Abstract
Patient: Female, 51
Final Diagnosis: Breast cancer with gastric metastasis
Symptoms: Epigastric pain
Medication: —
Clinical Procedure: Radical gastrectomy • both MRM
Specialty: Oncology
Objective:
Rare co-existance of disease or pathology
Background:
Metastasis to the stomach can be found as the first presentation of breast cancer, although it is very rare. The authors report an unusual case of metastasis to the stomach as the first presentation of breast cancer, which had a good prognosis.
Case Report:
A 51-year-old female underwent radical subtotal gastrectomy and chemotherapy because of gastric cancer with distant metastasis. At the time of diagnosis of gastric cancer, she had a negative result from routine mammography. One year later, a newly detected lesion on routine mammography was confirmed as breast cancer. Initial diagnosis of gastric cancer was changed to metastatic carcinoma from breast cancer through immunohistochemistry after bilateral mastectomy. After the completion of chemotherapy, she is currently receiving treatment with letrozole, without recurrence for 66 months.
Conclusions:
Considering metastasis from breast cancer might be needed when unusual presentation of gastric cancer is observed even though gastric cancer is still one of the most common malignancies in Korea. Immunohistochemical analysis is helpful for diagnosis. Surgery for metastatic carcinoma of the stomach could be another option for treatment.
MeSH Keywords: Breast Neoplasms, Gastrectomy, Immunohistochemistry, Neoplasm Metastasis, Prognosis
Background
Metastatic spread to the gastrointestinal tract, peritoneum, and retroperitoneum is infrequently reported with primary breast cancers [1]. Although there is considerable heterogeneity among individual tumors, clinical outcomes of breast cancer patients with gastric metastases is poor compared to that of stage IV breast cancer regardless of the site of disease. The median survival after diagnosis of gastric metastasis from primary breast cancer ranges from 10 to 28 months, compared with 36 months for stage IV breast cancer, according to an American Society of Clinical Oncology report of 2008 [2,3].
Gastric metastasis as the first presentation of primary breast cancer is rare [4]. We present an unusual case of gastric metastasis with simultaneous involvement of the ovaries and peritoneum as the first presentation one year before diagnosis of primary breast cancer, which had a good prognosis.
Case Report
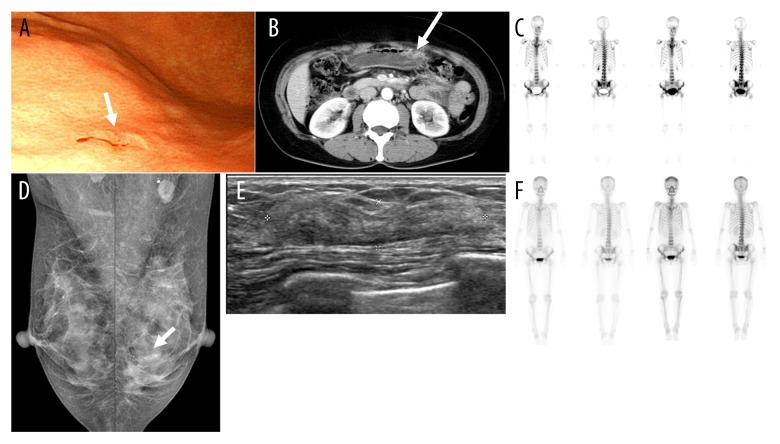
A 51-year-old female was referred to our hospital because of gastric cancer confirmed by biopsy in endoscopic screening (Figure 1A). The patient, with no significant comorbidity, reported a 1-month history of epigastric pain. She rarely drank alcohol, did not smoke, and had no allergies. She had no family history of cancer. She had a negative result from screening mammography at the time of diagnosis of gastric cancer. On examination, her abdomen was soft and non-distended, without palpable masses or tenderness. Also, no other masses were palpable in her breasts or axillae. Abdominal and pelvic computed tomography (APCT) revealed a suspicious gastric lesion as well as a large ovarian cyst and suspicious bone lesions, which were also noted on whole body bone scintigraphy (WBBS) (Figure 1B, 1C). However, there was no suspicious hypermetabolic lesion on whole body positron-emission tomography imaging.
Figure 1.
Clinical diagnostic evaluation of a patient with gastric and breast tumors, and response to chemotherapy. (A) Endoscopy for upper gastrointestinal cancer screening demonstrated an elevated mucosal lesion with central depression in the greater curvature of lower body (arrow). (B) CT of the abdomen and pelvis showed suspicious focal wall thickening in the greater curvature of the lower corpus (arrow). (C) WBBS revealed possible disseminated bone metastasis in the whole spine, pelvic bone, proximal extremities, and both ribs. This unusual clinical manifestation in primary gastric cancer made the patient undergo bone marrow biopsy. About 12 months after gastric surgery, annual screening mammography (D) Mammography showed focal asymmetry with mild parenchymal distortion in the lower outer quadrant of the left breast (arrow), which was not found 1 year previously. (E) Breast ultrasonography revealed focal heterogeneous bulging parenchyma with a probable benign nodule from the 3 to 4 o’clock position of the left breast, which was 3 cm in extent and was classified as category 4A according to the BI-RADS. Core needle biopsy of this lesion showed invasive lobular carcinoma. (F) After bilateral modified radical mastectomy, 8 cycles of chemotherapy and radiotherapy, WBBS showed near complete remission of multiple bone metastases at the time of gastric cancer diagnosis. CT – computed tomography; WBBS – whole body bone scan; BI-RADS – breast imaging-reporting and data system.
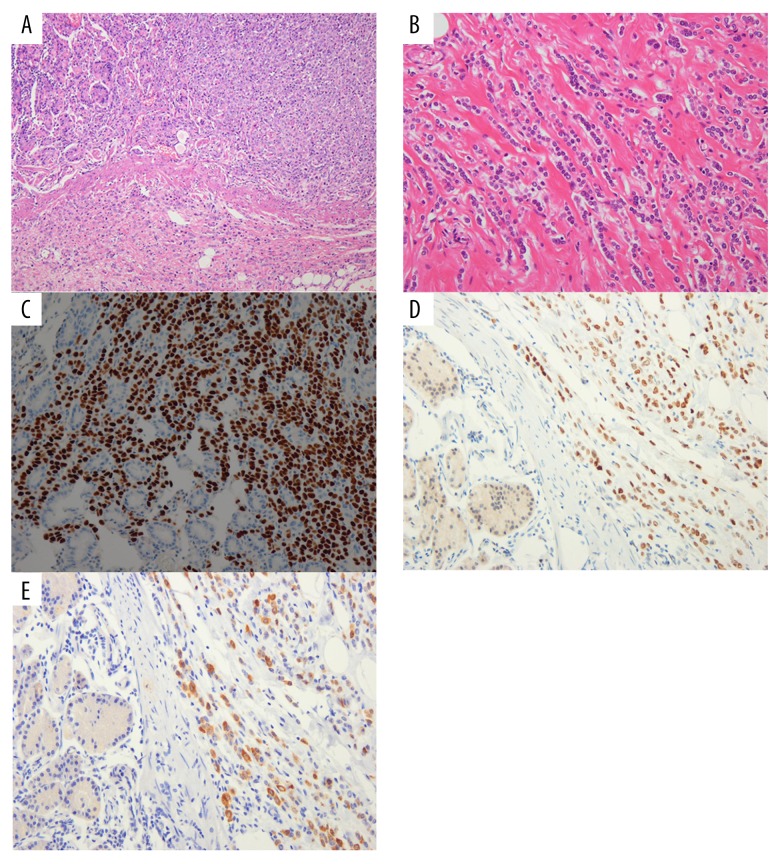
She underwent radical subtotal gastrectomy with Billroth II anastomosis and D2 lymph node dissection on December 10, 2009. Right salpingo-oophorectomy was simultaneously performed. Postoperative histology demonstrated a poorly differentiated gastric adenocarcinoma, penetrating the serosa, with multiple lymph nodes involved (pT4N3b) (Figure 2A). Diagnosis of metastatic adenocarcinoma was confirmed in specimens of omentum and right ovary, and bone marrow biopsy samples (pM1).
Figure 2.
Immunohistochemical staining in gastric and breast tumors of the patient. (A) Histology after gastrectomy demonstrated poorly differentiated gastric adenocarcinoma. (H & E stain, 200×). (B) Histology after mastectomy showed grade I invasive lobular carcinoma. (H & E stain, 200×) (C–E) Additional immunehistochemical staining of the gastric specimen obtained after gastrectomy exhibited ER, PR, and MGB positivity, consistent with metastatic breast carcinoma. Consequently, the diagnosis of stomach cancer was revised to metastatic breast cancer from primary gastric cancer. (H & E stain, 200×). H & E – hematoxylin and eosin; ER – estrogen receptor; PR – progesterone receptor; MGB – mammaglobin.
She was treated with 6 cycles of chemotherapy consisting of tegafur/gimeracil/oteracil potassium and cisplatin every 5 weeks for 5 months. Tegafur/gimeracil/oteracil potassium was orally administered at a dose of 80 mg/m2/day for 21 consecutive days, followed by a 14-day rest. Cisplatin was infused at a dose of 60 mg/m2 over a 2-hour period on the 8th day of chemotherapy. She experienced grade 2 sensory peripheral neuropathy by the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI CTCAE) grading system. She had constant numbness in her feet for 3 months after completing her chemotherapy. APCT after treatment completion revealed no evidence of tumor recurrence and no significant change in multiple bone metastases.
In November 2010, screening mammography revealed a new focal asymmetry in the lower outer quadrant of the left breast, which had not been noted on the last screening mammography at the time of gastric cancer diagnosis (Figure 1D). On examination, there was no palpable lesion in her breasts or axillae. A low-suspicious lesion on ultrasonography, which was correlated with mammographic abnormality, was diagnosed as invasive lobular carcinoma by core needle biopsy (Figure 1E).
After unilateral breast-conserving surgery was attempted, the patient underwent conversion to bilateral modified radical mastectomy because of left nipple involvement and sentinel lymph node metastasis on December 10, 2010. Also, a suspicious, small, ill-defined hypoechoic nodule in the periareolar region of the right breast, detected on second-look ultrasonography, was excised with preoperative localization. Invasive lobular carcinomas were found close to the right nipple and in palpable axillary lymph nodes by intraoperative frozen section analysis.
Postoperative histology showed grade I invasive lobular carcinoma with immunostaining positive for estrogen receptor (ER) and progesterone receptor (PR) and negative for human epidermal growth factor receptor 2 (HER2) (Figure 2B). Tumor presenting with diffuse and multifocal involvement of both breasts measured >7 cm [pT3(m)]. All 17 sampled lymph nodes in the left axilla and 23 out of 25 sampled lymph nodes in the right axilla contained metastasis (pN3). Additional immunohistochemistry on a previous gastric specimen was performed to distinguish primary adenocarcinoma from metastatic breast carcinoma. It was positive for ER, PR, and mammaglobin (MGB), consistent with metastatic breast carcinoma (Figure 2C–2E). Consequently, the diagnosis of stomach cancer was revised to metastatic breast cancer.
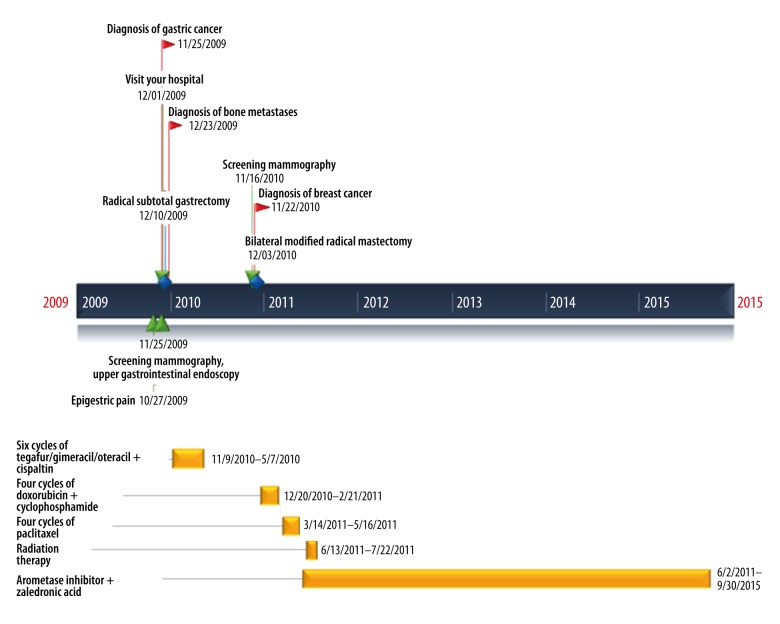
She received 4 cycles of chemotherapy consisting of doxorubicin 60 mg/m2 combined with cyclophosphamide 600 mg/m2 every 3 weeks followed by 4 cycles of paclitaxel 175 mg/m2 every 3 weeks. With every cycle of doxorubicin and cyclophosphamide chemotherapy grade 3/4 neutropenia occurred. Hospitalization was not required. She was also treated with radiotherapy to the chest wall and supraclavicular fossa, and boost radiation to the tumor bed was given. Adjuvant dose of 60 Gy was delivered in 30 fractions for each breast. Six months after breast surgery, follow-up studies showed no evidence of tumor recurrence. Furthermore, WBBS and APCT demonstrated nearly complete remission of multiple bone metastases (Figure 1F). She is currently receiving treatment with letrozole and zoledronic acid, without recurrence of cancer for 66 months (Figure 3)
Figure 3.
Timeline. This figure displays a list of medical events in chronological order.
Discussion
We present an unusual case of metastasis to the stomach as the first presentation of breast cancer, which had a good prognosis. The case was initially considered a primary gastric cancer case because a gastric lesion was detected one year before diagnosis of primary breast cancer. There was no doubt about the diagnosis because the gastric lesion was detected earlier without any evidence of primary breast cancer at that time despite breast cancer screening. An important point to note is that there is a high incidence of gastric cancer in Korea. According to a statistical review of the National Cancer Information Center in 2014, primary gastric cancer was the 4th most common malignancy in Korean women.
Bone metastasis in gastric cancer has been reported to be very rare [5]. We considered the possibility of other primary solid tumors such as breast cancer or lung cancer in which bone metastasis generally occurs, or hematologic malignancies such as multiple myeloma [6]. However, evaluations including mammography, chest CT, and bone marrow biopsy provided no evidence of other causes of disseminated bone lesions.
Metastatic patterns of lobular and ductal carcinoma of the breast are different. In a large retrospective study of 2605 cases of invasive breast cancer, gastrointestinal system (4.5% vs. 0.2%), gynecologic organs (4.5% vs. 0.8%), peritoneum-retroperitoneum (3.1% vs. 0.6%), and bone marrow (21.2% vs. 14.4%) metastases are markedly more prevalent in lobular carcinoma [7]. Taal et al. [8] showed that 20 out of 27 patients (83%) who developed gastric metastasis from primary breast carcinoma had lobular carcinoma. In our patient case, there was involvement not only of the stomach but also of the ovary and peritoneum synchronously from invasive lobular carcinoma of the breast, although the patient was initially diagnosed with primary gastric cancer and metastasis from the stomach.
The clinical manifestation of breast carcinoma metastasis to the stomach is unhelpful to distinguish it from primary gastric cancer. Although the incidence of breast cancer metastasis to the stomach is reported to be from 0.1% to 6%, the estimated rate is reported to be from 5% to 18% in autopsy series [2,8]. This underestimation may result from mild symptoms or lesser clinical signs [8]. Our patient also presented initially with mild epigastric pain despite the presence of advanced cancer. Metastatic gastric cancer from invasive lobular carcinoma of breast may manifest as several types: discrete and localized lesions, external compression at the cardia or pylorus, and diffuse infiltration which is the most common pattern [2].
Detailed immunohistochemical analysis will allow the most accurate diagnosis to differentiate between primary gastric cancer and gastric metastasis from breast cancer. Several studies reported the ER-positive rate to be in the range of 26.6% to 31.0% and the PR-positive rate to be in the range of 11.9% to 20.6% for female patients with gastric cancer [9]. ER-positive rates have been reported to be slightly higher in cases of poorly differentiated gastric cancer. Furthermore, metastatic carcinoma from the breast may show negative ER and PR status although the primary breast cancer expresses ER and PR [9,10].
In our case, MGB was used as an important marker. MGB is a 93-amino acid glycoprotein with homology with the rat prostatic steroid-binding protein subunit C3, human Clara cell 10-kd protein, and rabbit uteroglobin [11]. Sensitivity for the detection of breast cancer with MGB is 55.4% to 84.3%, which is higher than with GCDFP-15; and specificity with MGB is 90%, which is lower than with GCDFP-15 [11]. It is well-known that GCDFP-15 is a useful marker for metastatic breast cancer [12], but it was not expressed in the metastatic tumor of the stomach in this case.
Particularly interesting aspects of our case were the time sequence of primary breast cancer and gastrointestinal involvement, and the good prognosis. Previous studies have reported that the time interval ranges from synchronous presentation to be as long as 30 years, but the reverse is also true, with gastrointestinal manifestation preceding a diagnosis of breast cancer [1]. Only a few studies have reported breast cancers that were detected by physical examinations or routine mammography at the time of initial presentation of gastrointestinal metastasis or after gastrointestinal surgery with poor prognosis [1,4]. In our case, although the patient had been treated for primary gastric cancer with distant metastasis for over a period of 12 months, she actually had a stage IV breast cancer when the gastric lesion was found. At that time, routine mammography and clinical examination did not identify an abnormality. Despite incomplete removal of the malignant lesion (R2 resection) because of diffuse peritoneal nodularity, the patient has survived for 7 years after diagnosis of metastasis to the stomach. A recent study from the Mayo Clinic reported that in breast cancer patients with metastasis to the gastrointestinal tract and the peritoneum, advanced age at diagnosis and gastric metastatic disease had a negative effect on survival, whereas treatment with systemic chemotherapy or hormone therapy had a positive effect on survival [3]. The overall survival after diagnosis of gastrointestinal or peritoneal metastatic disease was reported to be 28 months [3]; which was not inferior to that of all women with metastatic disease secondary to breast cancer [13]. Surgical intervention did not significantly extend overall survival, although surgical intervention might be considered in a select group of patients with only gastrointestinal metastasis; gastric metastasis from primary breast cancer has been diversely treated with surgery, chemotherapy, or endocrine therapy [3].
Conclusions
If primary gastric cancer with unusual clinical manifestation such as multiple bone metastasis is diagnosed, the possibility of metastasis from breast cancer should be considered even though gastric cancer is still one of the most common malignancies in Korea and gastric metastasis is rare. Further evaluation of the breasts, such as ultrasonography as well as mammography, might be needed. It is important to determine appropriate treatment, including chemotherapy and selective surgery. Immunohistochemical analysis is useful for differentiating between a primary gastric cancer and metastatic breast cancer.
Footnotes
Conflicts of interests
None.
Source of suport: This work was supported by the Ewha Womans University Research Grant of 2017
References:
- 1.Savanis G, Simatos G, Tzaida O, et al. Gastrointestinal tract metastasis as first presentation of breast cancer. J BUON. 2006;11:79–81. [PubMed] [Google Scholar]
- 2.Taal BG, Peterse H, Boot H. Clinical presentation, endoscopic features, and treatment of gastric metastases from breast carcinoma. Cancer. 2000;89:2214–21. [PubMed] [Google Scholar]
- 3.McLemore EC, Pockaj BA, Reynolds C, et al. Breast cancer: Presentation and intervention in women with gastrointestinal metastasis and carcinomatosis. Ann Surg Oncol. 2005;12:886–94. doi: 10.1245/ASO.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 4.Eljabu W, Finch G, Nottingham J, Vaingankar N. Metastatic deposits of breast lobular carcinoma to small bowel and rectum. Int J Breast Cancer. 2011;2011:413949. doi: 10.4061/2011/413949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crivellari D, Carbone A, Sigon R, et al. Gastric cancer with bone marrow invasion at presentation: case-report and review of the literature. Tumori. 1995;81:74–76. doi: 10.1177/030089169508100117. [DOI] [PubMed] [Google Scholar]
- 6.Abrams HL, Spiro R, Goldstein N. Metastases in carcinoma; analysis of 1000 autopsied cases. Cancer. 1950;3:74–85. doi: 10.1002/1097-0142(1950)3:1<74::aid-cncr2820030111>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 7.Borst MJ, Ingold JA. Metastatic patterns of invasive lobular versus invasive ductal carcinoma of the breast. Surgery. 1993;114:637–41. discussion 641–42. [PubMed] [Google Scholar]
- 8.Taal BG, den Hartog Jager FC, Steinmetz R, Peterse H. The spectrum of gastrointestinal metastases of breast carcinoma: I. Stomach. Gastrointest Endosc. 1992;38:130–35. doi: 10.1016/s0016-5107(92)70377-0. [DOI] [PubMed] [Google Scholar]
- 9.Matsui M, Kojima O, Kawakami S, et al. The prognosis of patients with gastric cancer possessing sex hormone receptors. Surg Today. 1992;22:421–25. doi: 10.1007/BF00308791. [DOI] [PubMed] [Google Scholar]
- 10.Koike K, Kitahara K, Higaki M, et al. Clinicopathological features of gastric metastasis from breast cancer in three cases. Breast Cancer. 2014;21:629–34. doi: 10.1007/s12282-011-0284-3. [DOI] [PubMed] [Google Scholar]
- 11.Bhargava R, Beriwal S, Dabbs DJ. Mammaglobin vs. GCDFP-15: An immunohistologic validation survey for sensitivity and specificity. Am J Clin Pathol. 2007;127:103–13. doi: 10.1309/TDP92PQLDE2HLEET. [DOI] [PubMed] [Google Scholar]
- 12.O’Connell FP, Wang HH, Odze RD. Utility of immunohistochemistry in distinguishing primary adenocarcinomas from metastatic breast carcinomas in the gastrointestinal tract. Arch Pathol Lab Med. 2005;129:338–47. doi: 10.5858/2005-129-338-UOIIDP. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg PA, Hortobagyi GN, Smith TL, et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol. 1996;14:2197–205. doi: 10.1200/JCO.1996.14.8.2197. [DOI] [PubMed] [Google Scholar]