Abstract
Patient: Male, 62
Final Diagnosis: Enteritis
Symptoms: Diarrhea • enteritis
Medication: Nivolumab
Clinical Procedure: —
Specialty: Oncology
Objective:
Adverse events of drug therapy
Background:
Enterocolitis is an immune-related adverse event associated with nivolumab treatment. Although intravenous corticosteroids and infliximab are recommended as a first-line and second-line therapy, respectively, there is no established treatment for severe enterocolitis that is refractory to these drugs.
Case Report:
A 62-year-old male with non-small cell lung cancer, with multiple brain metastasis, received nivolumab as the eighth-line chemotherapy for his disease. A few days after nivolumab administration, grade 2–3 enterocolitis developed in the patient. The enterocolitis improved to grade 1 after careful observation; however, it was aggravated to grade 3 after resuming nivolumab treatment. After cessation of nivolumab, 3.3 mg of intravenous dexamethasone and 40 mg of methylprednisolone were administered for 16 days and subsequently 30–60 mg of oral prednisolone was administered for 50 days, with little improvement in the patient’s colitis.
A second-line treatment with 5 mg/kg of infliximab was twice attempted, but the patient had persistent diarrhea. Therefore, 50 mg of oral cyclosporine was started as a third-line therapy. Three days after the start of cyclosporine, the number of diarrhea events decreased, with resolution 2 weeks after cyclosporine administration.
Conclusions:
Oral cyclosporine treatment can be a third-line therapy for enterocolitis associated with immune-related adverse events.
MeSH Keywords: Cyclosporine, Drug-Related Side Effects and Adverse Reactions, Enterocolitis
Background
Nivolumab, an immune checkpoint inhibitor (ICI), is a humanized anti-PD-1 monoclonal antibody that is used for the treatment for various types of cancer after second-line chemotherapy. Nivolumab may activate T cells to develop anti-tumor effects, but this immune activation sometimes causes immune-related adverse events (irAE), such as pneumonitis, hepatitis, and enterocolitis [1].
Enterocolitis is one form of irAE that decreases the quality-of-life of patients with lung cancer and disrupts treatment with ICIs. Among patients with lung cancer, enterocolitis is common, affecting approximately 8% of treated patients [2,3], and can reach grade 3–4 severity among 1% of patients [3]. Enterocolitis can be managed with cessation of nivolumab and administration of intravenous corticosteroid therapy after hospitalization (methylprednisolone 1–2 mg/kg per day) or administration of 5 mg/kg of infliximab in severe cases [1]. However, there is no standard therapy for enterocolitis that is intractable with corticosteroid therapy or infliximab, and the description of such cases in the literature is scarce, although the use of tacrolimus or mycophenolate has been suggested [4]. Herein, we report a case of severe enterocolitis associated with nivolumab that was refractory to the recommended treatment course (corticosteroid and infliximab) [1], but resolved successfully after cyclosporine treatment.
Case Report
A 62-year-old male with non-small cell lung cancer and suffering from multiple brain metastases was examined at our clinic. Eleven years before presenting to us, he was diagnosed with stage IIIA (cT3N2M0) lung adenocarcinoma and received cisplatin-based chemoradiotherapy without surgery. Four years before presenting to us, his lung cancer recurred, and he received multiple courses of standard chemotherapy (such as platinum-based chemotherapy and pemetrexed/bevacizumab) after confirmation that the genetic mutation of his tumor was wild-type for the EGFR and the ALK fusion genes. Standard chemotherapy was unable to control the lung lesions and brain metastases; therefore, intravenous nivolumab therapy was started (3 mg/kg intravenous nivolumab for 2 weeks per 1 cycle) as the eighth-line therapy. He received 18 cycles of intravenous nivolumab, and during the treatment, his lung lesions and multiple brain metastases were stable, and the patient followed an uneventful course. At that time, the nineteenth cycle of 3 mg/kg of nivolumab was administered after hospitalization. Four days after this nivolumab administration, grade 2–3 enterocolitis developed in the patient (Figure 1). Endoscopic examination of the colon revealed intestinal inflammation accompanied by edema in the whole colon mucosa, which was consistent with enterocolitis associated with irAE (Figure 2). After improvement of the patient’s enterocolitis to grade 1, nivolumab was again administered. However, the day after resumption of nivolumab, the patient’s enterocolitis became aggravated and worsened to grade 3. Therefore, nivolumab treatment was discontinued, and the recommended treatment for enterocolitis was started: 3.3 mg of intravenous dexamethasone and 40 mg of methylprednisolone (equivalent to 70 mg of prednisolone). After administration of the previously described regimen for 16 days, the treatment was switched to 50 mg of oral prednisolone; however, the patient’s enterocolitis remained grade 2–3. On the 39th day after the onset of diarrhea, 5 mg/kg of infliximab was administered intravenously. However, 2 weeks after infliximab administration the patient’s diarrhea persisted. Thus, a second administration of infliximab was attempted; however, there was no improvement in severe diarrhea symptoms. His diarrhea was severe enough to cause sleep disturbance and depressive mood. Therefore, 50 mg of cyclosporine was started as a third-line therapy. Three days after starting oral cyclosporine (60th day after the onset of enterocolitis), the number of diarrhea events started to decrease, and his enterocolitis became grade 1–2. Two weeks after cyclosporine treatment initiation, diarrhea symptoms resolved, and his stool became solid. Following this, an additional 5 mg/kg of infliximab was administered once. After confirmation of the resolution of the patient’s enterocolitis, he was re-administered nivolumab and discharged to continue outpatient treatment. However, soon after receiving 2 courses of nivolumab, grade 3 diarrhea relapsed and nivolumab administration was withdrawn. Regarding oncologic outcome, computed tomography imaging conducted before patient discharge revealed stable disease, based on the unchanging cancerous lesions of the primary lung focus and multiple brain metastases, suggesting sustained immuno-modulatory effect on cancerous cells despite nivolumab cessation (Figure 3).
Figure 1.
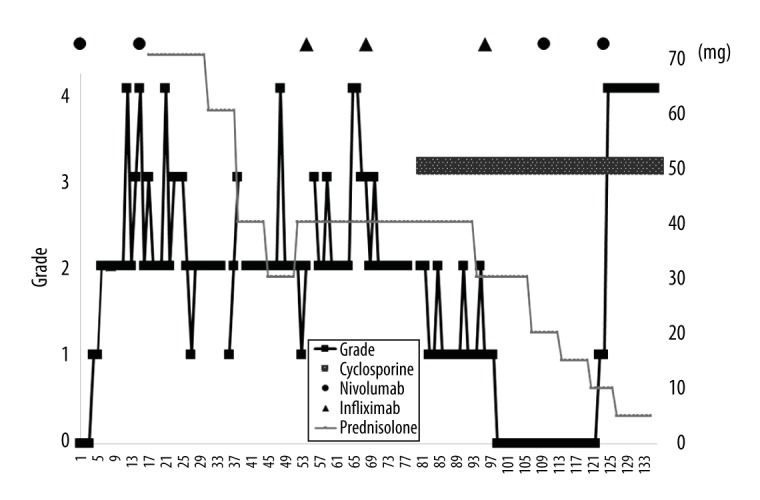
Time course of enterocolitis associated with nivolumab and subsequent treatment. The black solid line shows diarrhea grade, based on the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The thin light-gray solid line shows dose of prednisolone. The dark gray bar shows cyclosporine dose. The solid circle and triangle show administration of nivolumab and infliximab, respectively.
Figure 2.
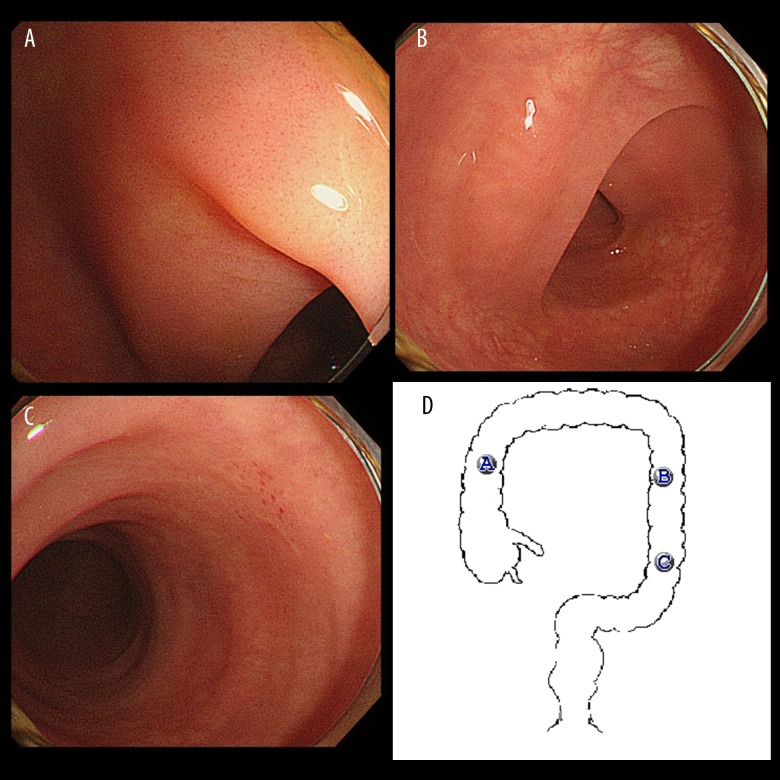
Endoscopic finding of the colon mucosa. (A–C) The endoscopic images of the ascending colon, the descending colon, and the sigmoid colon, respectively. There was enteritis with mild edema affecting the whole colon mucosa. However, ulcerative lesions were not observed at any site. The findings were compatible with enterocolitis associated with nivolumab. (D) Illustrates the sites of the colon where A–C were taken.
Figure 3.
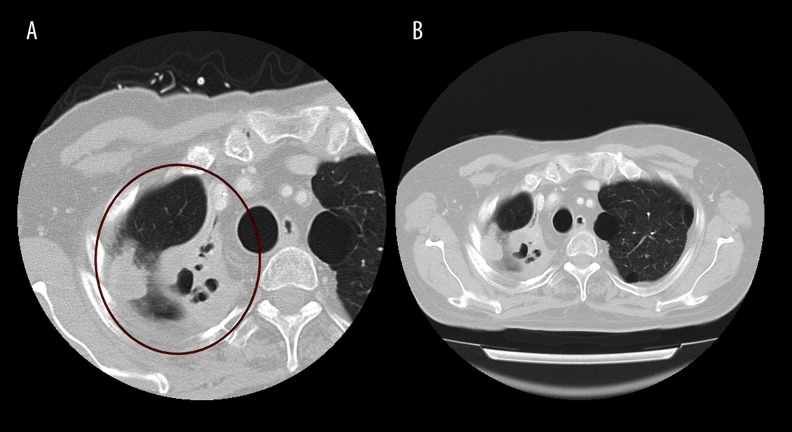
Computed tomography scans of the primary lung focus. (A) Indicates the cancerous lesions measured immediately after withdrawal of nivolumab administration. (B) Indicates the same lesions measured approximately 70 days after withdrawal of nivolumab administration. The size of the lesions remained unchanged.
Discussion
In this study, we reported the case of a non-small cell lung cancer patient with nivolumab-induced enterocolitis that was refractory to corticosteroid and infliximab, but improved after oral cyclosporine administration. Although his treatment was uneventful during the first 18 cycles of nivolumab administration, he seemed to develop enterocolitis as an irAE associated with nivolumab on the 19th cycle of nivolumab administration. While it is possible that enterocolitis associated with nivolumab could develop during early cycles of nivolumab therapy, studies have shown that there are lung cancer patients who developed enterocolitis associated with nivolumab around the 45th cycle of nivolumab administration [2,3]. Also, regarding the diagnostic accuracy of enterocolitis associated with irAE, the time course of its onset after nivolumab administration, as well as the relapse of enterocolitis after re-administration of nivolumab, support our final diagnosis. Although infectious enteritis [5], such as cytomegalovirus infection, should be a concern for patients with diarrhea during nivolumab therapy, this scenario was unlikely due to a lack of ulcerative lesions on colonoscopy (Figure 2), as well as no aggravation during cyclosporine treatment, which can also sustain immunosuppression. As cyclosporine is an immunosuppressive agent that blocks the transcription of cytokine genes in activated T cells [6], the drug may be effective for irAE involved by T-cell activation.
The strength of this report is the uniqueness of oral cyclosporine therapy and the subsequent resolution of the refractory enterocolitis. The enterocolitis of our patient seemed refractory, as he did not respond to corticosteroids and infliximab, and suffered from diarrhea during the day and night. However, diarrhea symptoms decreased and eventually subsided after 50 mg of oral cyclosporine. Thus, oral cyclosporine treatment can be a third-line therapy for enterocolitis associated with irAE, considering that treatment recommendations except for corticosteroids as first, and infliximab as second, choice therapies for enterocolitis do not exist [3]. In addition, the use of cyclosporine can be relatively cost effective compared with bio-engineered drugs. A study showed that vedolizumab [7], a humanized monoclonal IgG1 antibody against α4β7 integrin used for the treatment of inflammatory bowel disease, is effective against enterocolitis associated with irAE. In that study, 300 mg of vedolizumab was administered intravenously on days 1, 15, and 43, and then every 8 weeks thereafter. However, a single dose of vedolizumab costs about $5,000 (USD) [8].
Several limitations of this study need to be mentioned. First, the case report design does not guarantee the effectiveness of oral cyclosporine treatment for enterocolitis in all cases. Second, our patient may have responded to infliximab treatment in a delayed manner, as his enterocolitis improved to grade 1 to 2 soon after oral cyclosporine administration (i.e., after 3 days). In addition, his diarrhea became grade 0 immediately after the third administration of infliximab. Indeed, although a systematic review has reported that enterocolitis caused by irAE usually improves rapidly after the first infliximab administration, there are cases in which response took approximately 2 weeks after the second administration of infliximab [9]. Therefore, it is possible that cyclosporine and infliximab had a synergistic effect on the patient’s enterocolitis.
Conclusions
We reported a case of severe enterocolitis associated with nivolumab, which was refractory to corticosteroids and infliximab therapy, but improved after oral cyclosporine administration. Further study is warranted to build evidence of the effectiveness of oral cyclosporine for refractory enterocolitis caused by irAE.
Footnotes
Conflicts of interest
None.
References:
- 1.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: A review. JAMA Oncol. 2016;2:1346–53. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 2.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–35. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spain L, Diem S, Larkin J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat Rev. 2016;44:51–60. doi: 10.1016/j.ctrv.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Prieux-Klotz C, Dior M, Damotte D, et al. Immune checkpoint inhibitor-induced colitis: Diagnosis and management. Target Oncol. 2017;12:301–8. doi: 10.1007/s11523-017-0495-4. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda S, Koyasu S. Review mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–25. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 7.Bergqvist V, Hertervig E, Gedeon P, et al. Vedolizumab treatment for immune checkpoint inhibitor-induced enterocolitis. Cancer Immunol Immunother. 2017;66:581–92. doi: 10.1007/s00262-017-1962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erim DO, Mahendraratnam N, Okafor PN, Wheeler SB. The value of vedolizumab as rescue therapy in moderate-severe Crohn’s disease patients with adalimumab non-response in the USA. J Crohns Colitis. 2015;9:669–75. doi: 10.1093/ecco-jcc/jjv090. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, De Felice KM, Loftus EV, Khanna S. Systematic review: Colitis associated with anti-CTLA-4 therapy. Aliment Pharmacol Ther. 2015;42:406–17. doi: 10.1111/apt.13281. [DOI] [PubMed] [Google Scholar]