Abstract
Objectives
We set out to examine the prevalence and persistence of mutations conferring high-level nonnucleoside reverse transcriptase (NNRTI)-resistance in a cohort of HIV-infected children who had failed prophylaxis to prevent mother-to-child-transmission (PMTCT).
Design
A prospective observational cohort study at the Pediatric HIV Clinic at Kalafong Provincial Tertiary Hospital in Pretoria, South Africa.
Methods
Children referred for initiation of antiretroviral therapy (ART) were enrolled from July 2010 through February 2013. HIV drug resistance testing was performed using the oligonucleotide ligation assay (OLA) on dried blood spots (DBS) collected at enrolment and monthly follow-up visits for 2 years.
Results
South African children who failed HIV-prophylaxis had a high prevalence of NNRTI-resistant HIV (46/88; 52%). Among children with NNRTI-resistance, the frequency of the predominant resistant variant in each child’s HIV-quasispecies was high (median 96%) at study entry (median age 7.5 months), and in 26 out of 27 followed a median of 13 months persisted at a high frequency (median 89%).
Conclusion
Our finding that infants who fail HIV-prophylaxis frequently have long-lived NNRTI-resistant HIV suggests that resistance will likely persist through 36 months of age, when children qualify for NNRTI-based ART. These children may benefit from HIV drug resistance testing to guide selection of their treatment.
Keywords: children, HIV, HIV drug resistance, infants, nevirapine, non-nucleoside reverse transcriptase inhibitors, oligonucleotide ligation assay, transmitted resistance
Introduction
Antiretrovirals are used to prevent mother-to-child-transmission (PMTCT) of HIV-1. In 2000, the WHO recommended a single dose of nevirapine (NVP) be administered to HIV-infected women in labour and their newborns within 72 h of birth [1], as a simple inexpensive strategy for PMTCT. Single-dose NVP has been associated with transmission and selection of nonnucleoside reverse transcriptase inhibitor (NNRTI)-resistant HIV [2–4], which can lead to treatment failure when young infants are given NVP-based antiretroviral therapy (ART) [5,6]. Although decay of NVP-resistant HIV from infants’ plasma following single-dose NVP has been described [2], other studies have found that NNRTI-resistance can persist within infected infants [3,7]. The current 2015 WHO PMTCT Guidelines recommend life-long maternal ART throughout gestation and nursing, as well as infant NVP-prophylaxis for the first 6 weeks of breastfeeding [8]. HIV-infected infants who have taken 6 weeks of NVP-prophylaxis appear to have delayed decay of NNRTI-resistant HIV compared with single-dose NVP; however, this has been evaluated in only small cohorts (median n =10 individuals, range: 10–13) followed until 6–12 months of age [9–11].
Despite scale-up of ART in resource-limited settings, children have relatively few ART options. On the basis of clinical trials [6], protease inhibitor based ART (PI-ART) is recommended for HIV-infected children less than 3 years of age and NNRTI-based ART for older children. PI-ART is more costly, interacts with tuberculosis treatment, has long-term toxicities and suspensions are unpalatable. Virologic failure during PI-ART often selects lamivudine (3TC) resistance, which is part of nearly all NNRTI-ART regimens, and this resistance may contribute to the lower efficacy of NNRTI-ART following PI-ART. A better understanding is needed of the decay versus persistence of NNRTI-resistance; and if persistent NVP-resistance is cross-resistant to efavirenz (EFV)-based ART and diminishes suppression of HIV-replication in children at least 3 years of age. To address the former knowledge gap, we examined the prevalence of mutations conferring high-level NNRTI-resistance in a cohort of HIV-infected children who had failed MTCT-prophylaxis, and the persistence of these mutations over time. We hypothesized that the persistence of NNRTI-resistant HIV would correlate with a greater duration of NVP-prophylaxis.
Materials and methods
A prospective observational cohort study at the Pediatric HIV Clinic at Kalafong Provincial Tertiary Hospital in Pretoria, South Africa, enrolled children aged 0–5 years of age, referred for initiation of ART from July 2010 through February 2013. Children who had not received PMTCT prophylaxis, were severely ill or at a high risk of mortality and would not be available for prospective monthly follow-up were not eligible to participate in the study. The duration of NVP administration to infants varied due to changes in South Africa’s clinical guidelines for PMTCT in 2010. These guidelines recommended 6 weeks of daily NVP be given to infants born to HIV-infected mothers on lifelong ART, or if mothers were not taking ART, for the duration of breastfeeding. The study was approved by the Human Subjects Boards at Seattle Children’s Hospital and the University of Pretoria, with the consent of a guardian required for enrolment.
Whole blood was collected at enrolment and monthly for 2 years. Five-hundred microlitres of the specimen was divided across four sample-areas on Whatman FTA cards (GE Healthcare Life Sciences, Chicago, IL, USA) that contain a proprietary chemical-coated matrix that lyses cells and denatures proteins on contact, allowing DNA to adhere to the card. The remaining blood was separated into plasma and peripheral blood mononuclear cells (PBMCs). Plasma HIV RNA was quantified (Nuclisens EasyQ HIV-1 VL Assay v2.0; bioMéRIEUX, SA, Marcy-l’Etoile, France) according to the manufacturer’s instructions. Amplifiable HIV DNA templates within one 3-mm punch of a dried blood spot (DBS) were quantified by real-time PCR of HIV gag [3], and then at least 150 viral templates were amplified from one or more punches by nested HIV pol PCR [3], with amplicons from each specimen combined and tested for HIV-drug-resistance using an oligonucleotide ligation assay (OLA) for codons K103N, V106M, Y181C and G190A, optimized for HIV subtype C, the predominant subtype in South Africa [3]. A standard curve of 0, 2, 5, 10, 25, 50, 75, 100% mutant in each OLA plate allowed quantification of HIV-resistant subpopulations at least 2%. Clinical data, including infant feeding (breast or formula), were collected from each mother’s and child’s medical record.
Children with and without mutations at enrolment were compared by exposure groups (single-dose NVP versus extended NVP-prophylaxis) using the Fisher’s exact test, and by age using the Mann–Whitney test. A paired-sample t-test was used to compare the frequency of mutants at enrolment and last visit in the subpopulation analysis of the cohort.
Results
A total of 103 (87%) of 118 children referred for initiation of ART were enrolled into the study based on inclusion/ exclusion criteria, study nurse availability and refusal of three families due to collection of extra blood specimens. Eighty-eight HIV-infected children with a documented history of NVP-prophylaxis and with a DBS collected at least 1 time-point were included in this analysis. Their median age was 7.5 months [interquartile range (IQR): 4–15]. Their HIV-prophylaxis included single-dose NVP at birth in 40 (45%) and extended NVP-prophylaxis (median 6 weeks; IQR: 4–9) in 48 (55%) infants. During the study, 73 out of 88 (83%) infants initiated ART with lopinavir/ritonavir (LPV/r) along with abacavir (ABC) and 3TC; five out of 88 (6%) initiated NVP-ART or EFV-ART with 3TC and ABC or zidovudine (ZDV), and 10 out of 88 (11%) did not initiate ART.
At enrolment, NNRTI-associated mutations were detected by OLA in 46 out of 88 [52%; 95% confidence interval (95% CI), 42–64], with Y181C detected in 33%, K103N in 23%, G190A in 13% and V106M in 10%. A single mutant codon was detected in 27 out of 88 (31%) and at least two mutants in 19 out of 88 (22%). Children with mutant viruses were younger than those with exclusively wild-type virus (median 4.5 months of age, IQR: 2–8.5 versus 13.5 months, IQR: 7–24; P <0.0001). NNRTI-resistance was detected more frequently among those who took NVP-prophylaxis for a longer duration (17/40; 43% receiving single-dose NVP versus 29/48; 60% receiving extended NVP-prophylaxis), although this difference did not reach statistical significance (P =0.1334).
Among children with NNRTI-resistance at enrolment, the codon with the highest proportion of resistance represented a median of 96% (IQR: 82–100%) of the child’s HIV-quasispecies. Detection of low frequencies of NNRTI resistance (2–25%) within a child’s HIV-quasispecies was uncommon (8/88; 9%).
During the study, nearly half of children were lost-to-follow-up (LFTU) prior to study month 9; this included 17 out of 88 (20%) who participated only in the enrolment visit and 22 out of 88 (25%) who exited within 8 months of enrolment. A total of 49 out of 88 (56%) children participated in the study for at least 9 months. Follow-up in the latter group extended for a median of 13 months (IQR: 12–16) until children reached a median of 21 months of age (IQR: 17–32). This longitudinally followed subpopulation of 49 children was mostly breastfed [12/18 (66%) in the single-dose NVP group and 29 out of 31 (94%) in the extended-NVP group], and all were treated with LPV/r-ART, except one child (Child 49) who was given EFV-based ART. At enrolment, the age of the longitudinally followed subpopulation (median 8 months; IQR: 3–16), prevalence of NNRTI-resistant HIV (27/49; 55%) and mutant frequency did not differ from the greater study population, including those children LFTU. Among the 27 children with resistance in this subpopulation, NNRTI-resistant HIV persisted over the entire period in 26 out of 27 (96%). The single child with loss of resistance over time (Child 14) had received single-dose NVP; he was 26 months of age at enrolment when V106M was detected at a frequency of 3%, when LPV/r-ART was initiated. The frequency of the predominate NNRTI-resistant variant in each child’s quasispecies did not decay significantly during follow-up (medians and IQR: 98%; 84–100% versus 89%; 76–100%, P =0.2585, at enrolment and last visit, respectively).
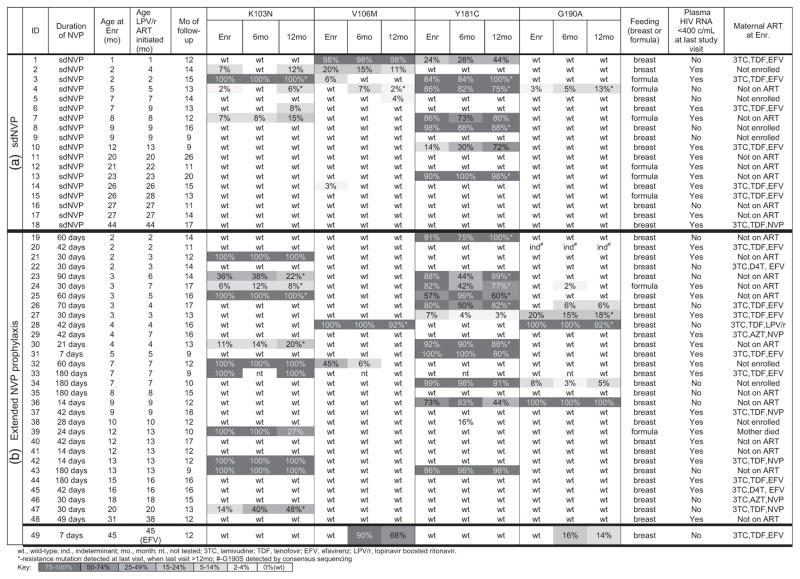
Of note, during the study, NNRTI-resistance was selected in only one child (Child 49). At enrolment, only wild-type virus was found, but 6 weeks after initiating EFV-ART, both V106M and G190A were detected (Fig. 1).
Fig. 1. HIV-nevirapine resistance genotypes and clinical parameters of 49 infants followed longitudinally.
Cohort (n =88) was composed of Pretorian infants and young children who failed NVP-prophylaxis for prevention of mother-to-children-transmission of HIV (PMTCT). NVP-resistance (K103N, V106M, Y181C and G190A) was evaluated by an oligonucleotide ligation assay (OLA) at enrolment (Enr), and ~6 (±3) months and ~12 (±3) months after enrolment. Study participants (n =49) followed for ≥9 months are shown grouped by duration of NVP exposure: (a) single-dose (sd) NVP (n =18) and (b) extended NVP prophylaxis (n =31). Within each group, participants are listed by increasing age at enrolment. These participants were followed from enrolment when their median age was 8 months (IQR: 3–16) for 13 (IQR: 12–16) months to a median age of 21 (IQR: 17–32) months. The median frequency of the predominate mutant variant within each individual’s HIV quasispecies at enrolment and last visit of follow-up was 98% (IQR: 84–100%) versus 89% (IQR: 76–100%), respectively (P =0.2585). Data on infants’ feeding, infants’ antiretroviral therapy (ART) regimen, and infant’s plasma HIV RNA (<400 copies/ml) (Nuclisens EasyQ HIV-1 VL Assay v2.0; bioMéRIEUX, SA, Marcy-l’Etoile, France) at last visit, as well as maternal ART are shown. Note all children received lopinavir/rt-based ART, except for Child-49, who received an efavirenz-based regimen; he was the only child who at enrolment had wild-type HIV and during ART selected HIV drug resistant variants.
Discussion
This study of ART-naive HIV-infected children who failed NVP-prophylaxis has three novel observations: The prevalence of NNRTI mutations was relatively high (>50%) among older infants/young children; NNRTI mutations predominated in these children’s HIV quasispecies; and NNRTI mutations persisted without significant decay across the cohort or within individual’s quasispecies, including those at least 3 years of age. Taken together, these findings suggest that infants who fail WHO-recommended MTCT prophylaxis have a high risk of NNRTI-resistant variants establishing long-lived viral reservoirs.
Our observation that NNRTI-resistant HIV prior to ART was more prevalent in younger children could be interpreted as suggesting that resistance decays as children age, as observed in previous studies [2,3, 9–11]. However, we rarely detected decay within a child’s quasispecies in this cohort. We suspect that the higher rate in younger children and the persistence of NNRTI-resistant HIV is due to the convergence of the following recent trends: a longer duration of NVP prophylaxis in more recently born infants; scale-up of PMTCT programmes, resulting in a greater proportion of infected infants receiving early diagnosis; and increasing rates of transmitted drug resistance to women of childbearing age [12].
The prevalence of NVP-associated mutations in our cohort is within the range of previous studies involving children who had been exposed to single-dose or extended-NVP prophylaxis, 27% [13] to 60% [3,14,15]. However, previous studies of single-dose NVP involved younger children and demonstrated that resistance decayed to clinically insignificant levels in most infants by 12 months [2,3,9–11]. In sharp contrast to the younger cohorts [9–11], decay of resistance was exceedingly rare in our cohort, suggesting a difference in HIV dynamics.
The persistence of NNRTI-resistant HIV DNA in the absence of selective drug pressure suggests that NNRTI-resistant HIV is a part of the long-lived viral reservoir thought to be established during acute infection, either through selection of randomly generated mutants by NVP [3] or as a result of maternal transmission of NNRTI-resistant HIV, which occurs more frequently during breastfeeding [4]. Among the 26 children with persistence of NNRTI-resistance over a median of 13 months of study follow-up, ongoing selection of NNRTI-resistant variants may have occurred in only five infants (19.2%; Children 1, 8, 26, 34 and 47) due to virus replication while breastfeeding from a mother receiving NNRTI-based ART (n =3; 11.5%) or from a mother with an unknown antiretroviral status (n =2; 7.7%). In the remaining 21 (80.7%) children, selection of NNRTI-resistance is not a likely mechanism of persistence, as their plasma HIV RNA was less than 400 copies/ml (n =12), or their mothers were receiving LPV/r-based ART (n =1) or untreated (n =8) (Fig. 1).
Persistence of NNRTI-resistant HIV has been described previously in smaller study populations [2,3,7,9–11]. Of concern is that high concentrations of archived NNRTI-resistance could result in treatment failure of the NNRTI-based ART, even at 3 or more years of age, when the WHO guidelines assume that NNRTI-resistant HIV has decayed. We contend that the stable frequency of NNRTI-resistant variants we observed in young children suggests that viral decay dynamics differ from past observations in infants who took single-dose NVP, and that NNRTI-resistance in children with longer periods of selection by NVP or with transmitted drug resistance may last for years or indefinitely, as observed in adults [16].
Limitations of our study include that maternal history was not available for all participants, limiting our assessment of ongoing selection for NNRTI resistance during breast-feeding of a few infants; because mothers and infants were not enrolled and followed prospectively from prior to birth, we could not determine the onset of HIV drug resistance and whether it appeared to have been transmitted to the infant or selected by NVP prophylaxis; and analyses of DBS by the OLA may have affected our estimates of the frequency of NNRTI-resistant DNA in a child’s quasispecies. Specifically, the occasional variation in mutant frequency in our longitudinal assessment of DBS may have been due to changes in the number of viral templates amplified from the DBS. Given validation of OLA quantification of mutant by ‘next-generation-sequencing’ [17], the variation in NNRTI-resistant DNA may alternatively been due to the turnover of HIV-infected and uninfected PBMC populations. Furthermore, we may have underestimated resistance due to primer and/or probe sequence biases affecting our assays on DBS.
In summary, NNRTI-resistant HIV was prevalent among older infants and young children who failed MTCT-prophylaxis and this resistance persisted at stable high levels without evidence of decay in nearly all children. If these data are confirmed in studies of children at least 3 years of age, the high prevalence of NNRTI-resistance would warrant testing for HIV drug resistance and evaluation of cross-resistance prior to prescription of NNRTI-ART regimens, or introduction of antiretroviral regimens for infant prophylaxis and/or therapy that has a higher genetic barrier to resistance, as observed with the integrase inhibitor dolutegravir [18].
Acknowledgments
We thank all children and their families for participation in this study, as well as the staff in the Kalafong Provincial Tertiary Hospital’s HIV Clinic. This work was supported by a grant from the Delegation of the European Union to South Africa: Drug Resistance Surveillance and Treatment Monitoring Network for the Public Sector HIV Antiretroviral Treatment Programme in the Free State-Sante 2007/147-790, National Research Council of South Africa, Unlocking the Future, 61509; NIH R01 AI058723; Clinical Research and Retrovirology Core of the Seattle Centers for AIDS Research (NIH P30 AI027757), NIH T32 HD007233-32, Seattle Children’s Research Institute Strategic Funds; NIH funding of the International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) Virology Developmental Laboratory award (U01 AI068632). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of interest
The authors do not have any conflicts of interest.
T.M.R., S.C., L.M.F. conceived and designed experiments. T.R. and U.D.F. provided patient samples and clinical data. R.K., S.O. performed experiments. R.K. analysed data and wrote first draft. All authors contributed to critical revision of the manuscript.
References
- 1.Dabis F, Sint TT, de Zoysa I. Consultative Meeting on the use of nevirapine for the prevention of mother-to-child transmission of HIV among women of unknown serostatus (2001: Geneva, Switzerland) prevention of mother-to-child transmission of HIV: use of nevirapine among women of unknown serostatus: report of a technical consultation. Geneva: Dec 5–6, 2001. [Google Scholar]
- 2.Eshleman SH, Mracna M, Guay LA, Deseyve M, Cunningham S, Mirochnick M, et al. Selection and fading of resistance mutations in women and infants receiving nevirapine to prevent HIV-1 vertical transmission (HIVNET 012) AIDS. 2001;15:1951–1957. doi: 10.1097/00002030-200110190-00006. [DOI] [PubMed] [Google Scholar]
- 3.Micek MA, Blanco AJ, Beck IA, Dross S, Matunha L, Montoya P, et al. Nevirapine resistance by timing of HIV type 1 infection in infants treated with single-dose nevirapine. Clin Infect Dis. 2010;50:1405–1414. doi: 10.1086/652151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micek MA, Dross S, Blanco AJ, Beck IA, Matunha L, Seidel K, et al. Transmission of nevirapine-resistant HIV type 1 via breast milk to infants after single-dose nevirapine in Beira, Mozambique. J Infect Dis. 2014;210:641–645. doi: 10.1093/infdis/jiu130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lockman S, Shapiro RL, Smeaton LM, Wester C, Thior I, Stevens L, et al. Response to antiretroviral therapy after a single, peripartum dose of nevirapine. N Engl J Med. 2007;356:135–147. doi: 10.1056/NEJMoa062876. [DOI] [PubMed] [Google Scholar]
- 6.Palumbo P, Lindsey JC, Hughes MD, Cotton MF, Bobat R, Meyers T, et al. Antiretroviral treatment for children with peripartum nevirapine exposure. N Engl J Med. 2010;363:1510–1520. doi: 10.1056/NEJMoa1000931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Persaud D, Palumbo P, Ziemniak C, Chen J, Ray SC, Hughes M, et al. Early archiving and predominance of nonnucleoside reverse transcriptase inhibitor-resistant HIV-1 among recently infected infants born in the United States. J Infect Dis. 2007;195:1402–1410. doi: 10.1086/513871. [DOI] [PubMed] [Google Scholar]
- 8.The World Health Organization. Guideline on when to start antiretroviral therapy and on preexposure prophylaxis for HIV. Geneva, Switzerland: WHO Press; 2015. [PubMed] [Google Scholar]
- 9.Persaud D, Bedri A, Ziemniak C, Moorthy A, Gudetta B, Abashawl A, et al. Slower clearance of nevirapine resistant virus in infants failing extended nevirapine prophylaxis for prevention of mother-to-child HIV transmission. AIDS Res Hum Retro-viruses. 2011;27:823–829. doi: 10.1089/aid.2010.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moorthy A, Gupta A, Bhosale R, Tripathy S, Sastry J, Kulkarni S, et al. Nevirapine resistance and breast-milk HIV transmission: effects of single and extended-dose nevirapine prophylaxis in subtype C HIV-infected infants. PLoS One. 2009;4:e4096. doi: 10.1371/journal.pone.0004096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Church JD, Omer SB, Guay LA, Huang W, Lidstrom J, Musoke P, et al. Analysis of nevirapine (NVP) resistance in Ugandan infants who were HIV infected despite receiving single-Dose (SD) NVP versus SD NVP plus daily NVP up to 6 weeks of age to prevent HIV vertical transmission. J Infect Dis. 2008;198:1075–1082. doi: 10.1086/591503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silverman R, Frenkel L, Yatich N, Beck I, Kiptiness C, Njoroge J, et al. Higher risk of pre-treatment HIV drug resistance among younger ART-naïve adults in Kenya. 2015 International HIV Drug Resistance Workshop; Seattle, WA. 2015. [Google Scholar]
- 13.Hunt GM, Coovadia A, Abrams EJ, Sherman G, Meyers T, Morris L, et al. HIV-1 drug resistance at antiretroviral treatment initiation in children previously exposed to single-dose nevirapine. AIDS. 2011;25:1461–1469. doi: 10.1097/QAD.0b013e3283492180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson JA, Fokar A, Hudgens MG, Compliment KJ, Hawkins JT, Tegha G, et al. Frequent nevirapine resistance in infants infected by HIV-1 via breastfeeding while on nevirapine prophylaxis. AIDS. 2015;29:2131–2138. doi: 10.1097/QAD.0000000000000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salou M, Butel C, Konou AA, Ekouevi DK, Vidal N, Dossim S, et al. High rates of drug resistance among newly diagnosed HIV-infected children in the National Prevention of Mother-To-Child Transmission Program in Togo. Pediatr Infect Dis J. 2016;35:879–885. doi: 10.1097/INF.0000000000001203. [DOI] [PubMed] [Google Scholar]
- 16.Ghosn J, Pellegrin I, Goujard C, Deveau C, Viard JP, Galimand J, et al. HIV-1 resistant strains acquired at the time of primary infection massively fuel the cellular reservoir and persist for lengthy periods of time. AIDS. 2006;20:159–170. doi: 10.1097/01.aids.0000199820.47703.a0. [DOI] [PubMed] [Google Scholar]
- 17.Beck IA, Deng W, Payant R, Hall R, Bumgarner RE, Mullins JI, et al. Validation of an oligonucleotide ligation assay for quantification of human immunodeficiency virus type 1 drug-resistant mutants by use of massively parallel sequencing. J Clin Microbiol. 2014;52:2320–2327. doi: 10.1128/JCM.00306-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner BG, Wainberg MA. Clinical benefit of dolutegravir in HIV-1 management related to the high genetic barrier to drug resistance. Virus Res. 2016 doi: 10.1016/j.virusres.2016.07.006. Epub ahead of print. [DOI] [PubMed] [Google Scholar]