Abstract
Aim
The aim of the study was to evaluate the effect of resin infiltration on colour changes and surface roughness of artificial white spot lesions (WSLs) on maxillary and mandibular premolar.
Materials and methods
Sixty (60) extracted sound Maxilla (Mx) and Mandibular (Mn) premolars were randomly divided into 2 groups (test and control). Artificial WSLs were produced on buccal surface of teeth and were immersed in artificial saliva for 8 weeks. Colour components (L∗, a∗, b∗) and surface roughness (Sa∗) were assessed on 40 teeth using colour difference meter RD-100 and Alicona® Infinite Focus profilometer respectively. The measurements were done at baseline (T1), directly after artificial WSLs (T2), after 24 hours immersed in saliva and application of resin (T3) and immersion in artificial saliva for 1 (T4), 2 (T5), 4 (T6), 6 (T7) and 8 (T8) weeks. SEM images analysis were carried out on 20 teeth in four time points.
Results
The values of L∗ (lightness), b∗ (yellow/blue) and Sa∗ (surface roughness) are gradually reduced to the baseline value. Whereas, the value of a∗ gradually increased with distinct treatment time to achieve the baseline value. The higher value of L∗ and Sa∗, the whiter the lesion suggesting higher degree of enamel demineralization and surface roughness. Lower L∗ values suggest a masking colour effect.
Conclusion
The material produced favorable esthetics on colour and the surface roughness of teeth at distinct treatment times. It is recommended to be used to improve WSL post orthodontic treatment.
Keywords: Esthetics, Infiltrant, Surface roughness, White spot lesion
1. Introduction
Demineralization of enamel resulted in a visible change in the appearance of tooth enamel. It starts to lose its gloss and shine and take an opaque and chalky-white appearance which is known as white spot lesions (WSLs; Thylstrup and Fejerskov, 1978). The demineralization surface characteristics of the enamel usually contribute to pigment precipitation, such as roughness and porosity causing enamel discolouration that can lead to unsatisfactory aesthetic (Heymann and Grauer, 2013, Murphy et al., 2007, Hattab et al., 1999). Furthermore, surface roughness plays an important role in plaque retention (Taher et al., 2012) and also bacterial adherence (Hattab et al., 1999, Hattab et al., 1999, An and Friedman, 1998, Chapman et al., 2010). Overtime, colonization of acidogenic bacteria resulted in an active caries lesions (Chapman et al., 2010, Chang et al., 1997).
Orthodontic patients developed significantly more WSLs than non-orthodontic patients (Ogaard, 1989, Hadler-Olsen et al., 2012). The highest incidence of WSLs was recorded on the gingival areas of maxillary lateral incisor, canine, premolars and first molar, as well as mandibular premolars and first molars (Khalaf, 2014). WSLs often occured during fixed appliance treatment partly due to irregular surfaces of attachments such as brackets, bands and wires which created a stagnant areas for plaque accumulation. These attachments made conventional plaque removal more difficult, especially at areas adjacent to the brackets. In addition, plaque clearance by saliva and cheeks were also reduced (Mattousch et al., 2007). These WSLs has always been a concern to the orthodontist, patients, and parents (Ballard et al., 2013, Maxfield et al., 2012). Some WSLs might remineralize and improve to a visually acceptable appearance. However, some WSLs may persist and resulted in an unacceptable esthetic appearance where restorative treatment might be required.
Several ways of remineralization have been suggested such as using saliva, mouth rinses, and toothpastes containing increased fluoride concentrations. Casein phosphopeptide amorphous calcium phosphate, and calcium sodium phosphosilicate glass have also been suggested to increase remineralization (Ballard et al., 2013). Nevertheless, WSLs that are left untreated after removal of a fixed orthodontic appliance can reduce in size naturally without intervention (Benson et al., 2005, Willmot, 2008). However, the lesions appear to be very resistant to complete remineralization that they often persist and remain visible both clinically and radiographically which still triggers esthetic concerns (Ogaard, 1989).
Due to the reversible nature of WSLs, less invasive options are more preferred (Paris et al., 2013). The caries infiltration technique was introduced to fill the intercrystalline spaces with a low-viscosity resin, to arrest lesions (Paris et al., 2007a) without drilling (Taher et al., 2012, Chapman et al., 2010, Mueller et al., 2006). Caries infiltration is an approach between preventive and restorative actions in the treatment of non-cavitated carious lesions (Ogaard et al., 1998). Resin infiltration creates a diffusion barrier within the enamel lesion and reinforced the weakened demineralized enamel structure with the resin matrix, preventing cavity formation (Paris et al., 2013, Paris et al., 2007b, Kielbassa et al., 2009). Moreover, resin infiltration method produces a positive effect in masking the colour of teeth. This resulted in the lesions losing their whitish appearance hence looking similar to sound enamel (Paris et al., 2013, Paris et al., 2007b, Kielbassa et al., 2009, Yetkiner et al., 2014, Torres et al., 2011). The masking effect of enamel caries is cause by infiltrating the lesions using resins with a similar refractive index (RI of infiltrant: 1.52) to sound enamel. Thus, light scattering is reduced and visual colour differences to enamel decreased (Paris et al., 2013).
Differences in incidence of WSL between the arch and teeth have been reported. Studies showed that incidence of WSLs is higher in maxillary teeth and symmetrical between right and left quadrant (Lovrov et al., 2007, Julien, et al., 2013). However, there is lack of literature which shows the differences of resin infiltration response between maxillary and mandibular teeth. Therefore, this study sought to test the hypothesis that under in vitro demineralizing (WSLs) characteristics, there will be difference between maxillary and mandibular teeth in comparison with controls in respond to the infiltration resins. The study aims to measure the colour component and surface roughness of maxillary and mandibular premolar teeth in distinct saliva immersion of time. The effect of the infiltrant resin on artificial WSLs was also compared between maxilla and mandibular teeth with control.
2. Materials and methods
2.1. Protocol Approval, study design, and samples
This study was approved by the UKM, Research Ethics Committee [Code DD/2013/002(2)]. Sixty (N = 60) extracted premolars; sound mandibular (n = 30) and maxillary (n = 30) premolars with no restoration, decalcification or obvious caries were collected from general practitioners and orthodontics clinics. The teeth were thoroughly cleaned with ultrasonic scaler and slurry pumice to remove all soft-tissue remnants, calculus and plaque and stored in normal saline.
The maxilla and mandibular teeth were randomly separated into two groups. Fifteen teeth of maxilla and mandible, each allocated into test and control group; maxilla test (MxT = 15), maxilla control (MxC = 15), mandibular test (MnT = 15) and mandibular control (MnC = 15). Each tooth was assigned with an identification number. All samples were demineralized with phosphoric etching acid material (Ormco, CA) as described by (Ogaard et al.,1998), to produce a WSL on the buccal surface. Prior the study, the WSL production method was verified by exposing 3 premolars to the demineralization solution for 14 days, followed by sectioning and measuring the lesion depth with polarized microscopy (Scanning Electron Microscope, SEM; QuantaTM 250 FEG, US). Lesion depth was found to be near 100 μm.
The samples (N = 60) were then immersed in artificial saliva (BioXtra Mouthrinse UK-ri/V2/18.4.12/MD) of pH7 at 37 °C for 24 hours to stimulate oral environment. After 24 hours, experimental resin infiltrant (Icon; DMG, Hamburg Germany) was applied on the buccal surface of the teeth in test group (MxT and MnT) which has WSL. No resin was applied in the control group (MxC and MnC). All of the samples were again reimmersed in artificial saliva for 8 weeks. The artificial saliva was changed once a day for the whole immersion duration. In each group, only 10 teeth were assessed for colour component (L*, a*, b*) and surface roughness (Sa*). Whereas 5 teeth were used for SEM images analysis.
2.2. Colour and surface roughness measurement
Colour and surface roughness of each tooth was assessed at 8 time points. The time point were at baseline before WSL (T1), after WSL (T2), 24 hours immersion in artificial saliva and application of resin (T3), and after immersion in the artificial saliva at weeks 1 (T4), 2 (T5), 4 (T6), 6 (T7) and at the end of 8 (T8) weeks.
Colour measurements of each specimen were assessed using spectrophotometer (Colour Difference Meter RD-100; Topcon, Japan). All measurements were performed in the same examination room with standardized lighting conditions. Prior to each measurement, the spectrophotometer was calibrated using a white calibration plate supplied by the manufacturer. The calibration process compensated for any deviation in the quantity of illumination output from the internal light source. The spectrophotometric data of each tooth were recorded twice by positioning, removing, and repositioning the measuring head on the buccal surface of each tooth. The instrument will display an absolute value for each tooth. The colour measurements were quantified using coordinate value L∗, a∗, b∗ established by the Commission International de l’Eclariage, 1978 (CIE) and the methods described by Johnston and Kao, 1989.
The InfiniteFocus G4 Microscope (IFM; Alicona Imaging, Grambach/Graz, Austria) which provides 3D surface measurement was used to measure the surface roughness of buccal surface of each tooth. The images were taken at the magnifications of 20× with vertical resolutions of 638 μm. Surface roughness was characterized by the average roughness (Sa∗; µm), which represents the arithmetical average value of 3D roughness. Surface roughness was determined using Surface Texture Software Measurement (INCA, Oxford Instruments Analytical UK)
2.3. Examiners
The operators' (WKH, LWJ) reliability of measuring the lesion depth using the spectrophotometer was carried out on 3 teeth at 2 weeks interval. The ICC result ranged from 0.995 to 1.0 (p < .01), for both consistency and absolute agreement, indicating an excellence level of agreement. The actual measurements were taken twice. If the differences between the two measurements were apparent, a third reading was made and the aberrant one was discarded. The mean of the two closest measurements was used in the calculation.
2.4. Infiltration treatment
The infiltration procedure was performed for each tooth in the test group (MxT and MnT) according to the manufacturer’s instructions.
2.5. Scanning electron microscope (SEM)
SEM images analysis was carried on 5 premolars of each group. The premolar was embedded in an epoxy resin and sectioned with a low-speed diamond saw (IsoMet® 4000 Linear Precision Saw, USA) in a buccolingual direction. Both buccal and cross sectional view of the specimens were examined under scanning electron microscope (SEM; QuantaTM FEG 250, FEI, Eidhoven, The Netherlands). Images were gathered at the following points: baseline (T1), after WSL (T2), after 24 hours immersion in saliva and with or without resin infiltrantion (T3), after immersion in saliva at week 1 (T4) and week 2 (T5).
2.6. Statistical analysis
Data was analyzed using the Statistical Package for Social Science Package Software (SPSS) version 21.0. Normal distribution was tested using the Shapiro-Wilk test prior to data analysis. As the data were not normally distributed, Kruskal-Wallis test was applied to analyse possible differences between the time points within maxilla and mandibular groups. Level of significance was set at p < .05 to determine significant change in parameter values of test group and control group and also between times points (T2, T3, T4, T5, T6, T7, T8) and T1 (baseline). The visible colour changes was calculate; ΔE = [(L1∗ − L2∗)2 + (a1∗ − a2∗)2 + (b1∗ − b2∗)2]1/2. ΔE was accepted clinically detectable when it exceeded 3.7 units (Johnston and Kao, 1989).
3. Results
Median values and interquartile range (IQR) of colour changes at baseline (T1), WSL formation (T1), after 24 hours immersion in saliva and infiltrant application (T2), immersion in saliva for 1 weeks (T3), 2 weeks (T4), 3 weeks (T5), 4 weeks (T6), 6 weeks (T7) and 8 weeks (T8) for maxillary (MxT and MxC) and mandibular (MnT and MnC) teeth are presented in Table 1, Table 2.
Table 1.
Median and interquartile range (IQR) value of colour measurements for maxilla.
| Time | Group | L* |
a* |
b* |
Sa* |
||||
|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | p | Median (IQR) | p | Median (IQR) | p | Median (IQR) | p | ||
| T1 | 1 | 48.85(44.85;53.66) | 0.890 | 13.43(10.54;16.27) | 0.012** | −80.53(−103.08;−67.06) | 0.783 | 4.99(3.67;5.21) | 0.039** |
| 2 | 49.49(43.07; 53.76) | 16.37(15.75;17.15) | −89.59(−104.44;−74.38) | 5.80(4.57;7.42) | |||||
| T2 | 1 | 56.29(52.22;61.20) | 0.679 | 6.63(5.67;7.19) | 0.008** | −77.56(−94.93;−67.89) | 0.124 | 6.47(5.13;7.93) | 0.039** |
| 2 | 56.57(52.85; 62.93) | 8.23(7.29;8.91) | −56.24(−95.83;−47.98) | 8.52(7.19;9.66) | |||||
| p (T2-T1) | 1 | 0.001** | 0.000** | 0.963 | 0.004** | ||||
| 2 | 0.001** | 0.000** | 0.098 | 0.002** | |||||
| T3 | 1 | 49.60(48.31;58.18) | 0.251 | 9.03(8.48;10.64) | 0.334 | −65.54(−85.61;−61.24) | 0.383 | 5.30(4.36;5.86) | 0.008** |
| 2 | 52.53(50.30;57.72) | 8.57(8.14;9.80) | −80.66(−95.24;−69.96) | 7.44(4.79;8.10) | |||||
| p (T3-T1) | 1 | 0.108 | 0.000** | 0.098 | 0.232 | ||||
| 2 | 0.048** | 0.000** | 0.312 | 0.060 | |||||
| T4 | 1 | 52.67(45.25;56.16) | 0.251 | 9.34(8.16;10.14) | 0.022** | −68.20(−92.70;−64.17) | 0.183 | 5.03(3.45;5.41) | 0.005** |
| 2 | 53.61(49.18;57.34) | 10.30(9.21;11.45) | −85.04(−93.03;−73.74) | 7.04(4.66;8.11) | |||||
| p (T4-T1) | 1 | 0.312 | 0.000** | 0.215 | 0.854 | ||||
| 2 | 0.060 | 0.000** | 0.435 | 0.270 | |||||
| T5 | 1 | 49.91(45.93;56.81) | 0.141 | 9.64(8.69;10.32) | 0.015** | −68.64(−82.98;−65.03) | 0.270 | 4.57(3.40;5.34) | 0.001** |
| 2 | 55.29(50.55;57.84) | 10.69(10.14;10.94) | −79.92(−99.13;−66.67) | 7.12(5.01;8.01) | |||||
| p (T5-T1) | 1 | 0.335 | 0.000** | 0.168 | 0.520 | ||||
| 2 | 0.027** | 0.000** | 0.491 | 0.168 | |||||
| T6 | 1 | 50.22(45.99;55.87) | 0.408 | 9.93(8.74;10.62) | 0.007** | −66.82(−73.60;−64.14) | 0.054 | 4.33(3.66;5.30) | 0.006** |
| 2 | 52.36(49.41;55.79) | 11.64(10.26;12.22) | −81.07(−91.38;−68.23) | 6.16(4.70;6.38) | |||||
| p (T6-T1) | 0.581 | 0.001** | 0.024** | 0.646 | |||||
| 0.183 | 0.000** | 0.198 | 0.462 | ||||||
| T7 | 1 | 49.50(45.72;55.50) | 0.662 | 9.74(8.35;10.90) | 0.006** | −67.39(−74.45;−61.77) | 0.054 | 4.38(3.61;5.20) | 0.015** |
| 2 | 51.29(49.76;53.77) | 11.74(10.31;12.40) | −78.12(−92.90;−68.37) | 5.99(4.10;6.48) | |||||
| p (T7-T1) | 1 | 0.550 | 0.001** | 0.043* | 0.408 | ||||
| 2 | 0.291 | 0.000** | 0.161 | 0.290 | |||||
| T8 | 1 | 49.60(46.67;56.66) | 0.818 | 9.54(8.70;10.76) | 0.015** | −66.95(−70.27;−63.21) | 0.024** | 4.37(3.51;5.08) | 0.019** |
| 2 | 49.85(47.49;53.62) | 11.07(10.29;12.13) | −75.04(−91.97;−68.56) | 5.76(3.95;5.99) | |||||
| p (T8-T1) | 1 | 0.491 | 0.001** | 0.019* | 0.232 | ||||
| 2 | 0.818 | 0.000** | 0.089 | 0.232 | |||||
L*-Lightness; a*-red/green; b*-yellow/blue; Sa*-Surface Roughness; T1-Baseline; T2-White Spot Lesion; T3-Infiltrant application; immersion in saliva; T4-1 week; T5-2 weeks; T6-4 weeks; T7-6 weeks; T8-8 weeks; Group 1-Test; Group 2-Control.
p < .05.
Table 2.
Median and interquartile range (IQR) value of colour measurements for mandibular teeth.
| Time | Group | L* |
a* |
b* |
Sa* |
||||
|---|---|---|---|---|---|---|---|---|---|
| Median (IQR) | p | Median (IQR) | p | Median (IQR) | p | Median (IQR) | p | ||
| T1 | 1 | 53.36(48.75;56.60) | 0.335 | 11.54(10.46;15.50) | 0.836 | −66.46(−79.64;−56.89) | 0.069 | 4.81(2.93;6.25) | 0.435 |
| 2 | 51.64(45.97;56.13) | 15.01(9.70;16.52) | −79.80(−119.61;−63.09) | 5.28(3.80;7.14) | |||||
| T2 | 1 | 63.94(59.96;67.99) | 0.004** | 7.20(5.31;9.32) | 0.421 | −58.09(−62.83;−49.09) | 0.408 | 6.48(5.08;7.38) | 0.073 |
| 2 | 54.44(51.25;62.89) | 7.64(5.99;10.30) | −56.68(−96.46;−52.94) | 7.34(5.82;10.29) | |||||
| p (T2-T1) | 1 | 0.000** | 0.000** | 0.081 | 0.027** | ||||
| 2 | 0.054 | 0.001** | 0.031** | 0.004** | |||||
| T3 | 1 | 57.86(52.36;61.89) | 0.323 | 12.31(10.40;13.95) | 0.002** | −63.86(−80.14;−54.13) | 0.383 | 5.84(3.85;6.26) | 0.009** |
| 2 | 52.76(50.54;61.20) | 8.19(6.93;10.78) | −67.89(−91.39;−55.54) | 7.00(5.72;7.47) | |||||
| p (T3-T1) | 1 | 0.066 | 0.713 | 0.613 | 0.462 | ||||
| 2 | 0.141 | 0.002** | 0.154 | 0.035** | |||||
| T4 | 1 | 52.94(50.43;56.42) | 0.854 | 12.24(9.91;13.59) | 0.054 | −73.15(−87.39;−57.04) | 0.730 | 5.63(3.811;6.13) | 0.015** |
| 2 | 52.92(49.37;60.30) | 10.49(7.29;11.64) | −75.44(−92.22;−68.22) | 6.48(5.77;7.36) | |||||
| p (T4-T1) | 1 | 0.927 | 0.383 | 0.613 | 0.520 | ||||
| 2 | 0.089 | 0.013** | 0.462 | 0.048** | |||||
| T5 | 1 | 53.77(50.60;59.46) | 0.462 | 12.03(10.63;13.61) | 0.015** | −60.66(−74.80;−53.94) | 0.141 | 5.70(3.78;6.20) | 0.039** |
| 2 | 50.89(48.42;60.11) | 10.68(7.68;11.75) | −72.27(−96.13;−58.38) | 6.36(5.66;7.37) | |||||
| p (T5-T1) | 1 | 0.581 | 0.909 | 0.383 | 0.550 | ||||
| 2 | 0.215 | 0.017** | 0.312 | 0.043** | |||||
| T6 | 1 | 53.49(49.91;58.10) | 0.945 | 12.21(10.24;13.99) | 0.060 | −63.83(−75.33;−56.18) | 0.154 | 5.56(3.73;6.17) | 0.089 |
| 2 | 52.18(49.60;58.96) | 11.03(7.84;12.14) | −73.65(−96.63;−60.25) | 5.90(5.28;6.95) | |||||
| p (T6-T1) | 1 | 0.505 | 0.713 | 0.613 | 0.890 | ||||
| 2 | 0.198 | 0.027** | 0.462 | 0.215 | |||||
| T7 | 1 | 52.26(49.73;56.32) | 0.890 | 12.24(10.48;15.06) | 0.035** | −65.33(−76.73;−56.54) | 0.291 | 5.48(3.84;5.97) | 0.312 |
| 2 | 52.48(49.04;58.47) | 10.77(8.91;12.32) | −73.24(−97.80;−59.35) | 5.56(5.02;6.71) | |||||
| p (T7-T1) | 1 | 0.963 | 0.890 | 0.783 | 0.748 | ||||
| 2 | 0.183 | 0.039** | 0.358 | 0.462 | |||||
| T8 | 1 | 52.16(49.622;55.24) | 0.890 | 12.60(10.49;15.02) | 0.054 | −66.32(−77.08;−57.52) | 0.291 | 5.38(3.67;5.91) | 0.646 |
| 2 | 52.37(48.93;58.71) | 11.06(8.52;12.51) | −73.18(−97.37;−61.17) | 5.47(4.06;6.68) | |||||
| p (T8-T1) | 1 | 0.679 | 0.890 | 0.927 | 0.927 | ||||
| 2 | 0.270 | 0.048** | 0.383 | 0.713 | |||||
L*-Lightness; a*-red/green; b*-yellow/blue; Sa*-Surface Roughness; T1-Baseline; T2-White Spot Lesion; T3-Infiltrant application; immersion in saliva; T4-1 week; T5-2 weeks; T6-4 weeks; T7-6 weeks; T8-8 weeks; Group 1-Test; Group 2-Control.
p < .05.
Table 1 shows that there are no significant difference (p > .05) in the lightness (L*) and yellow/blue (b*) values between MxT and MxC groups. However, there are significant differences (p<.05) in the red/green (a∗) and surface roughness (Sa∗) values at distinct treatment times. The formation of WSL at T2 increased the L* value significantly (p < .05) for both groups (MxT and MxC) in comparison to baseline (T1). For MxC, the L* value was significantly different at T2 (p = .001) and T3 (p = .048) in comparison to T1, due to WSL formation. However, the lightness (L*) differences were not significant (p > .05) after T5. For MxT group, there was no significant difference (p > .05) in the L* value at T3 compared to T1. This indicates that the infiltration of resin has diminished the whitish appearance of the WSL. This changes remained until T8, which may suggest that the infiltrant application is stable. Following the WSL treatment (T2), a∗ value decreased significantly (p = .000) in both groups (MxT and MxC) compared to T1. The application of the infiltrant (T3) increased a∗ value significantly (p = .000) for both groups (MxT and MxC) in comparison to T1. The changes in a∗ value remained significant (p < .05) for both groups (MxT and MxC) until T8. The formation of WSL (T2) increased the value of b∗ which later decreased by the application of infiltrant (T3), and it continued to reduce until T8 in both groups (MxT and MxC; p > .05). Both groups (MxT and MxC) showed significant increased (p < .05) in Sa* value with the formation of WSL (T2). Subsequently Sa* reduced with the application of infiltrant and immersion in saliva.
In general, there was no significant difference (p > .05) for L∗, a∗, b∗ and Sa∗ between MnT and MnC groups at distinct treatment times as shown in Table 2. Only during the formation of WSL (T2), there was a significant (p < .05) increased of L* value between MnT and MnC groups. After 24 hours in saliva, the infiltration treatment (T3) reduces the white appearance of the two groups (MnT and MnC), which are not significant (p > .05) as compared to baseline (T1) measurements. Then during subsequent treatment time, it was found that the L∗ value for both groups (MnT and MnC) was not significantly different (p > .05) compared to T1 value. This shows that the L∗ value has stabilized throughout the study period. Following the WSL treatment (T2), a∗ value decreased significantly (p < .05) in both groups (MnT and MnC) compared to T1. This later are followed by increased trend of a∗ values until T8. After application of the resin, there was no significant difference (p > .05) of a* value for the MnT group compared to T1. This was observed to be stable until T8. On the other hand, after the formation of WSL and subsequent experimental times, the MnC group showed a significant difference (p < .05) towards T1. Both groups (MnT and MnC) showed no significant difference (p > .05) for the value of b∗ after the formation of WSL (T2) towards T1. Surface roughness (Sa∗) for MnT and McC groups increased significantly (p < .05) with the formation of WSL (T2). However, there was a significant reduction (p < .05) for Sa∗ and this was observed throughout the immersion in saliva for MnT and MnC groups.
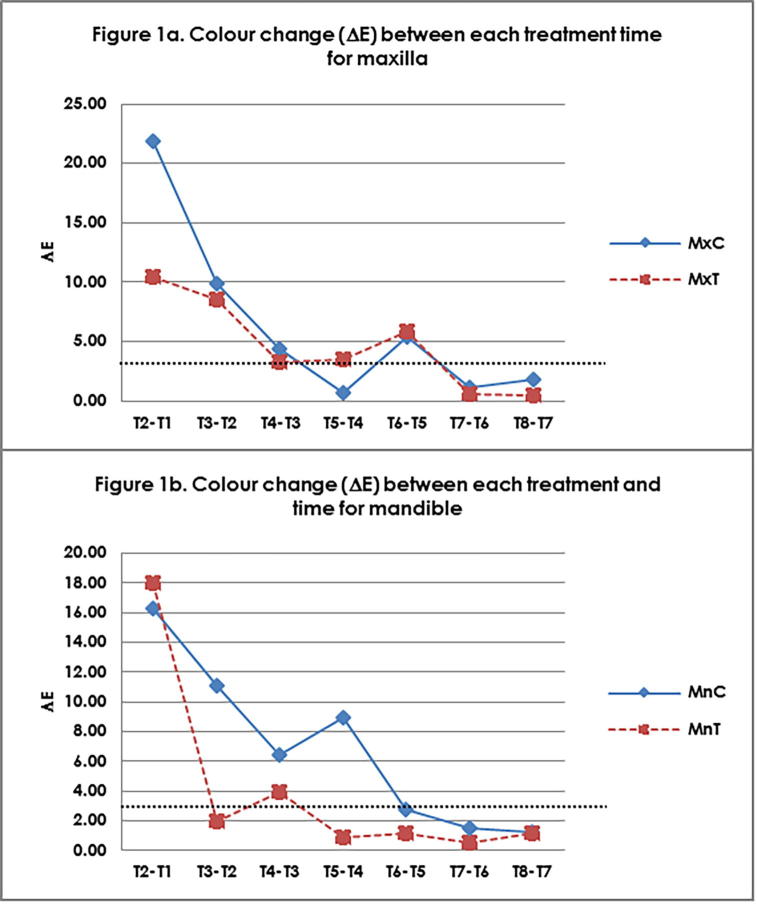
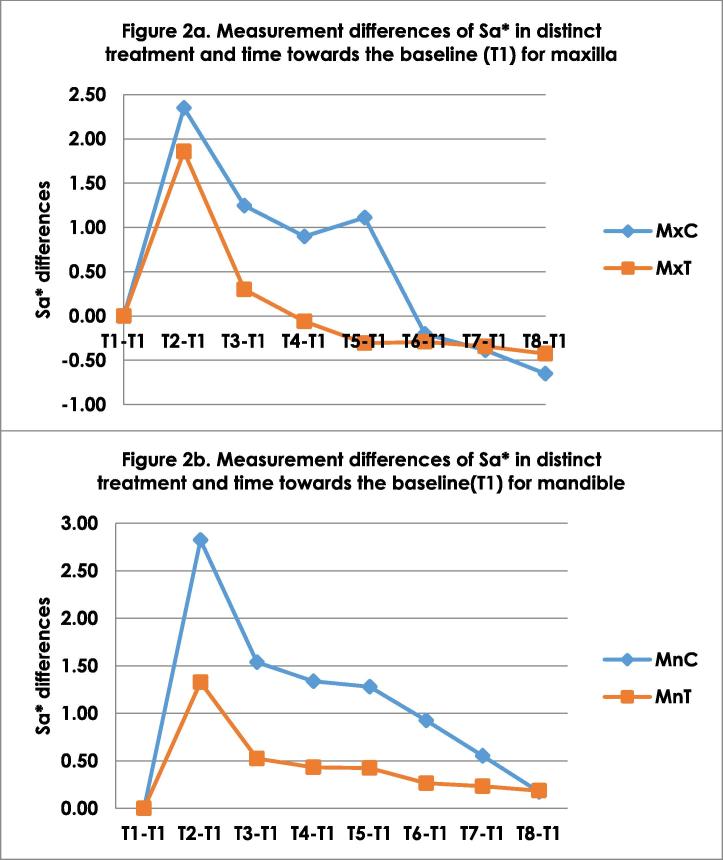
Colour change obtained between distinct treatment time of test and control groups are shown in Fig. 1a (maxilla) and 1b (mandible). The highest colour change was observed at T1 due to formation of WSL. Colour improvement of the WSLs achieved by infiltrant, was superior compared to the control group. However, this effect did not improve the colour components to the initial levels. Continuously, the WSL appearance reduced below the horizontal line of △E3.7. This line indicates that the colour change is no longer detectable by naked eye, hence showing further reduction in WSL. This reduction was also greater in the MnT group. However, there was not difference in the stability reduction pattern between maxilla and mandibular groups. Fig. 2 illustrate the reduction of Sa∗ in distinct treatment time for maxilla and mandible.
Fig. 1.
Colour change (△E) between each treatment time for (a) Maxilla (b) Mandible. T1 – Baseline, T2 – WSL; T3 – Infiltrant/non-infiltrant after 24 hours immersion in saliva; Immersion in saliva T4 – 1 week; T5 – 2 weeks; T6 – 4 weeks; T7 – 6 weeks; T8 – 8 weeks; Mx-Maxilla, Mn-Mandible; C-Control; T-Test.
Fig. 2.
Measurement differences of surface roughness (Sa*) in distinct treatment and time towards the baseline (T1) for a) Maxilla b) Mandible. T1-Baseline, T2 – WSL; T3 – Infiltrant/non-infiltrant after 24 hours immersion in saliva; Immersion in saliva T4 – 1 week; T5 – 2 weeks; T6 – 4 weeks; T7 – 6 weeks; T8 – 8 weeks; Mx-Maxilla, Mn-Mandible; C-Control; T-Test.
SEM analysis showed regular appearance of enamel rods for both test and control groups in maxillary (Fig. 3) and mandibular teeth (Fig. 4). However, treatment of WSLs with infiltrant resin resulted in the appearance of an irregular-shaped globular material compacted between regular enamel rods. The infiltrant had covered the porous structure. Through time the surface has been rough still, thus presenting an increased susceptibility to discoloration.
Fig. 3.
SEM buccal view in relation to distinct treatment and time for maxilla. T1-Baseline, T2 – WSL; T3 – Infiltrant/non-infiltrant after 24 hours immersion in saliva; Immersion in saliva T4 – 1 week; T5 – 2 weeks.
Fig. 4.
SEM buccal view in relation to distinct treatment and time for mandible. T1-Baseline, T2 – WSL; T3 – Infiltrant/non-infiltrant after 24 hours immersion in saliva; Immersion in saliva T4 – 1 week; T5 – 2 weeks.
4. Discussion
Demineralization process, which increased porosity at a subsurface level that affects how light, is absorbed in that region, resulting in the white chalky appearance or opaque in colour. Over time, the WSL may demineralize, but the opaque colour will usually remains and becomes stained making it less esthetic. In recent years, unique low viscosity infiltration light-cured resins were introduced which may drawn deep into the pore system of a lesion, like a sponge drawing up liquids (Meyer and Paris, 2008). The resin generally filled the pores, replacing lost tooth structure and impeded further demineralization progression. This process is in line with minimum intervention dentistry which aim for maximum conservation of demineralized, non-cavitated enamel and dentin. Others have also shown that the resins were able to stabilize and prevent the lesions from progressing while maintaining the anatomical shape and masked colour of the tooth (Mueller et al., 2006, Paris et al., 2007b).
In this study, we hypothesized that WSLs treated by means of resin infiltrant would exhibit different effect on colour components and surface roughness between maxillary and mandibular teeth in comparison with non-treated WSLs (control group). Our results generally indicate that there was gradual reduction of L∗, b∗ and Sa∗ values and gradual increased in the value of a∗ with distinct treatment times in both maxillary and mandibular premolars. Interestingly, colour improvement of the WSLs was achieved with the effect of infiltration resin for both maxilla and mandibular teeth. Although this effect did not totally bring the colour components back to the baseline level, the colour difference was not visible to the naked eye. Clinically, the colour changes have greatly improved which has a big impact on esthetics.
In this study, the teeth were immersed in the artificial saliva for 24 hours before application of the infiltrant. This may lead to infiltration of some water or air within the microporosities of WSLs and potentially causing less penetration of the resin infiltrant. Therefore, application of the resin infiltrant to the enamel surface had sealed the enamel porosities. This implied that an incomplete infiltration of the porous enamel may have greater effect in the maxillary teeth. Therefore, infiltrant should be applied as soon as the microporosities are present. From our study and others, we conclude that the factors that may affect the improvement of colour and surface roughness of the teeth are 1) Time of infiltrant application; 2) Lesion depth and; 3) Duration of the WSL presence. The techniques and time should be properly evaluated and control to gain the maximum effect. There is also a possibility that the mineral phases between the infiltrant and the enamel are different and as a result, the colour of the white spot area does not blend with the sound enamel. Furthermore, it has also been shown to change in a wet condition and after dehydration (Brodbelt et al., 1981).
The demineralized surface layer hampers resin infiltration into the underlying subsurface of the WSL and has to be removed before resin infiltration (Meyer-Lueckel et al., 2007, Paris et al., 2010, Paris et al., 2007a, Meyer and Paris, 2008). It has been shown that phosphoric acid does not adequately remove the demineralized surface layer. Whereas the 15% HCl for 90–120 s (Meyer-Lueckel et al., 2007) can adequately remove demineralized area enables resin infiltration into the body of the WSL. In this study, the changes in surface roughness in relation to distinct treatments and times showed gradually reduction of WSL with time. This result is similar with the one reported by Taher et al., 2012. This demonstrated that infiltrant application to the enamel surface may sealed the enamel porosities and the resulting product appeared smooth. These observations might explain the recorded surface roughness values of enamel when treated with the infiltrant resin. However, surface roughness in vitro may be quite different when compared to the dynamic complex biological system in the oral cavity in vivo.
Potentially, findings of this study may be due to differences in premolar morphology and the pattern of increasing enamel thickness distally throughout the dentition. In clinical practice, it is important to estimate the amount of labial/buccal enamel thickness that have been demineralized during orthodontic treatment. This may serve as parameters to perform different clinical preventative and therapeutic measures which would be different between the maxillary and mandibular teeth. Most studies investigated the enamel thickness on the mesial and distal surfaces of incisors, canines and premolars, which are relevant when performing stripping during orthodontic treatment (Sarig et al., 2015). Typically, the measurements from clinical radiographs are confined to marginal enamel from the mesial and distal interproximal regions of the crown. Little work has been done to quantify variation in enamel thickness across the labial/buccal of the dentition (Smith et al., 2008, Feeney et al., 2010). In populations comparison study, differences were found in average enamel thickness between maxillary and mandibular premolars, and between canines and premolars within both dental arches (Feeney et al., 2010). Further studies should be carried out to assess the association between WSL incidence and enamel thickness of the associated tooth. This will give insight in clinical management of the WSLs problem.
In summary, the current study provide valuable information on WSL and was completed in a simulated in vitro situation. This in vitro study was unable to completely simulate the intraoral condition which involved the dynamic and complex biological factor like saliva and plaque and bacterial biofilm. Therefore, direct extrapolations to clinical conditions must be exercised with caution (Taher et al., 2012).
5. Conclusion
This study shows that resin infiltration produced favourable esthetics results and improved surface roughness of the teeth in distinct treatment times, in comparison to control. The effect of resin infiltrant on WSLs on maxillary teeth is similar when compared to the mandibular teeth.
Conflict of interest
The authors have declared that no conflict of interest exists.
Footnotes
Peer review under responsibility of King Saud University.
References
- An Y.H., Friedman R.J. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J. Biomed. Mater. Res. 1998;43(3):338–348. doi: 10.1002/(sici)1097-4636(199823)43:3<338::aid-jbm16>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Ballard R.W., Hagan J.L. Evaluation of 3 commercially available materials for resolution of white spot lesions. Am. J. Orthod. Dentofacial. Orthop. 2013;143(4 Suppl):S78–S84. doi: 10.1016/j.ajodo.2012.08.020. [DOI] [PubMed] [Google Scholar]
- Benson P.E., Shah A.A. Fluorides, orthodontics and demineralization: a systematic review. J. Orthod. 2005;32(2):102–114. doi: 10.1179/146531205225021033. [DOI] [PubMed] [Google Scholar]
- Brodbelt R.H., O'Brien W.J. Translucency of human dental enamel. J. Dent. Res. 1981;60(10):1749–1753. doi: 10.1177/00220345810600100401. [DOI] [PubMed] [Google Scholar]
- Chang H.S., Walsh L.J. Enamel demineralization during orthodontic treatment: aetiology and prevention. Aust. Dent. J. 1997;42(5):322–327. doi: 10.1111/j.1834-7819.1997.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Chapman J.A., Roberts W.E. Risk factors for incidence and severity of white spot lesions during treatment with fixed orthodontic appliances. Am. J. Orthod. Dentofacial. Orthop. 2010;138(2):188–194. doi: 10.1016/j.ajodo.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Feeney R.N.M., Zermeno J.P. Enamel thickness in Asian human canines and premolars. Anthropol. Sci. 2010;118(3):191–198. [Google Scholar]
- Hadler-Olsen S., Sandvik K. The incidence of caries and white spot lesions in orthodontically treated adolescents with a comprehensive caries prophylactic regimen–a prospective study. Eur. J. Orthod. 2012;34(5):633–639. doi: 10.1093/ejo/cjr068. [DOI] [PubMed] [Google Scholar]
- Hattab F.N., Qudeimat M.A. Dental discoloration: an overview. J. Esthet. Dent. 1999;11(6):291–310. doi: 10.1111/j.1708-8240.1999.tb00413.x. [DOI] [PubMed] [Google Scholar]
- Heymann G.C., Grauer D. A contemporary review of white spot lesions in orthodontics. J. Esthet. Restor. Dent. 2013;25(2):85–95. doi: 10.1111/jerd.12013. [DOI] [PubMed] [Google Scholar]
- Johnston W.M., Kao E.C. Assessment of appearance match by visual observation and clinical colorimetry. J. Dent. Res. 1989;68(5):819–822. doi: 10.1177/00220345890680051301. [DOI] [PubMed] [Google Scholar]
- Julien K.C., Buschang P.H. Prevalence of white spot lesion formation during orthodontic treatment. Angle. Orthod. 2013;83(4):641–647. doi: 10.2319/071712-584.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalaf K. Factors affecting the formation, severity and location of white spot lesions during orthodontic treatment with fixed appliances. J. Oral. Maxillofac. Res. 2014;5(1):e4.1–e4.10. doi: 10.5037/jomr.2014.5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kielbassa A.M., Muller J. Closing the gap between oral hygiene and minimally invasive dentistry: a review on the resin infiltration technique of incipient (proximal) enamel lesions. Quintessence. Int. 2009;40(8):663–681. [PubMed] [Google Scholar]
- Lovrov S., Hertrich K. Enamel demineralization during fixed orthodontic treatment-incidence and correlation to various oral-hygiene parameters. J. Orofac. Orthop. 2007;68(5):353–363. doi: 10.1007/s00056-007-0714-1. [DOI] [PubMed] [Google Scholar]
- Mattousch T.J., van der Veen M.H. Caries lesions after orthodontic treatment followed by quantitative light-induced fluorescence: a 2-year follow-up. Eur. J. Orthod. 2007;29(3):294–298. doi: 10.1093/ejo/cjm008. [DOI] [PubMed] [Google Scholar]
- Maxfield B.J., Hamdan A.M. Development of white spot lesions during orthodontic treatment: Perceptions of patients, parents, orthodontists, and general dentists. Am. J. Orthod. Dentofacia.l Orthop. 2012;141(3):337–344. doi: 10.1016/j.ajodo.2011.08.024. [DOI] [PubMed] [Google Scholar]
- Meyer-Lueckel H., Paris S. Surface layer erosion of natural caries lesions with phosphoric and hydrochloric acid gels in preparation for resin infiltration. Caries. Res. 2007;41(3):223–230. doi: 10.1159/000099323. [DOI] [PubMed] [Google Scholar]
- Meyer-Lueckel H., Paris S. Improved resin infiltration of natural caries lesions. J Dent Res. 2008;87(12):1112–1116. doi: 10.1177/154405910808701201. [DOI] [PubMed] [Google Scholar]
- Mueller J., Meyer-Lueckel H. Inhibition of lesion progression by the penetration of resins in vitro: influence of the application procedure. Oper. Dent. 2006;31(3):338–345. doi: 10.2341/05-39. [DOI] [PubMed] [Google Scholar]
- Murphy T.C., Willmot D.R. Management of postorthodontic demineralized white lesions with microabrasion: a quantitative assessment. Am. J. Orthod. Dentofacial. Orthop. 2007;131(1):27–33. doi: 10.1016/j.ajodo.2005.04.041. [DOI] [PubMed] [Google Scholar]
- Ogaard B. Prevalence of white spot lesions in 19-year-olds: a study on untreated and orthodontically treated persons 5 years after treatment. Am. J. Orthod. Dentofacial. Orthop. 1989;96(5):423–427. doi: 10.1016/0889-5406(89)90327-2. [DOI] [PubMed] [Google Scholar]
- Ogaard B., Rølla G. Orthodontic appliances and enamel demineralization. Part 1. Lesion development. Am. J. Orthod. Dentofacial. Orthop. 1998;94(1):68–73. doi: 10.1016/0889-5406(88)90453-2. [DOI] [PubMed] [Google Scholar]
- Paris S., Dorfer C.E. Surface conditioning of natural enamel caries lesions in deciduous teeth in preparation for resin infiltration. J. Dent. 2010;38(1):65–71. doi: 10.1016/j.jdent.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Paris S., Meyer-Lueckel H. Penetration coefficients of commercially available and experimental composites intended to infiltrate enamel carious lesions. Dent. Mater. 2007;23(6):742–748. doi: 10.1016/j.dental.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Paris S., Meyer-Lueckel H. Resin infiltration of natural caries lesions. J. Dent. Res. 2007;86(7):662–666. doi: 10.1177/154405910708600715. [DOI] [PubMed] [Google Scholar]
- Paris S., Schwendicke F. Masking of white spot lesions by resin infiltration in vitro. J. Dent. 2013;41(Suppl 5):e28–e34. doi: 10.1016/j.jdent.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Sarig R., Vardimon A.D. Pattern of maxillary and mandibular proximal enamel thickness at the contact area of the permanent dentition from first molar to first molar. Am J Orthod Dentofacial Orthop. 2015;147(4):435–444. doi: 10.1016/j.ajodo.2014.11.026. [DOI] [PubMed] [Google Scholar]
- Smith T.M., Olejniczak A.J. Brief communication: enamel thickness trends in the dental arcade of humans and chimpanzees. Am. J. Phys. Anthropol. 2008;136(2):237–241. doi: 10.1002/ajpa.20796. [DOI] [PubMed] [Google Scholar]
- Taher N.M., Alkhamis H.A. The influence of resin infiltration system on enamel microhardness and surface roughness: An in vitro study. Saudi. Dent. J. 2012;24(2):79–84. doi: 10.1016/j.sdentj.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thylstrup A., Fejerskov O. Clinical appearance of dental fluorosis in permanent teeth in relation to histologic changes. Community. Dent. Oral. Epidemiol. 1978;6(6):315–328. doi: 10.1111/j.1600-0528.1978.tb01173.x. [DOI] [PubMed] [Google Scholar]
- Torres C.R.G., Borges A.B. Effect of caries infiltration technique and fluoride therapy on the colour masking of white spot lesions. J. Dent. 2011;39(3):202–207. doi: 10.1016/j.jdent.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Willmot D. White spot lesions after orthodontic treatment. Sem. Ortho. 2008;14(3):209–219. [Google Scholar]
- Yetkiner E., Wegehaupt F. Colour improvement and stability of white spot lesions following infiltration, micro-abrasion, or fluoride treatments in vitro. Eur. J. Orthod. 2014;36(5):595–602. doi: 10.1093/ejo/cjt095. [DOI] [PubMed] [Google Scholar]