Abstract
Glycosylated lipids (GLs) are added-value lipid derivatives of great potential. Besides their interesting surface activities that qualify many of them to act as excellent ecological detergents, they have diverse biological activities with promising biomedical and cosmeceutical applications. Glycolipids, especially those of microbial origin, have interesting antimicrobial, anticancer, antiparasitic as well as immunomodulatory activities. Nonetheless, GLs are hardly accessing the market because of their high cost of production. We believe that experience of metabolic engineering (ME) of microbial lipids for biofuel production can now be harnessed towards a successful synthesis of microbial GLs for biomedical and other applications. This review presents chemical groups of bacterial and fungal GLs, their biological activities, their general biosynthetic pathways and an insight on ME strategies for their production.
Keywords: Biosurfactant, Glycolipids biosynthesis, Glycosyl/acyl transferases, Glycosides, Physiological roles, Lipid biotechnology
1. Introduction
Lipid biotechnology research has focused to date on developing sustainable alternatives to depleting fossil fuels. One strategy was plant-derived fuel, biodiesel [1]. A main drawback of this approach is that oil and land allocated for biodiesel production compete with those allocated for human food consumption. Moreover, replacement of natural vegetations with plants used for biodiesel production generates long-term environmental concerns. Another strategy is to use lipids originating from microbes, called ‘‘single cell oil’’ (SCO) as substrates for biodiesel production. We believe that accumulating knowledge and developed biomolecular tools obtained from lipid engineering of oleogenic microbes can now be harnessed for the microbial production of lipid derivatives of added-value.
Glycosylation of organic molecules, including lipids, usually leads to derivatives of new and/or better physicochemical properties and biological activities [2], [3] that reflect in higher market prices. Metabolic engineering of lipid derivatives has previously investigated polyunsaturated fatty acids [4] and fatty acid derivatives that are used as substrates for oleochemical industries, e.g. heterologous production of ricinoleic acids by Y. lipolytica [5]. Other added-value lipid derivatives of commercial interest include wax esters, polyhydroxyalkanoates (bioplastics), hydroxylated fatty acids, carotenoids, polyenic polymers [6] and glycolipids.
This review focuses on simple glycolipids (SGLs) as an important family of glycolipids (GLs) class. The importance of SGLs stems from the fact that this family of GLs comprises a wide range of bioactive molecules with potential biomedical, pharmaceutical and cosmetic applications [7], [8]. Nonetheless, many simple GLs are limited commercially because of their still low yield and high cost of production, particularly of high purity simple GLs aimed for biopharmaceutical purposes.
We present the chemical groups of simple GLs, their microbial producers and their biological activities. Then, we describe the key biosynthetic enzymes and metabolic precursors involved in biosynthesis of simple GLs. Finally, we discuss metabolic engineering strategies for simple GLs production in native and heterologous hosts.
2. Definition and classification of simple glycolipids
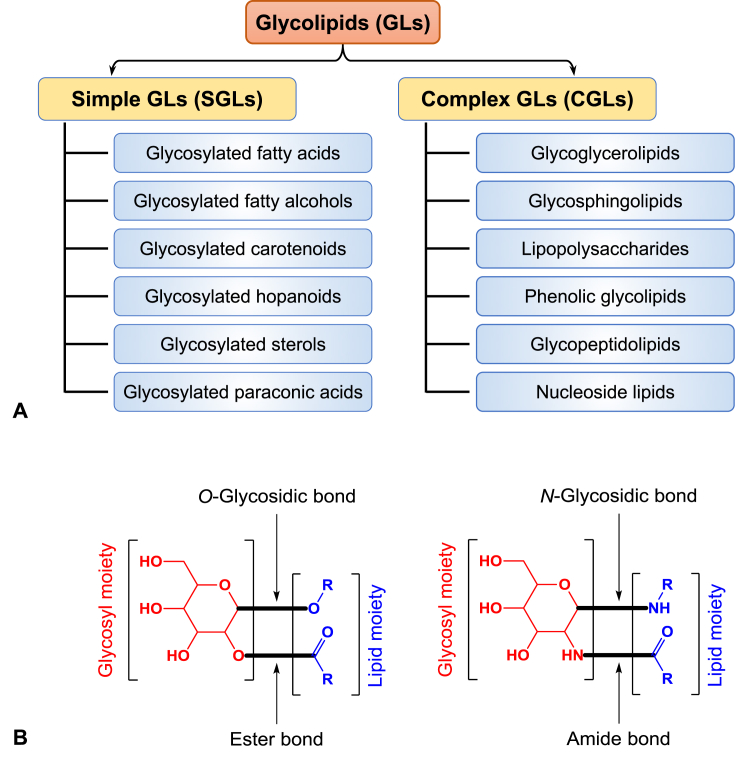
The term glycolipids (GLs), in general, encompasses a wide diversity of structurally heterogeneous biological compounds that are produced by microbes, plants, animals and humans [9]. As their names suggest, they are composed of glycosyl and lipid moieties. The IUPAC uses the term GLs to broadly designate any compound containing one or more monosaccharide residues bound by glycosidic linkage to a hydrophobic moiety [10]. Our definition of GLs is even broader to include glycoside and non-glycoside GLs in which the sugar and lipid residues are linked together via glycosidic (e.g. O- or N-glycosidic linkages) and non-glycosidic linkages (e.g. ester or amide linkages), respectively (Fig. 1). The glycosyl residue can be mono-, di-, oligo or polysaccharides (e.g. glucose, cellobiose or glycan, respectively), alcohol sugars/polyols (like mannitol, erythritol or arabinol, etc.), amino sugars (like desosamine, etc) or sugar acids (like glucuronic acids). The lipid residue of GLs ranges from fatty acids, fatty alcohols, fatty amino alcohols, polyketides, sterols, hopanoids and carotenoids with different substitutions, chain lengths, saturation levels, branching and di-/oligo-/polymerizations.
Fig. 1.
Classification of glycolipids and main types of linkages between their glycosyl and lipid residues. (A) Simple glycolipids (SGLs) comprise glycolipids consisting of glycosyl and lipid residues only, whereas, complex glycolipids (CGLs) contain glycerol, ceramide, phenolic, peptide, nucleoside or polysaccharide residues in addition to the glycosyl and lipid residues. (B) Glycosyl and lipid residues are mainly linked via O-glycosidic and/or ester bonds, and less frequently via N-glycosidic and/or amide bonds.
Numerous classifications exist for GLs [10], the most convenient of which is their classification into simple and complex GLs [11], [12], [13] (Fig. 1). Simple GLs (SGLs), sometimes called saccharolipids [14], are two-component (glycosyl and lipid moieties) GLs in which the glycosyl and lipid moieties are directly linked to each other. Complex glycolipids (CGLs) are, however, structurally more heterogeneous, as they contain, in addition to the glycosyl and lipid moieties, other residues like glycerol (glycoglycerolipids), peptide (glycopeptidolipids), acylated-sphingosine (glycosphingolipids), or other residues (Fig. 1). Polysaccharide-containing GLs, although containing no residues other than glycosyl and lipid moieties, are classified under complex glycolipids because of the complex nature of their polysaccharide residues; however, oligosaccharide-containing GLs are classified as simple GLs [13] (Fig. 1). Simple glycolipids addressed in this review are those of natural microbial origin, therefore, SGLs of synthetic or other biological origins are not mentioned.
3. Surfactant properties of simple glycolipids
Simple glycolipids (SGLs) are amphiphilic molecules as they comprise both the hydrophilic glycosyl and the lipophilic lipid residues. This amphiphilic nature confers surfactant activity to most GLs; those of which with pronounced surfactant activity are called biosurfactant. Compared to petroleum-derived (e.g. alkylbenzene sulfonates) or plant-based (e.g. alkyl polyglycosides) synthetic surfactants [15], microbially-produced SGL biosurfactants are mostly of higher surface activity, higher emulsifying power, lower critical micelle concentrations, higher biodegradability (compared to petroleum-derived surfactants), lower ecotoxicity [16] and lower protein denaturing potency [17], [18], [19]. The advanced properties of microbial SGLs are suggested to be attributed to a peculiar mosaic distribution of regions of polarity over the GL molecule, as well as to their branched or sometimes circular structures compared to synthetic surfactants [18]. Moreover, most SGLs are naturally produced as complex mixtures of congeners or homologues that vary in the number of glycosyl units and extent of their acylation, the number of conjugate lipid chains, their lengths, the extent of unsaturations and substitutions; these factors together contribute to their unique surfactant properties and behaviors [18].
Although the unique surface properties of some SGLs qualified some of them to be marketed as ecological surfactants [20], [21], yet, their competitiveness in the detergent market is limited because of their higher prices compared to alkyl polyglycosides synthetic surfactants which are at least 50% less expensive. For example, the estimated cost of large-scale production of the SGLs: sophorolipid and rhamnolipid biosurfactants, are about US$ 2.5–3/Kg [21], [22] and US$ 5–20/Kg [23], respectively, compared to US$ 1–3/Kg for the synthetic alkyl polyglycoside surfactants [23].
Aside from their surface activities, nearly all natural SGLs have interesting biological activities, as described later, that let them occupy market niches not approachable by synthetic surfactants [24]. Noteworthy, the biological activities of SGLs are thought to stem from their surface activities [25].
4. Chemical groups and origins of microbial simple glycolipids
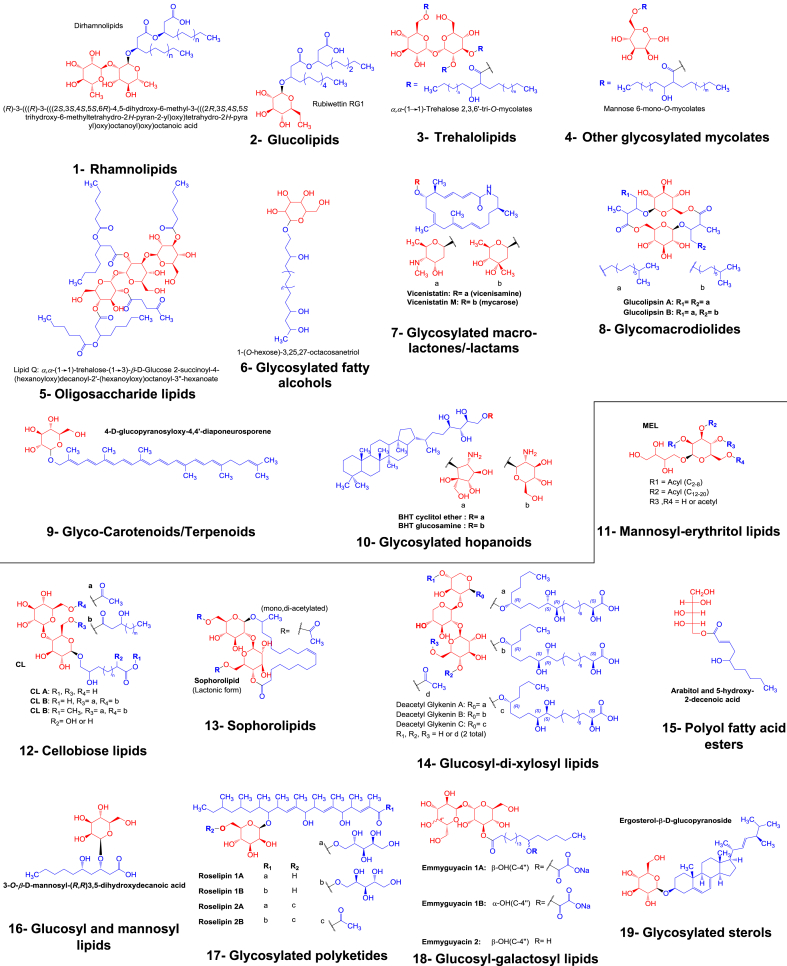
Microbially produced SGLs are classified in chemical groups based on their chemical structures so that every group comprises SGLs members sharing unique glycosyl and/or lipid moieties for SGLs produced by bacteria (Table 1) and fungi (Table 2). In this classification, some SGLs congeners are classified in separate groups when they originate from different microbial origins and vice versa. Under each SGL group, exhaustive list of its members, together with their chemical names, their microbial producers as well as their taxonomic phyla is mentioned (Table 1, Table 2). Furthermore, the confirmed chemical structures of representative or prototypic members of each SGL group are presented (Fig. 3).
Table 1.
Chemical groups and members of bacterial simple glycolipids as well as names and phyla of native producers.
| Common name: Chemical names (Cx: chain length of fatty acid chains) | Producer | Phylum |
|---|---|---|
| Bacteria | ||
| 1- Rhamnolipids | ||
| Monorhamnolipids: α-l-rhamnopyranosyl-R,R-3-(3′-hydroxyalkanoyloxy)alkanoate (C8-16) | Spp. of Pseudomonas and Burkholderia[29] | Proteobacteria |
| Dirhamnolipids: α-l-rhamnopyranosyl-(1–2)-α-l-rhamnopyranosyl-R,R-3-(3′-hydroxyalkanoyloxy)alkanoate (C8-16) | Spp. of Pseudomonas and Burkholderia[29] | Proteobacteria |
| 2- Glucolipids | ||
| Rubiwettin RG1: β-d-glucopyranosyl 3-(3′-hydroxytetradecanoyloxy)decanoate | Serratia rubidaea[30]. | Proteobacteria |
| 3- Trehalolipids | ||
| α,α-(1-1)-Trehalose 6-mono-O-mycolates | Rhodococcus erythropolis[31] | Actinobacteria |
| α,α-(1-1)-Trehalose 2,3-di-O-mycolates | Tsukamurella sp. [32] | Actinobacteria |
| Cord factor: α,α-(1-1)-Trehalose 6,6′-di-O-mycolates | Spp. of Mycobacterium, Rhodococcus, Arthrobacter, Nocardia and Gordonia[31], [33] | Actinobacteria |
| α,α-(1-1)-Trehalose 2,3,6′-tri-O-mycolates | Rhodococcus aurantiacus[34] | Actinobacteria |
| STL-1, α,α-(1-1)-Trehalose 2,2′-di-O-succinoyl-3,4-di-O-alkanoates | Rhodococcus erythropolis[35] | Actinobacteria |
| STL-2, α,α-(1-1)-Trehalose 2,3,4-mono-O-succinoyl-di-O-alkanoates | Rhodococcus erythropolis[35] | Actinobacteria |
| STL-3, α,α-(1-1)-Trehalose 2,3,4,2′-mono-O-succinoyl-tri-O-alkanoates | Spp. of Rhodococcus[36], [37], Arthrobacter[38] | Actinobacteria |
| 4- Other glycosylated (non-trehalose containing) mycolates | ||
| Sucrose 6-mono-O-mycolates | Spp. of Arthrobacter, Corynebacterium, Nocardia, Brevibacterium[39], [40] | Actinobacteria |
| Fructose 6-mono-O-mycolates | Spp. of Arthrobacter, Corynebacterium, Nocardia, Brevibacterium[39], [40] | Actinobacteria |
| Fructose 1,6-di-O-mycolates | Spp. of Arthrobacter, Corynebacterium, Nocardia, Brevibacterium[39], [40] | Actinobacteria |
| Glucose-6-β-hydroxy-α-hexadecenoyl-eicosenoate | Brevibacterium thiogenitalis[41] | Actinobacteria |
| Mannose 6-mono-O-mycolates | Arthrobacter sp. [42] | Actinobacteria |
| Maltose 6-mono-O-mycolates | Arthrobacter sp. [42] | Actinobacteria |
| Maltose 6,6′-di-O-mycolates | Arthrobacter sp. [42] | Actinobacteria |
| Maltotriose 6,6′,6″-tri-O-mycolates | Arthrobacter sp. [42] | Actinobacteria |
| Cellobiose 6-mono-O-mycolates | Arthrobacter sp. [42] | Actinobacteria |
| 5- Trehalose-containging Oligosaccharide lipids | ||
| Lipid Q: β-d-glucose-(1–3)-α,α-(1-1)-trehalose hexanoyl-succinoyl-3-(hexanoyloxy)octanoate-3-(hexanoyloxy)decanoate | Rhodococcus sp. [43], [44] | Actinobacteria |
| GL2: β-d-glucose-(1″-2′)-α,α-(1-1)-trehalose 4,6,2″,3″ tetra-O-alkanoates (C8-10) | Tsukamurella sp. [32] | Actinobacteria |
| GL3: β-d-glucose-(1″-2′)-α,α-(1-1)-trehalose-(6′-1‴)-β-d-galactose-4,6,2″,3″-tetra-O-alkanoates (C8-10) | Tsukamurella sp. [32] | Actinobacteria |
| β-d-glucose-(1–3)-α,α-(1-1)-trehalose-(6-1)-β-d-glucose-(6-1)-β-d-glucose mono-O-succinoyl-hepta-O-alkanoate (C2-8) | Nocardia corynebacteroides[45], [46], [47] | Actinobacteria |
| 4,6-(1-Carboxyethylidene)-3-O-Me-β-d-glucose-(1–3)-4,6-(1-carboxyethylidene)-β-d-glucose-(1–4)-β-d-glucose-(1–6)-α,α-(1″-1′)-trehalose-4″-O-alkanoyl-6′-O-alkenoate | Mycobacterium smegmatis[48], [49] | Actinobacteria |
| 6- Glycosylated fatty alcohols | ||
| Alkane 1,2-diol glycoside; Hexose 1-(O-hexose)alk-2-yl alkanoate (Diol = C19-20, alkanoate = C14-16) | Roseiflexus castenholzii[50] | Chloroflexi |
| 1-(O-hexose)-3,25-hexacosanediol and its homologue: 1-(O-hexose)-3,27-octacosanediol | Spp. of cyanobacteria e.g. Anabaena, Nodularia, Calothrix, Synechococcus[51] | Cyanobacteria |
| 1-(O-hexose)-3-keto-25-hexacosanol and its homologue: 1-(O-hexose)-3-keto-27-octacosanol | Cyanobacteria | |
| 1-(O-hexose)-3,25,27-octacosanetriol | Cyanobacteria | |
| 1-(O-hexose)-3-keto-25,27-octacosanediol OR its isomer: 1-(O-hexose)-27-keto-3,25-octacosanediol | Cyanobacteria | |
| 7- Glycosylated macro-lactones/-lactams | ||
| Barsilinolide A/B/C: 2-deoxy-α-l-fucopyranoside of C32-membered macrolactone | Nocardia brasiliensis[52], [53] | Actinobacteria |
| Fluvirucins: amino sugar glycosides of C14-membered macrolactam | Spp. of Actinomadura, Streptomyces, Microtetraspora and Saccharotrix mutabilis | Actinobacteria |
| Vicenistatin: amino sugar (vicenisamine) glycoside of C20-membered macrolactam | Streptomyces sp. [54], [55] | Actinobacteria |
| Vicenistatin M: d-mycarose glycoside of C20-membered macrolactam | Streptomyces sp. [54], [55] | Actinobacteria |
| Erythromycins A, B, D, C, E, F and Erythromycin esters (C14-membered macrolactam glycosides) | Streptomyces erythreus and Nocardia spp, and other Streptomyces spp. [56] | Actinobacteria |
| Oleandomycin (C14-membered macrolactam glycosides) | Streptomyces antibioticus[57] | Actinobacteria |
| Pikromycin, Narbomycin, 5-O-mycaminosyl-narbonolide (C14-membered macrolactam glycosides) | Streptomyces felleus and S. narbonensis[56] | Actinobacteria |
| 10,11-Dihydropikromycin, Kayamicin (C14-membered macrolactam glycosides) | Streptomyces narbonensis[56] | Actinobacteria |
| Spinosyns (Tetracyclic macrolide) containing forosamine (amino sugar) and tri-O-methyl rhamnose | Saccharopolyspora spinosa[56], [58] | Actinobacteria |
| Lepicidin A | Saccharopolyspora spinosa[56] | Actinobacteria |
| Leucomycins, Josamycin, Platenomycins, Medicamycin, Espinomycins | Streptomyces kitasatoensis[56] | Actinobacteria |
| Carbomycin B, platenomycins W1/W2, Niddamycin, Midecamycin A3/A4 | Streptomyces platensis[56] | Actinobacteria |
| Acumycin (cirramycin B), Cirramycin F and derivatives | Streptomyces griseoflavus, S. fradiae, S. flocculus[56] | Actinobacteria |
| Chalcomycin, Neutramycin | Streptomyces bikiniensis, S. rimosus, S. hirsutus[56] | Actinobacteria |
| Aldgamycin F, E and Swalpamycin | Streptomyces lavendulae, S. avidinii, S amandii (for swalpamycin) [56] | Actinobacteria |
| Spiramicins | Streptomyces ambofaciens[56], [59] | Actinobacteria |
| Tylosins | Streptomyces fradie, S. hygroscopicus[56], [60] | Actinobacteria |
| Concanamycins | Streptomyces diastatochromogenes[56] | Actinobacteria |
| Tetrins and related compounds, Maduralide | Streptomyces sp. [56] | Actinobacteria |
| Pimaricin | Streptomyces natalensis[56] | Actinobacteria |
| Colubricidin A | Streptomyces sp. [56], [61] | Actinobacteria |
| Nystatin | Streptomyces noursei[62] | Actinobacteria |
| Amphotericin B | Streptomyces nodosus[63] | Actinobacteria |
| Oasomycins, Desertomycins | Streptomyces cinnamoneus (previsouly Streptoverticillium baldacci) | Actinobacteria |
| Rapamycin | Streptomyces hygroscopicus[64] | Actinobacteria |
| Avermectins | Streptomyces avermetilis[65] | Actinobacteria |
| PM100117 and PM100118 | Streptomyces caniferus | Actinobacteria |
| 8- Glycomacrodiolides (glycosylated macrocyclic dilactones) | ||
| Glucolipsin A, B: dilactone of two glucosides of 3-hydroxy fatty acids C19/C19 | Streptomyces purpurogeniscleroticus, Nocardia vaccinii[66] | Actinobacteria |
| Fattiviracin A1: dilactone of two glucosides of 3-,17-, ω-1-trihydroxy fatty acids C24/C33 | Kibdelosporangium albatum[67] | Actinobacteria |
| Cycloviracin B1 and B2: dilactones glucosides of 3-,19-, ω-1-trihydroxy fatty acids (C22–28) and of 3-,17-, ω-1-trihydroxy fatty acids (C22–24) | Streptomyces microflavus[67] | Actinobacteria |
| Elaiophylins, Efomycin G | Streptomyces spp. [68] | Actinobacteria |
| Halichoblelides A, B, C | Streptomyces spp. [69], [70], [71] | Actinobacteria |
| Bispolides A1, A2, A3, B1, B2a, B2b and B3 | Microbispora species[72] | Actinobacteria |
| Macroviracins A-D: related to fattiviracin and cycolviracins | Streptomyces sp. [73] | Actinobacteria |
| 9- Glyco-carotenoids/-terpenoids: | ||
| 9.1-Acyclic glycocarotenoids | ||
| Rhodopsin glucoside | Halorhodospira abdelmalekii, H. halochloris[74] | Proteobacteria |
| Dihydroxylycopene mono-/di-glucosides and their acyl (C12:0 or C14:1) derivatives | Halorhodospira abdelmalekii, H. halochloris[74] | Proteobacteria |
| d-Glucosyl 4,4″-diapocarotene-6,6′-dioic acid | Pseudomonas rhodos[75], Rhizobium lupini[76], [77] | Proteobacteria |
| 1′-glucosyloxy-3′,4′-didehydro1′,2′-dihydro-ψ,ψ-carotene monoester | Chondromyces apiculatus[78], Myxococcus fulvus[79] | Proteobacteria |
| Staphyloxanthin: α-d-glucopyranosyl 1-O-(4,4′-diaponeurosporen-4-oate) 6-O-(12-methyltetradecanoate) | Staphylococcus spp. [80] | Firmicutes |
| 4-D-glucopyranosyloxy-4,4′-diaponeurosporene | Streptococcus faecium[81] | Firmicutes |
| Hydroxy-diaponeurosporene glucoside esters | Heliorestis sp. [82] | Firmicutes |
| Rhodopin β-D-glucoside, Rhodopinal β-D-glucoside | Rhodopseudomonas acidophila, Rhodospirillum tenue and Rhodocyclus purpureus[83] | Proteobacteria |
| Oscillaxanthin: 1,1′-dihydroxy-2,2′-di-β-l-rhanmosyl- 1,2,1′,2′-tetrahydro-3,4,3′,4′-tetradehydrolycopene | Oscillatoria rubescens[84] | Cyanobacteria |
| Bacterioruberin mono- and di-glycosides | Unidentified Halophilic bacterium[85] | Proteobacteria |
| Diapolycopenedioic acid xylosyl esters A, B, and C | Rubritalea squalenifaciens[86] | Verrucomicrobia |
| Methyl 5-glucosyl-5,6-dihydro-apo-4,4′-lycopenoate | Planococcus maritimus[87] | Firmicutes |
| Vancoresmycin | Amycolatopsis[88] | Actinobacteria |
| 9.2-Monocyclic glycocarotenoids | ||
| Salinixanthin | Salinibacter ruber[89], Rhodothermus marinus[90] | Bacteroidetes |
| Phleixanthophyll, 4-ketophleixanthophyll | Mycobacetrium phlei[91] | Actinobacteria |
| Phleixanthophyll palmitate: (2′-S)-1′-[(6-O-palmityl-β-d-glucopyranosyl)oxy]-3′,4′-didehydro-1′,2′-dihydro-β,ψ-caroten-2′-ol | Nocardia sp. [92] | Actinobacteria |
| 1′-[(6-O-acyl-β-d-glucopyranosyl)oxy]-1′,2′-dihydro-β,ψ-caroten-4-one | Rhodococcus rhodochrous[93], [94] | Actinobacteria |
| Myxobactone | Myxococcus fulvus[79], [95] | Proteobacteria |
| Myxobactin | Myxococcus fulvus[96] | Proteobacteria |
| Keto-myxocoxanthin glucoside ester (Myxobactone ester) | Roseiflexus castenholzii[97] | Chloroflexi |
| OH-γ-carotene glucoside laurate: 1'-[(6-O-lauryl-β-d-glucopyranosyl)oxy]-1′,2′-dihydro-β,ψ-carotene | Chlorobium tepidum[98] | Chlorobi |
| OH-chlorobactene glucoside laurate; 1'-[(6-O-lauryl-β-d-glucopyranosyl)oxy]-1′,2′-dihydro-ɸ,ψ-carotene | Chlorobium tepidum[98] | Chlorobi |
| OH-γ-carotene glucoside ester derivative | Chloroflexus aurantiacus[99] | Chloroflexi |
| 1′-β-glucopyranosyl-3,4,3′,4′-tetradehydro-1′,2′-dihydro-β,ψ-caroten-2-one | Meiothermus ruber[100] | Deinococcus-Thermus |
| Myxoxanthophyll like glycocarotenoid: (3R,2′S)-myxol-2′-(2,4-di-O-methyl-α-L-fucoside) | Synechocystis sp. [101] | Cyanobacteria |
| Sioxanthin; (2′S)-1′-(β-D-glucopyranosyloxy)-3′,4′didehydro-1′,2′-dihydro-φ,ψ-caroten-2′-ol | Salinispora sp. [102] | Actinobacteria |
| 9.3-Bicyclic glycocarotenoids | ||
| Corynexanthin monoglycoside | Corynebacterium sp. [103] | Actinobacteria |
| Corynexanthin diglycoside | Arthrobacter sp [104] | Actinobacteria |
| Sarcixanthin monoglucosides | Curtobacterium flaccumfaciens[105], Micrococcus luteus[106], M. yunnanensis[107]. | Actinobacteria |
| Sarcixanthin diglucosides | Micrococcus luteus[106], M. yunnanensis[107]. | Actinobacteria |
| Zeaxanthin mono- and di-glucosides | Erwinia herbicola, Rhodobacter sphaeroides[108] | Proteobacteria |
| Zeaxanthin mono- and di-rhamnosides (mainly Z-isomers), Zeaxanthin di-glucoside | Sulfolobus shibatae[109] | Archeabacteria |
| Zeaxanthin mono- and di-rhamnosides | Corynebacterium autotrophicum (Xanthobacter autotrophicus) [110] | Proteobacteria |
| Aastaxanthin dirhamnoside | Sphingomonas astaxanthinifaciens[111] | Proteobacteria |
| Myxocoxanthin rhamnoside | Sorangium compositum[112] | Proteobacteria |
| Thermozeaxanthin-13, -15, and -17 (Zeaxanthin mono-β-D-glucoside-branched fatty acid esters) | Thermus thermophilis[113] | Deinococcus-Thermus |
| Thermobiszeaxanthin-13-13, -13-15, and -15-15 (Zeaxanthin di-β-D-glucoside-branched fatty acid esters) | Thermus thermophilis[113] | Deinococcus-Thermus |
| Adonixanthin and astaxanthin glucosides | Agrobacterium aurantiacum[114], [115] | Proteobacteria |
| Decaprenoxanthin mono- and diglucoside (2R,6S,2′R,6′S)-(2,2′-bis(4-hydroxy-3-methyl-2-butenyl)ε,ε-carotene) di-β-D-glucoside | Corynebacterium glutamicum[116] | Actinobacteria |
| 10- Glycosylated hopanoids/sterols | ||
| Bacteriohopanetetrol cyclitol ether | Chloracidobacterium thermophilum[117], [118] | Acidobacteria |
| BHT cyclitol | Burkholderia cenocepacia[119], Zymomonas mobilis[120],[121] | Proteobacteria |
| BHT glucosamine | Burkholderia cenocepacia[119], Zymomonas mobilis[120], [121] | Proteobacteria |
| O-α-d-Glucuronopyranosyl BHT | Rhodospirillum rubrum[122] | Proteobacteria |
| Cholesteryl-α-d-glucopyranoside | Helicobacter pylori[123] | Proteobacteria |
| Cholesteryl-6-O-tetradecanoyl-α-d-glucopyranoside, Cholesteryl-6-O-dodecanoyl-α-d-glucopyranoside | Helicobacter pylori[123], H. felis, H. muridarum, H. mustelae, H. fennelliae, and H. cinaedi[124] | Proteobacteria |
| Cholesteryl-6-O-acyl-β-d-galactopyranoside | Borrelia burgdorferi, B. garinii and B. afzelii[125] | Spirochaetes |
| Cholesteryl-6-O-acyl-β-d-glucopyranoside | Borrelia hermsii[125] | Spirochaetes |
| Cholesteryl-β-d-glucopyranoside and its 3,4,6-triacyl derivatives | Mycoplasma gallinarum[126] | Tenericutes |
| Cholesteryl-α,α′-di-D-glucoside; α-d-glucopyranosyl-(1 → 3)-(O-acyl)-α-d-glucopyranosyl-(1 → 3)-cholesterol | Acholeplasma axanthum[127] | Tenericutes |
Table 2.
Chemical groups and members of fungal simple glycolipids as well as names and phyla of their native producers.
| Common name: Chemical names (Cx: chain length of fatty acid chains) | Producer | Phylum |
|---|---|---|
| Fungi | ||
| 1- Mannosyl-erythritol lipids (MEL, Ustilipids) and MEL congeners | ||
| MEL | Ustilago maydis, Pseudozyma (Candida) antarctica[128], [129], Kurtzmanomyces[130] | Basidiomycota |
| MEL | Geotrichum candidum[131] | Ascomycota |
| Mannosylmannitol lipids (MML), mannosylribitol lipids (MRL) and mannosylarabitol lipids (MAL) | Pseudozyma parantarctica[132] | Basidiomycota |
| 2- Cellobiose lipids (CL, Ustilagic acids) | ||
| Cellobiose (β-D-Glc-(1 → 4)-β-D-Glc) 2″-O-hexanoic acid 1-O-16-ω,ω-1-dihydroxyhexadecanoate or 1-O-16-ω,ω-1,α-trihydroxy hexadecanoate | Ustilago maydis[128] | Basidiomycota |
| Cellobiose 6′-O-acetyl-2″-O-β-hydroxyalkanoyl-1-O-16-ω,ω-1-dihydroxyhexadecanoate or 1-O-16-ω,ω-1,α-trihydroxy hexadecanoate methyl ester | Ustilago maydis[128], Pseudozyma fusiformata[133] | Basidiomycota |
| Cellobiose 16-O-ω,ω-1-dihydroxyhexadecanoate or 1-O-16-ω,ω-1,α-trihydroxy hexadecanoate | Sympodiomycopsis paphiopedili[134] | Basidiomycota |
| Microcin: cellobiose 2″,3″,4″,6″,6′-penta-O-acetyl-1-O-16-ω,α-dihydroxyhexadecanoate | Cryptococcus humicola[135] | Basidiomycota |
| Flocculosin: 2-(2′,4′-diacetoxy-5′-carboxy-pentanoyl) octadecyl cellobioside | Anthracocystis (Pseudozyma) flocculosa[136] | Basidiomycota |
| 3- Sophorolipids | ||
| Sophorose (β-D-Glc-(1 → 2)-β-D-Glc) -1-O-16-ω,ω-1-dihydroxyalkanoate or 1-O-16-ω,ω-1,α-trihydroxy alkanoate (C16-C18:0-2) | Starmerella (Candida) bombicola, Candida apicola and other spp.[137] | Ascomycota |
| Sophorose (β-D-Glc-(1 → 2)-β-D-Glc) -1-O-16-ω,ω-1-dihydroxyalkanoate or 1-O-16-ω,ω-1,α-trihydroxy alkanoate (C16-C18:0-2) | Cryptococcus curvatus[137] | Basidiomycota |
| Sophorose 6′-mono-O-acetyl or 6′,6″-di-O-acetyl -1-O-16-ω,ω-1-dihydroxyalkanoate or 1-O-16-ω,ω-1,α-trihydroxy alkanoate (C16-C20:1) | Starmerella (Candida) bombicola, Candida apicola and other spp.[137], Wickerhamiella domercqiae [138], [139] | Ascomycota |
| Sophorose lipid lactonic/ring form, lactonization of free carboxyl group with C-4″ or C-6'' (intramolecular ester bonds) | Starmerella (Candida) bombicola, Candida apicola and other spp.[137] | Ascomycota |
| Dimeric and trimeric sophorolipids (intermolecular ester bonds between carboxyl of one molecule to C-4″ of another molecule) | Candida spp. [140] | Ascomycota |
| 4- Glucosyl-di-xylosyl lipids (Glykenins) | ||
| Glykenins A, B, C: O-β-d-glucose-(1 → 2)-O-β-d-xylose-(1 → 2)-O-β-d-xylose tetrahydroxyhexacosanoic acids, mono-di or tri-acetylated | Basidiomycetous sp. [141] | Basidiomycota |
| 5- Polyol fatty acid esters (Liamocins and their congeners) | ||
| Liamocins | Aureobasidium pullulans | Ascomycota |
| Mannitol and pentitol esters of 3-D-hydroxypalmitic and 3-D-hydroxystearic acids | Rhodotorula glutinis and Rhodotorula graminis | Basidiomycota |
| 6- Glucosyl and mannosyl lipids | ||
| Monoglucosyloxyoctadecenoic acid | Aspergillus niger[142] | Ascomycota |
| Halymecin B: mannosylated tetramer of 3,5-dihydroxydecanoic acid | Fusarium sp. [143] | Ascomycota |
| Halymecins F: acetylated halemycin B, halymecin G: mannosylated trimer of 3,5-dihydroxydecanoic acid | Simplicillium lamellicola[144] | Ascomycota |
| (3R,5R)-3-O-β-d-mannosyl-3,5-dihydroxydecanoic acid | Simplicillium lamellicola[144] | Ascomycota |
| 7- Glycosylated polyketides | ||
| Roselipin 1, 2: 2,4,6,8,10,12,14,16,18-nonamethyl-5,9,13-trihydroxy-2E,6E,10E-icosenoic acid mannosylated (+-acetylated) at C-13, D-arabitol ester [145], [146] | Gliocladium[145], [146] | Ascomycota |
| TMC-151 A ∼ F: 2,4,6,8,10,12,14,16,18-nonamethyl-5,9,13-trihydroxy-2E,6E,10E-icosenoic acid mannosylated (+-acetylated) at C-13, D-mannitol ester [145], [146] | Gliocladium catenulatum[147] | Ascomycota |
| TMC-154: isolmeric form of roselipin 1 and TMC-171 A ∼ C: as roselipin 3 but esterified to mannitol | Gliocladium[148] | Ascomycota |
| Roselipins 3A to 3E: 14,15-dehydro derivatives of roselipin 1A/B | Clonostachys candelabrum[149] | Ascomycota |
| Cladionol A: 15-mannosyl-2,4,6,8,10,12,14,16,18,20-decamethyl-3,7,11,15-tetrahydroxy-4E,8E,12E-docosenoic acid arabitol ester | Gliocladium[150] | Ascomycota |
| 8- Glucosyl-galactosyl lipids | ||
| Emmyguyacin 1A: α-d-glucopyranosyl-α-d-galactopyranose 3′-O-hydroxydocosanoate with 17-((carboxycarbonyl)oxy) group of oxalate ester at OH of C-17 | Fungal species[151] | NA |
| Emmyguyacin 1B: Trehalose 3′-O-docosanoate with 17-((carboxycarbonyl)oxy) group of oxalate ester at OH of C-17 | Fungal species[151] | NA |
| Emmyguyacin 2: as emmyguyacin 1A without the oxalate ester | Fungal species[151] | NA |
| 9- Glycosylated sterols | ||
| Ergosterol-β-d-glucopyranoside | Pichia pastoris, Sordaria macrospora, Rhynchosporium secalis[152] | Ascomycota |
| 10- Glycosylated paraconic acids | ||
| Gobienines A/B/C (non-confirmed structure [28]) | Acarospora gobiensis (Lichen) [27] | Ascomycota |
Fig. 3.
Structures of prototypic members of bacterial and fungal simple glycolipid (SGL) groups. The glycosyl and lipid residues are colored in red and blue, respectively. Bacterial and fungal SGLs are represented in the upper and lower halves (separated by a line) of the figure, respectively. The representative structure of fungally produced glycosylated paraconic acids (20th group of SGLs) is not given as their structures have been debated [27], [28].
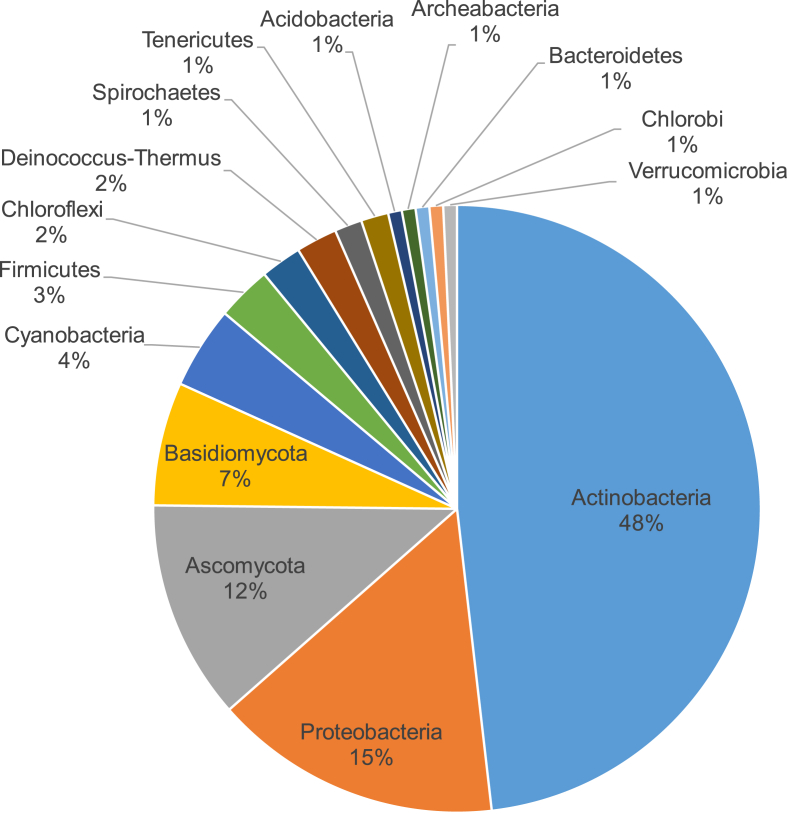
Based on our survey of microorganisms producing SGLs, we found that 50% of all known microbial SGLs are produced by microbes belonging to the phylum Actinobacteria (Fig. 2). Second in rank to Actinobacteria, comes phylum Proteobacteria followed by the two major fungal phyla, Ascomycota and Basidiomycota, consecutively (Fig. 2).
Fig. 2.
Approximate distribution of microbial producers of simple glycolipids in different bacterial and fungal phyla. Incidences of microbial production of chemically unique SGLs in every microbial phylum were counted. Homologues or stereoisomers of the same chemically unique SGLs did not add into these calculations to avoid false overestimations. As an example, rhamnolipids (RLs) exist in two unique structures known so far, mono-rhamnolipids and di-rhamnolipids containing one and two rhamnose moieties, respectively, and are produced by proteobacterial species. Although these two RL congeners has several homologues varying in chain length of their lipid moiety, they were counted as two chemically unique SGLs in our calculations. Only the phylum of the microbial producer and not its genus and species identity that was taken into account; for example, although di-rhamnolipids are produced by different species of the genus Pseudomonas and Burkholderia, all di-rhamnolipids scored one hit in our calculations because all these di-rhamnolipids producers belong to the same phylum, Proteobacteria.
4.1. Bacterial simple glycolipids
Overall, bacterially produced simple glycolipids (SGLs) outnumbers fungally-produced ones (Fig. 2). A previous survey of about 16000 pooled natural bacterial metabolites revealed that about 20% of them are glycosylated, about 30% of these glycosylated metabolites are glycosylated lipids of which glycosylated macro-lactones/-lactams take a share of about 20% and other glycosylated lipids (including SGLs) take a share of 10% [26]. Nearly all glycosylated macro-lactones/-lactams are produced by members of the phylum Actinobacteria. We classified baterially produced SGLs in 10 groups (Table 1).
4.2. Fungal simple glycolipids
Fungally produced simple glycolipids (SGLs) are less numerous than bacterially-produced ones (Fig. 2). Fungal SGLs are classified in 10 groups that are mainly produced by members of the phyla Ascomycota and Basidiomycota (Table 2).
5. Physiological roles of simple glycolipids
For most SGLs, the exact physiological roles to their native producers are not clearly known. Generally, SGLs are secondary metabolites that are not essential for cell viability. Nonetheless, given their antimicrobial properties, SGLs are suggested to help producing organism dominate environmental niches by inhibiting the growth of other organisms [153]. In addition, SGLs are required to coordinate multicellular or group behaviors (biofilm formation and swarming) and enhance growth of producing organisms on hydrophobic carbon sources [154], [155], [156]. Some additional roles are assigned to specific SGLs like rhamnolipids, which are considered as virulence factors that modulate host immune response [29]. Similarly to their unglycosylated counterparts, glycosylated carotenoids are postulated to act as photoprotectants and antioxidants to protect organisms from injuries caused by free radicals and active oxygen species [106]. In thermophiles, glycocarotenoids are thought to stabilize and reinforce cell membranes [113]. Hopanoids are sterol analogues in bacteria. Similarly to sterol in eukaryotes, hopanoids and their glycosylated derivatives are thought to help stabilize and regulate membrane fluidity and permeability particularly during shifts in pH and other physicochemical conditions [117], [157]. Sophorolipids are suggested to act as extracellular forms of carbon storage that can be recycled later under starvation conditions [154].
6. Bioactivities of simple glycolipids
Simple glycolipids (SGLs) have very interesting biological activities on other organisms ranging from viruses to human cells. Although the mechanism of these bioactivities is not definitively known, it is suggested that most of SGLs bioactivities arise from their surface activities. Collectively, many of them have antiviral, antimicrobial, anti-inflammatory and anticancer activities (Table 3). Many reviews are found in literature detailing the potential biomedical and cosmeceutical applications of biosurfactants in general, many of which are simple glycolipids [8], [158], [159], [160].
Table 3.
Biological activities of different chemical groups of microbial simple glycolipids.
| Chemical group of simple glycolipids (SGLs) | A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibacterial | Antifungal | Antiviral | Antiparasitic | Anticancer | ↑ Cell differentiationa | Immunomodulatory | Antioxidant | Antiadherent (Biofilm, wounds) | Neuronal activity | Sperm immobilizing activity | ↓ Diacylglycerol acyl transferase 2a | ||
| Bacterial SGLs | |||||||||||||
| 1 | Rhamnolipids | A1 | B1 | C1 | D1 | G1 | I1 | ||||||
| 2 | Glycolipids (Rubiwettin) | ||||||||||||
| 3 | Trehalolipids | C3 | E3 | F3 | G3 | ||||||||
| 4 | Other glycosylated mycolates | ||||||||||||
| 5 | Oligosaccharide lipids | E5 | |||||||||||
| 6 | Glycosylated fatty alcohols | ||||||||||||
| 7 | Glycosylated macro-lactones/-lactams | A7 | B7 | C7 | D7 | E7 | G7 | ||||||
| 8 | Glycomacrodiolides | A8 | B8 | C8 | D8 | E8 | G8 | ||||||
| 9 | Glyco-carotenoids/-terpenoids | G9 | H9 | ||||||||||
| 10 | Glycosylated hopanoids | ||||||||||||
| Fungal SGLs | |||||||||||||
| 11 | Mannosyl-erythritol lipids | A11 | E11 | F11 | H11 | J11 | |||||||
| 12 | Cellobiose lipids | A12 | B12 | ||||||||||
| 13 | Sophorolipids | C13 | F13 | G13 | I13 | K13 | |||||||
| 14 | Glucosyl-di-xylosyl lipids (Glykenins) | A14 | |||||||||||
| 15 | Polyol fatty acid esters | A15 | E15 | ||||||||||
| 16 | Glucosyl and mannosyl lipids | A16 | E16 | ||||||||||
| 17 | Glycosylated polyketides | C17 | D17 | L17 | |||||||||
| 18 | Glucosyl-galactosyl lipids | C18 | |||||||||||
| 19 | Glycosylated sterols | ||||||||||||
| 20 | Glycosylated paraconic acids | ||||||||||||
| References: | ||||
| A1: [23] | B8: [161], [162] | D7: [58], [61], [65] | F3: [36], [163], [164] | H9: [165] |
| A7: [56] | B12: [135], [166], [167], [168] | D8: [169], [170] | F11: [171], [172], [173], [174], [175] | H11: [129] |
| A8: [72], [176], [177] | C1: [178] | D17: [149] | F13: [171] | I1: [179], [180], [181] |
| A11: [182] | C3: [183] | E3: [47], [184] | G1: [185]. | I13: [186], [187]. |
| A12: [188], [189], [190] | C7: [191] | E5: [32], [47] | G3: [192], [193] | J11: [131] |
| A14: [194] | C8: [56], [73] | E7: [195] | G7: [53], [64], [196] | K13: [197] |
| A15: [198], [199], [200] | C13: [197] | E8: [70], [71], [176] | G8: [201] | L17: [202] |
| A16: [144] | C17: [203] | E11: [204], [205] | G9: [206] | |
| B1: [23], [158], [207], [208], [209], [210], [211], [212]. | C18: [151] | E15: [198] | G13: [213], [214] | |
The signs ↑ and ↓ denotes for stimulation and inhibition, respectively.
7. Biosynthesis of simple glycolipids
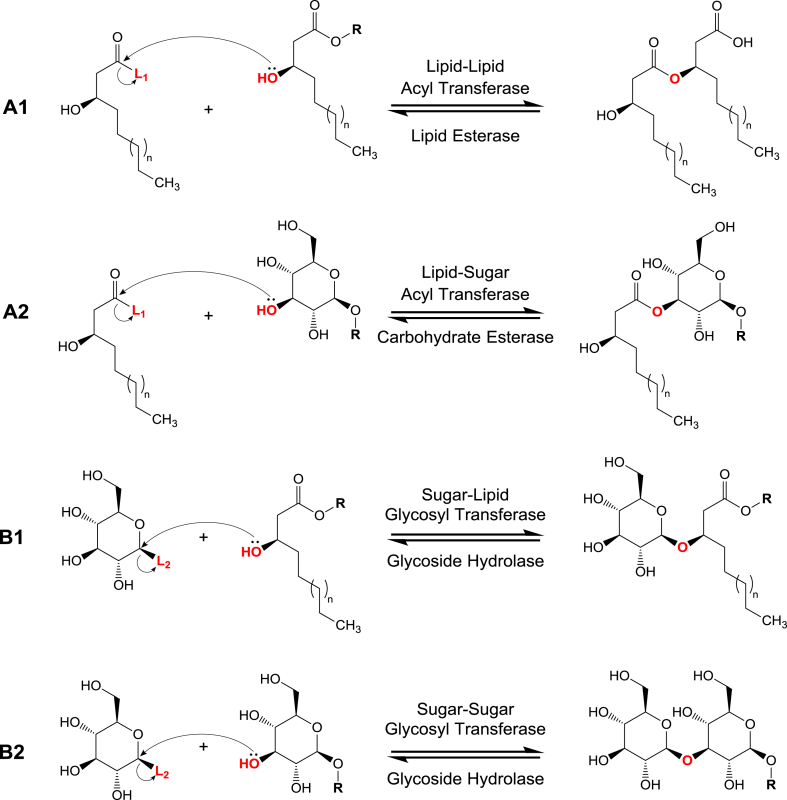
With few exceptions, the exact biosynthetic steps of majority of simple glycolipids (SGLs) are not yet fully understood. Generally however, biosynthesis of SGLs implicates the supply and linking of glycosyl and lipid precursors. Pathways supplying glycolipid precursors are depicted later (Fig. 5) and are thought to play an important role in regulation of SGLs biosynthesis. Linking of glycosyl and lipid precursors is mostly via O-glycosidic or ester bonds (Fig. 1B) that are formed by glycosyltransferases (GT) (Fig. 4 B1, B2) [215] or acyltransferases (AT) [216] (Fig. 4 A1, A2), respectively. Glycosyltransferases catalyze the transfer of the sugar moiety from an activated glycosyl donor, usually sugar-nucleotide (Leloir GTs) or –phosphate (non-Leloir GTs), to a lipid acceptor (or a sugar acceptor for extending the sugar backbone of glycolipids), by making glycosidic bonds between the hydroxyl groups (nucleophile) of the acceptor and the anomeric carbon of the sugar donor (Fig. 4 B1, B2) [215]. Acyltransferases (AT) catalyze the transfer of the lipid moiety from an activated acyl donor, mostly acyl-CoA or -ACP, to a glycosyl acceptor (or a lipid acceptor for extending the lipid backbone of the glycolipid) by making an ester bond between the hydroxyl group (nucleophile) of the acceptor and the acyl donor's carbonyl group [216] (Fig. 4 A1, A2).
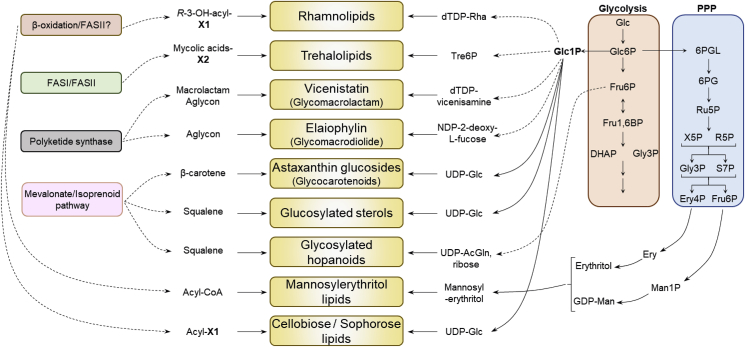
Fig. 5.
Sugar and lipid precursors of prominent members of simple glycolipid groups and their furnishing pathways. Biosynthesis of simple glycolipids that harbor glucoside units, like glucosides of astaxanthin [115] and zeaxanthin [223] as well as glucosylated sterols [224], cellobiose and sophorose lipids [225], require UDP-glucose as glycosyl donor. Peculiar glycosyl donors that are activated in other ways than UDP are required in case of rhamnolipids [226], trehalolipids [227], vicenistatin [228] and elaiophylin [68]. All glycosyl donors are derived from glucose -1-phosphate except that of glycosylated hopanoids whose glycosyl donors is derived from β-d-fructofuranose-6-phosphate and ribose [119]. Mannosylerythritol lipids are expected to derive the glycosyl unit, mannosylerythritol, from erythritol and GDP-mannose which originate from d-erythrose-4-phosphate and β-d-fructofuranose-6-phosphate intermediates of the pentose phosphate pathway as described in the yeast Yarrowia lipolytica[229], [230]. The lipid moiety originates mostly from fatty acid synthesis and/or β-oxidation except glycosylated macrolides, carotenoids and sterols/hopanoids whose lipid moiety is furnished from the polyketide for the former and from mevalonate/isoprenoid pathway for the latter two groups. 6PGL: 6-phosphogluconolactone; 6PG: 6-phosphogluconic acid; Ru5P: d-Ribulose-5-phosphate; R5P: d-Ribose-5-phosphate; X5P: d-Xylulose-5-phosphate; S7P: d-Sedoheptulose-7-phosphate; Gly3P: D-Glyceraldehyde-3-phosphate; DHAP: Dihydroxyacetone phosphate; Ery4P: d-Erythrose-4-phosphate; Fru6P: β-d-Fructofuranose-6-phosphate; Fru1,6BP: β-d-Fructofuranose-1,6-bisphosphate; dTDP-Rha: dTDP-rhamnose; Tre6P: Trehalose-6-phosphate; Glc: d-Glucopyranose; Glc1P: Glucose-1-phosphate; Glc6P: Glucose-6-phosphate; UDP-glc: Uridine diphosphate glucose; UDP-AcGln: Uridine diphosphate N-acetylglucosamine; Man1P: Mannose-1-phosphate; GDP-Man: GDP-mannose; R-3-OH-acyl-X1: R-3-hydroxy acyl-X1 (X1 = -CoA/-ACP); X2 = Mycolyl Carrier Protein. PPP: Pentose Phosphate Pathway. Dashed lines means that multiple biosynthetic steps are involved.
Fig. 4.
Key enzymes of glycolipid biosynthesis and hydrolysis. Last steps of glycolipid biosynthesis involves linking of sugar and lipid moieties via either or both Acyl Transferases (AT) (A1 and A2, forward reactions) and Glycosyl Transferases (GT) (B1 and B2, forward reactions) which catalyze the ester and glycosidic bonds formation, respectively. Glycolipids are catabolized or broken down by Lipid Esterase (LE), Carbohydrate Esterases (CE) and Glycoside Hydrolases (GH) that hydrolyze the bond between alkyl-alkanoate ester, acyl-sugar ester and glycosidic bonds, respectively (reverse reactions). L1: Coenzyme A (CoA-S-) or Acyl Carrier Protein (ACP-S-) activating groups on acyl donors; L2: Nucleotides or phosphates activating groups on glycosyl donors. R: any substitution that could be glycosyl, lipid, or glycolipid units. Notes: β-glucose and R-3-hydroxyalkanoate are used as examples of any sugar and hydroxyl fatty acid of any chain length (n), respectively. Hydrolysis reactions do not generate activated products.
Concerning the fate of SGLs, one report showed that the flocculosin GL can be degraded by its producing yeast, Pseudozyma flocculosa, which feeds on it under nutrient limitations [153]. Glycolipids could theoretically be hydrolyzed by one or more of the following enzymes. First, glycoside hydrolases (GH) that hydrolyze the sugar-sugar or sugar-lipid glycosidic bonds (Fig. 4 B1, B2) [153], [217]. Second, carbohydrate esterases (CE) hydrolyze the sugar-lipid ester bonds (Fig. 4 A2). Lipid esterases (LE), also known as lipases, hydrolyze lipid-lipid ester bonds (Fig. 4 A1) in glycolipids with multimeric hydroxyalkanoate lipid moieties e.g. rhamnolipids (Fig. 3). This hypothesis is corroborated by reports showing the hydrolysis of polymeric hydroxyalkanoates, which share the same lipid moieties as rhamnolipids, by the action of microbial lipases/esterases [218], [219], [220]. Nonetheless, the metabolic fate of SGLs is one of the subjects that require thorough investigations.
Among the poorly studied aspects in SGLs metabolism also are the transport SGLs across microbial membranes. Some SGLs require active transport for their exportation out of the cell, like cellobiose lipids, mannosylerythritol lipids [221] and sophorolipids [222], whereas, many other SGLs are thought to passively diffuse out of the cell.
To give a general overview, we present the general biosynthetic map of SGLs showing the diversity of the immediate glycosyl and lipid precursors of SGLs and the pathways furnishing them (Fig. 5).
8. Metabolic engineering of simple glycolipids
Metabolic engineering can be employed to satisfy demand for simple glycolipids (SGLs) by offering solutions to the main challenges facing their production and commercialization. The most important challenge is the high cost of production of SGLs at high purities to qualify for medical or cosmeceutical applications (usually >90–95% purities are required) [21], [22]. This high cost stems from a multiplicity of factors including the inherent low yield/productivity of microbial SGLs, costly raw nutritive materials, expensive biosafety containment measures when using pathogenic SGLs producers, expensive/laborious foam control and expensive downstream processing and purification. Futhermore, SGLs are in many cases naturally produced as mixture of homologues/congeners that are difficult to separate; this makes the study and attribution of a specific activity to a specific SGL homologue/congener unattainable. Lastly, there is accumulating evidence that SGLs biosynthesis is tightly regulated in native producers e.g. rhamnolipids production in Pseudomonas aeruginosa [226] and sophorolipids production in Starmerella bombicola [222]. These tight genetic and metabolic regulations possibly explain the limited improvement in SGL yields using simple optimization media components and process conditions in native SGL producers. One should not be misled, however, by the extraordinarily high GL yields reported in literature that are obtained through media optimization, particularly for rhamnolipids [231]. Such reports are questionable due to different quantification methods used that vary in their specificity and/or sensitivity. Standardized protocols for SGLs quantification were made recently available [232], [233] and are expected to profoundly minimize discrepancies in quantification values in glycolipid research.
Although their cost-effectiveness is still unclear, chemical synthesis of GL could overcome many of the problems of SGLs production. Nonetheless, chemical synthesis of SGLs is confronted also by many other limitations and concerns. First, the difficult stereoselective synthesis of glycolipids which are mostly chiral molecules. An attempt to chemically synthesize mono-rhamnolipid, that is naturally produced as α-l-rhamnopyranosyl-R-β-hydroxydecanoyl-R-β-hydroxydecanoate, resulted in the inevitable co-production of three other diastereomers with different configurations of the β-hydroxyl groups (R,R; R,S: S,S; S,R) and different surface activities [234], [235], [236]. Second, certain ecological/health issues are associated with synthetic approaches that most probably involve the use of non-sustainable petrochemical substrates and generate toxic waste products [237]. Thirdly, the biodegradability and toxicity issues of co-produced new-to-nature SGLs diastereomers require attention and investigation.
Genetic engineering and synthetic biology could offer promising ecological solutions to current challenges facing SGLs production, particularly after recent advances in metabolic engineering and tools for cloning and heterologous expression of large biosynthetic pathways. The following sections discuss some of the metabolic engineering strategies for SGLs production.
8.1. Engineering heterotrophic carbon source utilization
Raw nutritive materials accounts for approximately more than 85% of the total estimated production/operation costs of SGLs [21]. A wide range of low-cost renewable raw materials were suggested for SGLs production [238], [239], yet, the capacity of GL producers to utilize these raw materials should be investigated or genetically engineered in the selected production host. A successful example of the latter is the engineering of P. aeruginosa strain to utilize whey waste for RLs production via heterologous expression of E. coli lac genes [240]. Likewise, bacterial and fungal GL producers could be engineered to utilize cheap waste lignocellulosic wastes [241], [242], [243]. Although enhancing the utilization of waste oils by expression of lipid esterases seems a good strategy given the low cost and high GL yields [244], [245] associated with these oily carbon sources, these carbon sources are, however, cumbersome during recovery of glycolipids as they necessitate extra steps for their removal adding to the net cost of glycolipids recovery [246].
8.2. Heterologous expression of GL biosynthetic pathway
Containment of biosafety level 2 organisms contribute remarkably in the operational costs of simple glycolipids (SGLs) production. Moreover, working with pathogenic or opportunistic pathogens presents a health risk to manufacturing personnel as well as to public and environment. Heterologous expression of GL biosynthetic genes in hosts that are Generally Recognized As Safe (GRAS) is, therefore, a promising solution as it would require a less costly biosafety level 1 manufacturing facility.
Heterologous expression of rhamnolipids (RLs) in non-pathogenic hosts has received much attention because of the large commercial potential of RLs and because the main and best RLs producer is the opportunistic pathogenic bacterium Pseudomonas aeruginosa [247]. One example is the successful expression of rhamnolipids biosynthetic genes of P. aeruginosa in non-pathogenic bacteria, namely P. putida [246] and P. fluorescens [248] as well as in E. coli [249], [250], the best of which was recombinant P. putida [246], though, all recombinant strains produced RLs at much lower yields than the native producer. Interestingly, the non-pathogenic strain P. chlororaphis is naturally producing mono-rhamnolipids and not di-rhamnolipids as it lacks the gene coding for the second rhamnosyltransferase, rhlC [251]. Heterologous expression of rhlC from P. aeruginosa in P. chlororaphis resulted in production of di-RL at concentration more than twice that of mono-RL [251]. Burkholderia kururiensis is another nonpathogenic heterologous host that was successfully engineered for RLs production [252]. Further engineering strategies were reviewed for overproduction of RL [253].
Several glycosylated carotenoids (C40 and C50), e.g. glucosides of decaprenoxanthin and sarcinaxanthin as well as zeaxanthin, were successfully engineered in Corynebacterium glutamicum by overexpression of genes coding for lycopene cyclization, hydroxylation and glycosylation [254].
Interestingly, 17 genes were heterologously expressed in an E. coli strain that is already producing 6-deoxyerythronolide B precursor to produce the glycosylated macrolide, erythromycin C [255]. The cloned genes encoded the deoxysugar, desosamine, biosynthetic enzymes and the enzymes converting 6-deoxyerythronolide B to erythromycin C [255], [256].
Selection criteria for candidate hosts for heterologous glycolipid production should include, in addition to being non-pathogenic, to be natively tolerant to high concentrations of the target SGLs if high productivities are sought [246]. This is particularly important for SGLs which mostly demonstrate antimicrobial activities.
Moreover, the candidate host should, preferably, abundantly produce the precursors required for SGLs biosynthesis. A good approach would be starting with analysis of the intracellular concentration of lipid and glycosyl precursors. One example is the evaluation of the R-specific enoyl-CoA hydratase-2 (ECH-2) activity in crude cell lysate of target host organism as this predicts the potential of this host to synthesize R-3-hydroxyalkanoate precursors [257] that form the lipid part of many R-3-hydroxyfatty acid-containing glycolipids, e.g. rhamnolipids and rubiwettins. The ECH-2 activity was recently reported to be significantly implicated in rhamnolipids biosynthesis [258].
Oleaginous yeasts, like Yarrowia lipolytica and Rhodosporidium toruloides, are potential candidates in view of their already high lipid flux [259], [260]; therefore, they are supposed to have abundant lipid precursors for GLs biosynthesis.
8.3. Blocking competing pathways
Blocking competitive pathways is an important strategy that is expected to enhance GLs biosynthesis. One example is blocking polyhydroxyalkanoates (PHA) synthesis in P. aeruginosa that changed the distribution of produced rhamnolipids (RLs) congeners by doubling the amount of produced mono-rhamnolipids relative to di-rhamnolipids [258]. Also, PHA mutant of P. putida was used to enhance heterologous production of RLs [246].
8.4. Tailoring the GL pool composition
Most simple glycolipids (SGLs) are naturally produced in mixtures of congeners and homologues like rhamnolipids [29] and sophorolipids [222]. For studying the functions and properties of each GL species in these natural mixtures, engineered production of purified GL should be sought. This can be achieved by selectively knocking out the genes coding for biosynthesis of specific congeners or forms. A recent example is the production of sophorolipids pool enriched to 88% in the acidic non-lactonized free form by using a mutant strain of Starmerella bombicola [261] that is defective in lactone esterase [262].
9. Summary and perspectives
More than 5 decades of glycolipids research has led to the discovery of a huge number of simple microbial glycolipids, around 140 of which are cited in this review. In spite of the many publications demonstrating their great biomedical potential, the majority of discovered simple glycolipids are still unable to translate into commercial products because of their high cost of production mainly stemming from low biological yields. Metabolic engineering has the potential to overcome this cost problem particularly after the revolutionary developments in genetic engineering and synthetic biology techniques that were witnessed in the last 5 years.
This review is an attempt to structure the literature available on simple glycolipids aiming at providing metabolic engineers with an outlook on glycolipids and their biosynthesis. It highlights some of aspects and details that are still missing in the biosynthesis, transport and catabolism of glycolipids that need to be pursued and applied profitably in engineering cost-effective microbial glycolipid producers.
Acknowledgement
This work was funded by the United States Department of Energy - Chicago (DoE-Chicago) grant DE-SC0008744 to Professor Gregory Stephanopoulos. Dr. Ahmad M. Abdel-Mawgoud is funded by a postdoctoral fellowship from the Natural Sciences and Engineering Research Council of Canada (NSERC), funding reference number PDF-488195-2016, and partly by the US DoE grant DE-SC0008744 mentioned above. The authors would like to thank Ms. Nada Swedan for her generous shared contribution in drawing chemical structures and filling tables' data.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Schörken U., Kempers P. Lipid biotechnology: industrially relevant production processes. Eur J Lipid Sci Technol. 2009;111(7):627–645. [Google Scholar]
- 2.Herrera-González A. Functionalization of natural compounds by enzymatic fructosylation. Appl Microbiol Biotechnol. 2017;101(13):5223–5234. doi: 10.1007/s00253-017-8359-5. [DOI] [PubMed] [Google Scholar]
- 3.Gantt R.W., Peltier-Pain P., Thorson J.S. Enzymatic methods for glyco(diversification/randomization) of drugs and small molecules. Nat Product Rep. 2011;28(11):1811–1853. doi: 10.1039/c1np00045d. [DOI] [PubMed] [Google Scholar]
- 4.Zhang B. De novo synthesis of trans-10, cis-12 conjugated linoleic acid in oleaginous yeast Yarrowia Lipolytica. Microb Cell Factories. 2012;11(1):51. doi: 10.1186/1475-2859-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beopoulos A. Metabolic engineering for ricinoleic acid production in the oleaginous yeast Yarrowia lipolytica. Appl Microbiol Biotechnol. 2014;98(1):251–262. doi: 10.1007/s00253-013-5295-x. [DOI] [PubMed] [Google Scholar]
- 6.Sabirova J.S. The ‘LipoYeasts’ project: using the oleaginous yeast Yarrowia lipolytica in combination with specific bacterial genes for the bioconversion of lipids, fats and oils into high-value products. Microb Biotechnol. 2011;4(1):47–54. doi: 10.1111/j.1751-7915.2010.00187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varvaresou A., Iakovou K. Biosurfactants in cosmetics and biopharmaceuticals. Lett Appl Microbiol. 2015;61(3):214–223. doi: 10.1111/lam.12440. [DOI] [PubMed] [Google Scholar]
- 8.Lukic M., Pantelic I., Savic S. An overview of novel surfactants for formulation of cosmetics with certain emphasis on acidic active substances. Tenside Surfactants Deterg. 2016;53(1):7–19. [Google Scholar]
- 9.Holst O. Glycolipids: occurrence, significance, and properties. In: Fraser-Reid B.O., Tatsuta K., Thiem J., editors. Glycoscience: chemistry and chemical biology. Springer Berlin Heidelberg; Berlin, Heidelberg: 2008. pp. 1603–1627. [Google Scholar]
- 10.Chester M.A. IUPAC-IUB joint commission on biochemical nomenclature (JCBN) nomenclature of glycolipids. J Mol Biol. 1999;286(3):963–970. doi: 10.1006/jmbi.1998.2485. [DOI] [PubMed] [Google Scholar]
- 11.Leray C. Introduction to lipidomics. CRC Press; 2012. Complex lipids; pp. 211–212. [Google Scholar]
- 12.Leray C. Introduction to lipidomics. CRC Press; 2012. Simple lipids with two different components; pp. 169–210. [Google Scholar]
- 13.Leray C. Introduction to lipidomics. CRC Press; 2012. Complex glycolipids; pp. 247–294. [Google Scholar]
- 14.Merrill A.H., Jr., Vu M.N. Encyclopedia of cell biology. Academic Press; Waltham: 2016. Glycolipids; pp. 180–193. [Google Scholar]
- 15.Hill K., Rhode O. Sugar-based surfactants for consumer products and technical applications. Lipid/Fett. 1999;101(1):25–33. [Google Scholar]
- 16.Poremba K. Marine Biosurfactants, III. Toxicity testing with marine microorganisms and comparison with synthetic surfactants. Z für Naturforsch C. 1991:210. doi: 10.1515/znc-1991-3-409. [DOI] [PubMed] [Google Scholar]
- 17.Madsen J.K., Kaspersen J.D., Andersen C.B., Nedergaard Pedersen J., Andersen K.K., Pedersen J.S., Otzen D.E. Glycolipid biosurfactants activate, dimerize and stabilize T. lanuginosus lipase in a pH-dependent fashion. Biochemistry. 2017;56:4256–4268. doi: 10.1021/acs.biochem.7b00420. [DOI] [PubMed] [Google Scholar]
- 18.Otzen D.E. Biosurfactants and surfactants interacting with membranes and proteins: same but different? Biochim Biophys Acta (BBA) Biomembr. 2017;1859(4):639–649. doi: 10.1016/j.bbamem.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 19.Madsen J.K. The anionic biosurfactant rhamnolipid does not denature industrial enzymes. Front Microbiol. 2015;6 doi: 10.3389/fmicb.2015.00292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekhon K.K., Rahman P.K.S.M. Rhamnolipid biosurfactants – past, Present and future scenario of global market. Front Microbiol. 2014;5 doi: 10.3389/fmicb.2014.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ashby R.D. A process model for approximating the production costs of the fermentative synthesis of sophorolipids. J Surfactants Deterg. 2013;16(5):683–691. [Google Scholar]
- 22.Dhanarajan G., Sen R. Biosurfactants. CRC Press; 2014. Cost analysis of biosurfactant production from a scientist's perspective; pp. 153–162. [Google Scholar]
- 23.Lang S., Wullbrandt D. Rhamnose lipids – biosynthesis, microbial production and application potential. Appl Microbiol Biotechnol. 1999;51(1):22–32. doi: 10.1007/s002530051358. [DOI] [PubMed] [Google Scholar]
- 24.Singh A., Van Hamme J.D., Ward O.P. Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnol Adv. 2007;25(1):99–121. doi: 10.1016/j.biotechadv.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 25.Kitamoto D., Isoda H., Nakahara T. Functions and potential applications of glycolipid biosurfactants —from energy-saving materials to gene delivery carriers. J Biosci Bioeng. 2002;94(3):187–201. doi: 10.1263/jbb.94.187. [DOI] [PubMed] [Google Scholar]
- 26.Elshahawi S.I. A comprehensive review of glycosylated bacterial natural products. Chem Soc Rev. 2015;44(21):7591–7697. doi: 10.1039/c4cs00426d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Řezanka T., Guschina I.A. Macrolactone glycosides of three lichen acids from Acarospora gobiensis, a lichen of Central Asia. Phytochemistry. 2001;58(8):1281–1287. doi: 10.1016/s0031-9422(01)00388-0. [DOI] [PubMed] [Google Scholar]
- 28.Kondoh A. Total synthesis of nominal gobienine A. Chem A Eur J. 2013;19(24):7731–7738. doi: 10.1002/chem.201300827. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Mawgoud A.M., Lépine F., Déziel E. Rhamnolipids: diversity of structures, microbial origins and roles. Appl Microbiol Biotechnol. 2010;86(5):1323–1336. doi: 10.1007/s00253-010-2498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsuyama T. Surface-active novel glycolipid and linked 3-hydroxy fatty acids produced by Serratia rubidaea. J Bacteriol. 1990;172(6):3015–3022. doi: 10.1128/jb.172.6.3015-3022.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kretschmer A., Wagner F. Characterization of biosynthetic intermediates of trehalose dicorynomycolates from Rhodococcus erythropolis grown on n-alkanes. Biochim Biophys Acta (BBA) Lipids Lipid Metab. 1983;753(3):306–313. [Google Scholar]
- 32.Vollbrecht E. Production and structure elucidation of di- and oligosaccharide lipids (biosurfactants) from Tsukamurella sp. nov. Appl Microbiol Biotechnol. 1998;50(5):530–537. doi: 10.1007/s002530051330. [DOI] [PubMed] [Google Scholar]
- 33.Rapp P. Formation, isolation and characterization of trehalose dimycolates from Rhodococcus erythropolis grown on n-alkanes. Microbiology. 1979;115(2):491–503. [Google Scholar]
- 34.Tomiyasu I. Occurrence of a novel glycolipid, 'trehalose 2,3,6'-trimycolate' in a psychrophilic, acid-fast bacterium, Rhodococcus aurantiacus (Gordona aurantiaca) FEBS Lett. 1986;203(2):239–242. [Google Scholar]
- 35.Uchida Y. Extracellular accumulation of mono- and di-succinoyl trehalose lipids by a strain of Rhodococcus erythropolis grown on n-alkanes. Agric Biol Chem. 1989;53(3):757–763. [Google Scholar]
- 36.Sudo T. Induction of the differentiation of human HL-60 promyelocytic leukemia cell line by succinoyl trehalose lipids. Cytotechnology. 2000;33(1):259–264. doi: 10.1023/A:1008137817944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Espuny M.J. Nutritional requirements of a biosurfactant producing strain Rhodococcus sp 51T7. Biotechnol Lett. 1996;18(5):521–526. [Google Scholar]
- 38.Passeri A. Marine Biosurfactants, II. Production and characterization of an anionic trehalose tetraester from the marine bacterium Arthrobacter sp. EK 1. Z für Naturforsch C. 1991:204. doi: 10.1515/znc-1991-3-408. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T., Tanaka H., Itoh S. Sucrose lipids of arthrobacteria, corynebacteria and nocardia grown on sucrose. Agric Biol Chem. 1974;38(3):557–563. [Google Scholar]
- 40.Itoh S., Suzuki T. Fructose-lipids of arthrobacter, corynebacteria, nocardia and mycobacteria grown on fructose. Agric Biol Chem. 1974;38(8):1443–1449. [Google Scholar]
- 41.Okazaki H. L-Glutamic acid fermentation; Part VI. Structure of a sugar lipid produced by Brevibacterium thiogenitalis. Agric Biol Chem. 1969;33(5):764–770. [Google Scholar]
- 42.Li Z.-Y. Formation and identification of interfacial-active glycolipids from resting microbial cells. Appl Environ Microbiol. 1984;48(3):610–617. doi: 10.1128/aem.48.3.610-617.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esch S.W. A novel trisaccharide glycolipid biosurfactant containing trehalose bears ester-linked hexanoate, succinate, and acyloxyacyl moieties: NMR and MS characterization of the underivatized structure. Carbohydr Res. 1999;319(1):112–123. doi: 10.1016/s0008-6215(99)00122-6. [DOI] [PubMed] [Google Scholar]
- 44.Konishi M. Deep-sea Rhodococcus sp. BS-15, lacking the phytopathogenic fas genes, produces a novel glucotriose lipid biosurfactant. Mar Biotechnol. 2014;16(4):484–493. doi: 10.1007/s10126-014-9568-x. [DOI] [PubMed] [Google Scholar]
- 45.Kim J.-S. Microbial glycolipid production under nitrogen limitation and resting cell conditions. J Biotechnol. 1990;13(4):257–266. doi: 10.1016/0168-1656(90)90074-l. [DOI] [PubMed] [Google Scholar]
- 46.Powalla M., Lang S., Wray V. Penta- and disaccharide lipid formation by Nocardia corynebacteroides grown on n-alkanes. Appl Microbiol Biotechnol. 1989;31(5):473–479. [Google Scholar]
- 47.Palme O. Selected microbial glycolipids: production, modification and characterization. In: Sen R., editor. Biosurfactants. Springer New York; New York, NY: 2010. pp. 185–202. [DOI] [PubMed] [Google Scholar]
- 48.Kamisango K. Pyruvylated glycolipids from Mycobacterium smegmatis. Nature and location of the lipid components. J Biol Chem. 1985;260(7):4117–4121. [PubMed] [Google Scholar]
- 49.Saadat S., Ballou C.E. Pyruvylated glycolipids from Mycobacterium smegmatis. Structures of two oligosaccharide components. J Biol Chem. 1983;258(3):1813–1818. [PubMed] [Google Scholar]
- 50.van ver Meer M.T. Alkane-1,2-diol-based glycosides and fatty glycosides and wax esters in Roseiflexus castenholzii and hot spring microbial mats. Arch Microbiol. 2002;178(3):229–237. doi: 10.1007/s00203-002-0449-8. [DOI] [PubMed] [Google Scholar]
- 51.Bauersachs T. Distribution of heterocyst glycolipids in cyanobacteria. Phytochemistry. 2009;70(17):2034–2039. doi: 10.1016/j.phytochem.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Mikami Y. A new antifungal macrolide component, brasilinolide B, produced by Nocardia brasiliensis. J Antibiot (Tokyo) 2000;53(1):70–74. doi: 10.7164/antibiotics.53.70. [DOI] [PubMed] [Google Scholar]
- 53.Komatsu K. Absolute stereochemistry of immunosuppressive macrolide brasilinolide A and its new congener brasilinolide C. J Org Chem. 2004;69(5):1535–1541. doi: 10.1021/jo035773v. [DOI] [PubMed] [Google Scholar]
- 54.Shindo K. Vicenistatin, a novel 20-membered macrocyclic lactam antitumor antibiotic. J Antibiot (Tokyo) 1993;46(7):1076–1081. doi: 10.7164/antibiotics.46.1076. [DOI] [PubMed] [Google Scholar]
- 55.Matsushima Y. Isolation and structure elucidation of vicenistatin M, and importance of the vicenisamine aminosugar for exerting cytotoxicity of vicenistatin. J Antibiot (Tokyo) 2001;54(3):211–219. doi: 10.7164/antibiotics.54.211. [DOI] [PubMed] [Google Scholar]
- 56.Dembitsky V.M. Astonishing diversity of natural surfactants: 2. Polyether glycosidic ionophores and macrocyclic glycosides. Lipids. 2005;40(3):219–248. doi: 10.1007/s11745-005-1378-0. [DOI] [PubMed] [Google Scholar]
- 57.Vilches C. Biosynthesis of oleandomycin by Streptomyces antibioticus: influence of nutritional conditions and development of resistance. Microbiology. 1990;136(8):1447–1454. doi: 10.1099/00221287-136-8-1447. [DOI] [PubMed] [Google Scholar]
- 58.Mertz F.P., Yao R.C. Saccharopolyspora spinosa sp. nov. isolated from soil collected in a sugar mill rum still. Int J Syst Evol Microbiol. 1990;40(1):34–39. [Google Scholar]
- 59.Hara O., Hutchinson C.R. A macrolide 3-O-acyltransferase gene from the midecamycin-producing species Streptomyces mycarofaciens. J Bacteriol. 1992;174(15):5141–5144. doi: 10.1128/jb.174.15.5141-5144.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Baltz R.H. Biosynthesis of the macrolide antibiotic tylosin. A preferred pathway from tylactone to tylosin. J Antibiot (Tokyo) 1983;36(2):131–141. doi: 10.7164/antibiotics.36.131. [DOI] [PubMed] [Google Scholar]
- 61.Kong F. Colubricidin A, a novel macrolide antibiotic from a Streptomyces sp. Tetrahedron Lett. 1999;40(52):9219–9223. [Google Scholar]
- 62.Brautaset T. Biosynthesis of the polyene antifungal antibiotic nystatin in Streptomyces noursei ATCC 11455: analysis of the gene cluster and deduction of the biosynthetic pathway. Chem Biol. 2000;7(6):395–403. doi: 10.1016/s1074-5521(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 63.Gallis H.A., Drew R.H., Pickard W.W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12(2):308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 64.Park S.R. Biosynthesis of rapamycin and its regulation: past achievements and recent progress. J Antibiot. 2010;63(8):434–441. doi: 10.1038/ja.2010.71. [DOI] [PubMed] [Google Scholar]
- 65.Ikeda H., Ōmura S. Avermectin biosynthesis. Chem Rev. 1997;97(7):2591–2610. doi: 10.1021/cr960023p. [DOI] [PubMed] [Google Scholar]
- 66.Qian-Cutrone J. Glucolipsin A and B, two new glucokinase activators produced by Streptomyces purpurogeniscleroticus and Nocardia vaccinii. J Antibiot (Tokyo) 1999;52(3):245–255. doi: 10.7164/antibiotics.52.245. [DOI] [PubMed] [Google Scholar]
- 67.Yokomizo K. Fattiviracin A1, a novel antiviral agent produced by Streptomyces microflavus strain No. 2445. II. Biological properties. J Antibiot (Tokyo) 1998;51(11):1035–1039. doi: 10.7164/antibiotics.51.1035. [DOI] [PubMed] [Google Scholar]
- 68.Haydock S.F. The putative elaiophylin biosynthetic gene cluster in Streptomyces sp. DSM4137 is adjacent to genes encoding adenosylcobalamin-dependent methylmalonyl CoA mutase and to genes for synthesis of cobalamin. J Biotechnol. 2004;113(1):55–68. doi: 10.1016/j.jbiotec.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 69.Yamada T., Minoura K., Numata A. Halichoblelide, a potent cytotoxic macrolide from a Streptomyces species separated from a marine fish. Tetrahedron Lett. 2002;43(9):1721–1724. [Google Scholar]
- 70.Yamada T. Halichoblelides B and C, potent cytotoxic macrolides from a Streptomyces species separated from a marine fish. Tetrahedron Lett. 2012;53(23):2842–2846. [Google Scholar]
- 71.Han Y. Halichoblelide D, a new elaiophylin derivative with potent cytotoxic activity from Mangrove-derived Streptomyces sp. 219807. Molecules. 2016;21(8):970. doi: 10.3390/molecules21080970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Okujo N. Bispolides, novel 20-membered ring macrodiolide antibiotics from Microbispora. J Antibiot. 2007;60(3):216–219. doi: 10.1038/ja.2007.26. [DOI] [PubMed] [Google Scholar]
- 73.Takahashi S. Determination of absolute structure of macroviracins by chemical synthesis. Org Lett. 2003;5(9):1555–1558. doi: 10.1021/ol034338k. [DOI] [PubMed] [Google Scholar]
- 74.Takaichi S. Dihydroxylycopene diglucoside diesters: a novel class of carotenoids from the phototrophic purple sulfur bacteria Halorhodospira abdelmalekii and Halorhodospira halochloris. Arch Microbiol. 2001;175(3):161–167. doi: 10.1007/s002030000247. [DOI] [PubMed] [Google Scholar]
- 75.Kleinig H. New C30-carotenoic acid glucosyl esters from Pseudomonas rhodos. Z für Naturforsch C. 1979:181. [Google Scholar]
- 76.Kleinig H. Carotenoids of rhizobia. I. New Carotenoids from Rhizobium lupini. Helvetica Chim Acta. 1977;60(1):254–258. doi: 10.1002/hlca.19770600131. [DOI] [PubMed] [Google Scholar]
- 77.Kleinig H., Broughton W.J. Carotenoid pigments in a red strain of Rhizobium from Lotononis bainesii Baker. Arch Microbiol. 1982;133(2) 164–164. [Google Scholar]
- 78.Kleinig H., Reichenbach H. A new carotenoid glucoside ester from Chondromyces apiculatus. Phytochemistry. 1973;12(10):2483–2485. [Google Scholar]
- 79.Kleinig H., Reichenbach H. Biosynthesis of carotenoid glucoside esters in Myxococcus fulvus (Myxobacterales): inhibition by nicotine and carotenoid turnover. Biochim Biophys Acta (BBA) Lipids Lipid Metab. 1973;306(2):249–256. doi: 10.1016/0005-2760(73)90230-0. [DOI] [PubMed] [Google Scholar]
- 80.Marshall J.H., Wilmoth G.J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J Bacteriol. 1981;147(3):900–913. doi: 10.1128/jb.147.3.900-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Taylor R.F., Davies B.H. Triterpenoid carotenoids and related lipids. Triterpenoid monohydroxy- and monoglucosyloxy-carotenoids from Streptococcus faecium UNH 564P. Biochem J. 1974;139(3):761–769. doi: 10.1042/bj1390761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Takaichi S. Novel carotenoid glucoside esters from alkaliphilic heliobacteria. Arch Microbiol. 2003;179(2):95–100. doi: 10.1007/s00203-002-0504-5. [DOI] [PubMed] [Google Scholar]
- 83.Schmidt K. Carotenoids of purple nonsulfur bacteria. Arch für Mikrobiol. 1971;77(3):231–238. [PubMed] [Google Scholar]
- 84.Hertzberg S., Liaaen-Jensen S. The structure of oscillaxanthin. Phytochemistry. 1969;8(7):1281–1292. [Google Scholar]
- 85.Arpin N., Fiasson J.L., Liaaen-Jensen A. Bacterial carotenoids. XXXIX. C 50 -carotenoids. 10. Bacterioruberin mono- and diglucoside. Acta Chem Scand. 1972;26(6):2526–2528. doi: 10.3891/acta.chem.scand.26-2526. [DOI] [PubMed] [Google Scholar]
- 86.Shindo K. Diapolycopenedioic acid xylosyl esters A, B, and C, novel antioxidative glyco-C30-carotenoic acids produced by a new marine bacterium Rubritalea squalenifaciens. J Antibiot. 2008;61(3):185–191. doi: 10.1038/ja.2008.28. [DOI] [PubMed] [Google Scholar]
- 87.Shindo K., Misawa N. New and rare carotenoids isolated from marine bacteria and their antioxidant activities. Mar Drugs. 2014;12(3):1690. doi: 10.3390/md12031690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hopmann C. Isolation and structure elucidation of vancoresmycin—a new antibiotic from Amycolatopsis sp. ST 101170. Tetrahedron Lett. 2002;43(3):435–438. [Google Scholar]
- 89.Lutnaes B.F., Oren A., Liaaen-Jensen S. New C40-carotenoid acyl glycoside as principal carotenoid in Salinibacter ruber, an extremely halophilic eubacterium. J Nat Prod. 2002;65(9):1340–1343. doi: 10.1021/np020125c. [DOI] [PubMed] [Google Scholar]
- 90.Lutnaes B.F. Carotenoids of thermophilic bacteria—rhodothermus marinus from submarine Icelandic hot springs. Biochem Syst Ecol. 2004;32(5):455–468. [Google Scholar]
- 91.Hertzberg S., Liaaen Jensen S. Bacterial carotenoids XX. The carotenoids of Mycobacterium phlei strain Vera. 2. The structures of the phlei-xanthophylls–two novel tertiary glucosides. Acta Chem Scand. 1967;21(1):15–41. [PubMed] [Google Scholar]
- 92.Vacheron M.-J. Structural Investigations on cell walls of Nocardia sp. Eur J Biochem. 1972;29(1) doi: 10.1111/j.1432-1033.1972.tb01970.x. p. 156–106. [DOI] [PubMed] [Google Scholar]
- 93.Takaichi S., Ishidsu J.-i. Carotenoid glycoside ester from Rhodococcus rhodochrous. Methods Enzym. 1992;213:366–374. [Google Scholar]
- 94.Takaichi S. Carotenoid glucoside mycolic acid esters from the nocardioform actinomycetes, Rhodococcus rhodochrous. Phytochemistry. 1997;45(3):505–508. [Google Scholar]
- 95.Kleinig H. Membranes from Myxococcus fulvus (myxobacterales) containing carotenoid glucosides. Biochim Biophys Acta (BBA) Biomembr. 1972;274(2):489–498. doi: 10.1016/0005-2736(72)90194-0. [DOI] [PubMed] [Google Scholar]
- 96.Kleinig H., Reichenbach H., Achenbach H. Carotenoid pigments of stigmatella aurantiaca (Myxobacterales) Arch für Mikrobiol. 1970;74(3):223–234. doi: 10.1007/BF00408883. [DOI] [PubMed] [Google Scholar]
- 97.Takaichi S. Absence of carotenes and presence of a tertiary methoxy group in a carotenoid from a thermophilic filamentous photosynthetic bacterium huncastenholzii. Plant Cell Physiol. 2001;42(12):1355–1362. doi: 10.1093/pcp/pce172. [DOI] [PubMed] [Google Scholar]
- 98.Takaichi S. New carotenoids from the thermophilic green sulfur bacterium Chlorobium tepidum: 1′,2′-dihydro-γ-carotene, 1′,2′-dihydrochlorobactene, and OH-chlorobactene glucoside ester, and the carotenoid composition of different strains. Arch Microbiol. 1997;168(4):270–276. doi: 10.1007/s002030050498. [DOI] [PubMed] [Google Scholar]
- 99.Takaichi S. A monocyclic carotenoid glucoside ester is a major carotenoid in the green filamentous bacterium Chloroflexus aurantiacus. Plant Cell Physiol. 1995;36(5):773–778. [Google Scholar]
- 100.Burgess M.L. Carotenoid glycoside esters from the thermophilic bacterium Meiothermus ruber. J Nat Prod. 1999;62(6):859–863. doi: 10.1021/np980573d. [DOI] [PubMed] [Google Scholar]
- 101.Takaichi S., Maoka T., Masamoto K. Myxoxanthophyll in Synechocystis sp. PCC 6803 is myxol 2'-dimethyl-fucoside, (3R,2'S)-myxol 2'-(2,4-di-O-methyl-alpha-L-fucoside), not rhamnoside. Plant Cell Physiol. 2001;42(7):756–762. doi: 10.1093/pcp/pce098. [DOI] [PubMed] [Google Scholar]
- 102.Richter T.K.S., Hughes C.C., Moore B.S. Sioxanthin, a novel glycosylated carotenoid, reveals an unusual subclustered biosynthetic pathway. Environ Microbiol. 2015;17(6):2158–2171. doi: 10.1111/1462-2920.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dembitsky V.M. Astonishing diversity of natural surfactants: 3. Carotenoid glycosides and isoprenoid glycolipids. Lipids. 2005;40(6):535–557. doi: 10.1007/s11745-005-1415-z. [DOI] [PubMed] [Google Scholar]
- 104.Arpin N., Liaaen-Jensen S., Trouilloud M. Bacterial carotenoids. 38. C 50 -carotenoids. 9. Isolation of decaprenoxanthin mono- and diglucoside from an Arthrobacter sp. Acta Chem Scand. 1972;26(6):2524–2526. doi: 10.3891/acta.chem.scand.26-2524. [DOI] [PubMed] [Google Scholar]
- 105.Häberli A., Bircher C., Pfander H. Isolation of a new carotenoid and two new carotenoid glycosides from Curtobacterium flaccumfaciens pvar poinsettiae. Helvetica Chim Acta. 2000;83(2):328–335. [Google Scholar]
- 106.Osawa A. Characterization and antioxidative activities of rare C50 carotenoids-sarcinaxanthin, sarcinaxanthin monoglucoside, and sarcinaxanthin diglucoside obtained from Micrococcus yunnanensis. J Oleo Sci. 2010;59(12):653–659. doi: 10.5650/jos.59.653. [DOI] [PubMed] [Google Scholar]
- 107.Osawa A. Characterization and antioxidative activities of rare C(50) carotenoids-sarcinaxanthin, sarcinaxanthin monoglucoside, and sarcinaxanthin diglucoside-obtained from Micrococcus yunnanensis. J Oleo Sci. 2010;59(12):653–659. doi: 10.5650/jos.59.653. [DOI] [PubMed] [Google Scholar]
- 108.Hunter C.N. Introduction of new carotenoids into the bacterial photosynthetic apparatus by combining the carotenoid biosynthetic pathways of Erwinia herbicola and Rhodobacter sphaeroides. J Bacteriol. 1994;176(12):3692–3697. doi: 10.1128/jb.176.12.3692-3697.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kull D.R., Pfander H. Isolation and structure elucidation of carotenoid glycosides from the thermoacidophilic archaea Sulfolobus shibatae. J Nat Prod. 1997;60(4):371–374. [Google Scholar]
- 110.Hertzberg S., Borch G., Liaaen-Jensen S. Bacterial carotenoids. L. absolute configuration of zeaxanthin dirhamnoside. Arch Microbiol. 1976;110(1):95–99. doi: 10.1007/BF00416974. [DOI] [PubMed] [Google Scholar]
- 111.Asker D. Astaxanthin dirhamnoside, a new astaxanthin derivative produced by a radio-tolerant bacterium, Sphingomonas astaxanthinifaciens. J Antibiot. 2009;62(7):397–399. doi: 10.1038/ja.2009.50. [DOI] [PubMed] [Google Scholar]
- 112.Kleinig H. Carotenoid pigments of Sorangium compositum (Myxobacterales) including two new carotenoid glucoside esters and two new carotenoid rhamnosides. Arch für Mikrobiol. 1971;78(3):224–233. doi: 10.1007/BF00424896. [DOI] [PubMed] [Google Scholar]
- 113.Yokoyama A. Thermozeaxanthins, new carotenoid-glycoside-esters from thermophilic eubacterium Thermus thermophilus. Tetrahedron Lett. 1995;36(27):4901–4904. [Google Scholar]
- 114.Yokoyama A., Adachi K., Shizuri Y. New carotenoid glucosides, astaxanthin glucoside and adonixanthin glucoside, isolated from the astaxanthin-producing marine bacterium, Agrobacterium aurantiacum. J Nat Prod. 1995;58(12):1929–1933. [Google Scholar]
- 115.Yokoyama A., Shizuri Y., Misawa N. Production of new carotenoids, astaxanthin glucosides, by Escherichia coli transformants carrying carotenoid biosynthesis genes. Tetrahedron Lett. 1998;39(22):3709–3712. [Google Scholar]
- 116.Krubasik P. Detailed biosynthetic pathway to decaprenoxanthin diglucoside in Corynebacterium glutamicum and identification of novel intermediates. Arch Microbiol. 2001;176(3):217–223. doi: 10.1007/s002030100315. [DOI] [PubMed] [Google Scholar]
- 117.Garcia Costas A.M. Identification of the bacteriochlorophylls, carotenoids, quinones, lipids, and hopanoids of “Candidatus Chloracidobacterium thermophilum”. J Bacteriol. 2012;194(5):1158–1168. doi: 10.1128/JB.06421-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hallenbeck P.C. Draft genome sequence of the photoheterotrophic Chloracidobacterium thermophilum strain OC1 found in a Mat at Ojo Caliente. Genome Announc. 2016;4(1) doi: 10.1128/genomeA.01570-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Schmerk C.L. Elucidation of the Burkholderia cenocepacia hopanoid biosynthesis pathway uncovers functions for conserved proteins in hopanoid-producing bacteria. Environ Microbiol. 2015;17(3):735–750. doi: 10.1111/1462-2920.12509. [DOI] [PubMed] [Google Scholar]
- 120.Moreau R.A. Analysis of intact hopanoids and other lipids from the bacterium Zymomonas mobilis by high-performance liquid chromatography. Anal Biochem. 1995;224(1):293–301. doi: 10.1006/abio.1995.1043. [DOI] [PubMed] [Google Scholar]
- 121.Wu C.H. Quantitative hopanoid analysis enables robust pattern detection and comparison between laboratories. Geobiology. 2015;13(4):391–407. doi: 10.1111/gbi.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Llopiz P., Neunlist S., Rohmer M. Prokaryotic triterpenoids: O-α-D-glucuronopyranosyl bacteriohopanetetrol, a novel hopanoid from the bacterium Rhodospirillum rubrum. Biochem J. 1992;287(1):159–161. doi: 10.1042/bj2870159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hirai Y. Unique cholesteryl glucosides in Helicobacter pylori: composition and structural analysis. J Bacteriol. 1995;177(18):5327–5333. doi: 10.1128/jb.177.18.5327-5333.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Haque M. Steryl glycosides: a characteristic feature of the Helicobacter spp.? J Bacteriol. 1995;177(18):5334–5337. doi: 10.1128/jb.177.18.5334-5337.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stübs G. Acylated cholesteryl galactosides are specific antigens of Borrelia causing lyme disease and frequently induce antibodies in late stages of disease. J Biol Chem. 2009;284(20):13326–13334. doi: 10.1074/jbc.M809575200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smith P.F. Biosynthesis of cholesteryl glucoside by Mycoplasma gallinarum. J Bacteriol. 1971;108(3):986–991. doi: 10.1128/jb.108.3.986-991.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mayberry W.R., Smith P.F. Structures and properties of acyl diglucosylcholesterol and galactofuranosyl diacylglycerol from Acholeplasma axanthum. Biochim Biophys Acta. 1983;752(3):434–443. doi: 10.1016/0005-2760(83)90273-4. [DOI] [PubMed] [Google Scholar]
- 128.Spoeckner S. Glycolipids of the smut fungus Ustilago maydis from cultivation on renewable resources. Appl Microbiol Biotechnol. 1999;51(1):33–39. [Google Scholar]
- 129.Morita T. Production of mannosylerythritol lipids and their application in cosmetics. Appl Microbiol Biotechnol. 2013;97(11):4691–4700. doi: 10.1007/s00253-013-4858-1. [DOI] [PubMed] [Google Scholar]
- 130.Kakugawa K. Isolation of yeast Kurtzmanomyces sp. I-11, novel producer of mannosylerythritol lipid. Biosci Biotechnol Biochem. 2002;66(1):188–191. doi: 10.1271/bbb.66.188. [DOI] [PubMed] [Google Scholar]
- 131.Kurz M. Ustilipids, acylated β-D-mannopyranosyl D-erythritols from Ustilago maydis and Geotrichum candidum. J Antibiot (Tokyo) 2003;56(2):91–101. doi: 10.7164/antibiotics.56.91. [DOI] [PubMed] [Google Scholar]
- 132.Morita T. Formation of the two novel glycolipid biosurfactants, mannosylribitol lipid and mannosylarabitol lipid, by Pseudozyma parantarctica JCM 11752T. Appl Microbiol Biotechnol. 2012;96(4):931–938. doi: 10.1007/s00253-012-4230-x. [DOI] [PubMed] [Google Scholar]
- 133.Kulakovskaya T.V. Ustilagic acid secretion by Pseudozyma fusiformata strains. FEMS Yeast Res. 2005;5(10):919–923. doi: 10.1016/j.femsyr.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 134.Kulakovskaya T.V. Characterization of an antifungal glycolipid secreted by the yeast Sympodiomycopsis paphiopedili. FEMS Yeast Res. 2004;5(3):247–252. doi: 10.1016/j.femsyr.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 135.Puchkov E.O. The mycocidal, membrane-active complex of Cryptococcus humicola is a new type of cellobiose lipid with detergent features. Biochim Biophys Acta (BBA) Biomembr. 2002;1558(2):161–170. doi: 10.1016/s0005-2736(01)00428-x. [DOI] [PubMed] [Google Scholar]
- 136.Cheng Y. Insertional mutagenesis of a fungal biocontrol agent led to discovery of a rare cellobiose lipid with antifungal activity. Appl Environ Microbiol. 2003;69(5):2595–2602. doi: 10.1128/AEM.69.5.2595-2602.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Daniel H.-J. Production of sophorolipids from whey: development of a two-stage process with Cryptococcus curvatus ATCC 20509 and Candida bombicola ATCC 22214 using deproteinized whey concentrates as substrates. Appl Microbiol Biotechnol. 1999;51(1):40–45. doi: 10.1007/s002530051360. [DOI] [PubMed] [Google Scholar]
- 138.Ma X., Li H., Song X. Surface and biological activity of sophorolipid molecules produced by Wickerhamiella domercqiae var. sophorolipid CGMCC 1576. J Colloid Interface Sci. 2012;376(1):165–172. doi: 10.1016/j.jcis.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 139.Chen J. Production, structure elucidation and anticancer properties of sophorolipid from Wickerhamiella domercqiae. Enzyme Microb Technol. 2006;39(3):501–506. [Google Scholar]
- 140.Price N.P.J. Structural characterization of novel sophorolipid biosurfactants from a newly identified species of Candida yeast. Carbohydr Res. 2012;348:33–41. doi: 10.1016/j.carres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 141.Nishida F. Structure elucidation of glykenin, glycosidic antibiotics from Basidiomycetes sp. VII. Structure elucidation of the GK components using tandem mass spectrometry. J Mass Spectrom Soc Jpn. 1995;43(1):37–44. [Google Scholar]
- 142.Laine R.A. Monoglucosyloxyoctadecenoic acid, a glycolipid from Aspergillus Niger. Biochemistry. 1972;11(12):2267–2271. doi: 10.1021/bi00762a009. [DOI] [PubMed] [Google Scholar]
- 143.Chen C. Halymecins, new antimicroalgal substances produced by fungi isolated from marine algae. J Antibiot (Tokyo) 1996;49(10):998–1005. doi: 10.7164/antibiotics.49.998. [DOI] [PubMed] [Google Scholar]
- 144.Le Dang Q. Antimicrobial activities of novel mannosyl lipids isolated from the biocontrol fungus Simplicillium lamellicola BCP against phytopathogenic bacteria. J Agric Food Chem. 2014;62(15):3363–3370. doi: 10.1021/jf500361e. [DOI] [PubMed] [Google Scholar]
- 145.Tabata N. Structure elucidation of roselipins, inhibitors of diacylglycerol acyltransferase produced by Gliocladium roseum KF-1040. J Antibiot (Tokyo) 1999;52(9):815–826. doi: 10.7164/antibiotics.52.815. [DOI] [PubMed] [Google Scholar]
- 146.Tomoda H. Roselipins, inhibitors of diacylglycerol acyltransferase, produced by Gliocladium roseum KF-1040. J Antibiot (Tokyo) 1999;52(8):689–694. doi: 10.7164/antibiotics.52.689. [DOI] [PubMed] [Google Scholar]
- 147.Kohno J. Isolation and structure determination of TMC-151s: novel polyketide antibiotics from Gliocladium catenulatum Gilman & Abbott TC 1280. Tetrahedron. 1999;55(25):7771–7786. [Google Scholar]
- 148.Kohno J. TMC-171A, B,C and TMC-154, novel polyketide antibiotics produced by Gliocladium sp. TC 1304 and TC 1282. J Antibiot (Tokyo) 1999;52(12):1114–1123. doi: 10.7164/antibiotics.52.1114. [DOI] [PubMed] [Google Scholar]
- 149.Ayers S. Anthelmintic constituents of Clonostachys candelabrum. J Antibiot. 2010;63(3):119–122. doi: 10.1038/ja.2009.131. [DOI] [PubMed] [Google Scholar]
- 150.Kasai Y. Cladionol A, a polyketide glycoside from marine-derived fungus Gliocladium species. J Nat Prod. 2005;68(5):777–779. doi: 10.1021/np050046b. [DOI] [PubMed] [Google Scholar]
- 151.Boros C. Emmyguyacins A and B: Unusual glycolipids from a sterile fungus species that inhibit the low-pH conformational change of hemagglutinin A during replication of influenza virus. J Nat Prod. 2002;65(2):108–114. doi: 10.1021/np010345a. [DOI] [PubMed] [Google Scholar]
- 152.Sakaki T. Sterol glycosides and cerebrosides accumulate in Pichia pastoris, Rhynchosporium secalis and other fungi under normal conditions or under heat shock and ethanol stress. Yeast. 2001;18(8):679–695. doi: 10.1002/yea.720. [DOI] [PubMed] [Google Scholar]
- 153.Mimee B., Labbé C., Bélanger R.R. Catabolism of flocculosin, an antimicrobial metabolite produced by Pseudozyma flocculosa. Glycobiology. 2009;19(9):995–1001. doi: 10.1093/glycob/cwp078. [DOI] [PubMed] [Google Scholar]
- 154.Van Bogaert I.N.A. The biosynthetic gene cluster for sophorolipids: a biotechnological interesting biosurfactant produced by Starmerella bombicola. Mol Microbiol. 2013;88(3):501–509. doi: 10.1111/mmi.12200. [DOI] [PubMed] [Google Scholar]
- 155.Van Hamme J.D., Singh A., Ward O.P. Physiological aspects: Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol Adv. 2006;24(6):604–620. doi: 10.1016/j.biotechadv.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 156.Ron E.Z., Rosenberg E. Natural roles of biosurfactants. Environ Microbiol. 2001;3(4):229–236. doi: 10.1046/j.1462-2920.2001.00190.x. [DOI] [PubMed] [Google Scholar]
- 157.Kannenberg E.L., Poralla K. Hopanoid biosynthesis and function in bacteria. Naturwissenschaften. 1999;86(4):168–176. [Google Scholar]
- 158.Cortés-Sánchez A.d.J., Hernández-Sánchez H., Jaramillo-Flores M.E. Biological activity of glycolipids produced by microorganisms: new trends and possible therapeutic alternatives. Microbiol Res. 2013;168(1):22–32. doi: 10.1016/j.micres.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 159.Rodrigues L. Biosurfactants: potential applications in medicine. J Antimicrob Chemother. 2006;57(4):609–618. doi: 10.1093/jac/dkl024. [DOI] [PubMed] [Google Scholar]
- 160.Gudiña E.J. Potential therapeutic applications of biosurfactants. Trends Pharmacol Sci. 2013;34(12):667–675. doi: 10.1016/j.tips.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 161.Arcamone F.M. Melanosporin and elaiophylin, new antibiotics from Streptomyces melanosporus (sive melonsporofaciens) n. sp. G Microbiol. 1959;7(3):207–216. [Google Scholar]
- 162.Fang A., Wong G.K., Demain A.L. Enhancement of the antifungal activity of rapamycin by the coproduced elaiophylin and nigericin. J Antibiot (Tokyo) 2000;53(2):158–162. doi: 10.7164/antibiotics.53.158. [DOI] [PubMed] [Google Scholar]
- 163.Isoda H. Succinoyl trehalose lipid induced differentiation of human monocytoid leukemic cell line U937 into monocyte-macrophages. Cytotechnology. 1995;19(1):79–88. doi: 10.1007/BF00749758. [DOI] [PubMed] [Google Scholar]
- 164.Saita N. Trehalose 6,6′-dimycolate (cord factor) of Mycobacterium tuberculosis induces corneal angiogenesis in rats. Infect Immun. 2000;68(10):5991–5997. doi: 10.1128/iai.68.10.5991-5997.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Matsuno T., Miki W. Biological functions and activities of animal carotenoids. Pure Appl Chem. 1991;63(1):141–146. [Google Scholar]
- 166.Mimee B. Antifungal activity of flocculosin, a novel glycolipid isolated from Pseudozyma flocculosa. Antimicrob Agents Chemother. 2005;49(4):1597–1599. doi: 10.1128/AAC.49.4.1597-1599.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Puchkov E.O. Cytoplasmic membrane of a sensitive yeast is a primary target for Cryptococcus humicola mycocidal compound (microcin) Biochim Biophys Acta (BBA) Biomembr. 2001;1512(2):239–250. doi: 10.1016/s0005-2736(01)00325-x. [DOI] [PubMed] [Google Scholar]
- 168.Kulakovskaya T.V., Kulakovskaya E.V., Golubev W.I. ATP leakage from yeast cells treated by extracellular glycolipids of Pseudozyma fusiformata. FEMS Yeast Res. 2003;3(4):401–404. doi: 10.1016/S1567-1356(02)00202-7. [DOI] [PubMed] [Google Scholar]
- 169.Hammann P., Kretzschmar G., Seibert G. Secondary metabolites by chemical screening. 7: I. Elaiophylin derivatives and their biological activities. J Antibiot. 1990;43(11):1431–1440. doi: 10.7164/antibiotics.43.1431. [DOI] [PubMed] [Google Scholar]
- 170.Otoguro K. In vitro and in vivo antiprotozoal activities of bispolides and their derivatives. J Antibiot. 2010;63(5):275–277. doi: 10.1038/ja.2010.32. [DOI] [PubMed] [Google Scholar]
- 171.Isoda H. The neurite-initiating effect of microbial extracellular glycolipids in PC12 cells. Cytotechnology. 1999;31(1):165. doi: 10.1023/A:1008020121693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Shibahara M. Mannosylerythritol lipid increases levels of galactoceramide in and neurite outgrowth from PC12 pheochromocytoma cells. Cytotechnology. 2000;33(1):247–251. doi: 10.1023/A:1008155111024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wakamatsu Y. Mannosylerythritol lipid induces characteristics of neuronal differentiation in PC12 cells through an ERK-related signal cascade. Eur J Biochem. 2001;268(2):374–383. doi: 10.1046/j.1432-1033.2001.01887.x. [DOI] [PubMed] [Google Scholar]
- 174.Isoda H. Differentiation of human promyelocytic leukemia cell line HL60 by microbial extracellular glycolipids. Lipids. 1997;32(3):263–271. doi: 10.1007/s11745-997-0033-0. [DOI] [PubMed] [Google Scholar]
- 175.Isoda H. Microbial extracellular glycolipid induction of differentiation and inhibition of the protein kinase C activity of human promyelocytic leukemia cell line HL60. Biosci Biotechnol Biochem. 1997;61(4):609–614. doi: 10.1271/bbb.61.609. [DOI] [PubMed] [Google Scholar]
- 176.Lee S.-Y. Structure determination and biological activities of elaiophylin produced by Streptomyces sp. MCY-846. J Microbiol Biotechnol. 1996;6:245–249. [Google Scholar]
- 177.Ritzau M. New macrodiolide antibiotics, 11-O-monomethyl- and 11,11‘-O-dimethylelaiophylins, from Streptomyces sp. HKI-0113 and HKI-0114. J Nat Prod. 1998;61(11):1337–1339. doi: 10.1021/np9800351. [DOI] [PubMed] [Google Scholar]
- 178.Haferburg D. Antiphytovirale Aktivität von rhamnolipid aus Pseudomonas aeruginosa. Acta Biotechnol. 1987;7(4):353–356. [Google Scholar]
- 179.Janek T., Lukaszewicz M., Krasowska A. Identification and characterization of biosurfactants produced by the Arctic bacterium Pseudomonas putida BD2. Colloids Surf B Biointerfaces. 2013;110:379–386. doi: 10.1016/j.colsurfb.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 180.Rodrigues L.R. Interference in adhesion of bacteria and yeasts isolated from explanted voice prostheses to silicone rubber by rhamnolipid biosurfactants. J Appl Microbiol. 2006;100(3):470–480. doi: 10.1111/j.1365-2672.2005.02826.x. [DOI] [PubMed] [Google Scholar]
- 181.Stipcevic T., Pijac A., Pijac G. Enhanced healing of full-thickness burn wounds using di-rhamnolipid. Burns. 2006;32(1):24–34. doi: 10.1016/j.burns.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kitamoto D. Surface active properties and antimicrobial activities of mannosylerythritol lipids as biosurfactants produced by Candida Antarctica. J Biotechnol. 1993;29(1):91–96. [Google Scholar]
- 183.Azuma M. Role of interferon in the augmented resistance of trehalose-6,6′-dimycolate-treated mice to influenza virus infection. J General Virol. 1987;68(3):835–843. doi: 10.1099/0022-1317-68-3-835. [DOI] [PubMed] [Google Scholar]
- 184.Watanabe R. Inhibitory effect of trehalose dimycolate (TDM) and its stereoisometric derivatives, trehalose dicorynomycolates (TDCMs), with low toxicity on lung metastasis of tumour cells in mice. Vaccine. 1999;17(11):1484–1492. doi: 10.1016/s0264-410x(98)00367-3. [DOI] [PubMed] [Google Scholar]
- 185.Andra J. Endotoxin-like properties of a rhamnolipid exotoxin from Burkholderia (Pseudomonas) plantarii: immune cell stimulation and biophysical characterization. Biol Chem. 2006;387(3):301–310. doi: 10.1515/BC.2006.040. [DOI] [PubMed] [Google Scholar]
- 186.Díaz De Rienzo M.A. Sophorolipid biosurfactants: possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015;32(6):720–726. doi: 10.1016/j.nbt.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 187.Lydon H.L., Baccile N., Callaghan B., Marchant R., Mitchell C.A., Banat I.M. Adjuvant antibiotic activity of acidic sophorolipids with potential for facilitating wound healing. Antimicrob Agents Chemother. 2017 doi: 10.1128/AAC.02547-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Haskins R.H., Thorn J.A. Biochemistry of the ustilaginales: VII. Antibiotic activity of ustilagic acid. Can J Bot. 1951;29(6):585–592. [Google Scholar]
- 189.Mimee B., Pelletier R., Bélanger R.R. In vitro antibacterial activity and antifungal mode of action of flocculosin, a membrane-active cellobiose lipid. J Appl Microbiol. 2009;107(3):989–996. doi: 10.1111/j.1365-2672.2009.04280.x. [DOI] [PubMed] [Google Scholar]
- 190.Tulloch A.P. Glycosides of hydroxy fatty acids. In: Kates M., editor. Glycolipids, phosphoglycolipids, and sulfoglycolipids. Springer US; Boston, MA: 1990. pp. 463–487. [Google Scholar]
- 191.Naruse N. Fluvirucins A1, A2, B1, B2, B3, B4 and B5, new antibiotics active against influenza A virus. I. Production, isolation, chemical properties and biological activities. J Antibiot. 1991;44(7):733–740. doi: 10.7164/antibiotics.44.733. [DOI] [PubMed] [Google Scholar]
- 192.Besra G.S. Structural elucidation of a novel family of acyltrehaloses from Mycobacterium tuberculosis. Biochemistry. 1992;31(40):9832–9837. doi: 10.1021/bi00155a040. [DOI] [PubMed] [Google Scholar]
- 193.Saavedra R. Mycobacterial di-O-acyl-trehalose inhibits mitogen- and antigen-induced proliferation of murine T cells in vitro. Clin Diagn Lab Immunol. 2001;8(6):1081–1088. doi: 10.1128/CDLI.8.6.1081-1088.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nishida F. Structures of deacetyl glykenins-a, b, and c, glycosidic antibiotics from basidiomycetes sp. Tetrahedron Lett. 1988;29(41):5287–5290. [Google Scholar]
- 195.Perez M. PM100117 and PM100118, new antitumor macrolides produced by a marine Streptomyces caniferus GUA-06-05-006A. J Antibiot. 2016;69(5):388–394. doi: 10.1038/ja.2015.121. [DOI] [PubMed] [Google Scholar]
- 196.Tanaka Y. Brasilinolide A, a new macrolide antibiotic produced by Nocardia brasiliensis: producing strain, isolation and biological activity. J Antibiot (Tokyo) 1997;50(12):1036–1041. doi: 10.7164/antibiotics.50.1036. [DOI] [PubMed] [Google Scholar]
- 197.Shah V. Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrob Agents Chemother. 2005;49(10):4093–4100. doi: 10.1128/AAC.49.10.4093-4100.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Manitchotpisit P. Aureobasidium pullulans as a source of liamocins (heavy oils) with anticancer activity. World J Microbiol Biotechnol. 2014;30(8):2199–2204. doi: 10.1007/s11274-014-1639-7. [DOI] [PubMed] [Google Scholar]
- 199.Bischoff K.M. Liamocin oil from Aureobasidium pullulans has antibacterial activity with specificity for species of Streptococcus. J Antibiot. 2015;68(10):642–645. doi: 10.1038/ja.2015.39. [DOI] [PubMed] [Google Scholar]
- 200.Price N.P.J. Polyols, not sugars, determine the structural diversity of anti-streptococcal liamocins produced by Aureobasidium pullulans strain NRRL 50380. J Antibiot. 2017;70(2):136–141. doi: 10.1038/ja.2016.92. [DOI] [PubMed] [Google Scholar]
- 201.Lee S.-Y. Immunosuppressive activity of elaiophylins. J Microbiol Biotechnol. 1997;7(4):272–277. [Google Scholar]
- 202.Inokoshi J. Expression of two human acyl-CoA:diacylglycerol acyltransferase isozymes in yeast and selectivity of microbial inhibitors toward the isozymes. J Antibiot. 2009;62(1):51–54. doi: 10.1038/ja.2008.5. [DOI] [PubMed] [Google Scholar]
- 203.Singh S.B. Isolation, structure, and HIV-1-integrase inhibitory activity of structurally diverse fungal metabolites. J Ind Microbiol Biotechnol. 2003;30(12):721–731. doi: 10.1007/s10295-003-0101-x. [DOI] [PubMed] [Google Scholar]
- 204.Zhao X. Treatment of mouse melanoma cells with phorbol 12-myristate 13-acetate counteracts mannosylerythritol lipid-induced growth arrest and apoptosis. Cytotechnology. 2000;33(1):123–130. doi: 10.1023/A:1008129616127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhao X. Mannosylerythritol lipid is a potent inducer of apoptosis and differentiation of mouse melanoma cells in culture. Cancer Res. 1999;59(2):482–486. [PubMed] [Google Scholar]
- 206.Jyonouchi H., Zhang L., Tomita Y. Studies of immunomodulating actions of carotenoids. II. Astaxanthin enhances in vitro antibody production to T-dependent antigens without facilitating polyclonal B-cell activation. Nutr Cancer. 1993;19(3):269–280. doi: 10.1080/01635589309514258. [DOI] [PubMed] [Google Scholar]
- 207.Haba E. Physicochemical characterization and antimicrobial properties of rhamnolipids produced by Pseudomonas aeruginosa 47T2 NCBIM 40044. Biotechnol Bioeng. 2003;81(3):316–322. doi: 10.1002/bit.10474. [DOI] [PubMed] [Google Scholar]
- 208.Benincasa M. Chemical structure, surface properties and biological activities of the biosurfactant produced by Pseudomonas aeruginosa LBI from soapstock. Antonie Leeuwenhoek. 2004;85(1):1–8. doi: 10.1023/B:ANTO.0000020148.45523.41. [DOI] [PubMed] [Google Scholar]
- 209.Miao S. Rhamnolipids as platform molecules for production of potential anti-zoospore agrochemicals. J Agric Food Chem. 2015;63(13):3367–3376. doi: 10.1021/acs.jafc.5b00033. [DOI] [PubMed] [Google Scholar]
- 210.Stanghellini M.E., Miller R.M. Biosurfactants: Their identity and potential efficacy in the biological control of zoosporic plant pathogens. Plant Dis. 1997;81(1):4–12. doi: 10.1094/PDIS.1997.81.1.4. [DOI] [PubMed] [Google Scholar]
- 211.Yoo D.S., Lee B.S., Kim E.K. Characteristics of microbial biosurfactant as an antifungal agent against plant pathogenic fungus. J Microbiol Biotechnol. 2005;15(6):1164–1169. [Google Scholar]
- 212.Sanchez L. Rhamnolipids elicit defense responses and induce disease resistance against biotrophic, hemibiotrophic, and necrotrophic pathogens that require different signaling pathways in Arabidopsis and highlight a central role for salicylic acid. Plant Physiol. 2012;160(3):1630–1641. doi: 10.1104/pp.112.201913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fu S.L. Sophorolipid treatment decreases LPS induced inflammatory responses and NO production in macrophages. J Am Coll Surg. 2007;205(3):S44. [Google Scholar]
- 214.Hagler M. Sophorolipids decrease IgE production in U266 cells by downregulation of BSAP (Pax5), TLR-2, STAT3 and IL-6. J Allergy Clin Immunol. 2007;119(1):S263. [Google Scholar]
- 215.Williams G.J., Thorson J.S. Advances in enzymology and related areas of molecular biology. John Wiley & Sons, Inc; 2009. Natural product glycosyltransferases: properties and applications; pp. 55–119. [DOI] [PubMed] [Google Scholar]
- 216.Röttig A., Steinbüchel A. Acyltransferases in bacteria. Microbiol Mol Biol Rev. 2013;77(2):277–321. doi: 10.1128/MMBR.00010-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Davies G., Henrissat B. Structures and mechanisms of glycosyl hydrolases. Structure. 1995;3(9):853–859. doi: 10.1016/S0969-2126(01)00220-9. [DOI] [PubMed] [Google Scholar]
- 218.Mukai K. Substrate specificities in hydrolysis of polyhydroxyalkanoates by microbial esterases. Biotechnol Lett. 1993;15(6):601–604. [Google Scholar]
- 219.Jaeger K.E., Steinbüchel A., Jendrossek D. Substrate specificities of bacterial polyhydroxyalkanoate depolymerases and lipases: bacterial lipases hydrolyze poly(ω-hydroxyalkanoates) Appl Environ Microbiol. 1995;61(8):3113–3118. doi: 10.1128/aem.61.8.3113-3118.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ali Y.B., Verger R., Abousalham A. Lipases or esterases: does it really matter? Toward a new bio-physico-chemical classification. In: Sandoval G., editor. Lipases and phospholipases: methods and protocols. Humana Press; Totowa, NJ: 2012. pp. 31–51. [DOI] [PubMed] [Google Scholar]
- 221.Kulakovskaya E., Kulakovskaya T. Extracellular glycolipids of yeasts. Academic Press; 2014. Chapter 5-Metabolism of yeast extracellular glycolipids; pp. 65–74. [Google Scholar]
- 222.Van Bogaert I.N.A. Biosurfactants. CRC Press; 2014. Sophorolipids: microbial synthesis and application; pp. 19–36. [Google Scholar]
- 223.Hundle B.S. Functional expression of zeaxanthin glucosyltransferase from Erwinia herbicola and a proposed uridine diphosphate binding site. Proc Natl Acad Sci. 1992;89(19):9321–9325. doi: 10.1073/pnas.89.19.9321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Grille S. The functions of steryl glycosides come to those who wait: recent advances in plants, fungi, bacteria and animals. Prog Lipid Res. 2010;49(3):262–288. doi: 10.1016/j.plipres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 225.Teichmann B. A biosynthetic gene cluster for a secreted cellobiose lipid with antifungal activity from Ustilago maydis. Mol Microbiol. 2007;66(2):525–533. doi: 10.1111/j.1365-2958.2007.05941.x. [DOI] [PubMed] [Google Scholar]
- 226.Abdel-Mawgoud A.M. Rhamnolipids: detection, analysis, biosynthesis, genetic regulation & bioengineering of production. In: Soberón-Chávez G., editor. Biosurfactants: from genes to applications (microbiology monographs) Springer; Münster, Germany: 2011. pp. 13–55. [Google Scholar]
- 227.Marrakchi H., Lanéelle M.-A., Daffé M. Mycolic acids: structures, biosynthesis, and beyond. Chem Biol. 2014;21(1):67–85. doi: 10.1016/j.chembiol.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 228.Ogasawara Y. Cloning, sequencing, and functional analysis of the biosynthetic gene cluster of macrolactam antibiotic vicenistatin in Streptomyces halstedii. Chem Biol. 2004;11(1):79–86. doi: 10.1016/j.chembiol.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 229.Morita T. Genome and transcriptome analysis of the basidiomycetous yeast Pseudozyma Antarctica producing extracellular glycolipids, mannosylerythritol lipids. PLOS ONE. 2014;9(2) doi: 10.1371/journal.pone.0086490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Mirończuk A.M., Biegalska A., Dobrowolski A. Functional overexpression of genes involved in erythritol synthesis in the yeast Yarrowia lipolytica. Biotechnol Biofuels. 2017;10(1):77. doi: 10.1186/s13068-017-0772-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Irorere V.U. Microbial rhamnolipid production: a critical re-evaluation of published data and suggested future publication criteria. Appl Microbiol Biotechnol. 2017;101(10):3941–3951. doi: 10.1007/s00253-017-8262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Smyth T.J.P. Protocols for the detection and chemical characterisation of microbial glycolipids. In: McGenity T.J., Timmis K.N., Nogales B., editors. Hydrocarbon and lipid microbiology protocols : biochemical methods. Springer Berlin Heidelberg; Berlin, Heidelberg: 2016. pp. 29–60. [Google Scholar]
- 233.Abdel-Mawgoud A.M., Lépine F., Déziel E. Liquid chromatography/mass spectrometry for the identification and quantification of rhamnolipids. In: Filloux A., Ramos J.-L., editors. Pseudomonas methods and protocols. Springer New York; 2014. pp. 359–373. [Google Scholar]
- 234.Palos Pacheco R. Synthesis and characterization of four diastereomers of monorhamnolipids. J Am Chem Soc. 2017;139(14):5125–5132. doi: 10.1021/jacs.7b00427. [DOI] [PubMed] [Google Scholar]
- 235.Menhour B. A stereocontrolled synthesis of the hydrophobic moiety of rhamnolipids. Tetrahedron Lett. 2015;56(9):1159–1161. [Google Scholar]
- 236.Bauer J. Chemical synthesis of a glycolipid library by a solid-phase strategy allows elucidation of the structural specificity of immunostimulation by rhamnolipids. Chem – A Eur J. 2006;12(27):7116–7124. doi: 10.1002/chem.200600482. [DOI] [PubMed] [Google Scholar]
- 237.Fleurackers S. Biosurfactants. CRC Press; 2014. Biosurfactants versus chemically synthesized surface-active agents; pp. 37–48. [Google Scholar]
- 238.Makkar R.S., Cameotra S.S., Banat I.M. Advances in utilization of renewable substrates for biosurfactant production. AMB Express. 2011;1(1):5. doi: 10.1186/2191-0855-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Banat I.M. Cost effective technologies and renewable substrates for biosurfactants' production. Front Microbiol. 2014;5(697) doi: 10.3389/fmicb.2014.00697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Koch A.K. Genetic construction of lactose-utilizing strains of Pseudomonas aeruginosa and their application in biosurfactant production. Nat Biotech. 1988;6(11):1335–1339. [Google Scholar]
- 241.Meijnen J.-P., de Winde J.H., Ruijssenaars H.J. Engineering Pseudomonas putida S12 for efficient utilization of D-xylose and L-arabinose. Appl Environ Microbiol. 2008;74(16):5031–5037. doi: 10.1128/AEM.00924-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Zahoor A., Lindner S.N., Wendisch V.F. Metabolic engineering of Corynebacterium glutamicum aimed at alternative carbon sources and new products. Computational and Structural Biotechnology Journal. 2012;3(4) doi: 10.5936/csbj.201210004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Wei N. Simultaneous utilization of cellobiose, xylose, and acetic acid from lignocellulosic biomass for biofuel production by an engineered yeast platform. ACS Synth Biol. 2015;4(6):707–713. doi: 10.1021/sb500364q. [DOI] [PubMed] [Google Scholar]
- 244.Shah V., Jurjevic M., Badia D. Utilization of restaurant waste oil as a precursor for sophorolipid production. Biotechnol Prog. 2007;23(2):512–515. doi: 10.1021/bp0602909. [DOI] [PubMed] [Google Scholar]
- 245.Ozdal M., Gurkok S., Ozdal O.G. Optimization of rhamnolipid production by Pseudomonas aeruginosa OG1 using waste frying oil and chicken feather peptone. 3 Biotech. 2017;7(2):117. doi: 10.1007/s13205-017-0774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Wittgens A. Growth independent rhamnolipid production from glucose using the non-pathogenic Pseudomonas putida KT2440. Microb Cell Factories. 2011;10(1):80. doi: 10.1186/1475-2859-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Dobler L. Rhamnolipids in perspective: gene regulatory pathways, metabolic engineering, production and technological forecasting. New Biotechnol. 2016;33(1):123–135. doi: 10.1016/j.nbt.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 248.Ochsner U.A. Production of Pseudomonas aeruginosa rhamnolipid biosurfactants in heterologous hosts. Appl Environ Microbiol. 1995;61(9):3503–3506. doi: 10.1128/aem.61.9.3503-3506.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang Q.H. Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnol Bioeng. 2007;98(4):842–853. doi: 10.1002/bit.21462. [DOI] [PubMed] [Google Scholar]
- 250.Cabrera-Valladares N. Monorhamnolipids and 3-(3-hydroxyalkanoyloxy)alkanoic acids (HAAs) production using Escherichia coli as a heterologous host. Appl Microbiol Biotechnol. 2006;73(1):187–194. doi: 10.1007/s00253-006-0468-5. [DOI] [PubMed] [Google Scholar]
- 251.Solaiman D.Y. Dirhamnose-lipid production by recombinant nonpathogenic bacterium Pseudomonas chlororaphis. Appl Microbiol Biotechnol. 2015;99(10):4333–4342. doi: 10.1007/s00253-015-6433-4. [DOI] [PubMed] [Google Scholar]
- 252.Tavares L.F.D. Characterization of rhamnolipids produced by wild-type and engineered Burkholderia kururiensis. Appl Microbiol Biotechnol. 2013;97(5):1909–1921. doi: 10.1007/s00253-012-4454-9. [DOI] [PubMed] [Google Scholar]
- 253.Müller M.M., Hausmann R. Regulatory and metabolic network of rhamnolipid biosynthesis: traditional and advanced engineering towards biotechnological production. Appl Microbiol Biotechnol. 2011;91(2):251–264. doi: 10.1007/s00253-011-3368-2. [DOI] [PubMed] [Google Scholar]
- 254.Heider S.A.E. Production and glucosylation of C50 and C40 carotenoids by metabolically engineered Corynebacterium glutamicum. Appl Microbiol Biotechnol. 2014;98(3):1223–1235. doi: 10.1007/s00253-013-5359-y. [DOI] [PubMed] [Google Scholar]
- 255.Peirú S. Production of the potent antibacterial polyketide erythromycin C in Escherichia coli. Appl Environ Microbiol. 2005;71(5):2539–2547. doi: 10.1128/AEM.71.5.2539-2547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Baltz R.H. Molecular engineering approaches to peptide, polyketide and other antibiotics. Nat Biotech. 2006;24(12):1533–1540. doi: 10.1038/nbt1265. [DOI] [PubMed] [Google Scholar]
- 257.Abdel-Mawgoud A.M., Lépine F., Déziel E. A chiral high-performance liquid chromatography–tandem mass spectrometry method for the stereospecific analysis of enoyl-coenzyme A hydratases/isomerases. J Chromatogr A. 2013;1306:37–43. doi: 10.1016/j.chroma.2013.07.049. [DOI] [PubMed] [Google Scholar]
- 258.Abdel-Mawgoud A.M., Lépine F., Déziel E. A stereospecific pathway diverts β-oxidation intermediates to the biosynthesis of rhamnolipid biosurfactants. Chem Biol. 2014;21(1):156–164. doi: 10.1016/j.chembiol.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 259.Adrio J.L. Oleaginous yeasts: promising platforms for the production of oleochemicals and biofuels. Biotechnol Bioeng. 2017;114:1915–1920. doi: 10.1002/bit.26337. [DOI] [PubMed] [Google Scholar]
- 260.Ageitos J.M. Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol. 2011;90(4):1219–1227. doi: 10.1007/s00253-011-3200-z. [DOI] [PubMed] [Google Scholar]
- 261.Baccile N. Development of a cradle-to-grave approach for acetylated acidic sophorolipid biosurfactants. ACS Sustain Chem Eng. 2017;5(1):1186–1198. [Google Scholar]
- 262.Ciesielska K. Exoproteome analysis of Starmerella bombicola results in the discovery of an esterase required for lactonization of sophorolipids. J Proteomics. 2014;98(Supplement C):159–174. doi: 10.1016/j.jprot.2013.12.026. [DOI] [PubMed] [Google Scholar]