Abstract
This is the first surveillance study using methacrylate monolithic supports to concentrate environmental coastal water samples, prior to molecular target detection by RT-qPCR. Rotaviruses (RoV) and Noroviruses (NoV) were monitored in a polluted area at the Bay of Koper (Gulf of Trieste, Northern Adriatic Sea) and at a nearby bathing area and mussel farm areas.
RoV and NoV are released into the Bay of Koper, with higher rates close to the discharge of the wastewater treatment plant, however, they can be detected at recreational and mussel farming areas. Our results showed that water bodies considered safe based on FC concentrations, can still have low, yet potentially infective, concentrations of human viruses.
Keywords: Rotavirus, Norovirus, Faecal coliforms, RT-qPCR, Methacrylate monolithic columns, Enteric virus concentration
Highlights
-
•
First surveillance study of viral contamination in coastal waters at the Bay of Koper in Slovenia (Gulf of Trieste, Northern Adriatic Sea);
-
•
RoV and NoV were detected throughout the year, with highest detection rates at locations close to WWTP inputs;
-
•
RoV and NoV were occasionally detected at areas used for recreation and for mussel growth, and thus pose a threat to human health;
-
•
The use of faecal coliform bacteria alone as indicator of faecal pollution is insufficient to ensure safe use of coastal waters.
1. Introduction
Coastal areas are important climate change hotspots that are subjected to an increase of population and consequently an increase in economic activities that generate several environmental concerns (Giorgi, 2006; de Sherbinin, 2014). Nowadays, about 50% of world population resides in cities located near the coast, and the trend is expected to continue in the next decades. Estimations predict an increase in surface water temperatures, sea level rise and an intensification of inundation and flooding events (Brown et al., 2013; Small and Nicholls, 2003; WHO, 2015).
Pollution of coastal waters is a major global problem, since these areas are often recipients of waste waters and are used for recreation and aquaculture (Fiksdal et al., 1994; Goh et al., 2017). Contaminants that lead to pollution of coastal environments include feces and urine from humans, domesticated and wild animals (Bradshaw et al., 2016; Field and Samadpour, 2007). Pathogens discharged from wastewaters pose a health risk to everyone exposed to the polluted waters, mainly to recreational users (by swimming or participating in other water-related activities, such as surfing, snorkeling, sailboarding, among others) and consumers of harvested food from the contaminated area. The number of publications related to water quality and water-related diseases increased in the last decade and gastroenteritis is still a major health risk from exposure to contaminated waters (Sweileh et al., 2016).
Microbial quality of waters has been traditionally assessed by monitoring faecal indicator bacteria, such as Escherichia coli, intestinal enterococci and faecal coliform bacteria (FC) (Liang et al., 2015). They were selected as environmental indicators of human pathogens because they are abundant in surface waters, easy to culture under controlled conditions and widely available in the intestinal flora of humans and other warm-blooded animals (Bartram, 2001; Bradshaw et al., 2016; George et al., 2002). However, several studies have shown that the reliance on monitoring faecal coliform bacteria alone as indicators of faecal pollution is insufficient to protect human health (Bartram, 2001; Bradshaw et al., 2016; Gerba et al., 1979; Liang et al., 2015). One of the main limitations of coliform bacteria, as indicators of faecal pollution, is their poor correlation with the presence of non-bacterial pathogens, in particular with the presence of human viruses (Cook, 2013; Eslamian, 2016; Payment and Locas, 2011; Petterson et al., 2001). Viruses persist in marine environments for long periods (Suttle, 2005), which can lead to higher risk of human exposure (Updyke et al., 2015). Not surprisingly, human viruses are believed to cause the majority of waterborne diseases worldwide Griffin et al., 2001, Griffin et al., 2003.
A group of viruses that have significant impact on public health are enteric viruses (Griffin et al., 2003; Updyke et al., 2015). These pathogens are associated with a variety of human diseases, from ocular and respiratory infections to gastroenteritis, hepatitis, myocarditis and aseptic meningitis (Gerba et al., 1996). Infections by enteric viruses cause >2 million deaths each year (WHO, 2015), mainly in developing countries (Lin and Ganesh, 2013; Rezaeinejad et al., 2014; WHO, 2015). Rotavirus (RoV) and norovirus (NoV) are among the most commonly present enteric viruses in polluted coastal waters and the main agents of viral gastroenteritis worldwide (Bishop, 2009; Girones et al., 2010; Lin and Ganesh, 2013; Nwachcuku and Gerba, 2004; Robilotti et al., 2015).
Due to the low numbers of human viruses in natural waters and their low infection dose (as few as 10 particles), an efficient concentration step is critical for an effective detection (Balasubramanian et al., 2016; Bosch et al., 2005; Bosch, 2007; Gentry-Shields et al., 2013; Haas et al., 1993). Methods that use adsorption elution principle with electropositive or electronegative filters, sedimentation by flocculation, ultrafiltration and ultracentrifugation are some of the most widely used concentration methods for enteric viruses in environmental waters (Calgua et al., 2008; Fong and Lipp, 2005). However, these methods have poor recovery rates and slow processing times (Ikner et al., 2012). To overcome these limitations, in a recent study, CIM C4 hydrophobic interaction methacrylate monolithic columns, were successfully applied to concentrate RoV and NoV from water samples with different salinities, prior to molecular target detection with RT-qPCR. The procedure exhibited good recovery rates when compared with traditional methods, and allows fast concentration of enteric viruses in one step. The recovery rate of rotaviruses was 92% with a concentration factor of 307-fold. For noroviruses the recovery rate was 13% with a concentration factor of 43-fold (Balasubramanian et al., 2016).
In the current study, the presence of RoV and NoV genogroup II (from now on written as RoV and NoV, respectively) were monitored using a recently described CIM C4 concentration method, in combination with one-step RT-qPCR detection, to survey coastal water quality in the Gulf of Trieste (northern Adriatic Sea). During a period of one year, RoV and NoV were seasonally monitored, along a salinity/pollution gradient at the inner part of the Bay of Koper and at areas used for mussel farming and recreation. Possible correlations between the presence of RoV and NoV with the concentrations of FC and physicochemical parameters in coastal water samples were concurrently evaluated.
2. Materials and methods
2.1. Study site and sample collection
The Bay of Koper is a semi-closed and shallow area with an average depth of 16 m, located in the Gulf of Trieste (eastern northernmost part of Adriatic Sea). The oceanographic properties are dependent on pronounced seasonal variability of seawater temperature (5–26 °C) and salinity (25–38 psu). Despite the main anticlockwise circulation in the Gulf of Trieste, the circulation is additionally influenced by tides and wind, especially by eastern wind (Falcieri et al., 2016; Malačič and Petelin, 2009).
This area is under several anthropogenic pressures with the main pollution sources being freshwater inputs and sewage discharges. At the southern part of Slovenian coast (Bay of Koper), river Rižana is the main source of freshwater inputs. Rižana has small drainage basins (30–204 km2, length 9–28 km) with an average flow from 0.25 to 4.0 m3/s. The average discharges are highest in spring (from February to June) and lowest in summer (July and August). During the period of storms, the flow may increase up to 10 times. River Rižana receives urban and industrial wastewaters from inland, including the waste of Izola General Hospital, which is the main hospital in the area. Rižana also receives runoff water of unregulated sewage systems from the city and inland. The WWTP of Koper (84,500 PE - population equivalent) collects domestic wastewaters, industrial wastewater and runoff of the city of Koper and discharges them in the mouth of Rižana river (Cozzi et al., 2012; Malačič and Bogunovič, 2009; Olivotti et al., 1986; Turk et al., 2007).
The mouth of Rižana is located in the eastern part of the Bay of Koper. High discharges and sediment transport peaks occur during short periods of intense precipitation (Mandac et al., 2014). The ecological and sanitary quality of the whole bay strongly depends on yearly nutrient loads and sewage discharges from the WWTP of Koper (Cozzi et al., 2012; Olivotti et al., 1986; Turk et al., 2007). The Bay of Koper has a hilly coast in the east and a flat area in the west, with several hotels, camping sites and several beaches. The inner part has three basins from the port of Koper.
Tourism is the most important industry at the Slovenian coast. During summer months, a high density of population is present. From June 2015 till July 2016, >2.5 million overnight stays were registered in Koper, Piran, Izola and Ankaran (see Supplementary material).
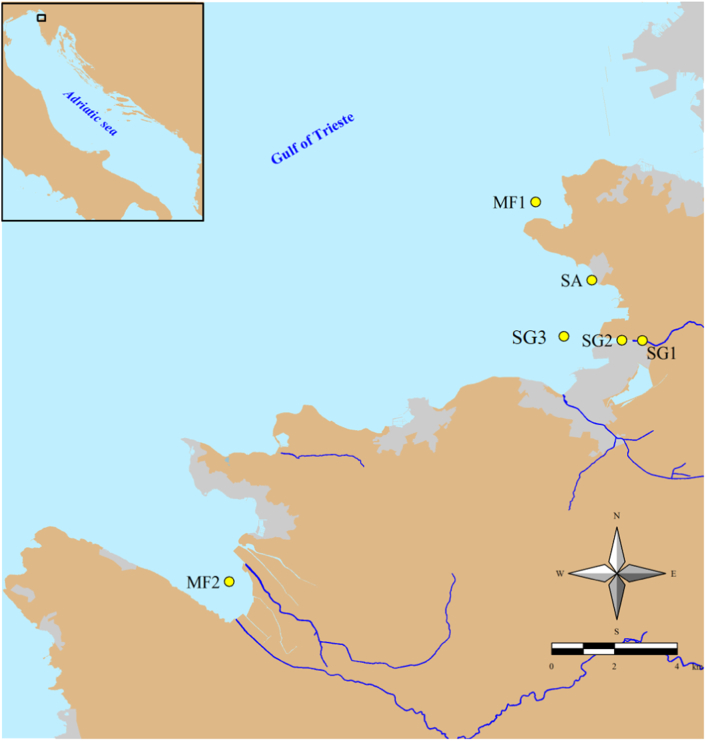
Samples were divided in two groups, based on their location (Fig. 1): Salinity/pollution gradient (SG1, SG2 and SG3) and areas used for human activities (MF1, MF2 and SA1). Within the salinity/pollution gradient, SG1 (13°45.608′E; 45°33.460′N) is located at the outlet of Koper WWTP in river Rižana, SG2 (13°44.622′E; 45°33.517′N) in the estuary at 700 m from WWTP discharge, and SG3 (13°43.199′E; 45°33.579′N) in the middle of the Bay of Koper and 2000 m from the WWTP discharge (Fig. 1). Sampling was performed when the flow rate of river Rižana and the level of seawater were close to the yearly average. Water samples were also collected at a nearby swimming area (station SA1–13°73.486′E;45°57.425′N), located at 3000 m from the discharge of WWTP of Koper, and at two mussel farming areas, one located in the Bay of Koper in front of the west end of Muggia peninsula Debeli Rtič (station MF1–13°42.501′E;45°35.899′N), and the second at the inner part of the Bay of Piran (station MF2–13°34.966′E; 45°29.353′N).
Fig. 1.
Sampling locations at the inner part of the Bay of Koper and Bay of Piran (Gulf of Trieste, northern Adriatic Sea). Salinity/pollution gradient: Outlet of Koper WWTP located in river Rižana mouth (SG1), Rižana estuary (SG2), located at 700 m from the WWTP discharge and middle of the Bay of Koper (SG3). MF1 and MF2 are two locations used for mussel farming and SA1 is in a beach used for swimming and other recreational activities.
Water samples were collected using Niskin bottles at the sub-surface level (≈0.3 m depth), except at the mussel farming areas, where a 10 m long tube was used to sample water from the entire water column. At each sampling campaign, water temperature and salinity were measured, using a CTD fine-scale probe (Microstructure Profiler MSS90, Sea & Sun Technology GmbH).
Sampling was performed in 7th of July, 5th of October, 25th of November of 2015 and 5th of February, 12th of April and 15th of June of 2016. In total, 36 water samples were collected during the period of one year.
2.2. Concentration of RoV and NoV from water samples using CIM C4 columns
Samples were filtered through cellulose-acetate membranes with a pore size of 0.8 μm (Whatman) to remove bigger particles and organisms. Concentration was performed using Fast Protein Liquid Chromatography (FPLC) (AKTA purifier 100, GE-Healthcare) with CIM 8 ml C4-HLD (High ligand density, Butyl Column, BIA Separations) as described by (Balasubramanian et al., 2016). Briefly, 4000 ml of each sample were pumped into a CIM 8 ml C4-HLD column using Buffer A (solution of 0.6 M NaCL and 50 mM HEPES; pH 7) as mobile phase. The elution was made using Buffer B (solution with 50 mM HEPES; pH 7) to 12 ml of concentrated sample. The elution peak was monitored by measuring UV absorption. After each measurement, CIM-HLD was sanitized with 1 M NaOH for 30 min between samples (between-run sanitization) and for 120 min at the end of each sampling set and before storage of the column (final sanitization). Elutions were collected using Fractionator 920 (GE-Healthcare). Aliquots of the load (L), flow-through (FC), wash (W) and elutions (E1, E2 and E3) were collected and immediately stored at −20 °C until further analysis.
At the end of each concentration round and after sanitization of the CIM C4 column with 1 M NaOH, both a negative concentration control (NCC) and a positive concentration control (PCC) were processed. NCC (4000 ml of tap water with 140 g of NaCl) was loaded into the column and treated in the same way as any real samples. In this way, we assessed for any potential carryover due to suboptimal column sanitization. For the PCC, RoV positive clarified stool samples were used to prepare artificially inoculated saline tap water, which was then concentrated in the same way as the samples to control the performance of the column (4000 ml of tap water plus 140 g of NaCl and 50 μl of clarified suspension of RoV). Clarified suspensions from clinical stool samples were obtained from hospitalized children diagnosed with acute gastroenteritis and characterized to be positive for RoV and NoV GII.
2.3. Viral RNA extraction
RNA was extracted from a 140 μl aliquot of the concentrated samples (12 ml) using QIAamp Viral RNA Mini Kit according to the manufacturer's instructions (QIAGEN, Chatsworth, CA, USA) to obtain 45 μl of isolated RNA. For all samples, a known concentration (2 ng) of luciferase RNA (Promega, Madison, WI, USA) was added as an internal control for RNA isolation and to check potential PCR inhibitory effects intrinsic to the samples (Toplak et al., 2004). For each RNA isolation procedure, a negative control of isolation (NCI) was included containing only buffers.
2.4. Molecular detection of RoV and NoV by RT-qPCR
RoV and NoV were detected using nucleic acid amplification by one-step RT-qPCR on an ABI PRISM 7900HT sequence detection system (Applied Biosystems) using Ag Path Kit (Life Technologies), with a final volume per reaction of 10 μl (8 μl of master-mix and 2 μl of extracted RNA). The qPCR assays for multiple RoV, NoV GII and luciferase were used according to Gutiérrez-Aguirre et al., 2008, Kageyama et al., 2003 and Toplak et al., 2004, respectively. The cycling conditions for RT-qPCR assays were: reverse transcriptase 48 °C, 10 min; denaturation 95 °C, 10 min; 45 cycles of denaturation at 95 °C for 15 s; and annealing/extension at 60 °C for 60 s. Reactions were run in triplicates. All the RT-qPCR reactions were planned using GENEIO qPCR workflow application (BioSistemika LLC, Ljubljana, Slovenia) and the microplates were pipetted with the assistance of PLATR smart pipetting assistant (BioSistemika LLC, Ljubljana, Slovenia). Quantification cycle (Cq) for each reaction was obtained using the software SDS 2.4 (AppliedBiosystems, CA, USA). The fluorescence thresholds were manually set for RoV and NoV according to the amplification curve. For luciferase, the threshold was set to 0.4. Non-template control (NTC) was used at each reaction to monitor potential contamination in the qPCR reagents during pipetting. A positive control (PC), for RoV and NoV, was added to monitor each amplification. A sample was considered positive when the software gave any Cq. In addition, each positive amplification curve was manually checked and only curves showing a significant slope increase, in contrast with the negative control curves (NTC, NCI, and NCC), were considered as real positives. The results were expressed as + or –, because the detected concentrations laid beyond the limit of quantification of the two assays (Cq ranged from 34.74 to 44.21 for RoV and from 34.69 to 44.72 for NoV).
2.5. Culture-based quantification of faecal coliform bacteria (FC)
FC were analyzed according to the guidelines of WHO/UNEP (WHO 2014), by membrane filtration method, using sterile membrane filters (0.45 μm pore size) (Millipore). After filtration, membrane filters were transferred onto solid mFC agar media plates (Merk) and incubated at 44 ± 0.5 °C for 24 h. The results are expressed as number of FC colonies per 100 ml (FC/100 ml).
2.6. Data analyses
Statistical analyses were performed using the free software R (R Core Team 2016, Vienna, Austria). Kruskal-Wallis rank sum test at 0.05 significance level was applied to determine if there are systematic differences in values among groups from the six sampling sites in the Gulf of Trieste. Sommers' D measure of association was calculated to evaluate the strength and direction of ordinal association between concentrations of FC and physicochemical parameters with the presence of RoV and NoV (Binary outcome). Spearman's rho2 rank correlation coefficients were calculated to access possible monotonic relationships between FC concentrations and physicochemical parameters. Different levels of correlation were defined (strong: r > 0.5; moderate: 0.3 < r < 0.5; and weak r < 0.3).
3. Results and discussion
In this study, we evaluated the use of CIM C4 columns to concentrate RoV and NoV during an environmental survey. Results of the detection of RoV and NoV were compared with results of FC and with in situ measurements of physical properties of water in a small faecal polluted estuary and in areas used for human activities at the inner part of the Bay of Koper.
To our knowledge, this is the first study in Slovenia in which coastal waters were sampled to monitor the presence of enteric viruses. It is also the first surveillance study using methacrylate monolithic chromatographic supports to concentrate enteric viruses from coastal waters, prior to detection with RT-qPCR. This combination was recently validated at the laboratory level using artificially inoculated samples and it proved to be an effective and efficient tool for detection of RoV and NoV in both seawater and brackish water (Balasubramanian et al., 2016).
3.1. Faecal coliform bacteria and background environmental parameters
FC were detected in 86% of the tested samples, which shows that contamination by human wastes is systematic in the area. Previous studies shown that the Bay of Koper has been suffering a major negative effect of improperly treated wastewaters (Turk et al., 2007).
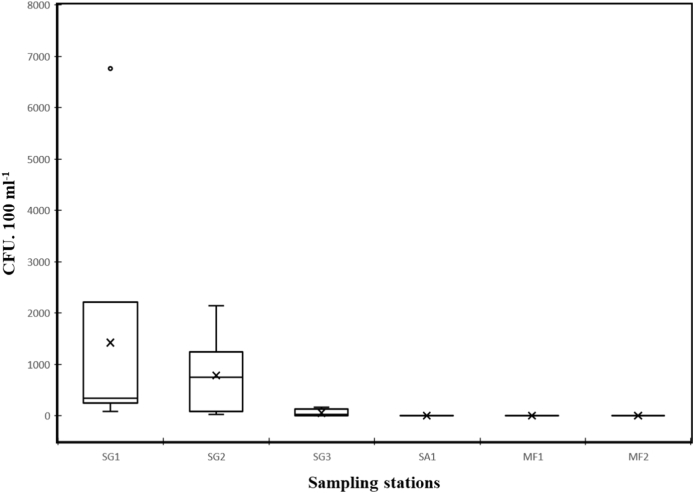
The levels of FC concentrations strongly varied among the tested locations (Fig. 2), with highest concentrations at the sampling site in the mouth of river Rižana (SG1) and at Rižana estuary (SG2). A clear decrease was observed as the distance to the WWTP discharge increased, suggesting that faecal pollution in the Bay of Koper is mainly originated by the WWTP effluent located in river Rižana. The observed variability among sites might be, not only because of the distance to sewage outputs, but due to a higher dispersion and dilution effects (Wyn-Jones et al., 2011). The observed variability among sampling sites was also observed in other studies in fresh and sea water (Marti et al., 2013; M. Oliveira et al., 2016; Sun et al., 2016).
Fig. 2.
Box plot with concentrations of faecal coliform bacteria by sampling location: Rižana river (SG1), Rižana estuary (SG2), Bay of Koper (SG3), swimming area (SA1) and mussel farming areas (MF1 and MF2). Mean values are represented with an X. Data from July 2015 till June 2016.
Concentrations of FC, in brackish and marine samples collected at the salinity/pollution gradient, were high and, except in July 2015, always exceeded the limit value of 100 FC/100 ml according to the guidelines of WHO (WHO, 2015). The highest concentrations occurred in February of 2016 at SG1 (6.76 × 103 FC/100 ml) (Fig. 3), when the lowest salinity was measured (2.0) and water temperature was low (10.4 °C) (Fig. 4).
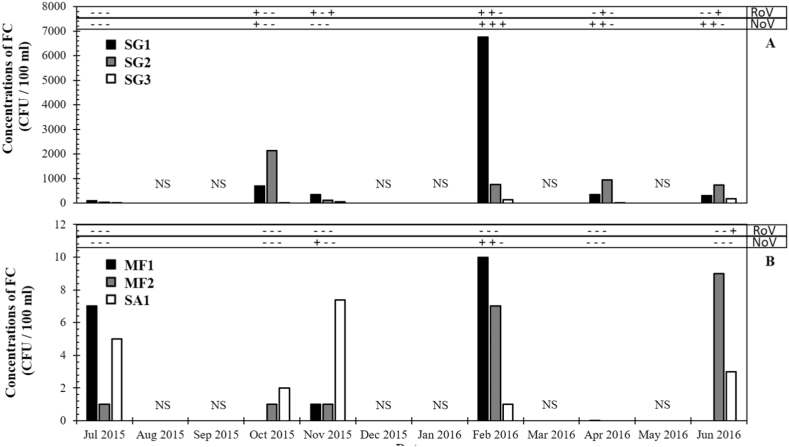
Fig. 3.
Seasonal concentrations of faecal coliform bacteria and presence/absence of RoV and NoV at the salinity pollution gradient (A) and at areas used for human activities (B): Rižana river (SG1), Rižana estuary (SG2), Bay of Koper (SG3), swimming area (SA1) and mussel farming areas (MF1 and MF2). Data from July 2015 till June 2016. NS indicates that samples were not collected in that month.
Fig. 4.
Seasonal variation of temperature at the six sampling locations (A) and seasonal variations of salinity at the locations closest to freshwater inputs (B). Salinity was plotted with the increase of distance to the mouth of river Rižana, where the WWTP discharge is located. SG1 corresponds to 0 m, SG2 to 700 m, SG3 to 2000 m and SA1 to 3000 m.
Average water temperature and salinity at the different locations are summarized in Table 1. Salinity was clearly lower at the locations closest to freshwater inputs with the highest fluctuations in SG2. Temperature was lower at the stations located in river Rižana (13.7 ± 2.8 ºC and 15.4 ± 4.4 ºC in SG1 and SG2, respectively) than in the other stations (17.5 ± 4.4 ºC, 17.4 ± 4.8 ºC, 17.5 ± 4.6 ºC and 17.3 ± 5.0 ºC in SG3, SA1, MF1 and MF2, respectively). Seasonal variations in water temperature followed the same pattern in all the locations (Fig. 4A).
Table 1.
Mean values of physicochemical data for each sampling time-point during the studied period (July 2015 to June 2016). Rižana river (SG1), Rižana estuary (SG2), Bay of Koper (SG3), swimming area (SA1) and mussel farming areas (MF1 and MF2).
| Parameters | SG1 |
SG2 |
SG3 |
SA1 |
MF1 |
MF2 |
|---|---|---|---|---|---|---|
| Surface water ± SD | Surface water ± SD | Surface water ± SD | Surface water ± SD | Water column ± SD | Water column ± SD | |
| Water temperature (°C) | 13.7 ± 2.8 | 15.4 ± 4.4 | 17.5 ± 4.4 | 17.4 ± 4.8 | 17.5 ± 4.6 | 17.3 ± 5.0 |
| Salinity | 6.4 ± 4.8 | 21.1 ± 10.5 | 31.6 ± 7.1 | 36.5 ± 0.9 | 36.5 ± 1.0 | 36.2 ± 0.8 |
± Standard deviation (SD); n = 6.
Salinity was rather constant during the studied year and higher than at the salinity/pollution gradient, which shows that less inputs of fresh water affect these locations and consequently less pollution is expected (George et al., 2002; S.S. Oliveira et al., 2016; Turk et al., 2007). In Fig. 4B, the salinity was plotted with the distance to the WWTP discharge (0, 700 and 2000 m, SG1, SG2 and SG3 respectively). SA1 was also included due to the proximity with the pollution source (3000 m). MF1 and MF2 had constant salinity values during the studied period, showing that these areas do not receive relevant freshwater inputs. As seen in Fig. 4B, the salinity varied a lot at 700 m from the WWTP (SG2). In February 2016, at 2000 m from the pollution source (station SG3), the salinity was the lowest compared to the other dates, which indicates that large inputs of freshwater reached this location.
As shown in Fig. 5A, except for February 2016, when the sea level was the lowest of all the year (163.4 cm), all the samples were collected in a range very close to the average yearly sea level (≃231 cm). Samples collected in July 2015 and April 2016 had the lowest flow rates (0.227 and 0.541 m3/s, respectively) (Fig. 5B). The highest flow rate was registered in February 2016 (4.74 m3/s), followed by November 2015 (1.61 m3/s) and October 2015 (1.375 m3/s). The mean flow rate was 3.18 m3/s and the sea level collected at the tide gauge in Koper had an average of 231.2 cm, ranging from 163.4 cm to 286.4 cm. The highest concentrations of FC in February 2016 can be related to the higher river flow rate, low sea level and the lowest salinity registered on that month, which could favour the spread of FC in the surface layer of the water column towards the middle of the bay (SG3) and towards the coastal swimming area (SA1).
Fig. 5.
Mean daily sea level (in cm) collected at the tide gauge of Koper. Horizontal line indicates the mean sea level for the studied year (≃231 cm) (A); and mean daily flow rate (in m3/s) of river Rižana (B). Red lines indicate the date of each sampling campaign.
At the mussel harvesting areas and swimming area, the mean concentrations of FC were significantly lower than at the salinity/pollution gradient (P < 0.001). Though the presence of FC was predominant, their concentrations were always lower than 100 FC/100 ml (Fig. 3), which classifies the waters as suitable for recreational usage (WHO, 2015).
3.2. Quality controls for CIM concentration, RNA extraction and RT-qPCR detection of RoV and NoV
Due to the novelty of the method and to evaluate the performance of the concentration step for environmental surveys, and as detailed in Section 2.2, we ran positive and negative controls for the CIM C4 concentration step, for each sampling campaign. There were no signs of carryover in the processed NCCs at the end of each set of concentrations, which demonstrates that sanitization with 1 M NaOH for 120 min after each run was reliable. The RoV concentration factors achieved in the processed PCCs in each column used in one round of concentrations were between 50-fold and 80-fold, indicating an optimal performance of CIM C4 columns. Additionally, the controls for RNA isolation, NCI (Section 2.3) and RT-qPCR, NTC (Section 2.4) were monitored for each sampling run and were observed to be negative, excluding any possibility of contamination during the RNA isolation and RT-qPCR detection. There was a low inter-sample variation in Cq values for the luciferase control of RNA, confirming the optimal performance of the RNA isolation procedure and excluding any inhibition during the amplification. Positive controls for RoV and NoV added to the RT-qPCR behaved always as expected. All the used controls demonstrate that CIM C4 hydrophobic interaction columns paired with RT-qPCR are an efficient and consistent tool to monitor enteric viruses from coastal environments.
3.3. Concentration of pathogenic enteric viruses using CIM C4 columns from coastal environmental water samples, prior to RT-qPCR detection
Results before the concentration step were always negative, however, after concentration, 42% (15/36) of the samples were positive for RoV and/or NoV: 22% (8/36) of samples were positive for RoV and 30% (11/36) for NoV (Due to low concentrations, the results are not quantitative and are expressed as positive (+) and negative (−)) (Table 2). The highest detection rate was observed in SG1 and decreased as the distance to the WWTP discharge increased and towards the middle of the Bay of Koper (83% in SG1, 50% in SG2 and 50% in SG3). The effect was especially notable for NoV (67%, 50% and 17% of positive samples in SG1, SG2 and SG3, respectively). The observed decrease might be due to a higher dispersion and dilution effects. In addition, other study reported that enteric viruses have lower stability in sea water than in freshwater (Wyn-Jones et al., 2011).
Table 2.
Detection of RoV and NoV at each sampling location during the study period (July 2015 to June 2016). Rižana river (SG1), Rižana estuary (SG2), Bay of Koper (SG3), swimming area (SA1) and mussel farming areas (MF1 and MF2).
| Sampling location | Target | Sampling date |
Detection rate per sampling location | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 7th July 2015 | 5th October 2015 | 25th November 2015 | 5th February 2016 | 12th April 2016 | 15th June 2016 | ||||
| Rižana river SG1 |
RoV (VP2) | − | + | + | + | − | − | 50% | 83% |
| NoV (GII) | − | + | − | + | + | + | 67% | ||
| Rižana estuary SG2 |
RoV (VP2) | − | − | − | + | + | − | 33% | 50% |
| NoV (GII) | − | − | − | + | + | + | 50% | ||
| Bay of Koper SG3 |
RoV (VP2) | − | − | + | − | − | + | 33% | 50% |
| NoV (GII) | − | − | − | + | − | − | 17% | ||
| Swimming area SA1 |
RoV (VP2) | − | − | − | − | − | + | 17% | 17% |
| NoV (GII) | − | − | − | − | − | − | 0% | ||
| Mussel farm 2 MF2 |
RoV (VP2) | − | − | − | − | − | − | 0% | 17% |
| NoV (GII) | − | − | − | + | − | − | 17% | ||
| Mussel farm 1 MF1 |
RoV (VP2) | − | − | − | − | − | − | 0% | 33% |
| NoV (GII) | − | − | + | + | − | − | 33% | ||
| Detection rate per sampling date | RoV (VP2) | 0% | 17% | 33% | 33% | 17% | 33% | ||
| NoV (GII) | 0% | 17% | 17% | 83% | 33% | 33% | |||
| 0% | 17% | 50% | 83% | 33% | 67% | ||||
At the mussel farm areas and the swimming area, the detection rate was expressively lower. Only NoV was detected at the mussel farming areas. NoV is the most important cause of viral gastroenteritis worldwide, and has been widely identified as the main pathogen associated with mussel-borne gastroenteritis in developed countries (Costantini et al., 2006; La Rosa et al., 2007; Loisy et al., 2005; Nenonen et al., 2008; Nishida et al., 2003; Smith et al., 1998). Several studies report that NoV are commonly detected in water samples from mussel farm areas (Boxman et al., 2006; Jothikumar et al., 2005; Loisy et al., 2005; Matthews et al., 2012). Another study, using PCR, performed in the north Adriatic Sea on the incidence of different strains of hepatitis A and NoV in shellfish samples, revealed that viral contamination was present on 22% of samples, precisely 6% for hepatitis A, 14% for norovirus and 2% for both (Croci et al., 2007). A study carried at the Slovenian coast also reported contamination of mussel samples with NoV (NoV was detected in 17.2% of mussel samples) (Henigman et al., 2015). The main reason for the wide presence of NoV in environmental waters is their high resistance to inactivation technologies and their high stability in the environment (Jothikumar et al., 2005; La Rosa et al., 2007). According to our results. at SA1, NoV was not detected and RoV was only detected in June 2016.
As seen in Fig. 3, even with low concentrations of FC (under 100 FC/100 ml), these locations still present occasionally low, yet possibly infective, concentrations of human virus, which suggests that the use of FC indicators alone is not enough to ensure a safe use of recreational waters (Petterson et al., 2001; Cook, 2013; Eslamian, 2016; Payment and Locas, 2011).
The highest occurrence of RoV and NoV was found in February, with 83% positive samples (33% for RoV and 83% for NoV), while the lowest occurrence was found in July 2015 (0%). This could suggest a negative effect of high temperatures on the occurrence of enteric viruses (Fig. 4A and Table 2), which was also recently observed for NoV in a non-coastal WWTP effluent (Steyer et al., 2015). NoV infections typically occur during winter months and are very sporadic throughout the year (Guyader et al., 2009; Rohayem, 2009), due to low temperatures, lack of UV light and frequent rainfall (Lowther et al., 2012). Similarly, disease due to RoV infection is more common during winter months in countries with temperate climate (Armah et al., 1994; Bosch et al., 2006, Bosch et al., 2008; Kudo et al., 1991).
At the sampling time of February 2016, the lowest sea water level of all year was measured (164,4 cm) and the flow rate of Rižana river was higher than at the other samplings (4.74 m3/s), which can explain the higher observed detection rate of enteric viruses and the highest concentrations of FC. On the other hand, the number of overnight stays was much lower in winter than in summer (see Supplementary material), which suggests that the highest rates of enteric viruses are not related with higher population, but could be related with a higher number of infections in winter, when the immunity of general population is lower. This is supported by the fact that the highest level of hospitalized patients in Izola General Hospital, diagnosed with NoV infection, was recorded during winter and for RoV infections during spring, while the lowest infections occurred during the warmest period (Fig. 6).
Fig. 6.
Percentage of hospitalized patients with infections of RoV and NoV for the period between July 2015 till June 2016. Data provided by Izola General Hospital.
Overall, both RoV and NoV were detected, which shows that they are released to the Bay of Koper. The presented results show that both RoV and NoV are more frequently detected close to WWTP discharges (with a detection rate of 83%) but they can, as well, be detected in areas used for recreation (17% of positive samples) and mussel's growth (17% and 33% of positive samples).
Other studies in the same location reported that pollution-related parameters were, in some cases, higher than the national regulations for bathing water (Mozetič et al., 2008). In another study, a constant increase of nitrogen and silicon from human origin was observed (Cozzi et al., 2012). These studies are in accordance with the presented results, showing that measures to reduce pollution should continue and be intensified in the area, to prevent potential threat to human health, not only by recreational activities during the bathing season, but also by seafood consumption throughout the year.
Additionally, climate change is expected to increase heavy rainfall events and floods, which can raise the risk of waterborne outbreaks (Delworth et al., 2016; Dye et al., 2017; Hunter, 2003). In the last decades the development of molecular techniques, such as qPCR, improved the reports of enteric viruses' occurrence in surface waters and in waters used for recreational activities (Symonds and Breitbart, 2015). Consequently, public and regulatory awareness also grew on enteric viruses as emerging waterborne pathogens (Nwachcuku and Gerba, 2004). Still, not many studies were made in coastal waters. Monitoring microbial water quality and sanitary risk assessment are priority tasks around the world (Gotkowska-Płachta et al., 2016; Karavoltsos et al., 2016).
3.4. Correlations between enteric viruses, faecal coliforms and environmental parameters
Associations between the presence of RoV and NoV with concentrations of FC were assessed by Sommers' D Rank association coefficient. Due to the low number of positive samples at the mussel farming areas and swimming area, Sommers' D rank association coefficient was only calculated for the salinity/pollution gradient. The presence of both RoV and NoV showed positive significant associations with concentrations of FC (S = 0.753 and 0.738 for RoV and NoV, respectively) (Table 3). Previous studies also demonstrated associations between viral pathogens and indicator bacteria in environments close to WWTP discharges (Bagordo et al., 2013; Ferguson et al., 2012; Liang et al., 2015, 2015). In this study, as well as in previous studies, the observed associations are not strong. Additionally, the presence of RoV was positively associated with the flow rate of river Rižana, which suggest that Rižana plays a key role in the spread of RoV in the area.
Table 3.
Somers' D Rank association coefficient between water temperature, salinity, flow rate of river Rižana, sea level, concentrations of FC with the presence/absence of enteric viruses (RoV and NoV). Analysis were performed for the locations along the salinity/pollution gradient: Rižana river (SG1), Rižana estuary (SG2) and Bay of Koper (SG3). Only significant correlations are shown (* < 0.05).
| Salinity/pollution gradient (00RI, ERI2 and 000 K) | ||
|---|---|---|
| Presence of RoV | Presence of NoV | |
| Temperature | – | – |
| Salinity | – | – |
| Flow rate | 0.791* | – |
| Sea level | – | – |
| FC | 0.753* | 0.738* |
Spearman's rho2 rank correlation coefficient was calculated to evaluate significant correlations between measured physicochemical parameters and concentrations of FC and the results are summarized in Table 4. At the salinity/pollution gradient, concentrations of FC showed moderate positive correlation with salinity (r = 0.320) and with seawater level (r = 0.454), suggesting that inputs of fresh water strongly affect the spreading of pollution in the area. At the swimming area (SA1) and mussel farm areas (MF1 and MF2) correlations between concentrations of FC and the measured physicochemical parameters were not found, which is due to the location of the sampling points (far from freshwater inputs).
Table 4.
Spearman's rank correlation coefficient between water temperature, salinity, flow rate of river Rižana, sea level with concentrations of FC. Only significant correlations are shown (* < 0.05 and ** < 0.01).
| Temperature | Salinity | Rižana flow rate | Sea level | |
|---|---|---|---|---|
| FC (SG1, SG2 and SG3) |
– | 0.323* | – | 0.454** |
| FC (SA1, MF1 and MF2) |
– | – | – | – |
4. Conclusion
CIM C4 hydrophobic interaction columns paired with molecular detection by RT-qPCR are an efficient tool to monitor enteric viruses in coastal waters with different salinity concentrations and their use is reliable and consistent to perform long surveys. Further studies are required to assess the use of CIM C4 columns for a broader range of enteric viruses, as already done for freshwater by Steyer et al. (Steyer et al., 2015).
The obtained data showed that pollution originated from human waste is widespread in the Bay of Koper. This was confirmed by the frequent detection rate of RoV and NoV, and by the measured concentrations of FC. RoV and NoV are released into the Bay of Koper, with higher rates close to the WWTP discharge of Koper, however, they can as well be detected at recreational waters and mussel harvesting areas. Therefore, water bodies that are considered safe based only on bacterial concentrations, may still have low, yet infective, concentrations of human viruses. A universal indicator of faecal pollution doesn't not exist, but the additional measurement of RoV and NoV is an important complementary choice to accurately access water quality. It is fundamental to improve water treatments to assure a safe use of water bodies in the Gulf of Trieste.
This study was the first to use CIM C4 hydrophobic interaction columns to concentrate RoV and NoV from saline coastal waters. It represents a successful pilot test that opens the door for future surveys with longer time series and more frequent sampling to better understand the relationships between human pathogenic viruses and faecal indicator bacteria. The gathered data will be useful to optimize monitoring programs that avoid potential exposure to illness risks.
Author's contributions
Study concept: Valentina Turk, Maja Ravnikar, Ion Gutiérrez-Aguirre and José Gonçalves.
Acquisition of data: José Gonçalves.
Analysis and data interpretation: José Gonçalves, Ion Gutiérrez-Aguirre, Mukundh N. Balasubramanian, Maja Zagorščak, Maja Ravnikar, Valentina Turk.
Drafting of manuscript: José Gonçalves.
Critical revision: José Gonçalves, Ion Gutiérrez-Aguirre, Mukundh N. Balasubramanian, Maja Zagorščak, Maja Ravnikar, Valentina Turk.
All the authors have read and approve the submission of the manuscript.
Competing interests
We do not have competing interests.
Funding
This research, and the fellowships awarded to José Gonçalves and Mukundh N. Balasubramanian, have been funded by the European Union's Seventh Framework Program for research, technological development and demonstration under grant agreement no. 607150 (FP7-PEOPLE-2013-ITN – INTERFACES - Ecohydrological interfaces as critical hotspots for transformations of ecosystem exchange fluxes and biogeochemical cycling); and by the research programs of Marine Biology Station Piran (ARRS Program P10237) and Department of Biotechnology and Systems Biology (ARRS Program P40165) of the National Institute of Biology, Slovenia.
Acknowledgments
Andrej Steyer (Institute of Microbiology and Immunology, Faculty of Medicine, University of Ljubljana) for virus material;
Martin Vodopivec (Slovenian Environmental Agency ARSO) to provide data on tides and seawater level;
Izabella Levpold (Tourist Information Center Koper) to provide data on overnight stays at the Slovenian coast;
Izola General Hospital to provide data on the number of infected patients with rotavirus and norovirus infections;
Janez Kosel (Faculty of Mechanical Engineering, University of Ljubljana) for critical revision and suggestions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.marpolbul.2018.01.040.
Appendix A. Supplementary data
Supplementary material
References
- Armah G.E., Mingle J.A.A., Dodoo A.K., Anyanful A., Antwi R., Commey J., Nkrumah F.K. Seasonality of rotavirus infection in Ghana. Ann. Trop. Paediatr. 1994;14:223–229. doi: 10.1080/02724936.1994.11747721. [DOI] [PubMed] [Google Scholar]
- Bagordo F., Grassi T., Idolo A., Serio F., Gabutti G., Donno A.D. Rotavirus occurrence in shellfish with low levels of E. Coli. Food Environ. Virol. 2013;5:169–175. doi: 10.1007/s12560-013-9119-z. [DOI] [PubMed] [Google Scholar]
- Balasubramanian M.N., Rački N., Gonçalves J., Kovač K., Žnidarič M.T., Turk V., Ravnikar M., Gutiérrez-Aguirre I. Enhanced detection of pathogenic enteric viruses in coastal marine environment by concentration using methacrylate monolithic chromatographic supports paired with quantitative PCR. Water Res. 2016;106:405–414. doi: 10.1016/j.watres.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Bartram J. ResearchGate. 2001. Water quality: guidelines, standards, and health: assessment of risk and risk management for water-related infectious disease. [Google Scholar]
- Bishop R. Discovery of rotavirus: implications for child health. J. Gastroenterol. Hepatol. 2009;24:S81–S85. doi: 10.1111/j.1440-1746.2009.06076.x. [DOI] [PubMed] [Google Scholar]
- Bosch A. Elsevier; 2007. Human Viruses in Water: Perspectives in Medical Virology. [Google Scholar]
- Bosch A., Abad F.X., Pintó R.M. Human pathogenic viruses in the marine environment. In: Belkin S., Colwell R.R., editors. Oceans and Health: Pathogens in the Marine Environment. Springer; US: 2005. pp. 109–131. [Google Scholar]
- Bosch A., Pintó R.M., Abad F.X. Survival and transport of enteric viruses in the environment. In: Goyal S.M., editor. Viruses in Foods, Food Microbiology and Food Safety. Springer; US: 2006. pp. 151–187. [Google Scholar]
- Bosch A., Guix S., Sano D., Pintó R.M. New tools for the study and direct surveillance of viral pathogens in water. Curr. Opin. Biotechnol. Energy Biotechnol./Environ. Biotechnol. 2008;19:295–301. doi: 10.1016/j.copbio.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxman I.L.A., Tilburg J.J.H.C., te Loeke N.A.J.M., Vennema H., Jonker K., de Boer E., Koopmans M. Detection of noroviruses in shellfish in the Netherlands. Int. J. Food Microbiol. 2006;108:391–396. doi: 10.1016/j.ijfoodmicro.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Bradshaw J.K., Snyder B.J., Oladeinde A., Spidle D., Berrang M.E., Meinersmann R.J., Oakley B., Sidle R.C., Sullivan K., Molina M. Characterizing relationships among fecal indicator bacteria, microbial source tracking markers, and associated waterborne pathogen occurrence in stream water and sediments in a mixed land use watershed. Water Res. 2016;101:498–509. doi: 10.1016/j.watres.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Brown S., Nicholls R.J., Woodroffe C.D., Hanson S., Hinkel J., Kebede A.S., Neumann B., Vafeidis A.T. Sea-level rise impacts and responses: a global perspective. In: Finkl C.W., editor. Coastal Hazards, Coastal Research Library. Springer; Netherlands: 2013. pp. 117–149. [Google Scholar]
- Calgua B., Mengewein A., Grunert A., Bofill-Mas S., Clemente-Casares P., Hundesa A., Wyn-Jones A.P., López-Pila J.M., Girones R. Development and application of a one-step low cost procedure to concentrate viruses from seawater samples. J. Virol. Methods. 2008;153:79–83. doi: 10.1016/j.jviromet.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Cook N. Elsevier; 2013. Viruses in Food and Water: Risks, Surveillance and Control. [Google Scholar]
- Costantini V., Loisy F., Joens L., Le Guyader F.S., Saif L.J. Human and animal enteric caliciviruses in oysters from different coastal regions of the United States. Appl. Environ. Microbiol. 2006;72:1800–1809. doi: 10.1128/AEM.72.3.1800-1809.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi S., Falconi C., Comici C., Čermelj B., Kovac N., Turk V., Giani M. Recent evolution of river discharges in the Gulf of Trieste and their potential response to climate changes and anthropogenic pressure. Estuar. Coast. Shelf Sci. 2012;115:14–24. https://doi.org/10.1016/j.ecss.2012.03.005 (Fluctuations and trends in the northern Adriatic marine systems: from annual to decadal variability) [Google Scholar]
- Croci L., Losio M.N., Suffredini E., Pavoni E., Di Pasquale S., Fallacara F., Arcangeli G. Assessment of human enteric viruses in shellfish from the northern Adriatic sea. Int. J. Food Microbiol. 2007;114:252–257. doi: 10.1016/j.ijfoodmicro.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Delworth T.L., Zeng F., Vecchi G.A., Yang X., Zhang L., Zhang R. The North Atlantic oscillation as a driver of rapid climate change in the northern hemisphere. Nat. Geosci. 2016;9:509–512. [Google Scholar]
- Dye S., Buckley P., Pinnegar J. 2017. Impacts of Climate Change on the Coastal and Marine Physical Environments of Caribbean Small Island Developing States (SIDS), in: Caribbean Marine Climate Change Report Card Scientific Reviews. Foreign & Commonwealth Office, GOV.UK. [Google Scholar]
- Eslamian S. CRC Press; 2016. Urban Water Reuse Handbook. [Google Scholar]
- Falcieri F.M., Kantha L., Benetazzo A., Bergamasco A., Bonaldo D., Barbariol F., Malačič V., Sclavo M., Carniel S. Turbulence observations in the Gulf of Trieste under moderate wind forcing and different water column stratification. Ocean Sci. 2016;12:433–449. [Google Scholar]
- Ferguson A.S., Layton A.C., Mailloux B.J., Culligan P.J., Williams D.E., Smartt A.E., Sayler G.S., Feighery J., McKay L.D., Knappett P.S.K., Alexandrova E., Arbit T., Emch M., Escamilla V., Ahmed K.M., Alam M.J., Streatfield P.K., Yunus M., van Geen A. Comparison of fecal indicators with pathogenic bacteria and rotavirus in groundwater. Sci. Total Environ. 2012;431:314–322. doi: 10.1016/j.scitotenv.2012.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field K.G., Samadpour M. Fecal source tracking, the indicator paradigm, and managing water quality. Water Res. 2007;41:3517–3538. doi: 10.1016/j.watres.2007.06.056. [DOI] [PubMed] [Google Scholar]
- Fiksdal L., Pommepuy M., Caprais M.P., Midttun I. Monitoring of fecal pollution in coastal waters by use of rapid enzymatic techniques. Appl. Environ. Microbiol. 1994;60:1581–1584. doi: 10.1128/aem.60.5.1581-1584.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong T.-T., Lipp E.K. Enteric viruses of humans and animals in aquatic environments: health risks, detection, and potential water quality assessment tools. Microbiol. Mol. Biol. Rev. 2005;69:357–371. doi: 10.1128/MMBR.69.2.357-371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry-Shields J., Wang A., Cory R.M., Stewart J.R. Determination of specific types and relative levels of QPCR inhibitors in environmental water samples using excitation–emission matrix spectroscopy and PARAFAC. Water Res. 2013;47:3467–3476. doi: 10.1016/j.watres.2013.03.049. [DOI] [PubMed] [Google Scholar]
- George I., Crop P., Servais P. Fecal coliform removal in wastewater treatment plants studied by plate counts and enzymatic methods. Water Res. 2002;36:2607–2617. doi: 10.1016/s0043-1354(01)00475-4. [DOI] [PubMed] [Google Scholar]
- Gerba C.P., Goyal S.M., LaBelle R.L., Cech I., Bodgan G.F. Failure of indicator bacteria to reflect the occurrence of enteroviruses in marine waters. Am. J. Public Health. 1979;69:1116–1119. doi: 10.2105/ajph.69.11.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerba C.P., Rose J.B., Haas C.N., Crabtree K.D. Waterborne rotavirus: a risk assessment. Water Res. 1996;30:2929–2940. [Google Scholar]
- Giorgi F. Climate change hot-spots. Geophys. Res. Lett. 2006;33:L08707. [Google Scholar]
- Girones R., Ferrús M.A., Alonso J.L., Rodriguez-Manzano J., Calgua B., Corrêa A. de A., Hundesa A., Carratala A., Bofill-Mas S. Molecular detection of pathogens in water–the pros and cons of molecular techniques. Water Res. 2010;44:4325–4339. doi: 10.1016/j.watres.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Goh S.G., Bayen S., Burger D., Kelly B.C., Han P., Babovic V., Gin K.Y.-H. Occurrence and distribution of bacteria indicators, chemical tracers and pathogenic vibrios in Singapore coastal waters. Mar. Pollut. Bull. 2017;114:627–634. doi: 10.1016/j.marpolbul.2016.09.036. [DOI] [PubMed] [Google Scholar]
- Gotkowska-Płachta A., Gołaś I., Korzeniewska E., Koc J., Rochwerger A., Solarski K. Evaluation of the distribution of fecal indicator bacteria in a river system depending on different types of land use in the southern watershed of the Baltic Sea. Environ. Sci. Pollut. Res. 2016;23:4073–4085. doi: 10.1007/s11356-015-4442-6. [DOI] [PubMed] [Google Scholar]
- Griffin D.W., Lipp E.K., Mclaughlin M.R., Rose J.B. Marine recreation and public health microbiology: quest for the ideal indicator this article addresses the historic, recent, and future directions in microbiological water quality indicator research. Bioscience. 2001;51:817–825. [Google Scholar]
- Griffin D.W., Donaldson K.A., Paul J.H., Rose J.B. Pathogenic human viruses in coastal waters. Clin. Microbiol. Rev. 2003;16:129–143. doi: 10.1128/CMR.16.1.129-143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Aguirre I., Steyer A., Boben J., Gruden K., Poljšak-Prijatelj M., Ravnikar M. Sensitive detection of multiple rotavirus genotypes with a single reverse transcription-real-time quantitative PCR assay. J. Clin. Microbiol. 2008;46:2547–2554. doi: 10.1128/JCM.02428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyader F.S.L., Parnaudeau S., Schaeffer J., Bosch A., Loisy F., Pommepuy M., Atmar R.L. Detection and quantification of noroviruses in shellfish. Appl. Environ. Microbiol. 2009;75:618–624. doi: 10.1128/AEM.01507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas C.N., Rose J.B., Gerba C., Regli S. Risk assessment of virus in drinking water. Risk Anal. Off. Publ. Soc. Risk Anal. 1993;13:545–552. doi: 10.1111/j.1539-6924.1993.tb00013.x. [DOI] [PubMed] [Google Scholar]
- Henigman U., Biasizzo M., Vadnjal S., Toplak I., Gombač M., Steyer A., Poljšak Prijatelj M., Ambrožič M., Fonda I., Kirbiš A., Barlič-Maganja D. Molecular characterisation of noroviruses detected in mussels (Mytilus galloprovincialis) from harvesting areas in Slovenia. New Microbiol. 2015;38:225–233. [PubMed] [Google Scholar]
- Hunter P.r. Climate change and waterborne and vector-borne disease. J. Appl. Microbiol. 2003;94:37–46. doi: 10.1046/j.1365-2672.94.s1.5.x. [DOI] [PubMed] [Google Scholar]
- Ikner L.A., Gerba C.P., Bright K.R. Concentration and recovery of viruses from water: a comprehensive review. Food Environ. Virol. 2012;4:41–67. doi: 10.1007/s12560-012-9080-2. [DOI] [PubMed] [Google Scholar]
- Jothikumar N., Lowther J.A., Henshilwood K., Lees D.N., Hill V.R., Vinjé J. Rapid and sensitive detection of noroviruses by using TaqMan-based one-step reverse transcription-PCR assays and application to naturally contaminated shellfish samples. Appl. Environ. Microbiol. 2005;71:1870–1875. doi: 10.1128/AEM.71.4.1870-1875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama T., Kojima S., Shinohara M., Uchida K., Fukushi S., Hoshino F.B., Takeda N., Katayama K. Broadly reactive and highly sensitive assay for Norwalk-like viruses based on real-time quantitative reverse transcription-PCR. J. Clin. Microbiol. 2003;41:1548–1557. doi: 10.1128/JCM.41.4.1548-1557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavoltsos S., Sakellari A., Kepenekos L., Tzafi A., Zafeiraki E., Dassenakis V., Scoullos M. 2016. Indicator Bacteria Concentrations in Coastal Areas of Central Evoikos Gulf (Greece): Preliminary Analysis Around a Sewage Outfall. [Google Scholar]
- Kudo H., Uyeda I., Shikata E. Viruses in the phytoreovirus genus of the Reoviridae family have the same conserved terminal sequences. J. Gen. Virol. 1991;72(Pt 12):2857–2866. doi: 10.1099/0022-1317-72-12-2857. [DOI] [PubMed] [Google Scholar]
- La Rosa G., Fontana S., Di Grazia A., Iaconelli M., Pourshaban M., Muscillo M. Molecular identification and genetic analysis of norovirus genogroups I and II in water environments: comparative analysis of different reverse transcription-PCR assays. Appl. Environ. Microbiol. 2007;73:4152–4161. doi: 10.1128/AEM.00222-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L., Goh S.G., Vergara G.G.R.V., Fang H.M., Rezaeinejad S., Chang S.Y., Bayen S., Lee W.A., Sobsey M.D., Rose J.B., Gin K.Y.H. Alternative fecal indicators and their empirical relationships with enteric viruses, Salmonella enterica, and Pseudomonas aeruginosa in surface waters of a tropical urban catchment. Appl. Environ. Microbiol. 2015;81:850–860. doi: 10.1128/AEM.02670-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Ganesh A. Water quality indicators: bacteria, coliphages, enteric viruses. Int. J. Environ. Health Res. 2013;23:484–506. doi: 10.1080/09603123.2013.769201. [DOI] [PubMed] [Google Scholar]
- Loisy F., Atmar R.L., Guillon P., Le Cann P., Pommepuy M., Le Guyader F.S. Real-time RT-PCR for norovirus screening in shellfish. J. Virol. Methods. 2005;123:1–7. doi: 10.1016/j.jviromet.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Lowther J.A., Gustar N.E., Powell A.L., Hartnell R.E., Lees D.N. Two-year systematic study to assess norovirus contamination in oysters from commercial harvesting areas in the United Kingdom. Appl. Environ. Microbiol. 2012;78:5812–5817. doi: 10.1128/AEM.01046-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malačič, Bogunovič . Il Nuovo Cimento. 2009. Circulation in the Gulf of Trieste: measurements and model results. [Google Scholar]
- Malačič V., Petelin B. Climatic circulation in the Gulf of Trieste (northern Adriatic) J. Geophys. Res. Oceans. 2009;114:C07002. [Google Scholar]
- Mandac R.S., Bogunović B., Žagar D., Faganeli J. Riverine Impact on the Thermohaline Properties, Turbidity and Suspended Solids in a Shallow Bay (Bay of Koper, Northern Adriatic Sea) Acta Adriat.: Int. J. Mar. Sci. 2014;55(2):195–212. (December 1) [Google Scholar]
- Marti R., Gannon V.P.J., Jokinen C., Lanthier M., Lapen D.R., Neumann N.F., Ruecker N.J., Scott A., Wilkes G., Zhang Y., Topp E. Quantitative multi-year elucidation of fecal sources of waterborne pathogen contamination in the South Nation River basin using Bacteroidales microbial source tracking markers. Water Res. 2013;47:2315–2324. doi: 10.1016/j.watres.2013.02.009. [DOI] [PubMed] [Google Scholar]
- Matthews J.E., Dickey B.W., Miller R.D., Felzer J.R., Dawson B.P., Lee A.S., Rocks J.J., Kiel J., Montes J.S., Moe C.L., Eisenberg J.N.S., Leon J.S. The epidemiology of published norovirus outbreaks: a review of risk factors associated with attack rate and genogroup. Epidemiol. Infect. 2012;140:1161–1172. doi: 10.1017/S0950268812000234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozetič P., Malačič V., Turk V. A case study of sewage discharge in the shallow coastal area of the Northern Adriatic Sea (Gulf of Trieste) Mar. Ecol. 2008;29:483–494. [Google Scholar]
- Nenonen N.P., Hannoun C., Horal P., Hernroth B., Bergström T. Tracing of norovirus outbreak strains in mussels collected near sewage effluents. Appl. Environ. Microbiol. 2008;74:2544–2549. doi: 10.1128/AEM.02477-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida T., Kimura H., Saitoh M., Shinohara M., Kato M., Fukuda S., Munemura T., Mikami T., Kawamoto A., Akiyama M., Kato Y., Nishi K., Kozawa K., Nishio O. Detection, quantitation, and phylogenetic analysis of noroviruses in Japanese oysters. Appl. Environ. Microbiol. 2003;69:5782–5786. doi: 10.1128/AEM.69.10.5782-5786.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nwachcuku N., Gerba C.P. Emerging waterborne pathogens: can we kill them all? Curr. Opin. Biotechnol. 2004;15:175–180. doi: 10.1016/j.copbio.2004.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira M., Serrano I., Harten S.V., Bessa L.J., Bernardo F., da Costa P.M. Fecal contamination of wastewater treatment plants in Portugal. Environ. Sci. Pollut. Res. 2016;23:14671–14675. doi: 10.1007/s11356-016-6962-0. [DOI] [PubMed] [Google Scholar]
- Oliveira S.S., Sorgine M.H.F., Bianco K., Pinto L.H., Barreto C., Albano R.M., Cardoso A.M., Clementino M.M. Detection of human fecal contamination by nifH gene quantification of marine waters in the coastal beaches of Rio de Janeiro, Brazil. Environ. Sci. Pollut. Res. 2016;23:25210–25217. doi: 10.1007/s11356-016-7737-3. [DOI] [PubMed] [Google Scholar]
- Olivotti R., Faganeli J., Malej A. Impact of ‘organic pollutants on coastal waters, gulf of Trieste. Water Sci. Technol. 1986;18:57–68. [Google Scholar]
- Payment P., Locas A. Pathogens in water: value and limits of correlation with microbial indicators. Ground Water. 2011;49:4–11. doi: 10.1111/j.1745-6584.2010.00710.x. [DOI] [PubMed] [Google Scholar]
- Petterson S.R., Ashbolt N.J., Sharma A. Microbial risks from wastewater irrigation of salad crops: a screening-level risk assessment. Water Environ. Res. 2001;73:667–672. doi: 10.2175/106143001x143402. [DOI] [PubMed] [Google Scholar]
- Rezaeinejad S., Vergara G.G.R.V., Woo C.H., Lim T.T., Sobsey M.D., Gin K.Y.H. Surveillance of enteric viruses and coliphages in a tropical urban catchment. Water Res. 2014;58:122–131. doi: 10.1016/j.watres.2014.03.051. [DOI] [PubMed] [Google Scholar]
- Robilotti E., Deresinski S., Pinsky B.A. Norovirus. Clin. Microbiol. Rev. 2015;28:134–164. doi: 10.1128/CMR.00075-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohayem J. Norovirus seasonality and the potential impact of climate change. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2009;15:524–527. doi: 10.1111/j.1469-0691.2009.02846.x. [DOI] [PubMed] [Google Scholar]
- de Sherbinin A. Climate change hotspots mapping: what have we learned? Clim. Chang. 2014;123:23–37. [Google Scholar]
- Small C., Nicholls R.J. A global analysis of human settlement in coastal zones. J. Coast. Res. 2003;19:584–599. [Google Scholar]
- Smith A.W., Skilling D.E., Cherry N., Mead J.H., Matson D.O. Calicivirus emergence from ocean reservoirs: zoonotic and interspecies movements. Emerg. Infect. Dis. 1998;4:13–20. doi: 10.3201/eid0401.980103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyer A., Gutiérrez-Aguirre I., Rački N., Glaser S.B., Humar B.B., Stražar M., Škrjanc I., Poljšak-Prijatelj M., Ravnikar M., Rupnik M. The detection rate of enteric viruses and Clostridium difficile in a waste water treatment plant effluent. Food Environ. Virol. 2015;7:164–172. doi: 10.1007/s12560-015-9183-7. [DOI] [PubMed] [Google Scholar]
- Sun H., He X., Ye L., Zhang X.-X., Wu B., Ren H. Diversity, abundance, and possible sources of fecal bacteria in the Yangtze River. Appl. Microbiol. Biotechnol. 2016:1–10. doi: 10.1007/s00253-016-7998-2. [DOI] [PubMed] [Google Scholar]
- Suttle C.A. Viruses in the sea. Nature. 2005;437:356–361. doi: 10.1038/nature04160. [DOI] [PubMed] [Google Scholar]
- Sweileh W.M., Zyoud S.H., Al-Jabi S.W., Sawalha A.F., Shraim N.Y. Drinking and recreational water-related diseases: a bibliometric analysis (1980–2015) Ann. Occup. Environ. Med. 2016;28 doi: 10.1186/s40557-016-0128-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds E.M., Breitbart M. Affordable enteric virus detection techniques are needed to support changing paradigms in water quality management. Clean (Weinh) 2015;43:8–12. doi: 10.1002/clen.201400235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak N., Okršlar V., Stanič-Racman D., Gruden K., Žel J. A high-throughput method for quantifying transgene expression in transformed plants with real-time PCR analysis. Plant Mol. Biol. Report. 2004;22:237–250. [Google Scholar]
- Turk V., Mozetič P., Malej A. Overview of eutrophication-related events and other irregular episodes in Slovenian Sea (Gulf of Trieste, Adriatic sea) Annales. 2007;2:197–216. [Google Scholar]
- Updyke E.A., Wang Z., Sun S., Connell C., Kirs M., Wong M., Lu Y. Human enteric viruses–potential indicators for enhanced monitoring of recreational water quality. Virol. Sin. 2015;30:344–353. doi: 10.1007/s12250-015-3644-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO World Health Stat. 2015:2015. [Google Scholar]
- Wyn-Jones A.P., Carducci A., Cook N., D'Agostino M., Divizia M., Fleischer J., Gantzer C., Gawler A., Girones R., Höller C., de Roda Husman A.M., Kay D., Kozyra I., López-Pila J., Muscillo M., José Nascimento M.S., Papageorgiou G., Rutjes S., Sellwood J., Szewzyk R., Wyer M. Surveillance of adenoviruses and noroviruses in European recreational waters. Water Res. 2011;45:1025–1038. doi: 10.1016/j.watres.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material