Abstract
Objectives
It was to analyse and compare the effect of different disinfectant systems on the dimensional stability of commonly used irreversible hydrocolloid and addition silicone impression materials from developing countries as compared to materials from developed countries.
Material and methods
Disinfectant systems used were glutaraldehyde, sodium hypochlorite and ultraviolet chamber. The stability after disinfection of commonly used alginate and addition silicone of native origin (Algin-Gum & Ad-Sil) was compared with similar impression materials from developed countries (Vignette and Aquasil) and results compared. A CAD/CAM manufactured stainless steel die simulating maxilla with four metal studs at canine and molar region was used. Impressions were made and disinfected after rinsing and drying and casts poured. The cross arch distance, interabutment distance and the occluso-gingival length of the studs was measured under traveling microscope and observations were recorded and compared. ANOVA test and Bonferroni test was applied.
Results
An increase in the interabutment and cross arch distance and decrease in occluso-gingival height was seen in the casts obtained. Glutaraldehyde immersion showed variation in the interabutment and cross arch distance for all materials studied. Ultraviolet chamber and sodium hypochlorite produced best results. Dimensional stability of impression materials like Vignette, Algin-Gum & Aquasil was found to within clinically acceptable limits after disinfection while maximum deviation was seen with Algin-Gum.
Conclusion
Evaluated materials can be safely disinfected with sodium hypochlorite and ultraviolet chamber. Addition silicone of native origin is at par with impression materials from developed countries but same cannot be said about alginate.
Keywords: Dimensional stability, Immersion systems, Ultraviolet chamber, Addition silicone, Alginate, Sodium hypochlorite, Glutaraldehyde
1. Introduction
The impression material can act as a vehicle for the transfer of bacteria and fungi (Minagi et al., 1986). In a healthy patient, the chances of cross contamination are minimal but in the diseased and debilitated patients, chances of cross infection to the dental personnel and patients are high and can pose a serious threat if proper precautions are not taken. Thus there is a need for an effective system for prevention of cross contamination without causing dimensional changes in the impression.
Studies have focused attention towards the elimination of micro-organisms with various disinfectants as regards to their duration without causing dimensional changes (Minagi et al., 1986). A change in the dimensions can have profound bearing on the success of the prosthesis being finally placed in the patient’s mouth. This has been the most important reason for the dental personnel being negligent towards disinfection of impressions so as not to lose the details.
Immersion method with various disinfection solutions and spray disinfection have been tested and proven to be effective for the purpose (Council, 1996). However, the most reliable method is immersion as the disinfectant solution comes in contact with all surfaces of the impression material and tray. In 1996, the American Dental Association Council on Dental Materials recommended spray disinfection for irreversible hydrocolloid, immersion disinfection for polysulphide and addition silicone whereas for polyether, spraying with chlorine compound was recommended for 2–3 min (Council, 1996). Merchant VA concluded that efficacy of disinfection by immersion is preferable as sprayed disinfectant tends to pool and thus all surfaces of the impression are not covered (Merchant, 1989). Irreversible hydrocolloid materials are organic and hydrophilic thus facilitating retention and growth of microorganisms. It was reported in several studies that disinfection of alginate impressions by immersion did not cause clinically significant changes on the dimensional stability of the resultant casts (Minagi et al., 1986, Herrara and Merchant, 1986, Durr and Novak, 1987, Setcos et al., 1984). Later studies support immersion disinfection for polyether, hydrophilic-addition silicone and irreversible hydrocolloid impressions with recommended times and disinfectants (Tullner et al., 1988, Thouati et al., 1996, Kern et al., 1993, Rios et al., 1996).
New methods to disinfect impressions have been introduced like the autoclave chamber, microwave and ultraviolet chamber and the results have been evaluated (Boylon et al., 1987, Ishida et al., 1991). Research is going on for a material which is easy to use and incorporates the disinfectant without affecting the dimensional stability of the impressions.
In market today, most of the dental products are from developed countries. Developing countries are also producing products of native origin and trying to be at par with international standards. Many studies have been conducted on the dimensional stability of the international products from developed countries after disinfection but evaluation of products from developing countries has not been carried out extensively to see their stability. Irreversible hydrocolloid is one of the impression materials frequently used in the making of a fixed prosthesis. Alginate impressions are used extensively in dentistry for making diagnostic casts, check impressions and making impressions for provisional crowns. The other material taken up in this study is addition silicone which is one of the most accurate impression materials. In this study, the effect of commercially available disinfectants like 2% glutaraldehyde, 5.25% sodium hypochlorite and the ultraviolet rays on the dimensional stability of commonly available and extensively used irreversible hydrocolloid and addition silicone from international brands and the same materials from developing countries was studied and the results were recorded and compared.
2. Material and methods
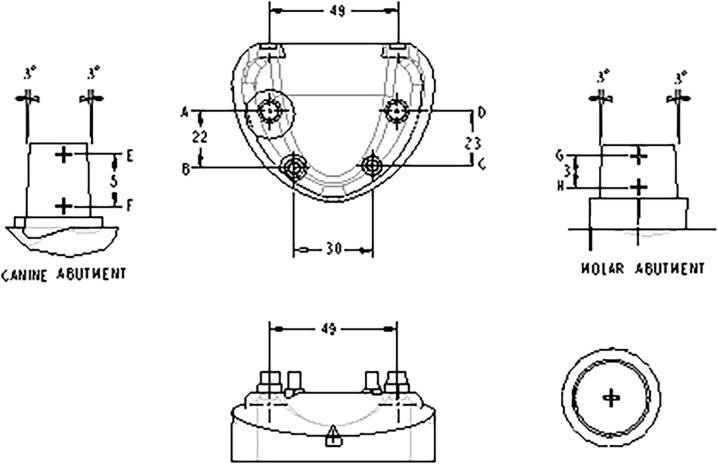
The study was conducted in the Prosthodontics Department. The dimensional stability of the impressions was assessed indirectly by measuring specific dimensions on gypsum casts which were recovered from impressions of a stainless steel metal die designed by CAD/CAM technology (Shivam Technology Lab., Pune) resembling human dental maxillary arch (Fig. 1). It was designed with four preparations designated A, B, C and D simulating a complete veneer crown preparation with total 6 degree taper and a butt joint finish line (3 degrees of negative taper/opposing surface) positioned symmetrically on the arch. Two studs were located in the region of right and left first molar (A & D) and two were in the region of canine (B & C). All the studs were in the same occlusal plane. Each stud had a CNC marking inscribed on the occlusal surface in the form of cross hair which served as a reference point to mark the centre of the preparation and facilitated future measurements. The markings were also inscribed on the buccal surface at two points as cross hair reference points along the length to evaluate change in occluso- gingival dimension (E-F, G-H) (Fig. 2).
Fig. 1.
CAD/CAM designed stainless steel die.
Fig. 2.
Schematic representation of the dimensions on the die.
The six dimensions selected for analysis of dimensional stability after disinfection were interabutment distance (A-B, C-D); cross arch distance (B-C, A-D) and the occluso-gingival length (E-F for the canine and G-H for the molar abutment).
Impressions of the master die were made using a modification of test device used by Holtan et al., 1991, Stauffer et al., 1976. A mounting jig (Shivam Technology Lab., Pune) was constructed with two fibre glass plates. The first plate had three parallel metal rods/guide posts fixed on it and the metal die was secured onto it with metal screws. The other fibre glass parallel plate had three holes that accommodated with the guide posts and a mandibular rigid and torsional resistant stainless steel stock metal tray (Jabbar-Jalandhar) was fixed onto it with cyanoacrylate cement closely approximating the metal die on the other fibre glass plate. Three metal cylinders of the same height were placed on the guide posts which allowed the minimum clearance of 3 mm for the impression material between the tray and the metal die. The guide posts and the metal cylinders allowed the impression tray to be positioned consistently over the master die.
The metal die was disinfected in the ultraviolet chamber for 30 min (254 nm wavelength). Impression materials used for the study are listed in Table 1. All impression materials were mixed according to the manufacturer’s instructions. The total amount of material used was constant with each impression material type as they were dispensed by weight and/or volume. Irreversible hydrocolloid (Algin–Gum, Vignette) was mixed at room temperature and placed within manufacturer’s recommended working time. In case of addition silicone (Ad-Sil, Aquasil), tray adhesive was applied onto the stock tray and allowed to air dry for 15 min. Impressions were made by one step putty wash technique. Putty material was mixed and loaded into the tray while light body addition silicone was injected onto the metal die through automatic dispenser.
Table 1.
Impression materials used.
| Product | Type | Manufacturer | Technique |
|---|---|---|---|
| Algin-Gum | Irreversible hydrocolloid | Prime Dental Pvt. Ltd., India | Single mix |
| Vignette | Irreversible hydrocolloid | Dentsply DeTrey GmbH Pvt. Ltd., Germany | Single mix |
| Ad-Sil | Addition silicone | Prime Dental Pvt. Ltd., India | One step putty-wash technique |
| Aquasil | Addition silicone | Dentsply DeTrey GmbH Pvt. Ltd., Germany | One step putty-wash technique |
The tray along with the fibre glass plate was guided over the metal die with the help of guide posts. Parallel guide posts ensured guided vertical removal of the impression tray which reduced lateral stresses on the set impression material. The tray was allowed to stay on the die for a period twice the recommended setting time to compensate for polymerisation at room temperature rather than mouth temperature as it was an in vitro study (Lepe and Johnson, 1997). Test device also allowed the cast to be poured with its base parallel to the occlusal surfaces of the preparations. The tray was removed with a blunt instrument and rinsed under distilled water for 15 s to simulate saliva removal. Excess water was shaken off by hand from irreversible hydrocolloid impressions while addition silicone impressions were dried with air blow from a three-way syringe.
Disinfection systems used in the study were the commonly used immersion systems (Glutaraldehyde 2%, Sodium hypochlorite 5.25%) and Ultraviolet chamber (Table 2). The fourth group was the control group. Forty impressions of the die were made with each impression material which were equally distributed amongst the disinfection systems selected. Each disinfection system and control group consisted of ten impressions with each impression material. Total one hundred and sixty impressions were made with all the four impression materials.
Table 2.
Disinfectant systems used.
| Product | Type | Group | Procedure |
|---|---|---|---|
| Korsolex | Glutaraldehyde 2% 1:19 dilution | G | Rinsed in distilled water for 15 s and dried & immersed in this solution for 10 min |
| Sodium Hypo Chlorite Sol (Shree Chemicals) | Sodium Hypochlorite 5.25% 1: 10 dilution | S | Rinsed in distilled water for 15 s and dried & immersed in this solution for 10 min |
| Ultraviolet chamber | Raysat 254 nm frequency | U | Rinsed in distilled water for 15 s and dried and placed in ultraviolet chamber at 254 nm for 3 min |
| Control | No disinfection | C | Rinsed in distilled water for 15 s and dried |
Two sets of impressions were immersed in freshly prepared immersion solutions for ten minutes. (Group G & S) The third set was placed in ultraviolet light chamber at 254 nm wavelength for three minutes on a rotating table. (Group U) This was done to avoid any shadowing effect and ensure there was proper disinfection from all sides. The fourth untreated sample acted as control group and was kept in a plastic bag after rinsing it with distilled water.
The impressions were poured with Type IV dental stone (Ultra Rock) which was vacuum -mixed according to the manufacturer’s instructions with water: powder ratio of 0.23. Irreversible hydrocolloid impressions were poured within 12 min of the impression making including the time for the particular disinfection group. The addition silicone impressions were poured after one hour of their making and disinfection. Bases were poured for each of the dies with type III stone and casts were thus prepared for the study. All measurements were made with the help of traveling microscope (Oriental Company) at the Bharati Vidyapeeth Jawaharlal Institute of Technology, Pune after one week of pouring of the impressions for the casts to gain adequate strength. The microscope had the accuracy of 10 µm.
All the dies were placed under traveling microscope and the distance between the CNC marks was measured. The measurements of the six dimensions selected were repeated three times for each stone cast by the same operator and the mean of three readings was used. Measurements of the master die were made in the similar way but 10 separate measurements for each dimension were taken and the mean was calculated as given in Table 3.
Table 3.
The various parameters measured on the metal die.
| Dimensions measured | Mean (mm) | SD | |
|---|---|---|---|
| 1. | Interabutment distance A-B (right side) | 22.04 mm | 0.008 mm |
| 2. | Interabutment distance C-D (left side) | 23.038 mm | 0.005 mm |
| 3. | Cross arch distance B-C | 30.3 mm | 0.005 mm |
| 4. | Cross arch distance A-D | 49.042 mm | 0.008 mm |
| 5. | Occluso-gingival length E-F (canine) | 5.024 mm | 0.000 mm |
| 6. | Occluso-gingival length G-H (molar) | 3.022 mm | 0.000 mm |
The statistical analysis was carried out using Statistical Package for Social Sciences (SPSS Inc., Chicago, IL, version 15.0 for Windows). All quantitative variables were estimated using measures of central location (mean) and measures of dispersion (standard deviation and standard error). Normality of data was checked by measures of skewness and Kolmogorov Smirnov tests of normality.
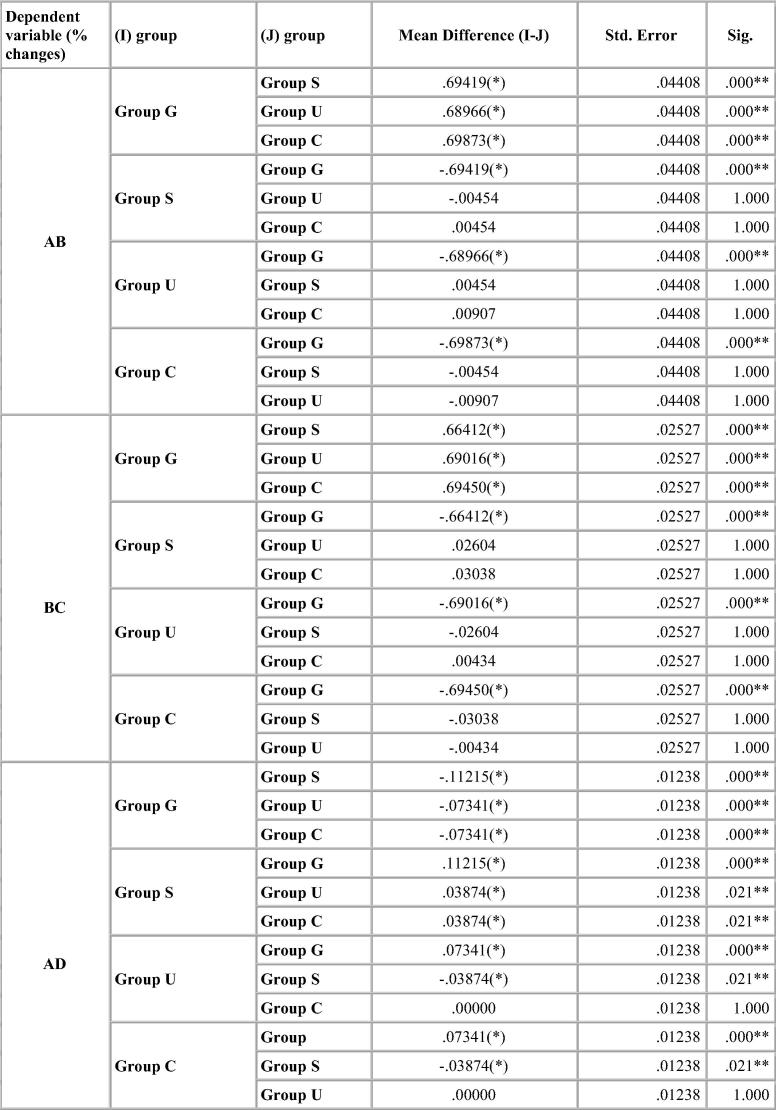
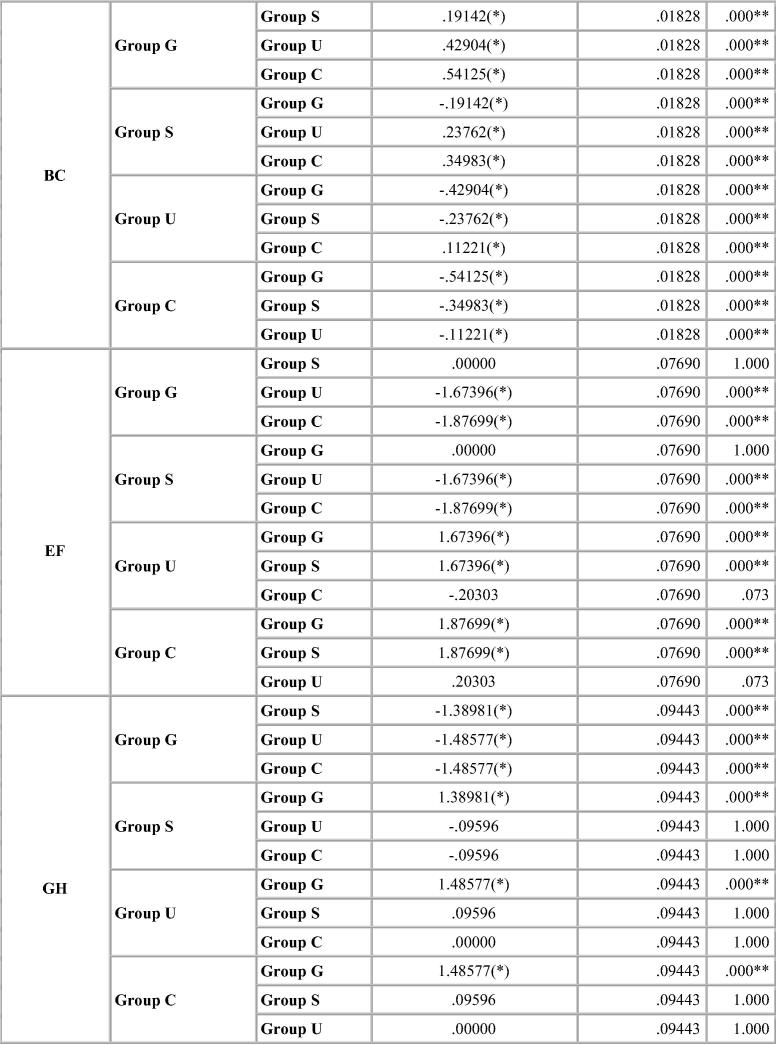
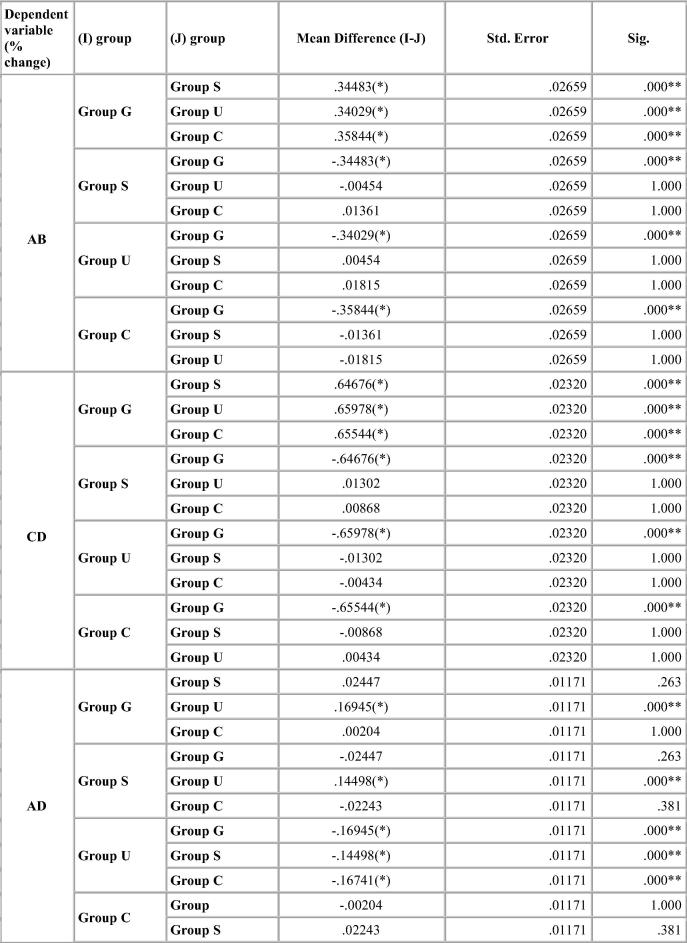
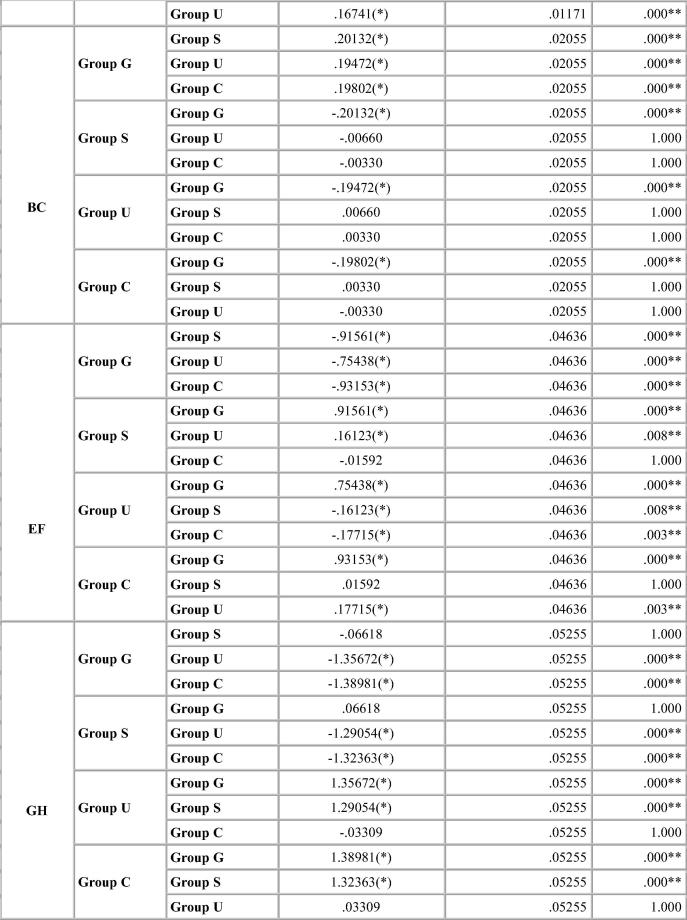
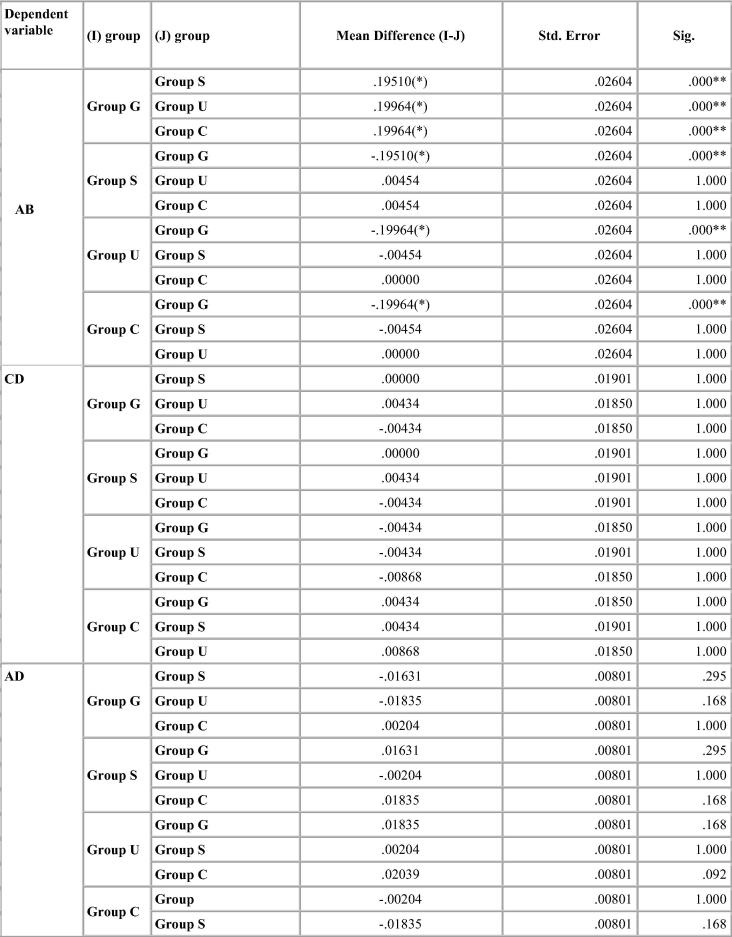
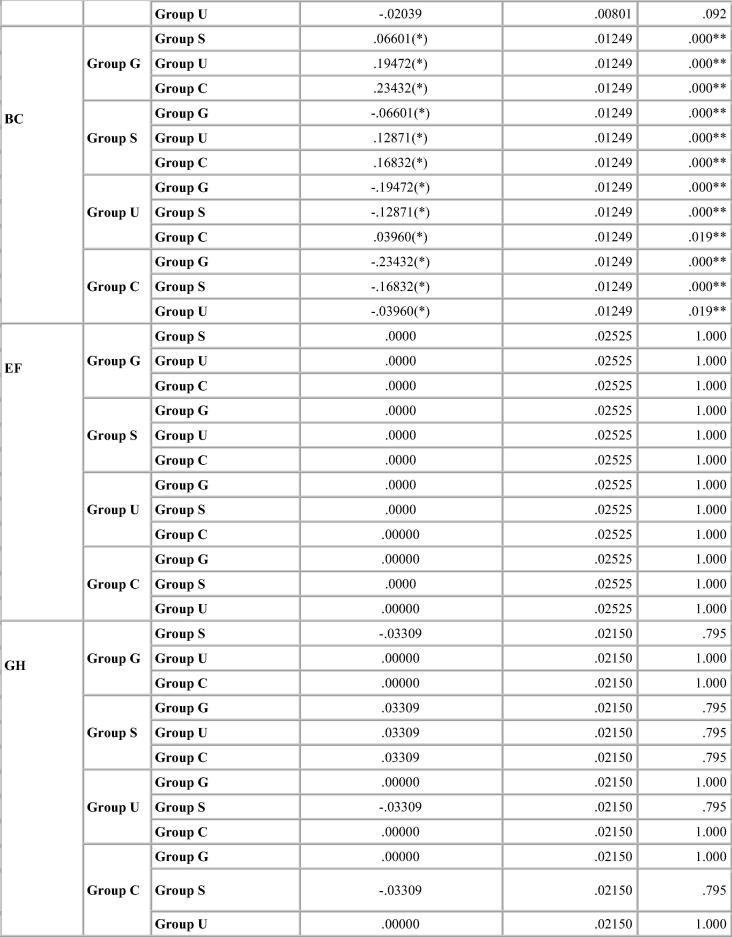
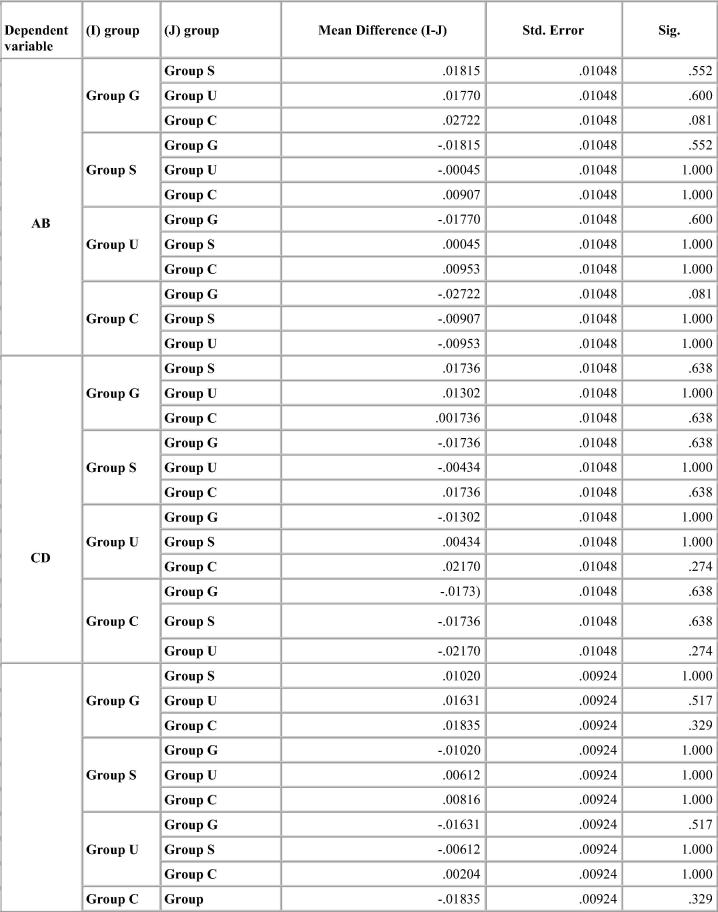
Dimensional change was expressed in micrometers and as percentage change from the standard mean. This facilitates comparison among the measurement of six dimensions selected because the distances on the model varied. A two-tailed t-test was carried out to test the significance in difference of the distances between the master model and the stone models (Table 4, Table 5, Table 6, Table 7). The two-tailed t test would document differences in either direction. One way analysis of variance (ANOVA) was used for multiple group comparison (Table 8) followed by the post hoc Bonferroni’test for pair wise comparison and the results were evaluated (Chart 1, Chart 2, Chart 3, Chart 4).
Table 4.
Statistical analysis of the dimensional variation in stone models produced after different disinfection systems for Algin-Gum.
| Test agents | Standard mean (mm) | Mean (mm) | % Deviation | Std. deviation | t value | df | Sig. (2-tailed) | Mean difference | |
|---|---|---|---|---|---|---|---|---|---|
| AB | Group G | 22.04 | 22.57 | 2.4183 | 0.16013 | 47.758 | 9 | 0.000 | 0.53300 |
| Group S | 22.42 | 1.7241 | 0.09323 | 58.481 | 9 | 0.000 | 0.38000 | ||
| Group U | 22.42 | 1.7287 | 0.03348 | 163.286 | 9 | 0.000 | 0.38100 | ||
| Group C | 22.41 | 1.7196 | 0.05838 | 93.147 | 9 | 0.000 | 0.37900 | ||
| CD | Group G | 23.038 | 23.60 | 2.4394 | 0.07088 | 108.831 | 9 | 0.000 | 0.56200 |
| Group S | 23.44 | 1.7753 | 0.07392 | 75.949 | 9 | 0.000 | 0.40900 | ||
| Group U | 23.44 | 1.7493 | 0.03203 | 172.714 | 9 | 0.000 | 0.40300 | ||
| Group C | 23.44 | 1.7449 | 0.03544 | 155.694 | 9 | 0.000 | 0.40200 | ||
| AD | Group G | 49.042 | 49.36 | 0.6586 | 0.03629 | 57.399 | 9 | 0.000 | 0.32300 |
| Group S | 49.42 | 0.7708 | 0.02884 | 84.523 | 9 | 0.000 | 0.37800 | ||
| Group U | 49.40 | 0.7320 | 0.01505 | 153.857 | 9 | 0.000 | 0.35900 | ||
| Group C | 49.40 | 0.7320 | 0.02624 | 88.231 | 9 | 0.000 | 0.35900 | ||
| BC | Group G | 30.30 | 30.84 | 1.8119 | 0.04246 | 134.927 | 9 | 0.000 | 0.54900 |
| Group S | 30.79 | 1.6205 | 0.03632 | 141.088 | 9 | 0.000 | 0.49100 | ||
| Group U | 30.71 | 1.3828 | 0.03282 | 133.242 | 9 | 0.000 | 0.41900 | ||
| Group C | 30.85 | 1.2706 | 0.04981 | 80.669 | 9 | 0.000 | 0.38500 | ||
| EF | Group G | 5.024 | 4.93 | −1.9506 | 0.26869 | −22.958 | 9 | 0.000 | −0.09800 |
| Group S | 4.93 | −1.9506 | 0.21397 | −28.829 | 9 | 0.000 | −0.09800 | ||
| Group U | 5.01 | −0.2767 | 0.00629 | −139.000 | 9 | 0.000 | −0.01390 | ||
| Group C | 5.01 | −0.0736 | 0.01639 | −14.212 | 9 | 0.000 | −0.00370 | ||
| GH | Group G | 3.022 | 2.97 | −1.5553 | 0.35742 | −13.760 | 9 | 0.000 | −0.04700 |
| Group S | 3.017 | −0.1655 | 0.22334 | −2.343 | 9 | 0.044 | −0.00500 | ||
| Group U | 3.019 | −0.0695 | 0.01878 | −11.699 | 9 | 0.000 | −0.00210 | ||
| Group C | 3.019 | −0.0695 | 0.01878 | −11.699 | 9 | 0.000 | −0.00210 |
Table 5.
Statistical analysis of the dimensional variation in stone models produced after different disinfection systems for vignette impression material.
| Groups | Standard mean (mm) | Mean (mm) | % Deviation | SD | t value | df | Sig. (2-tailed) | Mean difference | |
|---|---|---|---|---|---|---|---|---|---|
| AB | Group G | 22.04 | 22.3250 | 1.2931 | 0.08883 | 46.032 | 9 | 0.000 | 0.28500 |
| Group S | 22.2490 | 0.9483 | 0.03348 | 89.571 | 9 | 0.000 | 0.20900 | ||
| Group U | 22.2500 | 0.9528 | 0.03025 | 99.612 | 9 | 0.000 | 0.21000 | ||
| Group C | 22.2460 | 0.9347 | 0.06487 | 45.560 | 9 | 0.000 | 0.20600 | ||
| CD | Group G | 23.038 | 23.3810 | 1.4888 | 0.06924 | 67.998 | 9 | 0.000 | 0.34300 |
| Group S | 23.2320 | 0.8421 | 0.05715 | 46.597 | 9 | 0.000 | 0.19400 | ||
| Group U | 23.2290 | 0.8291 | 0.01388 | 188.913 | 9 | 0.000 | 0.19100 | ||
| Group C | 23.2300 | 0.8334 | 0.05012 | 52.581 | 9 | 0.000 | 0.19200 | ||
| AD | Group G | 49.042 | 49.331000 | 0.5893 | 0.02955 | 63.065 | 9 | 0.000 | 0.28900 |
| Group S | 49.319000 | 0.5648 | 0.02441 | 73.165 | 9 | 0.000 | 0.27700 | ||
| Group U | 49.247900 | 0.4198 | 0.01870 | 71.000 | 9 | 0.000 | 0.20590 | ||
| Group C | 49.330000 | 0.5873 | 0.03040 | 61.094 | 9 | 0.000 | 0.28800 | ||
| BC | Group G | 30.30 | 30.5100 | 0.6931 | 0.04667 | 46.957 | 9 | 0.000 | 0.21000 |
| Group S | 30.4490 | 0.4917 | 0.05914 | 26.294 | 9 | 0.000 | 0.14900 | ||
| Group U | 30.4510 | 0.4983 | 0.02435 | 64.714 | 9 | 0.000 | 0.15100 | ||
| Group C | 30.4500 | 0.4950 | 0.04667 | 33.541 | 9 | 0.000 | 0.15000 | ||
| EF | Group G | 5.024 | 4.9730 | −1.0151 | 0.18883 | −17.000 | 9 | 0.000 | −0.05100 |
| Group S | 5.0190 | −0.0995 | 0.06364 | −4.945 | 9 | 0.001 | −0.00500 | ||
| Group U | 5.0109 | −0.2607 | 0.05665 | −14.556 | 9 | 0.000 | −0.01310 | ||
| Group C | 5.0198 | −0.0836 | 0.00839 | −31.500 | 9 | 0.000 | −0.00420 | ||
| GH | Group G | 3.022 | 2.9880 | −1.125 | 0.13952 | −25.500 | 9 | 0.000 | −0.03400 |
| Group S | 2.9900 | −1.0589 | 0.15599 | −21.466 | 9 | 0.000 | −0.03200 | ||
| Group U | 3.0290 | 0.2316 | 0.10580 | 6.923 | 9 | 0.000 | 0.00700 | ||
| Group C | 3.0300 | 0.2647 | 0.01560 | 53.666 | 9 | 0.000 | 0.00800 |
Table 6.
Statistical analysis of the dimensional variation in stone models produced after different disinfection systems for Ad-Sil Impression material.
| Groups | Standard mean (mm) | Mean (mm) | % Deviation | SD | t value | df | Sig. (2-tailed) | Mean difference | |
|---|---|---|---|---|---|---|---|---|---|
| AB | Group G | 22.04 | 22.1840 | 0.6534 | 0.07771 | 26.588 | 9 | 0.000 | 0.14400 |
| Group S | 22.1410 | 0.4583 | 0.06217 | 23.308 | 9 | 0.000 | 0.10100 | ||
| Group U | 22.1400 | 0.4537 | 0.03025 | 47.434 | 9 | 0.000 | 0.10000 | ||
| Group C | 22.1400 | 0.4537 | 0.05239 | 27.386 | 9 | 0.000 | 0.10000 | ||
| CD | Group G | 23.038 | 23.1500 | 0.4862 | 0.04575 | 33.600 | 9 | 0.000 | 0.11200 |
| Group S | 23.1500 | 0.4862 | 0.03069 | 47.518 | 8 | 0.000 | 0.11200 | ||
| Group U | 23.1490 | 0.4818 | 0.03203 | 47.571 | 9 | 0.000 | 0.11100 | ||
| Group C | 23.1510 | 0.4905 | 0.05197 | 29.847 | 9 | 0.000 | 0.11300 | ||
| AD | Group G | 49.042 | 49.3210 | 0.5689 | 0.01505 | 119.571 | 9 | 0.000 | 0.27900 |
| Group S | 49.3290 | 0.5852 | 0.01785 | 103.652 | 9 | 0.000 | 0.28700 | ||
| Group U | 49.3300 | 0.5873 | 0.01359 | 136.610 | 9 | 0.000 | 0.28800 | ||
| Group C | 49.3200 | 0.5669 | 0.02355 | 76.133 | 9 | 0.000 | 0.27800 | ||
| BC | Group G | 30.30 | 30.4700 | 0.5611 | 0.02695 | 65.841 | 9 | 0.000 | 0.17000 |
| Group S | 30.4500 | 0.4950 | 0.02695 | 58.095 | 9 | 0.000 | 0.15000 | ||
| Group U | 30.4110 | 0.3663 | 0.02435 | 47.571 | 9 | 0.000 | 0.11100 | ||
| Group C | 30.3990 | 0.3267 | 0.03282 | 31.482 | 9 | 0.000 | 0.09900 | ||
| EF | Group G | 5.024 | 5.0020 | −0.4379 | 0.08392 | −16.500 | 9 | 0.000 | −0.02200 |
| Group S | 5.0018 | −0.4419 | 0.07553 | −18.500 | 9 | 0.000 | −0.02220 | ||
| Group U | 5.0100 | −0.2787 | 0.00000 | A | NS | ||||
| Group C | 5.0100 | −0.2787 | 0.00000 | A | NS | ||||
| GH | Group G | 3.022 | 3.0100 | −0.3971 | 0.01560 | −80.498 | 9 | 0.000 | −0.01200 |
| Group S | 3.0110 | −0.3640 | 0.09359 | −12.298 | 9 | 0.000 | −0.01100 | ||
| Group U | 3.0100 | −0.3971 | 0.00000 | A | NS | ||||
| Group C | 3.0100 | −0.3971 | 0.01560 | −80.498 | 9 | 0.000 | −0.01200 |
A: t cannot be computed because the standard deviation is 0.
NS: Not significant.
Table 7.
Statistical analysis of the dimensional variation in stone models produced after different disinfection systems for Aquasil impression material.
| Groups | Standard mean (mm) | Mean (mm) | % Deviation | SD | t value | Df | Sig. (2-tailed) | Mean difference | |
|---|---|---|---|---|---|---|---|---|---|
| AB | Group G | 22.04 | 22.0760 | 0.1633 | 0.02343 | 22.045 | 9 | 0.000 | 0.03600 |
| Group S | 22.0720 | 0.1452 | 0.02870 | 16.000 | 9 | 0.000 | 0.03200 | ||
| Group U | 22.0721 | 0.1456 | 0.02857 | 16.120 | 9 | 0.000 | 0.03210 | ||
| Group C | 22.0700 | 0.1361 | 0.00302 | 142.302 | 9 | 0.000 | 0.03000 | ||
| CD | Group G | 3.038 | 23.0780 | 0.1736 | 0.01830 | 30.000 | 9 | 0.000 | 0.04000 |
| Group S | 23.0740 | 0.1563 | 0.02242 | 22.045 | 9 | <0.001** | 0.03600 | ||
| Group U | 22.0750 | 0.1606 | 0.02288 | 15.179 | 9 | <0.001** | 0.03200 | ||
| Group C | 23.0700 | 0.1389 | 0.02894 | 15.179 | 9 | 0.000 | 0.03200 | ||
| AD | Group G | 49.042 | 49.1150 | 0.1489 | 0.01982 | 23.754 | 9 | 0.000 | 0.07300 |
| Group S | 49.1100 | 0.1387 | 0.02355 | 18.623 | 9 | 0.000 | 0.06800 | ||
| Group U | 49.1070 | 0.1325 | 0.02160 | 19.403 | 9 | 0.000 | 0.06500 | ||
| Group C | 49.1060 | 0.1305 | 0.01719 | 24.000 | 9 | 0.000 | 0.06400 | ||
| BC | Group G | 30.30 | 30.3770 | 0.2541 | 0.01594 | 50.408 | 9 | 0.000 | 0.07700 |
| Group S | 30.3840 | 0.2772 | 0.02783 | 31.500 | 9 | 0.000 | 0.08400 | ||
| Group U | 30.3800 | 0.2640 | 0.03112 | 26.833 | 9 | 0.000 | 0.08000 | ||
| Group C | 30.3820 | 0.2706 | 0.02087 | 41.000 | 9 | 0.000 | 0.08200 | ||
| EF | Group G | 5.024 | 5.0200 | −0.0796 | 0.00000 | A | NS | ||
| Group S | 5.0200 | −0.0796 | 0.00000 | A | NS | ||||
| Group U | 5.0200 | −0.0796 | 0.00000 | A | NS | ||||
| Group C | 5.0200 | −0.0796 | 0.00938 | −26.833 | 9 | 0.000 | −0.00400 | ||
| GH | Group G | 3.022 | 3.019 | −0.0728 | 0.01395 | A | NS | ||
| Group S | 3.0200 | −0.0662 | 0.00000 | A | 9 | 0.000 | −0.00200 | ||
| Group U | 3.0200 | −0.0662 | 0.00000 | A | NS | ||||
| Group C | 3.0200 | −0.0662 | 0.01560 | −13.416 | 9 | 0.000 | −0.00200 |
A No statistics are computed for one or more split files.
A: t cannot be computed because the standard deviation is 0.
NS: Not significant.
The mean difference is highly significant at the .05 level.
Table 8.
One-way ANOVA.
| Material | F | Sig. | |
|---|---|---|---|
| Algin Gum | A to B (mm) | 124.017 | 0.000 |
| C to D (mm) | 365.784 | 0.000 | |
| A to D (mm) | 28.683 | 0.000 | |
| B-C (mm) | 351.826 | 0.000 | |
| E-F (mm) | 357.663 | 0.000 | |
| G-H (mm) | 118.969 | 0.000 | |
| Vignette | A to B (mm) | 85.767 | 0.000 |
| C to D (mm) | 397.433 | 0.000 | |
| A to D (mm) | 95.844 | 0.000 | |
| B-C (mm) | 46.453 | 0.000 | |
| E-F (mm) | 180.894 | 0.000 | |
| G-H (mm) | 434.207 | 0.000 | |
| Ad-Sil | A to B (mm) | 28.953 | 0.000 |
| C to D (mm) | 0.073 | 0.974 | |
| A to D (mm) | 3.540 | 0.024 | |
| B-C (mm) | 153.349 | 0.000 | |
| E-F (mm) | 27.195 | 0.000 | |
| G-H (mm) | 1.184 | 0.329 | |
| Aquasil | A to B (mm) | 2.361 | 0.088 |
| C to D (mm) | 3.743 | 0.019 | |
| A to D (mm) | 1.589 | 0.209 | |
| B-C (mm) | 1.597 | 0.207 | |
| E-F (mm) | 0.000 | 1.000 | |
| G-H (mm) | 1.000 | 0.404 |
Chart 1.
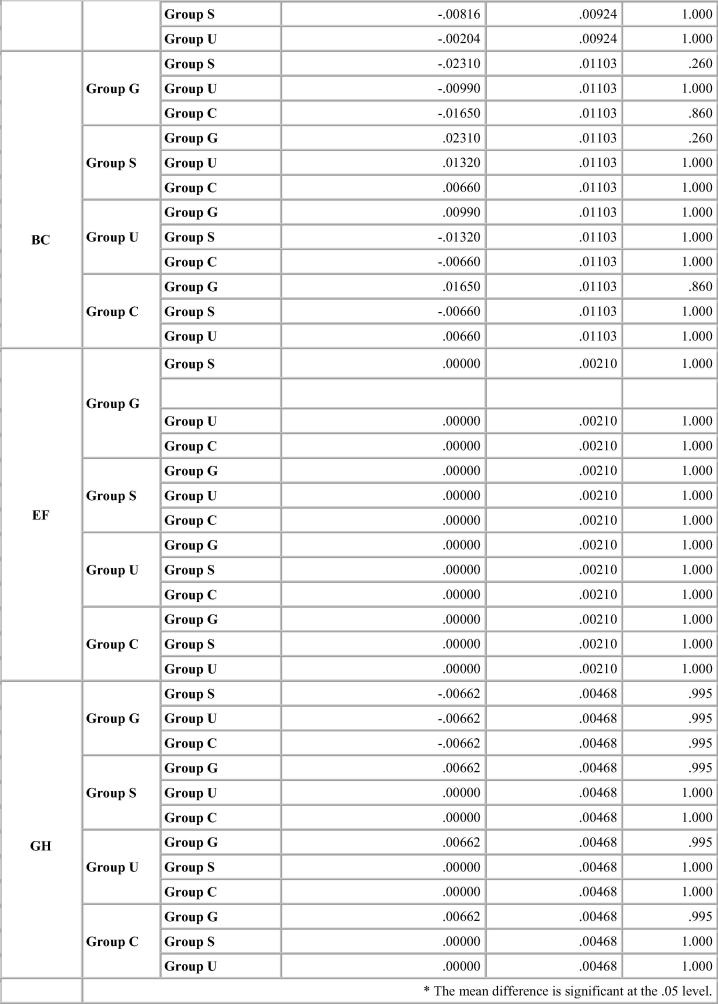
Bonferroni test for percentage deviation between different groups for Algin Gum.
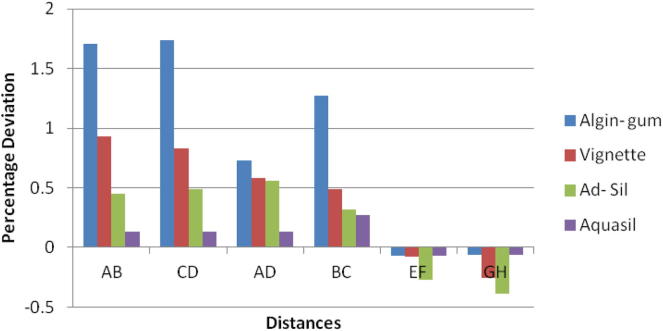
Chart 2.
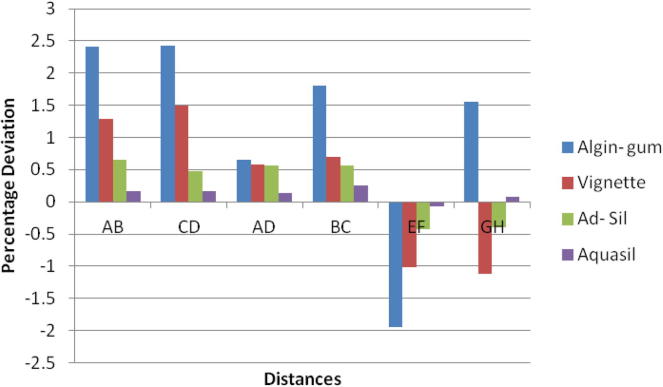
Bonferroni test for percentage deviation between different groups for Vignette.
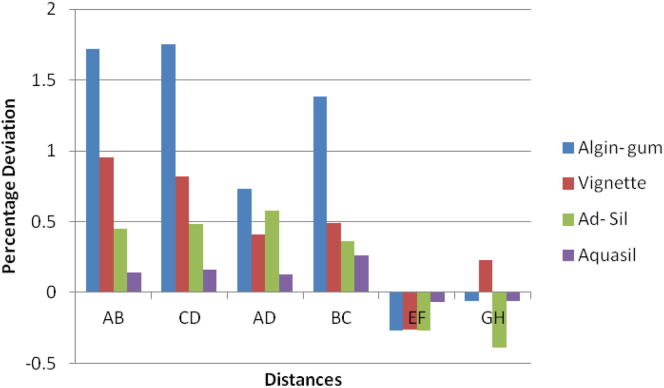
Chart 3.
Bonferroni test for percentage deviation between different groups for Ad-sil.
Chart 4.
Bonferroni test for percentage deviation between different groups for Aquasil.
3. Results
Changes in the interabutment and cross arch dimensions of the stone casts were compared with that of metal die for all impression materials studied (Table 4, Table 5, Table 6, Table 7). The effect of each disinfection system amongst the impression materials is depicted graphically in Graph 1, Graph 2, Graph 3, Graph 4. Expansion was noted amongst all impression materials for interabutment and cross arch measurements. Contraction was generally observed for occluso-gingival dimensions of the canine and molar abutments resulting in shorter dies.
Graph 1.
Multiple bar diagram showing group wise comparison of the percentage deviation of the selected dimensions for control group for different Impression materials taken.
Graph 2.
Multiple bar diagram showing group wise comparison of the percentage deviation of the selected dimensions for glutaraldehyde immersion system for different Impression materials taken.
Graph 3.
Multiple bar diagram showing group wise comparison of the percentage deviation of the selected dimensions for sodium hypochlorite immersion system for different Impression materials taken.
Graph 4.
Multiple bar diagram showing group wise comparison of the percentage deviation of the selected dimensions for ultraviolet disinfection system for different Impression materials taken.
For Algin–Gum, Glutaraldehyde immersion showed maximum percentage deviation from the standard values with expansion in the interabutment distance and cross arch measurements. Sodium hypochlorite immersion showed same results as the control group for the interabutment distance but showed expansion for the cross arch measurements. UV chamber showed minimum deviation with values close to that of control group. The change in vertical dimension for both canine and molar abutment varied significantly with the immersion systems resulting in shorter dies. The change was more noticeable for canine abutment than the molar. p-value was significant for all disinfection systems including the control group (Table 4).
Vignette impression material showed expansion in linear dimensions. Maximum deviation was seen in the glutaraldehyde group. Ultraviolet chamber disinfection showed results similar to the control group. There was decrease in the canine abutment for the control group by 4–10 µm. But there was an expansion of the molar abutment for the control group and the ultraviolet chamber group by 8 µm resulting in longer dies. Shorter dies were seen for the glutaraldehyde and sodium hypochlorite immersion. p-value was significant for the sodium hypochlorite immersion and the glutaraldehyde immersion group (Table 5).
With Ad-Sil impressions, Glutaraldehyde immersion caused expansion in linear measurements while sodium hypochlorite and UV chamber showed similar deviation. The change was same for canine abutment and the molar amongst all the disinfection systems resulting in shorter dies. Variation in p value is depicted in Table 6. p-value was significant for glutaraldehyde immersion for all dimensions measured and for cross arch dimension (A-D) for all disinfection systems. The variations were statistically significant for some dimensions but within clinically acceptable limits (Table 6).
Aquasil impressions had linear variations of almost similar magnitude with the different disinfection systems. All disinfection systems showed values well within clinically acceptable limits. UV chamber showed minimum deviation with values close to the control group. The change was same for canine abutment than the molar amongst all the disinfection systems resulting in shorter dies. These changes were clinically insignificant (Table 7).
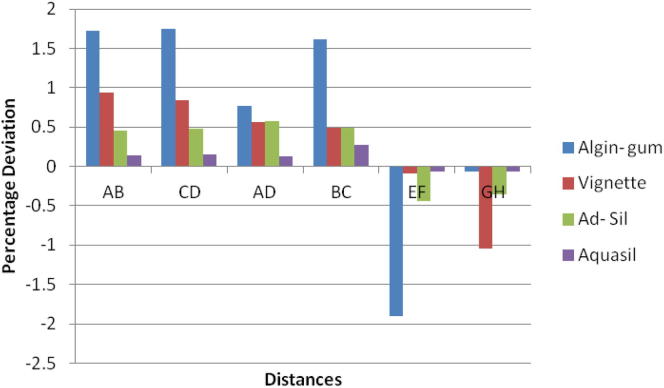
For the control Group, maximum deviation was seen for Algin Gum and minimum change in dimensions was noted for Aquasil for all dimensions. (Graph 1) Glutaraldehyde immersion showed significant change in values from control group for Algin Gum showing maximum deviation to 2.4% for AB and minimum change in dimensions was noted for Aquasil for all dimensions (Graph 2). Sodium hypochlorite immersion (Graph 3) and Ultraviolet disinfection (Graph 4) had similar values for values of distances selected for the impression materials studied.
For Algin-Gum and Vignette, significant value of unpaired t-test value was found between the control and the glutaraldehyde immersion for the interabutment (C-D) distance and also the cross arch distance (B-C) for control and sodium hypochlorite group. t-test was found to be significant with the disinfection systems used for the other three impression materials studied.
One way ANOVA (Table 8) for comparison of the distances between groups revealed a highly significant difference for each distance location for both Indian and International alginate brands. For Ad-Sil, value was significant for interabutment distance (A-B), intra-abutment distance (B-C) and canine abutment. For Aquasil impression material, all the values were insignificant (except for interabutment distance C-D).
Bonferroni’s test (Chart 1, Chart 2, Chart 3, Chart 4) for pairwise comparison of the mean distances between the different disinfection systems with the control group revealed insignificant differences in distances between sodium hypochlorite immersion and Ultraviolet disinfection system with values similar to that of the control group for all impression materials. Significant difference in distances between glutaraldehyde immersion and control group for Algin-Gum was noticed. For BC, almost all disinfection systems showed significant values. Vignette impression material showed significant values for glutaraldehyde immersion. Sodium hypochlorite immersion showed significant values for EF & GH (Chart 2).
Significant difference in interabutment distance AB between control group and glutaraldehyde immersion was seen for Ad-Sil impression material. Crossarch distance BC was significant for all groups (Chart 3).
There was no significant difference in mean distances studied for Aquasil for all disinfection systems and control group (Chart 4).
4. Discussion
In literature, two ways have been described to evaluate the dimensional stability of the impression materials: one by studying the stability of the impression material itself (Clancy et al., 1983, Valderhaug, 1984) and the other by measuring casts made from an impression (Chen et al., 2004, Purk et al., 1998, Lapria Faria et al., 2008, Marcinak et al., 1980, Federick and Caputo, 1997).
The latter should consider the setting expansion of the plaster which would compensate for part of the elastomer polymerization contraction. The ADA Council on Dental Materials and Devices No. 19 for elastomers has stipulated that dimensional stability of type I & III elastomers should not exceed 0.5% (Revised American Dental Association Specification, 1977). Materials used to fabricate the replica or working cast may also be subject to changes in dimension, such as gypsum expansion with setting. Although accuracy is affected by many factors, it should be realized that the magnitude of some of these changes may not be clinically significant (Johnson et al., 1998).
The results of this study showed that all the disinfection systems showed the dies to be shorter than the master model. A full arch model was preferred in order to simulate the intraoral conditions and the clinical procedures as opposed to the sectional model or the ruled die block (for evaluating dimensional changes) as recommended by the American Dental Association specification No. 19 (Revised American Dental Association Specification, 1977).
Implementation of infection control measures has become an essential part of the present day prosthodontic practice. As the impression materials used today were never designed to undergo disinfection or sterilisation, they might have untoward changes following the disinfection procedures as stated by Rios et al. (1996). Hence to maintain the precision in the made impressions, it is necessary to take care of disinfection procedures and study their effect on the dimensional stability of impression materials.
The test device used in the study differed from the one used by jig used by Holtan et al., 1991, Stauffer et al., 1976 as it had the metal die fixed onto a glass plate and not a metal plate with three guide posts along with metal cylinders. The other glass fibre plate had the mandibular stock tray fixed on to it and this was filled with impression material. This test device allowed for the placement of the stock tray over the metal die as it would have been in the patient’s mouth. The guide posts and the metal stops made sure that the amount of impression material over any particular aspect of the master die was uniform for every impression made.
One step putty wash impression technique was used for addition silicone, as it has been proven that the stability of the impressions made in metal stock tray was not inferior to the stability of the impressions made in custom made trays (Stauffer et al., 1976, Valderhaug, 1984, Hung et al., 1992).
In the past, the determination of accuracy was done by various means. They include the direct measurements on the impression itself or on the die. The use of die was preferred as it is easier to determine the dimensions especially the vertical dimensions even though it adds another variable of setting expansion. The accuracy of the die can be measured by the fit of a casting or by measuring the dimensions which can be a two dimensional analysis or a three dimensional analysis. The method of comparing linear measurements was preferred in this study. This may be done by contact or non contact methods. Contact methods include the use of micrometer, vernier caliper; dial gauge or linear variable differential transducers. The non contact methods include the use of traveling microscope, measuring microscope, profile projector and the laser digitizers. In this study, the linear dimensions were measured with the help of traveling microscope with an accuracy of 10 µm and the mean percentage deviation from the master model was used to compare the groups (Table 4, Table 5, Table 6, Table 7).
There was an increase in the inter-abutment and cross arch distance in the casts obtained from all the impression materials while there was decrease in the occluso-gingival height when compared to the standard mean. Compared with the controls, casts made from impressions with Vignette, Ad-Sil and Aquasil disinfected by sodium hypochlorite immersion and UV rays showed same dimensions for the interabutment and cross arch distance. Glutaraldehyde immersion caused maximum deviation with the Algin-Gum, Vignette and Ad-Sil impression material. For Aquasil, treatment with glutaraldehyde showed results similar to other disinfectants studied. Thus Aquasil could be disinfected with glutaradehyde, sodium hypochlorite immersion and UV rays without causing any significant dimensional changes.
For Algin-Gum, the values were statistically and clinically significant even in the control group. Percentage deviation from mean value for interabutment and cross arch was high with an average value of 1.7% and 1.3% respectively for the control group. Percentage deviation with both immersion systems was high. Glutaraldehyde immersion produced maximum change of 2.4% for the interabutment distance and 1.7% for the cross arch distance. Ultraviolet chamber showed results similar to that of the control group. Thus results clearly indicated that ultraviolet chamber disinfection was the recommended method of disinfection for this material. Results by Boylon et al. (1987) on disinfection by ultraviolet chamber also showed the same results as this study. They had advocated use of the ultraviolet chamber disinfection method as it reduced surface contamination and neither produced irritating vapours nor produced any dimensional changes.
Percentage deviation recorded with Vignette impression material was statistically significant for cross-arch dimensions and interabutment distance (A-D) but this was clinically insignificant for the purposes that irreversible hydrocolloid material is used. Vignette impression material can be immersed in sodium hypochlorite and disinfected with UV rays without compromising the dimensional accuracy required for casts for making diagnostic and opposing casts for fixed denture prosthesis; working casts for fabrication of occlusal splints and for removable prosthesis.
Percentage change with Vignette, Ad-Sil, Aquasil was in accordance to the study by Matyas et al. (1990). They had concluded that the standard deviation amongst the disinfected samples of irreversible hydrocolloids were high ranging to more than 1% while in case of elastomers, it was well within the clinically acceptable limits i.e. it was below 0.5% in all the treated samples. American Dental Association (ADA) specification No. 18 (dental alginate impression material) does not stipulate the maximum allowable percentage of dimensional change for alginate impression materials (ADA, 2009). Aquasil showed statistically and clinically insignificant values with all the disinfectant systems studied thus rendering it as a high precision material used routinely for impression of fixed partial dentures, precision attachments, implants and removable cast partial denture framework or complete dentures. This was also in accordance to a study done on different impression materials done by Jagger DC, Vowles RW, McNally L, Davis F, and O'Sullivan DJ where Aquasil putty/LV combination gave least percentage change for width in dimensional stability (Jagger et al., 2007).
The results of this study showed that all groups showed the dies to be shorter than the master model except for Vignette impression material where longer die was seen of the molar abutment for the control group and Ultraviolet rays disinfection. The shorter dies can be explained in accordance with the results of the study by Johnson et al. (1988). Stackhouse suggested that the models were shorter because the vertical component of contraction is in a direction towards the occlusal portion of the preparation where the impression adheres to the stock tray and the impression material provides a minimal yet uniform setting contraction. This could be expressed as percentage shrinkage towards the perforations in the stock tray or in the tray adhesive (Stackhouse, 1970). The second reason could be the use of a subgingival taper which would contribute to the reduced height by the incomplete elastic recovery.
The marginal fit acceptability ranges from 50 µm (Schillinburg et al., 1997) to 90 µm (Thongthammachat et al., 2002) as the distortion occurred within the limit of the periodontal ligament space (90–240 µm). Minute discrepancies of several microns are not considered clinically significant as the crystalline structure of die stone used does not reproduce details to that accuracy (Derrien and Le Menn, 1995), and the margin of error of the microscope is within this range. Marginal discrepancies of cemented artificial crowns as large as 50 µm are considered to be clinically acceptable (Abbate et al., 1989).
In this study, the interaction of disinfection systems and impression material was statistically significant according to the ANOVA (Table 5) except for Aquasil impression material. However, these statistical results represent extremely minute differences in the means and thus there should be no clinical relevance to these small deviations despite the statistically significant interactions.
An error on the positive side wherein the die is longer than the master model would be still preferable as a shorter die prepares a shorter casting which fails to fit leading to a greater marginal discrepancy (Jagger et al., 2007). However, the maximum variation which amounted to 15 µm in the addition silicone of International brand in this study could be taken care of by the die spacer which is normally kept 20–40 µm (Johnson et al., 1988).
Limitations in the study were that methodology of measurement used had an accuracy of 10 µm only. On each cast the occluso-gingival, inter-abutment and cross arch measurements were measured separately. When considering the possibility of twisting, bending and stretching deformation, the 2-Dimensional analysis is not sufficient as when an impression twists or bends or stretches, it may not appear to have changed in one plane but when all the planes were considered together, deformation may be apparent.
There are methods described for 3-Dimensional analysis with a microscope. However they are complicated, more time consuming and the operator errors still exist. The best way to analyse would be using an optic digitizer which can compare distortion over the surface of an arch and between distinct points, reduce operator error through robotic operation and automation and thus provide greater precision allowing 3 -dimensional measurements (Quick et al., 1992). But these are extremely expensive and there is need for customized software to compare the data.
5. Conclusion
-
•
Native alginate produced statistically and clinically significant deviation from the mean for all linear dimensions measured with all the disinfectant groups and the control group. Vignette irreversible hydrocolloid can be immersed in sodium hypochlorite and disinfected with UV rays without compromising the accuracy needed for diagnostic and opposing casts; check impressions and fabrication of provisional crowns.
-
•
Ad-Sil showed dimensional changes but within clinically acceptable limits. Aquasil was found to be most stable with all the disinfection systems used.
-
•
New disinfection methods such as Ultraviolet chamber can thus be the recommended method for disinfecting impressions without compromising their dimensional stability.
-
•
Addition silicone Ad- Sil from developing country showed acceptable results.
Conflict of interest
We have no conflict of interest to declare.
Ethical statement
The work has been approved by the appropriate ethical committees related to the institution(s) in which it was performed and it was an in Vitro study. This study was in accordance with institution guidelines.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abbate M.F., Tjan A.H., Fox W.M. Comparison of the marginal fit of various ceramic crown systems. J. Prosthet. Dent. 1989;61:527–531. doi: 10.1016/0022-3913(89)90270-9. [DOI] [PubMed] [Google Scholar]
- ADA Council on Scientific Affairs and ADA Council on Dental Practice Infection control recommendations for the dental office and the dental laboratory. J. Am. Dent. Assoc. 1996;127:672–680. doi: 10.14219/jada.archive.1996.0280. [DOI] [PubMed] [Google Scholar]
- ADA specification no. 18-1992. Dental alginate impression material. http://webstore.ansi.org/RecordDetail.aspx?sku=ADA+Specification+No.+18-1992. [accessed Nov. 19, 2009].
- Boylon R.J., Goldstein G.R., Schulman A. Evaluation of an ultraviolet disinfection unit. J. Prosthet. Dent. 1987;58:650–654. doi: 10.1016/0022-3913(87)90403-3. [DOI] [PubMed] [Google Scholar]
- Chen S.Y., Liang W.M., Chen F.N. Factors affecting the accuracy of elastomeric impression materials. J. Dent. 2004;32:603–609. doi: 10.1016/j.jdent.2004.04.002. [DOI] [PubMed] [Google Scholar]
- Clancy J.M., Scandrett F.R., Ettinger R.L. Long-term dimensional stability of three current elastomers. J. Oral. Rehabil. 1983;10:325–333. doi: 10.1111/j.1365-2842.1983.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Derrien G., Le Menn G. Evaluation of detail reproduction for three die materials by using scanning electron microscopy and two-dimensional protilometry. J. Prosthet. Dent. 1995;74:1–7. doi: 10.1016/s0022-3913(05)80221-5. [DOI] [PubMed] [Google Scholar]
- Durr D.P., Novak E.V. Dimensional stability of alginate impressions immersed in disinfecting solutions. ASDC. J. Dent. Child. 1987;54:45–48. [PubMed] [Google Scholar]
- Federick D.R., Caputo A. Comparing the accuracy of reversible hydrocolloid and elastomeric impression materials. J. Am. Dent. Assoc. 1997;128:183–188. doi: 10.14219/jada.archive.1997.0162. [DOI] [PubMed] [Google Scholar]
- Herrara S.P., Merchant V.A. Dimensional stability of dental impressions after immersion disinfection. J. Am. Dent. Assoc. 1986;113:419–422. doi: 10.14219/jada.archive.1986.0214. [DOI] [PubMed] [Google Scholar]
- Holtan J.R., Olin P.S., Rudney J.D. Dimensional stability of a polyvinylsiloxane impression material following ethylene oxide and steam autoclave sterilisation. J. Prosthet. Dent. 1991;65:519–525. doi: 10.1016/0022-3913(91)90292-5. [DOI] [PubMed] [Google Scholar]
- Hung S.H., Purk J.H., Tira D.E., Eick J.D. Accuracy of one-step versus two-step putty wash addition silicone impression technique. J. Prosthet. Dent. 1992;67:583–589. doi: 10.1016/0022-3913(92)90151-y. [DOI] [PubMed] [Google Scholar]
- Ishida H., Nahara Y., Tamamoto M., Hamada T. The fungicidal effect of ultraviolet light on impression materials. J. Prosthet. Dent. 1991;65:532–535. doi: 10.1016/0022-3913(91)90295-8. [DOI] [PubMed] [Google Scholar]
- Jagger D.C., Vowles R.W., McNally L., Davis F., O'Sullivan D.J. The effect of a range of disinfectants on the dimensional accuracy and stability of some impression materials. Eur. J. Prosthodont. Restor. Dent. 2007;15:23–28. [PubMed] [Google Scholar]
- Johnson G.H., Drennon D.G., Powell G.L. Accuracy of elastomeric impressions disinfected by immersion. J. Am. Dent. Assoc. 1988;116:525–530. doi: 10.14219/jada.archive.1988.0307. [DOI] [PubMed] [Google Scholar]
- Johnson G.H., Chellis K.D., Gordon G.E., Lepe X. Dimensional stability and detail reproduction of irreversible hydrocolloid and elastomeric impressions disinfected by immersion. J. Prosthet. Dent. 1998;79:446–453. doi: 10.1016/s0022-3913(98)70160-x. [DOI] [PubMed] [Google Scholar]
- Kern M., Rathmer R.M., Strub J.R. Three dimensional investigation of the accuracy of impression materials after disinfection. J. Prosthet. Dent. 1993;70:449–456. doi: 10.1016/0022-3913(93)90084-2. [DOI] [PubMed] [Google Scholar]
- Lapria Faria A.C., Silveira Rodriguez R.C., Macedo A.P., Chiarello M., Faria Ribeiro R. Accuracy of stone casts obtained by different impression materials. Braz. Oral. Res. 2008;22:293–298. doi: 10.1590/s1806-83242008000400002. [DOI] [PubMed] [Google Scholar]
- Lepe X., Johnson G.H. Accuracy of polyether and addition silicone after long term disinfection. J. Prosthet. Dent. 1997;78:245–249. doi: 10.1016/s0022-3913(97)70021-0. [DOI] [PubMed] [Google Scholar]
- Marcinak C.F., Young F.A., Draughn R.A., Flemming W.R. Linear dimensional changes in elastic impression materials. J. Dent. Res. 1980;59:1152–1155. doi: 10.1177/00220345800590071001. [DOI] [PubMed] [Google Scholar]
- Matyas J., Dao N., Caputo A.A., Lucatorto F.M. Effects of disinfectants on dimensional accuracy of impression materials. J. Prosthet. Dent. 1990;64:25–31. doi: 10.1016/0022-3913(90)90148-6. [DOI] [PubMed] [Google Scholar]
- Merchant V.A. Infection control and Prosthodontics. J. Calif. Dent. Assoc. 1989;17:49–53. [PubMed] [Google Scholar]
- Minagi S., Fukushima K., Maeda N., Satomi K., Ohkawa S., Akagawa Y. Disinfection methods for impression materials: Freedom from hepatitis B and acquired immunodeficiency syndrome. J. Prosthet. Dent. 1986;56:451–454. doi: 10.1016/0022-3913(86)90387-2. [DOI] [PubMed] [Google Scholar]
- Purk J.H., Willes M.G., Tira D.E., Eick J.D., Hung S.H. The effects of different storage conditions on polyether and polyvinylsiloxane impressions. J. Am. Dent. Assoc. 1998;129:1014–1021. doi: 10.14219/jada.archive.1998.0356. [DOI] [PubMed] [Google Scholar]
- Quick D.C., Holtan J.R., Ross G.K. Use of a scanning laser three-dimensional digitizer to evaluate dimensional accuracy of dental impression materials. J. Prosthet. Dent. 1992;68:229–235. doi: 10.1016/0022-3913(92)90319-6. [DOI] [PubMed] [Google Scholar]
- Revised American Dental Association Specification No. 19 for Non- Aqueous Elastomeric Dental Impression Materials. J. Am. Dent. Assoc. 1977; 94: 733–741. [DOI] [PubMed]
- Rios M.P., Morgano S.M., Stein R.S., Rose L. Effects of chemical disinfectant solutions on the stability and accuracy of the dental impression complex. J. Prosthet. Dent. 1996;76:356–362. doi: 10.1016/s0022-3913(96)90538-7. [DOI] [PubMed] [Google Scholar]
- Schillinburg H.T., Hobo S., Whitsett L.D., Jacobi R., Brackettn S.E. Quintessence Publication Co, Inc; Chicago, IL: 1997. Fundamentals of Fixed Prosthodontics. third ed., 281-307,313,392. [Google Scholar]
- Setcos J.C., Peng L., Palenik C.J. The effect of disinfection procedures on an alginate impression material. J. Dent. Res. 1984;63:235. [Google Scholar]
- Stackhouse J.A., Jr. The accuracy of stone dies made from rubber impression materials. J. Prosthet. Dent. 1970;24:377–386. doi: 10.1016/0022-3913(70)90078-8. [DOI] [PubMed] [Google Scholar]
- Stauffer J.P., Meyer J.M., Nally J.N. Accuracy of six elastic impression materials used for complete arch fixed partial dentures. J. Prosthet. Dent. 1976;35:407–415. doi: 10.1016/0022-3913(76)90008-1. [DOI] [PubMed] [Google Scholar]
- Thongthammachat S., Moore B.K., Barco M.T., 2nd, Hovijitra S., Brown D.T., Andres C.J. Dimensional accuracy of dental casts: influence of tray material, impression material and time. J. Prosthodont. 2002;11:98–108. [PubMed] [Google Scholar]
- Thouati A., Deveaux E., Iost A., Behin P. Dimensional stability of seven elastomeric impression materials immersed in disinfectants. J. Prosthet. Dent. 1996;76:8–14. doi: 10.1016/s0022-3913(96)90338-8. [DOI] [PubMed] [Google Scholar]
- Tullner J.B., Commette J.A., Moon P.C. Linear dimensional changes in dental impressions after immersion in disinfectant solutions. J. Prosthet. Dent. 1988;60:725–728. doi: 10.1016/0022-3913(88)90407-6. [DOI] [PubMed] [Google Scholar]
- Valderhaug J., Odont, Floystrand F. Dimensional stability of elastomeric impression materials in custom- made and stock trays. J. Prosthet. Dent. 1984;52:514–517. doi: 10.1016/0022-3913(84)90336-6. [DOI] [PubMed] [Google Scholar]