Abstract
Approximately 50 million children and adolescents in Latin America are affected by the childhood obesity pandemic. We present the case of a 5-year-old Mexican girl with obesity and gastro-oesophageal reflux disease (GORD), in whom prenatal, lifestyle and environmental risk factors were identified. Here, we demonstrate how childhood obesity is rooted since pregnancy and the perinatal stage, and how the social determinants of health like unsafe outdoor conditions, lack of infrastructure to exercise and a suboptimal physical activity curriculum in government schools strongly influence the development and maintenance of childhood obesity and complicate management.
Keywords: global health, obesity (nutrition), childhood nutrition, childhood nutrition (paediatrics)
Case presentation
A 5-year-old Mexican girl was brought to the gastroenterology and nutrition department by her mother to participate in a paediatric abdominal pain clinical trial. She was diagnosed with gastro-oesophageal reflux (GORD) and obesity.
She was born by caesarean section to a 32-year-old primigravida mother at 36 weeks 5 days of gestation due to severe preeclampsia. Her weight was 3080 g and length 50 cm. The mother’s prepregnancy weight was 100 kg, and during pregnancy she gained 15 kg. There were no complications in the postnatal period. The mother was supplemented with folic acid and iron; she did not have infections or smoked during pregnancy. Immunisations were up to date.
Family history
Thirty-eight year-old mother with obesity and type 2 diabetes (T2DM), under treatment with metformin. Forty-four-year-old father with T2DM, under treatment with metformin. Paternal grandmother with T2DM and paternal grandfather with hypertension.
Nutrition history
She was exclusively breast fed for the first 2 months of life, afterwards, antireflux formula was added until 6 months of age. At 6 months of age, complementary food was initiated in conjunction with a lactose-free formula. The 24-hour diet recall diet was the following: at 7:00, yoghurt and chocolate milk; at 10:00, a sandwich and sugar-sweetened beverage (SSB)—in fact, she drank one 125 mL SSB every day at school. At 14:30, soup, bread and meat; at 19:00 the same meal and at 22:00, a glass of milk. They denied taking vitamins. Her daily caloric requirements were 1225 kcal/day based on her ideal body weight.1 However, based on the 24-hour diet recall, her caloric intake was approximately 1700 kcal/day.
Social history
She is an only child. She lives with her mother and father in a two-bedroom apartment. The mother is a stay-at-home mom with a sedentary lifestyle. The father is a taxi driver, also with a sedentary lifestyle. Their monthly income was approximately MXN8000 (or US$431). They did not have pets. They denied household smoking exposure. They also denied difficulty affording healthy foods. She only exercised 1 hour per week in sports class at her school; they avoided going to the park because the unsafe outdoor conditions and the park’s poor infrastructure. She had a 1 hour nap every day. She went to bed at midnight every night and woke up at 7:00 to go to school. They revealed 5 hours of screen use daily—in fact, the mom watched television with her.
Vital signs were within normal limits. Anthropometric data were the following: weight, 29.9 kg (99th percentile); height, 121 cm (92th percentile); body mass index (BMI), 20.9 kg/m2 (2 SD above the mean). The WHO growth charts were used. Body composition bioelectrical impedance analysis (InBody) revealed the following: total body water, 13.9 kg (normal); lean body mass, 3.7 kg (normal); minerals, 1.27 kg (normal) and adipose tissue, 11.00 kg (high); BMI, 20.4 kg/m2 (high); body fat percentage, 36.7% (high); degree of obesity, 129% (high) and ideal body weight was 23.10 kg. Physical exam did not show abnormalities. Complete blood count, liver enzymes, liver function tests, urinalysis, fasting glucose and lipid panel were normal; stool ova and parasite exam was negative.
Management
First, the family was motivated, educated and provided with information about childhood and adult obesity. For GORD, lifestyle changes (weight reduction and diet modification (reduction of chocolate and spicy foods)) and esomeprazole (1 mg/kg/day) were indicated. For obesity, the family was instructed to lower (or quit if possible) the consumption of SSBs, chocolate milk, candy and refined carbohydrates and to increase fruit, vegetables, legumes and fibre; to reduce screen time to 1 hour per day of high-quality programmes; to exercise for 1 hour everyday of moderate-to-vigorous-intensity physical activity and to improve sleep hygiene to accomplish a minimum of 10 hours of good quality sleep daily.
Two-month follow-up appointment
Weight, 31.1 kg; height, 121. Body composition bioelectrical impedance analysis revealed the following: total body water, 14.9 kg (normal); lean body mass, 4 kg; minerals, 1.34 kg and adipose tissue, 10.9 kg (high); BMI, 20.7 (2 SD above the mean); body fat percentage, 35.1% (high).
Lifestyle modifications
She decreased candy consumption and the daily SSB but increased milk consumption (from one glass to three glasses daily). She got a bicycle and started riding it in her apartment patio, three times in the weekdays during 5 min and 30 min in the weekends. She continued to watch television 5 hours per day and continued to have the same hours of sleep per day (8 hours).
The aim of this case report is to demonstrate how childhood obesity is rooted since pregnancy and the perinatal stage, and how the social determinants of health, like unsafe outdoor conditions, lack of infrastructure to exercise and a suboptimal physical activity curriculum in government schools strongly influence the development and maintenance of childhood obesity and complicate management figure 1.
Figure 1.
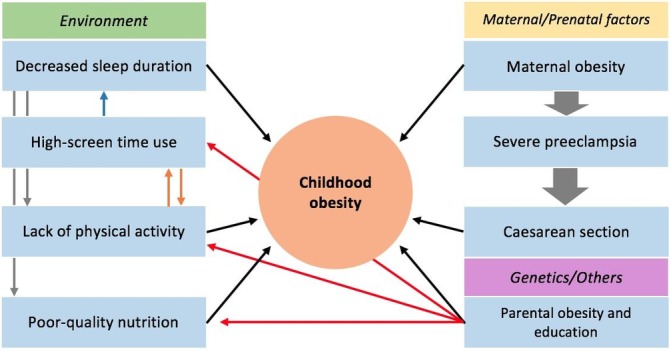
Shows the main factors contributing to the patient’s obesity and how they interact with each other.
Global health problem list
The childhood obesity pandemic and its short- and long-term consequences;
Maternal and prenatal factors as contributing factors for the development of childhood obesity;
Social determinants of health, environmental and lifestyle factors are strongly associated with childhood obesity;
Treatment of childhood obesity becomes even more complex when considering the social determinants of health in Mexico.
Global health problem analysis
Childhood obesity pandemic and its short and long-term consequences
To talk about childhood obesity, one must first define it. By using the WHO growth charts, childhood obesity is defined as weight for height greater that 3 SD above median in the 0 to 5-year-old age group; and in the 5 to 19-year-old age group, as a BMI for age greater than 2 SD above the mean.2 In the case of our patient, obesity was a clear diagnosis based on anthropometric and body composition bioelectrical impedance analysis data. Although the patient is growing proportionally in weight and height, the strong family history of obesity and type 2 diabetes mellitus (T2DM) places her at increased risk of developing these comorbidities and being obese later in life. Therefore, we aimed for weight reduction and family-centred lifestyle modifications; however, they were poorly accomplished.
A recent systematic review estimated that approximately 42 to 51 million children and adolescents (0 to 18 years) in Latin America are overweight or obese.3 According to the National Health and Nutrition Survey (ENSANUT-2012)—a national cross-sectional study performed in Mexico in 2012—the prevalence of overweight and obesity (national combined prevalence) in the 0 to 5-year-old age group was 9.7%; in the 5 to 11-year-old age group was 34.4% (19.8% for overweight and 14.6% for obesity); and in the 12 to 19-year-old age group, the national combined prevalence was 35%.4 Hernández-Cordero et al estimated the prevalence and trends of overweight and obesity in Mexican children and adolescents by comparing the previous National Nutrition Surveys from 1988, 1999 and 2006 with ENSANUT-2012 (most recent data); they found that in 2012, 28.8% of children 0 to 19 years old were overweight, obese or at risk of overweight. The highest prevalence was seen among children and adolescents in urban areas and those from the highest socioeconomic level. They also found that in the last 13–24 years, the prevalence of overweight and obesity increased at the highest rate among female adolescents and school-aged girls. The authors concluded that the prevalence of childhood obesity in Mexico is one the highest worldwide, and that the burden of childhood obesity is shifting towards groups with a lower socioeconomic level: in the case of our patient, her family belonged to the lower middle socioeconomic level.5 Abarca-Gómez et al estimated the BMI of 2416 population-based studies (128.9 million participants 5 years and older) from 1975 to 2016 in 200 countries and found that the mean BMI and prevalence of obesity increased worldwide in children and adolescents from 1975 to 2016. They also found that despite the increase in mean BMI and obesity, more children and adolescents are underweight than obese worldwide; however, the authors postulated that if trends continue, childhood obesity is expected to surpass moderate and severe underweight by 2022.6 The Organization for Economic Cooperation and Development projects that there will be a steady increase in obesity rates until at least 2030, particularly in the USA, Mexico and England.7 More recently, a study predicted that more than 50% of children aged 2 to 19 years will be obese by the age of 35 years, and found that half of the total prevalence of obesity begins in childhood; indeed, a 2-year old who is obese is more likely to be obese at 35 years than a 19-year old with overweight.8 Therefore, childhood comprehends a critical life stage for healthcare providers and parents to address obesity and prevent children from being obese in adulthood.
Multiple complications are associated with childhood obesity, such as: obstructive sleep apnoea; non-alcoholic fatty liver disease, gallstones and GORD; T2DM; polycystic ovary syndrome; pseudomotor cerebri; hypertension and hyperlipidaemia; depression; orthopaedic disorders and acanthosis nigricans (or insulin resistance).9 10 In 2016, non-communicable diseases contributed 60% to the global disability-adjusted life years (DALYs). In Mexico for instance, the three leading causes of all-age DALYs were: diabetes, ischaemic heart disease and chronic kidney disease; all of which are complications/comorbidities of obesity.11 In addition, childhood obesity is associated with an increased rate of death from endogenous causes during early adulthood.12 Based on the history, physical exam and laboratory tests our patient did not have other comorbidities apart from GORD.
Maternal and prenatal factors as contributing factors for the development of childhood obesity
Maternal obesity, severe preeclampsia and caesarean section
Offspring of obese women are at increased risk of being overweight or obese later in life.13 Multiple studies have found a strong association between maternal obesity and preeclampsia. Baeten et al found that women with a prepregnancy BMI of >30, 25–29.9 and 20–24.9 had a 3.3, 2.0 and 1.3 OR for preeclampsia, respectively.14 Paré et al found a ‘dose–response’ effect between BMI and preeclampsia: being overweight or obese was the most important risk factor for the development of preeclampsia and severe preeclampsia, with a BMI greater that 25 having an attributable risk per cent of 64.9%.15 According to multiple systematic reviews and meta-analyses, children who are born by caesarean section may be at higher risk of developing obesity in childhood.16–19 The patient’s mother had a prepregnancy weight of 100 kg and a pregnancy weight of 115 kg (BMI >30), thus, the development of severe preeclampsia was concordant with the ‘dose–response’ effect as indicated by Paré et al. As a result, the patient was born by caesarean section, which may potentially be associated with the development childhood obesity. Therefore, the process described above (maternal obesity → severe preeclampsia → caesarean section) could have been prevented if maternal BMI had been <25. Ultimately, this leads us to ask ourselves the following questions: Is this a vicious cycle? Will this cycle repeat once the patient reaches fertility years? Obesity in pregnancy negatively affects the mother and the offspring; for example, these women are at increased risk of gestational diabetes, hypertensive disorders and instrumental and caesarean section; consequently, longer duration of hospital stay and increased costs. The offspring on the other hand is at increased risk of large-for-gestational age, preterm birth, congenital anomalies and fetal death.20 Therefore, in addition to the cardiometabolic complications of obesity itself, obese pregnant women carry additional complications for them and the offspring.
Social determinants of health, environmental and lifestyle factors are strongly associated with childhood obesity
Lack of physical activity
More than 40% of adolescents (>15 years) in Latin America are categorised as inactive.21 22 The social determinants of health play a major role in this aspect. First, the fact that the family avoids going to the park because of the unsafe outdoor and poor park conditions are important impediments for the patient to exercise. As a consequence, she can only exercise either at her school or at home. These unequal living conditions clearly are not under the control of the patient but are the result of ineffective policy intervention. Second, this family lives in a small apartment with a small patio (3×4 m), so how much can this patient effectively exercise in this area? Although the patient got a bicycle and the parents encouraged her to ride it (in their patio), what is the impact of this on the patient’s caloric balance? When under normal circumstances, this girl could and should be riding her bicycle outdoors. Third, the suboptimal physical activity offered by government schools. The WHO recommends at least 60 min of moderate-to-vigorous-intensity physical activity daily for children 5 to 17 years old.23 Tremblay et al compared physical activity of children among 15 countries and found that less than 50% of schools in Mexico had active schools policies (ie, daily physical activity, recess, ‘everyone plays’ approach, bike racks at school, traffic calming on school property, outdoor time); less than 50% of students were taught by a physical activity specialist; and less than 50% of students were offered at least 150 min of physical activity per week.24 In this case, 300 min of physical activity were lacking per week, and the patient’s school only offered 60 min of physical activity per week, which is consistent with the latter study. In addition, the parental educational level,25 parental obesity and lack of exercise and the time spent in front of a television also contribute with the patient’s sedentary lifestyle.
Poor-quality nutrition
In the case of our patient, the total daily caloric intake surpassed her daily caloric requirements by approximately 500 kcal/day—most of which came from sweetened beverages, milk and candies. The family was instructed to lower or quit the daily SSB, chocolate milk and glass of milk and to eliminate the candies from her diet to achieve a negative caloric balance. At the follow-up visit, the parents reported an increase in milk consumption. She did decrease the amount of candy and stopped drinking the SSB and was eating more fruits; but overall, a negative caloric balance was not achieved.
Economic globalisation and the ‘nutrition transition’ have been major contributors to the growth of the childhood obesity epidemic in many developing countries. The ‘nutrition transition’ is defined as a high intake of refined carbohydrates, added sugars, fats and animal-source foods and a low intake of legumes, vegetables and grains. Food marketing exposure to children and the school food environment also have an impact on childhood obesity.21 26 The consumption of SSBs is increasing in most developing countries; in 2014, Mexico was the second country with the highest SSB consumption worldwide.27 In fact, a study found that SSBs comprehended 17.5% of the total daily caloric intake among Mexican children aged 1 to 19 years old.28 The rationale behind the link between high consumption of SSBs and childhood obesity is that liquid calories fail to trigger satiety, which ultimately leads to an increased total daily calorie intake. However, to date, this mechanism is controversial.29 30 As a result of the high SSBs consumption among Mexicans, in 2014, the government implemented an excise tax of 1 peso/L (10%) on several SSBs; a year later, the consumption of SSBs decreased by 6%, especially among households of lower socioeconomic status.29 31 Moreover, a recent study concluded that doubling the SSBs tax to 20% could lead to larger benefits.32 A recent meta-analysis concluded that there was a direct association between SSBs consumption and weight gain, overweight and obesity in children; although some discrepancies were identified in some studies.33 Furthermore, the link between SSBs and childhood obesity is supported by a double-blinded, randomised, controlled trial that showed a reduction in weight gain and fat accumulation in children (4 to 11 years) who consumed sugar-free beverages as compared with children who consumed a 104 kcal SSB/day.34 The front-of-package food labelling is also a promising intervention to promote healthy food choices among families and to decrease childhood obesity. This was implemented by the Mexican government to all foods and non-alcoholic beverages in 2015. To date, however, no studies have evaluated the impact of this on people’s food consumption and health outcomes.35 36
High screen time use
There is a clear association between high media use and childhood obesity,37 38 which has been attributed to the children’s sedentary lifestyle and possibly to food advertising.39 As per the American Academy of Pediatrics (AAP), for children 2 to 5 years, screen use should be limited to 1 hour per day of high-quality programmes.40 Multiple studies have found a strong association between high screen time use and childhood obesity; in fact, a large international study that included 77 000 children found a 20% to 27% increased risk of overweight and obesity in adolescents and children who watched 1 to 3 hours of television per day.38 In the case of our patient, 5 hours of screen use per day clearly exceed the recommended daily screen use hours by the AAP, which also affects the duration of sleep and contribute to the patient’s sedentary lifestyle.37 The family was instructed to decrease screen time to 1–2 hours per day of high-quality programmes. At the follow-up visit, however, they still reported 5 hours of screen use per day.
Decreased sleep duration
According to the National Sleep Foundation,41 preschoolers (3–5 years) should sleep from 10 to 13 hours per day, as the evidence shows that children who sleep less than 9 hours have greater odds of being obese than those who sleep more than 10 hours. A systematic review and bias-adjusted meta-analysis42 found that sleep duration was inversely associated with later BMI in children and adolescents. In addition, later bedtimes are associated with childhood obesity and weight gain.43 In the case of our patient, the parents reported a total of 8 hours of sleep per day (1 hour nap and 7 hours of sleep at night), which is clearly less than the minimum recommended hours of sleep for her age. The parents also reported a late bedtime (midnight), which is consistent with the latter study. Moreover, sleep duration positively influences healthy food choices among children,37 which also has an impact on the caloric balance.
Treatment of childhood obesity becomes even more complex when considering the social determinants of health in Mexico
‘Women and children in low socioeconomic groups are the most vulnerable and inequities in obesity are passed on from generation to generation’.44 As previously mentioned, childhood obesity in Mexico is shifting towards groups with a lower socioeconomic level6 in whom health inequities are more prevalent. Multiple guidelines for the management of childhood obesity exist,11 which mainly focus on lifestyle interventions (eg, dietary, physical activity and behavioural) to achieve a negative caloric balance; and in more severe cases, the use of medications or surgery. In clinical practice however, lifestyle modifications are extremely difficult to accomplish. When the social determinants of health (figure 2)46 are included in the equation, it certainly becomes a more complex problem. In the case of our patient for example, lifestyle interventions were instructed and close follow-up was warranted, however, being a lower middle class family in Mexico and the inequalities this implies clearly affects management. Unsafe streets, lack of infrastructure to exercise (ie, parks) and a suboptimal physical activity curriculum in government schools are some of the social determinants of health that require more attention and action by governmental authorities. Although Mexico City has witnessed various improvements in terms of infrastructure and transportation in recent years, most community parks have been built in popular areas that are located far away from the most vulnerable.45 Therefore, policy implementation in regard these aspects should also be a priority.
Figure 2.
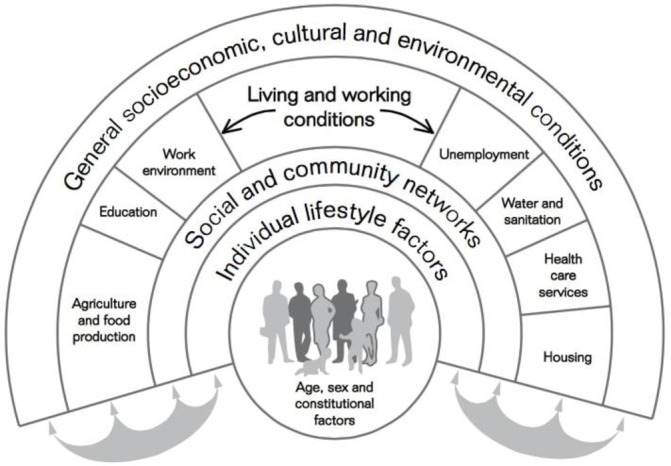
The main determinants of health. Reprinted from “European strategies for tackling social inequities in health: levelling up part 2, WHO Regional Office for Europe, Studies on social and economic determinants of population health, No.3. Göran Dahlgren, Margaret Whitehead. Copyright (2006). Page 20. Accessed on 14/02/2018 at http://www.euro.who.int/__data/assets/pdf_file/0018/103824/E89384.pdf
Patient’s perspective.
When we asked the parents about the long-term consequences of childhood obesity, they were aware of them: ‘diabetes and heart disease’ they said. We also asked them if they thought childhood obesity disappears on adolescence, but they said no, that ‘it is a matter of exercise and a healthy diet for it to disappear’.
We asked the patient about her self psychical perception, and she said she thought she was ‘chubby’; the parents stated the same. However, she seemed to think her body weight and image were normal. She did not report bullying at school.
They were afraid of going outdoors because of fear of getting robbed as there are many people doing drugs in the street. The patient mentioned that she would like to exercise and play outdoors with her friends more; “it is dangerous to walk to the park; I get bored at home, after finishing homework I can only play with my dolls or watch TV, so I get bored. That’s why every weekend my parents take me to the park ‘La Mexicana’—the new one—so I can ride my bicycle more. In my house I only ride it three times a week.”
Learning points.
Keeping maternal prepregnancy body mass index <25 is essential to decrease the risk of preeclampsia and caesarean section and to possibly reduce the risk of childhood obesity in the offspring.
Half of the total prevalence of obesity begins in childhood; therefore, this is a critical life stage to address it and prevent these children from being obese in adulthood.
In addition to the family-centred lifestyle modifications and interdisciplinary approach, one must keep in mind the social determinants of health and how these affect management.
Schools must offer more than 60 min of physical activity per week to achieve a negative caloric balance in children.
Construction of new parks in vulnerable neighbourhoods and tackling crime are priorities to promote outdoor physical activity in Mexico.
Acknowledgments
We would like to thank the patient and her family for sharing their private information.
Footnotes
Contributors: DAR and KRIA were involved in the patient’s care. They collected the information and images, carried out the literature review, wrote the manuscript and revised it extensively. EMTM and JRM also wrote and edited the manuscript and revised it extensively. All the authors approved the final version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Human energy requirements. Report of a Joint FAO/WHO/UNU expert consultation Rome, 17–24 October 2001. http://www.fao.org/3/a-y5686e.pdf (accessed 05 Dec 2017).
- 2.The World Health Organization. Obesity and overweight Factsheet. http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed 05 Dec 2017).
- 3.Rivera JÁ, de Cossío TG, Pedraza LS, et al. Childhood and adolescent overweight and obesity in Latin America: a systematic review. Lancet Diabetes Endocrinol 2014;2:321–32. 10.1016/S2213-8587(13)70173-6 [DOI] [PubMed] [Google Scholar]
- 4.Resultados nacionales. Encuesta nacional de salud y nutrición 2012. http://ensanut.insp.mx/informes/ENSANUT2012ResultadosNacionales.pdf (accessed 05 Dec 2017).
- 5.Hernández-Cordero S, Cuevas-Nasu L, Morales-Ruán MC, et al. Overweight and obesity in Mexican children and adolescents during the last 25 years. Nutr Diabetes 2017;7:e280 10.1038/nutd.2017.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abarca-Gómez L, Abdeen ZA, Hamid ZA, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. The Lancet 2017;390:2627–42. 10.1016/S0140-6736(17)32129-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Organization for Economic Cooperation and Development (OECD). Obesity update 2017. https://www.oecd.org/els/health-systems/Obesity-Update-2017.pdf (accessed 05 Dec 2017).
- 8.Ward ZJ, Long MW, Resch SC, et al. Simulation of growth trajectories of childhood obesity into adulthood. N Engl J Med 2017;377:2145–53. 10.1056/NEJMoa1703860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlow SE. Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 2007;120(Suppl 4):S164–92. 10.1542/peds.2007-2329C [DOI] [PubMed] [Google Scholar]
- 10.Styne DM, Arslanian SA, Connor EL, et al. Response to Letter: "Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline". J Clin Endocrinol Metab 2017;102:2123–4. 10.1210/jc.2017-00561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.GBD 2016 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1260–344. 10.1016/S0140-6736(17)32130-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franks PW, Hanson RL, Knowler WC, et al. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med 2010;362:485–93. 10.1056/NEJMoa0904130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams CB, Mackenzie KC, Gahagan S. The effect of maternal obesity on the offspring. Clin Obstet Gynecol 2014;57:508–15. 10.1097/GRF.0000000000000043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baeten JM, Bukusi EA, Lambe M. Pregnancy complications and outcomes among overweight and obese nulliparous women. Am J Public Health 2001;91:436–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paré E, Parry S, McElrath TF, et al. Clinical risk factors for preeclampsia in the 21st century. Obstet Gynecol 2014;124:763–70. 10.1097/AOG.0000000000000451 [DOI] [PubMed] [Google Scholar]
- 16.Kuhle S, Tong OS, Woolcott CG. Association between caesarean section and childhood obesity: a systematic review and meta-analysis. Obes Rev 2015;16:295–303. 10.1111/obr.12267 [DOI] [PubMed] [Google Scholar]
- 17.Pei Z, Heinrich J, Fuertes E, et al. Cesarean delivery and risk of childhood obesity. J Pediatr 2014;164:1068–73. 10.1016/j.jpeds.2013.12.044 [DOI] [PubMed] [Google Scholar]
- 18.Li HT, Zhou YB, Liu JM. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes 2013;37:893–9. 10.1038/ijo.2012.195 [DOI] [PubMed] [Google Scholar]
- 19.Yuan C, Gaskins AJ, Blaine AI, et al. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr 2016;170:e162385 10.1001/jamapediatrics.2016.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchi J, Berg M, Dencker A, et al. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev 2015;16:621–38. 10.1111/obr.12288 [DOI] [PubMed] [Google Scholar]
- 21.Corvalán C, Garmendia ML, Jones-Smith J, et al. Nutrition status of children in Latin America. Obes Rev 2017;18 Suppl 2(Suppl 2):7–18. 10.1111/obr.12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hallal PC, Andersen LB, Bull FC, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet 2012;380:247–57. 10.1016/S0140-6736(12)60646-1 [DOI] [PubMed] [Google Scholar]
- 23.The World Health Organization. Physical activity and young people. –accessed 05 Dec 2017 http://www.who.int/dietphysicalactivity/factsheet_young_people/en/.
- 24.Tremblay MS, Gray CE, Akinroye K, et al. Physical activity of children: a global matrix of grades comparing 15 countries. J Phys Act Health 2014;11(Suppl 1):S113–25. 10.1123/jpah.2014-0177 [DOI] [PubMed] [Google Scholar]
- 25.Chen YC, Chen PC, Hsieh WS, et al. Environmental factors associated with overweight and obesity in taiwanese children. Paediatr Perinat Epidemiol 2012;26:561–71. 10.1111/ppe.12001 [DOI] [PubMed] [Google Scholar]
- 26.Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 2012;70:3–21. 10.1111/j.1753-4887.2011.00456.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popkin BM, Hawkes C. Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diabetes Endocrinol 2016;4:174–86. 10.1016/S2213-8587(15)00419-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stern D, Piernas C, Barquera S, et al. Caloric beverages were major sources of energy among children and adults in Mexico, 1999-2012. J Nutr 2014;144:949–56. 10.3945/jn.114.190652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colchero MA, Popkin BM, Rivera JA, et al. Beverage purchases from stores in Mexico under the excise tax on sugar sweetened beverages: observational study. BMJ 2016;352:h6704 10.1136/bmj.h6704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almiron-Roig E, Chen Y, Drewnowski A. Liquid calories and the failure of satiety: how good is the evidence? Obes Rev 2003;4:201–12. 10.1046/j.1467-789X.2003.00112.x [DOI] [PubMed] [Google Scholar]
- 31.The Lancet Diabetes Endocrinology. Sweet success: will sugar taxes improve health? Lancet Diabetes Endocrinol 2017;5:235 10.1016/S2213-8587(17)30070-0 [DOI] [PubMed] [Google Scholar]
- 32.Barrientos-Gutierrez T, Zepeda-Tello R, Rodrigues ER, et al. Expected population weight and diabetes impact of the 1-peso-per-litre tax to sugar sweetened beverages in Mexico. PLoS One 2017;12:e0176336 10.1371/journal.pone.0176336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keller A, Bucher Della Torre S. Sugar-sweetened beverages and obesity among children and adolescents: a review of systematic literature reviews. Child Obes 2015;11:338–46. 10.1089/chi.2014.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Ruyter JC, Olthof MR, Seidell JC, et al. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med 2012;367:1397–406. 10.1056/NEJMoa1203034 [DOI] [PubMed] [Google Scholar]
- 35.Nuevo Etiquetado Frontal Nutrimental. Secretaria de salud. 2016. https://www.gob.mx/salud/en/documentos/nuevo-etiquetado-frontal-nutrimental (accessed 16 Mar 2018).
- 36.Instituto Nacional de Salud publica, UNICEF. Review of current labelling regulations and practices for food and beverage targeting children and adolescents in Latin America countries (Mexico, Childe, Costa Rica and Argentina) and recommendations for facilitating consumer information. 2016. https://www.unicef.org/lac/20161122_UNICEF_LACRO_Labeling_Report_LR(2).pdf (accessed 13 Mar 2018).
- 37.Börnhorst C, Wijnhoven TM, Kunešová M, et al. WHO European childhood obesity surveillance initiative: associations between sleep duration, screen time and food consumption frequencies. BMC Public Health 2015;15:442 10.1186/s12889-015-1793-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braithwaite I, Stewart AW, Hancox RJ, et al. The worldwide association between television viewing and obesity in children and adolescents: cross sectional study. PLoS One 2013;8:e74263 10.1371/journal.pone.0074263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reid Chassiakos YL, Radesky J, Christakis D, et al. Children and adolescents and digital media. Pediatrics 2016;138:e20162593 10.1542/peds.2016-2593 [DOI] [PubMed] [Google Scholar]
- 40.The American Academy of Pediatrics. American academy of pediatrics announces new recommendations for children’s media use. 2016. https://www.aap.org/en-us/about-the-aap/aap-press-room/Pages/American-Academy-of-Pediatrics-Announces-New-Recommendations-for-Childrens-Media-Use.aspx (accessed 05 Dec 2017).
- 41.Hirshkowitz M, Whiton K, Albert SM, et al. National sleep foundation’s updated sleep duration recommendations: final report. Sleep Health 2015;1:233–43. 10.1016/j.sleh.2015.10.004 [DOI] [PubMed] [Google Scholar]
- 42.Fatima Y, Doi SA, Mamun AA. Longitudinal impact of sleep on overweight and obesity in children and adolescents: a systematic review and bias-adjusted meta-analysis. Obes Rev 2015;16:137–49. 10.1111/obr.12245 [DOI] [PubMed] [Google Scholar]
- 43.Scharf RJ, DeBoer MD. Sleep timing and longitudinal weight gain in 4- and 5-year-old children. Pediatr Obes 2015;10:141–8. 10.1111/ijpo.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loring B, Robertson A. The World Health Organization. Obesity and inequities. Guidance for addressing inequities in overweight and obesity (2014). 2014. http://www.euro.who.int/en/publications/abstracts/obesity-and-inequities.-guidance-for-addressing-inequities-in-overweight-and-obesity-2014 (accessed 13 Feb 2018).
- 45.4to Informe de Gobierno. Ciudad de México. http://www.cdmx.gob.mx/storage/app/uploads/public/57d/c84/8ce/57dc848ceb3d0920333296.pdf (accessed 13 Feb 2018).


