Abstract
BACKGROUND: Increasing bodies of evidence suggest that metformin may be beneficial in the primary prevention of colorectal cancer (CRC), and a dose–response relationship has been reported. However, long-term epidemiological observations between the treatment period, cumulative dose, and intensity of metformin and CRC are rarely reported. The aim of this study was to identify the association between the effect of metformin and CRC development in a nationwide cohort study. METHODS: This nationwide population-based study examined a cohort of 1,000,000 patients randomly sampled from individuals enrolled in the Taiwan National Health Insurance system. Patients with newly diagnosed type 2 diabetes mellitus (DM) between 1997 and 2007 were enrolled. A statistical variables, including the demographic data, treatment period, cumulative dose, and intensity of metformin use, was compared between patients developing CRC and those without CRC. RESULTS: This study included 47,597 patients. The mean follow-time was 7.17 ± 3.21 years. After adjustment, metformin use was an independent protective factor against CRC development (P < .001). Although the protective ability of metformin against CRC development was reduced during long-term therapy, the risk of CRC decreased progressively with a higher cumulative dose or higher intensity of metformin use (both P < .001). CONCLUSION: This study revealed that metformin use significantly reduced the risk of CRC in a dose-dependent manner in patients with type 2 DM in the Taiwanese population. However, a gradual decline in medication adherence may reduce the protective ability of metformin against CRC development during long-term therapy.
Introduction
Colorectal cancer (CRC) is the third most commonly diagnosed cancer, with an estimated 1.4 million new cases and 693,900 deaths occurring in 2012 worldwide. According to the Ministry of Health and Welfare of Taiwan, since 2006, CRC has become the most common cancer and the third most common cause of cancer-related death. In 2015, the incidence of CRC was 44.3 per 100,000 population, with 24.2 deaths per 100,000 population and an average of 12.9 years of life lost in Taiwan (http://mohw.gov.tw/CHT/DOS/Index.aspx; accessed in December 2016) [1]. Various molecular markers and many clinical epidemiological factors, such as being overweight or obese, physical inactivity, certain types of diets, smoking, heavy alcohol use, a personal history of inflammatory bowel disease, colorectal polyps or CRC, and family history of adenomatous polyps or CRC, have been identified.
Increasing bodies of epidemiological evidence suggest that people with type 2 (usually noninsulin dependent) diabetes mellitus (DM) have an increased risk of CRC [2], with the incidence being to be 1.2 times higher than that in nondiabetic individuals [3]. Patients with DM also have higher rates of cancer mortality [3]. Moreover, both type 2 DM and CRC share some of the same risk factors (such as being overweight and physical inactivity). However, even after considering these factors, type 2 DM is an independent risk factor for CRC development, and type 2 DM tends to have a less favorable prognosis after CRC diagnosis [4]. The hyperinsulinemia hypothesis suggests that increased circulating insulin and insulin-like growth factor promote the proliferation of colon cells and lead to a survival benefit of transformed cells, ultimately resulting in CRC development [5].
Observational epidemiological studies have suggested that some anti-hyperglycemic agents could affect cancer risk [3], [6]. Patients with DM treated with sulphonylureas or insulin had a higher risk of CRC than other patients, whereas metformin use may be beneficial in primary prevention of CRC [3], [5], [6], [7], [8]. The dose-dependent relationship between metformin and CRC has been reported [9], [10], [11]; however, long-term epidemiological observations between metformin and CRC are rarely reported. Therefore, in the current investigation, we conducted a nationwide population-based study using the Taiwan National Health Insurance (NHI) database with a different study design to investigate the effect of metformin on the incidence of CRC in patients with type 2 DM.
Materials and Methods
Data Sources
In Taiwan, a government-run, single-payer NHI program was established in 1995. The program has a coverage rate of over 99% of Taiwan’s 23 million residents. More than 20,000 medical care facilities, including hospitals, clinics, pharmacies, and medical laboratories, which represent over 93% of all health care facilities in Taiwan, were contracted by the NHI scheme [12]. Under the universal health coverage program, virtually all health care services, including consulting and treatment expenses for inpatient and ambulatory care, dental services, traditional Chinese medicine therapies, physical rehabilitation, and home care, are covered. The dataset used for this study was a cohort of 1 million patients randomly selected from the Longitudinal Health Insurance Database 2005. The database includes all claims for ambulatory care, inpatient care, prescriptions, and registration entries. Patient identification numbers were scrambled for confidentiality protect; hence, researchers are blinded to patient identities. Using the scrambled personal identifier for each study patients, researchers can link the files to obtain sociodemographic information, such as sex, date of birth, occupation type, income level, and longitudinal medical history [13]. Our study protocol was approved by the institutional review board of our hospital (KMUHIRB-EXEMPT-20130056).
Criteria and Definitions of Variables in Diabetic Cohort
We identified DM diagnosis from both the ambulatory and the inpatient claim databases, between January 1, 1996, and December 31, 2008. Patients who had at least two claims in ambulatory or one inpatient claim were selected. The International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 250.xx or a code A181 was used for DM diagnosis. Patients with type 1 DM (ICD-9-CM code 250.x1 and 250.x3) were excluded. Patients with their first DM diagnosis before January 1, 1997, were excluded (n = 18,039) to ensure newly diagnosed patients were included; patients with their first DM diagnosis after December 31, 2007, were also excluded (n = 4,803) to ensure sufficient follow-up periods. Patients with diagnosis of any neoplasm (ICD-9-CM 140-239 or A-code A08x–A14x) before their first DM diagnosis were excluded (n = 14,133). We further excluded patients taking metformin before their DM diagnosis, diagnosed as having polycystic ovarian syndrome (ICD-9-CM 256.4), younger than 15 years at their first DM diagnosis, and with any cancer other than CRC.
Metformin Users and Metformin Nonusers and the Event
Patients who had used metformin anytime for more than 30 days were considered metformin users, whereas the rest of the patients in the cohort were considered metformin nonusers, because the usual prescription of oral hypoglycemic agents in Taiwan is a 28-day or 30-day supply. The outcome variable was CRC development, defined by the appearance of a CRC diagnosis (ICD-9-CM codes: 1530, 1531, 1532, 1533, 1534, 1535, 1536, 1537, 1538, 1539, 1540, 1541, 1542, 1543, 1548) at least twice in the ambulatory claims database or at least once in the inpatient claims database, which was further confirmed with the Catastrophic Illness Registry. Pathological confirmation is generally required for reporting a cancer diagnosis to the Catastrophic Illness Registry. The index date was defined as the date of the earliest DM diagnosis for each patient, and all patients were followed up to the development of CRC, end of the study period, or termination of the record because of death or withdrawal from the insurance program, whichever came first. To minimize immortal time bias, the follow-up time used for Cox regression analyses was calculated from a year after the index date [13].
A defined daily dose (DDD) of metformin of 2 g [14] was used to measure the medication unit. Three predictors were used to examine the association between metformin use and the incidence of CRC. The first predictor was years of use compared with no use. The second predictor was the cumulative dose of metformin use, which was calculated as the cumulative defined daily dose (cDDD). The third predictor was the intensity of metformin use during the treatment period, which was calculated by dividing the cDDD by the period of metformin use. In addition to the demographic variables, the modified Charlson Comorbidity Index (mCCI) score was calculated by subtracting two diabetic conditions from the original Charlson Comorbidity Index score [15], which were treated as covariates.
Statistical Methods
Descriptive analyses were performed to compare numbers and percentages between metformin use and nonuse. The distributions of all variables were presented for those who developed CRC and those who did not. The variables included demographic data, mCCI score, follow-up years, treatment period, cumulative dose, and intensity of metformin use. Student’s t test and the chi-squared test were used to compare continuous and categorical descriptive variables, respectively. Kaplan–Meier survival analyses with the log-rank test and multivariable Cox regression analyses were performed with adjustment for age, sex, and mCCI score. We used a sequential approach to estimate metformin use, including the treatment period (Model 1), cDDD of metformin (Model 2), and intensity of metformin use (Model 3). Microsoft SQL Server 2005 (Microsoft Corporation, Redmond, WA, USA) was used for data linkage, processing, and sampling. Results are expressed as the mean with a standard deviation or an effect and a 95% confidence interval (CI) where appropriate. A P value of <0.05 denoted statistical significance. All statistical analyses were performed using IBM SPSS statistical software, version 19.0 (SPSS Inc., Chicago, IL, USA).
Results
In total, 47,597 patients with newly diagnosed type 2 DM were identified initially and were followed from 1997 to 2008. The mean follow-time was 7.17 ± 3.21 years. The mean age was 54.39 ± 14.19 years, and 53.10% of the patients were men; 914 (1.90%) patients developed CRC during the follow-up period (Table 1). In the identified cohort, 19,082 patients (40.09%) had used metformin for more than a month (metformin users). Compared with metformin nonusers, metformin users involved a significantly higher percentage of men (55.50% vs. 51.40% in the rest of the patients; P < .001), had a lower incidence of CRC development (1.20% vs. 2.40%; P < .001), and had a lower mean mCCI score (0.29 ± 0.79 vs. 0.43 ± 1.00; P < .001) (Table 1).
Table 1.
Characteristics of the Diabetic Patients, Classified with the History of Metformin Use
| Variables | Total (n = 47597) |
Metformin Use |
P Value | |||||
|---|---|---|---|---|---|---|---|---|
| No (n = 28515) |
Yes (n = 19082) |
|||||||
| n | % | n | % | n | % | |||
| Age (year) | ||||||||
| Mean ± SD | 54.39±14.19 | 54.51 ±15.37 | 54.20 ±12.21 | .020 | ||||
| Grouping | 15 – 45 | 12186 | 25.60 | 7811 | 27.40 | 4375 | 22.90 | <.001 |
| 45 – 65 | 23728 | 49.90 | 12923 | 45.30 | 10805 | 56.60 | ||
| > 65 | 11683 | 24.50 | 7781 | 27.30 | 3902 | 20.40 | ||
| Gender | ||||||||
| Female | 22332 | 46.90 | 13847 | 48.60 | 8485 | 44.50 | <.001 | |
| Male | 25265 | 53.10 | 14668 | 51.40 | 10597 | 55.50 | ||
| CRC development | ||||||||
| No | 46683 | 98.10 | 27823 | 97.60 | 18860 | 98.80 | <.001 | |
| Yes | 914 | 1.90 | 692 | 2.40 | 222 | 1.20 | ||
| mCCI score | ||||||||
| Mean ± SD | 0.38±0.93 | 0.43±1.00 | 0.29±0.79 | <.001 | ||||
| Grouping | 0 | 37626 | 79.10 | 21864 | 76.70 | 15762 | 82.60 | <.001 |
| 1-2 | 7585 | 15.90 | 4989 | 17.50 | 2596 | 13.60 | ||
| ≥3 | 2386 | 5.00 | 1662 | 5.80 | 724 | 3.80 | ||
The modified Charlson Comorbidity Index (mCCI) score was calculated by subtracting two diabetic conditions from the original Charlson Comorbidity Index score.
Compared with patients without CRC (Table 2), the patients in the CRC group were older (61.54 ± 11.77 vs. 54.25 ± 14.19), with a higher percentage of them being men (53.83% vs. 46.17%; P < .001). In the patients developing CRC, we observed less use of metformin (24.29% vs. 40.40%, P < .001), a shorter period of metformin use (15.09 ± 32.11 vs. 18.26 ± 31.11 months, P = .002), lower cDDDs of metformin (70.75 ± 222 cDDD vs. 238.19 ± 565.48 cDDD, P < .001), and a lower intensity of metformin use (1.51 ± 4.49 DDD/month vs. 6.07 ± 14.12 DDD/month, P < .001).
Table 2.
Baseline Characteristics of the Diabetic Patients, Classified with The Development of Colorectal Cancer, and Their Use of Metformin
| Variables | Total (n =47597) |
CRC Development |
P Value | |||||
|---|---|---|---|---|---|---|---|---|
| No (n =46683) |
Yes (n =914) |
|||||||
| n | % | n | % | n | % | |||
| Age (year) | ||||||||
| Mean ± SD | 54.39±14.19 | 54.25±14.19 | 61.54±11.77 | <.001 | ||||
| Grouping | 15 – 45 | 12186 | 25.60 | 12102 | 25.92 | 84 | 9.19 | <.001 |
| 45 – 65 | 23728 | 49.85 | 23293 | 49.90 | 435 | 47.59 | ||
| > 65 | 11683 | 24.55 | 11288 | 24.18 | 395 | 43.22 | ||
| Gender | ||||||||
| Female | 22332 | 46.92 | 21910 | 46.93 | 422 | 46.17 | <.001 | |
| Male | 25265 | 53.08 | 24773 | 53.07 | 492 | 53.83 | ||
| mCCI score | ||||||||
| Mean ± SD | 0.38±0.93 | 0.38±0.93 | 0.36±0.96 | .499 | ||||
| Grouping | 0 | 37626 | 79.05 | 36877 | 78.99 | 749 | 81.95 | .104 |
| 1-2 | 7585 | 15.94 | 7466 | 15.99 | 119 | 13.02 | ||
| ≥3 | 2386 | 5.01 | 2340 | 5.01 | 46 | 5.03 | ||
| Ever use of metformin | ||||||||
| No | 28515 | 59.91 | 27823 | 59.60 | 692 | 75.71 | <.001 | |
| Yes | 19082 | 40.09 | 18860 | 40.40 | 222 | 24.29 | ||
| Follow-up time (year), mean ± SD | 7.17±3.21 | 7.22±3.2 | 4.81±2.69 | <.001 | ||||
| Period of metformin use | ||||||||
| Mean ± SD (months) | 18.2±31.13 | 18.26±31.11 | 15.09±32.11 | .002 | ||||
| Grouping (years) | Never use | 28515 | 59.91 | 27823 | 59.6 | 692 | 75.71 | <.001 |
| <5 | 13294 | 27.93 | 13186 | 28.25 | 108 | 11.82 | ||
| 5-10 | 5172 | 10.87 | 5072 | 10.86 | 100 | 10.94 | ||
| >10 | 616 | 1.29 | 602 | 1.29 | 14 | 1.53 | ||
| cDDD of metformin use | ||||||||
| Mean ± SD | 234.97±561.33 | 238.19±565.48 | 70.75±222 | <.001 | ||||
| Grouping | Never use | 28515 | 59.91 | 27823 | 59.6 | 692 | 75.71 | <.001 |
| ≤300 | 9121 | 19.16 | 8972 | 19.22 | 149 | 16.3 | ||
| 300-600 | 3898 | 8.19 | 3857 | 8.26 | 41 | 4.49 | ||
| 600-900 | 2159 | 4.54 | 2145 | 4.59 | 14 | 1.53 | ||
| >900 | 3904 | 8.2 | 3886 | 8.32 | 18 | 1.97 | ||
| Intensity of metformin use (DDD/month) | ||||||||
| Mean ± SD | 5.98±14.01 | 6.07±14.12 | 1.51±4.49 | <.001 | ||||
| Grouping | Never use | 28515 | 59.91 | 27823 | 59.60 | 692 | 75.71 | <.001 |
| ≤10 | 8658 | 18.19 | 8489 | 18.18 | 169 | 18.49 | ||
| 10-20 | 6639 | 13.95 | 6598 | 14.13 | 41 | 4.49 | ||
| >20 | 3785 | 7.95 | 3773 | 8.08 | 12 | 1.31 | ||
The modified Charlson Comorbidity Index (mCCI) score was calculated by subtracting two diabetic conditions from the original Charlson Comorbidity Index score. The cumulative dose of metformin use was presented as cumulative defined daily dose (cDDD). The intensity of metformin use during the treatment period was calculated with dividing cDDD by the period of metformin use.
Multivariable Cox regression models indicated that older age and the male sex were independent risk factors for CRC incidence (both P < .05) (Table 3). After adjustment, metformin use was an independent protective factor (P < .001, Table 3). For the treatment period predictor, the likelihood of developing CRC increased from 36% (95% CI, 0.29–0.44) in ≤5 years to 59% (0.34–1.00) in >10 years in metformin users compared with nonusers (Model 1). The cDDD of metformin (Model 2) and intensity of metformin use (Model 3) were both hierarchically associated with a diminished reduction in the incidence of CRC (both P < .001, Table 3). Consider, for example, the cumulative dose of metformin; in patients who had cDDDs of ≤300, 300–600, 600–900, and >900, the risk levels of developing CRC were 30%, 59%, 77%, and 86%, respectively, compared with nonusers. (P < .001, Table 3). Figure 1 present the results of Kaplan–Meier analyses for variables of the treatment period: the cumulative incidence and intensity of metformin use among patients differed significantly in the log-rank test (all P < .001).
Table 3.
Multivariable Cox Regression Analysis of the Related Factors for Developing Colorectal Cancer in Diabetic Patients
| Variables | Multivariable Cox Regression Analysis |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 |
Model 2 |
Model 3 |
||||||||||
| HR | 95% CI |
P Value | HR | 95% CI |
P Value | HR | 95% CI |
P Value | ||||
| Llower | Upper | Lower | Upper | Lower | Upper | |||||||
| Age (year) | ||||||||||||
| 15–45 | ||||||||||||
| 45–65 | 3.00 | 2.37 | 3.79 | <.001 | 3.03 | 2.39 | 3.82 | <0.001 | 3.00 | 2.37 | 3.79 | <.001 |
| >65 | 5.38 | 4.24 | 6.82 | <.001 | 5.27 | 4.16 | 6.68 | <0.001 | 5.27 | 4.16 | 6.68 | <.001 |
| Gender | ||||||||||||
| Female | ||||||||||||
| Male | 1.25 | 1.10 | 1.43 | .001 | 1.25 | 1.10 | 1.43 | 0.001 | 1.26 | 1.11 | 1.44 | .001 |
| mCCI score | ||||||||||||
| 0 | ||||||||||||
| 1–2 | 0.86 | 0.71 | 1.05 | .130 | 0.84 | 0.69 | 1.02 | 0.074 | 0.85 | 0.70 | 1.04 | .107 |
| >3 | 1.04 | 0.77 | 1.40 | .796 | 1.02 | 0.75 | 1.37 | 0.920 | 1.03 | 0.76 | 1.39 | .846 |
| Period of metformin use (year) | ||||||||||||
| never use | ||||||||||||
| <5 | 0.36 | 0.29 | 0.44 | <.001 | ||||||||
| 5-10 | 0.60 | 0.49 | 0.74 | <.001 | ||||||||
| >10 | 0.59 | 0.34 | 1.00 | .048 | ||||||||
| cDDD of metformin use | ||||||||||||
| never use | 0.70 | 0.59 | 0.84 | <0.001 | ||||||||
| ≤300 | 0.41 | 0.30 | 0.56 | <0.001 | ||||||||
| 300-600 | 0.23 | 0.13 | 0.38 | <0.001 | ||||||||
| 600-900 | 0.15 | 0.10 | 0.24 | <0.001 | ||||||||
| >900 | ||||||||||||
| Intensity of metformin use (DDD/month) | ||||||||||||
| never uses | 0.73 | 0.61 | 0.86 | <.001 | ||||||||
| ≤10 | 0.24 | 0.17 | 0.33 | <.001 | ||||||||
| 10–20 | 0.14 | 0.08 | 0.24 | <.001 | ||||||||
| >20 | ||||||||||||
The modified Charlson Comorbidity Index (mCCI) score was calculated by subtracting two diabetic conditions from the original Charlson Comorbidity Index score. The cumulative dose of metformin use was presented as cumulative defined daily dose (cDDD). The intensity of metformin use during the treatment period was calculated with dividing cDDD by the period of metformin use. The follow-up time was calculated from a year after the initial diagnosis of diabetes mellitus to either the development of cancer, end of the study period, or termination of the record because of death or withdrawal from the insurance program, whichever comes first.
Figure 1.
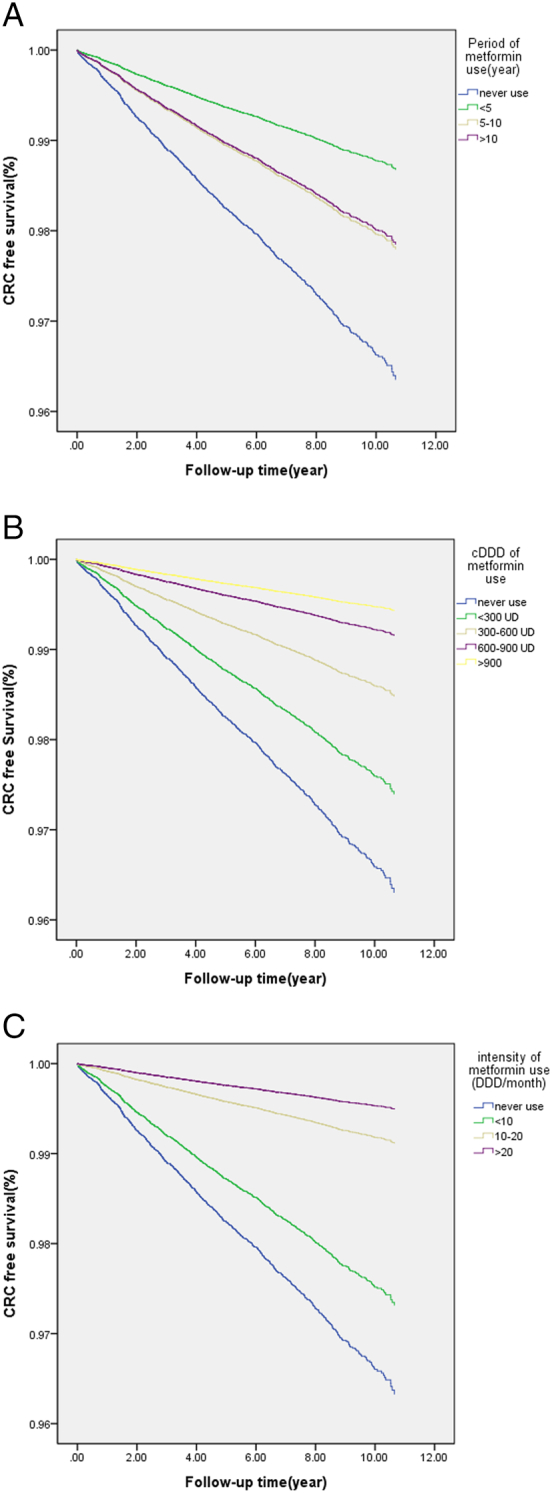
Colorectal cancer-free survival curves of patients with DM, classified by the treatment period of metformin (P < 0.001) (Model 1, Figure 1A), cumulative defined daily dose (cDDD) of metformin (P < 0.001) (Model 2, Figure 1B), and intensity of metformin use (DDD/month) (P < 0.001) (Model 3, Figure 1C).
Discussion
The high prevalence of type 2 DM in patients with CRC is a crucial public health concern worldwide [2], [16], and the high blood sugar levels also significantly affect the prognosis of patients with CRC [17], [18]. Emerging research on CRC risk for people with DM suggests that patients with DM with inadequate glycemic control may have an even higher risk of CRC and often receive polytherapy [19]. A national study in the United States of the trends in glucose-lowering medications determined that more patients were treated with multiple glucose-lowering medications; the mean number of antidiabetic agents per treated patient increased from 1.14 (95% CI, 1.06–1.22) in 1994 to 1.63 (1.54–1.72) in 2007 [20].
Metformin, a biguanide, remains the most widely used first-line medication for type 2 DM, being the oldest oral therapeutic class [6], [7]. Metformin use has almost doubled over the past decade, and approximately one in every two noninsulin antidiabetic drug prescriptions was for single-ingredient metformin in 2012 [6]. According to the guidelines issued by the American Diabetes Association, metformin monotherapy is the first choice at the early stage of the disease, and a second medication is concomitant to help maintain glycated hemoglobin levels at the target if the metformin monotherapy is insufficient. In the present study, 40.09% of the patients were metformin users, and they comprised a relatively healthy population as evidenced by the lower mCCI (metformin users, 0.29 ± 0.79 vs. metformin nonusers, 0.43 ± 1.00; P < .001).
Metformin may exert cancer chemopreventive effects by suppressing the transformative and hyperproliferative processes that initiate carcinogenesis [7]. Although the precise molecular mechanisms by which metformin affects various cancers have not been fully elucidated, suppression of mechanistic target of rapamycin (mTOR) signaling in AMP-activated protein kinase (AMPK)-dependent and AMPK-independent pathways, along with energy metabolism aberration, cell cycle arrest, and apoptosis or autophagy induction, has emerged as a crucial regulator in these processes [3]. Chemoresistant colon cancer cell lines were treated with incremental doses of metformin, which was determined to inhibit growth and migration in a dose-dependent manner [11]. Some investigators have hypothesized that the dose of metformin may determine the effect of this medication. In 2013, Yi et al. demonstrated that a low concentration of metformin induced p53-dependent senescence in hepatoma cells, whereas higher doses induced apoptotic cell death [21]. In ovarian cancer, metformin inhibits tumor growth in nude mice in a dose-dependent manner and reduces the number of lung metastases, proliferation (determined by Ki-67), vascular density, and angiogenesis, as measured using the vascular endothelial growth factor [22], [23]. Metformin may also reduce the levels of the human epidermal growth factor receptor 2 (HER2) and its expression in cell lines in a dose-dependent manner. At low doses (in the micromolar range), the tyrosine kinase activity of HER2 is blocked, but its expression levels are unaffected, whereas at higher concentrations (in the millimolar range), HER2 protein expression is down-regulated [24].
Results of numerous clinical studies have indicated that metformin use, and possibly the cumulative duration of therapy and cumulative dose, was associated not only with decreased incidence of cancer in the diabetic population but also with more favorable outcomes in patients with cancer [7], [25]. A decrease in cancer diagnoses in patients taking insulin and metformin was observed in another study [26]. Another meta-analysis reported a 31% reduction in the cancer rate in patients taking metformin and determined that this relationship was also dose-dependent [9]. In a retrospective study of patients with type 2 DM, reduced cancer rates were found in patients who had been treated with metformin, and this relationship was again dose-dependent [10]. A prospective observational trial followed patients who had used metformin and other sugar-lowering medications for 9.6 years [27]. The trial revealed that metformin use was associated with lower mortality due to cancer with an adjusted hazard ratio of 0.43, and this association was also dose-dependent, resulting in a 42% probability of mortality because of cancer for every 1 g increase in the metformin dose [27]. In the present study, the incidence of CRC decreased by more than 50% if the patients had been treated with metformin with a cumulative dose of >300 cDDD (Model 2) or an intensity of >10 DDD/month (Model 3). Our study indicated that the cumulative dose of metformin and intensity of metformin use are hierarchically associated with reductions in CRC development, consistent with high volume and intensity.
Compared with metformin nonusers, the HR for developing CRC in metformin users decreased by 64% to 51%, depending on the years of use (Model 1). In a longitudinal retrospective cohort study that evaluated early nonadherence in new-user cohorts by using electronic medical record data from 1992 to 2001, 90% of patients filled their first prescription for metformin or sulfonylurea within 30 days [16], [28]. High rates of early discontinuation were observed and, after 6 months, only 73% of patients continued therapy. In an extensive study of electronic records for various medications in patients with DM, among 8,191 patients prescribed glucose-lowering therapies, only 39.6% continued after 24 months, and 4% never filled their prescription (primary nonadherence), despite 53% having glycated hemoglobin ≥7% [16], [29]. Although the risk of developing CRC decreased progressively with a higher cumulative dose or a higher intensity of metformin use (both P < 0.001), the decline in medication adherence to long-term therapy for chronic illnesses may account for the decreasing protective ability in the present study.
A number of limitations in this study have been identified. First, some widely known potentially key clinical covariates, such as diet type, smoking, and alcohol use, are unavailable in the NHI database. Second, some recent epidemiological studies have provided conflicting conclusions about the anticancer effect of metformin [30]. However, the present results are similar to the results of several other observational studies that have been conducted using the Health Information Network database of the United Kingdom, the MarketScan® Commercial Claims and Encounters database of the United States, and the Taiwanese NHI database [26], [31], [32], [33]. In these studies, metformin was associated with a statistically significant risk reduction of 27% to 44% in CRC incidence. A dose-dependent protective effect between metformin use and reduced risk of CRC was demonstrated in the present study. However, to the best of our knowledge, the present study is the first to report long-term epidemiological evidence between metformin and CRC. Third, as in many observational studies on this topic, time-related biases were inevitable [13]. The inclusion of all patients with type 2 DM without metformin use and prior history of cancer as the control group not only avoided selection bias but also minimized the effects of time-related biases [13]. In addition, both the cumulative dose of metformin and the intensity of metformin use showed consistent results, and the effects of these biases were minimal [13]. Furthermore, the dose-dependent effect shown in the multivariable Cox regression analyses increases the reliability of our present results [13].
In summary, metformin use in patients with type 2 DM was associated with a decreased risk of CRC in a dose-dependent manner in this nationwide cohort study. However, a decline in medication adherence may reduce the protective ability of metformin during the treatment period. Preventing CRC development in healthy individuals by treating them with metformin would be a worthwhile study topic.
Acknowledgments
Acknowledgments
This manuscript was edited by Wallace Academic Editing.
Footnotes
Author Contributions: Yu-Tang Chang: writing-original draft. Hsiang-Lin Tsai: data curation and writing – original draft. Ya-Ting Kung: formal analysis. Yung-Sung Yeh: writing – original draft. Ching-Wen Huang: conceptualization and formal analysis. Cheng-Jen Ma: project administration. Herng-Chia: funding acquisition and writing – review and editing. Jaw-Yuan Wang: funding acquisition and writing – review and editing.
Declarations of Interest: none.
Funding: This work was supported by the Excellence for Cancer Research Center Grant through funding by the Ministry of Science and Technology [grant numbers MOST105-2325-B-037-001, MOST-101-2314-B-037-050-MY3]: the Ministry of Health and Welfare [grant numberMOHW106-TDU-B-212-144007]; Health and welfare surcharge of tobacco products, Taiwan, Republic of China; Kaohsiung Medical University Hospital [grant numbers KMUH100-0M14, KMUH105-5R26, KMUHS10608, KMUHS10601, KMUHA10664]: and the Grant of Biosignature in Colorectal Cancers, Academia Sinica, Taiwan, ROC [grant numberT106-002]
Contributor Information
Herng-Chia Chiu, Email: chiu@kmu.edu.tw.
Jaw-Yuan Wang, Email: cy614112@ms14.hinet.net.
References
- 1.Chang YT, Huang MY, Yeh YS, Huang CW, Tsai HL, Cheng TL, Wang JY. A prospective study of comparing multi-gene biomarker chip and serum carcinoembryonic antigen in the postoperative surveillance for patients with stage I-III colorectal cancer. PLoS One. 2016;11 doi: 10.1371/journal.pone.0163264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang CW, Sun LC, Shih YL, Tsai HL, Chen CW, Yeh YS, Ma CJ, Huang CJ, Wang JY. The impact on clinical outcome of high prevalence of diabetes mellitus in Taiwanese patients with colorectal cancer. World J Surg Oncol. 2012;10:76. doi: 10.1186/1477-7819-10-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujita Y, Inagaki N. Metformin: new preparations and nonglycemic benefits. Curr Diab Rep. 2017;17:5. doi: 10.1007/s11892-017-0829-8. [DOI] [PubMed] [Google Scholar]
- 4.Yuhara H, Steinmaus C, Cohen SE, Corley DA, Tei Y, Buffler PA. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am J Gastroenterol. 2011;106:1911–1921. doi: 10.1038/ajg.2011.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berster JM, Göke B. Type 2 diabetes mellitus as risk factor for colorectal cancer. Arch Physiol Biochem. 2008;114:84–98. doi: 10.1080/13813450802008455. [DOI] [PubMed] [Google Scholar]
- 6.Ahuja V, Chou CH. Novel therapeutics for diabetes: uptake, usage trends, and comparative effectiveness. Curr Diab Rep. 2016;16:47. doi: 10.1007/s11892-016-0744-4. [DOI] [PubMed] [Google Scholar]
- 7.Del Barco S, Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B, Menendez JA. Metformin: multi-faceted protection against cancer. Oncotarget. 2011;2:896–917. doi: 10.18632/oncotarget.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paulus JK, Williams CD, Cossor FI, Kelley MJ, Martell RE. Metformin, diabetes, and survival among U.S. veterans with colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2016;25:1418–1425. doi: 10.1158/1055-9965.EPI-16-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S. Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 2010;3:1451–1461. doi: 10.1158/1940-6207.CAPR-10-0157. [DOI] [PubMed] [Google Scholar]
- 10.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nangia-Makker P, Yu Y, Vasudevan A, Farhana L, Rajendra SG, Levi E, Majumdar AP. Metformin: a potential therapeutic agent for recurrent colon cancer. PLoS One. 2014;9 doi: 10.1371/journal.pone.0084369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiang JK, Lin CW, Wang CL, Koo M, Kao YH. Cancer studies based on secondary data analysis of the Taiwan's National Health Insurance Research Database: A computational text analysis and visualization study. Medicine (Baltimore) 2017;96:e6704. doi: 10.1097/MD.0000000000006704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsai MJ, Yang CJ, Kung YT, Sheu CC, Shen YT, Chang PY, Huang MS, Chiu HC. Metformin decreases lung cancer risk in diabetic patients in a dose-dependent manner. Lung Cancer. 2014;86:137–143. doi: 10.1016/j.lungcan.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Wellington K. Rosiglitazone/metformin. Drugs. 2005;65:1581–1592. doi: 10.2165/00003495-200565110-00013. [DOI] [PubMed] [Google Scholar]
- 15.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 16.García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4:175–194. doi: 10.1007/s13300-013-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma YS, Yang IP, Tsai HL, Huang CW, Juo SH, Wang JY. High glucose modulates antiproliferative effect and cytotoxicity of 5-fluorouracil in human colon cancer cells. DNA Cell Biol. 2014;33:64–72. doi: 10.1089/dna.2013.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang IP, Tsai HL, Huang CW, Lu CY, Miao ZF, Chang SF, Juo SH, Wang JY. High blood sugar levels significantly impact the prognosis of colorectal cancer patients through down-regulation of microRNA-16 by targeting Myb and VEGFR2. Oncotarget. 2016;7:18837–18850. doi: 10.18632/oncotarget.7719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson JE, Culpepper L. Associations between colorectal cancer screening and glycemic control in people with diabetes, Boston, Massachusetts, 2005-2010. Prev Chronic Dis. 2011;8:A82. [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander GC, Sehgal NL, Moloney RM, Stafford RS. National trends in treatment of type 2 diabetes mellitus, 1994–2007. Arch Intern Med. 2008;168:2088–2094. doi: 10.1001/archinte.168.19.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yi G, He Z, Zhou X, Xian L, Yuan T, Jia X, Hong J, He L, Liu J. Low concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the AMPK pathway. Int J Oncol. 2013;43:1503–1510. doi: 10.3892/ijo.2013.2077. [DOI] [PubMed] [Google Scholar]
- 22.Alimova IN, Liu B, Fan Z, Edgerton SM, Dillon T, Lind SE, Thor AD. Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro. Cell Cycle. 2009;8:909–915. doi: 10.4161/cc.8.6.7933. [DOI] [PubMed] [Google Scholar]
- 23.Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia. 2011;13:483–491. doi: 10.1593/neo.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vazquez-Martin A, Oliveras-Ferraros C, Menendez JA. The antidiabetic drug metformin suppresses HER2 (erbB-2) oncoprotein overexpression via inhibition of the mTOR effector p70S6K1 in human breast carcinoma cells. Cell Cycle. 2009;8:88–96. doi: 10.4161/cc.8.1.7499. [DOI] [PubMed] [Google Scholar]
- 25.Kasznicki J, Sliwinska A, Drzewoski J. Metformin in cancer prevention and therapy. Ann Transl Med. 2014;2:57. doi: 10.3978/j.issn.2305-5839.2014.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Currie CJ, Poole CD, Gale EA. The influence of glucose lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52:1766–1777. doi: 10.1007/s00125-009-1440-6. [DOI] [PubMed] [Google Scholar]
- 27.Landman GW, Kleefstra N, van Haterenm KJJ, Groenier KH, Gans ROB, Bilo HJG. Metformin associated with lower cancer mortality in type 2 diabetes: ZODIAC-16. Diabetes Care. 2010;33:322–326. doi: 10.2337/dc09-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trinacty CM, Adams AS, Soumerai SB, Zhang F, Meigs JB, Piette JD, Ross-Degnan D. Racial differences in long-term adherence to oral antidiabetic drug therapy: a longitudinal cohort study. BMC Health Serv Res. 2009;9:24. doi: 10.1186/1472-6963-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karter AJ, Parker MM, Moffet HH, Ahmed AT, Schmittdiel JA, Selby JV. New prescription medication gaps: a comprehensive measure of adherence to new prescriptions. Health Serv Res. 2009;44:1640–1661. doi: 10.1111/j.1475-6773.2009.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevens RJ, Ali R, Bankhead CR, Bethel MA, Cairns BJ, Camisasca RP, Crowe FL, Farmer AJ, Harrison S, Hirst JA. Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia. 2012;55:2593–2603. doi: 10.1007/s00125-012-2653-7. [DOI] [PubMed] [Google Scholar]
- 31.Lee MS, Hsu CC, Wahlqvist ML, Tsai HN, Chang YH, Huang YC. Type 2 diabetes increases and metformin reduces total, colorectal, liver and pancreatic cancer incidences in Taiwanese: a representative population prospective cohort study of 800,000 individuals. BMC Cancer. 2011;11:20. doi: 10.1186/1471-2407-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sehdev A, Shih Y-CT, Vekhter B, Bissonnette M, Olopade OI, Polite B. Metformin for primary colorectal cancer prevention in diabetic patients: a case-control study in a US population. Cancer. 2015;121:1071–1078. doi: 10.1002/cncr.29165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tseng CH. Diabetes, metformin use, and colon cancer: a population-based cohort study in Taiwan. Eur J Endocrinol. 2012;167:409–416. doi: 10.1530/EJE-12-0369. [DOI] [PubMed] [Google Scholar]


