Abstract
Objective
Carbohydrate staples such as pasta have been implicated in the obesity epidemic. It is unclear whether pasta contributes to weight gain or like other low-glycaemic index (GI) foods contributes to weight loss. We synthesised the evidence of the effect of pasta on measures of adiposity.
Design
Systematic review and meta-analysis using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Data sources
MEDLINE, Embase, CINAHL and the Cochrane Library were searched through 7 February 2017.
Eligibility criteria for selecting studies
We included randomised controlled trials ≥3 weeks assessing the effect of pasta alone or in the context of low-GI dietary patterns on measures of global (body weight, body mass index (BMI), body fat) and regional (waist circumference (WC), waist-to-hip ratio (WHR), sagittal abdominal diameter (SAD)) adiposity in adults.
Data extraction and synthesis
Two independent reviewers extracted data and assessed risk of bias. Data were pooled using the generic inverse-variance method and expressed as mean differences (MDs) with 95% CIs. Heterogeneity was assessed (Cochran Q statistic) and quantified (I2 statistic). GRADE assessed the certainty of the evidence.
Results
We identified no trial comparisons of the effect of pasta alone and 32 trial comparisons (n=2448 participants) of the effect of pasta in the context of low-GI dietary patterns. Pasta in the context of low-GI dietary patterns significantly reduced body weight (MD=−0.63 kg; 95% CI −0.84 to –0.42 kg) and BMI (MD=−0.26 kg/m2; 95% CI −0.36 to –0.16 kg/m2) compared with higher-GI dietary patterns. There was no effect on other measures of adiposity. The certainty of the evidence was graded as moderate for body weight, BMI, WHR and SAD and low for WC and body fat.
Conclusions
Pasta in the context of low-GI dietary patterns does not adversely affect adiposity and even reduces body weight and BMI compared with higher-GI dietary patterns. Future trials should assess the effect of pasta in the context of other ‘healthy’ dietary patterns.
Trial registration number
NCT02961088; Results.
Keywords: body weight, pasta, glycemic index, glycaemic index, systematic review and meta-analysis, weight loss
Strengths and limitations of this study.
The present systematic review and meta-analysis was based on a comprehensive search and included a large number of randomised controlled trials which provide the best protection against bias.
We used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to evaluate the strength and quality of the evidence.
There was evidence of unexplained inconsistency among the trial estimates for waist circumference and body fat.
The generalisability of our results is called into question for all body weight and adiposity outcomes, as the available trials only assessed pasta in the context of low-glycaemic index dietary patterns (none assessed the effect of pasta alone or in the context of other dietary patterns and most did not quantify the amount of pasta consumed).
Introduction
As the role of saturated fat in chronic disease has been called into question, carbohydrates have come under attack in the media,1 2 popular books,3–9 statements of health advocacy groups10 and commentaries in leading medical journals.11 12 Much of the attention has focused on sugars, but traditional carbohydrate staples like pasta, rice and breads are increasingly being implicated in the epidemics of overweight and obesity.2 7 Although systematic reviews and meta-analyses of randomised controlled trials (RCTs) of dietary patterns that include these foods but are low in glycaemic index (GI),13 14 high in whole grains15 16 and/or high in dietary fibre have shown advantages for weight-related outcomes,17 18 there has been a general lack of recognition of the importance of carbohydrate quality.
Pasta is an important example of a food that is considered a refined carbohydrate but has a low GI, a property that has been exploited extensively in studies of low-GI dietary patterns. It remains unclear whether pasta alone or in the context of a low-GI dietary pattern shares the advantages of other low-GI foods or on the contrary contributes to weight gain. We are not aware of any systematic reviews and meta-analyses that have synthesised the evidence of the effect of pasta on body weight outcomes. We undertook a systematic review and meta-analysis of RCTs using the GRADE approach to quantify the effect of pasta alone or in the context of low-GI dietary patterns on body weight and measures of adiposity relevant to the prevention and management of overweight and obesity.
Methods
Design
Our protocol followed the guideline of the Cochrane Handbook for Systematic Reviews of Interventions,19 and findings are reported according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses20 (online supplementary table S1). The protocol is registered at ClinicalTrials.gov (identifier, NCT02961088; Results).
bmjopen-2017-019438supp001.pdf (1.2MB, pdf)
Data sources and searches
We searched MEDLINE (http://www.nlm.nih.gov/bsd/pmresources.html), Embase (https://www.embase.com), CINAHL (https://health.ebsco.com/products/the-cinahl-database) and the Cochrane Library (http://www.cochranelibrary.com/) from inception through 7 February 2017. The full search terms used in this study are presented in online supplementary tables S2–S3. Briefly, we searched using variations of the terms pasta and glycaemic index and glycaemic load and body weight and body mass index (BMI). The search was limited to human studies and had no language restrictions. Reference lists of selected studies and reviews were also searched to identify additional articles.
Study selection
We included RCTs that investigated the effect of pasta consumed alone or in the context of low-GI dietary patterns that emphasised pasta in comparison with higher-GI diets that did not include pasta on body weight or other measures of global (BMI, body fat) or abdominal (waist circumference, waist-to-hip ratio, sagittal abdominal diameter or visceral adipose tissue as assessed by imaging modalities) adiposity in participants of all health backgrounds. Trials were included if the intervention arm assessed the effect of pasta consumed alone or assessed the effect of a low-GI diet that emphasised pasta as part of the low-GI dietary advice. Trials were excluded if they had diet duration of <3 weeks, did not intend to use a calorie-matched and macronutrient-matched comparator arm that was higher in GI, included pregnant or breastfeeding women or children, or did not provide suitable end-point data. When multiple publications existed for the same study, the article with the most information was included (n=6). Published abstracts were not included.
Data extraction
Two reviewers (LC, CRB) assessed the titles and abstracts of all identified studies and independently reviewed and extracted relevant data from each report, including study design, blinding, sample size, participant characteristics, follow-up duration, identification of pasta in the low-GI diet only, comparator diet, macronutrient profile, funding source and outcome data.
In those trials where the data were included in figures and not provided numerically, we extracted data using the software program Plot Digitizer V.2.6.8 (http://plotdigitizer.sourceforge.net/), a Java program that digitises scanned figures of X and Y plots from GIF, JPEG or PNG image file formats and allows one to calibrate the X and Y axes for the estimation data points. Additional information was requested from the authors of all included trials. Disagreement were resolved by consensus or where necessary by a third author (SBM).
Risk of bias assessment
Risk of bias for each included trial was assessed independently using the Cochrane Risk of Bias tool by the two reviewers.19 Assessment was done across 5 domains of bias (sequence generation, allocation concealment, blinding, incomplete outcome data and selective reporting) and assessed. The risk of bias was assessed as either low (proper methods taken to reduce bias), high (improper methods creating bias) or unclear (insufficient information provided to determine the bias level).
Outcomes
The primary outcome was body weight, and secondary outcomes included markers of global (BMI, body fat) and abdominal (waist circumference, waist to-hip ratio, sagittal abdominal diameter or visceral adipose tissue assessed by imaging modalities) adiposity. Change-from baseline differences were preferred over end differences and expressed as Mean±SD. When not provided, between-treatment differences in change-from-baseline or end differences were calculated by subtracting means, and SDs were calculated from the available data and statistics using published formulas.19 If there was insufficient information available to allow for these calculations, then missing SDs were imputed with the use of the pooled SD from other studies included in the analysis.19
Statistical analysis
Data analyses were conducted using Review Manager (RevMan) V.5.3 (Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014) for primary analyses and Stata V.13 (College Station, TX: StataCorp LP) for subgroup analyses. A generic inverse-variance method with random-effects models was used to calculate pooled mean differences and 95% CIs. Random-effects models were used even in the absence of statistically significant interstudy heterogeneity, as they yield more conservative summary effect estimates in the presence of residual heterogeneity. Paired analyses were applied to all cross-over trials with the use of a within-individual correlation coefficient between treatments of 0.5 as described by Elbourne et al.21
Interstudy heterogeneity was assessed by the Cochran Q statistic, where P <0.10 was considered statistically significant, and quantified by the I2 statistic, where I2 ≥50% indicates substantial heterogeneity.19 Sensitivity analyses were performed, which included the removal of each single study from the meta-analyses one at a time and recalculation of the summary effect. An influential study was considered a study whose removal changed the magnitude of the pooled effect by >10%. Sensitivity analyses were also conducted using different correlation coefficient values for cross-over trials (0.25 and 0.75) to test for the robustness of the effect size, conducting analyses using fixed-effects models and restricting analyses to those trials for which pasta intake could be quantified.
If ≥10 trial comparisons were available, then sources of heterogeneity were explored by subgroup analyses. A priori categorical subgroups analyses were assessed by meta-regression analyses. These included patient type (normal body weight, overweight or obese (average baseline BMI >27 kg/m2), diabetes, coronary heart disease), follow-up (<24 weeks, ≥24 weeks), baseline BMI (BMI ≤30, >30 kg/m2), design (parallel, cross-over), energy balance (negative on both arms (weight loss diets), neutral on both arms (weight maintaining diets)) and dose of pasta (based on the median). A priori categorical subgroup analyses also included the following dietary factors: GI (absolute level (≤55, >55; glucose scale), within-treatment change, between-treatment change), fat intake (absolute level (<30%, ≥30% energy), within-treatment change, between-treatment change), carbohydrate intake (absolute level (<50%, ≥50% energy), within-treatment change, between-treatment change), protein intake (absolute level (<20%, ≥20% energy), within-treatment change, between-treatment change), dietary fibre intake (absolute level (<28 g/day, ≥28 g/day), within-treatment change, between-treatment change) and risk of bias. A priori continuous meta-regression analyses were conducted on the absolute levels and within-treatment and between-treatment changes of these same dietary factors in the intervention arms of pasta in the context of low-GI dietary patterns. Linear and non-linear pasta intake dose–response analyses were assessed by using continuous meta-regression analyses and spline curve modelling (MKSPLINE procedure), respectively. Publication bias was assessed by visual inspection of funnel plots and the Egger22 and Begg23 tests, when ≥10 trial comparisons were available. If publication bias was suspected, then adjustment for funnel plot asymmetry was done by imputing missing study data using the Duval and Tweedie trim-and-fill method.24
Grading the evidence
The grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach was used to assess the certainty of the evidence.25 Evidence was graded as high, moderate, low or very low quality. The included RCTs were graded as high-quality evidence by default and downgraded based on prespecified criteria. Criteria to downgrade evidence included risk of bias (weight of studies show risk of bias assessed by the Cochrane Risk of Bias tool), inconsistency (substantial unexplained heterogeneity, I2>50%, P<0.10), indirectness (presence of factors that limited the generalisability of the results), imprecision (the 95% CI for effect estimates were wide or crossed prespecified minimally important differences (MIDs) for harm) and publication bias (significant evidence of small-study effects).
Patient involvement
No patients were directly involved in the development of the research question, selection of the outcome measures, design and implementation of the study, or interpretation of the results.
Results
Search results
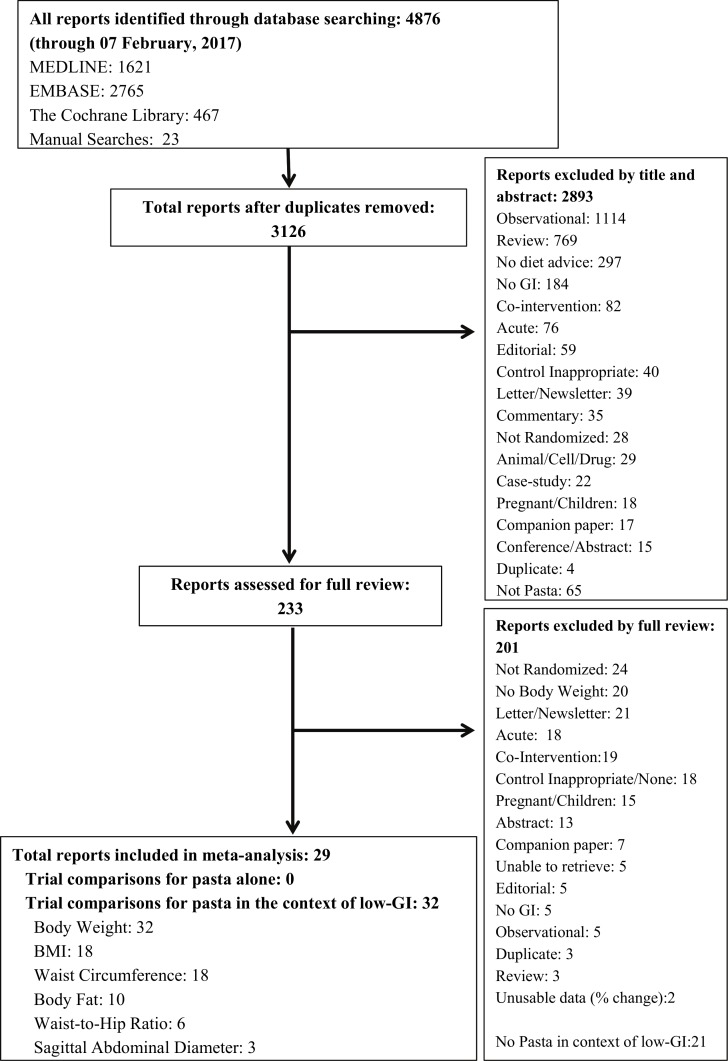
Figure 1 shows the flow of the literature. We identified 4876 reports of which 29 met eligibility criteria. No reports were identified that assessed the effect of pasta alone, while 29 reports (including 32 trial comparisons involving 2448 participants) were identified that assessed the effect of pasta in the context of low-GI dietary patterns on any adiposity outcome in adults.26–54 Of the 32 trial comparisons that assessed the effect of pasta in the context of a low-GI dietary pattern, there were 32 trial comparisons for body weight, 18 trial comparisons for BMI,27 28 31–33 35 36 39–41 43–46 48 49 52 53 18 trial comparisons for waist circumference,27 28 31 33 34 36 38–40 42 44–47 52 53 10 trial comparisons for body fat,27 28 31–33 36 38 41 43 53 6 trial comparisons for waist-to-hip ratio31 39 40 44 45 52 and 3 trial comparisons for sagittal abdominal diameter.34 39 There was only one trial comparison identified35 for visceral adipose tissue as assessed by imaging modalities, thus a meta-analysis could not be undertaken for this outcome.
Figure 1.
Literature search. BMI, body mass index; GI, glycaemic index.
Trial characteristics
Table 1 and online supplementary table S4 show the characteristics of all included trials of the effect of pasta in the context of low-GI dietary patterns. The majority of trials had a parallel design (26/32) with a median follow-up of 12 weeks (IQR 9–21 weeks) and a median number of participants per trial of 43 (IQR 21–112). Most participants were middle aged (median age, 50 years; IQR 40–58 years) men and women (median ratio of men to women, 0.4:1 in available trials). The median baseline BMI across studies was 30.4 kg/m2 (IQR 28.2–32.0). Regarding metabolic phenotype, 21 (66%) trials included participants who were overweight or obese (had a baseline BMI ≥27 kg/m2), 10 (31%) had diabetes and one trial (3%) with coronary heart disease (CHD). We did not retrieve any trials where participants had a normal BMI at baseline (≤25 kg/m2), although six trials did not include BMI >25 kg/m2 as part of criteria, the average baseline BMI was ≥27 kg/m2, therefore categorised as overweight.
Table 1.
Summary of trial characteristics
| Trial characteristics* | All | Neutral energy balance | Negative energy balance |
| Trial no (n) | 32 | 23 | 9 |
| Trial size (total, range) | 2448 (8–250) | 1989 (8–250) | 459 (13–123) |
| Male:female† (%) | 40:60 | 47:53 | 27:73 |
| Age (years) | 50 (40–58) | 52.0 (42.1–59.5) | 49.5 (34.2–53.0) |
| Metabolic phenotype (OW/OB:DM:CHD) (%) | 66:31:3 | 57:39:4 | 89:11:0 |
| Setting (IP:OP) (%) | 3:97 | 4:96 | 0:100 |
| Baseline body weight (kg)‡ | 85.5 (80.0–91.9) | 84.1 (79.5–87.5) | 92.5 (86.1–93.9) |
| Baseline BMI (kg/m2)§ | 30.4 (28.2–32.0) | 29.5 (27.4–31.4) | 31.7 (30.1–32.9) |
| Study design (C:P) (%) | 19:81 | 26:74 | 0:100 |
| Dose pasta (servings/week)¶ | 3.3 (2.3–3.5) | 3.4 (2.9–4.1) | 2.3 (2.3–3.5) |
| GI in pasta/LGI group | 49.0 (44.0–55.1) | 46.5 (49.9–55.5) | 44.0 (42.3–49.4) |
| GI in higher-GI group | 62.5 (61.6–63.2) | 63.3 (60.1–64.4) | 61.0 (59.2–66.6) |
| Calorie reduction in pasta/LGI group (kcal)** | −179 (−90 to −448) | −165 (−74 to −313) | −447 (−134 to −594) |
| Calorie reduction in higher-GI group (kcal)** | −181 (−93 to −401) | −160 (−40 to −248) | −470 (−172 to −561) |
| Feeding control (Met:Suppl:DA) (%) | 6:44:50 | 4:48:48 | 11:33:56 |
| Follow-up duration (weeks) | 12 (9–21) | 12 (6–24) | 12 (10–21) |
| Funding sources (A:I:AI:NR) (%) |
47:9:25:19 | 44:13:26:17 | 56:0:22:22 |
*Median (IQR), unless otherwise indicated.
†24/32 trials provided data on sex.
‡30/32 trials reported baseline body weight.
§28/32 trials reported baseline BMI.
¶11/32 trials provided data from which dose could be approximated.
**20/32 trials provided data from which to approximate changes in caloric intake.
A, agency; AI, agency and industry; BMI, body mass index; C, cross-over design; CHD, coronary heart disease; DA, dietary advice; DM, diabetes; GI, glycaemic index; I, industry; IP, inpatient; LGI, low glycaemic index; Met, metabolic; NR, not reported; OB, obese; OP, outpatient; OW, overweight; P, parallel design; Suppl, supplemented/provision of certain food.
Risk of bias
Online supplementary figures S1 and S2 show the individual Cochrane Risk of Bias tool assessments for each of the included trials of the effect of pasta in the context of low-GI dietary patterns. No serious risk of bias was detected.
Effect of pasta in the context of low-GI dietary patterns and body weight
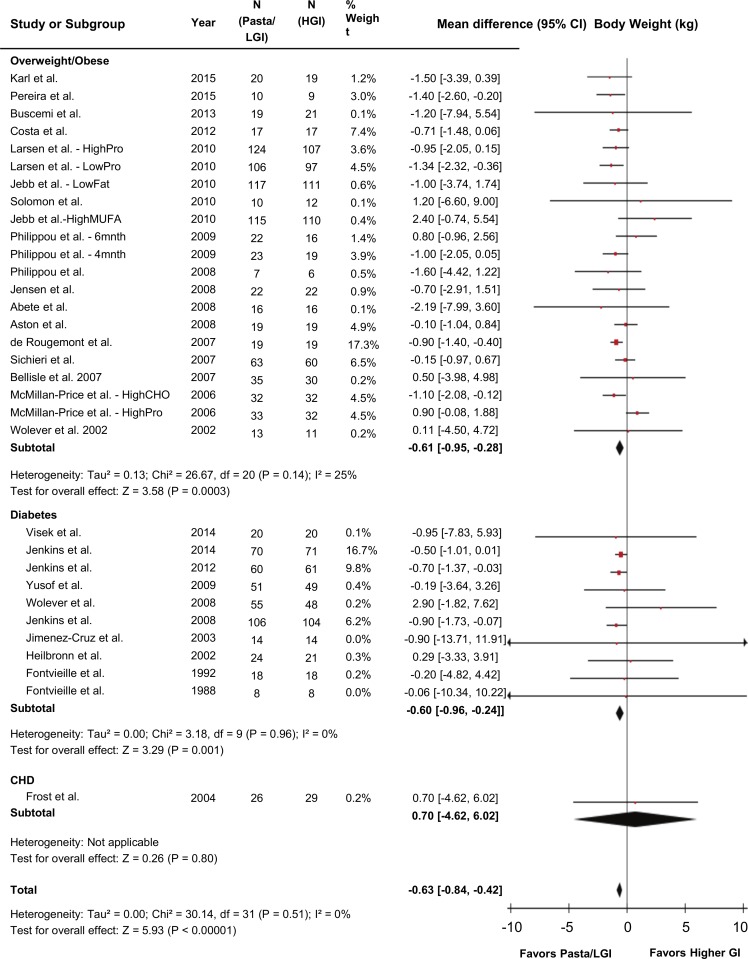
Figure 2 shows the effect of pasta in the context of low-GI dietary patterns on the primary outcome body weight. Pooled analyses showed pasta in the context of low-GI dietary patterns had the effect of reducing body weight by −0.63 kg (95% CI −0.84 to –0.42 kg; P<0.001) compared with higher-GI control diets with no evidence of heterogeneity (I2=0%, P-heterogeneity=0.51).
Figure 2.
Forest plot of randomised controlled trials investigating the effects of pasta in the context of low-GI dietary patterns on body weight (kg). n=2448. Data are expressed as mean differences represented by a square and 95% CIs by the line through the square. 95% CIs exceeding the plot’s bounds are represented by an arrowhead. Pooled effect estimates are represented by diamonds and were estimated with the use of generic inverse variance random effects models. Between-study heterogeneity was assessed by the Cochran Q statistic, where P<0.10 is considered statistically significant, and quantified by the I2 statistic, where I2≥50% is considered evidence of substantial heterogeneity. CHD, coronary heart disease; CHO, carbohydrate; GI, glycaemic index; HGI, higher-glycaemic index diet; LGI, low-glycaemic index diet; MUFA, monounsaturated fatty acid; Pro, protein.
Effect of pasta in the context of low-GI dietary patterns on markers of global adiposity
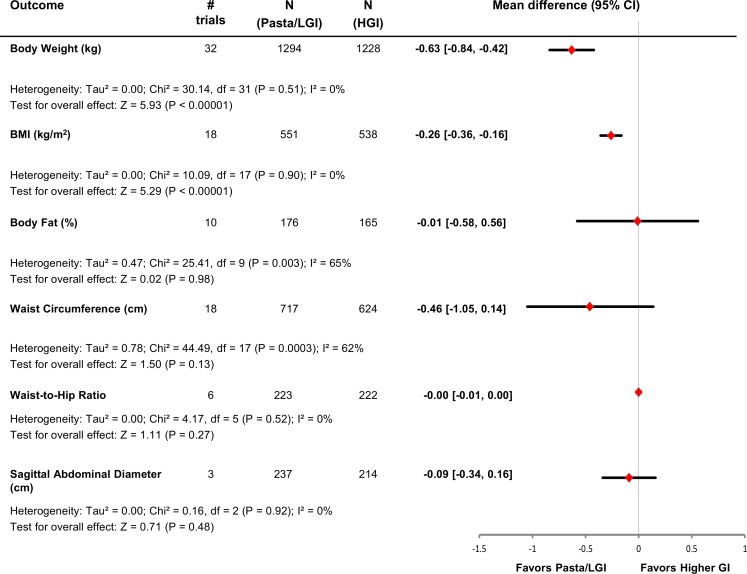
Figure 3 and online supplementary figures S3–S4 show the effect of pasta in the context of low-GI dietary patterns on markers of global adiposity. Pooled analyses showed that pasta in the context of low-GI dietary patterns had the effect of reducing BMI by −0.26 kg/m2 (n=18 trials; MD=−0.26 kg/m2; 95% CI −0.36 to –0.16 kg/m2; P<0.001) compared with higher-GI control diets with no evidence of heterogeneity (I2=0%, P-heterogeneity=0.90). There was no effect on body fat (n=10 trials; MD=−0.01%; 95% CI −0.58% to 0.56%; P=0.98) with evidence of substantial heterogeneity (I2=65%, P-heterogeneity <0.01).
Figure 3.
Plot of the pooled effect estimates from randomised controlled trials investigating the effects of pasta in the context of low-GI dietary patterns on global and abdominal markers of adiposity. Pooled effect estimates are represented by diamonds and were estimated with the use of generic inverse-variance random-effects models. Between-study heterogeneity was assessed by the Cochran Q statistic, where P<0.10 is considered statistically significant, and quantified by the I2 statistic where I2 ≥50% is considered evidence of substantial heterogeneity. BMI, body mass index; GI, glycaemic index; HGI, higher-glycaemic index diet; LGI, low-glycaemic index diet.
Effect of pasta in the context of low-GI dietary patterns on markers of abdominal adiposity
Figure 3 and online supplementary figures S5–S7 show the pooled estimates for the markers of abdominal adiposity. Pooled analyses did not show a significant effect of pasta in the context of low-GI dietary patterns compared with higher-GI control diets on waist circumference (n=18 trials, MD=−0.46 cm, 95% CI −1.05 to 0.14 cm; P=0.13), waist-to-hip ratio (n=6 trials, MD=−0.00, 95% CI −0.01 to 0.00; P=0.27) or sagittal abdominal diameter (n=3 trials, MD=−0.09 cm, 95% CI −0.34 to 0.16 cm; P=0.48). There was only evidence of substantial heterogeneity in the analyses for waist circumference (I2=62%, P-heterogeneity <0.01).
Sensitivity analyses
We conducted four sets of sensitivity analyses (online supplementary tables S5–6, S8–9). The systematic removal of each trial did not modify the direction or significance of the effect estimates or the evidence of heterogeneity for any of the outcomes with the exception of waist circumference (online supplementary table S5). In the sensitivity analysis for waist circumference, two studies were influential studies in that their removal altered the magnitude of the pooled effect in the remaining studies by >10%, where the removal of the studies of McMillan-Price et al (high protein comparison)42 and Jenkins et al44 rendered the results for waist circumference statistically significant (MD=−0.62 cm, 95% CI −1.19 to –0.05, P=0.03 and MD=−0.61 cm, 95% CI −1.18 to –0.04, P=0.04, respectively; forest plots not shown). Heterogeneity remained significant in both cases (I2=55%, P-heterogeneity <0.01 and I2=50%, P-heterogeneity=0.01, respectively). Sensitivity analyses using correlation coefficients of 0.25 and 0.75 for paired analyses of cross-over trials also did not modify the results (online supplementary table S6). In the sensitivity analyses where fixed-effects models were applied (online supplementary figure S8), the direction, magnitude and significance of the pooled estimates were very similar to those produced by the random-effects models with the exception of the sensitivity analysis for waist circumference, which was significant (MD=−0.62, 95% CI −0.93 to –0.32; P<0.001). Finally, restricting analyses to the 11 trials for which pasta intake could be quantified (median pasta intake, 3.33 servings/week (range, 1.75–7 servings/week)) showed a similar reduction in body weight (MD=−0.70 kg, 95% CI −1.10 to –0.29 kg; P<0.001) when pasta was consumed in the context of low-GI dietary patterns compared with the higher-GI control arms without evidence of heterogeneity (I2=0%, P-heterogeneity=0.68) (online supplementary figure S9).
Subgroup analyses
We were only able to conduct a priori categorical and continuous subgroup analyses for body weight, BMI, body fat and waist circumference. Subgroup analyses for waist-to-hip ratio and sagittal abdominal diameter could not be assessed, owing to <10 trial comparisons in each case. Online supplementary figures S10–S12 show the categorical a priori subgroup analyses for body weight. There was no evidence of significant effect modification in any of the subgroup analyses for body weight, including no effect modification of follow-up when comparing studies less than 24 weeks’ duration to those greater than or equal to 24 weeks (−0.63 kg vs −0.57 kg, respectively) (online supplementary figure S10). Neither was there evidence of significant effect modification in any of the subgroup analyses for BMI, body fat or waist circumference (online supplementary figures S13–20).
Online supplementary table S7 and figures S21–S22 show the continuous subgroup analyses for body weight. There was evidence of significant effect modification by carbohydrate and protein intake, where an increase in carbohydrate intake in the intervention group in which pasta was consumed in the context of low-GI dietary patterns was associated with weight loss (β=−0.07, 95% CI −0.12 to –0.01, I2=0.00%, P=0.02), and an increase in protein intake in the intervention group in which pasta was consumed in the context of low-GI dietary patterns was associated with weight gain (β=0.15, 95% CI 0.03 to 0.27, I2=0.00%, P=0.02). None of the other continuous subgroup analyses were significant. There was no evidence of significant effect modification in any of the continuous subgroup analyses for BMI (online supplementary table S8). For body fat, there was evidence of significant effect modification in the continuous meta-regression subgroup analysis of difference in GI between intervention and control groups, where greater difference in GI between the groups was associated with greater reduction in body fat in the intervention group (β=−0.09, 95% CI −0.15 to –0.03, I2=19.39%, P=0.01) (online supplementary table S9). None of the other continuous subgroup analyses were significant. For waist circumference, there was evidence of significant effect modification in the continuous meta-regression subgroup analysis of absolute carbohydrate level and absolute protein level, where greater carbohydrate level in the intervention group in which pasta was consumed in the context of low-GI dietary patterns was associated with greater loss in waist circumference (β=−0.11, 95% CI −0.19 to –0.04, I2=27.06%, P<0.01) and a lower protein level in the intervention group in which pasta was consumed in the context of low-GI dietary patterns was associated with an increase in waist circumference (β=0.20, 95% CI 0.01 to 0.38, I2=43.92%, P=0.04) (online supplementary table S10). None of the other continuous subgroup analyses were significant.
Dose-response analyses
Online supplementary tables S7, S11 and figure S23 show the dose-response analysis for the 11 trials for which pasta intake could be quantified. No evidence of a linear dose response was seen for pasta intake by meta-regression analyses (online supplementary table S8). There was also no evidence of a non-linear dose response by MKSPLINE (P=0.85) (online supplementary figure S23) or piecewise linear meta-regression analyses (online supplementary table S11).
Publication bias
Online supplementary figures S24–S27 shows the funnel plots for body weight, BMI, body fat and waist circumference. There was no evidence of funnel-plot asymmetry. Formal testing with the Egger and Begg tests did not show evidence of small-study effects (P>0.05 for both). Publication bias was not assessed for waist-to-hip ratio and sagittal abdominal diameter, owing to <10 trial comparisons.
GRADE assessment
Online supplementary table S12 shows a summary of the GRADE assessments for the effect of pasta in the context of low-GI dietary patterns on body weight and measures of adiposity. The evidence was graded as moderate for body weight, BMI, waist-to-hip ratio and sagittal abdominal diameter, owing to a downgrade for indirectness, and low for waist circumference and body fat, owing to downgrades for indirectness and inconsistency (I2=59%, P-heterogeneity <0.001; I2=66%, P-heterogeneity <0.01, respectively).
Discussion
The present systematic review and meta-analysis was undertaken to quantify the effect of pasta alone and pasta in the context of low-GI dietary patterns on body weight and other markers of adiposity. We failed to identify any trial comparisons for the effect of pasta alone but did identify 32 trial comparisons for the effect of pasta in the context of low-GI dietary patterns in 2448 participants who were predominantly middle-aged and overweight or obese. The primary pooled analysis demonstrated that pasta in the context of low-GI dietary patterns did not contribute to weight gain, resulting in a significant weight loss of −0.63 kg when compared with diets higher in GI over a median follow-up of 12 weeks. The lack of harm was reflected in the established clinical secondary outcome measures of global (BMI and body fat) and abdominal adiposity (waist circumference, waist-to-hip ratio and sagittal abdominal diameter). These findings were robust across subgroups. The findings did not differ by metabolic phenotype in those who were overweight or obese or had diabetes, which is noteworthy since these are populations who would benefit from weight management strategies. There was also no effect modification by the energy balance of the design such that the weight loss was seen even under conditions of neutral energy balance (in which participants were instructed to consume dietary advice ad libitum), suggesting that encouragement of the consumption of pasta in the context of a low-GI dietary patterns does not cause harm and may even lead to spontaneous weight loss. There was also no effect modification by follow-up either in continuous meta-regression or categorical, where the 24 trials with <24 weeks’ follow-up had a weight reduction similar to those eight trials with ≥24 weeks’ follow-up (−0.63 kg vs −0.57 kg, respectively). This finding is of particular relevance since many dietary studies are successful in demonstrating weight loss in the short term but not over the long term.
Findings in the context of existing studies
We are not aware of any RCTs directly assessing the effect of pasta intake on any health parameters including body weight. Our findings, however, agree with earlier systematic reviews and meta-analyses of RCTs of the effect of low-GI dietary patterns irrespective of pasta intake on body weight and adiposity. The systematic review and meta-analysis by Thomas et al in 2007 found a significant −1.1 kg weight loss and −1.3 kg/m2 reduction in BMI favouring low-GI or glycaemic load (GL) diets compared with control diets in six RCTs of 5 weeks to 6 months in duration in overweight or obese individuals.13 Another systematic review and meta-analysis by Schwingshackl and Hoffmann in 2013 found a −0.62 kg weight loss favouring low-GI/GL diets compared with higher GI/GL diets in 14 RCTs with greater than 6 months’ duration in overweight individuals (BMI >25 kg/m2).14
Our findings also agree with trials in which pasta was emphasised in the context of other healthy dietary patterns. One trial done in children in Spain given Mediterranean dietary advice which included increasing the intake of pasta found that approximately 11.3% of the participants in the Mediterranean diet group who were classified as overweight and obese changed their weight status to normal weight compared with only approximately 2.6% of the participants in the control group.55
Other lines of evidence from observational studies have demonstrated benefits of pasta consumption on body weight and adiposity. Pasta intake was recently assessed in the Moli-sani study and the Italian Nutrition and Health Survey, a cross-sectional study of over 20 000 Italians from all over Italy.56 The study demonstrated that higher pasta consumption was associated with lower BMI, waist circumference and waist-to-hip ratio and with a lower prevalence of overweight and obesity.56 Furthermore, greater pasta intake was associated with better adherence to the Mediterranean diet, a dietary pattern which has a demonstrated cardiovascular benefit.57 Similar associations between greater pasta intake and lower body weight have also been observed in prospective US cohorts.58 A pooled analysis of the three Harvard cohorts also showed that higher-GI diets (which would preclude pasta) were associated with weight gain.59
Although the product form of pasta can vary widely, including in shape (eg, macaroni, spaghetti, linguine), ingredients (eg, type of wheat, egg content) and processing technique (eg, drying temperature), studies have demonstrated that when comparing pastas varying in these parameters, despite slight variations in glycaemic response among pastas, glycaemic responses are still lower compared with a control, for example, white bread.60 61 One concern of the choice of pasta as a carbohydrate food is that is it a refined food low in fibre. Although there are whole-grain pasta options available, studies have demonstrated that fibre added to pasta does not significantly affect the glucose or insulin response, the secretion of gut hormones or satiety.62 63 Furthermore, pasta has a similar GI compared with many fibre-rich carbohydrates, including barley, legumes and steel cut oats, and still a lower GI compared with other fibre-rich foods including whole-wheat bread, breakfast cereals like bran flakes and potatoes with skin.64 The typically consumed white wheat pasta also has a higher micronutrient content compared with other white wheat products like bread since it contains the aleurone layer, which is preserved as a result of the use of harder wheats (durum wheat); even when durum wheats are used in breads, pasta retains a lower glycaemic response primarily because of the processing techniques used in pasta making, which give pasta a compact structure and reduced starch hydrolysis.61
The mechanism by which pasta in the context of low-GI dietary patterns lead to weight loss even under conditions of ad libitum dietary advice is unclear. Lower-GI diets may result in greater body weight reduction compared with higher-GI diets because lower-GI foods have been shown to be more satiating65 and delay hunger and decrease subsequent energy intake.13 Low-GI dietary patterns are also characterised by high fibre content,64 66 which may contribute to improvements in satiety and hunger.17 Furthermore, studies that have compared ad libitum low-GI dietary patterns with standard energy-restricted low-fat diets have demonstrated similar or better weight loss with low-GI dietary patterns, despite the fact that participants were free to consume as much as they desired.13 67 Thus, voluntary energy intake may be lower after low-GI meals, as has been previously demonstrated.68
Strengths and limitations
The strengths of the present systematic review and meta-analysis include that it is comprehensive, includes RCTs, a design which provides the best protection against bias, and uses the GRADE approach to evaluate the quality of evidence. Additionally, a large number of trials were identified (32 trials) for the primary outcome of body weight; the median follow-up period was 12 weeks, which allows for the assessment of a moderate duration of intervention; none of the trials were rated as having a serious risk of bias; and there was no evidence of publication bias.
There are several limitations. First, we downgraded the certainty of the evidence for serious inconsistency in the treatment estimates across trials for some of the outcomes assessed. There was evidence of unexplained heterogeneity in waist circumference (I2=62%) and in body fat (I2=65%). Although the inconsistency in these outcomes may have related to measurement error69 in the different techniques for measuring waist circumference and body fat, we were unable to conduct sensitivity or subgroup analyses to explore this source of heterogeneity. Second, we downgraded the certainty of the evidence for serious indirectness. Most of the available trials did not quantify the amount of pasta consumed in the context of the low-GI dietary patterns. Although sensitivity analyses in which analyses were restricted to the 11 trials that did quantify (providing a median 3.33 servings/week) pasta intake did not meaningfully alter our estimates (−0.70 kg vs −0.63 kg), it is difficult to quantify the effect of pasta in these diets. There is also the question of indirectness in the translation to other background diets. None of the available trials evaluated the effect of pasta alone or in the context of other dietary patterns. Whether the observed effect of pasta in the context of low-GI dietary patterns will hold in the context of other healthy dietary patterns, such as Mediterranean and vegetarian dietary patterns, is unclear. Although there is no biological reason to doubt that the findings would hold across different dietary patterns, there was no direct evidence to support this conclusion. If the question had been asked from the perspective of benefit as opposed to that of harm, then the moderate duration of the included trials might be another reason to downgrade for serious indirectness. In the absence of long-term trials (>1-year diet duration), it is difficult to conclude with certainty that there is a sustainable weight loss benefit of consuming pasta. Finally, there was some evidence of imprecision for benefit but not harm. Whereas the 95% CI of the pooled estimates did not overlap with our prespecified MID for harm (that is, they did not contain evidence for harm) and so were not downgraded for imprecision, the upper bound of the 95% CI did overlap with the lower margin of the same MID to assess the precision of the evidence for benefit for some outcomes.
Balancing these strengths and limitations, the GRADE approach assessed the overall certainty of the available evidence as moderate for the primary outcome of body weight and the secondary outcomes of BMI, waist-to-hip ratio and sagittal abdominal diameter, owing to downgrades for indirectness. The evidence was assessed as low for the other secondary outcomes of body fat and waist circumference, owing to downgrades for indirectness and inconsistency.
Implications
These results are important given the negative messages with which the public has been inundated regarding carbohydrates, messages which appear to be influencing their food choices, as evidenced by recent reductions in carbohydrate intake,70–72 especially in pasta intake.70 73–76 Contrary to these concerns, the available evidence shows that when pasta is consumed in the context of low-GI dietary patterns, there is no weight gain but rather marginally clinically significant weight loss (>0.5 kg).77
Although we were able to approximate the amount of pasta consumed in one-third of included trials, it is unclear what the effect of pasta would be in the context of other dietary patterns. A Low-GI dietary pattern, however, shares many similarities with a Mediterranean dietary pattern, which emphasises many low-GI foods and has demonstrated a cardiovascular benefit.57
Current clinical practice guidelines already suggest the replacement of high-GI foods with low-GI foods for improvement of glycaemic control and cardiovascular risk factors.78 79 The present evidence means that pasta may be highlighted as an important example of a low-GI food that can contribute to a low-GI dietary pattern, a pattern which in turn may potentially improve cardiometabolic risk without an adverse effect on weight control.
Conclusions
In conclusion, the available evidence from RCTs does not allow us to conclude that pasta consumed in the context of low-GI dietary patterns has an adverse effect on body weight and adiposity outcomes of importance in the prevention and management of overweight and obesity. On the contrary, pasta in the context of low-GI dietary patterns reduces body weight and BMI compared with higher-GI dietary patterns. The results are generalisable in the context of a high carbohydrate dietary pattern composed of low-GI foods with or without the intention of weight loss in middle-aged individuals who are overweight or obese or have diabetes. Although the clinical significance of the observed weight loss is debatable, this finding increases our confidence that pasta in the context of low-GI dietary patterns does not result in weight gain. Further research is needed to improve our estimates. There is also a need for more randomised trials of >1-year diet duration to clarify whether the lack of harm for pasta in the context of low-GI dietary patterns will translate into meaningful long-term benefits. Other randomised trials should focus on whether pasta will have similar effects in the context of other ‘healthy’ dietary patterns such as a Mediterranean diet.
Supplementary Material
Acknowledgments
We wish to thank Teruko Kishibe, an information specialist in the Scotiabank Health Sciences Library, St Michael’s Hospital, for her help in the development of search terms used. Aspects of this work were presented at the Società Italiana di Nutrizione Umana (SINU) Congress in Parma, Italy, on 12 April 2017 and at the 35th International Symposium on Diabetes and Nutrition, of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD) in Skagen, Denmark, on 20–21 June 2017.
Footnotes
Contributors: All authors had full access to all of the data (including statistical reports and tables) in this study and take full responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: CWCK, JLS. Analysis and interpretation of the data: LC, CWCK, CRB, SBM, LAL, DJAJ, JLS. Drafting of the article: LC, JLS. Critical revision of the article for important intellectual content: LC, CWCK, CRB, SBM, LAL, DJAJ, JLS. Final approval of the article: LC, CWCK, CRB, SBM, LAL, DJAJ, JLS. Obtaining of funding: CWCK, DJAJ, JLS. Administrative, technical or logistic support: SBM. Collection and assembly of data: LC, CRB, SBM. Guarantor: JLS. Transparency declaration: As guarantor, JLS affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Funding: This work was funded by the Canadian Institutes of Health Research (funding reference no. 129920) through the Canada-wide Human Nutrition Trialists’ Network (NTN). The Diet, Digestive tract, and Disease (3D) Centre, funded through the Canada Foundation for Innovation (CFI) and the Ministry of Research and Innovation’s Ontario Research Fund (ORF), provided the infrastructure for the conduct of this project. LC was funded by a Toronto 3D Postdoctoral Fellowship Award. DJAJ was funded by the Government of Canada through the Canada Research Chair Endowment. JLS was funded by a PSI Graham Farquharson Knowledge Translation Fellowship, Canadian Diabetes Association (CDA) Clinician Scientist award, CIHR INMD/CNS New Investigator Partnership Prize, and Banting and Best Diabetes Centre Sun Life Financial New Investigator Award.
Disclaimer: None of the sponsors had a role in any aspect of the present study, including design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review, approval of the manuscript or decision to publish.
Competing interests: All authors have completed the Unified Competing Interest form (available on request from the corresponding author) and declare: LC has worked as a clinical research coordinator at Glycaemic Index Laboratories, Toronto, Ontario, Canada. CWCK has received research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), Almond Board of California, American Pistachio Growers, Barilla, California Strawberry Commission, Calorie Control Council, Canadian Institutes of Health Research (CIHR), Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, Pulse Canada, Saskatchewan Pulse Growers and Unilever. He has received in-kind research support from the Almond Board of California, California Walnut Council, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Kellogg Canada, WhiteWave Foods. He has received travel support and/or honoraria from the American Peanut Council, American Pistachio Growers, Barilla, Bayer, California Walnut Commission, Canola Council of Canada, General Mills, International Tree Nut Council, Loblaw Brands Ltd, Nutrition Foundation of Italy, Oldways Preservation Trust, Orafti, Paramount Farms, Peanut Institute, Pulse Canada, Sabra Dipping Co., Saskatchewan Pulse Growers, Sun-Maid, Tate & Lyle, Unilever and White Wave Foods. He has served on the scientific advisory board for the International Tree Nut Council, McCormick Science Institute, Oldways Preservation Trust, Paramount Farms and Pulse Canada. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. DJAJ has received research grants from Saskatchewan Pulse Growers, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd., Unilever, Barilla, the Almond Board of California, Agriculture and Agri-food Canada, Pulse Canada, Kellogg’s Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd., Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit (INC), Soy Foods Association of North America, the Coca-Cola Company (investigator initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, the Canola and Flax Councils of Canada, the Calorie Control Council (CCC), the CIHR, the Canada Foundation for Innovation and the Ontario Research Fund. He has received in-kind supplies for trial as a research support from the Almond Board of California, Walnut Council of California, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Kellogg Canada, and WhiteWave Foods. He has been on the speaker’s panel, served on the scientific advisory board and/or received travel support and/or honoraria from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system, the Coca-Cola Company, EPICURE, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, True Health Initiative, Institute of Food Technologists (IFT), Saskatchewan Pulse Growers, Sanitarium Company, Orafti, the Almond Board of California, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamental for Health, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, the Coca-Cola Company, the Griffin Hospital, Abbott Laboratories, the Canola Council of Canada, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi-Bred International, DuPont Nutrition and Health, Spherix Consulting and WhiteWave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, the Nutritional Fundamentals for Health, Agri-Culture and Agri-Food Canada, the Canadian Agri-Food Policy Institute, Pulse Canada, the Saskatchewan Pulse Growers, the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), Nutra-Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael’s Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), the American Society of Nutrition (ASN), Arizona State University, Paolo Sorbini Foundation and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the United States Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association (CDA). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, ALJ, is a director and partner of Glycemic Index Laboratories, Inc., and his sister received funding through a grant from the St. Michael’s Hospital Foundation to develop a cookbook for one of his studies. JLS has received research support from the Canadian Institutes of health Research (CIHR), Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), Canadian Nutrition Society (CNS), American Society for Nutrition (ASN), Calorie Control Council, INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, and The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers). He has received in-kind research support from the Almond Board of California, California Walnut Commission, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Kellogg Canada, WhiteWave Foods. He has received travel support, speaker fees and/or honoraria from Diabetes Canada, Canadian Nutrition Society (CNS), Mott’s LLP, Dairy Farmers of Canada, Sprim Brasil, WhiteWave Foods, Rippe Lifestyle, mdBriefcase, Alberta Milk, FoodMinds LLC, Memac Ogilvy & Mather LLC, PepsiCo, The Ginger Network LLC, International Sweeteners Association, Nestlé Nutrition Institute, Pulse Canada, Canadian Society for Endocrinology and Metabolism (CSEM), Barilla Centre for Food and Nutrition (BCFN) Foundation, and GI Foundation. He has ad hoc consulting arrangements with Winston & Strawn LLP, Perkins Coie LLP, and Tate & Lyle. He is a member of the European Fruit Juice Association Scientific Expert Panel. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Canadian Obesity Network. He serves as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of the International Life Science Institute (ILSI) North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of Unilever Canada. No competing interests were declared by CRB, SBM and LAL.
Patient consent: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data available.
References
- 1.Malhotra A. Eating MORE fat while cutting carbs and quitting sugar can help you lose weight and be happier, says top cardiologist: Mail Online, 2016. [Google Scholar]
- 2.Taubes G. Diet advice that ignores hunger. Sunday Review ed: The New York Times, 2015. [Google Scholar]
- 3.Ludwig D. Always hungry? Conquer cravings, retrain your fat cells and lose weight permanetly. New York: Grand Central Life & Style, 2016. [Google Scholar]
- 4.Taubes G. Why we get fat and what to do about it. New York: Alfred A Knopf, 2011. [Google Scholar]
- 5.Atkins R. Dr Atkins’ diet revolution: the high calorie way to stay thin forever: Bantam Books, 1973. [Google Scholar]
- 6.Perlmutter D. Grain Brain: the surprising truth about wheat, carbs, and sugar—your brain’s silent killers: Little, Brown and Company, 2013. [Google Scholar]
- 7.David W. Wheat belly: HarperCollins, 2012. [Google Scholar]
- 8.Cordain L. The Paleo Diet revised: lose weight and get healthy by eating the foods you were designed to eat: Houghton Mifflin Harcourt, 2010. [Google Scholar]
- 9.Teicholz N. The big fat surprise: why butter, meat and cheese belong in a healthy diet: Simon and Schuster, 2014. [Google Scholar]
- 10.National Obesity Forum, The Public Health Collaboration. Eat fat, cut the carbs and avoid snacking to reverse obesity and type 2 diabetes. http://www.nationalobesityforum.org.uk/index.php/136-news_/746-%E2%80%9Ceat-fat,-cut-the-carbs-and-avoid-snacking-to-reverse-obesity-and-type-2-diabetes-%E2%80%9D.html (accessed 05 Mar 2018).
- 11.Malhotra A. Saturated fat is not the major issue. BMJ 2013;347:f6340 10.1136/bmj.f6340 [DOI] [PubMed] [Google Scholar]
- 12.Malhotra A. Author’s reply to Lichtenstein, Tedstone and Pyne, Mann and colleagues, Lim, and Clifton. BMJ 2013;347:f6855 10.1136/bmj.f6855 [DOI] [PubMed] [Google Scholar]
- 13.Thomas DE, Elliott EJ, Baur L. Low glycaemic index or low glycaemic load diets for overweight and obesity. Cochrane Database Syst Rev 2007:CD005105 10.1002/14651858.CD005105.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis 2013;23:699–706. 10.1016/j.numecd.2013.04.008 [DOI] [PubMed] [Google Scholar]
- 15.Pol K, Christensen R, Bartels EM, et al. Whole grain and body weight changes in apparently healthy adults: a systematic review and meta-analysis of randomized controlled studies. Am J Clin Nutr 2013;98:872–84. 10.3945/ajcn.113.064659 [DOI] [PubMed] [Google Scholar]
- 16.Harland JI, Garton LE. Whole-grain intake as a marker of healthy body weight and adiposity. Public Health Nutr 2008;11:554–63. 10.1017/S1368980007001279 [DOI] [PubMed] [Google Scholar]
- 17.Slavin JL. Dietary fiber and body weight. Nutrition 2005;21:411–8. 10.1016/j.nut.2004.08.018 [DOI] [PubMed] [Google Scholar]
- 18.Dahl WJ, Stewart ML. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J Acad Nutr Diet 2015;115:1861–70. 10.1016/j.jand.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Green S, Cochrane handbook for systematic reviews of interventions [internet]. Version 5.1.0: The Cochrane Collaboration, 2011. www.cochrane-handbook.org (accessed 23 Sep 2015). [Google Scholar]
- 20.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 21.Elbourne DR, Altman DG, Higgins JPT, et al. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol 2002;31:140–9. 10.1093/ije/31.1.140 [DOI] [PubMed] [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB. A measure to aid in the interpretation of published clinical trials. Stat Med 1985;4:1–9. 10.1002/sim.4780040103 [DOI] [PubMed] [Google Scholar]
- 24.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455–63. 10.1111/j.0006-341X.2000.00455.x [DOI] [PubMed] [Google Scholar]
- 25.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 26.Jebb SA, Lovegrove JA, Griffin BA, et al. Effect of changing the amount and type of fat and carbohydrate on insulin sensitivity and cardiovascular risk: the RISCK (Reading, Imperial, Surrey, Cambridge, and Kings) trial. Am J Clin Nutr 2010;92:748–58. 10.3945/ajcn.2009.29096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Philippou E, Bovill-Taylor C, Rajkumar C, et al. Preliminary report: the effect of a 6-month dietary glycemic index manipulation in addition to healthy eating advice and weight loss on arterial compliance and 24-hour ambulatory blood pressure in men: a pilot study. Metabolism 2009;58:1703–8. 10.1016/j.metabol.2009.05.026 [DOI] [PubMed] [Google Scholar]
- 28.Philippou E, McGowan BM, Brynes AE, et al. The effect of a 12-week low glycaemic index diet on heart disease risk factors and 24 h glycaemic response in healthy middle-aged volunteers at risk of heart disease: a pilot study. Eur J Clin Nutr 2008;62:145–9. 10.1038/sj.ejcn.1602688 [DOI] [PubMed] [Google Scholar]
- 29.Sichieri R, Moura AS, Genelhu V, et al. An 18-mo randomized trial of a low-glycemic-index diet and weight change in Brazilian women. Am J Clin Nutr 2007;86:707–13. [DOI] [PubMed] [Google Scholar]
- 30.Wolever TMS, Mehling C. High-carbohydrate–low-glycaemic index dietary advice improves glucose disposition index in subjects with impaired glucose tolerance. Br J Nutr 2002;87:477–87. 10.1079/BJN2002568 [DOI] [PubMed] [Google Scholar]
- 31.Pereira EV, Costa JA, Alfenas RC. Effect of glycemic index on obesity control. Arch Endocrinol Metab 2015;59:245–51. 10.1590/2359-3997000000045 [DOI] [PubMed] [Google Scholar]
- 32.Karl JP, Roberts SB, Schaefer EJ, et al. Effects of carbohydrate quantity and glycemic index on resting metabolic rate and body composition during weight loss. Obesity 2015;23:2190–8. 10.1002/oby.21268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buscemi S, Cosentino L, Rosafio G, et al. Effects of hypocaloric diets with different glycemic indexes on endothelial function and glycemic variability in overweight and in obese adult patients at increased cardiovascular risk. Clin Nutr 2013;32:346–52. 10.1016/j.clnu.2012.10.006 [DOI] [PubMed] [Google Scholar]
- 34.Larsen TM, Dalskov SM, van Baak M, et al. Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 2010;363:2102–13. 10.1056/NEJMoa1007137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Solomon TP, Haus JM, Kelly KR, et al. A low-glycemic index diet combined with exercise reduces insulin resistance, postprandial hyperinsulinemia, and glucose-dependent insulinotropic polypeptide responses in obese, prediabetic humans. Am J Clin Nutr 2010;92:1359–68. 10.3945/ajcn.2010.29771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Philippou E, Neary NM, Chaudhri O, et al. The effect of dietary glycemic index on weight maintenance in overweight subjects: a pilot study. Obesity 2009;17:396–401. 10.1038/oby.2008.533 [DOI] [PubMed] [Google Scholar]
- 37.Abete I, Parra D, Martinez JA. Energy-restricted diets based on a distinct food selection affecting the glycemic index induce different weight loss and oxidative response. Clin Nutr 2008;27:545–51. 10.1016/j.clnu.2008.01.005 [DOI] [PubMed] [Google Scholar]
- 38.Aston LM, Stokes CS, Jebb SA. No effect of a diet with a reduced glycaemic index on satiety, energy intake and body weight in overweight and obese women. Int J Obes 2008;32:160–5. 10.1038/sj.ijo.0803717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jensen L, Sloth B, Krog-Mikkelsen I, et al. A low-glycemic-index diet reduces plasma plasminogen activator inhibitor-1 activity, but not tissue inhibitor of proteinases-1 or plasminogen activator inhibitor-1 protein, in overweight women. Am J Clin Nutr 2008;87:97–105. [DOI] [PubMed] [Google Scholar]
- 40.Bellisle F, Dalix AM, De Assis MA, et al. Motivational effects of 12-week moderately restrictive diets with or without special attention to the Glycaemic Index of foods. Br J Nutr 2007;97:790–8. 10.1017/S0007114507450309 [DOI] [PubMed] [Google Scholar]
- 41.de Rougemont A, Normand S, Nazare JA, et al. Beneficial effects of a 5-week low-glycaemic index regimen on weight control and cardiovascular risk factors in overweight non-diabetic subjects. Br J Nutr 2007;98:1288–98. 10.1017/S0007114507778674 [DOI] [PubMed] [Google Scholar]
- 42.McMillan-Price J, Petocz P, Atkinson F, et al. Comparison of 4 diets of varying glycemic load on weight loss and cardiovascular risk reduction in overweight and obese young adults: a randomized controlled trial. Arch Intern Med 2006;166:1466–75. 10.1001/archinte.166.14.1466 [DOI] [PubMed] [Google Scholar]
- 43.Visek J, Lacigova S, Cechurova D, et al. Comparison of a low-glycemic index vs standard diabetic diet. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2014;158:112–6. 10.5507/bp.2012.103 [DOI] [PubMed] [Google Scholar]
- 44.Jenkins DJ, Kendall CW, Vuksan V, et al. Effect of lowering the glycemic load with canola oil on glycemic control and cardiovascular risk factors: a randomized controlled trial. Diabetes Care 2014;37:1806–14. 10.2337/dc13-2990 [DOI] [PubMed] [Google Scholar]
- 45.Jenkins DJ, Kendall CW, Augustin LS, et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: a randomized controlled trial. Arch Intern Med 2012;172:1653–60. 10.1001/2013.jamainternmed.70 [DOI] [PubMed] [Google Scholar]
- 46.Yusof BN, Talib RA, Kamaruddin NA, et al. A low-GI diet is associated with a short-term improvement of glycaemic control in Asian patients with type 2 diabetes. Diabetes Obes Metab 2009;11:387–96. 10.1111/j.1463-1326.2008.00984.x [DOI] [PubMed] [Google Scholar]
- 47.Wolever TM, Gibbs AL, Mehling C, et al. The Canadian Trial of Carbohydrates in Diabetes (CCD), a 1-y controlled trial of low-glycemic-index dietary carbohydrate in type 2 diabetes: no effect on glycated hemoglobin but reduction in C-reactive protein. Am J Clin Nutr 2008;87:114–25. [DOI] [PubMed] [Google Scholar]
- 48.Jenkins DJ, Kendall CW, McKeown-Eyssen G, et al. Effect of a low-glycemic index or a high-cereal fiber diet on type 2 diabetes: a randomized trial. JAMA 2008;300:2742–53. 10.1001/jama.2008.808 [DOI] [PubMed] [Google Scholar]
- 49.Jimenez-Cruz A, Bacardi-Gascon M, Turnbull WH, et al. A flexible, low-glycemic index Mexican-style diet in overweight and obese subjects with type 2 diabetes improves metabolic parameters during a 6-week treatment period. Diabetes Care 2003;26:1967–70. 10.2337/diacare.26.7.1967 [DOI] [PubMed] [Google Scholar]
- 50.Heilbronn LK, Noakes M, Clifton PM. The effect of high- and low-glycemic index energy restricted diets on plasma lipid and glucose profiles in type 2 diabetic subjects with varying glycemic control. J Am Coll Nutr 2002;21:120–7. 10.1080/07315724.2002.10719204 [DOI] [PubMed] [Google Scholar]
- 51.Fontvieille AM, Acosta M, Rizkalla SW, et al. A moderate switch from high to low glycemic-index foods for 3 weeks improves metabolic control of type I (IDDM) diabetic subjects. Diabetes Nutr Metab 1988;1:139–43. [Google Scholar]
- 52.Frost GS, Brynes AE, Bovill-Taylor C, et al. A prospective randomised trial to determine the efficacy of a low glycaemic index diet given in addition to healthy eating and weight loss advice in patients with coronary heart disease. Eur J Clin Nutr 2004;58:121–7. 10.1038/sj.ejcn.1601758 [DOI] [PubMed] [Google Scholar]
- 53.de Assis Costa J, de Cássia Gonçalves Alfenas R. The consumption of low glycemic meals reduces abdominal obesity in subjects with excess body weight. Nutr Hosp 2012;27:1178–83. 10.3305/nh.2012.27.4.5845 [DOI] [PubMed] [Google Scholar]
- 54.Fontvieille AM, Rizkalla SW, Penfornis A, et al. The use of low glycaemic index foods improves metabolic control of diabetic patients over five weeks. Diabet Med 1992;9:444–50. 10.1111/j.1464-5491.1992.tb01815.x [DOI] [PubMed] [Google Scholar]
- 55.Bibiloni MDM, Fernández-Blanco J, Pujol-Plana N, et al. [Improving diet quality in children through a new nutritional education programme: INFADIMED]. Gac Sanit 2017;31 10.1016/j.gaceta.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 56.Pounis G, Castelnuovo AD, Costanzo S, et al. Association of pasta consumption with body mass index and waist-to-hip ratio: results from Moli-sani and INHES studies. Nutr Diabetes 2016;6:e218 10.1038/nutd.2016.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Estruch R, Ros E, Salas-Salvadó J, et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med 2013;368:1279–90. 10.1056/NEJMoa1200303 [DOI] [PubMed] [Google Scholar]
- 58.Shay CM, Van Horn L, Stamler J, et al. Food and nutrient intakes and their associations with lower BMI in middle-aged US adults: the International Study of Macro-/Micronutrients and Blood Pressure (INTERMAP). Am J Clin Nutr 2012;96:483–91. 10.3945/ajcn.111.025056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith JD, Hou T, Ludwig DS, et al. Changes in intake of protein foods, carbohydrate amount and quality, and long-term weight change: results from 3 prospective cohorts. Am J Clin Nutr 2015;101:1216–24. 10.3945/ajcn.114.100867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Granfeldt Y, Björck I. Glycemic response to starch in pasta: a study of mechanisms of limited enzyme availability. J Cereal Sci 1991;14:47–61. 10.1016/S0733-5210(09)80017-9 [DOI] [Google Scholar]
- 61.Granfeldt Y, Bjorck I, Hagander B. On the importance of processing conditions, product thickness and egg addition for the glycaemic and hormonal responses to pasta: a comparison with bread made from ’pasta ingredients'. Eur J Clin Nutr 1991;45:489–99. [PubMed] [Google Scholar]
- 62.Frost GS, Brynes AE, Dhillo WS, et al. The effects of fiber enrichment of pasta and fat content on gastric emptying, GLP-1, glucose, and insulin responses to a meal. Eur J Clin Nutr 2003;57:293–8. 10.1038/sj.ejcn.1601520 [DOI] [PubMed] [Google Scholar]
- 63.Korczak R, Timm D, Ahnen R, et al. High protein pasta is not more satiating than high fiber pasta at a lunch meal, nor does it decrease mid-afternoon snacking in healthy men and women. J Food Sci 2016;81:S2240–S2245. 10.1111/1750-3841.13406 [DOI] [PubMed] [Google Scholar]
- 64.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diabetes Care 2008;31:2281–3. 10.2337/dc08-1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ludwig DS. Dietary glycemic index and obesity. J Nutr 2000;130:280S–3. 10.1093/jn/130.2.280S [DOI] [PubMed] [Google Scholar]
- 66.Riccardi G, Rivellese AA, Giacco R. Role of glycemic index and glycemic load in the healthy state, in prediabetes, and in diabetes. Am J Clin Nutr 2008;87:269S–74. [DOI] [PubMed] [Google Scholar]
- 67.Saris WH, Astrup A, Prentice AM, et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: the CARMEN study. The Carbohydrate Ratio Management in European National diets. Int J Obes Relat Metab Disord 2000;24:1310–8. [DOI] [PubMed] [Google Scholar]
- 68.Ludwig DS, Majzoub JA, Al-Zahrani A, et al. High glycemic index foods, overeating, and obesity. Pediatrics 1999;103:e26 10.1542/peds.103.3.e26 [DOI] [PubMed] [Google Scholar]
- 69.Wang J, Thornton JC, Bari S, et al. Comparisons of waist circumferences measured at 4 sites. Am J Clin Nutr 2003;77:379–84. 10.1093/ajcn/77.2.379 [DOI] [PubMed] [Google Scholar]
- 70.Makarem N, Scott M, Quatromoni P, et al. Trends in dietary carbohydrate consumption from 1991 to 2008 in the Framingham Heart Study Offspring Cohort. Br J Nutr 2014;111:2010–23. 10.1017/S0007114513004443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rehm CD, Peñalvo JL, Afshin A, et al. Dietary intake among US adults, 1999–2012. JAMA 2016;315:2542–53. 10.1001/jama.2016.7491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Golzarand M, Mirmiran P, Jessri M, et al. Dietary trends in the Middle East and North Africa: an ecological study (1961 to 2007). Public Health Nutr 2012;15:1835–44. 10.1017/S1368980011003673 [DOI] [PubMed] [Google Scholar]
- 73.Leclercq C, Arcella D, Piccinelli R, et al. The Italian National Food Consumption Survey INRAN-SCAI 2005–06: main results in terms of food consumption. Public Health Nutr 2009;12:2504–32. 10.1017/S1368980009005035 [DOI] [PubMed] [Google Scholar]
- 74.Bonaccio M, Di Castelnuovo A, Bonanni A, et al. Decline of the Mediterranean diet at a time of economic crisis. Results from the Moli-sani study. Nutr Metab Cardiovasc Dis 2014;24:853–60. 10.1016/j.numecd.2014.02.014 [DOI] [PubMed] [Google Scholar]
- 75.Sofi F, Innocenti G, Dini C, et al. Low adherence of a clinically healthy Italian population to nutritional recommendations for primary prevention of chronic diseases. Nutr Metab Cardiovasc Dis 2006;16:436–44. 10.1016/j.numecd.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 76.Ferdman RA. This is what happens when everyone is terrified of carbs: The Washington Post 2015, 2015. [Google Scholar]
- 77.Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA 2014;312:923–33. 10.1001/jama.2014.10397 [DOI] [PubMed] [Google Scholar]
- 78.Stone NJ, Robinson JG, Lichtenstein AH, et al. ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2013;2014:S1–45. [DOI] [PubMed] [Google Scholar]
- 79.Anderson TJ, Grégoire J, Hegele RA, et al. 2012 update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol 2013;29:151–67. 10.1016/j.cjca.2012.11.032 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2017-019438supp001.pdf (1.2MB, pdf)