Abstract
Previous studies utilizing PUGNAc, the most widely used β-N-acetylglucosaminidase (OGA) inhibitor to increase global O-N-acetylglucosamine (GlcNAc) levels, have reported a variety of effects including insulin resistance as a direct result of elevated O-GlcNAc levels. The notion of OGA inhibition causing insulin resistance was not replicated in studies in which elevated global O-GlcNAc levels were achieved using two other OGA inhibitors. Related to insulin action, work by others has suggested that O-GlcNAc elevation may inhibit the anti-apoptotic action of insulin. Thus, we examined the pro-survival action of insulin upon serum deprivation in the presence of PUGNAc as well as two selective OGA inhibitors (GlcNAcstatin-g and Thiamet-G), and a selective lysosomal hexosaminidase inhibitor (INJ2). We established that PUGNAc inhibits the pro-survival action of insulin but this effect is not recapitulated by the selective OGA inhibitors suggesting that elevation in O-GlcNAc levels alone is not responsible for PUGNAc's effect on the anti-apoptotic action of insulin. Further, we demonstrate that a selective hexosaminidase A/B (HexA/B) inhibitor does not impact insulin action suggesting that PUGNAc's effect is not due to inhibition of lysosomal hexosaminidase. Finally, we tested a combination of selective OGA and lysosomal hexosaminidase inhibitors but were not able to recapitulate the inhibition of insulin action generated by PUGNAc alone. These results strongly suggest that the defect in insulin action upon PUGNAc treatment does not derive from its inhibition of OGA or HexA/B, and that there is an unknown target of PUGNAc that is the likely culprit in inhibiting the protective effect of insulin from apoptosis.
Keywords: apoptosis, HexA/B, OGA, O-GlcNAc, insulin
Introduction
Dynamic O-linked β-N-acetylglucosamine (O-GlcNAc) modification is a ubiquitous post-translational modification (PTM) found on the hydroxyl group of serine and threonine residues on a large number of intracellular proteins (Hart et al. 2007). The O-GlcNAc modification has been reported in most eukaryotic model organisms, including apicomplexan protozoan parasites (Banerjee et al. 2009; Perez-Cervera et al. 2011), nematodes (Hanover et al. 2005), fruit flies (Kelly and Hart 1989), and mammals (Torres and Hart 1984; Holt and Hart 1986), with baker's yeast being the notable exception. The spatio-temporal aspects of the O-GlcNAc modification resemble that of phosphorylation, and cumulative reports indicate that both PTMs act as regulatory switches to modulate the functionalities of proteins, which in turn fine-tune many cellular processes (Hart et al. 2011). Unlike phosphorylation, in which large families of kinases and phosphatases modify their targets in a consensus sequence-dependent manner, the reversible nature of O-GlcNAc modification is orchestrated by a pair of cycling enzymes, O-GlcNAc transferase (OGT; Haltiwanger et al. 1990, 1992; Kreppel et al. 1997, for the attachment) and neutral β-N-acetylglucosaminidase (OGA; Dong and Hart 1994; Gao et al. 2001, for the removal), without strict sequons in their substrates.
The degree of intracellular O-GlcNAc is correlated with the availability of UDP-GlcNAc, the obligatory donor substrate of OGT (Boehmelt et al. 2000), as well as many other intra- and extra-cellular stimuli (Hart et al. 2011). UDP-GlcNAc is the end product of the hexosamine biosynthetic pathway (HBP), one of the immediate metabolic routes of glucose (Buse 2006; Teo et al. 2010). Since O-GlcNAc is inducible and reversible, it is poised as a nutrient sensor downstream of the HBP (Wells et al. 2003). Following the seminal findings for the link between excessive HBP flux and the development of insulin resistance (Marshall et al. 1991; Virkamaki et al. 1997; Nelson et al. 2000; Nakamura et al. 2001; Buse et al. 2002), many studies have proceeded to demonstrate increased O-GlcNAc levels as the bridge between the two events (Hebert et al. 1996; Buse et al. 2002; McClain et al. 2002; Vosseller et al. 2002; Clark et al. 2003; Hanover et al. 2005; Hu et al. 2005; Forsythe et al. 2006; Dentin et al. 2008; D'Apolito et al. 2010; Duran-Reyes et al. 2010; Lee et al. 2010; Love et al. 2010; Rahman et al. 2010; Sekine et al. 2010; Mondoux et al. 2011). The first direct study on O-GlcNAc was established in an immortal murine adipocyte cell line (3T3-L1), whereby using PUGNAc (PUGNAc, the first generation of OGA inhibitors; Dong and Hart 1994; Haltiwanger et al. 1998) to elevate global O-GlcNAc levels lead to an impairment of acute insulin-stimulated glucose uptake and signal transmission through the IRS/PI3K/Akt cascade (Vosseller et al. 2002). Complementary to PUGNAc administration, transgenic mice overexpressing OGT in adipose and other peripheral tissues displayed insulin resistant phenotypes despite normal blood glucose levels (McClain et al. 2002), a condition that closely resembles transgenic mice overexpressing GFAT, the rate-limiting enzyme in the HBP (Hebert et al. 1996; McClain et al. 2000). Moreover, overexpression of OGA in diabetic db/db mice was reported to alleviate the whole-body insulin resistant condition (Dentin et al. 2008). In addition to mammalian models, the implication of O-GlcNAc in the insulin signaling pathway has been further supported with studies using two other model organisms, Drosophila melanogaster (Sekine et al. 2010) and Caenorhabditis elegans (Hanover et al. 2005; Forsythe et al. 2006; Lee et al. 2010; Love et al. 2010; Rahman et al. 2010; Mondoux et al. 2011), in which genetic perturbation of O-GlcNAc cycling enzymes results in distinct phenotypes that recapitulate their corresponding insulin signaling mutant phenotypes: body size in fruit flies and life span/dauer regulation in nematodes.
While PUGNAc has been routinely used for the past decades as an OGA inhibitor to manipulate O-GlcNAc levels in vivo (Dong and Hart 1994; Haltiwanger et al. 1998), recent available information on the structure and catalytic mechanism of OGA has opened the possibility for obtaining more selective OGA inhibitors than PUGNAc (Macauley and Vocadlo 2010). Several groups have undertaken this rational design challenge and generated various more selective and potent OGA inhibitors (Macauley et al. 2005; Dorfmueller et al. 2006, 2009, 2010; Whitworth et al. 2007; Macauley et al. 2008; Yuzwa et al. 2008; Macauley, Shan, et al. 2010). Unexpectedly, when Vocadlo's laboratory treated cultured adipocytes with NButGT (one of the more selective OGA specific inhibitors) to augment global O-GlcNAc levels, they did not observe any negative effect in insulin-stimulated glucose uptake or Akt phosphorylation as demonstrated in PUGNAc-treated adipocytes (Macauley et al. 2008). Additionally, animals subjected to NButGT regime remain insulin sensitive with a normal whole-body glucose homeostasis profile (Macauley, Shan, et al. 2010). In order to rule out the potential side effect derived from NButGT treatment, Vocadlo's group also utilized a structurally unrelated and less selective OGA inhibitor, termed 6-Ac-Cas, and examined its effect on insulin action in adipocytes. In line with their findings with NButGT, global elevation in O-GlcNAc levels upon 6-Ac-Cas treatment does not lead to insulin resistance (Macauley, He, et al. 2010). Collectively, these studies initiated a debate for the role of O-GlcNAc in insulin-mediated signal transduction and the development of insulin resistance.
In addition to its anabolic function, insulin also plays a significant pro-survival role in various tissues (Wick and Liu 2001; Duronio 2008). Hence, insulin resistance not only manifests in the dysregulation of glucose homeostasis but also results in programmed cell death in multiple organs, leading to complications such as retinopathy (Reiter and Gardner 2003) and nephropathy (De Cosmo et al. 2013) in diabetic individuals. Given that excessive HBP flux has been implicated in the impairment of the pro-survival role of insulin upon serum-deprivation in a retinal cell line via disrupting the IRS/PI3K/Akt signaling cascade (Barber et al. 2001; Nakamura et al. 2001), we set out to initially test the hypothesis that insulin's pro-survival function could be inhibited by O-GlcNAc elevation. Based on our initial findings, we began to scrutinize PUGNAc's action in the inhibition of insulin action. Toward this end, we use PUGNAc as well as two more selective OGA inhibitors, GlcNAcstatin-G (GNSg, developed by van Aalten's group; Dorfmueller et al. 2010) and Thiamet-G (TMG, a more stable version of NButGT synthesized by Vocadlo's group; Yuzwa et al. 2008) in our research. Since PUGNAc was previously shown to also affect lysosomal hexosaminidase A/B (HexA/B) (Stubbs et al. 2009), we also included a selective HexA/B inhibitor, INJ2 (generated in Lin's group; Ho et al. 2010), in our studies in an attempt to narrow down PUGNAc's role in hampering insulin action. Using these tools, we demonstrate that blocking either OGA or HexA/B using more selective inhibitors does not recapitulate PUGNAc treatment, suggesting that a yet unknown target is likely responsible for PUGNAc-mediated inhibition of insulin action.
Results
Elevation of global O-GlcNAc levels does not affect the pro-survival action of insulin
We chose Chinese hamster ovary cells ectopically overexpressing human insulin receptor (CHO-IR; Ebina et al. 1985) as our cell line of choice since this model has been extensively used to study insulin-mediated signal transduction (Sun et al. 1992; Wilden et al. 1992; Myers et al. 1993). Under serum-withdrawal condition, adding insulin into CHO-IR culture can prevent serum deprivation-induced apoptosis via activating the insulin-mediated mitogenic pathway (Bertrand et al. 1998; Lee-Kwon et al. 1998), a phenomenon observed in the retinal neuron cell model as well (Barber et al. 2001). In contrast to the retinal neuron model, CHO-IR cells do not require laborious differentiation steps that are imperative for culturing retinal neurons, hence allowing us to streamline the experimental workflow. To evaluate the protection by insulin against serum-withdrawal induced apoptosis, we implemented two parallel experiments in our workflow: the first approach monitors the formation of internucleosomal DNA fragments, a well-established signature for cells undergoing programmed cell death, using propidium iodine (PI) to stain and then quantify of the subG1 distribution of the intracellular DNA content. The second approach employs immunoblot detection of activated/cleaved caspase-3 (an executioner of apoptosis) and the cleavage product of its downstream substrate, poly-ADP ribose polymerase-1 (PARP-1). The formation of cleaved caspase-3 and cleaved PARP-1 precedes the formation of DNA fragments during the course of apoptosis (Hengartner 2000).
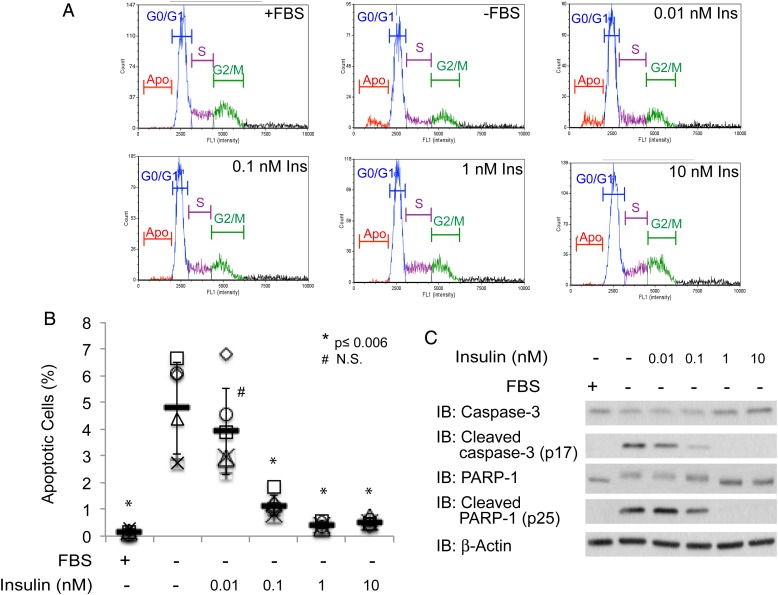
CHO-IR cells were susceptible to apoptosis after culturing in serum-withdrawal conditions for 24 h as represented by the appearance of apoptotic DNA fragments detected by PI staining (∼5%, Figure 1A and B). Likewise, cleavage products from caspase-3 and PARP-1 were also readily detected in cell lysate prepared from cells that were serum deprived, but not from those that were cultured in the presence of serum (Figure 1C). To establish the lowest concentration of insulin that is sufficient for its anti-apoptotic function, we cultured CHO-IR in the serum free medium supplemented with 0.01, 0.1, 1 or 10 nM of insulin. We did not detect any of the apoptosis markers (fragmented DNA, cleaved caspase-3 or cleaved PARP-1) with 1 or 10 nM insulin treatment, indicating that 1 nM of insulin is sufficient to effectively rescue CHO-IR cells from undergoing serum-withdrawal-induced apoptosis (Figure 1). While the percentage of apoptotic cells is lower than reported in Bertrand et al., our observation is otherwise in agreement with their findings in the same type of cells (Bertrand et al. 1998).
Fig. 1.
Insulin rescues CHO-IR cells from undergoing serum-withdrawal induced programmed cell death. (A) Distribution of fluorescence intensity of PI stained CHO-IR cells that were cultured in the presence or absence of serum and 0.01, 0.1, 1 and 10 nM insulin for 24 h. (B) Scatter plots represent the percentage of apoptotic cells from each condition (n = 6). Independent experiments were plotted with an identical symbol for different conditions. Black bars indicate the average percentage from six independent experiments, and the error bars indicate ± STDEV. Student's t-test (paired, one-tailed) was used to evaluate statistical significance using serum-deprived sample as the control. * P ≤ 0.006, # NS, not significant. (C) Western blots of apoptotic markers, cleaved caspase-3 and cleaved PARP-1. Antibodies against full-length caspase-3 and PARP-1, as well as β-actin, are included as controls. This figure is available in black and white in print and in colour at Glycobiology online.
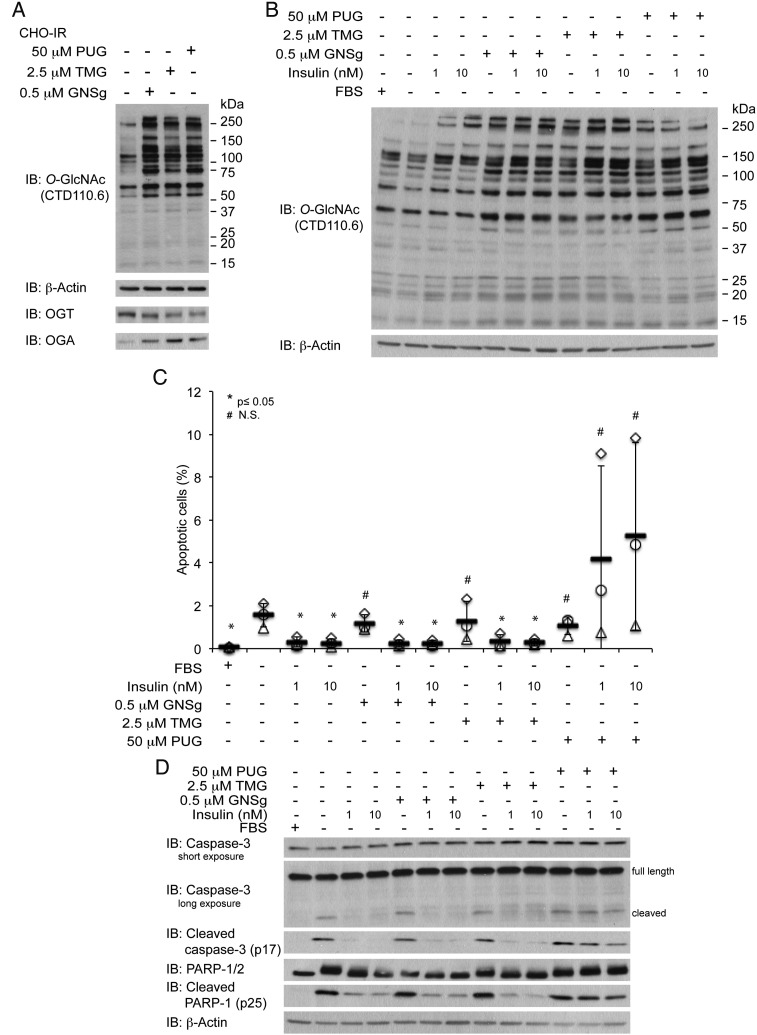
To increase the global O-GlcNAc levels, we cultured CHO-IR cells in the presence of GNSg (0.5 µM; Dorfmueller et al. 2009), TMG (2.5 µM; Yuzwa et al. 2008) or PUGNAc (50 µM) for 24 h (Figure 2A). Since GNSg and TMG were rationally designed based on the structural and catalytic information of OGA and its bacterial homologs, both inhibitors are more potent and showed greater selectivity against OGA over HexA/B in comparison to PUGNAc (Macauley and Vocadlo 2010). As a result, instead of treating cells with the same concentration for all three inhibitors, we selected concentrations for each inhibitor that gave us consistent and comparable global O-GlcNAc levels as detected by a one-dimensional immunoblot using a pan-O-GlcNAc-specific antibody, CTD110.6 (Figure 2A).
Fig. 2.
PUGNAc (PUG), but not elevated O-GlcNAc levels via OGA inhibition, blocks the pro-survival function of insulin. (A) O-GlcNAc western blots of cell lysate from CHO-IR cells treated with 0.5 µM GNSg, 2.5 µM TMG or 50 µM PUG in the absence or presence of insulin (1 nM or 10 nM). (B) Scatter plots represent the percentage of apoptotic cells from each condition (n = 3). Independent experiments were plotted with an identical symbol for different conditions. Black bars indicate the average percentage from three independent experiments, and the error bars indicate ± STDEV. Student's t-test (paired, one tailed) was used to evaluate statistical significance using serum-deprived sample as the control. * P ≤ 0.05, # NS, not significant. (C) Western blots of apoptotic markers, cleaved caspase-3 and cleaved PARP-1, and their full-length counterparts as well as β-actin of cell lysates treated with the same conditions as in (A) and (B).
When CHO-IR cells were treated with either GNSg or TMG in the presence of insulin, we did not observe the formation of apoptotic DNA fragment (Figure 2B), or caspase-3 and PARP-1 cleavage products (Figure 2C). On the other hand, all of the apoptotic markers were readily detectable when cells were cultured in the presence of both insulin and PUGNAc (Figure 2B and C). Collectively, these results indicate that unlike PUGNAc, GNSg and TMG do not lead to any detrimental effect on the anti-apoptotic action of insulin. Since both GNSg and TMG are more potent and selective OGA inhibitor than PUGNAc, our observation suggests that elevated global O-GlcNAc levels alone are not responsible for dampening the protective action of insulin. In agreement with Macauley et al. whose reports primarily focused on the glucose homeostasis aspect of insulin function (Macauley et al. 2008; Macauley, He, et al. 2010; Macauley, Shan, et al. 2010), our data also indicate that PUGNAc impinges on insulin actions via a secondary target rather than the inhibition of OGA.
Lysosomal hexosaminidase B inhibition is not responsible for PUGNAc-mediated inhibition of insulin action
After ruling out O-GlcNAc elevation alone in interfering with the protective action of insulin, we were curious to understand the inhibitory effect of PUGNAc on insulin action. In mammals, there are two additional enzymes, namely lysosomal hexosaminidases A and B (HexA and HexB or HexA/B), that hydrolyze terminal GlcNAc structure via the same substrate-assisted catalytic mechanism utilized by OGA (Davies and Martinez-Fleites 2010; Slamova et al. 2010). Although OGA and HexA/B belong to different CAZy families and reside in distinct cellular compartments, PUGNAc was found to inhibit HexA/B leading to an accumulation of glycosphingolipids (GSLs) in various neuronal cell lines (Stubbs et al. 2009; Ho et al. 2010). We reasoned that one possible scenario to explain the discrepancy between PUGNAc and other selective OGA inhibitors may be due to PUGNAc's inhibition of lysosomal HexA/B activity. To test our hypothesis, we utilized a highly selective HexA/B inhibitor, INJ2, which is a GlcNAc-type iminocyclitiol derivative synthesized by Lin's group (Ho et al. 2010), to specifically inhibit lysosomal HexA/B activity without altering the intracellular O-GlcNAc profile.
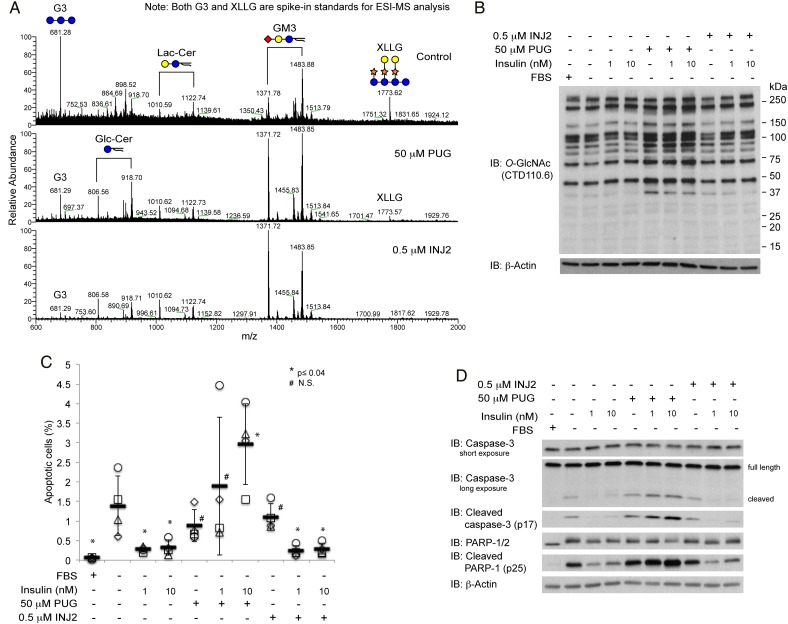
To confirm PUGNAc and INJ2 display a similar inhibitory effect on lysosomal HexA/B and yield a comparable GSL profile, we performed an electrospray ionization mass spectrometry analysis on permethylated GSLs isolated from CHO-IR cells with vehicle control (DMSO), PUGNAc or INJ2 treatments. In the vesicle control treatment, our GSL profiling reveals GM3 as the predominant GSL expressed in CHO-IR cells (Figure 3A, top panel), which agrees with previous reports in the parental CHO cell line (Briles et al. 1977; Young et al. 1990). Based on the relative peak intensities of two spiked-in MS standards (G3 and XLLG) to GSL peaks, both PUGNAc (Figure 3A, middle panel) and INJ2 (Figure 3A, bottom panel) treatment lead to a global increase of GM3 as wells as lactose-ceramide (Lac-Cer) and glucose-ceramide (Glc-Cer) in a similar manner. Unlike PUGNAc, however, INJ2 does not result in any change in global O-GlcNAc levels (Figure 3B).
Fig. 3.
PUGNAc (PUG), but not elevated GSL via HexA/B inhibition, blocks the protective action of insulin. (A) Mass spectrometry analysis on permethylated GSL reveals a relative increase in global GM3, Lac-Cer and Glc-Cer levels in 50 µM PUG or 0.5 µM INJ2-treated cells to spiked in standards. G3 and XLLG are spiked in as standards after permethylated GSLs from each treatment was normalized according to the BCA assay readout from the initial starting materials. Since all the spectra are shown based on the relative ion intensity, the relative abundance of internal standards to GSLs reflects an inverse correlation in which sample with higher ion intensities for GSLs has lower ion peaks for both G3 and XLLG. (B) O-GlcNAc western blots of cell lysates from CHO-IR cells treated with 50 µM PUG or 0.5 µM INJ2 in the absence or presence of insulin (1 or 10 nM). (C) Scatter plots represent the percentage of apoptotic cells from each condition (n = 4). Independent experiments were plotted with an identical symbol for different conditions. Black bars indicate the average percentage from four independent experiments, and the error bars indicate ± STDEV. Student's t-test (paired, one tailed) was used to evaluate statistical significance using serum-deprived sample as the control. * P ≤ 0.04, # N.S., not significant. (D) Western blots of apoptotic markers, cleaved caspase-3 and cleaved PARP-1, and their full-length counterparts as well as β-actin of cell lysates treated with the conditions as described in (B) and (C). This figure is available in black and white in print and in colour at Glycobiology online.
We did not observe any increase in the apoptotic DNA fragments in cells treated with both insulin and INJ2 (Figure 3C). Likewise, comparable levels of cleaved products from caspase-3 and cleaved PARP-1 were detected when CHO-IR cells were cultured in insulin alone or in the presence of both insulin and INJ2 (Figure 3D). Thus, according to our observations, targeted inhibition of lysosomal HexA/B does not explain the inhibitory effect of PUGNAc on the protection of insulin upon serum-withdrawal-induced apoptosis.
An unknown target of PUGNAc is causing the inhibitory effect on insulin
Having excluded the scenarios in which targeting either OGA or HexA/B alone impinge on insulin's anti-apoptotic function, we wanted to explore the possibility that a simultaneous perturbation of both OGA and HexA/B activities resulting from PUGNAc treatment is perhaps essential for the detrimental outcome. To address this question, we compared the protective action of insulin with PUGNAc alone and, with the combination of OGA and HexA/B selective inhibitors. We hypothesized that if the concurrent inhibition on both OGA and HexA/B is required to impinge on insulin action, the presence of GNSg or TMG in conjunction to INJ2 would mimic PUGNAc treatment.
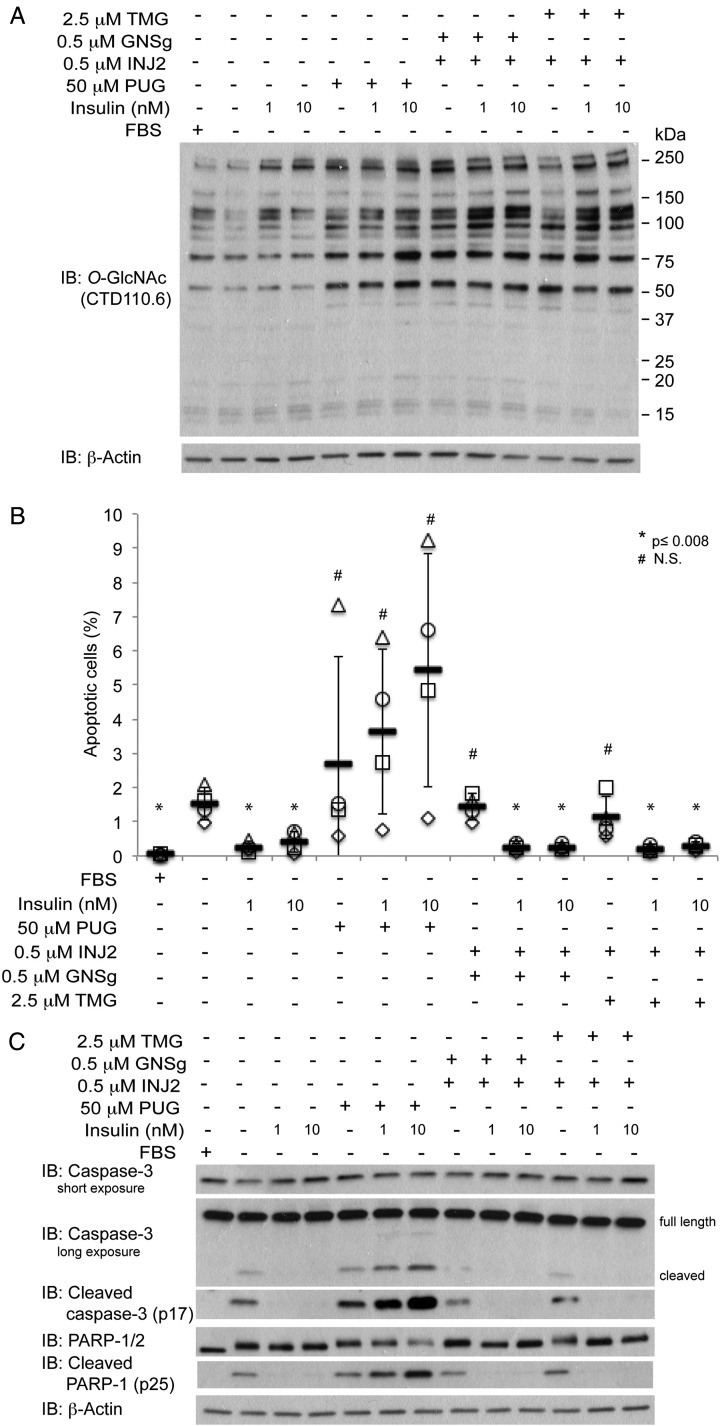
In Figure 4A, we showed that treating cells with both OGA and HexA/B inhibitors lead to an increase in the levels of O-GlcNAc modified proteins which is comparable with that of PUGNAc treatment (Figure 4A). Counter to our hypothesis, when CHO-IR cells were treated with either GNSg/INJ2 or TMG/INJ2 combinations in conjunction with insulin, we were not able to detect any significant increase in the apoptotic cell population (Figure 4B) or the cleavage products of caspase-3 and PARP-1 (Figure 4C). Since none of the treatments recapitulated the negative effect of PUGNAc on the pro-survival role of insulin, we conclude that the inhibition of OGA and HexA/B by PUGNAc are irrelevant in PUGNAc-induced inhibition of insulin action. In conclusion, our study strongly suggests that PUGNAc has an unknown target, which is responsible for its role in compromising insulin action.
Fig. 4.
A combination of elevated O-GlcNAc and GSL via specific inhibitors of OGA and HexA/B do not recapitulate PUGNAc action. (A) O-GlcNAc western blots of cell lysates from CHO-IR cells treated with 50 µM PUG or 0.5 µM INJ2 in the absence or presence of insulin (1 or 10 nM). (B) Scatter plots represent the percentage of apoptotic cells from each condition (n = 4). Independent experiments were plotted with an identical symbol for different conditions. Black bars indicate the average percentage from four independent experiments, and the error bars indicate ± STDEV. Student's t-test (paired, one-tailed) was used to evaluate statistical significance using serum-deprived sample as the control. * P ≤ 0.008, # NS, not significant. (C) Western blots of apoptotic markers, cleaved caspase-3 and cleaved PARP-1, and their full-length counterparts as well as β-actin of cell lysates treated with the same conditions as in (A) and (B).
Discussion
Controversy surrounding the role of O-GlcNAc in regulating insulin action using PUGNAc and two other OGA inhibitors (NButGT and 6-Ac-Cas) prompted us to compare the effect of PUGNAc to GNSg and TMG on insulin action. In contrast to PUGNAc, which lacks inhibitory selectivity of OGA over lysosomal hexosaminidases, GNSg and TMG are structurally distinctive yet more selective and potent OGA inhibitors. Unlike Vocadlo and colleagues whose studies on NButGT (Macauley et al. 2008; Macauley, Shan, et al. 2010) and 6-Ac-Cas (Macauley, He, et al. 2010) focused on the impact of O-GlcNAc on insulin-mediated glucose uptake in adipocytes, our primary goal was to investigate the effect of O-GlcNAc on the anti-apoptotic action of insulin. This is because insulin not only serves as a regulator for glucose homeostasis but also acts as a survival factor in some target tissues and cell types (Figure 1; Reiter and Gardner 2003; Barber et al. 2011). We also wanted to follow-up on studies by Gardener and Buse that had implicated increased flux through the HBP in inhibition of the pro-survival role of insulin (Nakamura et al. 2001). We observed that while GNSg, TMG and PUGNAc all lead to an elevation of global O-GlcNAc levels, only PUGNAc treatment results in an attenuation of the pro-survival function of insulin (Figure 2). As a side note, we routinely detected a slight increase in global O-GlcNAc when CHO-IR cells were exposed to the insulin regardless of the presence of additional inhibitors (Supplementary data, Figure S2A, S3B and S4A). While we do not comprehend the biological significance of such phenomenon, it has also been shown in 3T3-L1 adipocytes that insulin stimulation is linked to enhancing OGT activity potentially via insulin-dependent tyrosine phosphorylation of OGT (Whelan et al. 2008). Since GNSg and TMG are specifically designed to target OGA, our results are resonant with those in adipocytes by Macauley et al. (2008), Macauley, He, et al. (2010) and Macauley, Shan, et al. (2010), in which increased O-GlcNAc levels does not directly participate in hampering specific insulin functions.
Having ruled out the direct involvement of OGA in PUGNAc-mediated inhibitory effect of anti-apoptotic function of insulin, we further examined the role of PUGNAc in interfering with insulin action. The second obvious PUGNAc target is lysosomal HexA/B, since previous studies have established that PUGNAc can also block lysosomal HexA/B activity and results in a buildup in GSL levels (Stubbs et al. 2009; Ho et al. 2010) and free oligosaccharides (Mehdy et al. 2012) by hampering GSL and N-glycan turnover, respectively. Indeed, dysregulation in GSL metabolism has been proposed to be one of the causative factors in the development of insulin resistance (Inokuchi 2014). For example, using a cytokine-induced insulin resistant adipocyte model, Inokuchi and colleagues detected an upregulation in GM3 synthase expression, an accumulation in GM3 levels and a disruption in insulin-mediated signal transmission (Kabayama et al. 2005; Tagami et al. 2002). Additionally, Tanabe et al. documented a drastic shift in the GSL profile of high-fat/sugar-diet-induced diabetic mice compared with that of control animals, with an increase in GM2, GM1 and GD1a levels (Tanabe et al. 2009). In order to investigate whether PUGNAc-mediated alteration in GSL contributes to the impingement of insulin action, we chose a selective and potent HexA/B inhibitor, INJ2, for the comparison study. We reasoned that, by using INJ2 alone or INJ2 in conjunction with either GNSg or TMG, it would be possible to unveil the culprit behind PUGNAc-mediated insulin resistance: if the inhibition of HexA/B by INJ2 alone is sufficient to recapitulate PUGNAc effect, then blocking HexA/B activity is the main event in PUGNAc-induced insulin resistance; otherwise, if the presence of both HexA/B and OGA selective inhibitors are required to impinge on insulin action, then PUGNAc-induced insulin inhibition entails perturbations of two independent glycoconjugate catabolic pathways. MS analysis of GSL profile reveals that the expression of GSL in CHO-IR cells resembles its parental CHO cell line, in which GM3 is the predominant species (Young et al. 1990). This is because CHO cells do not express GM2 synthase that is responsible for the biosynthesis of GM2 (Rosales Fritz et al. 1997). While we did not detect any GM2 even after PUGNAc or INJ2 treatments, we observed significantly higher levels of GM3, as wells as the precursors of GM3 (Lac-Cer and Glc-Cer) in the GSL biosynthesis pathway, in both PUGNAc and INJ2 treated samples compared with a sample treated with vehicle control (DMSO) (Figure 3A). Surprisingly, neither selective HexA/B inhibitor alone nor the presence of both selective HexA/B and OGA inhibitors lead to any attenuation in insulin action (Figures 3 and 4), suggesting that concurrent blocking OGA and HexA/B activities is not sufficient to induce insulin resistance.
Other than OGA and lysosomal HexA/B, an additional obvious PUGNAc's target is α-N-acetylglucosaminidase (NAGLU) that participates in the lysosomal degradation of heparin sulfate (Ficko-Blean et al. 2008). Enzymatic characterization of recombinant NAGLU from different species has demonstrated that NAGLU activity is susceptible to inactivation by various non-selective N-acetylglucosaminidase inhibitors, including PUGNAc and 6-Ac-Cas (Zhao and Neufeld 2000; Ficko-Blean et al. 2008). Interestingly, when Macauley and colleagues treated 3T3-L1 adipocytes with 6-Ac-Cas, they observed a drastic increase in global O-GlcNAc levels while the cells remain insulin sensitive (Macauley, He, et al. 2010). Since PUGNAc and 6-Ac-Cas are both potent OGA and NAGLU inhibitors, the differential outcomes from these two inhibitors suggest that NAGLU is unlikely a contributor to PUGNAc-mediated attenuation of insulin action. Collectively, our and others' results strongly implicate that PUGNAc has an additional elusive target that is causing insulin resistance.
While we are still puzzled by PUGNAc's effect on insulin resistance, we propose the following less obvious culprits that might be responsible for this outcome that warrant further investigations. Given that the structure of PUGNAc is based on an N-acetylglucosamine (GlcNAc) scaffold, one can speculate that PUGNAc may affect the functions of enzymes or other proteins that interact with hexosamine-containing molecules. These potential PUGNAc targets include (i) a nucleocytoplasmic hexosaminidase (HexD), whose in vivo substrates have yet to be unambiguously determined (Gutternigg et al. 2009); (ii) ENGase, an enzyme that participates in cytosolic free oligosaccharides processing (Suzuki et al. 2002; Huang et al. 2015); (iii) sugar and nucleotide sugar transporters; (4) metabolic enzymes; (5) GlcNAc transferases for complex glycosylations; and (6) lectins that bind GlcNAc containing structures.
A mass spectrometry-based attempt to address PUGNAc-mediated insulin resistance was recently reported by Baek and coworkers (Lee et al. 2013), in which a targeted quantitative proteomics approach was taken to identify nucleotide-binding proteins whose expressions differ in control and PUGNAc treated adipocytes and myotubes. Many of the differently expressed nucleotide-binding proteins identified in this study are functionally associated with the protein ubiquitination pathway (Lee et al. 2013). However, it remains unclear whether such phenomenon is specific to PUGNAc administration, since cells treated with PUGNAc and 6-Ac-Cas both result in an increase in global ubiquitin levels compared with that of the control treatment (Lee et al. 2013). Further in-depth examination is warranted to delineate the exact mechanism of PUGNAc in the modulating insulin action as well as registering all of the PUGNAc targets.
Given that PUGNAc has been used extensively as an OGA inhibitor to implicate O-GlcNAc levels in multiple biological processes, it is prudent to scrutinize those conclusions that were made solely based on PUGNAc treatment and future studies should utilize the more selective inhibitors and/or a combination of both genetic and pharmacological approaches to study O-GlcNAc biology.
Materials and methods
Reagents
PUGNAc [O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino N-phenyl carbamate] was purchased from Toronto Research Chemicals, Inc. (ON, Canada). Thiamet-G, PI and RNAse A were purchased from Sigma-Aldrich (St. Louis, MO). GlcNAcstatin G was a kind gift from Dr. Daan van Aalten (University of Dundee, UK). INJ2 was a kind gift from Dr. Chun-Hung Lin (Academia Sinica, Taiwan). Protease inhibitor and phosphatase inhibitor cocktails were purchased from Calbiochem (Gibbstown, NJ). Human insulin was purchased from Roche (Indianapolis, IN).
Cell culture and treatments
CHO-IR cells (Chinese hamster ovary cells overexpressing human insulin receptor, a gift from Dr. Richard Roth) were maintained in Ham's F-12 medium (Corning/Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (Life Technologies/Invitrogen, Carlsbad, CA) as previously described (Ebina et al. 1985). Cells were seeded in 12-well plates (5 × 104 cells per well, for PI staining), 35 mm dishes (1.5 × 105 cells per dish, for western blotting) or 100 mm dishes (1 × 106 cells per dish, for GSL analysis) and cultured for 48 h before treatments. For each treatment, the cells were washed twice with PBS, and re-fed for an additional 24 h with Ham's F-12 medium with or without supplemented 10% FBS, insulin (1 or 10 nM) or inhibitors (0.5 µM GNSg, 2.5 µM TMG, 50 µM PUGNAc or 0.5 µM INJ2) as indicated in the figure legends.
PI staining and the measurement of apoptotic cells
At the end of the incubation period, treated cells (∼5 × 104 cells per sample) were washed twice with PBS and incubated with 60 mM EDTA/PBS for 5 min at room temperature. Dislodged cells were transferred into 15 mL centrifuge tubes and centrifuged at 800 × g for 8 min at 4°C. Cell pellets were resuspended in 200 µL of 60 mM EDTA/PBS, mixed with 1.8 mL of 70% pre-chilled ethanol and incubated at −20°C for overnight. The cells were centrifuged (800 × g for 15 min at 4°C), resuspended in the staining buffer (50 µg/mL PI, 200 µg/mL RNAse A, 1% Triton X-100). After incubating at 37°C for 1 h, the cells were pelleted, resuspended in 20 µL of PBS and analyzed on a Nexcelom automatic cell counter (Nexcelom Bioscience, Lawrence, MA) using the default setting for cell cycle analysis (CBA_Cell Cycle_PI595 nm). Percentage of apoptotic cells from each analysis was compiled and graphed into the scatter plot format. Each identical symbol in different conditions is a data point collected from the same experiment. T-test (paired, one-tailed) was used to determine statistical significance.
Whole cell lysate preparation and immunoblotting
At the end of the incubation period, treated cells were washed twice with PBS and incubated with 60 mM EDTA/PBS for 5 min at room temperature. Dislodged cells were transferred into 15 mL centrifuge tubes and centrifuged at 800 × g for 8 min at 4°C. Cell pellets were resuspended in TNS lysis buffer (10 mM Tris, pH 7.5, 150 mM NaCl, 1% Igepal CA-630, 0.1% SDS, 4 mM EDTA, 1 mM DTT, 100 µM PUGNAc, protease inhibitor cocktail and phosphatase inhibitor cocktail) and incubated on ice for 15 min. Cell lysates were then transferred into microtubes and centrifuged at 12,000 × g for 15 min at 4°C. Clarified supernatants were transferred into fresh tubes and protein concentration from each sample was quantified with Bradford protein assay according to manufacturer's instructions (Bio-Rad, Hercules, CA) using bovine serum albumin (BSA; from Thermo Fisher Scientific, Pittsburgh, PA) as a standard. Equal amount of protein from each lysate was mixed with 5× Laemmli sample buffer, heated at 80°C for 15 min, and stored at −20°C until further immunoblot analyses. Equal amount of total protein (typically 5–10 µg per lane) was subjected to SDS–PAGE analysis using Mini-PROTEAN TGX precast gels (7.5%, 4–15% or 4–20% gels, Bio-Rad, electrophoresis settings: 200 V, 30–35 min). The gels were subsequently electrotransferred (Trans-Blot SD semi-dry apparatus, Bio-Rad, electrotransfer settings: 20 V, 30 min) onto Immobilon-P membranes (0.45 µm, EMD Millipore Corporation, Bedford, MA) and the membranes were subjected to immunoblotting according to the standard procedure. The membranes were blocked with either 3% BSA (CTD110.6) or 5% non-fat milk (the rest of the blots) in TBST (TBS supplemented with 0.1% Tween 20). The primary antibodies used included (source and dilution factor as indicated): anti-caspase-3 (Cell Signaling Technology, Beverly, MA, 1:1000), anti-cleaved caspase-3 (Cell Signaling Technology, 1:2500), PARP-1/2 (Santa Cruz Biotechnology, Santa Cruz, CA, 1:4000) and cleaved PARP-1/p25 (EMD Millipore, 1:20,000). All the membranes were incubated in primary antibodies for overnight at 4°C. The secondary antibody-HRP conjugates were used in accordance to the species in which the primary antibodies were generated [sheep anti-mouse IgG-HRP (GE Healthcare, Piscataway, NJ, 1:20,000), goat anti-rabbit IgG-HRP (GE Healthcare, 1:20,000)] and the final detection of HRP activity was performed using Pierce ECL western blotting substrate (Thermo Fisher Scientific/Pierce, Rockford, IL). The only exception was the cleaved caspase-3 blot, in which stabilized goat anti-rabbit IgG (H + L)-HRP (Thermo/Pierce, 1:5000) and SuperSignal west Femto (Thermo Fisher Scientific/Pierce) were used as the secondary antibody and ECL substrate, respectively. Finally, the membranes were exposed to HyBlotCL films (Denville Scientific, Metuchen, NJ). Upon the completion of the aforementioned procedure, all the blots were subsequently stripped (either with 0.1 M glycine, pH 2.5, or 30% H2O2) and reprobed with β-actin (Sigma-Aldrich, 1:50,000) antibody as loading control.
Glycospingolipid analysis
CHO-IR cell (treated with DMSO, 50 µM PUGNAc or 0.5 µM INJ2 for 24 h; 4 × 100 mm dishes per condition) pellets were subjected to organic solvent extraction, alkaline saponification, C18 solid-phase extraction and permethylation, as described in Boccuto et al. (2014), to obtain permethylated GSL. The protein-containing insoluble fraction from the initial organic solvent extraction step was subjected to trypsin digestion in the presence of 40 mM ammonium bicarbonate at 37°C for overnight and the total peptide contents were quantified with bicinchoninic acid (BCA) assay according to manufacturer's instructions (Thermo Fisher Scientific/Pierce) using BSA (from Thermo Fisher Scientific, Pittsburgh, PA) as a standard. Permethylated GSLs from each treatment were dissolved in 1 mM sodium hydroxide prepared in methanol–water (1:1) after normalizing the amount to the readout of the BCA assay. Each sample was spiked in with two internal standards for relative quantification of peaks compared with the standards: permethylated cellotriose (m/z 681) and permethylated xyloglugan XLLG oligosaccharide (m/z 1773.85), see (York et al. 1990; Tuomivaara et al. 2015) for the structures and preparation. All samples were directly infused into a linear ion trap mass spectrometer (Thermo Finnigan, San Jose, CA) using an electrospray source connected to a syringe with a flow rate of 3 µL/min. All the spectra were acquired in the positive mode, with a spray voltage of 5 kV and capillary temperature of 275°C.
Supplementary data
Supplementary data for this article are available online at http://glycob.oxfordjournals.org/.
Funding
The GSL data were collected on an LTQ mass spectrometer funded by the BioEnergy Science Center (to Dr. William S. York, Complex Carbohydrate Research Center, University of Georgia). This work is supported in part by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (R01DK075069 to L.W.) and National Institute of General Medical Sciences at the National Institutes of Health (P41GM103490, L.W. senior investigator). C.F.T. is an American Heart Association Pre-doctoral Fellow (Southeast affiliate, 0715377B) and a Cousins Foundation Fellow (Complex Carbohydrate Research Center, University of Georgia)
Conflict of interest statement
None declared.
Supplementary Material
Acknowledgements
We are indebted to Dr. Sami T. Tuomivaara for in-depth discussions, critical reading of this manuscript and providing highly purified cellotriose (G3) and XLLG xyloglucan oligosaccharides and Drs. Kazuhiro Aoki and Tadahiro Kumagai for technical advice on the GSL analysis. We thank Drs. Richard A. Roth (Stanford University, Stanford, CA), Daan van Aalten (University of Dundee, UK) and Chun-Hung Lin (Academia Sinica, Taiwan) for sharing CHO-IR cells, GlcNAcstatin G, and INJ2, respectively. We are grateful to Dr. Gerald W. Hart (Johns Hopkins University, Baltimore, MD) for providing pan-O-GlcNAc specific (CTD110.6) antibody.
Abbreviations
BCA, bicinchoninic acid; BSA, bovine serum albumin; CHO-IR, Chinese hamster ovary cells expressing human insulin receptor; Glc-Cer, glucose-ceramide; GlcNAc, N-acetylglucosamine; GNSg, GlcNAcstatin g; GSL, glycosphingolipid; HBP, hexosamine biosynthetic pathway; Hex, lysosomal hexosaminidase; HexA/B, hexosaminidase A/B; Lac-Cer, lactose-ceramide; NAGLU, α-N-acetylglucosaminidase; OGA, β-N-acetylglucosaminidase; O-GlcNAc, O-linked β-N-acetylglucosamine; OGT, O-GlcNAc transferase; PARP-1, poly-ADP ribose polymerase-1; PI, propidium iodine; PTM, post-translational modification; PUGNAc, O-(2-acetamido-2-deoxy-d-glucopyranosylidene)amino N-phenyl carbamate/PUGNAc; TMG, thiamet-G.
References
- Banerjee S, Robbins PW, Samuelson J. 2009. Molecular characterization of nucleocytosolic O-GlcNAc transferases of Giardia lamblia and Cryptosporidium parvum. Glycobiology. 19:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Gardner TW, Abcouwer SF. 2011. The significance of vascular and neural apoptosis to the pathology of diabetic retinopathy. Invest Ophthalmol Vis Sci. 52:1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Nakamura M, Wolpert EB, Reiter CE, Seigel GM, Antonetti DA, Gardner TW. 2001. Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J Biol Chem. 276:32814–32821. [DOI] [PubMed] [Google Scholar]
- Bertrand F, Atfi A, Cadoret A, L'allemain G, Robin H, Lascols O, Capeau J, Cherqui G. 1998. A role for nuclear factor kappaB in the antiapoptotic function of insulin. J Biol Chem. 273:2931–2938. [DOI] [PubMed] [Google Scholar]
- Boccuto L, Aoki K, Flanagan-Steet H, Chen CF, Fan X, Bartel F, Petukh M, Pittman A, Saul R, Chaubey A et al. 2014. A mutation in a ganglioside biosynthetic enzyme, ST3GAL5, results in salt & pepper syndrome, a neurocutaneous disorder with altered glycolipid and glycoprotein glycosylation. Hum Mol Genet. 23:418–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmelt G, Wakeham A, Elia A, Sasaki T, Plyte S, Potter J, Yang Y, Tsang E, Ruland J, Iscove NN et al. 2000. Decreased UDP-GlcNAc levels abrogate proliferation control in EMeg32-deficient cells. EMBO J. 19:5092–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briles EB, Li E, Kornfeld S. 1977. Isolation of wheat germ agglutinin-resistant clones of Chinese hamster ovary cells deficient in membrane sialic acid and galactose. J Biol Chem. 252:1107–1116. [PubMed] [Google Scholar]
- Buse MG. 2006. Hexosamines, insulin resistance, and the complications of diabetes: Current status. Am J Physiol Endocrinol Metab. 290:E1–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buse MG, Robinson KA, Marshall BA, Hresko RC, Mueckler MM. 2002. Enhanced O-GlcNAc protein modification is associated with insulin resistance in GLUT1-overexpressing muscles. Am J Physiol Endocrinol Metab. 283:E241–E250. [DOI] [PubMed] [Google Scholar]
- Clark RJ, Mcdonough PM, Swanson E, Trost SU, Suzuki M, Fukuda M, Dillmann WH. 2003. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 278:44230–44237. [DOI] [PubMed] [Google Scholar]
- D'apolito M, Du X, Zong H, Catucci A, Maiuri L, Trivisano T, Pettoello-Mantovani M, Campanozzi A, Raia V, Pessin JE et al. 2010. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J Clin Invest. 120:203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies GJ, Martinez-Fleites C. 2010. GlaxoSmithKline Award Lecture. The O-GlcNAc modification: Three-dimensional structure, enzymology and the development of selective inhibitors to probe disease. Biochem Soc Trans. 38:1179–1188. [DOI] [PubMed] [Google Scholar]
- De Cosmo S, Menzaghi C, Prudente S, Trischitta V. 2013. Role of insulin resistance in kidney dysfunction: Insights into the mechanism and epidemiological evidence. Nephrol Dial Transplant. 28:29–36. [DOI] [PubMed] [Google Scholar]
- Dentin R, Hedrick S, Xie J, Yates J III, Montminy M. 2008. Hepatic glucose sensing via the CREB coactivator CRTC2. Science. 319:1402–1405. [DOI] [PubMed] [Google Scholar]
- Dong DL, Hart GW. 1994. Purification and characterization of an O-GlcNAc selective N-acetyl-beta-D-glucosaminidase from rat spleen cytosol. J Biol Chem. 269:19321–19330. [PubMed] [Google Scholar]
- Dorfmueller HC, Borodkin VS, Schimpl M, Shepherd SM, Shpiro NA, Van Aalten DM. 2006. GlcNAcstatin: A picomolar, selective O-GlcNAcase inhibitor that modulates intracellular O-glcNAcylation levels. J Am Chem Soc. 128:16484–16485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfmueller HC, Borodkin VS, Schimpl M, Van Aalten DM. 2009. GlcNAcstatins are nanomolar inhibitors of human O-GlcNAcase inducing cellular hyper-O-GlcNAcylation. Biochem J. 420:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfmueller HC, Borodkin VS, Schimpl M, Zheng X, Kime R, Read KD, Van Aalten DM. 2010. Cell-penetrant, nanomolar O-GlcNAcase inhibitors selective against lysosomal hexosaminidases. Chem Biol. 17:1250–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Reyes G, Pascoe-Lira D, Garcia-Macedo R, Medina-Navarro R, Rosales-Torres AM, Vergara-Onofre M, Foyo-Niembro E, Gutierrez-Rodriguez ME, Garcia-Gutierrez MT, Valladares-Salgado A et al. 2010. O-GlcNAc-selective-N-acetyl-beta-D-glucosaminidase activity and mRNA expression in muscle is related to glucosamine-induced insulin resistance. Pharmacology. 85:121–130. [DOI] [PubMed] [Google Scholar]
- Duronio V. 2008. The life of a cell: Apoptosis regulation by the PI3K/PKB pathway. Biochem J. 415:333–344. [DOI] [PubMed] [Google Scholar]
- Ebina Y, Edery M, Ellis L, Standring D, Beaudoin J, Roth RA, Rutter WJ. 1985. Expression of a functional human insulin receptor from a cloned cDNA in Chinese hamster ovary cells. Proc Natl Acad Sci USA. 82:8014–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficko-Blean E, Stubbs KA, Nemirovsky O, Vocadlo DJ, Boraston AB. 2008. Structural and mechanistic insight into the basis of mucopolysaccharidosis IIIB. Proc Natl Acad Sci USA. 105:6560–6565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ME, Love DC, Lazarus BD, Kim EJ, Prinz WA, Ashwell G, Krause MW, Hanover JA. 2006. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc Natl Acad Sci USA. 103:11952–11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Wells L, Comer FI, Parker GJ, Hart GW. 2001. Dynamic O-glycosylation of nuclear and cytosolic proteins: Cloning and characterization of a neutral, cytosolic beta-N-acetylglucosaminidase from human brain. J Biol Chem. 276:9838–9845. [DOI] [PubMed] [Google Scholar]
- Gutternigg M, Rendic D, Voglauer R, Iskratsch T, Wilson IB. 2009. Mammalian cells contain a second nucleocytoplasmic hexosaminidase. Biochem J. 419:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltiwanger RS, Blomberg MA, Hart GW. 1992. Glycosylation of nuclear and cytoplasmic proteins. Purification and characterization of a uridine diphospho-N-acetylglucosamine:polypeptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 267:9005–9013. [PubMed] [Google Scholar]
- Haltiwanger RS, Grove K, Philipsberg GA. 1998. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O-(2-acetamido-2-deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem. 273:3611–3617. [DOI] [PubMed] [Google Scholar]
- Haltiwanger RS, Holt GD, Hart GW. 1990. Enzymatic addition of O-GlcNAc to nuclear and cytoplasmic proteins. Identification of a uridine diphospho-N-acetylglucosamine:peptide beta-N-acetylglucosaminyltransferase. J Biol Chem. 265:2563–2568. [PubMed] [Google Scholar]
- Hanover JA, Forsythe ME, Hennessey PT, Brodigan TM, Love DC, Ashwell G, Krause M. 2005. A Caenorhabditis elegans model of insulin resistance: Altered macronutrient storage and dauer formation in an OGT-1 knockout. Proc Natl Acad Sci USA. 102:11266–11271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Housley MP, Slawson C. 2007. Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature. 446:1017–1022. [DOI] [PubMed] [Google Scholar]
- Hart GW, Slawson C, Ramirez-Correa G, Lagerlof O. 2011. Cross talk between O-GlcNAcylation and phosphorylation: Roles in signaling, transcription, and chronic disease. Annu Rev Biochem. 80:825–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LF Jr, Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, Mcclain DA. 1996. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest. 98:930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO. 2000. The biochemistry of apoptosis. Nature. 407:770–776. [DOI] [PubMed] [Google Scholar]
- Ho CW, Popat SD, Liu TW, Tsai KC, Ho MJ, Chen WH, Yang AS, Lin CH. 2010. Development of GlcNAc-inspired iminocyclitiols as potent and selective N-acetyl-beta-hexosaminidase inhibitors. ACS Chem Biol. 5:489–497. [DOI] [PubMed] [Google Scholar]
- Holt GD, Hart GW. 1986. The subcellular distribution of terminal N-acetylglucosamine moieties. Localization of a novel protein-saccharide linkage, O-linked GlcNAc. J Biol Chem. 261:8049–8057. [PubMed] [Google Scholar]
- Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. 2005. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 96:1006–1013. [DOI] [PubMed] [Google Scholar]
- Huang C, Harada Y, Hosomi A, Masahara-Negishi Y, Seino J, Fujihira H, Funakoshi Y, Suzuki T, Dohmae N, Suzuki T. 2015. Endo-beta-N-acetylglucosaminidase forms N-GlcNAc protein aggregates during ER-associated degradation in Ngly1-defective cells. Proc Natl Acad Sci USA. 112:1398–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi JI. 2014. GM3 and diabetes. Glycoconj J. 31:193–197. [DOI] [PubMed] [Google Scholar]
- Kabayama K, Sato T, Kitamura F, Uemura S, Kang BW, Igarashi Y, Inokuchi J. 2005. TNFalpha-induced insulin resistance in adipocytes as a membrane microdomain disorder: Involvement of ganglioside GM3. Glycobiology. 15:21–29. [DOI] [PubMed] [Google Scholar]
- Kelly WG, Hart GW. 1989. Glycosylation of chromosomal proteins: Localization of O-linked N-acetylglucosamine in Drosophila chromatin. Cell. 57:243–251. [DOI] [PubMed] [Google Scholar]
- Kreppel LK, Blomberg MA, Hart GW. 1997. Dynamic glycosylation of nuclear and cytosolic proteins. Cloning and characterization of a unique O-GlcNAc transferase with multiple tetratricopeptide repeats. J Biol Chem. 272:9308–9315. [DOI] [PubMed] [Google Scholar]
- Lee J, Kim KY, Paik YK. 2010. Regulation of Dauer formation by O-GlcNAcylation in Caenorhabditis elegans. J Biol Chem. 285:2930–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Park JH, Moon PG, Baek MC. 2013. Identification of differentially expressed proteins by treatment with PUGNAc in 3T3-L1 adipocytes through analysis of ATP-binding proteome. Proteomics. 13:2998–3012. [DOI] [PubMed] [Google Scholar]
- Lee-Kwon W, Park D, Baskar PV, Kole S, Bernier M. 1998. Antiapoptotic signaling by the insulin receptor in Chinese hamster ovary cells. Biochemistry. 37:15747–15757. [DOI] [PubMed] [Google Scholar]
- Love DC, Ghosh S, Mondoux MA, Fukushige T, Wang P, Wilson MA, Iser WB, Wolkow CA, Krause MW, Hanover JA. 2010. Dynamic O-GlcNAc cycling at promoters of Caenorhabditis elegans genes regulating longevity, stress, and immunity. Proc Natl Acad Sci USA. 107:7413–7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley MS, Bubb AK, Martinez-Fleites C, Davies GJ, Vocadlo DJ. 2008. Elevation of global O-GlcNAc levels in 3T3-L1 adipocytes by selective inhibition of O-GlcNAcase does not induce insulin resistance. J Biol Chem. 283:34687–34695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley MS, He Y, Gloster TM, Stubbs KA, Davies GJ, Vocadlo DJ. 2010. Inhibition of O-GlcNAcase using a potent and cell-permeable inhibitor does not induce insulin resistance in 3T3-L1 adipocytes. Chem Biol. 17:937–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley MS, Shan X, Yuzwa SA, Gloster TM, Vocadlo DJ. 2010. Elevation of Global O-GlcNAc in rodents using a selective O-GlcNAcase inhibitor does not cause insulin resistance or perturb glucohomeostasis. Chem Biol. 17:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macauley MS, Vocadlo DJ. 2010. Increasing O-GlcNAc levels: An overview of small-molecule inhibitors of O-GlcNAcase. Biochim Biophys Acta. 1800:107–121. [DOI] [PubMed] [Google Scholar]
- Macauley MS, Whitworth GE, Debowski AW, Chin D, Vocadlo DJ. 2005. O-GlcNAcase uses substrate-assisted catalysis: Kinetic analysis and development of highly selective mechanism-inspired inhibitors. J Biol Chem. 280:25313–25322. [DOI] [PubMed] [Google Scholar]
- Marshall S, Bacote V, Traxinger RR. 1991. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 266:4706–4712. [PubMed] [Google Scholar]
- Mcclain DA, Alexander T, Cooksey RC, Considine RV. 2000. Hexosamines stimulate leptin production in transgenic mice. Endocrinology. 141:1999–2002. [DOI] [PubMed] [Google Scholar]
- Mcclain DA, Lubas WA, Cooksey RC, Hazel M, Parker GJ, Love DC, Hanover JA. 2002. Altered glycan-dependent signaling induces insulin resistance and hyperleptinemia. Proc Natl Acad Sci USA. 99:10695–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehdy A, Morelle W, Rosnoblet C, Legrand D, Lefebvre T, Duvet S, Foulquier F. 2012. PUGNAc treatment leads to an unusual accumulation of free oligosaccharides in CHO cells. J Biochem. 151:439–446. [DOI] [PubMed] [Google Scholar]
- Mondoux MA, Love DC, Ghosh SK, Fukushige T, Bond M, Weerasinghe GR, Hanover JA, Krause MW. 2011. O-Linked-N-acetylglucosamine cycling and insulin signaling are required for the glucose stress response in Caenorhabditis elegans. Genetics. 188:369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG Jr, Sun XJ, Cheatham B, Jachna BR, Glasheen EM, Backer JM, White MF. 1993. IRS-1 is a common element in insulin and insulin-like growth factor-I signaling to the phosphatidylinositol 3’-kinase. Endocrinology. 132:1421–1430. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Barber AJ, Antonetti DA, Lanoue KF, Robinson KA, Buse MG, Gardner TW. 2001. Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J Biol Chem. 276:43748–43755. [DOI] [PubMed] [Google Scholar]
- Nelson BA, Robinson KA, Buse MG. 2000. High glucose and glucosamine induce insulin resistance via different mechanisms in 3T3-L1 adipocytes. Diabetes. 49:981–991. [DOI] [PubMed] [Google Scholar]
- Perez-Cervera Y, Harichaux G, Schmidt J, Debierre-Grockiego F, Dehennaut V, Bieker U, Meurice E, Lefebvre T, Schwarz RT. 2011. Direct evidence of O-GlcNAcylation in the apicomplexan Toxoplasma gondii: A biochemical and bioinformatic study. Amino Acids. 40:847–856. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Stuchlick O, El-Karim EG, Stuart R, Kipreos ET, Wells L. 2010. Intracellular protein glycosylation modulates insulin mediated lifespan in C.elegans. Aging (Albany NY). 2:678–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter CE, Gardner TW. 2003. Functions of insulin and insulin receptor signaling in retina: Possible implications for diabetic retinopathy. Prog Retin Eye Res. 22:545–562. [DOI] [PubMed] [Google Scholar]
- Rosales Fritz VM, Daniotti JL, Maccioni HJ. 1997. Chinese hamster ovary cells lacking GM1 and GD1a synthesize gangliosides upon transfection with human GM2 synthase. Biochim Biophys Acta. 1354:153–158. [DOI] [PubMed] [Google Scholar]
- Sekine O, Love DC, Rubenstein DS, Hanover JA. 2010. Blocking O-linked GlcNAc cycling in Drosophila insulin-producing cells perturbs glucose-insulin homeostasis. J Biol Chem. 285:38684–38691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamova K, Bojarova P, Petraskova L, Kren V. 2010. Beta-N-acetylhexosaminidase: What's in a name…. Biotechnol Adv. 28:682–693. [DOI] [PubMed] [Google Scholar]
- Stubbs KA, Macauley MS, Vocadlo DJ. 2009. A selective inhibitor Gal-PUGNAc of human lysosomal beta-hexosaminidases modulates levels of the ganglioside GM2 in neuroblastoma cells. Angew Chem Int Ed Engl. 48:1300–1303. [DOI] [PubMed] [Google Scholar]
- Sun XJ, Miralpeix M, Myers MG Jr, Glasheen EM, Backer JM, Kahn CR, White MF. 1992. Expression and function of IRS-1 in insulin signal transmission. J Biol Chem. 267:22662–22672. [PubMed] [Google Scholar]
- Suzuki T, Yano K, Sugimoto S, Kitajima K, Lennarz WJ, Inoue S, Inoue Y, Emori Y. 2002. Endo-beta-N-acetylglucosaminidase, an enzyme involved in processing of free oligosaccharides in the cytosol. Proc Natl Acad Sci USA. 99:9691–9696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y et al. 2002. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J Biol Chem. 277:3085–3092. [DOI] [PubMed] [Google Scholar]
- Tanabe A, Matsuda M, Fukuhara A, Miyata Y, Komuro R, Shimomura I, Tojo H. 2009. Obesity causes a shift in metabolic flow of gangliosides in adipose tissues. Biochem Biophys Res Commun. 379:547–552. [DOI] [PubMed] [Google Scholar]
- Teo CF, Wollaston-Hayden EE, Wells L. 2010. Hexosamine flux, the O-GlcNAc modification, and the development of insulin resistance in adipocytes. Mol Cell Endocrinol. 318:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres CR, Hart GW. 1984. Topography and polypeptide distribution of terminal N-acetylglucosamine residues on the surfaces of intact lymphocytes. Evidence for O-Linked GlcNAc. J Biol Chem. 259:3308–3317. [PubMed] [Google Scholar]
- Tuomivaara ST, Yaoi K, O'neill MA, York WS. 2015. Generation and structural validation of a library of diverse xyloglucan-derived oligosaccharides, including an update on xyloglucan nomenclature. Carbohydr Res. 402:56–66. [DOI] [PubMed] [Google Scholar]
- Virkamaki A, Daniels MC, Hamalainen S, Utriainen T, Mcclain D, Yki-Jarvinen H. 1997. Activation of the hexosamine pathway by glucosamine in vivo induces insulin resistance in multiple insulin sensitive tissues. Endocrinology. 138:2501–2507. [DOI] [PubMed] [Google Scholar]
- Vosseller K, Wells L, Lane MD, Hart GW. 2002. Elevated nucleocytoplasmic glycosylation by O-GlcNAc results in insulin resistance associated with defects in Akt activation in 3T3-L1 adipocytes. Proc Natl Acad Sci USA. 99:5313–5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells L, Vosseller K, Hart GW. 2003. A role for N-acetylglucosamine as a nutrient sensor and mediator of insulin resistance. Cell Mol Life Sci. 60:222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan SA, Lane MD, Hart GW. 2008. Regulation of the O-linked beta-N-acetylglucosamine transferase by insulin signaling. J Biol Chem. 283:21411–21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth GE, Macauley MS, Stubbs KA, Dennis RJ, Taylor EJ, Davies GJ, Greig IR, Vocadlo DJ. 2007. Analysis of PUGNAc and NAG-thiazoline as transition state analogues for human O-GlcNAcase: Mechanistic and structural insights into inhibitor selectivity and transition state poise. J Am Chem Soc. 129:635–644. [DOI] [PubMed] [Google Scholar]
- Wick KL, Liu F. 2001. A new molecular target of insulin action: Regulating the pivotal PDK1. Curr Drug Targets Immune Endocr Metabol Disord. 1:209–221. [DOI] [PubMed] [Google Scholar]
- Wilden PA, Siddle K, Haring E, Backer JM, White MF, Kahn CR. 1992. The role of insulin receptor kinase domain autophosphorylation in receptor-mediated activities. Analysis with insulin and anti-receptor antibodies. J Biol Chem. 267:13719–13727. [PubMed] [Google Scholar]
- York WS, Van Halbeek H, Darvill AG, Albersheim P. 1990. Structural analysis of xyloglucan oligosaccharides by 1H-n.m.r. spectroscopy and fast-atom-bombardment mass spectrometry. Carbohydr Res. 200:9–31. [DOI] [PubMed] [Google Scholar]
- Young WW Jr, Lutz MS, Mills SE, Lechler-Osborn S. 1990. Use of brefeldin A to define sites of glycosphingolipid synthesis: GA2/GM2/GD2 synthase is trans to the brefeldin A block. Proc Natl Acad Sci USA. 87:6838–6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuzwa SA, Macauley MS, Heinonen JE, Shan X, Dennis RJ, He Y, Whitworth GE, Stubbs KA, Mceachern EJ, Davies GJ et al. 2008. A potent mechanism-inspired O-GlcNAcase inhibitor that blocks phosphorylation of tau in vivo. Nat Chem Biol. 4:483–490. [DOI] [PubMed] [Google Scholar]
- Zhao KW, Neufeld EF. 2000. Purification and characterization of recombinant human alpha-N-acetylglucosaminidase secreted by Chinese hamster ovary cells. Protein Expr Purif. 19:202–211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.