Abstract
Objective
Truncal vagotomy is associated with a decreased risk of subsequent Parkinson disease (PD), although the effect of vagotomy on dementia is unclear. In response, we investigated the risk of dementia in patients who underwent vagotomy.
Setting
Population-based cohort study.
Participants
A total of 155 944 patients who underwent vagotomy (vagotomy cohort) and 155 944 age-matched, sex-matched and comorbidity-matched controls (non-vagotomy cohort) were identified between 2000 and 2011.
Primary and secondary outcome measures
All patient data were tracked until the diagnosis of dementia, death or the end of 2011. The cumulative incidence of subsequent dementia and HRs were calculated.
Results
The mean ages of the study patients in the vagotomy and non-vagotomy cohorts were 56.6±17.4 and 56.7±17.3 years, respectively. The overall incidence density rate for dementia was similar in the vagotomy and non-vagotomy cohorts (2.43 and 2.84 per 1000 person-years, respectively). After adjustment for age, sex and comorbidities such as diabetes, hypertension, hyperlipidaemia, stroke, depression, coronary artery disease and PD, the patients in the vagotomy cohort were determined to not be at a higher risk of dementia than those in the non-vagotomy cohort (adjusted HR=1.09, 95% CI 0.87 to 1.36). Moreover, the patients who underwent truncal vagotomy were not associated with risk of dementia (adjusted HR=1.04, 95% CI 0.87 to 1.25), compared with the patients who did not undergo vagotomy.
Conclusion
Vagotomy, either truncal or selective, is not associated with risk of dementia.
Keywords: parkinson disease, dementia, vagotomy, cohort study
Strengths and limitations of this study.
The strengths of our study are its population-based design, and generalisability of findings with a very large sample size including study and control cohorts.
All insurance claims should be scrutinised by medical reimbursement specialists and peer review.
Although we have considered the major surrogate variables, information on individual-based risk factors for dementia, including smoking, genetic mutation, family history, vitamin D consumption, sleep patterns, caffeine use and education level, was unavailable in this database.
Introduction
Dementia, a syndrome representing a cluster of disturbances in cognitive functioning, is currently the leading chronic cause of irreversible disability among elderly patients.1 According to a WHO estimate, more than 30 million people are living with dementia worldwide, and this number is expected to increase by more than three times by 2050.2 3
The most common causes of dementia are Alzheimer disease (AD), vascular dementia, Parkinson disease (PD) and neurodegenerative diseases; these conditions have different pathogeneses that lead to a decline in cognition.4 Because dementia is the end result of a complex process involving genetic defects,5 hypoperfusion, oxidative stress,6 mitochondrial dysfunction7 and protein deposition,8 the aetiology of dementia is multifactorial.
Typically, accumulation of fibrous amyloid-β (Aβ) plaques or protein fibrils of α-synuclein is the hallmark of AD, whereas aggregation of α-synuclein in the Lewy bodies is the hallmark of PD.9 10 The levels of Aβ are associated with the severity of AD.11 Bachhuber et al12 reported that α-synuclein is also associated with the inhibition of Aβ plaque formation; these findings suggest an overlap between the pathogeneses of AD and PD.
In murine models, intragastric injection of rotenone-initiated α-synuclein can reproduce the progression of PD pathology13 and vagotomy can stop the spread of PD pathology.14 Vagotomy is the surgical resection of the vagus nerve, and it is performed to reduce acid secretion for managing complicated peptic ulcer disease (PUD).15 Two surgical strategies of vagotomy are available: truncal vagotomy, in which the trunk of the vagus nerve is cut to innervate the abdomen, and selective vagotomy, in which the vagus nerve is resected to innervate the fundus and body of the stomach.16 Liu et al17 used a Swedish database to investigate the association between vagotomy and the risk of PD, and found that vagotomy was not associated with the risk of PD overall. However, a study by Svensson et al,18 which was conducted using a database from Denmark, revealed an association between complete truncal vagotomy and a decreased risk for subsequent PD. Thus, the association between vagotomy and PD remains inconclusive. The aforementioned observations also suggest that vagotomy may reduce the risk of other neurodegenerative diseases, such as AD. However, clinical evidence in the form of data supporting this hypothesis is not available. Because dementia is the most common clinical sign and the result of most neurodegenerative diseases, we used the National Health Insurance (NHI) registration database to determine the association between vagotomy and dementia risk.
Methods
Data source
The Taiwan NHI programme, launched in March 1995, currently covers more than 99% of the 23.72 million people in Taiwan.19 The inpatient database was used for this nationwide population-based retrospective cohort study. The details of the programme and inpatient databases have been discussed in previous studies.20 21
Diseases were coded according to the 2001 International Classification of Disease, Revision 9, Clinical Modification (ICD-9-CM). The Institutional Review Board also specifically waived the consent requirement.
Sampled participants
Patients aged more than 20 years old who had undergone vagotomy (ICD-9-OP codes 44.0) formed the vagotomy cohort. The dates of the first hospitalisation for vagotomy were defined as the index dates of the vagotomy patients. Patients with a history of dementia (ICD-9-CM codes 290, 294.1 and 331.0) before the index date were excluded.
Patients who had undergone vagotomy were matched (1:1 ratio) with those who did not undergo vagotomy according to their propensity score through nearest neighbour matching, initially to the eighth digit and then as required to the first digit.22 Therefore, matches were first made within a calliper width of 0.0000001, and then the calliper width was increased for unmatched cases to 0.1. We considered the matching criteria and performed a rematch (greedy algorithm) without replacement. For each vagotomy patient, the corresponding comparisons were selected based on the nearest propensity score. The date of enrolment in non-vagotomy cohort was matched with the same year and month of the vagotomy cohort, by the random assignment method. The propensity scores were calculated using logistic regression to estimate the probability of the surgery status using baseline variables of sex, age, and comorbidities such as diabetes, hypertension, hyperlipidaemia, stroke, coronary artery disease, chronic kidney disease, liver disease, osteoarthritis, chronic obstructive pulmonary disease, depression, head injury, PD, cancer or PUD. The samples were matched with sex, age, comorbidities and index year to reduce confounding effects of sex, age and comorbidities, and to have a valid measure of follow-up person-years. All of the study participants were followed up from the index date to the occurrence of dementia, withdrawal from the NHI programme or the end of 2011, whichever occurred first. Most important of all, there would be no any event of vagotomy in the control cohort during the follow-up period.
Statistical analysis
The frequency and percentage for categorical variables, as well as the mean and SD for continuous variables, were calculated for the vagotomy and non-vagotomy cohorts. The differences in distribution between the two cohorts were assessed using standardised mean differences. A standardised mean difference of ≤0.1 indicated a negligible difference between the two cohorts.23 The follow-up time in person-years estimated the incidence density of dementia among different risk factors and incidence density stratified by age, sex, comorbidity and follow-up period. Cox proportional hazards models stratifying on the matched pairs were performed to estimate the HR and 95% CI of developing dementia associated with vagotomy cohort, compared with non-vagotomy cohort. When the patients were stratified according to sex, age, comorbidity and follow-up period (using the first quartile and second quartile as a cut-off), the relative risk of dementia in the vagotomy and non-vagotomy cohorts was also compared using Cox regression models. To address the concern of constant proportionality, we examined the proportional hazard model assumption using a test of scaled Schoenfeld residuals. All analyses were performed using SAS V.9.4 and statistical significance was set at P<0.05.24
Results
Table 1 lists the characteristics of the study cohort. We identified 155 944 patients with vagotomy; hence, 155 944 patients were recruited for the non-vagotomy cohort. Men accounted for approximately 79% of the patients in each cohort and most of the patients were aged ≥50 years (61.7% vs 61.7%). Specifically, the mean age of the patients was 56.6±17.4 years for the vagotomy cohort and 56.7±17.3 years for the non-vagotomy cohort. The major comorbidities in both cohorts were PUD (96.2% vs 96.2%), hypertension (21.1% vs 21.1%) and diabetes (15.2% vs 15.1%). The mean follow-up periods were 5.88±3.80 and 6.58±3.35 years for the vagotomy and non-vagotomy cohorts, respectively. The incidence of dementia was 2.43 and 2.84 per 1000 person-years in the vagotomy and non-vagotomy cohorts, respectively. Results showed that there was no significant relationship between Schoenfeld residuals for vagotomy and follow-up time (P=0.07) in the model evaluating the dementia risk. The vagotomy cohort was not associated with dementia compared with the non-vagotomy cohort (HR=1.09, 95% CI 0.87 to 1.36). The risk of dementia in the patients who were ≥65 years old was 74.4-fold higher than in those who were ≤49 years old (95% CI 36.8 to 150.5). Furthermore, the multivariable models showed that dementia was independently associated with comorbidities, particularly stroke (HR=1.84, 95% CI 1.47 to 2.29), head injury (HR=1.66, 95% CI 1.23 to 2.24) and PD (HR=2.00, 95% CI 1.19 to 3.38) (table 2).
Table 1.
Demographic characteristics and comorbidities in the vagotomy and non-vagotomy cohorts
| Vagotomy | Standard mean difference* | ||
| No (n=155 944) | Yes (n=155 944) | ||
| Sex | |||
| Women | 3409 (21.4) | 3397 (21.3) | 0.002 |
| Men | 12 535 (78.6) | 12 547 (78.7) | 0.002 |
| Age-stratified | |||
| ≤49 | 6115 (38.4) | 6114 (38.4) | 0.000 |
| 50–64 | 3938 (24.7) | 3939 (24.7) | 0.000 |
| 65+ | 5891 (37.0) | 5891 (37.0) | 0.000 |
| Age, mean±SD | 56.7±17.3 | 56.6±17.4 | 0.01 |
| Comorbidity | |||
| Diabetes | 2399 (15.1) | 2421 (15.2) | 0.004 |
| Hypertension | 3359 (21.1) | 3360 (21.1) | 0.000 |
| Hyperlipidaemia | 584 (3.66) | 613 (3.84) | 0.01 |
| Stroke | 1334 (8.37) | 1340 (8.40) | 0.001 |
| Coronary artery disease | 1379 (8.65) | 1381 (8.66) | 0.000 |
| Chronic kidney disease | 613 (3.84) | 632 (3.96) | 0.01 |
| Liver disease | 1834 (11.5) | 1846 (11.6) | 0.002 |
| Osteoarthritis | 498 (3.12) | 521 (3.27) | 0.01 |
| Chronic obstructive pulmonary disease | 158 (0.99) | 153 (0.96) | 0.003 |
| Depression | 191 (1.20) | 190 (1.19) | 0.001 |
| Head injury | 976 (6.12) | 994 (6.23) | 0.01 |
| Parkinson’s disease | 117 (0.73) | 108 (0.68) | 0.01 |
| Cancer | 597 (3.74) | 619 (3.88) | 0.01 |
| Peptic ulcer disease | 15 343 (96.2) | 15 343 (96.2) | 0.000 |
*A standardised mean difference of ≤0.10 indicates a negligible difference between the two cohorts.
Table 2.
Incidences of and risk factors for dementia
| Variable | Event | Person-years | Rate† | HR (95% CI) |
| Vagotomy | ||||
| No | 298 | 104 932 | 2.84 | 1.00 |
| Yes | 228 | 93 707 | 2.43 | 1.09 (0.87 to 1.36) |
| Age group, year | ||||
| ≤49 | 8 | 91 483 | 0.09 | 1.00 |
| 50–64 | 51 | 51 513 | 0.99 | |
| 65+ | 467 | 55 643 | 8.39 | |
| Sex | ||||
| Female | 143 | 39 468 | 3.62 | 1.00 (0.06 to 16.0) |
| Male | 383 | 159 171 | 2.41 | 1.00 |
| Comorbidity | ||||
| Diabetes | ||||
| No | 405 | 177 247 | 2.28 | 1.00 |
| Yes | 121 | 21 391 | 5.66 | 1.01 (0.82 to 1.25) |
| Hypertension | ||||
| No | 289 | 169 471 | 1.71 | 1.00 |
| Yes | 237 | 29 168 | 8.13 | |
| Hyperlipidaemia | ||||
| No | 497 | 193 049 | 2.57 | 1.00 |
| Yes | 29 | 5590 | 5.19 | 3.00 (0.31 to 28.8) |
| Stroke | ||||
| No | 400 | 188 269 | 2.12 | 1.00 |
| Yes | 126 | 10 370 | 12.2 | |
| Coronary artery disease | ||||
| No | 416 | 187 358 | 2.22 | 1.00 |
| Yes | 110 | 11 281 | 9.75 | 2.00 (0.18 to 22.1) |
| Chronic kidney disease | ||||
| No | 494 | 194 635 | 2.54 | 1.00 |
| Yes | 32 | 4004 | 7.99 | 1.33 (0.93 to 1.92) |
| Liver disease | ||||
| No | 442 | 180 865 | 2.44 | 1.00 |
| Yes | 84 | 17 774 | 4.73 | 1.20 (0.95 to 1.52) |
| Osteoarthritis | ||||
| No | 502 | 194 322 | 2.58 | 1.00 |
| Yes | 24 | 4317 | 5.56 | 0.76 (0.50 to 1.15) |
| Chronic obstructive pulmonary disease | ||||
| No | 513 | 197 076 | 2.60 | 1.00 |
| Yes | 13 | 1562 | 8.32 | 1.67 (0.96 to 2.90) |
| Depression | ||||
| No | 420 | 196 769 | 2.64 | 1.00 |
| Yes | 6 | 1869 | 3.21 | 1.03 (0.46 to 2.32) |
| Head injury | ||||
| No | 478 | 188 290 | 2.54 | 1.00 |
| Yes | 48 | 10 349 | 4.64 | |
| Parkinson’s disease | ||||
| No | 511 | 197 771 | 2.58 | 1.00 |
| Yes | 15 | 868 | 17.3 | |
| Cancer | ||||
| No | 516 | 194 585 | 2.65 | 1.00 |
| Yes | 10 | 4054 | 2.47 | 0.52 (0.05 to 5.51) |
| Peptic ulcer disease | ||||
| No | 10 | 6542 | 1.53 | 1.00 |
| Yes | 516 | 192 096 | 2.69 | 2.11 (1.13 to 3.95)* |
*P<0.05
†Incidence rate per 1000 person-years.
Table 3 compares the risk of dementia between the vagotomy and non-vagotomy cohorts and also presents the risk stratified by sex, age, comorbidity and follow-up period. Female vagotomy patients had a 1.56-fold higher risk of dementia than female patients from the non-vagotomy cohort did (HR=1.56, 95% CI 1.00 to 2.44). When compared with the non-vagotomy patients, the vagotomy patients had higher risk of dementia in those aged ≤64 years (HR=2.07, 95% CI 1.12 to 3.83).
Table 3.
Incidence densities of dementia HRs between the vagotomy and non-vagotomy cohorts based on demographic characteristics and comorbidities
| Vagotomy | HR (95% CI) | ||||||
| No | Yes | ||||||
| Event | PY | Rate† | Event | PY | Rate† | ||
| Sex | |||||||
| Women | 77 | 21 364 | 3.60 | 66 | 18 104 | 3.65 | 1.56 (1.00 to 2.44)* |
| Men | 221 | 83 568 | 2.64 | 162 | 75 603 | 2.14 | 0.96 (0.74 to 1.24) |
| Stratify age | |||||||
| ≤64 | 24 | 73 656 | 0.33 | 35 | 69 339 | 0.50 | 2.07 (1.12 to 3.83)* |
| ≥65 | 274 | 31 275 | 8.76 | 193 | 24 367 | 7.92 | 0.97 (0.76 to 1.24) |
| Comorbidity‡ | |||||||
| No | 1 | 2156 | 0.46 | 1 | 1493 | 0.67 | – |
| Yes | 297 | 102 776 | 2.89 | 227 | 92 214 | 2.46 | 1.08 (0.86 to 1.35) |
| Follow-up period | |||||||
| <4 | 131 | 43 502 | 3.01 | 116 | 38 634 | 3.00 | 1.24 (0.94 to 1.63) |
| 4–7 | 119 | 34 038 | 3.50 | 87 | 30 274 | 2.87 | 0.91 (0.59 to 1.40) |
| ≥7 | 48 | 19 534 | 2.46 | 25 | 17 780 | 1.41 | 0.50 (0.17 to 1.46) |
*P<0.05.
†Incidence rate per 1000 person-years.
‡Comorbidity: patients with any one of the listed comorbidities were classified as the comorbidity group.
PY, person-years.
We further assessed the association between vagotomy and dementia risk stratified by sex and age (table 4). Among the men, the effects of vagotomy on dementia risk were not associated with age (HR=1.77, 95% CI 0.90 to 3.49 for subgroup aged ≤64 years; HR=0.85, 95% CI 0.64 to 1.14 for subgroup aged ≥65 years).
Table 4.
Incidence and HRs of dementia, stratified by age and sex, between the vagotomy and non-vagotomy cohorts
| Variables | Men | HR (95% CI) | Women | HR (95% CI) | ||||||
| Vagotomy | Vagotomy | |||||||||
| No | Yes | No | Yes | |||||||
| Event | Rate* | Event | Rate* | Event | Rate* | Event | Rate* | |||
| Age | ||||||||||
| ≤64 | 19 | 0.31 | 27 | 0.46 | 1.77 (0.90 to 3.49) | 5 | 0.42 | 8 | 0.73 | 4.00 (0.85 to 18.8) |
| ≥65 | 202 | 9.23 | 135 | 7.87 | 0.85 (0.64 to 1.14) | 72 | 7.68 | 58 | 8.05 | 1.40 (0.88 to 2.24) |
*Incidence rate per 1000 person-years.
Furthermore, the truncal vagotomy patients exhibited no association with risk of dementia, compared with the non-vagotomy patients (HR=1.05, 95% CI 0.87 to 1.25) (table 5). Similarly, compared with non-vagotomy cohort, the types of vagotomy, including trunk, highly selective and other selective vagotomy were not associated with risk of dementia (table 5). Figure 1 shows the cumulative incidence for vagotomy group compared with non-vagotomy cohort (log-rank test P=0.08).
Table 5.
Incidence and HRs of dementia among the vagotomy and non-vagotomy cohorts
| Variable | n | Event | Person-years | Rate* | HR (95% CI) |
| Control | 15 944 | 298 | 104 932 | 2.84 | 1 (reference) |
| Vagotomy | |||||
| Truncal vagotomy | 12 999 | 192 | 77 219 | 2.49 | 1.04 (0.87 to 1.25) |
| Highly selective | 1035 | 8 | 6141 | 1.30 | 0.84 (0.42 to 1. 69) |
| Other selective vagotomy | 101 | 1 | 529 | 1.89 | 0.80 (0.11 to 5.73) |
*Rate, incidence rate per 1000 person-years.
Figure 1.
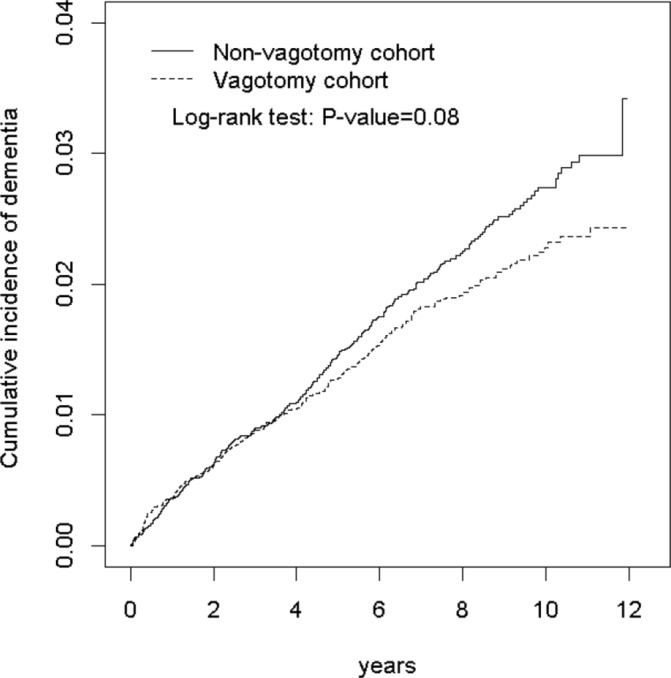
Cumulative incidence of dementia in the vagotomy and non-vagotomy cohorts.
To address the concern of constant proportionality, we examined the proportional hazard model assumption using a test of scaled Schoenfeld residuals. Results showed that there was no significant relationship between Schoenfeld residuals for vagotomy and follow-up time (P=0.07) in the model evaluating the dementia risk.
Discussion
Recent studies have reported that the vagus nerve is associated with the transport of α-synuclein and progression of PD.14 25 A nationwide population-based study by Svensson et al revealed that truncal vagotomy is associated with a decreased risk of subsequent PD.18 However, Liu et al17 reported that neither truncal nor selective vagotomy is associated with a protective effect against PD. Similar to the findings of Liu et al,17 our study revealed that vagotomy is not associated with overall risk of dementia. However, vagotomy, in those women or patients aged ≤64 years old, is associated with higher risk of dementia.
Several explanations for these results are possible. First, dementia has several aetiologies, including PD, AD, vascular dementia and other neurodegenerative diseases. Braak et al26 proposed that the pathogenic process of PD originates when an environmental insult enters the body and is transmitted to the brain. Furthermore, the pathogenic process is mediated by the neurontransport of α-synuclein. Sevenson et al reported that truncal vagotomy is associated with a decreased risk of PD,16 which thus supported the hypothesis of Braak et al.26 A major component of Lewy bodies in PD, α-synuclein, was first isolated from plaques in the brains of patients with AD.27 Although α-synuclein is associated with the development of PD and AD, the enteric route might be a spread source for neurodegenerative diseases.28 Thus, vagotomy might not be associated directly with the risk of dementia.
Another possible explanation is the microbiome effect of vagotomy. In animal models of sepsis, the vagal nerve has been proposed to be involved in the regulation of inflammatory responses.29 Li et al30 described a case of impaired intestinal microbiota barrier following vagotomy and subsequent rescue after microbiota transplantation. Recently, it has been recognised that the human microbiome of the gastrointestinal tract may affect the brain and behaviour, an association that has been named the gut–brain axis.31 The brains of patients with Parkinson-dementia and AD were found to have elevated levels of beta-N-methylamino-L-alanine.32 Schwartz et al33 proposed that the host bacteria amyloids contribute to misfolding and amyloidogenic diseases such as AD. Thus, vagotomy might alter the microbiome of the gastrointestinal tract. However, the negative effects of an altered microbiome following vagotomy may attenuate the possible positive effects discussed by Braak et al. The effect of vagotomy on neurological dysfunction therefore remains only vaguely defined. One interesting finding of this study is that vagotomy, in those women or patients aged ≤64 years-old, is associated with higher risk of dementia. Further studies are needed to clarify whether and why vagotomy are associated with the risk of dementia in a group of women or aged ≤64 years old.
This study has several limitations. First, we defined dementia as an outcome instead of a specific neurodegenerative disease. Dementia is the most common presentation of neurodegenerative diseases, including AD and PD. The diagnosis of AD is stricter than that of dementia and requires distinct clinical and laboratory features.34 35 Thus, in this study, we used dementia as an outcome and adjusted cardiovascular-related comorbidities for vascular dementia to avoid the possible bias of underdiagnosis related to AD and other neurodegenerative diseases. The discrimination and validity for subtypes of dementia are difficult in this study based on coding. We did not analyse the association between vagotomy and subtypes of dementia. Second, although we have considered the major surrogate variables, information on individual-based risk factors for dementia, including smoking, genetic mutation, family history, vitamin D consumption, sleep patterns, caffeine use and education level, was unavailable in this database. Further, the vagotomy cohort might have more medical visits and more chances to be diagnosed with dementia, and possible surveillance and detection bias should be mentioned here. The follow-up period of this study is relatively short. Since our data showed that vagotomy had no association with dementia before 7 years, but may provide protection for dementia after 7 years, it would be possible that vagotomy might be protective for dementia once the follow-up duration is longer. Further, the number of dementia events is small in this study; thus, the conclusion and presumption would not be precise and valid. Longitudinal cohort studies of longer follow-up duration are necessary to clarify the possible protective role of vagotomy for dementia. Finally, most of the study population is Taiwanese; thus, it needs caution to translate and generalise our findings to other population. The generalisability of our results might be limited.
In conclusion, the current nationwide cohort study revealed that vagotomy, either truncal or selective, was not associated with risk of dementia. Although one study showed that truncal vagotomy reduces the risk of PD,17 our study offers preliminary evidence that the association between vagotomy and dementia is not direct.
Supplementary Material
Footnotes
Contributors: Conceptualisation: S-YL, C-HK. Methodology: C-LL, C-HK. Software: C-LL, C-HK. Validation: S-YL, C-LL, I-KW, C-CL, C-HL, W-HH, C-HK. Formal analysis: S-YL, C-LL, I-KW, C-CL, C-HL, W-HH, C-HK. Investigation: C-LL, C-HK. Resources: C-LL, C-HK. Data curation: S-YL, C-LL, I-KW, C-CL, C-HL, W-HH, C-HK. Writing (original draft preparation): S-YL, C-LL, I-KW, C-CL, C-HL, W-HH, C-HK. Writing (review and editing): S-YL, C-LL, I-KW, C-CL, C-HL, W-HH, C-HK. Visualisation: S-YL, C-LL, I-KW, C-CL, C-HL, W-HH, C-HK. Supervision: C-HK. Project administration: C-HK. Funding acquisition: C-HK.
Funding: This work was supported by grants from the Ministry of Health and Welfare, Taiwan (MOHW107-TDU-B-212-123004), China Medical University Hospital; Academia Sinica Stroke Biosignature Project (BM10701010021); MOST Clinical Trial Consortium for Stroke (MOST 106-2321-B-039-005-); Tseng-Lien Lin Foundation, Taichung, Taiwan;and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. No additional external funding received for this study.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: None declared.
Patient consent: Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval: The Research Ethics Committee of China Medical University and Hospital in Taiwan approved the study (CMUH104-REC2-115-CR2).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The data set used in this study is held by the Taiwan Ministry of Health and Welfare (MOHW). The Ministry of Health and Welfare must approve our application to access this data. Any researcher interested in accessing this data set can submit an application form to the Ministry of Health and Welfare requesting access. Please contact the staff of MOHW (email: stcarolwu@mohw.gov.tw) for further assistance. Taiwan Ministry of Health and Welfare address: No 488, Sec 6, Zhongxiao E Rd, Nangang Dist, Taipei City 115, Taiwan (ROC). Phone: +886-2-8590-6848. All relevant data are within the paper.
References
- 1.Wimo A1, Jönsson L, Bond J, et al. . Alzheimer Disease International. The worldwide economic impact of dementia 2010. Alzheimers Dement 2013;9:1–11. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Dementia: a public health priority. http://www.who.int/mental_health/publications/dementia_report_2012/en/
- 3.Prince M, Bryce R, Albanese E, et al. . The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75. 10.1016/j.jalz.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 4.Irvine GB, El-Agnaf OM, Shankar GM, et al. . Protein aggregation in the brain: the molecular basis for Alzheimer’s and Parkinson’s diseases. Mol Med 2008;14:1–64. 10.2119/2007-00100.Irvine [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spillantini MG, Murrell JR, Goedert M, et al. . Mutation in the tau gene in familial multiple system tauopathy with presenile dementia. Proc Natl Acad Sci U S A 1998;95:7737–41. 10.1073/pnas.95.13.7737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aliev G, Smith MA, Obrenovich ME, et al. . Role of vascular hypoperfusion-induced oxidative stress and mitochondria failure in the pathogenesis of Azheimer disease. Neurotox Res 2003;5:491–504. 10.1007/BF03033159 [DOI] [PubMed] [Google Scholar]
- 7.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006;443:787–95. 10.1038/nature05292 [DOI] [PubMed] [Google Scholar]
- 8.Thal DR, Capetillo-Zarate E, Del Tredici K, et al. . The development of amyloid beta protein deposits in the aged brain. Sci Aging Knowledge Environ 2006;2006:re1 10.1126/sageke.2006.6.re1 [DOI] [PubMed] [Google Scholar]
- 9.Kelly JW. Structural biology: proteins downhill all the way. Nature 2006;442:255–6. 10.1038/442255a [DOI] [PubMed] [Google Scholar]
- 10.Knowles TP, Vendruscolo M, Dobson CM. The amyloid state and its association with protein misfolding diseases. Nat Rev Mol Cell Biol 2014;15:384–96. 10.1038/nrm3810 [DOI] [PubMed] [Google Scholar]
- 11.McLean CA, Cherny RA, Fraser FW, et al. . Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol 1999;46:860–6. [DOI] [PubMed] [Google Scholar]
- 12.Bachhuber T, Katzmarski N, McCarter JF, et al. . Inhibition of amyloid-β plaque formation by α-synuclein. Nat Med 2015;21:802–7. 10.1038/nm.3885 [DOI] [PubMed] [Google Scholar]
- 13.Pan-Montojo F, Anichtchik O, Dening Y, et al. . Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 2010;5:e8762 10.1371/journal.pone.0008762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan-Montojo F, Schwarz M, Winkler C, et al. . Environmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci Rep 2012;2:898 10.1038/srep00898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston D, Wilkinson AR. Highly selective vagotomy without a drainage procedure in the treatment of duodenal ulcer. Br J Surg 1970;57:289–96. 10.1002/bjs.1800570414 [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson AR, Johnston D. Effect of truncal, selective and highly selective vagotomy on gastric emptying and intestinal transit of a food-barium meal in man. Ann Surg 1973;178:190–3. 10.1097/00000658-197308000-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu B, Fang F, Pedersen NL, et al. . Vagotomy and Parkinson disease: A Swedish register-based matched-cohort study. Neurology 2017;88:1996–2002. 10.1212/WNL.0000000000003961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svensson E, Horváth-Puhó E, Thomsen RW, et al. . Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 2015;78:522–9. 10.1002/ana.24448 [DOI] [PubMed] [Google Scholar]
- 19. Database NHIR. 2015. http://nhird.nhri.org.tw/en/index.html
- 20.Peng YC, Lin CL, Yeh HZ, et al. . Diverticular disease and additional comorbidities associated with increased risk of dementia. J Gastroenterol Hepatol 2016;31:1816–22. 10.1111/jgh.13389 [DOI] [PubMed] [Google Scholar]
- 21.Chen YT, Su JS, Tseng CW, et al. . Inflammatory bowel disease on the risk of acute pancreatitis: A population-based cohort study. J Gastroenterol Hepatol 2016;31:782–7. 10.1111/jgh.13171 [DOI] [PubMed] [Google Scholar]
- 22.Parsons LS, Seattle W; Ovation Research Group,. Performing a 1:N Case-Control Match on Propensity Score. SUGI 2009;29:165. [Google Scholar]
- 23.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons LS, Group OR, Seattle W. Performing a 1:N Case-Control Match on Propensity Score. SUGI 2001;29:165–29. [Google Scholar]
- 25.Phillips RJ, Walter GC, Wilder SL, et al. . Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson’s disease? Neuroscience 2008;153:733–50. 10.1016/j.neuroscience.2008.02.074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braak H, Rüb U, Gai WP, et al. . Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 2003;110:517–36. 10.1007/s00702-002-0808-2 [DOI] [PubMed] [Google Scholar]
- 27.Masliah E, Rockenstein E, Veinbergs I, et al. . Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 2000;287:1265–9. 10.1126/science.287.5456.1265 [DOI] [PubMed] [Google Scholar]
- 28.Visanji NP, Brooks PL, Hazrati LN, et al. . The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol Commun 2013;1:2 10.1186/2051-5960-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borovikova LV, Ivanova S, Zhang M, et al. . Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 2000;405:458–62. 10.1038/35013070 [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Wang C, Tang C, et al. . Successful treatment of severe sepsis and diarrhea after vagotomy utilizing fecal microbiota transplantation: a case report. Crit Care 2015;19:37 10.1186/s13054-015-0738-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cryan JF, Dinan TG. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 2012;13:701–12. 10.1038/nrn3346 [DOI] [PubMed] [Google Scholar]
- 32.Brenner SR. Blue-green algae or cyanobacteria in the intestinal micro-flora may produce neurotoxins such as Beta-N-Methylamino-L-Alanine (BMAA) which may be related to development of amyotrophic lateral sclerosis, Alzheimer’s disease and Parkinson-Dementia-Complex in humans and Equine Motor Neuron Disease in horses. Med Hypotheses 2013;80:103 10.1016/j.mehy.2012.10.010 [DOI] [PubMed] [Google Scholar]
- 33.Schwartz K, Boles BR. Microbial amyloids-functions and interactions within the host. Curr Opin Microbiol 2013;16:93–9. 10.1016/j.mib.2012.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dubois B, Feldman HH, Jacova C, et al. . Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol 2007;6:734–46. 10.1016/S1474-4422(07)70178-3 [DOI] [PubMed] [Google Scholar]
- 35.Chi NF, Chien LN, Ku HL, et al. . Alzheimer disease and risk of stroke: a population-based cohort study. Neurology 2013;80:705–11. 10.1212/WNL.0b013e31828250af [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


