Abstract
Scleroderma, known also as systemic sclerosis (SSc), is a severe disease associated with high mortality rates, and right ventricular (RV) remodeling and dysfunction, along with pulmonary artery hypertension (PAH), are among the most important internal organ manifestations of this disease. PAH has a higher prevalence in patients with SSc compared to the general population and represents a significant predictor of mortality in SSc. In patients with SSc, the morphological remodeling and alteration of RV function begin even before the setting of PAH and lead to development of a specific adaptive pattern of the RV which is different from the one recorded in patients with IAPH. These alterations cause worse outcomes and increased mortality rates in SSc patients. Early detection of RV dysfunction and remodeling is possible using modern imaging tools currently available and can indicate the initiation of specific therapeutic measures before installation of PAH. The aim of this review is to summarize the current knowledge related to mechanisms involved in the remodeling and functional alteration of the RV in SSc patients.
1. Introduction
Scleroderma, known also as systemic sclerosis (SSc), is a severe disease associated with high mortality rates, and right ventricular (RV) remodeling and dysfunction, along with pulmonary artery hypertension (PAH), are among the most important internal organ manifestations of this disease [1].
The mortality of SSc depends primary on the burden of the internal organ involvement. Pulmonary hypertension (PH), pulmonary fibrosis, and kidney damage are the most frequent causes of death in SSc [2, 3]. Myocardial, pericardial, and pulmonary artery (PA) involvement is observed in 1 out of 4 patients and serve as predictors of mortality in SSc, as approximately 30% of the SSc deaths are attributed to cardiac causes, followed by respiratory causes (17%) [4].
SSc is a complex autoimmune disease characterized by a marked chronic inflammation, which leads to increased connective tissue fibrosis and vascular involvement causing severe damage of internal organs (mainly the heart, lungs, gastrointestinal tract, kidneys, and muscles) and the skin [5].
Activation of the immune system, linked with fibroblast dysfunction, T lymphocyte, macrophage, and mast cell disturbances induce the alteration of the extracellular matrix by secretion of cytokines, chemokines, growth factors, and other potent mediators resulting in excessive collagen deposit in the tissues [6]. The exact etiology and trigger of the disease still remain unknown, but many studies suggest that genetic and environmental factors (silica, solvent, or radiation exposure) play an important role in the pathophysiology of SSc, generating changes in the expression of deoxyribonucleic acid (DNA) and microribonucleic acid (miRNA), which ultimately lead to inadequate activation of the immune system with phenotypic transformation of various cell types [7, 8]. Infiltration of the microvasculature accompanied by endothelial dysfunction and fibrosis initiates platelet activation and thrombosis causing ischemia of the surrounding tissues [9, 10]. It has been suggested that eNOS G894T polymorphism is correlated with an increased risk of SSc and PAH as well [11, 12].
The various clinical form of SSc varies from limited cutaneous systemic sclerosis (lcSSc) or CREST (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasias) syndrome, to diffuse, sometimes fulminant forms with extended involvement of internal organs. The incidence of SSc greatly varies between different geographical regions and ethnic groups, with higher numbers among the African American population [13, 14]. The risk of developing SSc is significantly higher in woman than in men, with a 3–6 : 1 female to male ratio [15].
The aim of this review is to summarize the current knowledge related to mechanisms involved in the remodeling and functional alteration of the RV in SSc patients.
2. Systemic Sclerosis-Related Pulmonary Artery Hypertension (SSc-PAH)
PAH has a higher prevalence in patients with SSc compared to the general population, being more commonly observed in patients with lcSSc form, and represents a significant predictor of mortality in SSc [16, 17]. Current literature data suggests that the prevalence of PAH in this patient category reaches 12% and, despite the introduction of modern optimized treatments, the 3-year survival rate is still 52%, compared to 94% for SSc patients without PAH [18, 19]. At the same time one-year mortality rate of patients with SSc-PAH is around 30% versus 15% in patients with IPAH [20, 21]. Despite the fact that hemodynamic changes are less pronounced in SSc-PAH compared to idiopathic PAH (IPAH), SSc-related PAH is associated with twofold to threefold higher mortality risk, due to the systemic nature and complexity of the disease, added to a limited efficiency of the administered therapy [22, 23].
Argula et al. evaluated the functional changes of the RV during treatment in patients with SSc-PAH and IPAH at 3.8 and 1.95 years of follow-up. While the IPAH group showed significant improvement of the tricuspid annular plane systolic excursion (TAPSE [p = 0.01]), no such gain was observed in SSc-PAH patients, who also exhibited a worsening trend in tricuspid regurgitation jet velocity, right atrium (RA), and ventricular size [24].
In a comparative study Tedford et al. evidenced worse RV systolic function and prognosis in patients with SSc-PAH, compared to IPAH patients at similar RV afterload settings, suggesting the presence of an intrinsic systolic dysfunction in SSc patients [1]. The increase of the pulmonary artery pressure is a result of the alteration of the pulmonary microvascular bed triggered by the chronic inflammation, vasoconstriction, endothelial dysfunction, microthromboses, hypoxia, and excessive fibrous deposits at the level of the arterioles, causing increase of the pulmonary resistance [25, 26].
Hassoun et al. performed endomyocardial biopsies of the RV in SSc patients with and without PAH and compared these with samples from preserved ejection fraction heart failure (HFpEF) patients. Unpublished data from this study showed a significant decrease of the capillary density in SSc-PAH samples compared to the HFpEF and SSc without PAH samples. Furthermore, myocardial capillary density was significantly lower in samples from SSc patients with increased right atrial pressure (RAP), compared with lower RAP SSc samples, supporting the theory of structural RV alteration in these patients [27].
3. Right Ventricular Involvement in Scleroderma: A Distinctive Pattern
3.1. Right Ventricular Involvement in Scleroderma Patients without PAH
Cardiac manifestation of SSc includes involvement of the myocardium, pericardium, and the electrical conduction system, which may lead to ischemia, heart failure, pericardial effusion, and arrhythmias. RV failure can represent an important cause of mortality in SSc patients. It was believed that RV involvement is mainly linked to PAH, but recent studies suggest that SSc may have a direct impact on the RV structure and function, as alteration of RV function is more expressed in SSc-associated PAH than in non-SSc-PAH [28, 29]. The extent of cardiac involvement is likely to be underestimated, as autopsy studies identified considerable fibrotic changes of the myocardium in 70% of the examined patients [30]. Focal recurrent ischemia because of microvascular thrombosis, vasospasm thickening of the vascular wall, and fibrous deposits lead to irreversible functional and structural modifications of the myocardium [31, 32]. It is hard to differentiate the primary heart involvement from secondary development of these alterations due to PAH and kidney injuries. These changes may remain silent for long time (thus frequently underdiagnosed), but when clinically manifested it is described to represent an important negative prognostic factor [33].
Several studies tried to characterize RV involvement that occurs in SSc patients before development of PAH. Proper echocardiographic assessment of the RV function and volume estimation has been bound by its crescent shape, presence of intense trabeculations, and different contraction pattern, but novel techniques overcame these limitations [34]. Pigatto et al. evaluated the RV function of 45 SSc patients without any signs or symptoms of heart disease of PAH using three-dimensional echocardiography (3DE) and two-dimensional speckle-tracking echocardiography (2DSTE) and compared these findings with the similar parameters of 43 healthy subjects. A significant increase in RV size was observed in SSc patients with higher end-systolic volume [ESV, (p < 0.0001)], end-diastolic volume [EDV, (p = 0.049)], and reduced ejection fraction (p < 0.0001) determined by 3DE. Doppler measurements showed increased systolic pulmonary artery pressure (sPAP) and pulmonary vascular resistance (tPVR) in SSc patients compared to the control group. These changes were more pronounced in patients with lcSSc form [35].
Durmus et al. used 2DSTE for the assessment of RV function in patients with SSc without PAH. Significantly higher sPAP (within normal limits) was recorded in SSc patients (p = 0.002), accompanied by decreased RV systolic function with significantly lower TAPSE and tissue Doppler maximum systolic myocardial velocity (RVS′), but no correlation was observed between these parameters. Right ventricle global longitudinal strain (RVGLS) was also significantly lower in SSc patients compared to the control group (p < 0.001). An inverse correlation was observed between duration of the disease and TAPSE and RVGLS [36]. These findings consolidate the earlier results published by Schattke et al. who reported significantly decreased TAPSE and RVS′ determined by 2DSTE in SSc patients without PAH and identified isovolumetric acceleration (IVA) as the best predictor of early RV systolic impairment in this patient category [37].
Another early predictor of RV systolic dysfunction in SSc patients determined by 2DSTE may be represented by elevated longitudinal strain rates of the RV, which may serve as an adaptive response to even subtle elevation of the sPAP [38]. Furthermore these early systolic dysfunctions of the RV and RA may presage the sPAP elevation; thus it is important to detect them for the optimization of the further treatment of SSc patients, as recent trials show support for an early aggressive therapy [39–42].
3.2. The Distinctive Pattern of RV Remodeling and Function in Scleroderma Patients
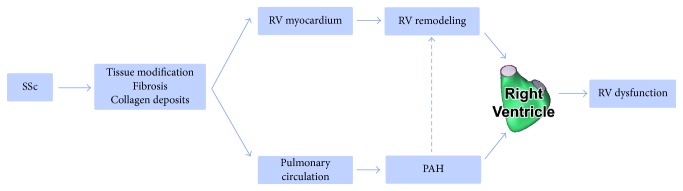
Functional and structural alterations of the RV develop even before the setting of PAH in SSc patients, suggesting a remodeling pattern different from the one usually recorded in IPAH. Poor response to optimal therapy was observed in SSc-PAH, ultimately leading to RV failure and death, generating higher mortality rates compared to IPAH patients [1, 43, 44]. These findings led to the emergence of a novel theory about a different adaptive functional and structural remodeling of the RV in SSc patients. The complex interrelation between the mechanisms involved in development of RV dysfunction and remodeling is summarized in Figure 1. Different clinical studies tried to characterize the clinical condition of patients with SSc with or without PAH and to identify predictors of RV involvement and dysfunction. The main clinical studies and their findings are presented in Table 1.
Figure 1.
The complex interrelation between the mechanisms involved in development of RV dysfunction and remodeling.
Table 1.
Main clinical studies addressing Ssc in patients with or without PAH.
| Imaging method | Author (year) | Number of patients | Clinical setting | Analyzed parameter | SSc | Controls | p |
|---|---|---|---|---|---|---|---|
| Echocardiography | Pigatto et al. (2015) [35] |
n = 88 | SSc versus healthy subjects | sPAP (mmHg) | 33 ± 14.0 | 22 ± 5.0 | <0.0001 |
| TAPSE (mm) | 23 ± 3.0 | 26 ± 2.0 | <0.0001 | ||||
| PVR (WU) | 1.9 ± 0.6 | 1.4 ± 0.3 | 0.001 | ||||
| global RVLS (%) | −24.8 ± 4.0 | −25.6 ± 3 | n.s. | ||||
| Mukherjee et al. (2016) [58] |
n = 178 | SSc versus healthy subjects | sPAP (mmHg) | 31.4 ± 13.3 | 22.6 ± 4.4 | 0.0001 | |
| TAPSE (mm) | 21.6 ± 4.7 | 22.5 ± 4.0 | 0.307 | ||||
| PVR (WU) | 1.48 ± 0.45 | 1.24 ± 0.26 | 0.002 | ||||
| global RVLS (%) | −17.7 ± 5.9 | −20.4 ± 2.4 | 0.005 | ||||
| Durmus et al. (2015) [36] |
n = 80 | SSc versus healthy subjects | sPAP (mmHg) | 24.2 ± 5.7 | 19.8 ± 6.2 | 0.002 | |
| TAPSE (mm) | 21.1 ± 3.2 | 24.3 ± 3.4 | <0.001 | ||||
| global RVLS (%) | −18.5 ± 4.9 | −21.8 ± 2.4 | <0.001 | ||||
|
| |||||||
| cMRI | Hachulla et al. (2009) [69] |
n = 52 | SSc-PAH versus SSc without PAH | RV hypertophy, n (%) | 2 (17) | 0 (0) | 0.04 |
| RV dilation, n (%) | 4 (33) | 7 (17) | 0.25 | ||||
| Mean RV EF (%) | 54 (13) | 50 (11) | 0.20 | ||||
| Mean RV EDV index (ml/mm2) | 75 (9) | 79 (23) | 0.67 | ||||
| Delayed contrast enhancement, n (%) | 1 (8) | 10 (26) | 0.42 | ||||
| Tzelepis et al. (2007) [70] |
n = 36 | Abnormal versus normal 24-h ECG in SSc | Delayed contrast enhancement, n (%) | 15 (78.9) | 9 (52.9) | 0.098 | |
| Number of enhancing segments, n | 5.4 ± 4.8 | 2.5 ± 2.9 | 0.035 | ||||
| Enhancement at RV insertion points, n (%) | 4 (21.1) | 2 (11.8) | 0.66 | ||||
| Kelemen et al. (2015) [46] |
n = 53 | SSc-PAH versus IPAH | RV mass (g) | 58.8 | 65.9 | 0.47 | |
| RV EDV index (ml/mm2) | 88.1 | 90.1 | 0.83 | ||||
| RV EF (%) | 46.0 | 41.6 | 0.29 | ||||
|
| |||||||
| Scintigraphy | Papagoras et al. (2014) [65] | n = 35 | SSc patients | Reversible myocardial perfusion defects, n of pts (%) | 21 (60) | - | - |
sPAP: systolic pulmonary artery pressure; TAPSE: tricuspid annular plane systolic excursion; PVR: pulmonary vascular resistance; RVLS: right ventricle longitudinal strain; EF: ejection fraction; EDV: end diastolic volume.
In an autopsy study Overbeek et al. analyzed the histological samples of the RV from SSc-PAH and IPAH patients and compared them with samples taken from healthy controls. Significantly more inflammatory cells were observed in interstitium of the RV from SSc-PAH patients compared to IPAH patients and controls; however the quantity of fibrosis did not show significant differences between the groups, suggesting that the underlying mechanism of RV dysfunction may have multiple factors [45].
Kelemen et al. assessed the RV remodeling of SSc-PAH and IPAH patients using cardiac magnetic resonance imaging (cMRI). No significant differences were recorded in terms of RV mass, RV-EDV, RV ejection fraction, stroke volume, or TAPSE between the two groups [46]. It has been demonstrated that SSc-PAH patients exhibit significantly less pronounced increase of the RV mass (assessed by right ventricular modeling index (RVMI) and volume mass index (VMI)), in response to increased RV load (assessed by PVR and (mean) mPAP) compared to IPAH patients, evidencing a different adaptive hypertrophy mechanism of these patients [46]. However, the benefits and disadvantages gained from RV hypertrophy in this patient category are still under debate [47]. In a recently published article Ramjug et al. did not confirm the aforementioned findings, as no significant differences of RV mass were recorded between SSc-PAH and IPAH patients at increased RV load. Nevertheless, they found a notable correlation between the VMI and PVR in SSc-PAH through the entire range of PVR, which may be a predictor for survival, as earlier studies suggested [48, 49].
The functional reserve of RV may also play an important role in the adaptive remodeling of the RV in SSc. Hsu et al. assessed RV function and morphology during exercise (or atrial pacing) testing in 15 SSc-PAH and 9 IPAH patients with comparable resting RV parameters. The RV contractility of the IPAH group was significantly increased during exercise, while the SSc-PAH group did not display improved contractility leading to important increase of RV-EDV and RV-ESV. The authors attribute these changes to decreased calcium cycling in the myocytes of SSc-PAH group (p = 0.03). The abnormal RV-PA coupling and diminished force-frequency responsiveness (FFR) of these patients during exercise, along with reduced contractile and diastolic reserve, plead for the depletion of RV functional reserve of SSc-PAH patients. These results highlight the importance of intrinsic RV dysfunction, which ultimately leads to RV failure in SSc-PAH patients [50].
Kovacs et al. determined the pulmonary exercise hemodynamics of SSc patients with a 4-year follow-up period, observing significant increase of mPAP (p = 0.02) and PVR (p = 0.002) during exercise, with no changes in resting mPAP values. These findings illustrate the progressive nature of the disease and point out that alterations of exercise hemodynamic parameters precede the routinely determined resting values. The aforementioned changes might be related to the distinctive morphological and functional remodeling pattern of the RV in SSc patients [51].
4. Imaging Tools for Characterization of the RV in Scleroderma
Different imaging technologies are currently available for characterization of RF function and morphology, the most used ones being represented by echocardiography, scintigraphy, and cardiac magnetic resonance imaging (cMRI) (Table 1).
4.1. Echocardiography
Given the high prevalence of RV impairment in scleroderma patients, current guidelines recommend early echocardiographic screening of SSc patients at risk for PAH. Immediate initiation of optimal therapy and prevention of right heart failure can improve the prognosis and survival of patients with SSc-PAH [26]. Transthoracic echocardiography (TTE) is a widely available imaging method used for a proper functional and morphological assessment of the RV; however, a considerable number of patients remain undiagnosed until late stages of heart failure, presumably because of the complex geometry of the RV. Conventional 2D echocardiography along with Doppler technology is able to evaluate RV diameters, areas, and volumes, as well as pressures, velocities, and gradients across the cardiac chambers and valves [52].
Different echocardiographic biomarkers have been proposed for characterization of RV function and can serve for evaluation of ventricular dysfunction in patients with SSc. TAPSE is an easily determinable parameter which is considered the most accurate in the assessment of the global RV contractility (albeit it is influenced by load and structure). It correlates with the parameters obtained from right heart catheterization and was proved as a prognostic factor in SSc-PAH [53, 54]. Another useful parameter for the assessment of RV and global ventricular function is represented by the Tei index, or myocardial performance index (expressed as isovolumetric contraction time and isovolumetric relaxation time divided ejection time), which uses pulsed wave Doppler velocities of ventricular inflow and outflow to calculate ventricular performance. Several studies validated its correlation with lower survival rates in SSc patients [55–57]. Tissue Doppler imaging (TDI) has the advantage to determine myocardial velocities, allowing detection of systolic and diastolic deformations (defined as strain); therefore it is able to assess segmental and global RV function. The major drawback of this method is the dependence of the Doppler beam angle [34, 39].
Novel echocardiographic modalities based on speckle tracking technology allow a better identification of subclinical heart failure. The technique is a software-based method that allows the calculation of tissue velocity and deformations of the myocardium derived from 2D images, tracking pixels (speckles) of a certain myocardial sector along the cardiac cycle. It can detect the changes in length of the tracked segment during systole and diastole with calculation of strain and strain rate (SR) parameters. These parameters offer an accurate, operator, and angle-independent view of the segmental and global (systolic and diastolic) function of the ventricles. Multiple studies suggest that RV longitudinal strain values and patterns are helpful in the assessment of RV function in SSc and PAH patients and correlate well with the prognosis of this patient category [57–61].
4.2. Scintigraphy
The evaluation of the right ventricular function with nuclear imaging and scintigraphy can determine myocardial damage caused by SSc in early stages by identification of small perfusion defects. A ten-year survival study conducted by Steen et al. showed that SSc patients that presented perfusion defects upon scintigraphy examination with thallium presented a higher rate of cardiac disease and mortality [62]. Furthermore, 99 m Technetium ventriculography performed on 42 patients without PAH revealed a significantly lower RV ejection fraction compared to controls, both at baseline and at 2 hours from administration of 40 mg of oral Nicardipine [63]. Additionally, patients with SSc may present the so-called “myocardial Raynaud's phenomenon” that leads to transient ischemic episodes induced during the cold pressor test. Lekakis et al. performed dipyridamole-thallium-201 scintigraphy during cold pressor testing and found that scleroderma patients presented transient myocardial ischemia induced by cold and that subjects with Raynaud's phenomenon of under 5 years did not present any defects [64]. A combined echocardiography and scintigraphy research study by Papagoras et al. reported that 60% of patients presented reversible myocardial perfusion defects in spite of the lack of clinically active heart disease, even in younger patients [65].
Although scintigraphy has been proven to be a sensitive method for detection of early perfusion defects in systemic sclerosis patients with no cardiac symptomatology, further studies are needed to elucidate its role in establishing prognosis and therapeutic management, which may be a hard task, since nuclear imaging methods present high costs and are not widely available.
4.3. Cardiac MRI
cMRI is a noninvasive imaging modality that faced a tremendous development in the last decade, being able to provide high quality images on the anatomy and function of the heart [66–68]. cMRI has been shown to offer a significant aid in the diagnosis of several inflammatory processes affecting the myocardium as well as in fibrosis detection and quantification in a great number of pathologies, including SSc [69–73]. cMRI currently is the gold standard method for evaluation of RV parameters, and several studies have focused on the role of cardiac magnetic resonance for assessing right and left ventricular function, the extent and pattern of myocardial fibrosis, and perfusion abnormalities in SSc subjects [69, 70, 74]. Bezante et al. aimed to research the myocardial effects of SSc by cMRI imaging in 50 patients with scarce or no clinical signs of heart failure. The study showed that both the right and left ventricular ejection fractions were reduced compared to controls (p < 0.001 and p < 0.009, resp.), and the RV ejection fraction matched for body surface area was significantly reduced in subjects with diffuse compared to limited cutaneous SSc [74]. Tzelepis et al. sought to evaluate the distribution and pattern of the fibrotic involvement of the myocardium on 41 patients with SSc with the use of Delayed-Enhancement cMRI (DE-CMR) with gadolinium [70]. Their results revealed that 66% of the enrolled patients presented late enhancement, predominantly in the middle region of the myocardium wall, with a sparing of the endo- and epicardial regions, predominantly in the middle and basal areas of the left ventricle, with a noncoronary distribution. As for the right ventricular involvement, 17% of patients presented globular, intermittent enhanced areas in the RV insertion points, independent of the presence of PAH, upon echocardiographic assessment [70].
Hachulla et al. found that 75% of SSc patients presented cMRI abnormalities in their study population and that 21% of patients presented an altered RV ejection fraction, while 17% showed RV dilation in the absence of PAH. Moreover, they observed the thinning of the left ventricular myocardium that affected primarily patients with diffuse cutaneous SSc and those with no PAH, suggesting that this might be caused by disease's chronic effect on the microvasculature of the heart, being similar to the thinning that occurs during the ventricular remodeling process secondary to infarction [69].
Magnetic resonance imaging of the heart is also a useful tool in evaluating the effects of various therapies in patients with PAH, as well as for prognosis implications. Allanore et al. have evaluated the effect of bosentan on the perfusion and function of the myocardium by performing cMRI and Tissue Doppler Echocardiography on 18 SSc patients with no PA and no symptoms of impaired cardiac function. The study found that cMRI perfusion index and the echocardiography parameters were improved after 4 weeks of bosentan administration [75]. This shows that an improved myocardial function and perfusion can be revealed in SSc patients by highly sensitive imaging methods such as cMRI, which is a valuable noninvasive, nonirradiating, and highly reproducible imaging method useful in this patient population.
5. Conclusions
In patients with SSc, the morphological remodeling and alteration of RV function begin even before the setting of PAH and lead to development of a specific adaptive pattern of the RV which is different from the one recorded in patients with IAPH. These alterations cause worse outcomes and increased mortality rates in SSc patients. Early detection of RV dysfunction and remodeling is possible using modern imaging tools currently available and can indicate the initiation of specific therapeutic measures before installation of PAH.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this article.
References
- 1.Tedford R. J., Mudd J. O., Girgis R. E., et al. Right ventricular dysfunction in systemic sclerosis-associated pulmonary arterial hypertension. Circulation: Heart Failure. 2013;6(5):953–963. doi: 10.1161/CIRCHEARTFAILURE.112.000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung L., Farber H. W., Benza R., et al. Unique Predictors of Mortality in Patients With Pulmonary Arterial Hypertension Associated With Systemic Sclerosis in the REVEAL Registry. CHEST. 2014;146(6):1494–1504. doi: 10.1378/chest.13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winstone T. A., Assayag D., Wilcox P. G., et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: A systematic review. CHEST. 2014;146(2):422–436. doi: 10.1378/chest.13-2626. [DOI] [PubMed] [Google Scholar]
- 4.Elhai M., Meune C., Boubaya M., et al. Mapping and predicting mortality from systemic sclerosis. Annals of the Rheumatic Diseases. 2017 doi: 10.1136/annrheumdis-2017-211448. [DOI] [PubMed] [Google Scholar]
- 5.Romanowska-Próchnicka K., Walczyk M., Olesińska M. Recognizing systemic sclerosis: Comparative analysis of various sets of classification criteria. Reumatología Clínica. 2016;54(6):296–305. doi: 10.5114/reum.2016.64906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sakkas L. I. New developments in the pathogenesis of systemic sclerosis. Autoimmunity. 2005;38(2):113–116. doi: 10.1080/16066350500095415. [DOI] [PubMed] [Google Scholar]
- 7.Barsotti S., Stagnaro C., d'Ascanio A., Della Rossa A. One year in review 2016: Systemic sclerosis. Clinical and Experimental Rheumatology. 2016;34:3–13. [PubMed] [Google Scholar]
- 8.Gilbane A. J., Denton C. P., Holmes A. M. Scleroderma pathogenesis: A pivotal role for fibroblasts as effector cells. Arthritis Research & Therapy. 2013;15(3, article no. 215) doi: 10.1186/ar4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matucci-Cerinic M., Kahaleh B., Wigley F. M. Review: evidence that systemic sclerosis is a vascular disease. Arthritis & Rheumatology. 2013;65(8):1953–1962. doi: 10.1002/art.37988. [DOI] [PubMed] [Google Scholar]
- 10.Jimenez S. A. Role of endothelial to mesenchymal transition in the pathogenesis of the vascular alterations in systemic sclerosis. ISRN Rheumatology. 2013;2013:15. doi: 10.1155/2013/835948.835948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fatini C., Gensini F., Sticchi E., et al. High prevalence of polymorphisms of angiotensin-converting enzyme (I/D) and endothelial nitric oxide synthase (Glu298Asp) in patients with systemic sclerosis. American Journal of Medicine. 2002;112(7):540–544. doi: 10.1016/S0002-9343(02)01069-0. [DOI] [PubMed] [Google Scholar]
- 12.Togǎnel R., Muntean I., Duicu C., Fǎgǎrǎşan A., Gozar L., Bǎnescu C. The role of eNOS and AGT gene polymorphisms in secondary pulmonary arterial hypertension in Romanian children with congenital heart disease. Revista Romana de Medicina de Laborator. 2013;21(3-4):267–274. doi: 10.2478/rrlm-2013-0031. [DOI] [Google Scholar]
- 13.Barnes J., Mayes M. D. Epidemiology of systemic sclerosis: Incidence, prevalence, survival, risk factors, malignancy, and environmental triggers. Current Opinion in Rheumatology. 2012;24(2):165–170. doi: 10.1097/BOR.0b013e32834ff2e8. [DOI] [PubMed] [Google Scholar]
- 14.Gelber A. C., Manno R. L., Shah A. A., et al. Race and association with disease manifestations and mortality in scleroderma : A 20-year experience at the johns hopkins scleroderma center and review of the literature. Medicine (United States) 2013;92(4):191–205. doi: 10.1097/MD.0b013e31829be125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen C., Bérezné A., Baubet T., et al. Association of gender with clinical expression, quality of life, disability, and depression and anxiety in patients with systemic sclerosis. PLoS ONE. 2011;6(3) doi: 10.1371/journal.pone.0017551.e17551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Pavec J., Humbert M., Mouthon L., Hassoun P. M. Systemic sclerosis-associated pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2010;181(12):1285–1293. doi: 10.1164/rccm.200909-1331PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mullin C. J., Mathai S. C. New insights into the recognition, classification and management of systemic sclerosis-associated pulmonary hypertension. Current Opinion in Rheumatology. 2017;29(6):561–567. doi: 10.1097/BOR.0000000000000440. [DOI] [PubMed] [Google Scholar]
- 18.Lefèvre G., Dauchet L., Hachulla E., et al. Survival and prognostic factors in systemic sclerosis–associated pulmonary hypertension: a systematic review and meta-analysis. Arthritis & Rheumatology. 2013;65(9):2412–2423. doi: 10.1002/art.38029. [DOI] [PubMed] [Google Scholar]
- 19.Tyndall A. Systemic sclerosis in Europe: first report from the EULAR Scleroderma Trials And Research (EUSTAR) group database. Annals of the Rheumatic Diseases. 2005;64(7):1107–1107. doi: 10.1136/ard.2005.036038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinchcliff M., Fischer A., Schiopu E., Steen V. D. Pulmonary hypertension assessment and recognition of outcomes in scleroderma (PHAROS): Baseline characteristics and description of study population. The Journal of Rheumatology. 2011;38(10):2172–2179. doi: 10.3899/jrheum.101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonneau G., Gatzoulis M., Adatia I. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology. 2013;62(25, supplement) doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 22.Fisher M. R., Mathai S. C., Champion H. C., et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis & Rheumatology. 2006;54(9):3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 23.Campo A., Mathai S. C., Le Pavec J., et al. Hemodynamic predictors of survival in scleroderma-related pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2010;182(2):252–260. doi: 10.1164/rccm.200912-1820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Argula R. G., Karwa A., Lauer A., et al. Differences in right ventricular functional changes during treatment between systemic sclerosis-associated pulmonary arterial hypertension and idiopathic pulmonary arterial hypertension. Annals of the American Thoracic Society. 2017;14(5):682–689. doi: 10.1513/AnnalsATS.201608-655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin V., Channick R. N., Ghofrani H.-A., et al. Bosentan added to sildenafil therapy in patients with pulmonary arterial hypertension. European Respiratory Journal. 2015;46(2):405–413. doi: 10.1183/13993003.02044-2014. [DOI] [PubMed] [Google Scholar]
- 26.Galiè N., Humbert M., Vachiery J. L., et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) European Heart Journal. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 27.Hassoun P. M. The right ventricle in scleroderma (2013 Grover conference series) Pulmonary Circulation. 2015;5(1):3–14. doi: 10.1086/679607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathai S. C., Bueso M., Hummers L. K., et al. Disproportionate elevation of N-terminal pro-brain natriuretic peptide in scleroderma-related pulmonary hypertension. European Respiratory Journal. 2010;35(1):95–104. doi: 10.1183/09031936.00074309. [DOI] [PubMed] [Google Scholar]
- 29.Overbeek M. J., Lankhaar J.-W., Westerhof N., et al. Right ventricular contractility in systemic sclerosis-associated and idiopathic pulmonary arterial hypertension. European Respiratory Journal. 2008;31(6):1160–1166. doi: 10.1183/09031936.00135407. [DOI] [PubMed] [Google Scholar]
- 30.Follansbee W. P., Miller T. R., Curtiss E. I., et al. A controlled clinicopathologic study of myocardial fibrosis in systemic sclerosis (scleroderma) The Journal of Rheumatology. 1990;17(5):656–662. [PubMed] [Google Scholar]
- 31.Meune C., Vignaux O., Kahan A., Allanore Y. Heart involvement in systemic sclerosis: evolving concept and diagnostic methodologies. Archives of Cardiovascular Diseases. 2010;103(1):46–52. doi: 10.1016/j.acvd.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 32.Fernandes F., Ramires F. J. A., Arteaga E., Ianni B. M., Bonfá E. S. D. O., Mady C. Cardiac remodeling in patients with systemic sclerosis with no signs or symptoms of heart failure: An endomyocardial biopsy study. Journal of Cardiac Failure. 2003;9(4):311–317. doi: 10.1054/jcaf.2003.51. [DOI] [PubMed] [Google Scholar]
- 33.Lambova S. Cardiac manifestations in systemic sclerosis. World Journal of Cardiology. 2014;6(9):p. 993. doi: 10.4330/wjc.v6.i9.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kjærgaard J. Assessment of right ventricular systolic function by tissue Doppler echocardiography. Danish Medical Journal. 2012;59(3):p. B4409. [PubMed] [Google Scholar]
- 35.Pigatto E., Peluso D., Zanatta E., et al. Evaluation of right ventricular function performed by 3d-echocardiography in scleroderma patients. Reumatismo. 2015;66(4):259–263. doi: 10.4081/reumatismo.2014.773. [DOI] [PubMed] [Google Scholar]
- 36.Durmus E., Sunbul M., Tigen K., et al. Right ventricular and atrial functions in systemic sclerosis patients without pulmonary hypertension: Speckle-tracking echocardiographic study. Herz. 2015;40(4):709–715. doi: 10.1007/s00059-014-4113-2. [DOI] [PubMed] [Google Scholar]
- 37.Schattke S., Knebel F., Grohmann A., et al. Early right ventricular systolic dysfunction in patients with systemic sclerosis without pulmonary hypertension: A Doppler Tissue and Speckle Tracking echocardiography study. Cardiovascular Ultrasound. 2010;8(1, article no. 3) doi: 10.1186/1476-7120-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matias C., Isla L. P. D., Vasconcelos M., et al. Speckle-tracking-derived strain and strain-rate analysis: A technique for the evaluation of early alterations in right ventricle systolic function in patients with systemic sclerosis and normal pulmonary artery pressure. Journal of Cardiovascular Medicine. 2009;10(2):129–134. doi: 10.2459/JCM.0b013e32831af028. [DOI] [PubMed] [Google Scholar]
- 39.D'Alto M., Cuomo G., Romeo E., et al. Tissue Doppler imaging in systemic sclerosis: A 3-year longitudinal study. Seminars in Arthritis and Rheumatism. 2014;43(5):673–680. doi: 10.1016/j.semarthrit.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Hassoun P. M., Zamanian R. T., Damico R., et al. Ambrisentan and tadalafil up-front combination therapy in scleroderma-associated pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine. 2015;192(9):1102–1110. doi: 10.1164/rccm.201507-1398oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kovacs G., Olschewski H. Borderline pulmonary pressures in scleroderma—a pre-pulmonary arterial hypertension condition? Arthritis Research & Therapy. 2015;17(1):p. 123. doi: 10.1186/s13075-015-0649-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Andrea A., D’Alto M., Di Maio M., et al. Right atrial morphology and function in patients with systemic sclerosis compared to healthy controls: a two-dimensional strain study. Clinical Rheumatology. 2016;35(7):1733–1742. doi: 10.1007/s10067-016-3279-9. [DOI] [PubMed] [Google Scholar]
- 43.Rubenfire M., Huffman M. D., Krishnan S., Seibold J. R., Schiopu E., McLaughlin V. V. Survival in systemic sclerosis with pulmonary arterial hypertension has not improved in the modern era. CHEST. 2013;144(4):1282–1290. doi: 10.1378/chest.12-0653. [DOI] [PubMed] [Google Scholar]
- 44.Fisher M. R., Mathai S. C., Champion H. C., et al. Clinical differences between idiopathic and scleroderma-related pulmonary hypertension. Arthritis & Rheumatology. 2006;54(9):3043–3050. doi: 10.1002/art.22069. [DOI] [PubMed] [Google Scholar]
- 45.Overbeek M. J., Mouchaers K. T. B., Niessen H. M., et al. Characteristics of interstitial fibrosis and inflammatory cell infiltration in right ventricles of systemic sclerosis-associated pulmonary arterial hypertension. International Journal of Rheumatology. 2010;2010 doi: 10.1155/2010/604615.604615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelemen B. W., Mathai S. C., Tedford R. J., et al. Right ventricular remodeling in idiopathic and scleroderma-associated pulmonary arterial hypertension: Two distinct phenotypes. Pulmonary Circulation. 2015;5(2):327–334. doi: 10.1086/680356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrison A., Hatton N., Ryan J. J. The right ventricle under pressure: Evaluating the adaptive and maladaptive changes in the right ventricle in pulmonary arterial hypertension using echocardiography (2013 Grover conference series) Pulmonary Circulation. 2015;5(1):29–47. doi: 10.1086/679699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ramjug S., Hussain N., Hurdman J., et al. Idiopathic and Systemic Sclerosis-Associated Pulmonary Arterial Hypertension: A Comparison of Demographic, Hemodynamic, and MRI Characteristics and Outcomes. CHEST. 2017;152(1):92–102. doi: 10.1016/j.chest.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 49.Hagger D., Condliffe R., Woodhouse N., et al. Ventricular mass index correlates with pulmonary artery pressure and predicts survival in suspected systemic sclerosis-associated pulmonary arterial hypertension. Rheumatology. 2009;48(9):1137–1142. doi: 10.1093/rheumatology/kep187. [DOI] [PubMed] [Google Scholar]
- 50.Hsu S., Houston B. A., Tampakakis E., et al. Right ventricular functional reserve in pulmonary arterial hypertension. Circulation. 2016;133(24):2413–2422. doi: 10.1161/CIRCULATIONAHA.116.022082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovacs G., Avian A., Wutte N., et al. Changes in pulmonary exercise haemodynamics in scleroderma: A 4-year prospective study. European Respiratory Journal. 2017;50(1) doi: 10.1183/13993003.01708-2016.1601708 [DOI] [PubMed] [Google Scholar]
- 52.Hachulla E., Launay D., Yaici A., et al. Pulmonary arterial hypertension associated with systemic sclerosis in patients with functional class II dyspnoea: mild symptoms but severe outcome. Rheumatology. 2010;49(5):940–944. doi: 10.1093/rheumatology/kep449. [DOI] [PubMed] [Google Scholar]
- 53.Forfia P. R., Fisher M. R., Mathai S. C., et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine. 2006;174(9):1034–1041. doi: 10.1164/rccm.200604-547oc. [DOI] [PubMed] [Google Scholar]
- 54.Mathai S. C., Sibley C. T., Forfia P. R., et al. Tricuspid annular plane systolic excursion is a robust outcome measure in systemic sclerosis-associated pulmonary arterial hypertension. The Journal of Rheumatology. 2011;38(11):2410–2418. doi: 10.3899/jrheum.110512. [DOI] [PubMed] [Google Scholar]
- 55.Tei C. New non-invasive index for combined systolic and diastolic ventricular function. Journal of Cardiology. 1995;26(2):135–136. [PubMed] [Google Scholar]
- 56.Frea S., Capriolo M., Marra W. G., et al. Echo doppler predictors of pulmonary artery hypertension in patients with systemic sclerosis. Journal of Echocardiography. 2011;28(8):860–869. doi: 10.1111/j.1540-8175.2011.01467.x. [DOI] [PubMed] [Google Scholar]
- 57.Blessberger H., Binder T. Two dimensional speckle tracking echocardiography: Basic principles. Heart. 2010;96(9):716–722. doi: 10.1136/hrt.2007.141002. [DOI] [PubMed] [Google Scholar]
- 58.Mukherjee M., Chung S., Ton V. K., et al. Unique abnormalities in right ventricular longitudinal strain in systemic sclerosis patients. Circulation: Cardiovascular Imaging. 2016;9, article e003792(6) doi: 10.1161/CIRCIMAGING.115.003792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meris A., Faletra F., Conca C., et al. Timing and magnitude of regional right ventricular function: A speckle tracking-derived strain study of normal subjects and patients with right ventricular dysfunction. Journal of the American Society of Echocardiography. 2010;23(8):823–831. doi: 10.1016/j.echo.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Rudski L. G., Lai W. W., Afilalo J., et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography. 2010;23(7):685–713. doi: 10.1016/j.echo.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Muntean I., Benedek T., Melinte M., Suteu C., Togãnel R. Deformation pattern and predictive value of right ventricular longitudinal strain in children with pulmonary arterial hypertension. Cardiovascular Ultrasound. 2016;14(1, article no. 27) doi: 10.1186/s12947-016-0074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Steen V. D., Follansbee W. P., Conte C. G., Medsger T. A., Jr. Thallium perfusion defects predict subsequent cardiac dysfunction in patients with systemic sclerosis. Arthritis & Rheumatology. 1996;39(4):677–681. doi: 10.1002/art.1780390421. [DOI] [PubMed] [Google Scholar]
- 63.Meune C., Allanore Y., Devaux J.-Y., et al. High prevalence of right ventricular systolic dysfunction in early systemic sclerosis. The Journal of Rheumatology. 2004;31(10):1941–1945. [PubMed] [Google Scholar]
- 64.Lekakis J., Mavrikakis M., Emmanuel M. Cold-induced coronary Raynaud's phenomenon in patients with systemic sclerosis. Clinical and Experimental Rheumatology. 1998;16(2):135–140. [PubMed] [Google Scholar]
- 65.Papagoras C., Achenbach K., Tsifetaki N., Tsiouris S., Fotopoulos A., Drosos A. A. Heart involvement in systemic sclerosis: A combined echocardiographic and scintigraphic study. Clinical Rheumatology. 2014;33(8):1105–1111. doi: 10.1007/s10067-014-2666-3. [DOI] [PubMed] [Google Scholar]
- 66.Hendel R. C., Patel M. R., Kramer C. M., et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. Journal of the American College of Cardiology. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 67.Ridgway J. P. Cardiovascular magnetic resonance physics for clinicians: Part i. Journal of Cardiovascular Magnetic Resonance. 2010;12(1, article no. 71) doi: 10.1186/1532-429X-12-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Biglands J. D., Radjenovic A., Ridgway J. P. Cardiovascular magnetic resonance physics for clinicians: Part II. Journal of Cardiovascular Magnetic Resonance. 2012;14(1, article no. 66) doi: 10.1186/1532-429X-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hachulla A.-L., Launay D., Gaxotte V., et al. Cardiac magnetic resonance imaging in systemic sclerosis: A cross-sectional observational study of 52 patients. Annals of the Rheumatic Diseases. 2009;68(12):1878–1884. doi: 10.1136/ard.2008.095836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tzelepis G. E., Kelekis N. L., Plastiras S. C., et al. Pattern and distribution of myocardial fibrosis in systemic sclerosis: A delayed enhanced magnetic resonance imaging study. Arthritis & Rheumatology. 2007;56(11):3827–3836. doi: 10.1002/art.22971. [DOI] [PubMed] [Google Scholar]
- 71.Vignaux O., Allanore Y., Meune C., et al. Evaluation of the effect of nifedipine upon myocardial perfusion and contractility using cardiac magnetic resonance imaging and tissue Doppler echocardiography in systemic sclerosis. Annals of the Rheumatic Diseases. 2005;64(9):1268–1273. doi: 10.1136/ard.2004.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Olimulder M. A., van Es J., Galjee M. A. The importance of cardiac MRI as a diagnostic tool in viral myocarditis-induced cardiomyopathy. Netherlands Heart Journal. 2009;17(12):481–486. doi: 10.1007/BF03086308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heydari B., Jerosch-Herold M., Kwong R. Y. Assessment of Myocardial Ischemia with Cardiovascular Magnetic Resonance. Progress in Cardiovascular Diseases. 2011;54(3):191–203. doi: 10.1016/j.pcad.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bezante G. P., Rollando D., Sessarego M., et al. Cardiac magnetic resonance imaging detects subclinical right ventricular impairment in systemic sclerosis. The Journal of Rheumatology. 2007;34:2431–2437. [PubMed] [Google Scholar]
- 75.Allanore Y., Meune C., Vignaux O., Weber S., Legmann P., Kahan A. Bosentan increases myocardial perfusion and function in systemic sclerosis: A magnetic resonance imaging and Tissue-Doppler echography study. The Journal of Rheumatology. 2006;33(12):2464–2469. [PubMed] [Google Scholar]