Abstract
Heroin and oxycodone abuse occurs over a wide range of drug doses and by various routes of administration characterized by differing rates of drug absorption. The current study addressed the efficacy of a heroin vaccine [morphine hapten conjugated to keyhole limpet hemocyanin (M-KLH)] or oxycodone vaccine [oxycodone hapten conjugated to keyhole limpet hemocyanin (OXY-KLH)] for reducing drug distribution to brain after intravenous heroin or oxycodone, or subcutaneous oxycodone. Rats immunized with M-KLH or keyhole limpet hemocyanin (KLH) control received an intravenous bolus dose of 0.26 or 2.6 mg/kg heroin. Vaccination with M-KLH increased retention of heroin and its active metabolites 6-acetylmorphine (6-AM) and morphine in plasma compared with KLH controls, and reduced total opioid (heroin + 6-AM + morphine) distribution to brain but only at the lower heroin dose. Immunization also protected against respiratory depression at the lower heroin dose. Rats immunized with OXY-KLH or KLH control received 0.22 or 2.2 mg/kg oxycodone intravenously, the molar equivalent of the heroin doses. Immunization with OXY-KLH significantly reduced oxycodone distribution to brain after either oxycodone dose, although the magnitude of effect of immunization at the higher oxycodone dose was small (12%). By contrast, vaccination with OXY-KLH was more effective when oxycodone was administered subcutaneously rather than intravenously, reducing oxycodone distribution to brain by 44% after an oxycodone dose of 2.3 mg/kg. Vaccination also reduced oxycodone-induced antinociception. These data suggest that the efficacy of OXY-KLH and M-KLH opioid vaccines is highly dependent upon opioid dose and route of administration.
Introduction
Opioid use disorders are a public health burden affecting over 30 million people worldwide (United Nations Office on Drugs and Crime, 2016). In the United States, over 2.5 million people are dependent on heroin and prescription opioids and opioid use disorder was associated with over 33,000 overdose deaths in 2015 (Paulozzi, 2012; National Survey on Drug Use and Health, 2014; Rudd et al., 2016; United Nations Office on Drugs and Crime, 2016; Dowell et al., 2017). Medications to treat opioid abuse are available and effective, but less than 30% of individuals with opioid use disorder are receiving them (National Survey on Drug Use and Health, 2014). Treatment with the opioid receptor agonists methadone and buprenorphine is complicated due to their abuse liability, potential diversion, and strict administrative regulations (Rosenberg and Phillips, 2003; Appel et al., 2004). Treatment with the opioid antagonist naltrexone can complicate pain management and requires detoxification prior to initiation of treatment. Immunization against abused opioids is being considered as an alternative or complementary option to pharmacotherapy. Opioid vaccines act by producing antibodies that bind the targeted opioid in blood and extracellular fluid, and by reducing their distribution to brain. The potential advantages of vaccination over current pharmacotherapies include being long-acting, non-addictive, and devoid of the side effects associated with opioid receptor ligands.
Therapeutic vaccines for heroin and prescription opioid abuse have shown efficacy in a wide range of preclinical models, demonstrating that immunization can reduce opioid distribution to the brain and attenuate opioid-related behaviors including self-administration, locomotor activation, and analgesia in mice, rats, and non-human primates (Bonese et al., 1974; Anton and Leff, 2006; Li et al., 2011, 2014; Stowe et al., 2011; Pravetoni et al., 2012b,c, 2013, 2014a; Kosten et al., 2013; Raleigh et al., 2013, 2014; Schlosburg et al., 2013; Laudenbach et al., 2015; Bremer et al., 2017). However, vaccine efficacy is often tested in animals using immunization protocols involving routes of administration (e.g., intraperitoneal) and adjuvants (e.g., Freund’s complete adjuvant) that are not used in humans, or at opioid doses that are suitable for the animal models chosen but are at the lower end of the range that may be abused by humans. In addition, many studies employ only a single opioid dose size such that the impact of opioid dose on vaccine efficacy is difficult to assess.
The primary goal of this study was to compare the efficacy of two vaccines directed against heroin [morphine hapten conjugated to keyhole limpet hemocyanin (M-KLH)] or oxycodone [oxycodone hapten conjugated to keyhole limpet hemocyanin (OXY-KLH)] in rats challenged with either a small or a large intravenous dose of the targeted opioid. The intravenous route for opioid dosing was examined because this is a common route of administration for abused heroin and occasionally for oxycodone as well. The intravenous route also represents the most rapid means of drug delivery and therefore the most rigorous test of vaccine efficacy. The highest opioid dose used (2.6 mg/kg) was chosen because it is within the range reportedly abused via the intravenous route by humans (Oviedo-Joekes et al., 2010), and this was contrasted with an opioid dose one-tenth of that. For oxycodone, the subcutaneous route was also examined because oxycodone is most commonly abused by the oral route, which is characterized by slower drug absorption than the intravenous route. The oral route was not used to study oxycodone because its oral bioavailability in rats is low (Chan et al., 2008). The current study showed marked opioid dose-dependent efficacy for both M-KLH and OXY-KLH vaccines, as well as greater efficacy of OXY-KLH after subcutaneous rather than intravenous oxycodone dosing.
Materials and Methods
Animals
Male Holtzman rats (Harlan Laboratories, Madison, WI) weighing between 325 and 350 g at arrival were double housed under a 12/12-hour standard light/dark cycle and free fed. Testing occurred during the light phase. These studies were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Animal protocols were approved by the Minneapolis Medical Research Foundation Animal Care and Use Committee. Surgery was performed under ketamine (75 mg/kg) and dexmedetomidine (0.05 mg/kg) anesthesia, animals were euthanized by CO2 inhalation using Association for Assessment and Accreditation of Laboratory Animal Care approved chambers, and all efforts were made to minimize suffering.
Drugs
Oxycodone and heroin were obtained through the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD) or Sigma-Aldrich (St. Louis, MO). Drug doses and concentrations are expressed as the weight of the base.
Vaccines against Heroin and Oxycodone
The study tested the efficacy of two analogous vaccines consisting of morphine or oxycodone-based haptens conjugated via a tetraglycine linker to keyhole limpet hemocyanin (KLH) and adsorbed to alum (Pravetoni et al., 2012b,c; Raleigh et al., 2013). Experiments 1 and 2 used vaccines consisting of opioid haptens conjugated to native (didecamer) KLH (Thermo Fisher Scientific, Waltham, MA); experiment 3 used a vaccine consisting of an oxycodone hapten conjugated to KLH subunit dimer (Stellar Biotechnologies, Port Hueneme, CA) because of greater ease of formulation. We have found that native and dimer KLH conjugates of oxycodone elicit equivalent antibody responses and comparable effects on opioid distribution (Pravetoni et al., 2014b). M-KLH generates antibodies specific for heroin, 6-acetylmorphine (6-AM), morphine, and morphine-6-glucuronide, but do not appreciably cross react with methadone, buprenorphine, naloxone, naltrexone, oxycodone, or leu-enkephalin (Raleigh et al., 2013). OXY-KLH generates antibodies specific for oxycodone and oxymorphone, but do not appreciably cross react with naloxone, naltrexone, methadone, buprenorphine, or leu-enkephalin (Pravetoni et al., 2012b).
Experimental Design
Experiment 1: M-KLH Vaccine Efficacy Following Intravenous Heroin Dosing.
Groups of eight rats each were vaccinated intramuscularly with 25 μg of M-KLH (groups 1–3) or unconjugated KLH (groups 4–6) with 0.5 mg aluminum as aluminum hydroxide (Alhydrogel; Brenntag Biosector, Frederikssund, Denmark) in a volume of 0.3 ml on days 0, 21, and 42 (Table 1). One week after the final vaccine dose, all groups were anesthetized and cannulated via the jugular vein, and blood was removed for antibody characterization. To prevent potentially fatal heroin-induced respiratory depression, groups 2, 3, 5, and 6 then received naloxone 0.1 mg/kg i.v., and 1 minute later groups 2 and 5 received 0.26 mg/kg (0.7 µmol/kg) heroin and groups 3 and 6 received 2.6 mg/kg (7.0 µmol/kg) heroin intravenously. We have previously shown that antibodies elicited by vaccination with M-KLH do not bind naloxone (Raleigh et al., 2013). However, to ensure that naloxone did not affect heroin and metabolite distribution groups 1 and 4, receiving the lower heroin dose of 0.26 mg/kg heroin, were pretreated with saline rather than naloxone prior to administration of heroin. Pretreatment with saline in groups 1 and 4 also allowed assessment of vaccine effects on arterial oxygen saturation (SaO2), which was measured every minute after saline administration by percutaneous oximetry (Nellcor OxiMax N-65; Medtronic, Minneapolis, MN). Rats were sacrificed 4 minutes after heroin administration, decapitated, and trunk blood and brain were obtained to quantitate heroin, 6-AM, and morphine levels.
TABLE 1.
Experiment 1 treatment groups
| Group | Vaccine | Pretreatment | Heroin Dose |
|---|---|---|---|
| mg/kg | |||
| 1 | M-KLH | Saline | 0.26 |
| 2 | M-KLH | 0.1 mg/kg naloxone | 0.26 |
| 3 | M-KLH | 0.1 mg/kg naloxone | 2.6 |
| 4 | KLH | Saline | 0.26 |
| 5 | KLH | 0.1 mg/kg naloxone | 0.26 |
| 6 | KLH | 0.1 mg/kg naloxone | 2.6 |
Experiment 2: OXY-KLH Vaccine Efficacy Following Intravenous Oxycodone Dosing.
This experiment was identical to that of experiment 1 except as follows. 1) Groups of eight rats were immunized intramuscularly with 25 μg of OXY-KLH (groups 1 and 2) or unconjugated KLH (groups 3 and 4) on days 0, 21, 42, and 63. The additional vaccine booster dose was used, based on our prior experience with this vaccine, to achieve satisfactory antibody levels. 2) Rats received 0.22 or 2.2 mg/kg oxycodone intravenously, the molar equivalents of the heroin doses used in experiment 1. Because naloxone had no effect on vaccine efficacy or opioid distribution in M-KLH-treated animals, naloxone was administered only to the high oxycodone dose groups to prevent respiratory compromise.
Experiment 3: OXY-KLH Efficacy Following Subcutaneous Oxycodone Dosing.
Two groups of six rats each were vaccinated with OXY-KLH or KLH as in experiment 2. On day 70, blood was collected via tail vein for antibody characterization. On day 77, both groups received 2.3 mg/kg oxycodone subcutaneously and were tested for hot-plate nociception 30 minutes later. Immediately following testing, blood and brain samples were collected to measure oxycodone concentrations.
Drug Level Analysis
Heroin, 6-AM, and morphine concentrations were measured by liquid chromatography/mass spectrometry (Jones et al., 2013). Samples were analyzed within 3 days of extraction under conditions that minimized their degradation (Jones et al., 2013). In brief, trunk blood was collected in a syringe containing 4 mg/ml of ice-cold sodium fluoride and 100 IU/ml heparin (for ease of sample handling) and centrifuged immediately at 3100g for 3 minutes at 4°C. Plasma was diluted 1:1 with ice-cold 10 mM formate buffer (pH 3.0) prior to extraction. Brains were rinsed with 10 mM formate buffer (pH 3.0) and four parts (by weight) of 10 mM formate buffer (pH 3.0) was added to each sample. Samples were homogenized for 30–40 seconds and stored no longer than 60 minutes at −20°C until extraction.
Oxycodone concentrations were measured by gas chromatography/mass spectrometry (Pravetoni et al., 2013). Trunk blood was centrifuged at 3100g for 3 minutes at 4°C and serum was collected. Brain was rinsed with distilled H2O and patted dry. Serum and brain were stored at −20°C until analysis.
Antibody Characterization
Antibody Titers.
To measure drug-specific serum IgG antibody titers, morphine or oxycodone haptens conjugated to bovine serum albumin were coated on enzyme-linked immunosorbent assay plates at 5 ng/well in carbonate buffer (pH 9.6) and blocked with 1% gelatin (Pravetoni et al., 2012c; Raleigh et al., 2013, 2017). Goat anti-rat IgG conjugated to horseradish peroxidase was used as secondary antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA).
Antibody Affinity and Concentration in Serum.
Serum antibody affinity for oxycodone was measured by ultrafiltration. A 20-fold dilution of serum was mixed 1:1 (v:v) with oxycodone at various concentrations in 0.9% NaCl. Samples were mixed by gentle rotation (5–10 rpm) for 4 hours at room temperature and then placed in 10 kDa molecular weight cutoff ultrafiltration tubes (PALL Nanosep with Omega membrane; Pall Corporation, Port Washington, NY) and spun at 10,000g for 15 minutes. The total oxycodone concentration was measured from an aliquot obtained prior to ultrafiltration, and the unbound concentration was measured from the ultrafiltrate. Bound oxycodone was calculated as the total minus unbound drug. The same procedure was used to measure morphine-specific antibody affinity. Because heroin is not stable in serum (Jones et al., 2013), morphine was used instead. Antibody affinity (Kd) was measured as half-maximum binding at equilibrium and converted to molar concentrations using Prism version 7.0 (GraphPad Software, Inc., La Jolla, CA). Opioid-specific antibody concentrations were calculated from the Bmax value using a molecular weight of 150 kDa for IgG (Bazin-Redureau et al., 1997; Pravetoni et al., 2012b) and two binding sites per IgG.
Stoichiometry
The total number of moles per kilogram of opioid-specific IgG in rats vaccinated with M-KLH or OXY-KLH was estimated as the product of the measured antibody concentration in serum and the reported IgG volume of distribution (131 ml/kg) in rats (Bazin-Redureau et al., 1997; Pravetoni et al., 2012b).
Protein Binding
The percentage of oxycodone bound to protein in serum or morphine bound to protein in plasma (because blood collected for analysis had to be heparinized) in experiments 1 and 2 was measured by ultrafiltration. Serum or plasma from vaccinated animals was placed in 10 kDa molecular weight cutoff ultrafiltration tubes and spun at 10,000g for 1 hour. The total drug concentration was measured from an aliquot of serum or plasma before ultrafiltration, and the unbound concentration was measured as the ultrafiltrate. Bound opioid was determined as the total opioid minus unbound opioid concentrations. Because of technical issues, samples from two rats in the KLH group in experiment 2 were removed from analysis.
Saturation of Serum Opioid-Specific Antibody
The percentage of serum morphine-specific antibody that was occupied by total opioid (heroin + 6-AM + morphine) was calculated from the ratio of the molar bound opioid concentration in serum (as determined by the protein binding assay described previously) divided by the antibody binding site concentration in serum (measured by the affinity assay). This measure was obtained in experiment 1 for group 3 (M-KLH vaccinated rats receiving 2.6 mg/kg heroin intravenously). The same measure was obtained in experiment 2 for group 2 (OXY-KLH vaccinated rats receiving 2.2 mg/kg oxycodone intravenously).
Thermal Nociception
Rats were habituated for 1 hour to the testing environment and nociception assessed on a hot-plate (Columbus Instruments, Columbus, OH) set to 54°C ± 0.2°C (Raleigh et al., 2013). A nociceptive response of hindpaw lick or jumping was measured as the latency to respond. Baseline hot-plate responses were obtained 2 hours prior to drug dosing. A maximum cutoff of 60 seconds was used to avoid injury. Vaccine efficacy in blocking opioid-induced antinociception was expressed as percentage of maximum possible effect calculated as (postdrug latency − predrug latency/maximum latency − predrug latency) × 100.
Statistics
All statistical analyses were performed using Prism version 7.0 (GraphPad Software, Inc.). Mean antibody titers, opioid concentrations, and percentage of maximum possible effect were compared using unpaired t tests. When variances were significantly different, Welsh’s correction was applied. One-site-specific binding was used to calculate Bmax and Kd from ultrafiltration data. For protein binding, log-transformed unbound oxycodone concentrations were used to compare controls versus vaccine that received the low oxycodone dose due to the large difference in variance between groups. The SaO2 levels were compared using two-way analysis of variance and Sidak’s multiple comparisons test.
Results
Experiment 1: M-KLH Vaccine Efficacy Following Intravenous Heroin Dosing.
Because the two groups pretreated with naloxone versus saline prior to receiving heroin 0.26 mg/kg did not differ with respect to any measures of plasma or brain opioid concentrations (Supplemental Fig. 1), only the group pretreated with saline was used for further analyses. Morphine-specific antibody titers were comparable between the low and high heroin dose groups (180 ± 100 vs. 160 ± 80 × 103, mean ± S.D., respectively; t = 0.441, P > 0.1). The morphine-specific antibody concentration in pooled serum was 787 μg/ml (10.5 μM), and affinity for morphine was 13.9 nM (Supplemental Fig. 2). The molar ratio of the low dose of heroin (0.26 mg/kg) to estimated antibody binding sites in vaccinated rats was 0.51, while the ratio of the high dose of heroin (2.6 mg/kg) to estimated antibody binding sites was 5.1.
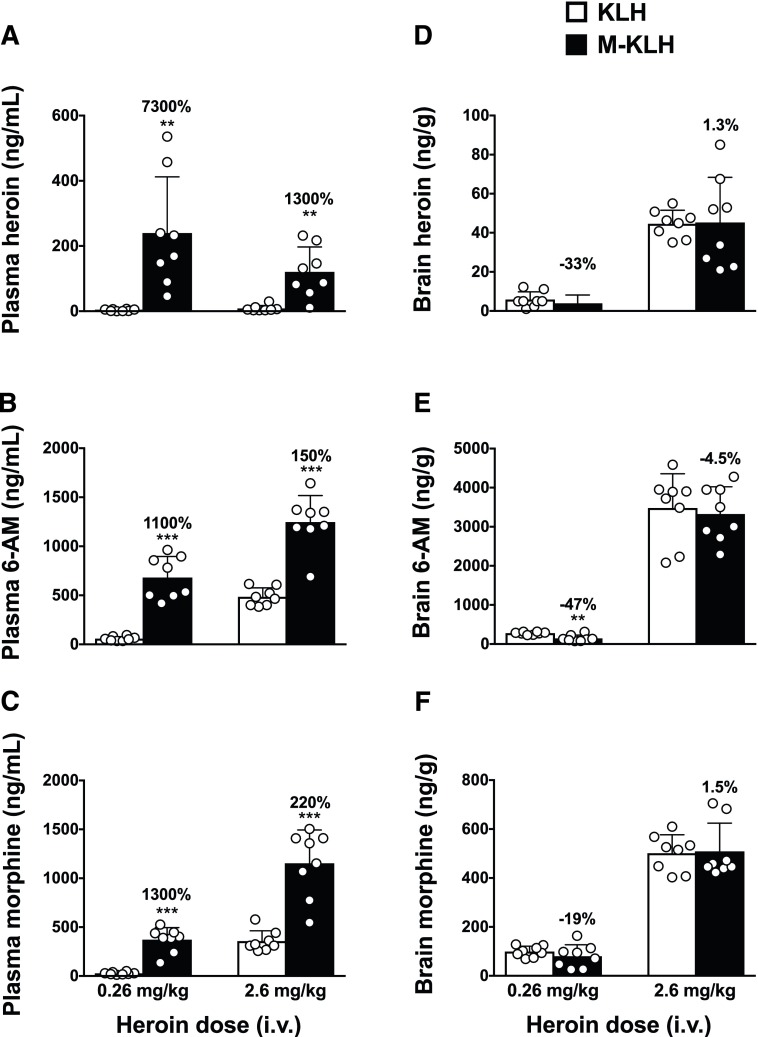
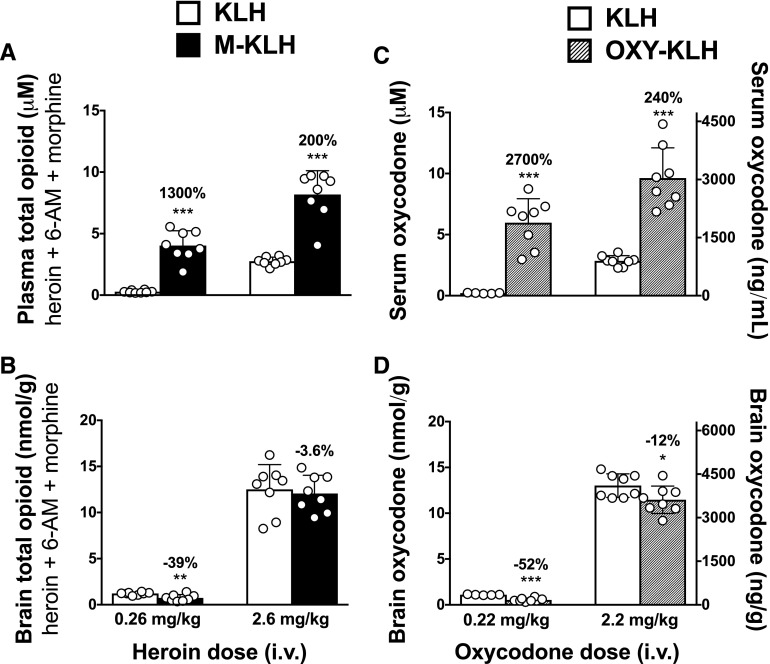
M-KLH vaccinated rats from both the low and high heroin dose groups had significantly higher plasma concentrations of heroin and active metabolites compared with KLH controls (Fig. 1, A–C). M-KLH vaccinated rats that received the low heroin dose had significantly lower 6-AM (t = 4.232, P < 0.01), but not heroin or morphine, concentrations in brain compared with controls (Fig. 1, D–F). Vaccination with M-KLH did not alter heroin or metabolite brain concentrations in rats that received the high heroin dose compared with KLH controls. Total plasma opioid concentrations (heroin + 6-AM + morphine) were significantly increased in rats vaccinated with M-KLH following either the low or high heroin doses compared with KLH controls (Fig. 2A). Brain total opioid concentration was significantly decreased in rats vaccinated with M-KLH after the low heroin dose compared with KLH controls, but did not significantly differ from KLH controls after the high heroin dose (Fig. 2B, t = 3.429, P < 0.01).
Fig. 1.
Effect of vaccination with M-KLH on heroin, 6-AM, and morphine concentrations after intravenous administration of a low or high heroin dose. Plasma (A–C) and brain (D–F) samples were collected 4 minutes following the end of 1-minute infusion of heroin. **P < 0.01; ***P < 0.001 for the difference between M-KLH and KLH control groups. Numbers above bars represent the percentage of difference from controls. Unpaired t tests with Welch’s correction were used to compare groups. Mean ± S.D., n = 8/group.
Fig. 2.
Effect of vaccination with M-KLH or OXY-KLH on opioid concentrations after intravenous administration of low or high heroin or oxycodone doses. Plasma (A) and brain (B) samples were collected 4 minutes following the end of 1-minute infusion of heroin, and serum (C) and brain (D) samples were collected 4 minutes following the end of 1-minute infusion of oxycodone. Plasma and brain drug concentrations after heroin administration represent total opioid (heroin + 6-AM + morphine); converted data from Fig. 1 and presented here for comparison. *P < 0.05; **P < 0.01; ***P < 0.001 for the difference between vaccinated rats and KLH controls. Numbers above bars represent the percentage of difference from controls. Unpaired t tests with Welch’s correction were used to compare groups. Mean ± S.D., n = 8/group.
Vaccination with M-KLH increased the plasma protein binding of total opioid (Tables 2 and 3). The unbound total opioid concentration in both low and high heroin dose groups was not changed by vaccination with M-KLH but the associated variances were high. The calculated saturation of opioid-specific antibody by total opioid (heroin + 6-AM + morphine) in serum was 68.8% in M-KLH vaccinated rats given the high heroin dose of 2.6 mg/kg.
TABLE 2.
Protein binding data for experiments 1 and 2
Drug concentration in plasma (total opioid; heroin + 6-AM + morphine) 4 minutes after intravenous administration of a low or high opioid dose. *P < 0.05 for the difference between vaccine and control groups given the same dose. Unpaired t tests were used to compare groups. Mean ± S.D., n = 6–8/group.
| Heroin Dose |
Drug Concentration in Plasma |
||
|---|---|---|---|
| Total |
Unbound |
Bound |
|
| μmol/kg (mg/kg) | μM | μM | % |
| KLH | |||
| 0.7 (0.26) | 0.26 ± 0.09 | 0.12 ± 0.06 | 52.9 ± 11.7 |
| 7.0 (2.6) | 2.75 ± 0.33 | 0.82 ± 0.17 | 70.0 ± 6.7 |
| M-KLH | |||
| 0.7 (0.26) | 4.03 ± 1.20* | 0.08 ± 0.13 | 97.0 ± 5.8* |
| 7.0 (2.6) | 8.17 ± 1.94* | 0.87 ± 0.23 | 88.5 ± 5.1* |
TABLE 3.
Protein binding data for experiments 1 and 2
Drug concentration in serum (oxycodone) 4 minutes after intravenous administration of a low or high opioid dose. *P < 0.05 for the difference between vaccine and control groups given the same dose. Unpaired t tests were used to compare groups. Mean ± S.D., n = 6–8/group.
| Oxycodone Dose |
Drug Concentration in Serum |
||
|---|---|---|---|
| Total |
Unbound |
Bound |
|
| μmol/kg (mg/kg) | μM | μM | % |
| KLH | |||
| 0.7 (0.22) | 0.21 ± 0.04 | 0.17 ± 0.03 | 18.8 ± 2.5 |
| 7.0 (2.2) | 2.85 ± 0.43 | 2.37 ± 0.26 | 15.1 ± 15.2 |
| OXY-KLH | |||
| 0.7 (0.22) | 5.97 ± 1.97* | 0.10 ± 0.10* | 97.9 ± 2.1* |
| 7.0 (2.2) | 9.63 ± 2.48* | 2.34 ± 0.51 | 74.1 ± 9.8* |
Experiment 2: OXY-KLH Vaccine Efficacy Following Intravenous Oxycodone Dosing.
Oxycodone-specific antibody titers were comparable between the low and high oxycodone dose groups (190 ± 50 and 145 ± 52 × 103, mean ± S.D., respectively; t = 1.795 P = 0.094). The oxycodone-specific antibody concentration from pooled sera was 757 μg/ml (10 μM), and affinity for oxycodone was 14.5 nM (Supplemental Fig. 2). The molar ratio of low dose of oxycodone (0.22 mg/kg) to estimated antibody binding sites in OXY-KLH vaccinated rats was 0.53, while the ratio of the high dose of oxycodone (2.2 mg/kg) to estimated antibody binding sites was 5.3.
Serum oxycodone concentrations were significantly increased in OXY-KLH vaccinated rats following intravenous dosing with 0.22 or 2.2 mg/kg oxycodone compared with KLH controls (Fig. 2C). Oxycodone concentrations in brain were significantly reduced in OXY-KLH vaccinated rats (by 52%) following the 0.22 mg/kg oxycodone dose compared with KLH controls (t = 8.531, P < 0.001), and to a lesser extent (12%) following the 2.2 mg/kg oxycodone dose (Fig. 2D, t = 2.22, P < 0.05).
Vaccination with OXY-KLH increased the serum protein binding of oxycodone (Tables 2 and 3). The unbound oxycodone concentration was significantly reduced by vaccination with OXY-KLH in the low oxycodone dose group compared with KLH controls, but was not altered by vaccination in the high oxycodone dose group. The calculated saturation of oxycodone-specific antibody by oxycodone in serum was 70.6% in OXY-KLH vaccinated rats given the high oxycodone dose of 2.2 mg/kg.
Experiments 1 and 2: Opioid-Induced Respiratory Depression.
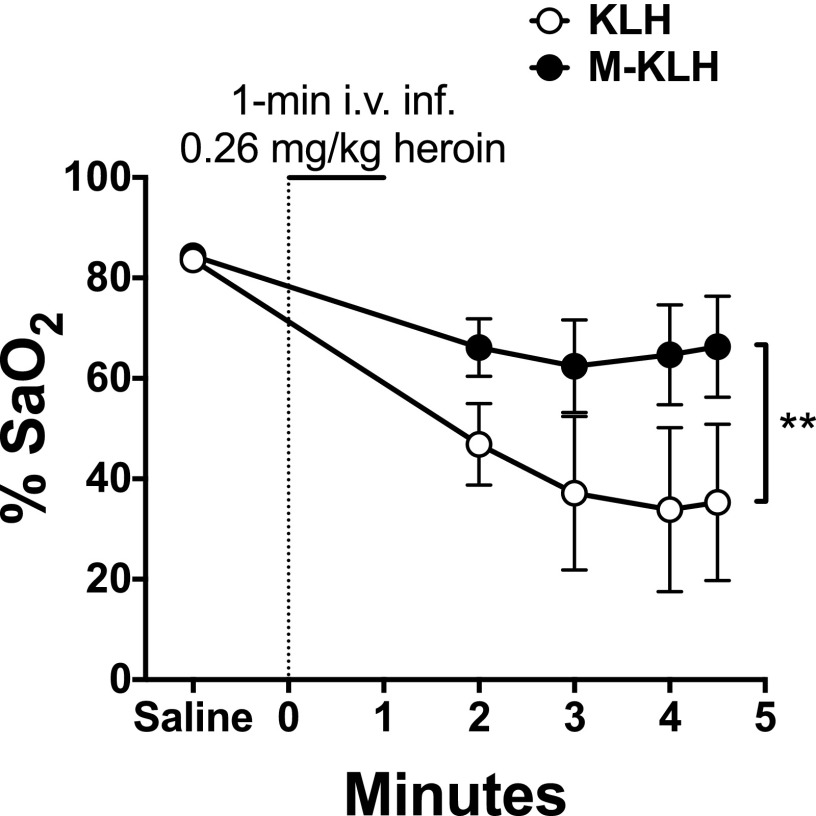
Vaccination with M-KLH prevented the decrease in arterial SaO2 produced by the 0.26 mg/kg heroin dose (Fig. 3). Effects of vaccination on SaO2 were not analyzed in the low-dose oxycodone group because this oxycodone dose did not produce significant respiratory depression in KLH controls (data not shown). The SaO2 values in the high-dose oxycodone or heroin groups were not compared because respiratory depression was prevented by pretreatment with naloxone.
Fig. 3.
Effect of vaccination with M-KLH on SaO2 after intravenous administration of 0.26 mg/kg heroin. Heroin was infused intravenously for 1-minute following saline pretreatment. Vaccination with M-KLH significantly blunted the decrease in SaO2 seen in the KLH control group. Both groups had significantly lower %SaO2 levels following heroin administration (P < 0.01). **P < 0.01 for the difference between M-KLH and KLH control groups. Two-way analysis of variance with Sidak’s multiple comparisons test was used to compare groups. Mean ± S.E.M., n = 8/group.
Experiment 3: OXY-KLH Vaccine Efficacy Following Subcutaneous Oxycodone Dosing.
Oxycodone-specific antibody titers (210 ± 51 × 103, mean ± S.D.) were comparable to those elicited in experiment 2, which examined vaccine effects on oxycodone administered intravenously. Serum oxycodone concentrations were increased (by 620%) and brain oxycodone concentrations were decreased (by 44%) compared with KLH controls (Fig. 4A; serum, t = 10.06, P < 0.001; brain, t = 3.934, P < 0.01). Vaccination with OXY-KLH also significantly reduced oxycodone-induced antinociception compared with controls by 60% (Fig. 4B; t = 4.19, P < 0.01).
Fig. 4.
Effect of vaccination with OXY-KLH on oxycodone concentrations following subcutaneous administration of a high oxycodone dose. Serum and brain oxycodone concentrations (A) and oxycodone-induced antinociception (B) 30 minutes following subcutaneous administration of 2.3 mg/kg oxycodone. **P < 0.01; ***P < 0.001 for the difference between OXY-KLH and KLH control groups. Numbers above bars represent the percentage of difference from controls. Unpaired t tests with Welch’s correction were used to compare groups. Mean ± S.D., n = 6/group.
Discussion
The key findings from this study were that: 1) both OXY-KLH and M-KLH reduced drug distribution to the brain after intravenous challenges with low opioid doses, but vaccines were only minimally effective (OXY-KLH) or ineffective (M-KLH) against the higher intravenous opioid doses; 2) OXY-KLH was effective in blocking oxycodone distribution to brain, even with a high oxycodone dose, when immunized rats were challenged with oxycodone subcutaneously rather than intravenously; and 3) M-KLH had a protective effect against heroin-induced respiratory depression. The data highlight the dose- and route-dependent efficacy of vaccination against opioids.
M-KLH and OXY-KLH produced very high serum antibody concentrations of 0.75 μg/ml, consistent with other reports of heroin and oxycodone vaccine immunogenicity in rats (Anton and Leff, 2006; Pravetoni et al., 2012c, 2014a; Kosten et al., 2013; Raleigh et al., 2013; Jalah et al., 2015), although one report found even higher concentrations of up to 2.8 mg/ml (Stowe et al., 2011). These concentrations are higher than have generally been reported with nicotine or cocaine vaccines using similar linkers and carrier proteins, suggesting that opioid-based haptens are particularly immunogenic, perhaps due to their larger size (Kantak et al., 2000; Keyler et al., 2008; Roiko et al., 2008; Pravetoni et al., 2012a). In clinical trials of nicotine or cocaine vaccines, serum antibody concentrations have been considerably lower than those elicited in animals and this likely contributed to their limited efficacy in reducing smoking or cocaine use (Martell et al., 2009; Hatsukami et al., 2011; Esterlis et al., 2013). If opioid vaccines prove more immunogenic than nicotine or cocaine vaccines in humans this could substantially improve their chances of showing clinical efficacy against opioid abuse, but this has to be addressed in humans.
In the current study, the molar ratio of the oxycodone or heroin doses to antibody binding sites in vaccinated rats was approximately 0.5:1 at the lower opioid dose and 5:1 at the higher dose. The large excess of drug relative to antibody drug-binding capacity readily explains the lesser efficacy of the OXY-KLH and M-KLH vaccines at high drug doses. However, OXY-KLH was effective in reducing oxycodone distribution to brain when 2.3 mg/kg oxycodone was administered subcutaneously, a dose similar to the high dose administered intravenously. OXY-KLH also reduced opioid-induced antinociception in the hot-plate assay at this same oxycodone dose given subcutaneously, as previously reported (Pravetoni et al., 2014b). The expected slower absorption of oxycodone into blood after subcutaneous compared with intravenous administration was likely responsible for the increased vaccine efficacy despite the high oxycodone dose. In addition, the bioavailability of oxycodone administered subcutaneously to rats is 57% (Huang et al., 2005; Chan et al., 2008), similar to the bioavailability of oral oxycodone in humans (Pöyhiä et al., 1992), which would reduce the amount of drug presented to serum antibody compared with intravenous dosing. Because oxycodone is most commonly abused by the oral or intranasal routes (Jones et al., 2011), vaccination with OXY-KLH might be clinically useful for blocking oxycodone effects. On the other hand, heroin is most often abused by the intravenous route. Efficacy of M-KLH against heroin will likely depend heavily upon the heroin dose used. Extrapolation of rat data to humans is made more difficult for heroin because the main active components are its metabolites 6-AM and morphine. These metabolites are formed at different rates in humans compared with rodents (Comer et al., 1999; Rentsch et al., 2001; Gottås et al., 2013) and their rates of distribution to brain may also differ. These considerations underscore the need to advance opioid vaccines to human studies to properly assess their therapeutic potential.
Antibody affinity toward morphine (13.9 nM) or oxycodone (14.5 nM) was within the range of affinities measured by other groups (0.4–24 nM) using similar immunogens (Anton and Leff, 2006; Stowe et al., 2011; Torres et al., 2016; Kimishima et al., 2017). It is not clear whether higher affinity antibodies would have performed substantially better. The calculated saturation of serum morphine-specific antibody with total opioid in rats receiving the higher heroin dose was already quite high (68.8%), as was the saturation of oxycodone-specific antibody with oxycodone (70.6%), such that enhancing this percentage by generating higher affinity antibodies would have contributed relatively little to the amount of drug bound in serum and the ability of these vaccines to reduce drug distribution to brain. By contrast, the total opioid or oxycodone concentrations in brain varied inversely with the drug-specific antibody titers, a surrogate for antibody concentration (Supplemental Fig. 3). Enhancing the efficacy of vaccination would seem to require generating higher serum antibody concentrations more so than enhancing antibody affinity.
Heroin doses used in humans vary considerably. Heroin abusers report significant drug-liking effects in a clinical laboratory setting after single intravenous heroin doses as low as 0.09–0.36 mg/kg (Comer et al., 1999), which is similar to the low heroin dose used in the current study. In support of heroin’s reinforcing effects at relatively low doses, subjects in a clinical laboratory study consumed 0.3–0.57 mg/kg/session when each heroin dose of 10 mg (approximately 0.14 mg/kg) was administered by pressing a lever at a fixed ratio of 300 (Mello and Mendelson, 1980; Mello et al., 1981). These doses are at the lower end of the range examined in the current study. On the other hand, the typical intravenous heroin dose range used in heroin maintenance programs, presumably reflecting typical abuse rates, is 150–600 mg divided into two or three intravenous injections (Oviedo-Joekes et al., 2010; Strang et al., 2010). This wide range of doses from different settings may reflect individual differences in opioid tolerance. Thus, M-KLH could be effective in reducing heroin effects in some individuals with heroin use disorder but is likely to be less effective in those abusing very high doses.
Abuse patterns for oxycodone differ from those of heroin. In a clinical laboratory study of nondependent opioid users, 20 mg (0.29 mg/kg) oxycodone administered intravenously (Backonja et al., 2016) or 30 mg (0.43 mg/kg) administered as intranasal powder (Harris et al., 2014) produced significant drug-liking effects. These doses are at the lower end of the range administered to rats intravenously in the current study. Self-report of orally abused oxycodone doses in one survey were somewhat higher at 0.3–1.2 mg/kg, most often taken orally (Jones et al., 2011) but still within the range at which OXY-KLH showed efficacy in the current study against oxycodone administered subcutaneously. Drug absorption following subcutaneous oxycodone, as in the current study, is likely more rapid than with oral dosing, suggesting that OXY-KLH would be at least as effective against orally dosed oxycodone. Overall, the current data suggest that OXY-KLH could be effective in reducing the effects of oxycodone at the doses and routes typically used by those with oxycodone use disorder.
Vaccination with M-KLH protected against heroin-induced respiratory depression in the low-dose heroin group. M-KLH effects on respiratory depression were not studied in the high-dose heroin group because they had received naloxone to prevent respiratory arrest and OXY-KLH effects were not reported for the low-dose oxycodone group because controls did not show significant respiratory depression. However, OXY-KLH has been shown to reduce oxycodone-induced respiratory depression following a cumulative subcutaneous oxycodone dose of 9 mg/kg (Raleigh et al., 2017). Protection against opioid-induced respiratory depression is of note because respiratory depression is the principal mechanism of death from opioid overdose. While the opioid vaccines studied are not likely to protect against respiratory depression from very large opioid doses, vaccination might provide some protection to occasional or infrequent heroin users who have little or no opioid tolerance and use lower doses.
It should be noted that slightly different vaccine formulations were used in the different experiments, with oxycodone or heroin haptens conjugated to either native KLH (a didecamer) or dimer KLH, the latter used because it is available as a cGMP product. However, we have previously shown that native KLH and dimer KLH are equally effective as carrier proteins for the development of oxycodone vaccines (Pravetoni et al., 2014b), and in the current study both vaccine formulations elicited similar serum opioid-specific antibody concentrations.
In summary, these data highlight the potential efficacy of OXY-KLH or M-KLH for reducing the effects of oxycodone or heroin but also point out the dose and route dependence of their efficacy. Because oxycodone is often abused at doses within the range examined in this study, and by the oral or intranasal routes, which are characterized by slower absorption than intravenous dosing, OXY-KLH could be helpful in treating oxycodone use disorder and merits further study in clinical trials. The very high heroin doses that are often abused, and the predominance of the intravenous route of abuse, make the treatment of heroin abuse disorder with a vaccine more challenging. However, there is a very wide range of abused heroin doses, and heroin pharmacokinetics in humans differ from those of rodents, thus further study of M-KLH and similar heroin vaccines remains of interest.
Acknowledgments
We thank Danielle M. Burroughs, Theresa M. Harmon, and Jennifer R. Vigliaturo for technical support.
Abbreviations
- 6-AM
6-acetylmorphine
- KLH
keyhole limpet hemocyanin
- M-KLH
morphine hapten conjugated to keyhole limpet hemocyanin
- OXY-KLH
oxycodone hapten conjugated to keyhole limpet hemocyanin
- SaO2
arterial oxygen saturation
Authorship Contributions
Participated in research design: Raleigh, Winston, Pentel, Pravetoni.
Conducted experiments: Raleigh, Laudenbach, Baruffaldi, Peterson, Roslawski.
Contributed new reagents or analytic tools: Roslawski, Birnbaum, Carroll, Runyon.
Performed data analysis: Raleigh, Roslawski, Birnbaum, Pentel, Pravetoni.
Wrote or contributed to the writing of the manuscript: Raleigh, Laudenbach, Baruffaldi, Peterson, Birnbaum, Carroll, Winston, Pentel, Pravetoni.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant U01DA038876] (to M.P. and P.R.P.).
Part of this work was presented previously: Raleigh MD, Laudenbach M, Baruffaldi F, Peterson SJ, Roslawski MJ, Birnbaum AK, Carroll FI, Runyon SP, Winston S, Pentel PR, et al. (2017) Efficacy of heroin and oxycodone vaccines for reducing opioid distribution to brain over a range of drug doses in rats. 79th annual meeting of the College on Problems of Drug Dependence; 2017 Jun 17–22; Montréal, Canada.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Anton B, Leff P. (2006) A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine 24:3232–3240. [DOI] [PubMed] [Google Scholar]
- Appel PW, Ellison AA, Jansky HK, Oldak R. (2004) Barriers to enrollment in drug abuse treatment and suggestions for reducing them: opinions of drug injecting street outreach clients and other system stakeholders. Am J Drug Alcohol Abuse 30:129–153. [DOI] [PubMed] [Google Scholar]
- Backonja M, Webster LR, Setnik B, Bass A, Sommerville KW, Matschke K, Malhotra BK, Wolfram G. (2016) Intravenous abuse potential study of oxycodone alone or in combination with naltrexone in nondependent recreational opioid users. Am J Drug Alcohol Abuse 42:539–549. [DOI] [PubMed] [Google Scholar]
- Bazin-Redureau MI, Renard CB, Scherrmann JM. (1997) Pharmacokinetics of heterologous and homologous immunoglobulin G, F(ab′)2 and Fab after intravenous administration in the rat. J Pharm Pharmacol 49:277–281. [DOI] [PubMed] [Google Scholar]
- Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR. (1974) Changes in heroin self-administration by a rhesus monkey after morphine immunisation. Nature 252:708–710. [DOI] [PubMed] [Google Scholar]
- Bremer PT, Schlosburg JE, Banks ML, Steele FF, Zhou B, Poklis JL, Janda KD. (2017) Development of a clinically viable heroin vaccine. J Am Chem Soc 139:8601–8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan S, Edwards SR, Wyse BD, Smith MT. (2008) Sex differences in the pharmacokinetics, oxidative metabolism and oral bioavailability of oxycodone in the Sprague-Dawley rat. Clin Exp Pharmacol Physiol 35:295–302. [DOI] [PubMed] [Google Scholar]
- Comer SD, Collins ED, MacArthur RB, Fischman MW. (1999) Comparison of intravenous and intranasal heroin self-administration by morphine-maintained humans. Psychopharmacology (Berl) 143:327–338. [DOI] [PubMed] [Google Scholar]
- Dowell D, Arias E, Kochanek K, Anderson R, Guy GP, Jr, Losby JL, Baldwin G. (2017) Contribution of opioid-involved poisoning to the change in life expectancy in the United States, 2000–2015. JAMA 318:1065–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterlis I, Hannestad JO, Perkins E, Bois F, D’Souza DC, Tyndale RF, Seibyl JP, Hatsukami DM, Cosgrove KP, O’Malley SS. (2013) Effect of a nicotine vaccine on nicotine binding to β2*-nicotinic acetylcholine receptors in vivo in human tobacco smokers. Am J Psychiatry 170:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottås A, Øiestad EL, Boix F, Vindenes V, Ripel Å, Thaulow CH, Mørland J. (2013) Levels of heroin and its metabolites in blood and brain extracellular fluid after i.v. heroin administration to freely moving rats. Br J Pharmacol 170:546–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SC, Perrino PJ, Smith I, Shram MJ, Colucci SV, Bartlett C, Sellers EM. (2014) Abuse potential, pharmacokinetics, pharmacodynamics, and safety of intranasally administered crushed oxycodone HCl abuse-deterrent controlled-release tablets in recreational opioid users. J Clin Pharmacol 54:468–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatsukami DK, Jorenby DE, Gonzales D, Rigotti NA, Glover ED, Oncken CA, Tashkin DP, Reus VI, Akhavain RC, Fahim RE, et al. (2011) Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin Pharmacol Ther 89:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Edwards SR, Smith MT. (2005) Comparison of the pharmacokinetics of oxycodone and noroxycodone in male dark agouti and Sprague–Dawley rats: influence of streptozotocin-induced diabetes. Pharm Res 22:1489–1498. [DOI] [PubMed] [Google Scholar]
- Jalah R, Torres OB, Mayorov AV, Li F, Antoline JF, Jacobson AE, Rice KC, Deschamps JR, Beck Z, Alving CR, et al. (2015) Efficacy, but not antibody titer or affinity, of a heroin hapten conjugate vaccine correlates with increasing hapten densities on tetanus toxoid, but not on CRM197 carriers. Bioconjug Chem 26:1041–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Vosburg SK, Manubay JM, Comer SD. (2011) Oxycodone abuse in New York city: characteristics of intravenous and intranasal users. Am J Addict 20:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JM, Raleigh MD, Pentel PR, Harmon TM, Keyler DE, Remmel RP, Birnbaum AK. (2013) Stability of heroin, 6-monoacetylmorphine, and morphine in biological samples and validation of an LC-MS assay for delayed analyses of pharmacokinetic samples in rats. J Pharm Biomed Anal 74:291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. (2000) Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl) 148:251–262. [DOI] [PubMed] [Google Scholar]
- Keyler DE, Roiko SA, Earley CA, Murtaugh MP, Pentel PR. (2008) Enhanced immunogenicity of a bivalent nicotine vaccine. Int Immunopharmacol 8:1589–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimishima A, Wenthur CJ, Zhou B, Janda KD. (2017) An advance in prescription opioid vaccines: overdose mortality reduction and extraordinary alteration of drug half-life. ACS Chem Biol 12:36–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TA, Shen XY, O’Malley PW, Kinsey BM, Lykissa ED, Orson FM, Kosten TR. (2013) A morphine conjugate vaccine attenuates the behavioral effects of morphine in rats. Prog Neuropsychopharmacol Biol Psychiatry 45:223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenbach M, Baruffaldi F, Vervacke JS, Distefano MD, Titcombe PJ, Mueller DL, Tubo NJ, Griffith TS, Pravetoni M. (2015) The frequency of naive and early-activated hapten-specific B cell subsets dictates the efficacy of a therapeutic vaccine against prescription opioid abuse. J Immunol 194:5926–5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QQ, Luo YX, Sun CY, Xue YX, Zhu WL, Shi HS, Zhai HF, Shi J, Lu L. (2011) A morphine/heroin vaccine with new hapten design attenuates behavioral effects in rats. J Neurochem 119:1271–1281. [DOI] [PubMed] [Google Scholar]
- Li QQ, Sun CY, Luo YX, Xue YX, Meng SQ, Xu LZ, Chen N, Deng JH, Zhai HF, Kosten, et al. (2014) A conjugate vaccine attenuates morphine- and heroin-induced behavior in rats. Int J Neuropsychopharmacol 18:pyu093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR. (2009) Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry 66:1116–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH. (1980) Buprenorphine suppresses heroin use by heroin addicts. Science 207:657–659. [DOI] [PubMed] [Google Scholar]
- Mello NK, Mendelson JH, Kuehnle JC, Sellers MS. (1981) Operant analysis of human heroin self-administration and the effects of naltrexone. J Pharmacol Exp Ther 216:45–54. [PubMed] [Google Scholar]
- National Survey on Drug Use and Health (2014) Results from the 2013 National Survey on Drug Use and Health: detailed tables.
- Oviedo-Joekes E, March JC, Romero M, Perea-Milla E. (2010) The Andalusian trial on heroin-assisted treatment: a 2 year follow-up. Drug Alcohol Rev 29:75–80. [DOI] [PubMed] [Google Scholar]
- Paulozzi LJ. (2012) Prescription drug overdoses: a review. J Safety Res 43:283–289. [DOI] [PubMed] [Google Scholar]
- Pöyhiä R, Seppälä T, Olkkola KT, Kalso E. (1992) The pharmacokinetics and metabolism of oxycodone after intramuscular and oral administration to healthy subjects. Br J Clin Pharmacol 33:617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Keyler DE, Pidaparthi RR, Carroll FI, Runyon SP, Murtaugh MP, Earley CA, Pentel PR. (2012a) Structurally distinct nicotine immunogens elicit antibodies with non-overlapping specificities. Biochem Pharmacol 83:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Le Naour M, Harmon TM, Tucker AM, Portoghese PS, Pentel PR. (2012b) An oxycodone conjugate vaccine elicits drug-specific antibodies that reduce oxycodone distribution to brain and hot-plate analgesia. J Pharmacol Exp Ther 341:225–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Le Naour M, Tucker AM, Harmon TM, Hawley TM, Portoghese PS, Pentel PR. (2013) Reduced antinociception of opioids in rats and mice by vaccination with immunogens containing oxycodone and hydrocodone haptens. J Med Chem 56:915–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Pentel PR, Potter DN, Chartoff EH, Tally L, LeSage MG. (2014a) Effects of an oxycodone conjugate vaccine on oxycodone self-administration and oxycodone-induced brain gene expression in rats. PLoS One 9:e101807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Raleigh MD, Le Naour M, Tucker AM, Harmon TM, Jones JM, Birnbaum AK, Portoghese PS, Pentel PR. (2012c) Co-administration of morphine and oxycodone vaccines reduces the distribution of 6-monoacetylmorphine and oxycodone to brain in rats. Vaccine 30:4617–4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pravetoni M, Vervacke JS, Distefano MD, Tucker AM, Laudenbach M, Pentel PR. (2014b) Effect of currently approved carriers and adjuvants on the pre-clinical efficacy of a conjugate vaccine against oxycodone in mice and rats. PLoS One 9:e96547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD, Pentel PR, LeSage MG. (2014) Pharmacokinetic correlates of the effects of a heroin vaccine on heroin self-administration in rats. PLoS One 9:e115696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD, Peterson SJ, Laudenbach M, Baruffaldi F, Carroll FI, Comer SD, Navarro HA, Langston TL, Runyon SP, Winston S, et al. (2017) Safety and efficacy of an oxycodone vaccine: addressing some of the unique considerations posed by opioid abuse. PLoS One 12:e0184876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh MD, Pravetoni M, Harris AC, Birnbaum AK, Pentel PR. (2013) Selective effects of a morphine conjugate vaccine on heroin and metabolite distribution and heroin-induced behaviors in rats. J Pharmacol Exp Ther 344:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch KM, Kullak-Ublick GA, Reichel C, Meier PJ, Fattinger K. (2001) Arterial and venous pharmacokinetics of intravenous heroin in subjects who are addicted to narcotics. Clin Pharmacol Ther 70:237–246. [DOI] [PubMed] [Google Scholar]
- Roiko SA, Harris AC, Keyler DE, Lesage MG, Zhang Y, Pentel PR. (2008) Combined active and passive immunization enhances the efficacy of immunotherapy against nicotine in rats. J Pharmacol Exp Ther 325:985–993. [DOI] [PubMed] [Google Scholar]
- Rosenberg H, Phillips KT. (2003) Acceptability and availability of harm-reduction interventions for drug abuse in American substance abuse treatment agencies. Psychol Addict Behav 17:203–210. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, Scholl L. (2016) Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. MMWR Morb Mortal Wkly Rep 65:1445–1452. [DOI] [PubMed] [Google Scholar]
- Schlosburg JE, Vendruscolo LF, Bremer PT, Lockner JW, Wade CL, Nunes AA, Stowe GN, Edwards S, Janda KD, Koob GF. (2013) Dynamic vaccine blocks relapse to compulsive intake of heroin. Proc Natl Acad Sci USA 110:9036–9041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe GN, Vendruscolo LF, Edwards S, Schlosburg JE, Misra KK, Schulteis G, Mayorov AV, Zakhari JS, Koob GF, Janda KD. (2011) A vaccine strategy that induces protective immunity against heroin. J Med Chem 54:5195–5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang J, Metrebian N, Lintzeris N, Potts L, Carnwath T, Mayet S, Williams H, Zador D, Evers R, Groshkova T, et al. (2010) Supervised injectable heroin or injectable methadone versus optimised oral methadone as treatment for chronic heroin addicts in England after persistent failure in orthodox treatment (RIOTT): a randomised trial. Lancet 375:1885–1895. [DOI] [PubMed] [Google Scholar]
- Torres OB, Antoline JF, Li F, Jalah R, Jacobson AE, Rice KC, Alving CR, Matyas GR. (2016) A simple nonradioactive method for the determination of the binding affinities of antibodies induced by hapten bioconjugates for drugs of abuse. Anal Bioanal Chem 408:1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (2016) World drug report 2016.