Abstract
Pain in infants is under-treated and poorly understood, representing a significant clinical problem. In part, this is due to our inability to objectively measure pain in non-verbal populations. Here, we present an EEG-based measure of infant nociceptive brain activity that is evoked following acute noxious stimulation and is sensitive to analgesic modulation. This measure will be valuable for both mechanistic investigations and for testing analgesic efficacy in the infant population.
Introduction
The implicit challenges associated with the measurement of infant pain have led to poor analgesic provision in infants (1). As pain exposure in early life is associated with both short-term and long-term negative consequences (2), re-evaluation of analgesic provision is urgently required, and crucial in infants receiving intensive care who are exposed to a high burden of pain (1). However, analgesics that are effective in adults may not be suitable for use in infants, and even when adult analgesics are efficacious, dose scaling remains a significant, non-trivial issue due to differences in pharmacokinetics and pharmacodynamics, resulting from differences in absorption, clearance, body composition and receptor expression (3).
It is therefore imperative that a developmentally sensitive approach is used to assess analgesic efficacy and dosing requirements in infants. However, as infants cannot describe their pain, its measurement is reliant on observing indirect noxious-evoked changes in behavior and physiology, such as changes in facial expression, crying and heart rate (4). While these observations may be useful in clinical practice, their utility as an outcome measure in clinical trials and research investigations is limited as these physiological and behavioral observations are not uniquely evoked by nociceptive input (4).
More recently, changes in infant electrophysiological and hemodynamic brain activity have been identified and characterized in response to noxious stimulation (5, 6). As the perception of pain manifests in the brain, it is plausible that in the absence of language, brain-derived approaches may provide the best surrogate measures of infant pain and be more directly linked to pain experience (7). In adults, noxious-evoked changes in brain activity are correlated with individual subjective pain intensity and can be modulated by analgesic administration (8, 9). Brain-derived approaches have therefore been proposed as key methods to optimize drug discovery (10) and to identify targeted analgesic treatments (8, 9).
The potential utility of developing brain-derived measures in pre-verbal infants is considerable. Here, using EEG (electroencephalography) recordings we aim to identify and validate a template of nociceptive brain activity that is sensitive to analgesic administration and suitable for use in clinical trials and research investigations. To this end, a total of 72 infants were included across five studies. The template was derived in Study 1 and then subsequently validated in four independent samples of infants. In Study 2 the specificity of the template was examined in response to noxious, visual, auditory and tactile stimuli in term infants; in Study 3 the applicability of the template to preterm infants aged 34 – 36 weeks’ gestation was investigated; in Study 4 the relationship between noxious-evoked brain activity characterized by the template and pain-related behaviour was established; and in Study 5 the sensitivity of the template to analgesic modulation was assessed.
Results
Study 1: Deriving a template of nociceptive brain activity
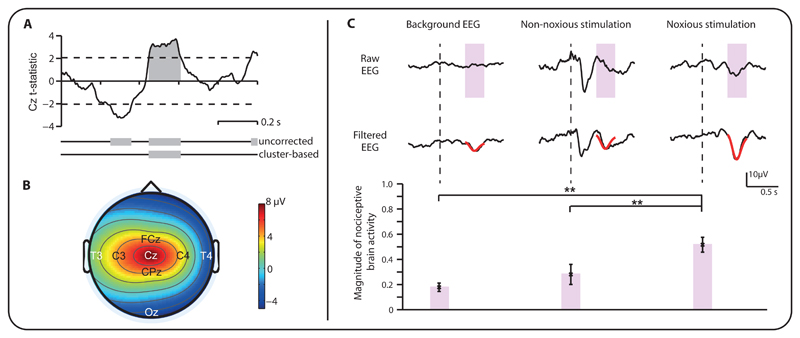
We identified the template by contrasting the patterns of brain activity, evoked by noxious (clinically-required heel lance and experimental noxious stimulation) and non-noxious stimulation (heel lance control procedure and tactile stimulation) in 18 term infants. The topography and time-course of the response was established by considering activity across all electrodes in the 1000 ms post-stimulation period. Noxious-evoked activity was maximal at Cz, and, at this electrode site, significantly different from the activity evoked by the non-noxious stimuli in the time-window 446 - 611 ms post-stimulation (p=0.003, cluster-corrected non-parametric test (11); Figure 1A,B). To obtain a representative waveform of the noxious-evoked brain activity, Principal Component Analysis (PCA) was applied in this time window (400 – 700 ms post-stimulation) at Cz. A principal component was identified that was significantly greater in response to the noxious stimuli compared with the non-noxious stimuli and background activity (p<0.01, Figure 1C). This principal component is comparable to that identified in previous research investigating noxious-evoked brain activity in infants (6, 12–16) and was defined as the template of nociceptive brain activity. The template is scaled so that a magnitude of 1 represents the average response evoked by a stimulus of equivalent intensity to a heel lance performed in a term infant. Activity evoked following experimental noxious stimuli (up to a maximum force of 128 mN) and in younger infants is expected to be smaller in magnitude (13, 14).
Fig 1. Deriving the template of nociceptive brain activity.
(A) Time course of the t-statistics in the 1000 ms period post-stimulation at the Cz electrode comparing the response to noxious (medically required heel lance and experimental noxious stimuli) and non-noxious (control heel lance and tactile) stimulation in 18 term infants. Dashed lines indicate the t-statistic threshold, equal to an uncorrected α of 0.05. The grey bars indicate time points that are above the t-statistic threshold and the time points that remain significant after cluster-based correction (11). A significant difference in response between non-noxious and noxious stimuli is identified by the grey shaded region. (B) Scalp map showing the topography of the mean amplitude response following noxious stimulation in the time-window from 400 – 852 ms post-stimulation. This time-window represents the time where significant differences in response to noxious and non-noxious stimulation were identified. (C) Principal component analysis was conducted at the Cz electrode 400 – 700 ms post stimulus (pink boxes) to derive the nociceptive template, by comparing background brain activity with activity evoked by noxious and non-noxious stimulation. The (Woody) filtered EEG is shown overlaid with the template (in red). The magnitude of template activity was significantly higher following noxious, compared with non-noxious stimulation and background activity and was therefore considered to be evoked by the noxious stimulation (**: p < 0.01, error bars indicate mean ± standard error of the mean).
The morphology of the noxious-evoked brain activity was not dependent on the modality of the noxious stimulus. This was demonstrated by independently calculating the noxious-evoked brain activity following the clinically-required heel lance and the experimental noxious stimuli. The principal components relating to the noxious-evoked brain activity in response to the heel lance and the experimental noxious stimuli were highly correlated with each other (r2=0.88, p<0.001), and both were highly correlated with the template of nociceptive brain activity (experimental noxious stimuli: r2=0.99, p<0.001; clinical heel lance: r2=0.91, p<0.001, Figure S1). This provides confirmation that the morphology of the noxious-evoked brain activity characterized by the template is common to both the clinical and the experimental noxious stimulus modalities.
Study 2: Testing template specificity
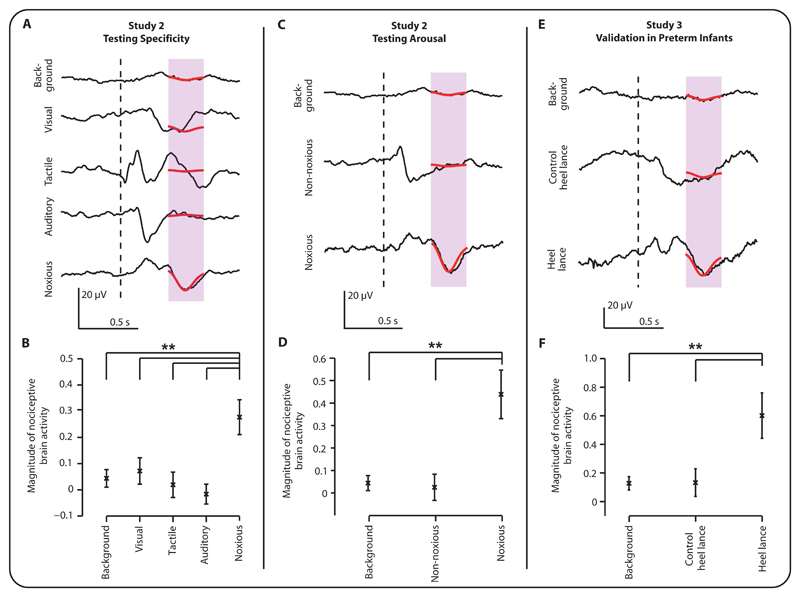
In Study 2 the nociceptive specificity of the template was tested in an independent sample of 14 term infants. For each infant, the template was projected onto the activity evoked by experimental noxious, visual, auditory and tactile stimulation, and onto background activity (Figure 2A). Visual, auditory and tactile stimulation did not evoke a pattern of activity, characterized by the template, which was significantly greater than background activity (p>0.05, Figure 2B). Whereas, activity evoked by the experimental noxious stimulation was significantly greater than that observed in the background and in response to all non-noxious stimuli (p<0.01, Figure 2B). This cannot be attributed to the non-noxious stimuli being too low intensity as all stimuli evoked shorter latency vertex responses that were significantly different from background (Figure 2A, p<0.005 cluster-corrected non-parametric test (11)), which in infants have been attributed to an arousal response (6), and may relate to stimulus saliency as proposed in adults (17).
Fig 2. Template validation.
(A) Average background EEG activity and the average evoked activity following visual, tactile, auditory and experimental noxious stimulation at the Cz electrode in 14 term infants. (B) Magnitude of the nociceptive brain activity characterized by the template during the background activity and in response to each experimental stimulus modality (visual, tactile, auditory and experimental noxious stimulation). (C) Average background EEG activity and the average evoked activity following experimental noxious and non-noxious stimulation for all stimuli that evoked a change in heart rate that was greater than the average change in heart rate evoked by the experimental noxious stimulation. (D) Magnitude of the nociceptive brain activity during background activity and following the experimental noxious and non-noxious stimulation that evoked larger than average changes in heart rate. (E) Average background EEG activity at the Cz electrode in 12 preterm infants (from 34 – 36 weeks’ gestation) and the average evoked activity following control heel lance stimulation and a medically required heel lance. (F) Magnitude of the nociceptive brain activity during the background activity and in response to control heel lance stimulation and a medically required heel lance. In panels (A,C,E) average EEG activity is overlaid with the template (in red). (B,D,F) The magnitude of the projected template is shown for each condition with mean ± standard error of the mean (**: p < 0.01).
It is plausible that application of the experimental noxious stimuli is more arousing than the auditory, visual or tactile stimuli. The experimental noxious stimulation evoked an increase in heart rate of 5.8±9.3 beats per minute (bpm), which is significantly higher than the change observed in the background activity (p<0.001). While this change in heart rate is relatively small compared with that evoked by a clinically-required heel lance, which on average evoked an increase in heart rate of 15.6±13.4 bpm (p<0.001, n=11), it is significantly greater than that evoked by the other non-noxious stimulus modalities (p<0.001; average evoked change in heart rate: tactile: 1.6±8.8 bpm; auditory: -1.3±10.8 bpm; visual: -0.4±8.5 bpm). However, the increase in heart rate evoked by the tactile stimulation was also significantly greater than background activity (p=0.018), even though concomitant noxious brain activity was not evoked (Figure 2B).
Importantly, when only considering the evoked brain activity following the most physiologically arousing stimuli, where the change in heart rate was greater than the average heart rate change evoked by the experimental noxious stimuli (n=140 of 513 stimuli; 22 auditory, 23 visual, 35 tactile and 60 experimental noxious), only the experimental noxious stimulation evoked nociceptive brain activity that was significantly greater than background activity (p<0.001). The non-noxious stimuli that evoked a comparable change in heart rate did not evoke noxious brain activity that was significantly different to background activity (p=0.85, Figure 2C,D). This suggests that the differences in the morphology of the brain activity evoked by the noxious and non-noxious stimuli is not related to differences in physiological arousal associated with the different stimulus modalities.
Study 3: Testing the validity of the template in premature infants
Patterns of brain activity evoked by noxious stimulation change across the preterm period (14), with infants younger than 34 weeks’ gestation more likely to exhibit non-specific neuronal bursts than nociceptive-specific activity (12). The suitability of the template for premature infants aged from 34 – 36 weeks’ gestation was demonstrated in a sample of 12 infants in Study 3. Projecting the template onto activity evoked by a noxious procedure (clinically-required heel lance) showed a significantly greater response compared with activity evoked by a non-noxious control procedure (control heel lance, p=0.008) or during background activity (p=0.006, Figure 2E,F).
Study 4: Investigating the relationship between noxious-evoked brain activity characterized by the template and pain-related behaviour
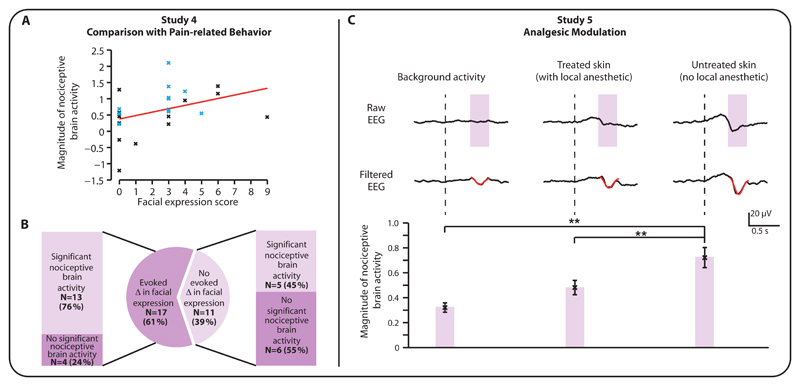
Clinical pain assessment tools, which primarily rely on pain-related changes in behavior, are the current standard method used to quantify pain in infants. In Study 4, we considered how the magnitude of the noxious-evoked brain activity characterized by the template compared with the evoked changes in pain-related behavior in a sample of 28 infants aged from 34 – 42 weeks’ gestation where a clinically-required heel lance was performed (the 12 preterm infants in Study 3 were included in this analysis). There was a significant correlation between the magnitude of noxious-evoked brain activity and pain-related facial grimacing (p=0.038, r=0.39, Figure 3A). However, similar to previous observations (18), in 5 of 11 infants (45 %) where pain-related behavioral changes were not observed there was still a significant increase in noxious-evoked brain activity (Figure 3B). Overall, 11 of the 12 preterm infants had a significant increase in noxious-evoked brain activity. Pain-related behavioral changes were recorded in 9 of these infants.
Figure 3. Magnitude of noxious-evoked brain activity is correlated with pain-related behavior and sensitive to analgesic modulation.
(A) The magnitude of the noxious-evoked brain activity was significantly correlated with the facial expression score in response to a clinically-required heel lance (r = 0.39, p = 0.038, n = 28) (preterm infants: blue; term infants: black). (B) Clinically-required heel lancing did not evoke a change in facial expression in 39 % of infants, yet significant nociceptive brain activity was recorded in 45 % of these infants. (C) Average background EEG activity and noxious-evoked brain activity recorded at the Cz electrode in 12 infants. In each infant the experimental noxious stimuli were applied to each foot – one that was treated with topical local anesthetic and one that was untreated. The (Woody) filtered EEG is shown overlaid with the template (in red). The magnitude of the noxious-evoked brain activity in response to experimental noxious stimuli applied to the untreated foot was significantly higher than the response evoked when the stimuli were applied to the treated foot and in the background activity (**: p < 0.01, error bars indicate mean ± standard error of the mean).
Study 5: Testing template sensitivity to analgesic modulation
The aim of Study 5 was to determine whether the noxious-evoked brain activity characterized by the template was sensitive to analgesic modulation. This was investigated in 12 infants who underwent venipuncture to obtain a clinically-required blood sample. In accordance with the clinical protocol, topical local anesthetic (4% Tetracaine gel) was applied to the dorsal surface of one foot. Experimental noxious stimuli were then applied to each foot (prior to venipuncture) and the evoked brain activity measured. When the experimental noxious stimuli were applied to the untreated foot the noxious evoked activity was significantly greater than background activity (p<0.001, Figure 3C). Whereas, when the experimental noxious stimuli were applied to the foot treated with local anesthetic the evoked activity was not significantly different from the background activity (p=0.26), and was significantly lower than the activity evoked in the untreated foot (p=0.002). In all but one infant the magnitude of the noxious-evoked brain activity was greater when the stimuli were applied to the untreated foot compared with the foot treated with local anesthetic. Figure S2 shows the noxious-evoked brain activity following stimulation on the treated foot and untreated foot for each individual infant included in the study.
Sensitivity and specificity
While the template has the ability to identify group differences, it is also of interest to consider the sensitivity and specificity of the measure to characterize noxious-evoked activity within individual infants. For the infants requiring a clinical heel lance (Studies 3 and 4), the magnitude of the noxious-evoked brain activity was compared with background brain activity. 18 of the 28 infants had a response to the heel lance that was higher than threshold (threshold derived from the 80th percentile of the background activity, see Materials and Methods), demonstrating 64 % sensitivity. In comparison 17 out of 26 infants had a response to the control heel lance that was less than threshold, demonstrating 65 % specificity. When the lower intensity experimental noxious stimuli were applied to the infants in Study 2 the sensitivity was 57 % (8 of the 14 infants had an average evoked response that was above threshold), and the specificity was 68 % (27 out of 40 non-noxious stimuli evoked average responses below the threshold). The receiver operating characteristic (ROC) curve calculated across all stimulus modalities supports this observation (Figure S3).
Discussion
Here, a template representing the temporal pattern of nociceptive brain activity in infants has been characterized at the Cz electrode site and validated across a range of studies. The template was derived using Principal Component Analysis, which is an approach that has previously been used in multiple publications to characterize infant nociceptive brain activity (6, 12–16). Predefining a template has several important advantages over current methodology. Firstly, it provides a more robust analytical approach due to the independence of the data sample. This avoids previous study limitations where noxious-evoked brain activity has been characterized and evaluated within the same population (12, 15), and is particularly important in randomized controlled trials where the precedent is to predefine outcome measures. Secondly, using a predefined template prevents the need for the evoked activity to be re-characterized in a subset of data within each new study. This will directly benefit smaller scale investigations where there is less power to robustly characterize the evoked activity across a range of infant demographics and stimulus conditions. Moreover, using all the available data to address questions pertaining to infant pain is of particular importance due to practical and ethical considerations, whereby data collection primarily relies on the need for clinically essential painful procedures to be performed in study participants. Thirdly, defining a template of nociceptive activity allows for a standardized approach to be adopted and will facilitate comparisons between future research investigations and study populations.
To date, the analgesic efficacy of topical local anesthetics in infants for acute tissue breaking clinical procedures has been investigated using a variety of clinical pain assessment tools (19). While some studies conclude that application of topical local anesthetics is effective (20–22), conflicting reports suggest a lack of analgesic efficacy for intramuscular injections (23, 24), heel lancing (25, 26), peripherally inserted central catheters (27–29), and venipuncture (30). Here we demonstrate that application of topical local anesthetic in infants does significantly reduce the peripheral afferent nociceptive input transmitted to the brain following the application of a mildly noxious acute experimental stimulus. While it is clear that further studies using these brain-derived measures are warranted during clinical procedures, numerous factors may explain the apparent lack of analgesic efficacy that has previously been reported. In some clinical procedures, the tissues affected by the nociceptive input will be deeper than the superficial anesthesia provided by topical local anesthetic. For example, following an injection, the nociceptive input caused by the needle prick may be alleviated, but not the subsequent pain associated with the injection of fluid into the muscle. For heel lancing, where there is substantial evidence for the lack of analgesic efficacy (25, 26) it is plausible that the vasoconstriction caused by some topical local anesthetics (31, 32), may lead to more vigorous squeezing of the limb to obtain an adequate blood sample, conversely resulting in increased nociceptive input in the treated infants compared with the untreated infants. In addition, behavioural measures may not be sensitive enough to discriminate between pain and distress, and so, the reported lack of analgesic efficacy may arise because of unpleasant features associated with the clinical procedure that are not necessarily related to the nociceptive input. For example, the need to physically restrain the limb during clinical procedures may be distressing and evoke a behavioural response even in the absence of nociceptive input. Moreover, even in this study where the nociceptive input was comparatively mild, the local anesthetic only partially reduced the noxious-evoked brain activity in some participants.
The template derived here will be used to characterize evoked nociceptive activity in infants from 34 to 42 weeks’ gestation in a randomised controlled trial that will test the analgesic efficacy of morphine for procedural pain in infants (33, 34). The Procedural Pain in Premature Infants (Poppi) Trial Protocol, which has been published as an Accepted Protocol Summary in The Lancet (33), provides a direct example of how these measures can be used to assess analgesic efficacy in the clinical setting. The advantage of characterizing activity at a single site is that the template can be used when only a single recording electrode is applied, for example, where excess handling in critically ill patients may not be appropriate or in situations where time is limited. While the highest amplitude activity was recorded at the Cz electrode site, this does not imply that noxious-evoked activity cannot be recorded elsewhere. Indeed, if these methods were to be developed such that they could be used to characterize an individual infant’s nociceptive sensitivity it would be important to consider the temporal and spatial activity across the other electrode sites. This study has demonstrated that the template can characterize noxious-evoked brain activity in infants from 34 – 42 weeks’ gestation. However, the template does not take into account that there is an increased likelihood that noxious stimulation may evoke non-modality specific bursts of neuronal activity in younger infants (12). The brain undergoes rapid functional and structural development during this period (35), and further refinement of the template could also take additional developmentally relevant information into account. For example, changes in the magnitude or latency of the noxious-evoked activity are known to occur with gestational age (14, 36), and there is evidence that infants born prematurely who reach term-age have greater magnitude noxious-evoked brain activity compared with age-matched term-born infants following the same nociceptive input (16).
The evoked brain activity characterized by the template cannot be interpreted as a direct measure of infant pain but rather it quantifies the degree of the noxious input that is reaching the infant’s brain. Various factors will contribute to the experience of pain (37) and the template does not reflect all nociceptive activity that takes place across the infant brain (5). Furthermore, genetic and environmental differences will contribute towards an individual’s nociceptive sensitivity and may explain why noxious-evoked brain activity and behavioral changes were not observed in all participants. Previous research has suggested that lack of pain-related facial grimacing may be related to factors such as postnatal-age and time since the last painful procedure (38). While the occurrence of pain-related facial grimacing and the likelihood of observing noxious-evoked brain activity increases with gestational age, lack of response, across both modalities, can occur in infants of any gestational age (12, 39), which is consistent with the results of this study. Further work is needed to understand why noxious-evoked brain activity and pain-related behavior is not observed in some infants.
While the specificity and sensitivity of the template is reasonably high, these brain-derived methods have not been developed to quantify an individual infant’s nociceptive brain activity, but rather to consider differences in nociceptive-evoked activity at the group level. The measures presented here could potentially provide the first step towards the development of a validated tool that may be useful for individual pain assessment, but in the current form the template cannot be used for this purpose. Nevertheless, this nociceptive response is validated here in several independent samples, has been observed across a number of previous research studies (6, 12–16), is strongly correlated with the degree of noxious input (13), is correlated with clinical measures of pain-related behavior and likely reflects a key component of acute nociceptive processing in infants. The validity of the template in older infants and across a range of stimulus modalities, for example, following cannulation or following more prolonged noxious procedures, is still to be established. Studies of this type will require careful consideration of latency differences that will arise between studies, for example, as a result of differences in stimulus location, time-locking approaches and the participants’ age.
In future investigations analytical approaches similar to those described in Study 1 can be used to appropriately identify the time window of interest. Age-dependent differences in the latency and morphology of noxious-evoked brain activity have been observed (12, 14), which means it cannot be assumed that the template derived here is appropriate for use in infants of different ages. Indeed, while similar noxious-evoked brain activity has been observed in both infants and adults, temporal and spatial differences in the patterns of activity also exist (5, 40, 41). Importantly, the template cannot be projected onto EEG activity in different time windows without characterizing the morphology of the evoked activity and confirming that it is related to the noxious input. Failure to apply the template appropriately could, for example, lead to the erroneous conclusions that non-noxious stimuli are perceived as nociceptive or that potentially effective treatments are not analgesic.
In summary, a template of nociceptive brain activity that is sensitive to the administration of analgesics has been identified in infants. The template provides an objective method to quantify infant nociceptive brain activity following acute procedural pain. In the absence of language this represents an essential surrogate measure of infant pain, which may complement other pain-related behavioural and physiological observations. This methodology can be used in future research studies of infant nociceptive processing. It provides a new approach to assess analgesic efficacy and may help to establish dose response curves of effective analgesics, thus identifying minimum effective analgesic doses and lowering the risk of adverse side effects (3). Rigorous analgesic assessment is essential if we are to help provide effective pain relief in the infant population.
Materials and Methods
Study Design
72 infants were included in five observational studies. Electrophysiological brain activity was recorded and characterized in response to noxious and non-noxious stimuli in order to derive and validate a template of nociceptive brain activity. The template was validated in term and preterm infants (from 34 weeks’ gestation), compared with clinical pain assessment scores, and demonstrated to be sensitive to analgesic modulation.
Participants
Infants were recruited from the Maternity Unit, Special Care Baby Unit and Neonatal Outpatients Clinics at the John Radcliffe Hospital, Oxford University Hospitals NHS Foundation Trust. Infants were eligible for inclusion in the study if they had no history of neurological problems and were clinically stable and not receiving any analgesics at the time of study (except for topical local anesthetic as described in Study 5). Infant demographic characteristics and experimental details are described in Table 1.
Table 1.
Infant demographics. Values given are median (lower quartile, upper quartile). * All the prematurely-born infants included in Study 4 (n=12) were also included in Study 3. The term infants in Study 4 were an independent sample of infants not included in the other studies (n=16). **Infants did not receive morphine for a minimum of 12 days prior to inclusion in the study.
| Study 1 Deriving the template |
Study 2 Testing specificity |
Study 3 Validation in preterm infants |
Study 4 Comparison with facial expression |
Study 5 Analgesic modulation |
|
|---|---|---|---|---|---|
| Number of infants | 18 | 14 | 12* | 28* | 12 |
| Gestational age at time of study (weeks) | 39.9 (37.9, 41.7) |
39.8 (37.8, 41.3) |
36.1 (35.1, 36.6) |
38.6 (36.4, 40.9) |
42.1 (41.1, 43.6) |
| Gestational age at birth (weeks) | 39.3 (37.5, 41.4) |
39.6 (37.2, 41.2) |
33.0 (31.9, 36.1) |
36.1 (31.7, 40.4) |
38.7 (38.0, 39.8) |
| Postnatal age at time of study (days) | 3 (2, 5) |
2 (1, 2) |
18 (2.8, 25) |
4.5 (1.8, 25.3) |
24.5 (21.5, 27.3) |
| Birthweight (g) | 3437 (3236, 3638) |
3127 (2532, 3649) |
2001 (1772, 2478) |
2595 (1622,3808) |
3330 (3125, 3488) |
| Number of males | 11 | 3 | 7 | 16 | 5 |
| Number of infants who have previously received morphine** | 0 | 0 | 4 | 7 | 0 |
| Applied stimuli | Heel lance, Control heel lance, Experimental noxious, Tactile |
Experimental noxious, Visual, Auditory, Tactile |
Heel lance, Control heel lance |
Heel lance, Control heel lance |
Experimental noxious |
Research Governance
Studies were carried out in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. Ethical approval was obtained from the National Research Ethics Service (reference: 12/SC/0447 & 11/LO/0350) and informed written parental consent was obtained prior to each study.
Experimental Design
Study 1: Deriving a template of nociceptive brain activity
EEG activity was recorded in 18 term infants in response to noxious and non-noxious stimuli. Clinically-required heel lance (n=8 infants) and experimental noxious stimuli (n=9) were compared with background activity and activity recorded in response to non-noxious stimuli (control heel lance (n=8) and experimental tactile stimuli (n=6)). Some infants received more than one stimulus type, see Table S1.
Using a nonparametric statistical approach described by Maris and Oostenveld (11), time courses evoked by the noxious and non-noxious stimuli were compared across all electrodes and significant differences in the evoked activity identified. The morphology of the noxious-evoked activity was then characterized in the time window of interest (identified from the nonparametric test) using Principal Component Analysis, and a template of nociceptive brain activity was defined.
A previous paper that demonstrates that noxious-evoked brain activity is correlated with the magnitude of spinal reflex withdrawal activity and graded with stimulus intensity (13) reports data from 17 infants that are used to characterize the nociceptive activity in Study 1.
Nonparametric statistical analysis
Using the nonparametric statistical analysis described by Maris and Oostenveld (11), time periods with significant differences in the response to noxious and non-noxious stimuli were identified. Briefly, a t-statistic was calculated at each time point for each electrode site, which represented the statistical difference in the brain activity evoked by the noxious and non-noxious stimuli. When the t-statistic was above threshold (set at 97.5 percentile of the t-distribution), clusters of significant activity were identified based on temporal and spatial adjacency of the activity. As EEG activity was only recorded in 8 electrodes, all electrodes were considered as neighbours. A single cluster therefore included any electrode sites where above threshold activity was concurrently recorded. The start of the activity cluster was defined as the earliest time point (in any electrode site) where an above-threshold t-statistic was identified. The end of the cluster was defined as the time point when no electrodes were identified with an above-threshold t-statistic. The cluster-based test statistic was calculated from 1000 random permutations of the data and the threshold for cluster significance was set as the 97.5 percentile of the permuted data.
Using this approach across all electrodes, significant activity, that was maximal at the Cz electrode site, was identified in the time-window from 400 – 852 ms post-stimulation. When considering only the Cz electrode site, significant activity was identified 446 - 611 ms post-stimulation (Figure 1A). Consistent with previous studies (6, 12–16), subsequent analysis was conducted at the Cz electrode site in the 400 – 700 ms post-stimulus time window. The scalp map in Figure 1B was produced using the ‘topoplot’ function in EEGLAB (Swartz Center for Computational Neuroscience, University of California San Diego).
Principal Component Analysis
To characterize the nociceptive brain activity waveform, Principal Component Analysis was conducted on the activity evoked by noxious and non-noxious stimulation, and on the background data. The first two principal components accounted for 89 % of the variance in the data and were therefore the only components considered. Noxious-evoked brain activity was identified as the component with significantly higher weights in response to the noxious stimuli compared with the non-noxious stimuli and background. The first principal component was not nociceptive-specific as the weight of the component evoked by the noxious stimulation was not significantly greater than the background activity (p=0.06). In contrast, the second principal component weight was significantly greater following noxious stimulation compared with non-noxious stimulation and background activity (Figure 1C), and was therefore considered to characterize the activity generated by the nociceptive stimulation.
To define the template of nociceptive brain activity, the second principal component was scaled so that the mean weight in response to the noxious heel lance in term infants was 1. This template of nociceptive brain activity in infants is available as a downloadable file in the Supplementary Material.
To confirm that the morphology of the template was not dependent on the modality of the noxious stimulus, principal component analysis was also conducted with the responses to the clinical heel lance and the experimental noxious stimulation separately, and the components compared with the template.
Study 2 : Testing template specificity
The aim of Study 2 was to determine whether nociceptive brain activity characterized using the template was specific to noxious stimulation in an independent sample of infants, i.e. whether the magnitude of the template activity in EEG data evoked by noxious stimulation was significantly greater than activity evoked by other sensory modalities. EEG activity was recorded in 14 term infants during a period of background activity and in response to a train of approximately 10 auditory, visual, tactile and experimental noxious stimuli (which were applied in a randomized order). In two infants either the visual or the tactile stimuli were not applied due to technical difficulties. Following rejection of epochs with gross movement artefact (see EEG data preparation), a total of 131 auditory, 126 visual, 123 tactile and 133 experimental noxious stimuli were analysed across the 14 infants (an average of 9.5 ± 1.2 stimuli per train). The template was projected onto the individual trials at the Cz electrode site in the 400 – 700 ms post-stimulation time window. The magnitude of the template was calculated for each trial and compared across stimulus modality.
Checking stimulus saliency
To test whether the non-noxious stimuli were sufficiently salient to evoke event-related patterns of brain activity the nonparametric statistical analysis approach described above was used (11). Differences in the activity evoked by each non-noxious stimulus modality at the Cz electrode site were compared with the background activity. As the activity evoked by the tactile stimulation crossed zero, t-statistics were calculated on the amplitude envelopes (the absolute value of the Hilbert transform). Evoked responses (significant clusters) with a latency less than 400 ms were identified at the Cz electrode in response to all non-noxious stimuli compared with background activity (visual: p=0.009, auditory: p=0.002, tactile: p<0.001).
Physiological arousal
To investigate whether the stimuli were equally physiologically arousing, evoked changes in heart rate were examined. R-R intervals were identified from the ECG trace in the 5 seconds before and after each individual stimulus (42), and the change in heart rate between the two periods calculated. Changes in heart rate evoked by the experimental stimuli were compared with the evoked change in heart rate recorded following a medically essential heel lance.
Study 3: Testing the validity of the template in premature infants
The aim of Study 3 was to determine whether the template could identify noxious-evoked activity in younger premature infants who were aged 34 to 36 weeks’ gestation at the time of study. This age range was chosen because previous data suggests that after approximately 34 weeks’ gestation noxious-specific evoked activity is likely to be generated (12).
EEG activity was recorded in a period of background activity, and in response to a medically required heel lance and a control heel lance in 12 infants. The nociceptive template was projected onto individual trials at the Cz electrode 400 – 700 ms post-stimulus. To determine whether the weight was significantly greater following the noxious heel lance stimulation the magnitude of the template was calculated for each trial and compared between the noxious and non-noxious stimuli and background activity.
Study 4: Investigating the relationship between noxious-evoked brain activity characterized by the template and pain-related behaviour
The aim of Study 4 was to investigate the relationship between noxious-evoked brain activity and pain-related behaviour in infants’ aged 34 – 42 weeks’ gestation. EEG activity and changes in facial expression were recorded in response to a clinically-required heel lance in 28 infants. The 12 preterm infants in Study 3 were also included in this study. The magnitude of the noxious-evoked brain activity was characterized by the template and correlated with the facial expression scores, as these behavioural measures are often used to quantify pain experience in the clinical setting (4).
Study 5: Testing template sensitivity to analgesic modulation
Study 5 aimed to test the sensitivity of the template to analgesic modulation. Term-born neonates (infants with a maximum post-natal age of 28 days) were eligible for inclusion in the study if they required topical local anesthetic to be applied to the dorsal surface of either the left or right foot prior to medically-required venipuncture. Topical local anesthetic (tetracaine 4% w/w – Ametop Gel; Smith and Nephew Healthcare, UK) was applied to the infant’s foot (and two other sites) as required by the clinical team and removed after approximately 30 minutes. EEG electrodes were positioned on the head and a train of approximately 10 experimental noxious stimuli were applied to each foot. The stimulus order (i.e. right verses left foot) was randomly selected. The template was projected onto individual trials at the Cz electrode and the magnitude of the projected template was calculated and compared in each foot and with the background activity.
To determine the sample size required in Study 5, a power calculation was performed using the data from Study 1. We anticipated that application of the local anesthetic would reduce the magnitude of the noxious-evoked brain activity to a magnitude equivalent to that observed in the background data. A sample size of 12 infants would be required to observe a reduction in the noxious-evoked brain activity of this order of magnitude for a power of 95 % at a two-sided significance level of 5 %.
Projecting the template of nociceptive brain activity onto new EEG data
The magnitude of the evoked nociceptive template activity was calculated by projecting the template onto the EEG data using singular value decomposition (12–14).
Let X0 be the template. The singular value decomposition of X0 is given by:
Given a new data set, X1, the corresponding weights, U1, are given by:
Thus, the corresponding weight for each new EEG trace was obtained, which represents the magnitude of the noxious-evoked brain activity. The MATLAB code to project the template onto new EEG data is available as a downloadable file within the Supplementary Material.
Filtered EEG signal
To ensure that the template provided the best morphological representation of the evoked responses in the individual trials, the EEG traces in Study 1 were Woody filtered in the 0 – 1000 ms interval post-stimulation with a maximum jitter of ±50 ms. Maximum correlation between the individual traces within subjects and the data average was achieved. In addition, once the time window of interest was identified (400 – 700 ms post-stimulation), the individual traces were also Woody filtered within this window to maximise the alignment with the data average.
Due to the wider age range of the participants included in Studies 4 and 5, individual responses were first Woody filtered with a maximum jitter of ±50 ms in the region 400 – 700 ms after the stimulus to achieve maximum correlation with the template. This approach accounts for the expected increase in latency variation that will be present in study participants with a greater gestational age range. As the age range in Studies 2 and 3 was comparatively small, Woody filtering was not applied to the data for these studies presented in the main paper. Nevertheless, to establish whether the results in Studies 2 and 3 would be affected if the data were corrected for individual latency variation, Woody filtering was applied to the data and the template was reapplied. The data was Woody filtered in the region 400 – 700 ms after stimulus onset, with a maximum jitter of ±50 ms, optimising the correlation with the template response. Allowing for this latency variation did not alter the results reported for the validation studies.
Comparison of the template with current standard analysis
The current standard analytical approach that would be used to analyse noxious-evoked brain activity would be to perform Principal Component Analysis (PCA). The template defined in Study 1 is no less sensitive than the standard approach (Figure S4), but has the benefit of being independent from the acquired data.
Sensitivity and specificity analysis
To investigate the sensitivity and specificity of the template response the magnitude of the response to the experimental and clinical stimuli was compared to a threshold set at 80 % of the distribution of background weights (i.e. the magnitude of the template response within the background data, 80 % of the distribution is equal to a magnitude of 0.48). The distribution of background data included data from all infants in Studies 2 and 4, which in total consisted of 175 background samples. The number of background samples included in the analysis was equivalent to the number of noxious stimuli applied in each participant. Therefore, for infants where trains of approximately 10 experimental noxious stimuli were applied, 10 background samples were included in the analysis. For the infants where a single clinically-required heel lance was performed, a single background sample was included in the analysis. An infant’s average response to individual stimuli were then defined as nociceptive if the magnitude was above this threshold. Sensitivity could therefore be assessed as the number of evoked responses defined as nociceptive when the stimulus applied was noxious (experimental noxious or heel lance), and specificity was assessed as the number of evoked responses that were correctly identified as below threshold when a non-noxious stimulus (control heel lance, auditory, visual or tactile) was applied. In addition, the threshold was varied between -2 and 2 (in increments of 0.001) and the sensitivity and specificity assessed using a receiver operating characteristic (ROC) curve. To maximise the alignment of the data with the template, the data (including the background brain activity) was Woody filtered for sensitivity and specificity analysis.
Stimulation Techniques
EEG activity was recorded in response to clinically-required heel lancing or experimental stimulation. All experimental stimuli (noxious, auditory, tactile, visual) were applied in trains of approximately 10 stimuli with a minimum inter-stimulus interval of 10 seconds. The inter-stimulus interval was extended if the infant was unsettled. During stimulus presentation infants were lying supine in an open cot or on an examination table. Studies took place in the Newborn Care Unit, Postnatal Ward (in a private room) or for Study 5, in an outpatient clinic. 2 of the 12 infants in Study 5 were held by their mother following parental request. All infants were asleep or quietly awake during stimulus presentation and during the background recording.
Clinical heel lancing and control heel lance
A single heel lance was performed in each infant to obtain a clinically-required blood sample, as part of the infants’ routine clinical care. The foot chosen for heel lancing was based on clinical judgment and not controlled during the experiment. Heel lances were performed on the medial or lateral plantar surface of the heel. In term infants, a BD Microtainer Quikheel Infant Lancet (Becton, Dickinson and Company, New Jersey, USA) with a penetration depth of 1.0 mm was used, and in preterm infants, a BD Quikheel Preemie Lancet with a penetration depth of 0.85 mm was used. Each infant was studied during a single heel lance performed in order to get the required blood sample. Heel lances were only performed if clinically required and the procedure was not performed for the purpose of the study. None of the infants were studied on more than one occasion. Consistent with previous reports, we anticipated that noxious-evoked brain activity would be recorded within the first 1000 ms following the application of the stimulus (6, 12–16). Release of the lancet blade was time-locked to the EEG recordings using event-detection interfaces that have been previously described (14, 43). A control heel lance was performed prior to heel lancing - the lancet was rotated by 90° and held against the infant’s foot, so that when the blade was released it did not make contact with the infant’s foot.
Experimental Noxious stimulation
Acute experimental noxious stimuli (PinPrick, MRC Systems, Germany) were applied perpendicular to the infant’s foot at a force of 128 mN. The stimulator is a weighted device that was used to apply a pre-specified force (128 mN) to the surface of the foot. It does not pierce or damage the skin and, in adults it is described as mildly painful (5). In Studies 1 and 2 the stimuli were applied to the infant’s heel, whilst in Study 5 the stimuli were applied to the dorsal surface of the infant’s foot.
In Studies 1 and 2 the experimental noxious stimuli were time-locked to the EEG recordings using a high-speed camera (220 frames per second, Firefly MV, Point Grey Research Inc.) that was directly linked to the recordings at the time of acquisition (42). The video recordings were reviewed post-acquisition and the time of stimulation manually event-marked as the point where the barrel of the stimulator was first depressed (13). In Study 5 the experimental noxious stimuli were time-locked using a contact trigger device (MRC Systems, Germany), which was directly linked to the EEG recordings allowing the point of stimulation to be marked on the recordings during acquisition.
Experimental tactile stimulation
Tactile stimuli were applied to the heel of the infant’s foot. Stimuli were applied using a modified tendon hammer with a built-in force transducer (Brüel & Kjær, Denmark) that sent a trigger pulse that event-marked the EEG recordings at the point of stimulation (43).
Visual stimulation
Flash stimulation was delivered to infants using an LED light (Maxima-84 Hybrid, Manfotto, Italy). The flash was held 50 cm behind the infant’s head and approximately 45° up from the cot, pointing towards the infant. Stimuli were initiated by a push-button that also event-marked the EEG recordings within 1 ms of the point of stimulation. Infant eye gaze was not controlled during the study.
Auditory stimulation
Single tones (500 Hz, 100 ms, approximately 80 dB) were delivered to infants using a MP3 player (Zen Style M300, Creative, Singapore) and speakers (X-mini Max 2 Portable Speakers, XMI, Singapore). The speakers were positioned approximately 5 cm away from the infant’s ears. An output trigger pulse from the MP3 player event-marked the EEG recordings within 1 ms of the point of stimulation.
Background activity
Background EEG activity was recorded in all infants prior to stimulation. During this period the experimenter gently held the infant’s foot but no stimuli were applied. Periods of background EEG activity were manually event marked by the experimenter at the time of recording.
Electrophysiological recordings and analysis
EEG and ECG recordings
Electrophysiological activity from DC to 400 Hz was acquired with a SynAmps RT 64-channel EEG/EP system (Compumedics Neuroscan). Activity was sampled at 2 kHz with a resolution of 24 bit. CURRYscan7 neuroimaging suite (Compumedics Neuroscan) was used to record the activity.
Reference and ground electrodes were placed at Fz and on the forehead respectively. For 3 infants the reference electrode was placed at Fpz and re-referenced to Fz post-acquisition. In Studies 1, 2, 3 and 4, EEG recording electrodes (Ambu Neuroline disposable Ag/AgCl cup electrodes) were placed on the surface of the scalp at Cz, CPz, C3, C4, T3, T4, FCz, and Oz. To limit the study time to approximately 15 minutes a reduced electrode montage, which always included the Cz electrode, was used in all infants in Study 5 and in 3 infants in Study 1. To optimise contact with the scalp the skin was gently rubbed with EEG preparation gel (NuPrep gel, D.O. Weaver and Co.) prior to electrode placement and EEG conductive paste (Elefix EEG paste, Nihon Kohden) was used. ECG activity was recorded using an ECG electrode (Ambu Neuroline 700 solid gel surface electrodes) that was placed on the chest.
EEG data preparation
To construct the template (Study 1) the EEG signals were filtered from 0.5 – 8 Hz. For all other studies the EEG signals were filtered 0.5 – 70 Hz, with a notch filter at 50 Hz. All signals were extracted from the recordings in 1500 ms epochs, with 500 ms before the stimulus. Epochs were baseline corrected to the pre-stimulus mean. EEG epochs with gross movement artefact were rejected from the analysis.
Facial expression scores
Infant’s facial expression was recorded using a video camera for 15 seconds before and 30 seconds after the heel lance and control heel lance procedures. An LED was synchronised to flash when the experimenter pressed a foot pedal at the point of stimulation. Videos were reviewed after the procedures and the facial expression component of the Premature Infant Pain Profile (PIPP) was calculated in the 30 seconds after the procedure, by identifying the presence of nasolabial furrow, brow bulge and eye squeeze (44, 45). This gave a maximum total facial expression score of 9. The researcher who viewed the videos and calculated the PIPP scores was blinded to the stimulation and did not know the magnitude of the noxious-evoked brain activity.
Statistical analysis
Statistical analysis was carried out using R (The R Project for Statistical Computing). For comparisons of the brain activity across different stimulus modalities, the data was fit with linear mixed-effects models (using the nlme R package) with stimulus modality set as a fixed effect and individual subjects taken as random effects. Comparisons were made between the noxious stimulation and all other stimulus modalities. Linear correlations and Pearson’s correlation coefficients were calculated to compare the noxious-evoked brain activity and facial expression score in Study 4, and to compare the morphology of the template activity with principal components derived from the responses to the clinical heel lance and the experimental noxious stimuli in Study 1.
Supplementary Material
Template of nociceptive brain activity in infants
MATLAB code to project the template onto new EEG data
One Sentence Summary.
A measure of nociceptive brain activity that can be used to assess analgesic efficacy in infants is derived and validated.
Acknowledgments
We would like to thank Amy Hoskin, Sezgi Goksan, Fiona Moultrie, and Ravi Poorun, Eleri Adams and Pam Whitehead for help conducting this study. We would also like to thank the children and their parents for taking part in this study.
Funding: Supported by The Wellcome Trust, UK.
Footnotes
Author contributions: R.S., C.H., R.R., and A.W. designed the study; G.G, G.S.M., and C.H. performed the experiments; C.H., E.D., R.S., and R.R. analyzed and interpreted the data; C.H. and R.S. wrote the paper. All authors critically revised the manuscript.
Competing interests: The authors declare no conflicts of interest.
References
- 1.Roofthooft DW, Simons SH, Anand KJ, Tibboel D, van Dijk M. Eight years later, are we still hurting newborn infants? Neonatology. 2014;105:218–226. doi: 10.1159/000357207. [DOI] [PubMed] [Google Scholar]
- 2.Grunau RE. Neonatal pain in very preterm infants: long-term effects on brain, neurodevelopment and pain reactivity. Rambam Maimonides Med J. 2013;4:e0025. doi: 10.5041/RMMJ.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349:1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 4.Ranger M, Johnston CC, Anand KJS. Current controversies regarding pain assessment in neonates. Semin Perinatol. 2007;31:283–288. doi: 10.1053/j.semperi.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Goksan S, Hartley C, Emery F, Cockrill N, Poorun R, Moultrie F, Rogers R, Campbell J, Sanders M, Adams E, Clare S, et al. fMRI reveals neural activity overlap between adult and infant pain. eLife. 2015;4 doi: 10.7554/eLife.06356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slater R, Worley A, Fabrizi L, Roberts S, Meek J, Boyd S, Fitzgerald M. Evoked potentials generated by noxious stimulation in the human infant brain. Eur J Pain. 2010;14:321–326. doi: 10.1016/j.ejpain.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Hartley C, Slater R. Neurophysiological measures of nociceptive brain activity in the newborn infant--the next steps. Acta Paediatr. 2014;103:238–242. doi: 10.1111/apa.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Truini A, Panuccio G, Galeotti F, Maluccio MR, Sartucci F, Avoli M, Cruccu G. Laser-evoked potentials as a tool for assessing the efficacy of antinociceptive drugs. Eur J Pain. 2010;14:222–225. doi: 10.1016/j.ejpain.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368:1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duff EP, Vennart W, Wise RG, Howard MA, Harris RE, Lee M, Wartolowska K, Wanigasekera V, Wilson FJ, Whitlock M, Tracey I, et al. Learning to identify CNS drug action and efficacy using multistudy fMRI data. Sci Transl Med. 2015;7:274ra216. doi: 10.1126/scitranslmed.3008438. [DOI] [PubMed] [Google Scholar]
- 11.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 12.Fabrizi L, Slater R, Worley A, Meek J, Boyd S, Olhede S, Fitzgerald M. A shift in sensory processing that enables the developing human brain to discriminate touch from pain. Curr Biol. 2011;21:1552–1558. doi: 10.1016/j.cub.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hartley C, Goksan S, Poorun R, Brotherhood K, Schmidt Mellado G, Moultrie F, Rogers R, Adams E, Slater R. The relationship between nociceptive brain activity, spinal reflex withdrawal and behaviour in newborn infants. Sci Rep. 2015;5:12519. doi: 10.1038/srep12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartley C, Moultrie F, Gursul D, Hoskin A, Adams E, Rogers R, Slater R. Changing Balance of Spinal Cord Excitability and Nociceptive Brain Activity in Early Human Development. Curr Biol. 2016;26:1998–2002. doi: 10.1016/j.cub.2016.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slater R, Cornelissen L, Fabrizi L, Patten D, Yoxen J, Worley A, Boyd S, Meek J, Fitzgerald M. Oral sucrose as an analgesic drug for procedural pain in newborn infants: a randomised controlled trial. Lancet. 2010;376:1225–1232. doi: 10.1016/S0140-6736(10)61303-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Slater R, Fabrizi L, Worley A, Meek J, Boyd S, Fitzgerald M. Premature infants display increased noxious-evoked neuronal activity in the brain compared to healthy age-matched term-born infants. NeuroImage. 2010;52:583–589. doi: 10.1016/j.neuroimage.2010.04.253. [DOI] [PubMed] [Google Scholar]
- 17.Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J Neurophysiol. 2009;101:3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- 18.Slater R, Cantarella A, Franck L, Meek J, Fitzgerald M. How well do clinical pain assessment tools reflect pain in infants? Plos Med. 2008;5:928–933. doi: 10.1371/journal.pmed.0050129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehr VT, Taddio A. Topical anesthesia in neonates: Clinical practices and practical considerations. Semin Perinatol. 2007;31:323–329. doi: 10.1053/j.semperi.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Jain A, Rutter N. Does topical amethocaine gel reduce the pain of venepuncture in newborn infants? A randomised double blind controlled trial. Arch Dis Child. 2000;83:F207–F210. doi: 10.1136/fn.83.3.F207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long CP, McCafferty DF, Sittlington NM, Halliday HL, Woolfson AD, Jones DS. Randomized trial of novel tetracaine patch to provide local anaesthesia in neonates undergoing venepuncture. Brit J Anaesth. 2003;91:514–518. doi: 10.1093/bja/aeg216. [DOI] [PubMed] [Google Scholar]
- 22.Moore J. No more tears: a randomized controlled double-blind trial of Amethocaine gel vs. placebo in the management of procedural pain in neonates. J Adv Nurs. 2001;34:475–482. doi: 10.1046/j.1365-2648.2001.01776.x. [DOI] [PubMed] [Google Scholar]
- 23.Newby B, Faschoway G, Soukoroff C. Tetracaine (ametop) compared to placebo for reducing pain associated with intramuscular injection of palivizumab (synagis) J Pediatr. 2009;24:529–533. doi: 10.1016/j.pedn.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Shah VS, Taddio A, Hancock R, Shah P, Ohlsson A. Topical amethocaine gel 4% for intramuscular injection in term neonates: A double-blind, placebo-controlled, randomized trial. Clin Ther. 2008;30:166–174. doi: 10.1016/j.clinthera.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Jain A, Rutter N, Ratnayaka M. Topical amethocaine gel for pain relief of heel prick blood sampling: a randomised double blind controlled trial. Arch Dis Child. 2001;84:F56–F59. doi: 10.1136/fn.84.1.F56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson BA, Jylli L, Lagercrantz H, Olsson GL. Does a Local-Anesthetic Cream (Emla) Alleviate Pain from Heel-Lancing in Neonates. Acta Anaesth Scand. 1995;39:1028–1031. doi: 10.1111/j.1399-6576.1995.tb04223.x. [DOI] [PubMed] [Google Scholar]
- 27.Ballantyne M, McNair C, Ung E, Gibbins S, Stevens B. A randomized controlled trial evaluating the efficacy of tetracaine gel for pain relief from peripherally inserted central catheters in infants. Adv Neonatal Care. 2003;3:297–307. doi: 10.1016/j.adnc.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Lemyre B, Sherlock R, Hogan D, Gaboury I, Blanchard C, Moher D. How effective is tetracaine 4% gel, before a peripherally inserted central catheter, in reducing procedural pain in infants: a randomized double-blind placebo controlled trial [ISRCTN75884221] BMC Med. 2006;4:11. doi: 10.1186/1741-7015-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taddio A, Lee C, Yip A, Parvez B, McNamara PJ, Shah V. Intravenous morphine and topical tetracaine for treatment of pain in preterm neonates undergoing central line placement. JAMA. 2006;295:793–800. doi: 10.1001/jama.295.7.793. [DOI] [PubMed] [Google Scholar]
- 30.Lemyre B, Hogan D, Gaboury I, Sherlock R, Blanchard C, Moher D. How effective is tetracaine 4% gel, before a venipuncture, in reducing procedural pain in infants: a randomized double-blind placebo controlled trial. BMC Pediatr. 2007;7:7. doi: 10.1186/1471-2431-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bjerring P, Andersen PH, Arendt-Nielsen L. Vascular response of human skin after analgesia with EMLA cream. Br J Anaesth. 1989;63:655–660. doi: 10.1093/bja/63.6.655. [DOI] [PubMed] [Google Scholar]
- 32.Mcintosh N, Vanveen L, Brameyer H. Alleviation of the Pain of Heel Prick in Preterm Infants. Arch Dis Child. 1994;70:F177–F181. doi: 10.1136/fn.70.3.f177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartley C, Moultrie F, Juszczak E, Adams E, Slater R. Protocol 15PRT/5747:A blinded randomised placebo-controlled trial investigating the efficacy of morphine analgesia for procedural pain in infants - ISRCTN82342359. Lancet. 2016 doi: 10.12688/wellcomeopenres.10005.1. http://www.thelancet.com/doi/story/10.1016/html.2016.1001.1019.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slater R, Hartley C, Moultrie F, Adams E, Juszczak E, Rogers R, Norman J, Patel C, Stanbury K, Hoskin A, Green G, et al. A blinded randomised placebo-controlled trial investigating the efficacy of morphine analgesia for procedural pain in infants: Trial protocol. Wellcome Open Res. 2016;1:7. doi: 10.12688/wellcomeopenres.10005.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kostovic I, Jovanov-Milosevic N. The development of cerebral connections during the first 20-45 weeks' gestation. Semin Fetal Neonatal Med. 2006;11:415–422. doi: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Slater R, Cantarella A, Gallella S, Worley A, Boyd S, Meek J, Fitzgerald M. Cortical pain responses in human infants. J Neurosci. 2006;26:3662–3666. doi: 10.1523/JNEUROSCI.0348-06.2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tracey I, Mantyh PW. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 38.Johnston CC, Stevens BJ, Franck LS, Jack A, Stremler R, Platt R. Factors explaining lack of response to heel stick in preterm newborns. J Obstet Gynecol Neonatal Nurs. 1999;28:587–594. doi: 10.1111/j.1552-6909.1999.tb02167.x. [DOI] [PubMed] [Google Scholar]
- 39.Slater R, Cantarella A, Yoxen J, Patten D, Potts H, Meek J, Fitzgerald M. Latency to facial expression change following noxious stimulation in infants is dependent on postmenstrual age. Pain. 2009;146:177–182. doi: 10.1016/j.pain.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 40.Fabrizi L, Verriotis M, Williams G, Lee A, Meek J, Olhede S, Fitzgerald M. Encoding of mechanical nociception differs in the adult and infant brain. Sci Rep. 2016;6:28642. doi: 10.1038/srep28642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fabrizi L, Williams G, Lee A, Meek J, Slater R, Olhede S, Fitzgerald M. Cortical activity evoked by an acute painful tissue-damaging stimulus in healthy adult volunteers. J Neurophysiol. 2013;109:2393–2403. doi: 10.1152/jn.00990.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartley C, Poorun R, Goksan S, Worley A, Boyd S, Rogers R, Ali T, Slater R. Noxious stimulation in children receiving general anaesthesia evokes an increase in delta frequency brain activity. Pain. 2014;155:2368–2376. doi: 10.1016/j.pain.2014.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Worley A, Fabrizi L, Boyd S, Slater R. Multi-modal pain measurements in infants. J Neurosci Methods. 2012;205:252–257. doi: 10.1016/j.jneumeth.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clin J Pain. 1996;12:13–22. doi: 10.1097/00002508-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Stevens BJ, Gibbins S, Yamada J, Dionne K, Lee G, Johnston C, Taddio A. The Premature Infant Pain Profile-Revised (PIPP-R) Initial Validation and Feasibility. Clin J Pain. 2014;30:238–243. doi: 10.1097/AJP.0b013e3182906aed. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Template of nociceptive brain activity in infants
MATLAB code to project the template onto new EEG data