19].
For clinical oncologic routine, it might be essential to predict cellularity in tumor patients. Firstly, it might aid in the primary diagnosis because malignant tumors most often have a higher cellularity as benign lesions [2]. Thereby, ADC values are able to discriminate between malignant and benign entities, as it was widely shown [2]. Secondly, it might aid in prediction in tumor treatment because tumor cell death is induced by radiotherapy and chemotherapy, and therefore, ADC values will be higher under therapy, which might be a very promising biomarker [2], [20], [21]. Thirdly, nowadays, histopathology specimens are acquired with progressively smaller bioptic portions, and therefore, they might not be able to reflect the whole tumor, whereas imaging studies can provide information of the whole tumor. Finally, contrary to histopathology, imaging can be obtained noninvasively and serially.
As mentioned above, Atuewegu et al. proposed two formulas for cellularity calculation based on ADC values (formula 1) and ADC and Ve values (formula 2). Recently, results of cellularity calculation according to formula 1 were compared with histopathological data in different tumors [13]. It could be identified that the formula may be used for prediction of tumor cellularity in cerebral lymphomas and rectal cancer, but not in uterine cervical cancer, meningioma, and thyroid cancer [13].
In the present study, we compared results of both formulas for tumor cell calculation with histopathological findings in HNSCC. As seen, formula 2, using ADC and Ve values, was more sensitive than formula 1, using ADC values only. Therefore, formula 2 may be recommended for clinical studies for prediction of cellularity.
This study has some limitations to address. Firstly, it is of retrospective nature with possible known bias. Secondly, the patient sample is relatively small. Thirdly, only one tumor entity was investigated in this study, and therefore, the results are not transferable to other tumor entities. Fourthly, tumor volume fractions vTC were calculated from the extravascular extracellular fraction (Ve) only due to the TM analysis of DCE data available on the scanner workstation. Taking into account of plasma volume (vp) fraction that could be estimated using ETM would possibly improve the accuracy of calculated cell density and has to be further evaluated. However, for poorly vascularized tissues, which also include head and neck tumors, the TM analysis of DCE data can be applied, and thus, negligible plasma volume can be assumed [22].
In conclusion, the present study identified a moderate positive correlation between the histopathologically estimated cell count and cell count calculated by the formula including ADC and Ve values. There was no significant correlation between the histopathologically estimated cell count and cell count calculated by the formula including ADC values only.
References
- 1.Jordan BF, Runquist M, Raghunand N, Baker A, Williams R, Kirkpatrick L, Powis G, Gillies RJ. Dynamic contrast-enhanced and diffusion MRI show rapid and dramatic changes in tumor microenvironment in response to inhibition of HIF-1alpha using PX-478. Neoplasia. 2005;7(5):475–485. doi: 10.1593/neo.04628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11(2):102–125. doi: 10.1593/neo.81328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schob S, Meyer HJ, Dieckow J, Pervinder B, Pazaitis N, Höhn AK, Garnov N, Horvath-Rizea D, Hoffmann KT, Surov A. Histogram analysis of diffusion weighted imaging at 3T is useful for prediction of lymphatic metastatic spread, proliferative activity, and cellularity in thyroid cancer. Int J Mol Sci. 2017;18(4) doi: 10.3390/ijms18040821. [pii: E821] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surov A, Meyer HJ, Wienke A. Correlation between apparent diffusion coefficient (ADC) and cellularity is different in several tumors: a meta-analysis. Oncotarget. 2017;8(35):59492–59499. doi: 10.18632/oncotarget.17752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampath D, Oeh J, Wyatt SK, Cao TC, Koeppen H, Eastham-Anderson J, Robillard L, Ho CC, Ross J, Zhuang G. Multimodal microvascular imaging reveals that selective inhibition of class I PI3K is sufficient to induce an antivascular response. Neoplasia. 2013;15(7):694–711. doi: 10.1593/neo.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szafer A, Zhong J, Gore JC. Theoretical model for water diffusion in tissues. Magn Reson Med. 1995;33(5):697–712. doi: 10.1002/mrm.1910330516. [DOI] [PubMed] [Google Scholar]
- 7.Surov A, Meyer HJ, Wienke A. Correlation between minimum apparent diffusion coefficient (ADCmin) and tumor cellularity: a meta-analysis. Anticancer Res. 2017;37(7):3807–3810. doi: 10.21873/anticanres.11758. [DOI] [PubMed] [Google Scholar]
- 8.Yan Y, Sun X, Shen B. Contrast agents in dynamic contrast-enhanced magnetic resonance imaging. Oncotarget. 2017;8(26):43491–43505. doi: 10.18632/oncotarget.16482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Surov A, Meyer HJ, Gawlitza M, Höhn AK, Boehm A, Kahn T, Stumpp P. Correlations between DCE MRI and histopathological parameters in head and neck squamous cell carcinoma. Transl Oncol. 2017;10(1):17–21. doi: 10.1016/j.tranon.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aryal MP, Nagaraja TN, Keenan KA, Bagher-Ebadian H, Panda S, Brown SL, Cabral G, Fenstermacher JD, Ewing JR. Dynamic contrast enhanced MRI parameters and tumor cellularity in a rat model of cerebral glioma at 7 T. Magn Reson Med. 2014;71(6):2206–2214. doi: 10.1002/mrm.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer HJ, Leifels L, Schob S, Garnov N, Surov A. Histogram analysis parameters identify multiple associations between DWI and DCE MRI in head and neck squamous cell carcinoma. Magn Reson Imaging. 2017;45:72–77. doi: 10.1016/j.mri.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Atuegwu NC, Arlinghaus LR, Li X, Chakravarthy B, Abramson VG, Sanders ME, Yankeelov TE. Parameterizing the logistic model oftumor growth by DW-MRI and DCE-MRI data to predict treatment response and changes in breast cancer cellularity during neoadjuvant chemotherapy. Transl Oncol. 2013;6(3):256–264. doi: 10.1593/tlo.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Surov A, Garnov N. Proving of a mathematical model of cell calculation based on apparent diffusion coefficient. Transl Oncol. 2017;10(5):828–830. doi: 10.1016/j.tranon.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoyagi T, Shuto K, Okazumi S, Hayano K, Satoh A, Saitoh H, Shimada H, Nabeya Y, Kazama T, Matsubara H. Apparent diffusion coefficient correlation with oesophageal tumour stroma and angiogenesis. Eur Radiol. 2012;22(6):1172–1177. doi: 10.1007/s00330-011-2359-0. [DOI] [PubMed] [Google Scholar]
- 15.Kakkad S, Zhang J, Akhbardeh A, Jacob D, Krishnamachary B, Solaiyappan M, Jacobs MA, Raman V, Leibfritz D, Glunde K. Collagen fibers mediate MRI-detected water diffusion and anisotropy in breast cancers. Neoplasia. 2016;18(10):585–593. doi: 10.1016/j.neo.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin YC, Lin G, Hong JH, Lin YP, Chen FH, Ng SH, Wang CC. Diffusion radiomics analysis of intratumoral heterogeneity in a murine prostate cancer model following radiotherapy: pixel wise correlation with histology. J Magn Reson Imaging. 2017;46(2):483–489. doi: 10.1002/jmri.25583. [DOI] [PubMed] [Google Scholar]
- 17.Barnes SL, Sorace AG, Loveless ME, Whisenant JG, Yankeelov TE. Correlation of tumor characteristics derived from DCE-MRI and DW-MRI with histology in murine models of breast cancer. NMR Biomed. 2015;28(10):1345–1356. doi: 10.1002/nbm.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arlinghaus LR, Li X, Rahman AR, Welch EB, Xu L, Gore JC, Yankeelov TE. On the relationship between the apparent diffusion coefficient and extravascular extracellular volume fraction in human breast cancer. Magn Reson Imaging. 2011;29(5):630–638. doi: 10.1016/j.mri.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mills SJ, Soh C, Rose CJ, Cheung S, Zhao S, Parker GJ, Jackson A. Candidate biomarkers of extravascular extracellular space: a direct comparison of apparent diffusion coefficient and dynamic contrast-enhanced MR imaging-derived measurement of the volume of the extravascular extracellular space in glioblastoma multiforme. AJNR Am J Neuroradiol. 2010;31(3):549–553. doi: 10.3174/ajnr.A1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park SH, Moon WK, Cho N, Song IC, Chang JM, Park IA, Han W, Noh DY. Diffusion-weighted MR imaging: pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer. Radiology. 2010;257(1):56–63. doi: 10.1148/radiol.10092021. [DOI] [PubMed] [Google Scholar]
- 21.Kyriazi S, Collins DJ, Messiou C, Pennert K, Davidson RL, Giles SL, Kaye SB, Desouza NM. Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging-value of histogram analysis of apparent diffusion coefficients. Radiology. 2011;261(1):182–192. doi: 10.1148/radiol.11110577. [DOI] [PubMed] [Google Scholar]
- 22.Sourbron SP, Buckley DL. On the scope and interpretation of the Tofts models for DCE-MRI. Magn Reson Med. 2011;66(3):735–745. doi: 10.1002/mrm.22861. [DOI] [PubMed] [Google Scholar]