Abstract
The Beckwith-Wiedemann syndrome (BWS) is a rare genetic syndrome. However, this is one of the most common overgrowth syndromes. This is a genetically and clinically heterogeneous syndrome. Here, we report a case of Beckwith-Weidemann syndrome without macrosomia, visceromegaly and hemihyperplasia but having macroglossia, omphalocele and anterior linear ear lobe creases. The diagnosis was confirmed by gene analysis suggestive of imprinting centre 2 (KvDMR1) hypomethylation defect.
Keywords: gastrointestinal system, genetic screening / counselling, neonatal intensive care
Background
The Beckwith-Wiedemann syndrome (BWS) is a rare genetic syndrome. However, this is one of the most common overgrowth syndromes. This is a genetically and clinically heterogeneous syndrome. Sometimes, the obvious features of macrosomia, visceromegaly and hemihypertrophy may not be present at birth but still BWS needs to be considered in infants with omphalocele present with other minor signs. These patients may subsequently develop overgrowth in the postnatal period. Prognosis of BWS largely depends on the mutation involved, and imprinting centre 2 (IC2) (KvDMR1) hypomethylation is one such mutation with low risk for future malignancy and therefore carries a good long-term prognosis.
Case presentation
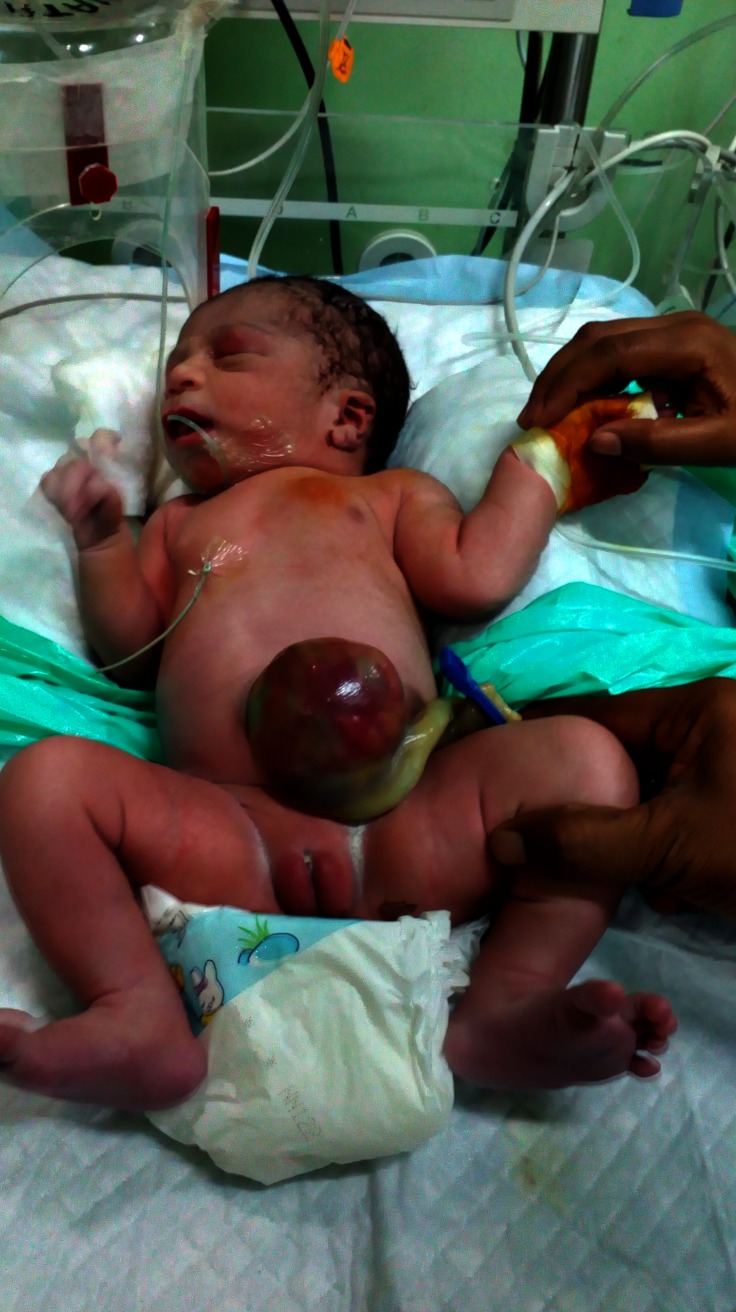
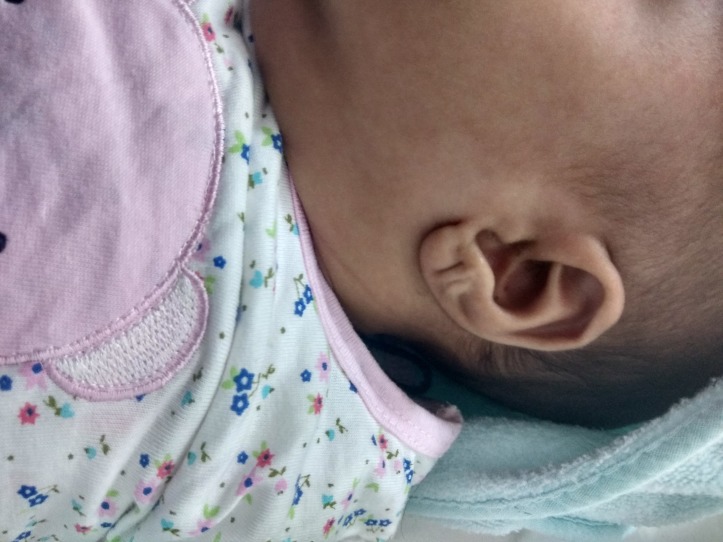
A 38+5 weeker, term, female infant with a birth weight of 3.39 kg (63 percentile) was born to a 26-year-old mother, gravid 3, para 1 abortion 2, living 0, by elective caesarean section in view of antenatal diagnosis of omphalocele. Infant was born out of non-consanguineous marriage. There was no history of any teratogen intake or exposure to any radiation during antenatal period. Targeted imaging for fetal anomaly done at 20 weeks was suggestive of omphalocele. Subsequent fetal karyotype done was normal. Baby cried immediately after birth and had Apgar score of 7, 9, 9 at 1, 5 and 10 min, respectively. On physical examination, birth weight was 3.39 kg (50–90th percentile), length 52 cm (50–90th percentile) and head circumference was 35 cm (10–50th percentile) according to Fenton chart. There was impressively large tongue protruding (figure 1) out of the oral cavity and about 7×7 cm omphalocele (figure 2), linear creases on ear lobule (figure 3), haemangioma on both upper eyelids but no obvious dysmorphic features, hemihypertrophy and organomegaly. Macroglosia did not interfere with feeding and there were no features of obstructive apnoea or respiratory distress. Blood sugar at birth was 56 mg/dL. There was no episode of hypoglycaemia during hospital stay.
Figure 1.
Macroglosia presenting as protruding tongue.
Figure 2.
Omphalocele at birth.
Figure 3.
Transverse ear pit seen at birth.
Investigations
Alpha-fetoprotein (AFP) level on day 9 was 9750.0 ng/mL and on follow-up, on day 30 it was 1738.0 ng/mL (decreasing trend).
Ultrasonogram, abdomen—normal.
Gene analysis—KvDMR1 IC2 hypomethylation defect.
Differential diagnosis
Hypothyroidism
Trisomy 21
Simpson-Golabi-Behmel syndrome
Costello syndrome
Perlman syndrome
Sotos syndrome
Mucopolysaccharidosis type VI (Maroteaux-Lamy syndrome)
Mosaicism for trisomy 8.
Treatment
Omphalocele was covered with saline-soaked gauze and baby was started on intravenous fluids at birth. Omphalocele was operated bed side in neonatal intensive care unit on day 3 of life and baby was started on feeds on day 4 of life. Postoperative period was uneventful. Feeds were subsequently increased to ad lib by day 5.
Outcome and follow-up
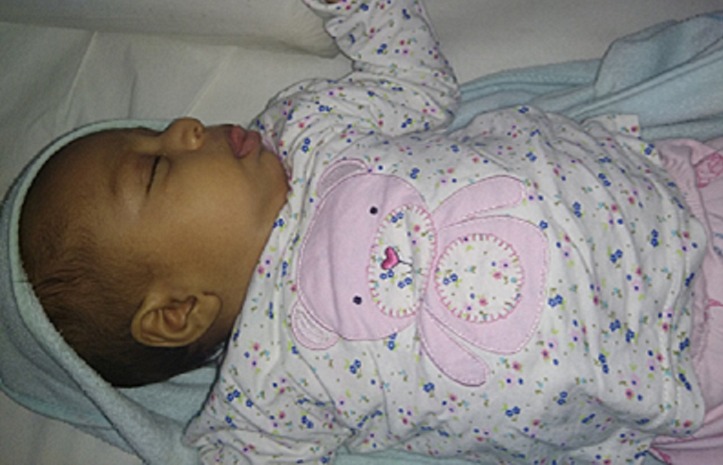
Baby was discharged on breast feeds with a plan to do abdomen ultrasonography every 3 month for at least 7–8 year of age and blood test for AFP every 6 week at least till 4 year of age. On follow-up at 58 days, weight was 5.886 kg (94 percentile), length was 60 cm (>98 percentile) and head circumference was 40 cm (98 percentile). At 9 months of age weight was 10.02kg, length 80cm and occipitofrontal circumference 45cm (figure 4). Ultrasonography of abdomen was suggestive of mild hepatosplenomegaly.
Figure 4.

Photo of the female infant.
Discussion
Beckwith-Wiedemann syndrome is a rare genetic syndrome. It is also known as EMG syndrome to describe exomphalos, macroglossia and gigantism. This syndrome was first time described independently by Beckwith in 1963 and by Wiedemann in 1964.1 2 The incidence of BWS is about 1:13 700 births, with an equal sex distribution. It is a clinically and genetically heterogeneous syndrome. Most of the cases (85%) are sporadic and few cases (15%) are familial with autosomal dominant inheritance.3 There was no family history suggestive of BWS in our case. There are two separate imprinted subdomain. The telomeric subdomain includes IGF2 and H19 genes, and the centromeric subdomain includes p57KIP2, LIT1 and KvLQT1 genes.2 There are two imprinting centres known as IC1 (H19DR) and IC2 (KvDMR1). BWS is a result of imprinting abnormality of various genes over the area of 11p15 in majority of cases and only 1%–2% cases are as a result of cytogenetic abnormalities and comprise translocations or inversions, microdeletions of KvDMR1 or H19DMR (maternally inherited) and paternal duplications. In our index case, KvDMR1 IC2 hypomethylation defect was noted which is the most common defect and is associated with better prognosis than most of other variants. Furthermore, the tumours in this molecular subgroup do not include Wilms tumour. Lastly, individuals with mutations in CDKN1C seem to have the lowest risk with only a small number of cases reported. In cases with CDKN1C mutation, only neuroblastoma has been reported to date.4–6 Clinically, BWS may present with various combination of features. These patients are genetically predisposed to overgrowth both prenatally and postnatally. Postnatally, overgrowth is most marked in the first few years. The common presenting features are macroglossia (97%–100%), the abdominal wall defects as omphalocele or gastroschisis (77%–80%), hypoglycaemia (63%) and macrosomy (68%).7 8 Elliott and Maher defined a criteria for diagnosis of BWS based on clinical features. The criteria includes major features such as prenatal and/or postnatal overgrowth (>90th centile), macroglossia, abdominal wall defects and some minor features such as ear signs (anterior linear lobe creases and posterior helical pits), facial nevus flammeus, hypoglycaemia, organomegaly and hemihypertrophy. For diagnosis, there should be either all three major features or any two major plus three or more minor features.9 However, phenotypic presentation of BWS may be subtle also called as incomplete form of BWS and may be diagnosed later in life secondary to a malignancy.3 10 In our case, omphalocele, macroglossia and ear lobe creases were present. It is important to follow-up cases who have features suggestive but not conclusive of BWS because of risk of malignancies. In our case, although infant was not having any overgrowth features at birth but subsequently on follow-up at 2 months of age overgrowth was observed. There was no prenatal overgrowth, hypoglycaemia, organomegaly, polycythaemia, hemihypertrophy and facial nevus flammeus. Wilms tumour is the most common tumour in patients with BWS followed by hepatoblastoma. In our patient, ultrasound of abdomen at 9 months of life was normal and AFP levels were normal. Furthermore, abnormal facies have been described which include prominent eyes with infraorbital creases, facial nevus flammeus, midfacial hypoplasia, full lower face with a prominent mandible, anterior earlobe creases and posterior helical pits. The BWS facies often normalises across childhood so that evaluation of adolescents or adults suspected to have BWS benefits from assessment of early childhood photographs.11 Prenatal diagnosis has been described in literature based on phenotypic features.12 Other associated features reported in literature include
embryonal tumour (eg, hepatoblastoma, neuroblastoma, rhabdomyosarcoma) in childhood;
visceromegaly involving liver, spleen, kidneys, adrenal glands and/or pancreas;
cytomegaly of the fetal adrenal cortex (pathognomonic);
renal abnormalities including structural abnormalities, nephromegaly, nephrocalcinosis and/or later development of medullary sponge kidney;
posterior helical ear pits;
placental mesenchymal dysplasia;
cleft palate;
cardiomyopathy;
positive family history (≥1 family members with a clinical diagnosis of BWS or a history or features suggestive of BWS).
A diagnosis of BWS can be confirmed by molecular/cytogenetic testing. Cytogenetically, detectable abnormalities involving chromosome 11p15 are found in 1% or fewer of affected individuals. Molecular genetic testing can identify epigenetic and genomic alterations of chromosome 11p15 in individuals with BWS. Loss of methylation on the maternal chromosome at IC2 is seen in 50% of affected individuals, paternal uniparental disomy for chromosome 11p15 in 20% and gain of methylation on the maternal chromosome at IC1 in 5%. Sequence analysis of CDKN1C identifies a heterozygous maternally inherited pathogenic variant in approximately 40% of familial cases and 5%–10% of cases with no family history of BWS.13
Learning points.
Beckwith-Weidemann syndrome (BWS) is genetically and clinically a heterogeneous syndrome; therefore, all the major features may or may not be present at birth.
Some features like overgrowth may not be present at birth but may develop subsequently.
Patients with BWS are prone to hypoglycaemia and polycythaemia.
Follow-up is important in these cases to look for any abdominal tumour like Wilms tumour.
Genetic counselling is important to know the risk in subsequent pregnancy.
Footnotes
Contributors: Aakash, SG, Astha wrote the manuscript. GG did the corrections. GG, Aakash did the final assessment and comilation. All authors before submission approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent: Parental/guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Beckwith JB. Extreme cytomegaly of the adrenal fetal cortex, omphalocele, hyperplasia of kidneys and pancreas, and Leydig-cell hyperplasia: another syndrome. Western Soc Pediatr Res (Abstr) 1963:20. [Google Scholar]
- 2.Wiedemann H-R. [Familial malformation complex with umbilical hernia and macroglossia – a "new syndrome"?]. J Genet Hum 1964;13:223–32. [PubMed] [Google Scholar]
- 3.Sotelo-Avila C, Gonzalez-Crussi F, Fowler JW. Complete and incomplete forms of Beckwith-Wiedemann syndrome: their oncogenic potential. J Pediatr 1980;96:47–50. 10.1016/S0022-3476(80)80322-2 [DOI] [PubMed] [Google Scholar]
- 4.Niemitz EL, DeBaun MR, Fallon J, et al. . Microdeletion of LIT1 in familial Beckwith-Wiedemann syndrome. Am J Hum Genet 2004;75:844–9. 10.1086/425343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riccio A, Sparago A, Verde G, et al. . Inherited and sporadic epimutations at the IGF2-H19 locus in Beckwith-Wiedemann syndrome and Wilms' tumor. Endocr Dev 2009;14:1–9. 10.1159/000207461 [DOI] [PubMed] [Google Scholar]
- 6.Reik W, Brown KW, Slatter RE, et al. . Allelic methylation of H19 and IGF2 in the Beckwith-Wiedemann syndrome. Hum Mol Genet 1994;3:1297–301. 10.1093/hmg/3.8.1297 [DOI] [PubMed] [Google Scholar]
- 7.Reish O, Lerer I, Amiel A, et al. . Wiedemann-Beckwith syndrome: further prenatal characterization of the condition. Am J Med Genet 2002;107:209–13. 10.1002/ajmg.10143 [DOI] [PubMed] [Google Scholar]
- 8.Li M. Molecular genetics of Wiedemann Beckwith Syndrome (BWS) a case report and literature review. Am J Med Gen 1998;79:253–9. [PubMed] [Google Scholar]
- 9.Elliott M, Maher ER. Beckwith-Wiedemann syndrome. J Med Genet 1994;31:560–4. 10.1136/jmg.31.7.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan KC, Cheung WK, Chen YC. Incomplete forms of Beckwith-Wiedemann syndrome: report of a case. J Formos Med Assoc 1994;93:813–5. [PubMed] [Google Scholar]
- 11.Weksberg R, Shuman C, Beckwith JB. Beckwith-Wiedemann syndrome. Eur J Hum Genet 2010;18:8–14. 10.1038/ejhg.2009.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kumar N, Agarwal S, Das V, et al. . Prenatal sonographic diagnosis of Beckwith-Wiedemann syndrome in a fetus with omphalocoele. BMJ Case Rep 2016. doi: 10.1136/bcr-2016-217993 [Epub ahead of print 2 Nov 2016]. 10.1136/bcr-2016-217993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuman C, Beckwith JB, Smith AC, et al. . Beckwith-Wiedemann syndrome. Gene reviews. http://www.ncbi.nlm.nih.gov/books/NBK1394/ (Retrieved 11 Aug 2016).