Summary
Tooth agenesis in the reduction of tooth number which includes hypodontia, oligodontia and anodontia is caused by disturbances and gene mutations that occur during odontogenesis. To date, several genetic mutations that unlock the causes of non-syndromic tooth agenesis are being discovered; these have been associated with certain illnesses because tooth development involves the interaction of several genes for tooth epithelium and mesenchyme odontogenesis. Mutation of candidate genes PAX9 and MSX1 have been identified as the main causes of hypodontia and oligodontia; meanwhile, AXIN2 mutation is associated with anodontia. Previous study using animal models reported that PAX9-deficient knockout mice exhibit missing molars due to an arrest of tooth development at the bud stage. PAX9 frameshift, missense and nonsense mutations are reported to be responsible; however, the most severe condition showed by the phenotype is caused by haploinsufficiency. This suggests that PAX9 is dosage-sensitive. Understanding the mechanism of genetic mutations will benefit clinicians and human geneticists in future alternative treatment investigations.
Keywords: Hypodontia, Oligodontia, Mutation, PAX9
1. Introduction
Tooth agenesis involving reduction of tooth number also known as congenitally missing teeth, is due to a craniofacial malformation. The teeth are fail to erupt in the oral cavity and mineralization is not visible in radiographs [1], [2]. It can be subdivided into three types which are hypodontia, oligodontia and anodontia, as summarized in Table 1.
Table 1.
Summary on type of tooth agenesis and its associated criteria.
| Tooth agenesis | Number of missing tooth | Level of severity | Classification | Type of inheritance |
|---|---|---|---|---|
| Hypodontia | 1–6 teeth (excluding third molar) | Mild to moderate | Syndromic and non-syndromic | Sporadic or familial |
| Oligodontia | More than 6 teeth | Severe | Syndromic and non-syndromic | Sporadic or familial |
| Anodontia | Complete absence of teeth | Severe | Syndromic | Sporadic or familial |
A person is diagnosed with hypodontia when there is an absence of one to six teeth excluding the third molar, while oligodontia refers to the absence of more than six teeth excluding the third molar. However, the most severe condition is anodontia, which refers to the absence of all teeth [3], [4]. Anodontia is normally present alongside syndromic conditions such as Witkop tooth–nail syndrome, Fried syndrome, Böök syndrome (PHC), hair–nail–skin–teeth dysplasias, Rieger syndrome, Holoprosencephaly, Down’s syndrome (trisomi 21), Wolf–Hirschhorn syndrome (deletion 4p) and Kabuki syndrome [5], [6], [7], [8]. Meanwhile, other types of tooth agenesis conditions may appear as syndromic or non-syndromic [3], [8], [9].
Since oral health plays a significant role in one’s life, tooth agenesis may cause the affected person to have improper masticatory function, suffer from speech alteration and develop aesthetic problems especially where teeth at the anterior region are missing. Tooth agenesis also causes some other problems, including affecting a person’s emotions [9], [10], [11].
To date, the mechanism of tooth development is well understood, with the aetiology of tooth agenesis being linked to both environmental and genetic factors [2], [8], [12]. This paper discusses the mutation gene candidate PAX9 that is responsible for early tooth development and the assertion that its mutations are associated with several types of tooth agenesis.
2. Prevalence, distribution and pattern of tooth agenesis
Severe tooth agenesis (absence of 4 or more teeth excluding the third molars) has an estimated prevalence of 0.25% in the general population [4]. However, hypodontia is identified as the most common dental anomaly in humans and affects almost 20% of the current population [10], [13]. As reported by Nik Hussein, hypodontia is the most common tooth anomaly in Malaysia with a prevalence of 2.8%; meanwhile the updated prevalence (including the third molar) is 3.2% [14], [15], however there are no published reports on the prevalence of oligodontia and anodontia in Malaysia.
Several studies have found that females are more affected with tooth agenesis compared to males. The highest prevalence (7.7% in female and 6.1% in male) was found in Chinese population, while one meta-analysis reported a female to male ratio of 1:1.4 [14], [16], [17]. The pattern of tooth agenesis varies by population. Previous studies conducted among Caucasians report that the lower second premolars and upper lateral incisors are the most common missing teeth after third molars, whilst American blacks have a lower prevalence of congenitally missing teeth than American whites [18], [19]. In Asians, the most common missing teeth after the third molar are second premolars and mandibular lateral incisors; however, in Malaysia, upper lateral incisors (1.7%) followed by lower and upper second premolars (1.5%) were identified as the most common missing teeth [11], [14].
3. Embryonic tooth development
Tooth development results from several interactions which act synergistically and antagonistically, leading to tooth epithelium and mesenchyme formation during embryonic odontogenesis. The process is governed by several components and mechanisms involving expression of several transcription factors, Sonic hedgehog (Shh), Wingless (Wnt) signaling families, fibroblast growth factors (FGFs), bone morphogenic proteins (BMP) and also cellular matrix molecules [9], [20], [21], [22], [23], [24]. This complex mechanism consisting of the continuous and progressive stages of odontogenesis have been divided into bud, cap and bell stages [25], [26].
During embryonic tooth development, dental lamina is resulted from thickening of the oral epithelium and invagination into the surrounding ectomesenchymal cells, forming the tooth bud. The epithelium proliferates and condenses to form the dental papilla and dental sac. Later, the bud further proliferates to form a cap-shaped enamel organ that surrounds the mesenchymal papilla. Continued growth and differentiation of adjacent epithelium and mesenchymal cells lead to formation of ameloblast and odontoblast cells which later develop into the enamel and dentin respectively [21], [22], [25].
In 2008, more than 300 genes were listed in the database created by Pekka Nieminen from Helsinki University, Finland (http://bite-it.helsinki.fi) containing compilations of expression patterns at various stages of odontogenesis [27], [28]. Several genes, transcription factors, growth factors and extracellular matrix molecules involved in odontogenesis are listed in Table 2 [20], [22], [29].
Table 2.
List of odontogenesis components and genes at each development stage.
| Stages | Components/genes | ||||
|---|---|---|---|---|---|
| Bud stage | MSX1 | FGF | Glil | Ptc | LEF1 |
| PAX9 | Shh | BMP4 | EGF | ||
| Cap stage | AXIN2 | Pitx2 | BMP2 | TGF | |
| MSX1 | Shh | EDA | |||
| Bell stage | PAX9 | TGF | BMP2 | ||
| MSX1 | BMP4 | AXIN2 | |||
4. Genetic basis of tooth agenesis
Advancement in genetics and molecular biology technology has allowed us to better understand the aetiology of tooth agenesis. Tooth morphogenesis is monitored under strict genetic control during embryonic development and these genes are being discovered at an increasing rate. Any disturbances and gene mutations that occur during odontogenesis are believed to cause missing teeth and also dental defects such as changes in tooth size, morphology, and cytodifferentiation [28], [30], [31].
Mutation of genes PAX9 (further discussed in the next part) and MSX1 have been identified as the main causes of hypodontia and oligodontia; meanwhile, AXIN2 mutation is associated with anodontia [2], [32], [33], [34]. PAX9 and MSX1 are members of paired-box and homeobox transcription factors respectively, which are involved in the early stages of tooth development. Mutation of MSX1 is associated with missing premolars and certain syndromic conditions such as cleft lip/palate and Witkop syndrome [35], [36]. The MSX1 gene located at chromosome 4 is important for determination of the shape and position of teeth [37].
AXIN2 located at chromosome 7 is the gene responsible for encoding axis inhibition protein 2 that regulates the stability of beta-catenin. This gene is associated with familial oligodontia and anodontia; additionally, the affected person has a higher susceptibility to colorectal cancer [38], [39], [40]. AXIN2 acts as a regulator in the Wnt signaling pathway and its expression is found in the enamel knot [20]. However, this paper will focus on mutations that occur within the PAX9 gene which has been reported by several studies (Table 3).
Table 3.
List of mutations occurring within the PAX9 gene according to type of mutation.
| Type of mutation | Classification | Nucleotide | Molecular consequence | Phenotype | References |
|---|---|---|---|---|---|
| Frameshift | Deletion > insertion | 175_188 (del8inse288) | Disruption of C-terminal binding region | Hypodontia | [43] |
| Deletion > insertion | 619_621 (del3ins24) | Premature termination of protein translation | Oligodontia | [41] | |
| Insertion | 218_219insG | Extension of several G-series | Oligodontia | [44], [45] | |
| Insertion | 792_793insC | Protein truncation and termination | Oligodontia | [46] | |
| Deletion | 230_242del13 | Disruption of protein translation | Oligodontia | [38] | |
| Deletion | 465delG | Disruption of protein translation | Hypodontia | [47] | |
| Deletion | 462delT | Disruption of protein translation | Hypodontia | [47] | |
| Insertion | 624_625insA | Disruption of protein translation | Hypodonita | [47] | |
| Nonsense | Deletion | 14q locus | Abolished protein function (Haploinsufficiency) | Hypodontia | [48] |
| Transition | 1A > G | Abolished protein function (Haploinsufficiency) | Oligodontia | [49] | |
| Transition | 2T > G | Abolished protein function (Haploinsufficiency) | Oligodontia | [50] | |
| Substitution | 340A > T | PAX9 premature termination at N-terminal DNA binding region | Oligodontia | [51] | |
| Substitution | 480C > G | Premature stop codon | Hypodontia | [45] | |
| Missense | Substitution | 62T > C | Reduction of DNA binding ability and specificity | Hypodontia | [43] |
| 271A > G | Hypodontia | [43] | |||
| 76C > T | Oligodontia | [53] | |||
| 83G > C | Oligodontia | [52] | |||
| 238A > G | Reduction of DNA binding ability and specificity | Oligodontia | [38] | ||
| 428A > G | |||||
| 152G > C | |||||
| 718G > C | Structural PAX9 protein sequence (polymorphism) | Hypodontia | [54] | ||
| Silent | Substitution | 717C > T | No significant changes in sequences | Hypodontia | [54] |
5. PAX9 (paired box gene 9) mutations experimental evidences
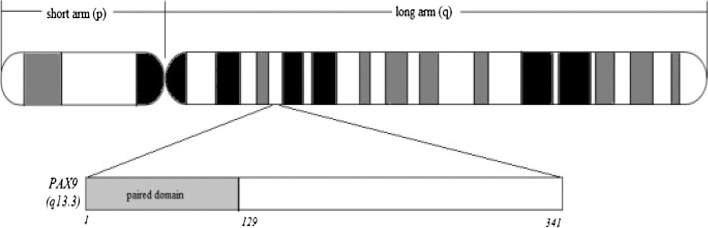
PAX9 is a member of the paired box transcription factor protein which contains an octapeptide, a pair of box domains and a 128-amino acid long paired-type homeodomain that plays a critical role in dental mesenchyme formation at all stages of odontogenesis [41]. This gene is found on chromosome 14 at cytogenetic location 14q13.3 (Fig. 1) and is strongly expressed in the oral mesenchyme during the early stages of tooth development [9], [22].
Figure 1.
Location of PAX9 gene within chromosome 14.
PAX9 is responsible for BMP4 expression which further regulates expression of MSX1. Thus, mutations of PAX9 cause abnormal or reduced downstream protein regulation function which is important for tooth development [20], [21]. Animal model studies have proven that PAX9-deficient knockout mice exhibit missing molars due to an arrest of tooth development at the bud stage [42].
PAX9 mutations occur from the range of single nucleotide substitutions: by changing one amino acid, premature termination and abolished protein function to haploinsufficiency. This suggests that PAX9 is dosage-sensitive whereby greater mutations showing more severe tooth agenesis phenotypes [42], [43]. Mutations in both coding and non-coding regions have been reported involving exons 1, 2, 3 and 4; exon 2 which is a highly conserved area containing paired-domain regions showed the most mutations [2]. All these mutations are associated with oligodontia and hypodontia, particularly causing missing molars. In most cases, the mutation is inherited as autosomal dominant with incomplete penetrance, though an autosomal recessive inheritance mode has also been reported in a Pakistani family [2], [37].
5.1. Frameshift mutation
Changes in amino acid sequences affect protein function and expression. Several frameshift mutations have been reported occurring at exon 2 and exon 4 involving insertion and deletion mutations. Das et al. reported that deletion of 8 nucleotides followed by insertion of 288 foreign nucleotides (175_188, del8ins288) within exon 2 lead to disruption of the C-terminal DNA binding domain of PAX9 paired-domain. The affected person was a twin with missing permanent molars [43]. Deletion of several nucleotides (619_621, del3ins24) followed by 24 bp foreign insertion and several duplications of 5′splicing site sequences occurring at the end of exon 2 caused premature termination of translation at aa 210. Aberrant splicing lead to frameshift mutation and premature termination of translation at aa 314. The proband had molar oligodontia [41].
Other than that, an oligodontia patient with missing molars, premolars and incisors showed insertion of a single guanine nucleotide (218_219insG) within the exon 2 PAX9 paired-domain region, causing a frameshift between N- and C-terminal DNA binding domains due to extension of several guanine series [44]. The same mutation was also found in a female Chinese oligodontia patient with 15 missing permanent teeth [45].
A single cytosine nucleotide insertion (792_793insC) at the N-terminal DNA binding region caused PAX9 protein truncation and lead to premature termination of translation at aa 315. This mutation occurred within exon 4 and the patient showed an autosomal dominant trait of missing molars and premolars [46].
Recently, Bergandal et al. reported that mutational analysis of an oligodontia patient with 13 missing teeth showed deletion of 13 nucleotides (230_242del13) at the PAX9 paired-domain upstream region [38].
Another two frameshift mutations within PAX9 exon 3 reported in two female Malaysian hypodontia patients who are missing first molar and lateral incisor showed deletion of single nucleotide, c.465delG and c.462delT respectively [47]. The same study also reported another insertion mutation c.624_625insA as the affected person is also hypodontia female patient with missing a canine tooth [47].
5.2. Nonsense mutation
Nonsense mutations mostly cause changes in amino acids that code for stop codon. A study reported an infant hypodontia patient has normal anterior teeth but missing both maxillary and mandibular molars and premolars. PAX9 gene in the patient showed deletion of the 14q locus. This mutation suggests haploinsufficiency, since only one copy of the PAX9 gene was able to function normally [48].
Transition mutation M1V (1A > G) at the start codon of exon 1 caused haploinsufficiency where only one copy of the gene functioned normally, while the other was not expressed due to abolished protein function. The affected person was diagnosed with oligodontia, inherited as autosomal dominant as permanent incisors, premolars and molars were missing [49]. Another transition mutation was found recently at the start codon (2T > G) which resulted in an ATG initiation start codon instead of ACG. The mutation was detected in mutational analysis of a 12-year old Chinese oligodontia patient [50].
Premature termination of PAX9 gene caused by substitution of Lys114stop (340A > T) occurring at the A340T switch lead to truncation and termination of protein at the N-terminal end of DNA binding region [51].
Mutational analysis from a recent study conducted by Zhu et al. reported a sporadic Chinese infant hypodontia patient showing nucleotide substitution (480C > G) within exon 2, causing nonsense mutation. This mutation suggested haploinsufficiency due to a premature stop codon which reduced the transcriptional activity of PAX9. The affected person was congenitally missing 6 primary molars and 20 permanent teeth [45].
5.3. Missense mutation
Nucleotide substitution is the most reported missense mutation. Two studies show nucleotide substitution occurring within the DNA binding region of PAX9 paired-domain which involves substitution of Leu21Pro (62T > C) and Lys91glu (271A > G), respectively. The affected person showed an oligodontia phenotype with missing molars, premolars and incisors [43].
Meanwhile, substitution mutation occurring at the N-terminal DNA binding region of the PAX9 paired-domain was reported in two studies. Substitution of Arg26Tyr (76C > T) and Arg28Pro (83G > C) affect DNA binding specificity, thus lead to reduction in DNA binding ability. The phenotype of the affected person showed oligodontia with missing molars, premolars and incisors [52], [53].
Polymorphism 718G > C leading to substitution of Ala240Pro and causing structural changes in PAX9 protein sequences has been reported in Portugese families. The probands showed missing lateral incisors [54]. Recently, Bergendal et al. reported three substitution mutations in Swedish oligodontia patients which are Thr80Ala (238A > G), Tyr143Cys (428A > G) and Gly51Ala (152G > C) [38].
5.4. Silent mutation
One silent mutation occurring within exon 3 involving substitution (717C > T) has been reported. It was found in a Portugese hypodontia proband who was missing a lateral incisor. No significant changes were observed in the PAX9 protein sequence but the phenotype of the proband was nevertheless affected [54].
6. Conclusion
Improper masticatory function and aesthetic problems caused by tooth agenesis may affect the patients’ daily life. Because embryonic tooth development involves complex signaling cascades and the expression of several genes, disturbances that occur during the process may lead to tooth agenesis. At present, several candidate genes have been identified as genetic causative agents for tooth agenesis such as MSX1 and AXIN2; however, the highest number of mutations was found in the PAX9 gene. Experimental evidence shows how PAX9 mutations ranging from single nucleotide substitutions to changes in amino acid and premature termination to haploinsufficiency. This suggests that the phenotype of the affected person is dosage-sensitive. A comprehensive understanding of the genetic mutations and mechanisms that cause tooth agenesis will benefit patients, clinicians and geneticists in providing alternative treatment plans in the future.
Conflict of interest
The authors declare that no conflicts of interest concerned in this study.
References
- 1.Bailleul-Forestier I., Molla M., Verloes A., Berdal A. The genetic basis of inherited anomalies of the teeth. Part 1: clinical and molecular aspects of non-syndromic dental disorders. Eur J Med Genet. 2008;51(4):273–291. doi: 10.1016/j.ejmg.2008.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Pemberton T., Das P., Patel P. Hypodontia: genetics and future perspectives. Braz J Oral Sci [Internet] 2005 [cited 2016 February 23]; Available from: http://bioline.org.br/request?os05011. [Google Scholar]
- 3.Shimizu T., Maeda T. Prevalence and genetic basis of tooth agenesis. Jpn Dent Sci Rev [Internet] 2009;45(1):52–58. Available from: http://dx.doi.org/10.1016/j.jdsr.2008.12.001. [Google Scholar]
- 4.Vastardis H. The genetics of human tooth agenesis: new discoveries for understanding dental anomalies. Am J Orthod Dentofac Orthop. 2000;117(6):650–656. [PubMed] [Google Scholar]
- 5.Chaitra T.R., Singh A.P., Singh S.P. Anodontia of permanent teeth—a case report. Pak Oral Dent J. 2010;30(1):165–167. [Google Scholar]
- 6.Larmour C.J., Mossey P.A., Thind B.S., Forgie A.H., Stirrups D.R. Hypodontia—a retrospective review of prevalence and etiology. Part I. Quintessence Int. 2005;36(4):263–270. [PubMed] [Google Scholar]
- 7.Jorgenson R.J. Clinician’s view of hypodontia. J Am Dent Assoc [Internet] 1980;101(2):283–286. doi: 10.14219/jada.archive.1980.0186. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6995515. [DOI] [PubMed] [Google Scholar]
- 8.Cobourne M. Familial human hypodontia—is it all in the genes? Br Dent J. 2007;203:203–208. doi: 10.1038/bdj.2007.732. [DOI] [PubMed] [Google Scholar]
- 9.Nieminen P. Genetic basis of tooth agenesis. J Exp Zool Part B Mol Dev Evol. 2009;312(4):320–342. doi: 10.1002/jez.b.21277. [DOI] [PubMed] [Google Scholar]
- 10.Liu H., Zhang J., Song S., Zhao H., Han D., Feng H. A case-control study of the association between tooth-development gene polymorphisms and non-syndromic hypodontia in the Chinese Han population. Eur J Oral Sci. 2012;120(13):378–385. doi: 10.1111/j.1600-0722.2012.00986.x. [DOI] [PubMed] [Google Scholar]
- 11.Alshahrani I., Togoo R.A., Alqarni M.A. A review of hypodontia: classification, prevalence, etiology, associated anomalies, clinical implications and treatment options. JP J. 2013;4(June):117–125. [Google Scholar]
- 12.Brook A.H. A unifying aetiological explanation for anomalies of human tooth number and size. Arch Oral Biol. 1984;29(5):373–378. doi: 10.1016/0003-9969(84)90163-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhang W., Qu H.C., Zhang Y. PAX-9 polymorphism may be a risk factor for hypodontia: a meta-analysis. Genet Mol Res. 2014;13(4):9997–10006. doi: 10.4238/2014.November.28.4. [DOI] [PubMed] [Google Scholar]
- 14.Mani S.A., Mohsin W.S.Y., John J. Prevalence and patterns of tooth agenesis among Malay children. Southeast Asian J Trop Med Public Health. 2014;45(2):490–498. [PubMed] [Google Scholar]
- 15.Nik-Hussein Hypodontia in the permanent dentition: a study of its prevalence in Malaysian children. Aust Orthod J. 1989;11(93):5. [PubMed] [Google Scholar]
- 16.Khalaf K., Miskelly J., Voge E., Macfarlane T.V. Prevalence of hypodontia and associated factors: a systematic review and meta-analysis. J Orthod [Internet] 2014;41(4):299–316. doi: 10.1179/1465313314Y.0000000116. Available from: http://www.maneyonline.com/doi/abs/10.1179/1465313314Y.0000000116. [DOI] [PubMed] [Google Scholar]
- 17.Polder B.J., Van’t Hof M.A., Van Der Linden F.P.G.M., Kuijpers-Jagtman A.M. A meta-analysis of the prevalence of dental agenesis of permanent teeth. Community Dent Oral Epidemiol. 2004;32(3):217–226. doi: 10.1111/j.1600-0528.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- 18.Kirkham J., Kaur R., Stillman E.C., Blackwell P.G., Elcock C., Brook A.H. The patterning of hypodontia in a group of young adults in Sheffield, UK. Arch Oral Biol. 2005;50(2 SPEC. ISS):287–291. doi: 10.1016/j.archoralbio.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Harris E., Clark L. Hypodontia: an epidemiologic study of American black and white people. Am J Orthod Dentofac Orthop [Internet] 2008;134:761–767. doi: 10.1016/j.ajodo.2006.12.019. [cited 2016 February 23] Available from: http://www.sciencedirect.com/science/article/pii/S088954060800718X. [DOI] [PubMed] [Google Scholar]
- 20.Cudney S.M., Vieira A.R. Molecular factors resulting in tooth agenesis and contemporary approaches for regeneration: a review. Eur Arch Paediatr Dent [Internet] 2012;13(6):297–304. doi: 10.1007/BF03320830. Available from: http://www.scopus.com/inward/record.url?eid=2-s2.0-84874154955&partnerID=tZOtx3y1. [DOI] [PubMed] [Google Scholar]
- 21.Kapadia H., Mues G., D’Souza R. Genes affecting tooth morphogenesis. Orthod Craniofac Res [Internet] 2007;10(November(4)):237–244. doi: 10.1111/j.1601-6343.2007.00407.x. [cited 2016 March 25] Available from: http://www.ncbi.nlm.nih.gov/pubmed/17973693. [DOI] [PubMed] [Google Scholar]
- 22.Scarel-Caminaga R.M., Pasetto S., Ribeiro da Silva E., Peres R.C.R. Genes and tooth development: reviewing the structure and function of some key players. Braz J Oral Sci. 2003;2(7):339–347. [Google Scholar]
- 23.Bei M. Molecular genetics of tooth development. Curr Opin Genet Dev. 2009;19(5):504–510. doi: 10.1016/j.gde.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galluccio G., Castellano M., La Monaca C. Genetic basis of non-syndromic anomalies of human tooth number. Arch Oral Biol [Internet] 2012;57(7):918–930. doi: 10.1016/j.archoralbio.2012.01.005. Available from: http://dx.doi.org/10.1016/j.archoralbio.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Bhalajhi S. Dental anatomy, histology and development. 2nd ed. Arya (Medi) Publishing House; 2005. Development of teeth; pp. 215–228. [Google Scholar]
- 26.Bath-Balogh M., Fehrenbach M.J. Dental embryology, histology and anatomy. 2nd ed. Elsevier Inc.; USA: 2006. Tooth development and eruption; pp. 61–83. [Google Scholar]
- 27.Nieminen P., Pekkanen M., Åberg T., Thesleff I. A graphical WWW-database on gene expression in tooth. Eur J Oral Sci. 1998;106:7–11. doi: 10.1111/j.1600-0722.1998.tb02146.x. [DOI] [PubMed] [Google Scholar]
- 28.Bloch-zupan A. Dento/oro/craniofacial anomalies and genetics. 1st ed. Elsevier Inc.; USA: 2012. Missinf teeth (hypodontia and oligodontia) pp. 9–74. [Google Scholar]
- 29.Suryadeva S., Begum M. Role of homeobox genes in tooth morphogenesis: a review. J Clin Diagn Res. 2015;9(2):ZE09–ZE12. doi: 10.7860/JCDR/2015/11067.5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borie E., Fuentes R., Beltran V. Multiple tooth agenesis in non-syndromic patient: a rare case report. Int J Morphol. 2012;30(2):634–636. [Google Scholar]
- 31.Goya H.A., Tanaka S., Maeda T., Akimoto Y. An orthopantomographic study of hypodontia in permanent teeth of Japanese pediatric patients. J Oral Sci [Internet] 2008;50(2):143–150. doi: 10.2334/josnusd.50.143. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18587203. [DOI] [PubMed] [Google Scholar]
- 32.Mostowska A., Biedziak B., Trzeciak W.H. A novel c.581C > T transition localized in a highly conserved homeobox sequence of MSX1: is it responsible for oligodontia? J Appl Genet. 2006;47(2):159–164. doi: 10.1007/BF03194616. [DOI] [PubMed] [Google Scholar]
- 33.Mostowska A., Kobielak A., Trzeciak W.H. Molecular basis of non-syndromic tooth agenesis: mutations of MSX1 and PAX9 reflect their role in patterning human dentition. Eur J Oral Sci. 2003;111(20):365–370. doi: 10.1034/j.1600-0722.2003.00069.x. [DOI] [PubMed] [Google Scholar]
- 34.Kamamoto M., Machida J. Clinical and functional data implicate the Arg (151) Ser variant of MSX1 in familial hypodontia. Eur J Hum Genet [Internet] 2011:1–7. doi: 10.1038/ejhg.2011.47. [cited 2016 February 23] Available from: http://www.nature.com/ejhg/journal/v19/n8/abs/ejhg201147a.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mostowska A., Biedziak B., Jagodzinski P.P. Novel MSX1 mutation in a family with autosomal-dominant hypodontia of second premolars and third molars. Arch Oral Biol [Internet] 2012;57(6):790–795. doi: 10.1016/j.archoralbio.2012.01.003. Available from: http://dx.doi.org/10.1016/j.archoralbio.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 36.Xuan K., Jin F., Liu Y.-L., Yuan L.-T., Wen L.-Y., Yang F.-S. Identification of a novel missense mutation of MSX1 gene in Chinese family with autosomal-dominant oligodontia. Arch Oral Biol. 2008;53:773–779. doi: 10.1016/j.archoralbio.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Matalova E., Fleischmannova J., Sharpe P., Tucker A. Tooth agenesis: from molecular genetics to molecular dentistry. J Dent Res. 2008;87(7):617–623. doi: 10.1177/154405910808700715. [DOI] [PubMed] [Google Scholar]
- 38.Bergendal B., Klar J., Stecksrn-Blicks C., Norderyd J., Dahl N. Isolated oligodontia associated with mutations in EDARADD, AXIN2, MSX1, and PAX9 genes. Am J Med Genet Part A. 2011;155(7):1616–1622. doi: 10.1002/ajmg.a.34045. [DOI] [PubMed] [Google Scholar]
- 39.Wang J., Jian F., Chen J., Wang H., Lin Y., Yang Z. Sequence analysis of PAX9, MSX1 and AXIN2 genes in a Chinese oligodontia family. Arch Oral Biol [Internet] 2011;56(October(10)):1027–1034. doi: 10.1016/j.archoralbio.2011.03.023. [cited 2016 January 28] Available from: http://www.sciencedirect.com/science/article/pii/S000399691100118X. [DOI] [PubMed] [Google Scholar]
- 40.Lammi L., Arte S., Somer M., Ja H., Thesleff I. Mutations in AXIN2 cause familial tooth agenesis and predispose to colorectal cancer. Am J Hum Genet. 2004;74:1043–1050. doi: 10.1086/386293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mostowska A., Biedziak B., Trzeciak W.H. A novel mutation in PAX9 causes familial form of molar oligodontia. Eur J Hum Genet. 2006;14(2):173–179. doi: 10.1038/sj.ejhg.5201536. [DOI] [PubMed] [Google Scholar]
- 42.Peters H., Neubüser A., Kratochwil K., Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12(17):2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das P., Hai M., Elcock C., Suzanne M., Donald T., Alan H. Novel missense mutations and a 288-bp exonic insertion in pax9 in families with autosomal dominant hypodontia. Am J Med Genet [Internet] 2003:35–42. doi: 10.1002/ajmg.a.10011. [cited 2016 February 23] Available from: http://onlinelibrary.wiley.com/doi/10.1002/ajmg.a.10011/full. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stockton D., Das P. Mutation of PAX9 is associated with oligodontia. Nat Genet [Internet] 2000;24(1):18. doi: 10.1038/71634. [cited 2016 April 13] Available from: http://search.ebscohost.com/login.aspx?direct=true&profile=ehost&scope=site&authtype=crawler&jrnl=10614036&AN=8815838&h=MAkN0EY9UkGHaKeTwUHZp2jiKL1%2FhrhwktD3c6TXmCgDY1UTP2zAIEsp%2B6cZ7SucZXYblrrIj5FZNmn1a8m9gQ%3D%3D&crl=c. [DOI] [PubMed] [Google Scholar]
- 45.Zhu J., Yang X., Zhang C., Ge L., Zheng S. A novel nonsense mutation in PAX9 is associated with sporadic hypodontia. Mutagenesis. 2011:1–5. doi: 10.1093/mutage/ger080. [DOI] [PubMed] [Google Scholar]
- 46.Frazier-Bowers S.A., Guo D.C., Cavender A., Xue L., Evans B., King T. A novel mutation in human PAX9 causes molar oligodontia. J Dent Res [Internet] 2002;81(February(2)):129–133. [cited 2016 July 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/11827258. [PubMed] [Google Scholar]
- 47.Nur Farahiyah M.I., Nur Syahira R., Nining Irfanita I., Yunita Dewi Ardini, Solachuddin A.I. PAX9 mutation of non-syndromic hypodontia in a Malysian family. UI Proceeding on Health and Medicine. 2017;1:108–111. [Google Scholar]
- 48.Das P., Stockton D.W., Bauer C., Shaffer L.G., D’Souza R.N., Wright J.T. Haploinsufficiency of PAX9 is associated with autosomal dominant hypodontia. Hum Genet. 2002;110(4):371–376. doi: 10.1007/s00439-002-0699-1. [DOI] [PubMed] [Google Scholar]
- 49.Klein M.L., Nieminen P., Lammi L., Niebuhr E., Kreiborg S. Novel mutation of the initiation codon of PAX9 causes oligodontia. J Dent Res [Internet] 2005;84(January(1)):43–47. doi: 10.1177/154405910508400107. [cited 2016 July 29] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15615874. [DOI] [PubMed] [Google Scholar]
- 50.Liang J., Qin C., Yue H., He H., Bian Z. A novel initiation codon mutation of PAX9 in a family with oligodontia. Arch Oral Biol [Internet] 2016;61:144–148. doi: 10.1016/j.archoralbio.2015.10.022. Available from: http://www.sciencedirect.com/science/article/pii/S0003996915300704. [DOI] [PubMed] [Google Scholar]
- 51.Nieminen P., Arte S., Tanner D., Paulin L., Alaluusua S., Thesleff I. Identification of a nonsense mutation in the PAX9 gene in molar oligodontia. Eur J Hum Genet. 2001;9(10):743–746. doi: 10.1038/sj.ejhg.5200715. [DOI] [PubMed] [Google Scholar]
- 52.Jumlongras D., Lin J.Y., Chapra A., Seidman C.E., Seidman J.G., Maas R.L. A novel missense mutation in the paired domain of PAX9 causes non-syndromic oligodontia. Hum Genet. 2004;114(3):242–249. doi: 10.1007/s00439-003-1066-6. [DOI] [PubMed] [Google Scholar]
- 53.Lammi L., Halonen K., Pirinen S., Thesleff I., Arte S., Nieminen P. A missense mutation in PAX9 in a family with distinct phenotype of oligodontia. Eur J Hum Genet. 2003;11(11):866–871. doi: 10.1038/sj.ejhg.5201060. [DOI] [PubMed] [Google Scholar]
- 54.Pinho T., Silva-Fernandes A., Bousbaa H., MacIel P. Mutational analysis of MSX1 and PAX9 genes in Portuguese families with maxillary lateral incisor agenesis. Eur J Orthod [Internet] 2010;32(5):582–588. doi: 10.1093/ejo/cjp155. [cited 2016 February 23] Available from: http://ejo.oxfordjournals.org/content/32/5/582.short. [DOI] [PubMed] [Google Scholar]